Abstract
Fritillaria naturally grows in the temperate region of Northern Hemisphere and mainly distributes in Central Asia, Mediterranean region, and North America. The dried bulbs from a dozen species of this genus have been usually used as herbal medicine, named Beimu in China. Beimu had rich sources of phytochemicals and have extensively applied to respiratory diseases including coronavirus disease (COVID-19). Fritillaria species have alkaloids that act as the main active components that contribute multiple biological activities, including anti-tussive, expectorant, and anti-asthmatic effects, especially against certain respiratory diseases. Other compounds (terpenoids, steroidal saponins, and phenylpropanoids) have also been identified in species of Fritillaria. In this review, readers will discover a brief summary of traditional uses and a comprehensive description of the chemical profiles, biological properties, and analytical techniques used for quality control. In general, the detailed summary reveals 293 specialized metabolites that have been isolated and analyzed in Fritillaria species. This review may provide a scientific basis for the chemical ecology and metabolomics in which compound identification of certain species remains a limiting step.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13020-021-00450-1.
Keywords: Fritillaria, Alkaloids, Chemical components, Ethnopharmacology, Respiratory disease
Background
The global climate change increased the spread of respiratory diseases, resulting in a health-care burden to the human beings, especially in some developing countries. Respiratory diseases contain acute respiratory distress syndrome, chronic obstructive pulmonary disease (COPD), respiratory infections, etc. [1, 2]. More than 545 million individuals live with a chronic respiratory condition, which is the third leading cause of death following cardiovascular diseases and neoplasms; meanwhile, chronic diseases affected 7.4% of the world’s population in 2017 [3]. Coronavirus disease 19 (COVID-19) pandemic is a brachychronic respiratory distress syndrome with cough and fever symptoms, and it has caused more than 114 million confirmed cases and 2,534,520 confirmed deaths as of March 2021 [4]. Traditional Chinese medicine has been playing positive role for the cure and prevention of the epidemic [5, 6]; an example of such traditional medicine is Jinhua Qinggan Granule (comprising Fritillaria thunbergii, Artemisia annua, etc.) which exhibits a curative effect via attenuating cytokine storms, thus inhibiting the activity of severe acute respiratory syndrome coronavirus 2 and enhancing antiviral immunity [7, 8]. F. thunbergii is one of the main ingredients in Jinhua Qinggan Granule possessing anti-tussive and antiasthmatic properties. In addition, Fritillaria species, which are consumed as household medicines, have potential properties with rich pharmacological history.
The crude materials from Fritillaria were first showed in Shen Nong Ben Cao Jing, with efficacy of moistening dryness and clearing lung heat. Pharmacological studies transformed these traditional efficacies into scientific pharmacological activities, including anti-tussive, expectorant, anti-asthmatic effects because of the presence of alkaloids and other metabolites [9]. Alkaloids, the largest class of photochemical components of Fritillaria species and accounting for approximate 42.32% of all authenticated components, are regarded as potential agents that reduce lung injury induced in various ways [10]. Cough treatment using natural products from Fritillaria have significant advantages compared with the usual drugs, such as codeine, and display less or no side effects [11]. F. cirrhosa, F. delavayi, and F. wabuensis, and other five species, collectively named as “Chuan Bei Mu”, have evident ability to treat dry coughs without phlegm and chronic cough due to Yin deficiency [9, 12]. In addition, bulbs from Fritillaria process cold properties in terms of the theory of traditional Chinese medicine. These characteristics may be suitable for the symptoms caused by the COVID-19 virus.
Statistical analysis indicated that 1529 Chinese patent medicines contain crude materials consisting of Fritillaria species that are used as anti-tussive agents, accounting for 19.28% of all cough-related products. However, the destruction in wild resources and crude cultivation methods led to the imbalance between the supply and demand for producing these patent medicines, especially the alpine Himalayan species of Fritillaria, such as F. cirrhosa and F. delavayi. Furthermore, combined with these obstacles, the growth periods of 3–5 years resulted in high manufacturing cost, which extremely restricted the industrial application of Fritillaria species. Awareness of the export amount of herbal medicine made from Fritillaria increased from 195,700 kg in 2015 to 321,800 kg in 2019, whereas while the export value increased by 133% within 5 years. The commercial medicinal plant harvest supports 50–100% of households living at high elevations from 2700 to 3400 m [13]. Therefore, it is extremely necessary for the researchers to conduct the chemical and biological investigations, for which consider that the increasing demand for Fritillaria is leading to their over-collection in the market and decline in the wild [14].
Previous review articles focused on the chemical composition and molecular biological techniques [9], resource situation [15], breeding technology [16], and classification of Fritillaria species [17, 18]. Nevertheless, the scientific concern and broad research of the genus are lacking. A systematic and comprehensive review focusing on these contents should be conducted for in-depth studies of Fritillaria species to determine the possible correlation between their biological activities and metabolic profiles. In this report, we provide a critical summary of the detailed phytochemical and pharmacological analyses of crude extracts or authenticated components, traditional use, and botanical description of taxa belonging to Fritillaria.
Botanical characterizations and traditional use
Natural resource and botanical description
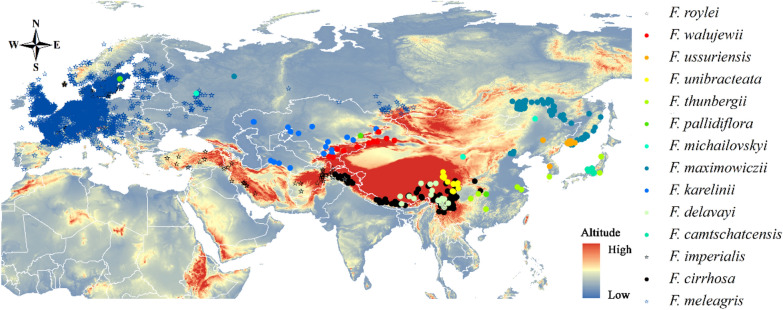
In general, there are 130 species of Fritillaria (Liliaceae), cultivated or distributed in the world as some ornamental and medicinal plants, and the crude material or extracted components exists diverse phytochemical properties. Parts of species distributed in the Northern Hemisphere, and the high-altitude species (such as F. cirrhosa and F. delavayi) alongside the Himalayan Mountain have been the most intensively exploited (Fig. 1). The extensive demand of Fritillaria species resulted in a situation that they are regarded as a Class III protected species; especially, their bulbs are registered as an active ingredient in medicinal preparations in Oceania, North America, and Asia [19]. The IUCN Red List of Threatened Species contains eighteen Fritillaria species and the document is an accessible system used for determining Fritillaria species as global extinction, because these plants are vulnerable to unsustainable collection after their bulbs have been dug out. Nine Fritillaria species are traded in Europe, which are commonly harvested from China with high volume of business transactions. F. roylei is included in the Ayurvedic Pharmacopeia, whereas the Korean Pharmacopoeia contain four species of the genus within its “Fritillariae Cirrhosae Bulbus” monograph. Meanwhile, Chinese Pharmacopeia contains ten species and one cultivation variation of Fritillaria from the genus used for different medicinal properties.
Fig. 1.
The distribution of main medicinal Fritillaria species in the Asian and Europe
The medicinal plants of Fritillaria species are generally characterized as perennial herbs with 2–3 fleshy and farinaceous (after dried) bulbs covering a translucent tunic. The stems are erect and without branches with petiolate basal and sessile cauline leaves. The arrangement of leaves is spirally alternate, opposite, or whorled, and the leaf blade is oblong or lanceolate. One or several flowers form a racemose or umbellate inflorescence, and the bracts are present after fading. The bisexual flowers are usually nodding with a campanulate or saucer-shape tessellation, with dark and light-color spots, and a nectary near the base adaxially. Six free stamens, and the anthers of stamens are basifixed, rarely dorsifixed. The three-lobed or subentire stigma is linear or extremely short. The winged or wingless capsule is erect with three locules and six angles. The seeds, which are generated from loculicidal dried fruits, are flatly arranged in two rows in each valve.
The Plant List, as an authoritative working list guided by the 2002–2010 Global Strategy for Plant Conservation [20], mentions 551 scientific plant names of Fritillaria. 156 scientific records are accepted as species names among of them, which indicates vast potential for the development of the Fritillaria. The Flora of China subdivided Fritillaria into three sections (Sect. Fritillaria, Sect. Liliorhiza, and Sect. Theresia) based on morphological traits. Most of the collected species in this review, such as F. cirrhosa, F. delavayi, and F. hupehensis, belong to Sect. Fritillaria. One species, F. maximowiczii, belongs to Sect. Liliorhiza, whereas F. karelinii belongs to Sect. Theresia. To date, several species have been successfully cultivated by herbal farmers in China, inspired by the vast demand and high profits. The main cultivated species include, F. cirrhosa, F. unibracteata, F. thunbergii, and F. ussuriensis, all of which belong to the Sect. Fritillaria. However, the whole cultivation period from seeds to commercial forms lasts for at latest four years, which seriously restricts the deep resource utilization and causes the destruction of wild Fritillaria resources, especially the original plants of Chuan Bei Mu. The plant grows one leaf and a weeny bulb within the first two years. The mature plants form fertile flowers and fruits after a five-year cultivation period. Bulbs are the confirmed the standard for medicinal purpose.
Ethnopharmacological properties
The dried bulbs of Fritillaria species (Bei Mu in Chinese) are consumed as an anti-tussive agent. The first record of the plant emerged in Wan Wu, an ancient herb book, under the name of Bei Mu, under without detailed botanical description, in the dynasty of Chunqiu Zhanguo Dynasty (BC 770–BC 221), followed by Shen Nong Ben Cao Jing between BC 221 and BC 202 [21]. The tuber of Bolbostemma paniculatum (Maxim) Frank. is used as Bei Mu and named as Tu Bei Mu. Two kinds of Bei Mu existed, namely, the crude materials Zhe Bei Mu and Tu Bei Mu with the clinical usage as Chinese medicine from AD 220 to AD 498; they were recorded in Ben Cao Jing Ji Zhu and Ming Yi Bie Lu [21]. The original plants were authenticated as species of Fritillaria in Xin Xiu Ben Cao and Ben Cao Tu Jing, which are two official herbal books. These Bei Mu materials were divided into Chuan Bei Mu and Zhe Bei Mu based on their geographical origins in Ben Cao Hui Yan in AD 1624 [21]. Ben Cao Cong Xin contains the morphological traits observed in Qing Dynasty (AD 1757), in which the base of the bulbs from Sichuan Province were flat-bottomed, whereas those produced from Zhejiang Province of China were the biggest [21]. In general, these original plants of Bei Mu were gradually defined with the development of plant taxonomy, and the conception of Bei Mu material and origin of Fritillaria species, have been formed since Ming Dynasty.
Nine Fritillaria species native to China are largely employed against several ailments in 11 national minorities, such as Tibetan, Mongolian, Miao, Lisu, Tujia, Miao, Kazakh, Uighur, Jingpo, De’ang, and Korean, with ethno-pharmacological reports from traditional practitioners. The bulbs, leaves, and seeds are equally utilized as herbal medicine in the Tibetan national minority, in which the bulbs are used for curing tracheitis and menometrorrhagia. The leaves are consumed for impetigo, and the seeds are effective remedy for the head and heat deficiency symptoms. The other 10 national minorities use the bulbs as a medicinal component given its different pharmacological properties; management of cough and phlegm is the main observation among these records of national minorities in China (Additional file 1: Table S1). In addition to China, other endemic species of Fritillaria contain similar alkaloids components, which may have similar pharmacological activities. F. michailovskyi is an endemic species in Turkey contain common steroidal alkaloids [22]. Additional file 1: Table S2 summarizes the traditional or ethnopharmacological records of the species included in this review. Coughs, asthma, and bronchitis are the main efficacies observed not only in Chinese Bei Mu but other congeneric species in other countries.
The medicinal potential of Fritillaria in the national minorities of China or other countries may present huge development capability in modern pharmacological research, especially certain high-altitude species threatened with extinction, which is caused by expanding human activities and habitat degradation. The further research of these species will provide scientific explanation for their traditional efficacy and be beneficial for the economic development of the regions.
Structure and properties of phytochemicals in Fritillaria species
Fritillaria is rich in multiple secondary metabolites, whereas the alkaloids are the main compounds isolated and identified from the crude extracts in the bulbs [12], in addition to terpenoids, steroidal saponins, and phenylpropanoids. These extracted and authenticated constituents are summarized in Additional file 1: Tables S3–S9 and the representatives are illustrated with their chemical structure.
Alkaloids
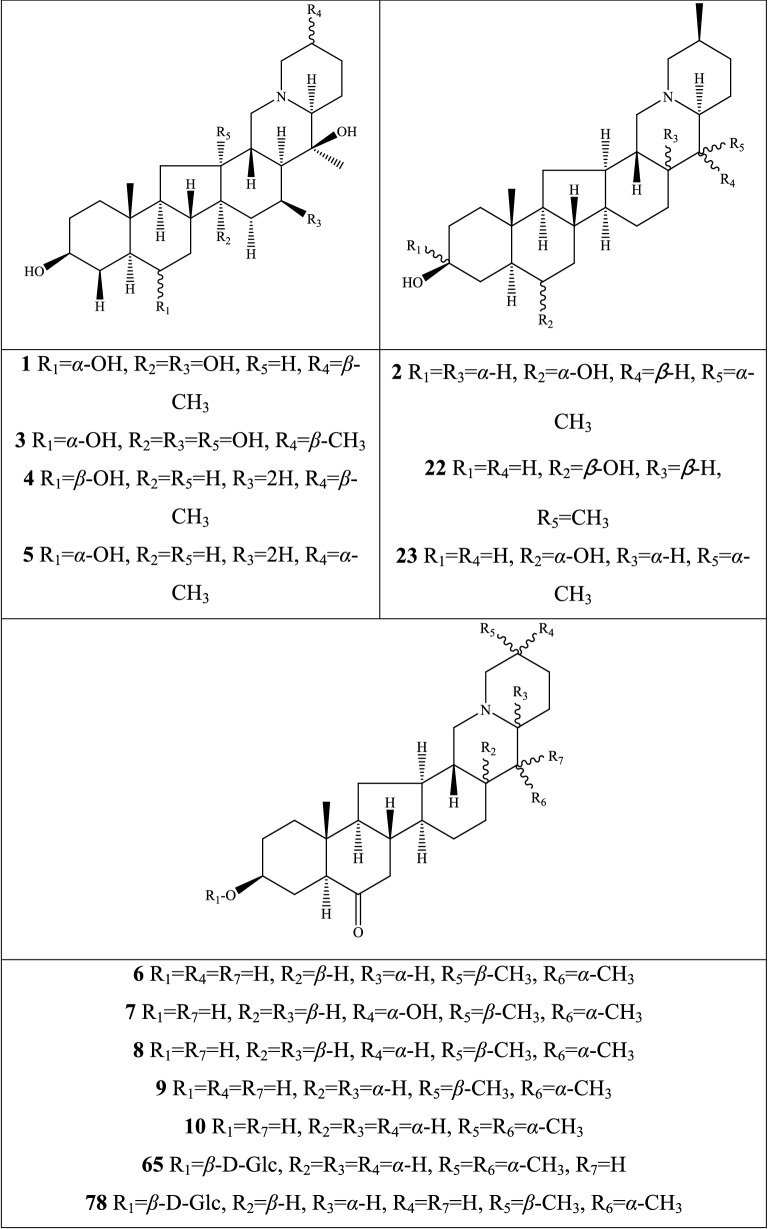
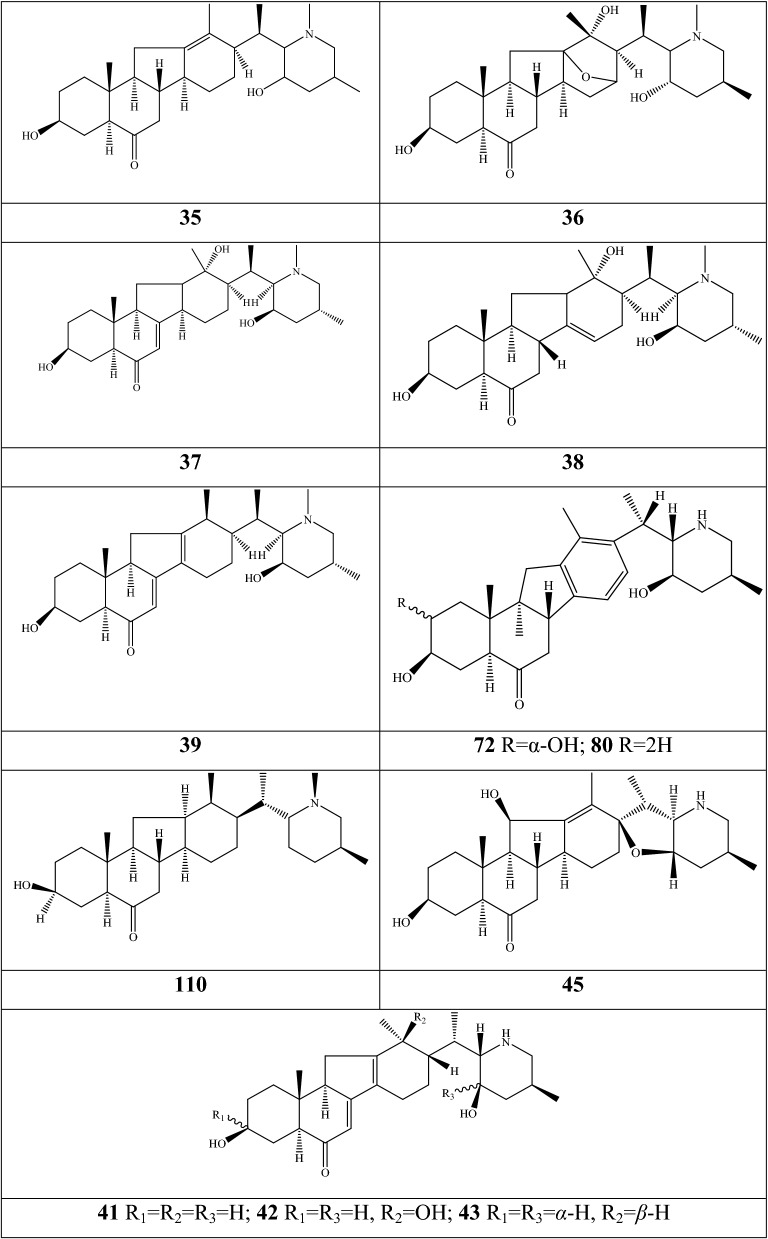
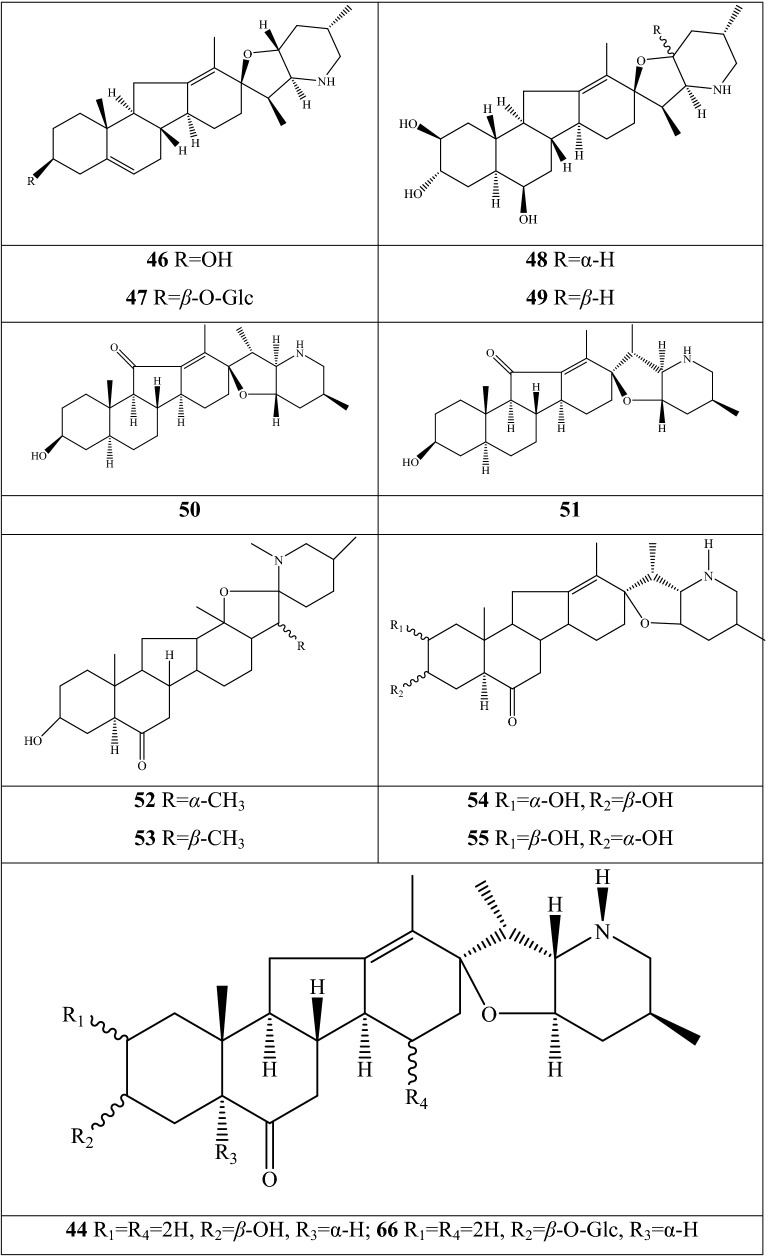
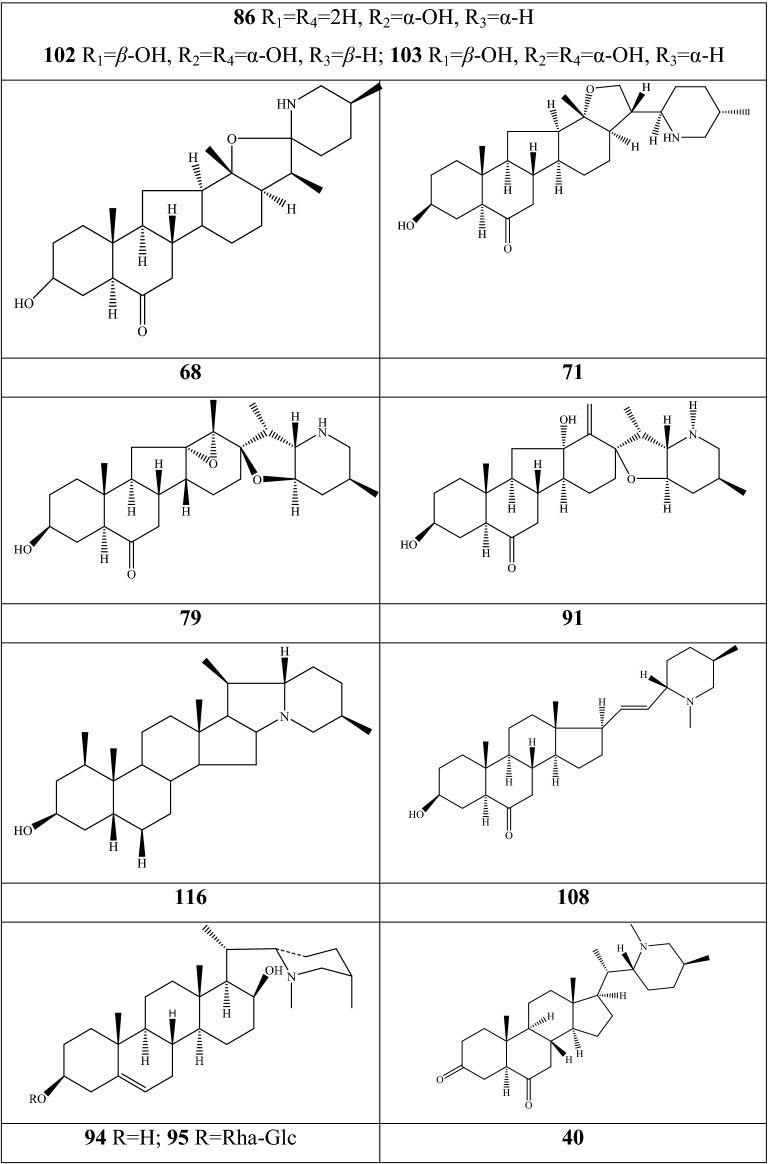
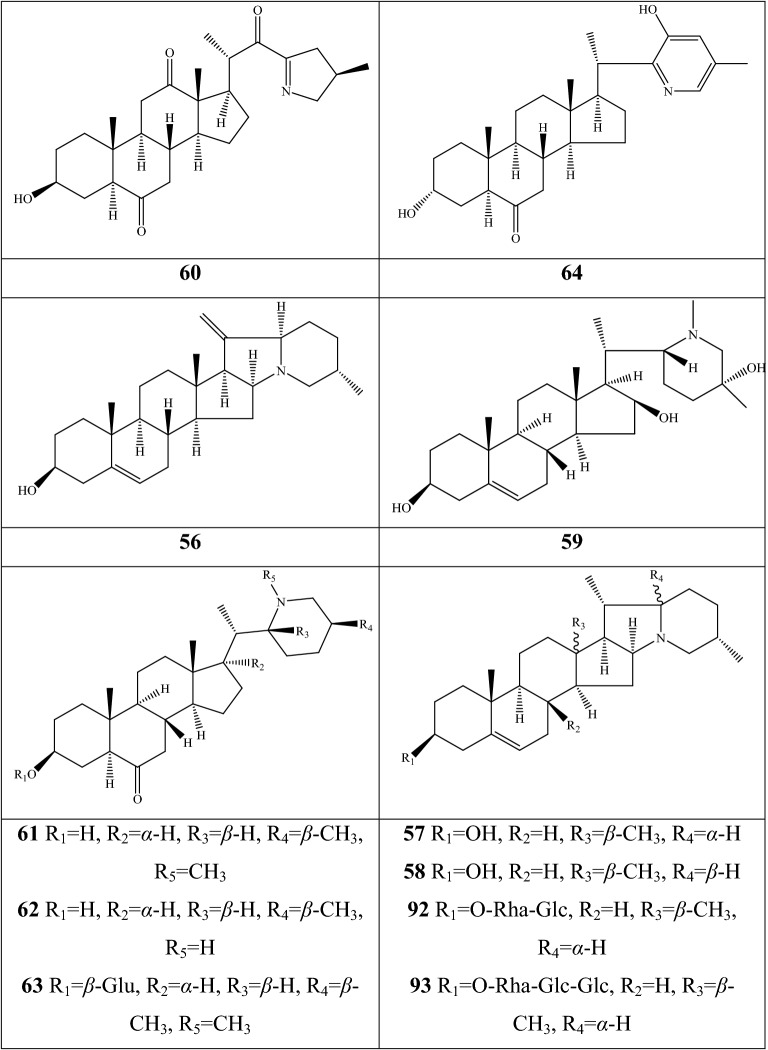
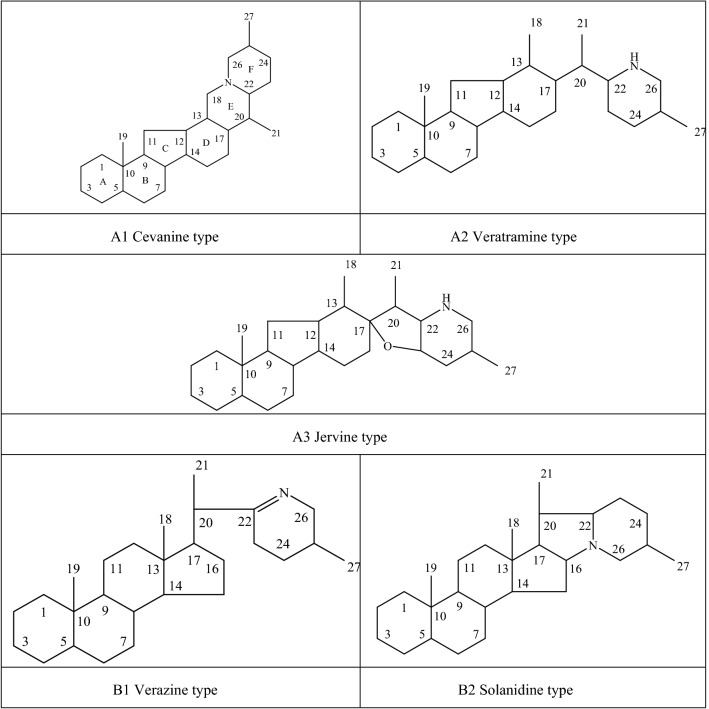
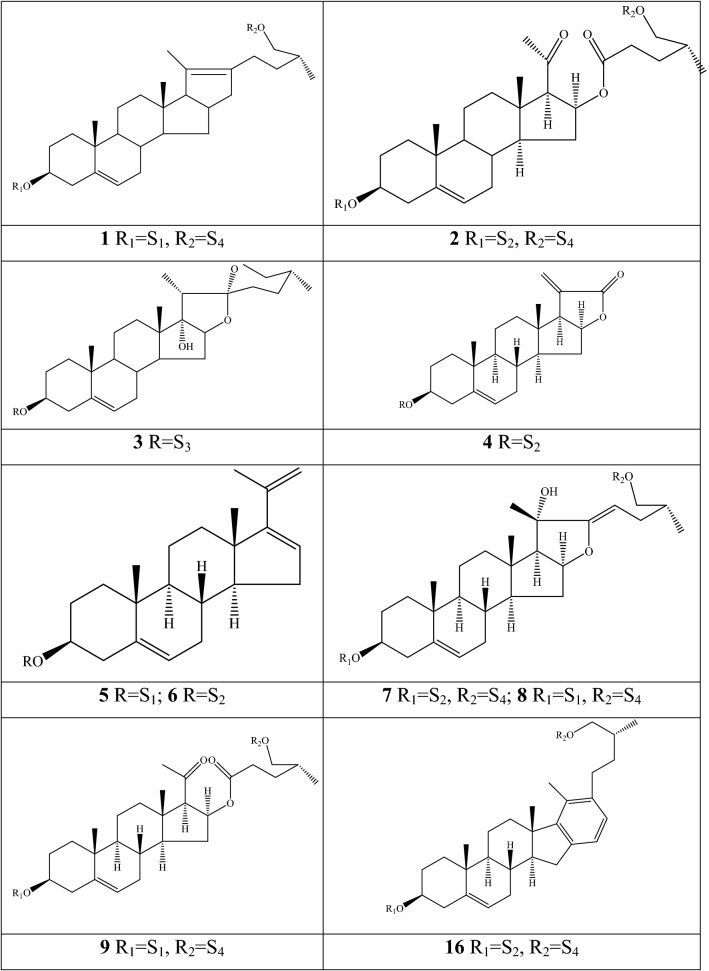
Alkaloids are organic components characterized by the basic nitrogen atoms. Based on the carbon framework, possessing a C27 cholestane carbon skeleton with carbocyclic and heterocyclic rings, the alkaloids extracted from Fritillaria species mainly consist of isosteroidal and steroidal types [12]. The isosteroidal alkaloids (Veratrum steroids), are further divided into cevanine (A1), veratramine (A2), and jervine (A3) types according to the linkage patterns between the E and F rings (Fig. 3). These alkaloids have a five-membered carbocycle. Meanwhile, Solanum steroidal alkaloids (comprise of solanidine B1 and verazine B2 type), which the nitrogen atom of the former is at the indolizidine ring, and that of the latter is in the piperidine ring (Fig. 2). Alkaloids have a six-membered carbocycle, a peculiar chemo-taxonomical significance, and are regarded as valuable markers in Liliaceae. The detailed chemical structures of 129 alkaloids (1–129) is shown in Fig. 3, and Additional file 1: Table S3 displays their occurrence in single taxa and botanical parts along with their trivial or semi-systematic names.
Fig. 3.
Structures of alkaloids in Fritillaria species
Fig. 2.
Basic skeletons of Fritillaria steroidal alkaloids
Isosteroidal alkaloids
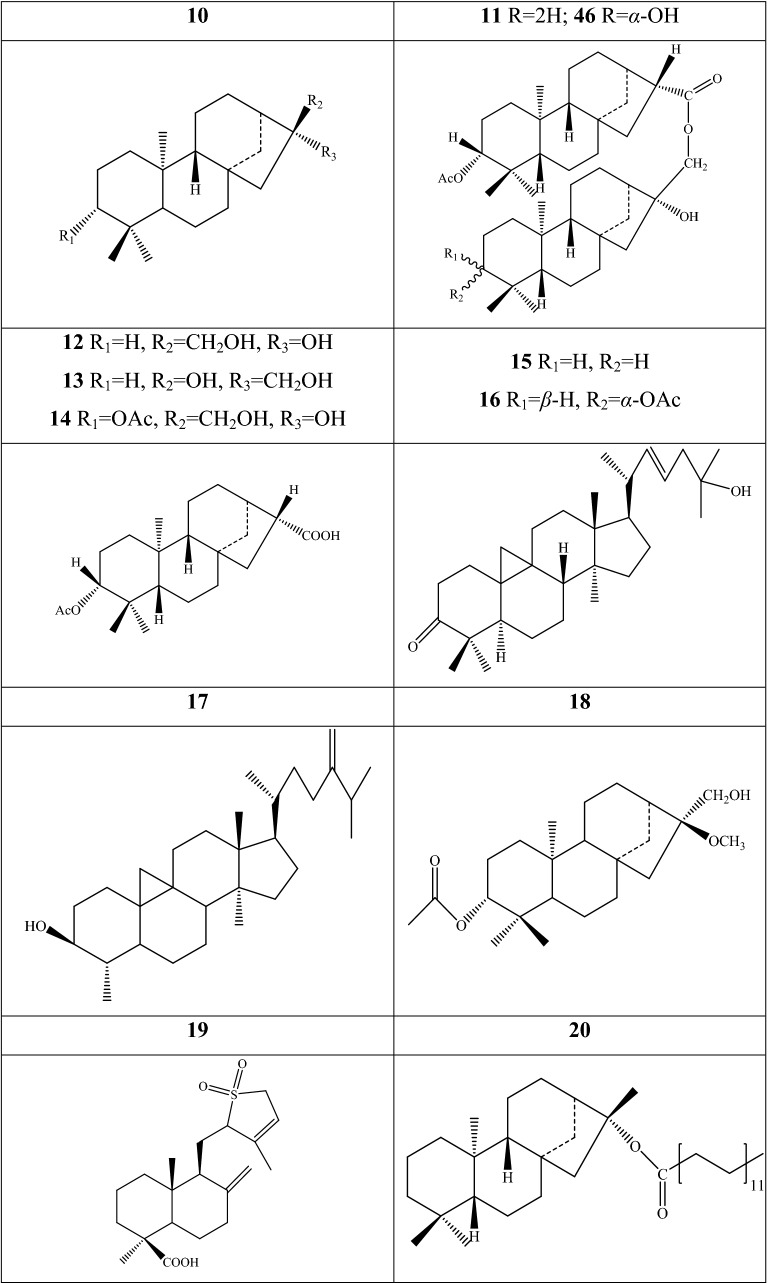
More than 100 isosteroidal alkaloids, which account for 85% of the total alkaloids, have been isolated from the complex blend of secondary metabolites in different botanical parts of Fritillaria species. The results indicate that isosteroidal alkaloids are the main phytochemical profiles in the genus. Four chemical components (peimine 2, sipeimine 11, peiminine 13, and peimisine 44) exist in more than 10 Fritillaria species, and these four constituents can be regarded as indexes of the genus. The combination of peimine and peiminine is used as an evaluation index of Zhe Bei Mu, whereas peiminine is regarded as a key component for the quality control of Ping Bei Mu and Hu Bei Bei Mu, with different content criteria indicated in the Chinese Pharmacopeia. Verticinone 13 is easily oxidized from verticine, and the synthesized cholic acid-verticinone ester showed more potent activity than codeine phosphate [23]. Sipeimine is used for the quality assessment of Chuan Bei Mu, whereas the contents of the component combined with sipeimine-3β-d-Glc is an analytical marker for Yi Bei Mu. Three of the four index components are cevanine-type isosteroidal alkaloids.
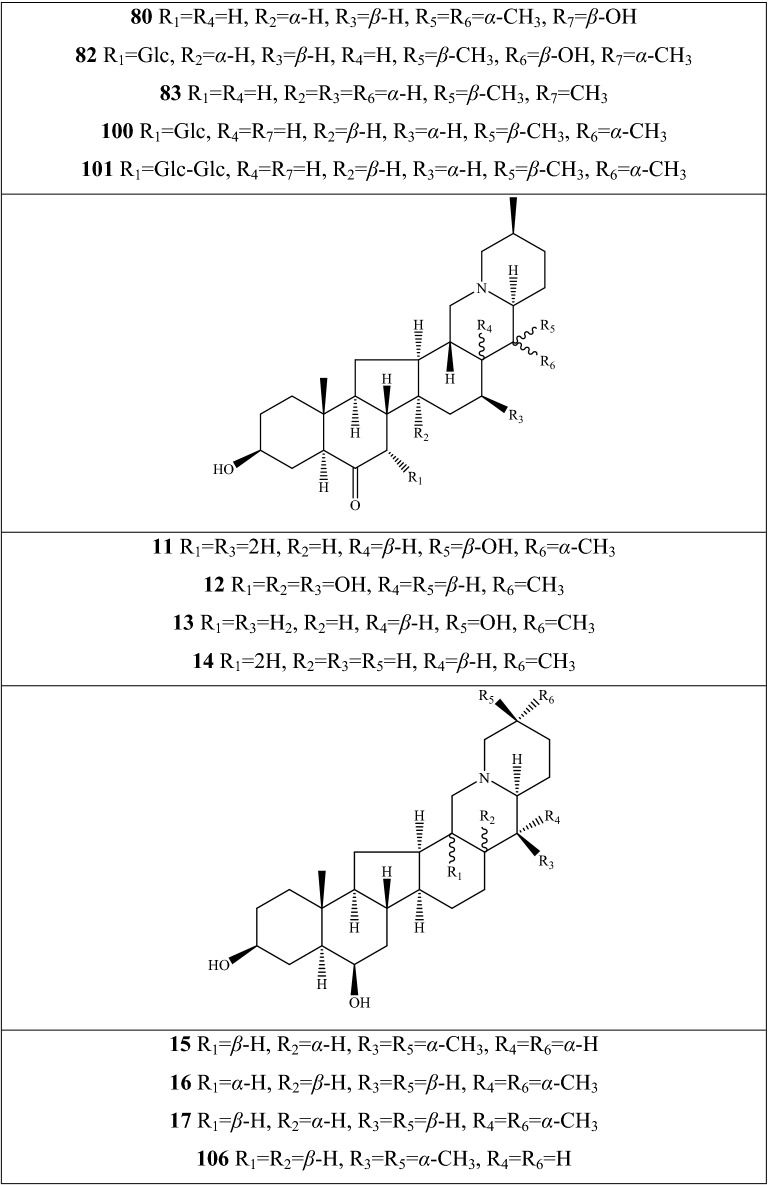
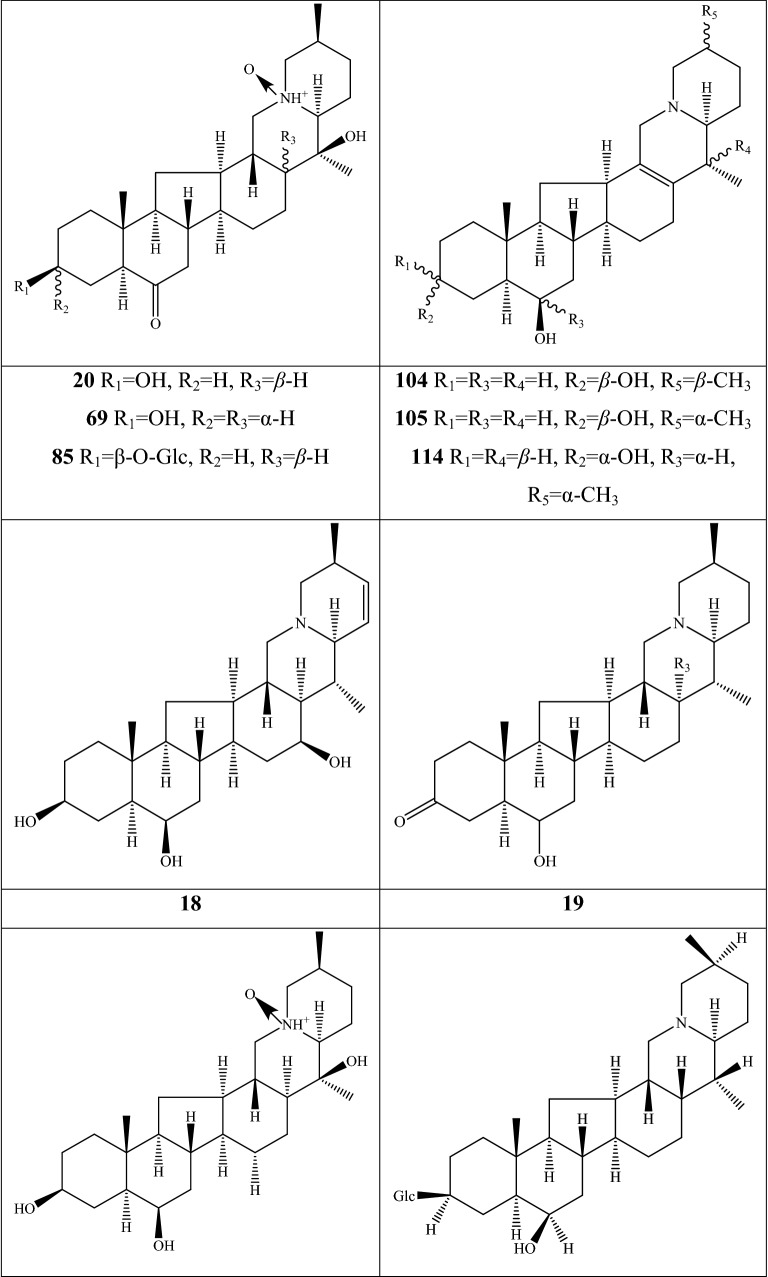
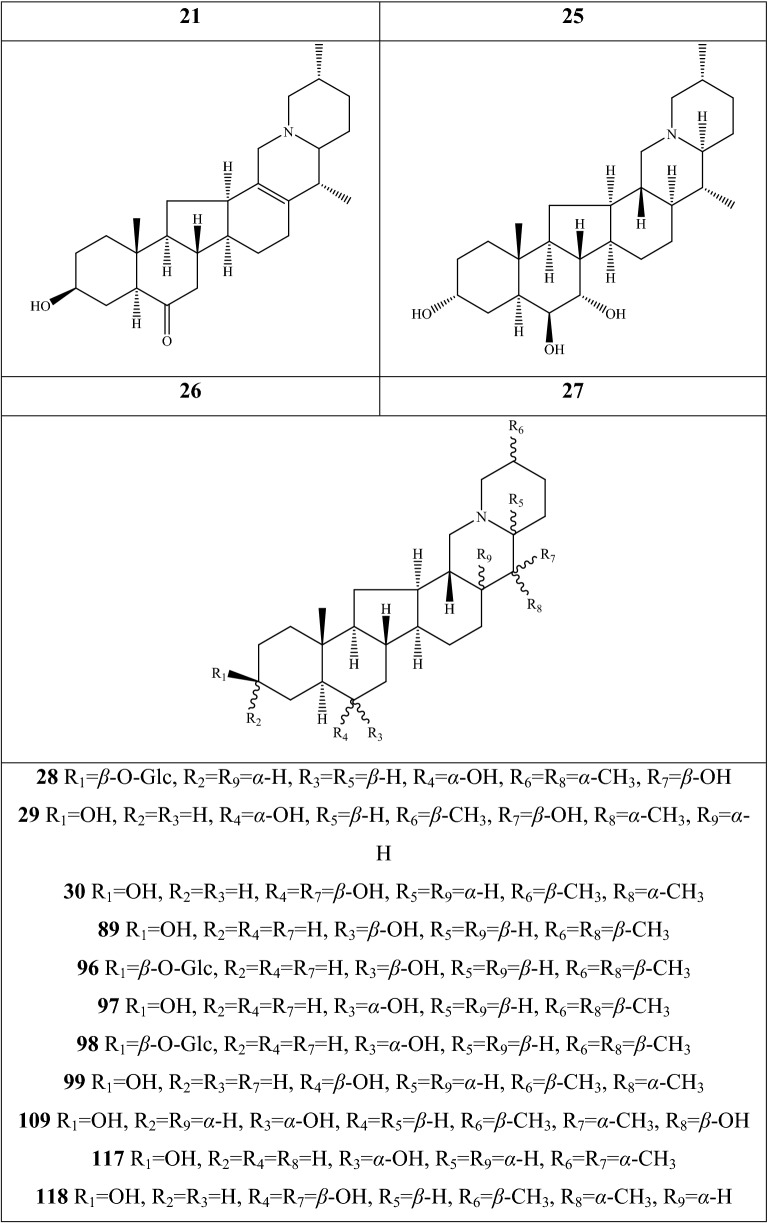
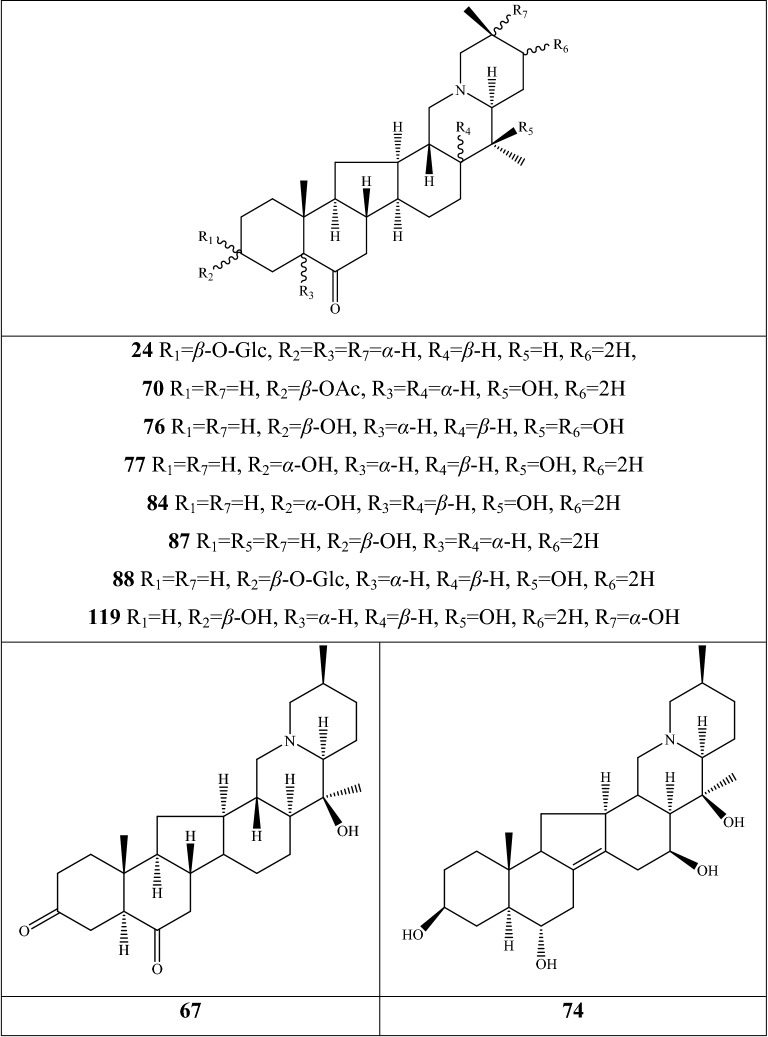
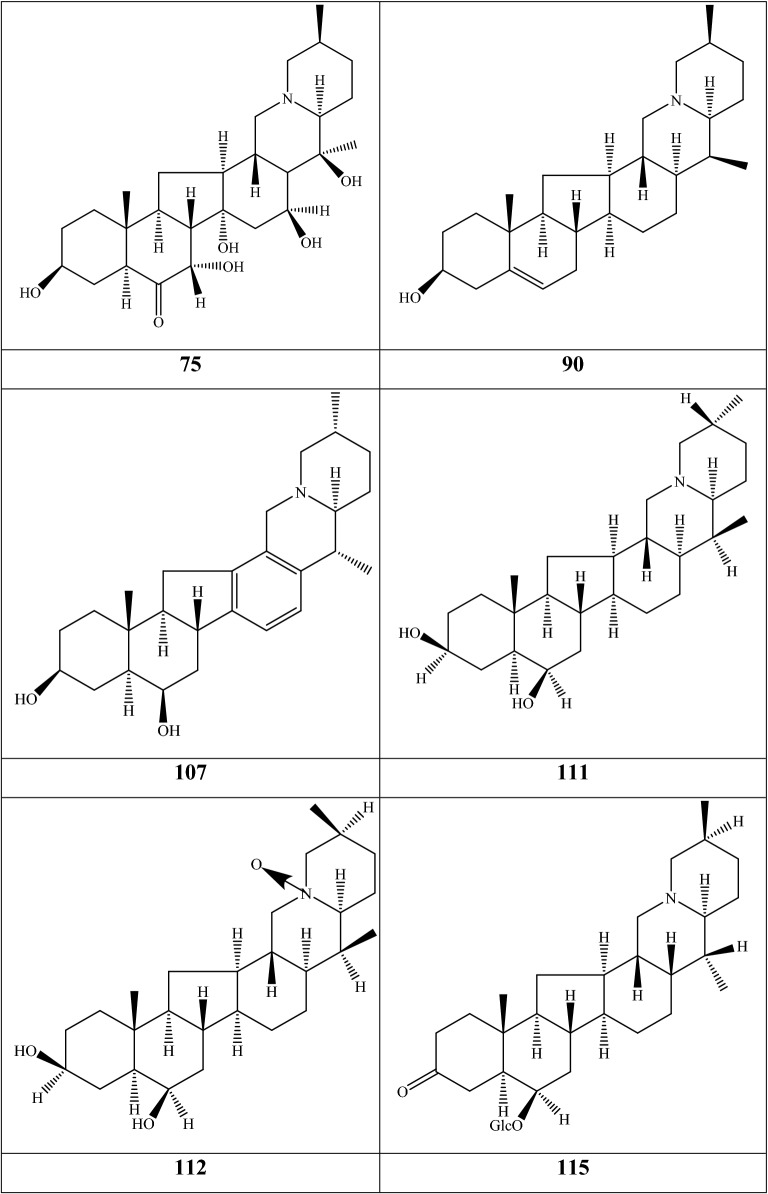
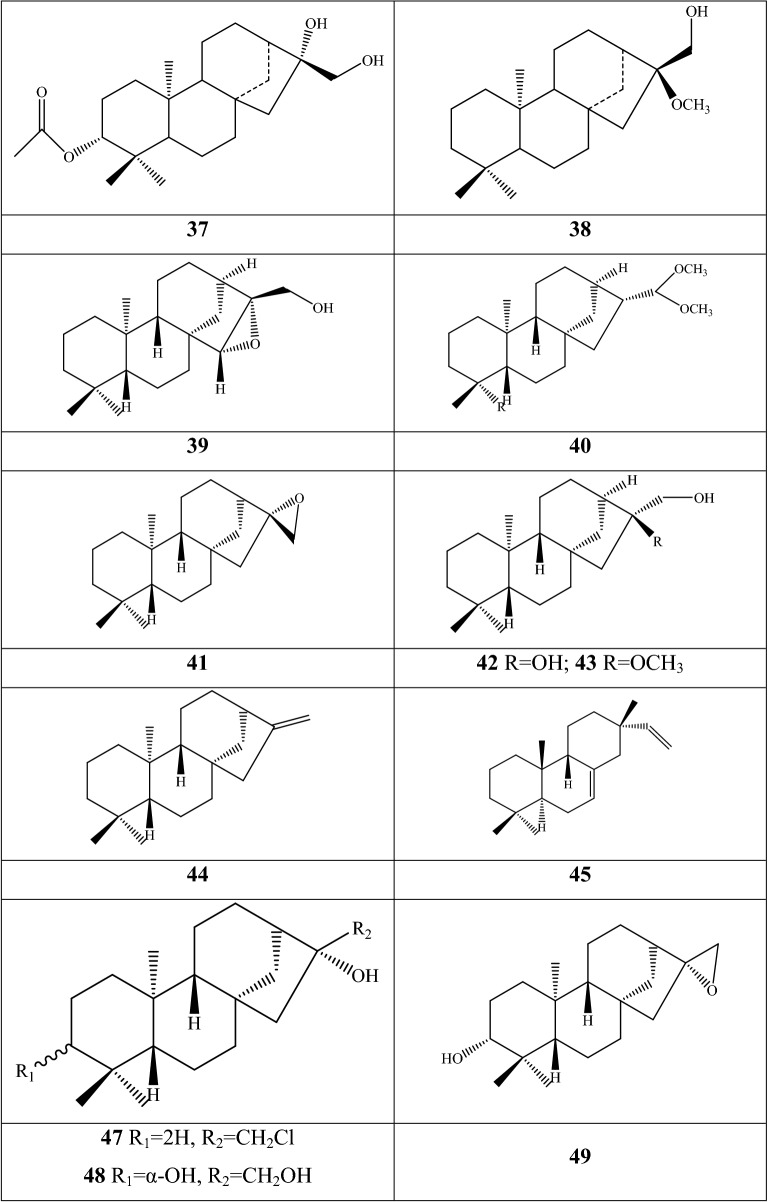
A total of 24 species and 3 varieties (F. taipaiensis var. ningxiaensis, F. unibracteata var. wabuensis, and F. ebeiensis var. purpurea) contain cevanine-type isosteroidal alkaloids (Additional file 1: Table S1). These types of constituents have a trans configuration between that of A and B rings, except shinonomenine 90 (no hydrogen at the C5 position) [24] and fritirorine A 84 (cis configuration) [25] that are isolated from the bulbs of F. tortifolia, an endemic herb in the Xinjiang Province of China. The configuration of all cevanine-type isosteroidal alkaloids, which is currently authenticated currently between B and C, is the same as that of A and B rings except for benzo(7,8) fluoreno(2,1-β)quinolizine cevane-3,6,16,20-tetrol 74 in the bulbs of F. ussuriensis [26]. Inversely, rings C and D have cis configuration as long as hydrogen exists at C12 and C14, respectively. Six components (ussuriedine 73 and 74, heilonine 107, ussurienine 120, ussurienone 121, and ussuriedinone 122) with saturated bonds exist between C12 and C14, and they are mostly extracted from F. ussuriensis, a cultivated species in Northeast China. Moreover, the steric configuration of methyl at the C19, C21, and C27 positions varies with different constituents. C3 and C6 are common positions with a hydroxide radical or carbonyl, whereas the hydroxide radical at the C3 position is often connected with a glucopyranosyl, such as that in hupeheninoside 24 and zhebeininoside 28, or multiple molecules, such as that walujewine E 101 that is only found in F. walujewii, which is distributed in the Xinjiang Uygur Autonomous Region of China [27]. 3-O-acetoxyverticinone 70, as a new isosteroidal cevan-based alkaloid, was firs extracted from the bulbs of F. hupehensis, it contains a carboxyl connected to the hydroxide radical at the C3 position [28]. Additionally, three isosteroidal alkaloids, including hupehenizine 19, verticinedinone 67, and yubeiside 115, were isolated and authenticated from F. imperialis, F. anhuiensis, and F. yuminensis, respectively, with a carbonyl at the C3 position [29–31]. Imperialine-N-oxide 20, a nitric oxide, was the first chemical component extracted from the natural herbs of F. pallidiflora [32], followed by the isolation and authentication of four other similar constituents, namely, isoverticine-β-N-oxide 21 from F. unibracteata var. wabuensis [33], verticinone-N-oxide 69 from F. shuchengensis [34], and lichuanisinine 112 from F. lichuanensis [35]. Hydroxide radical and methyl are common substituents at the C21 position.
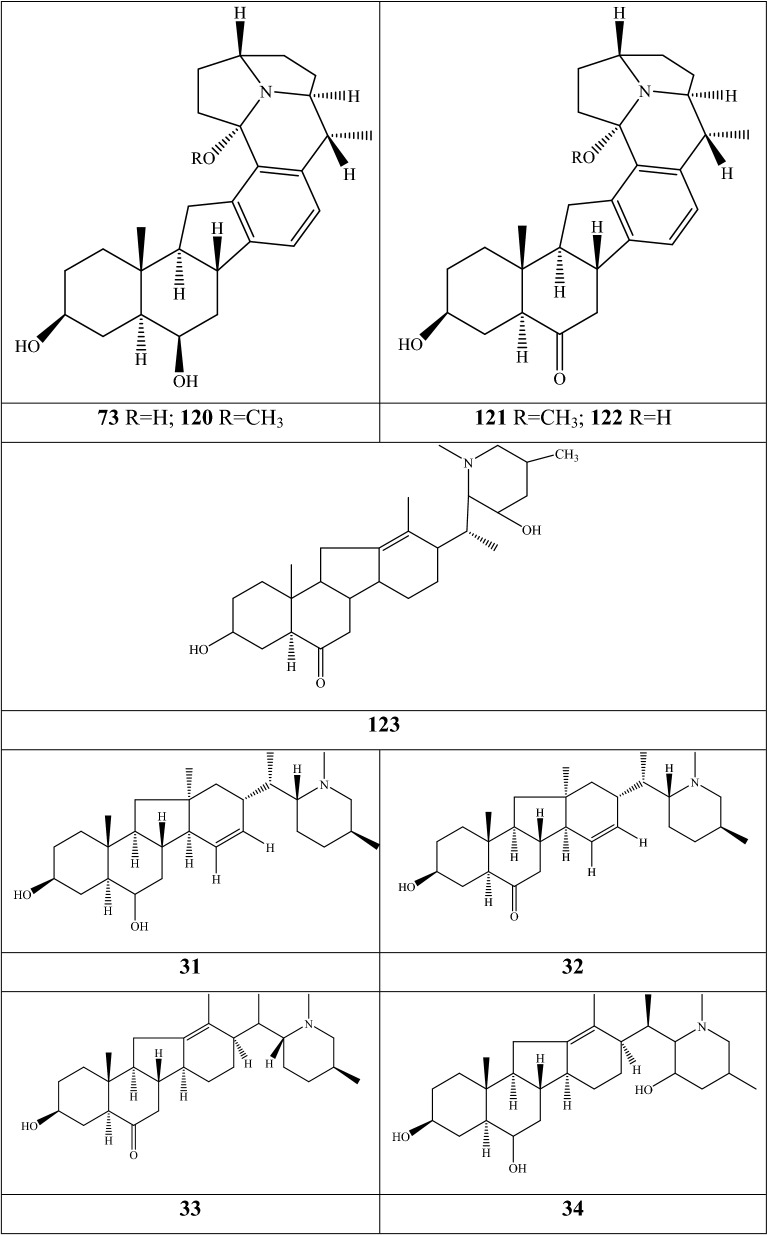
Bulbs from seven Fritillaria species were subjected to isolation and authentication research, Veratramine-type isosteroidal alkaloids from the bulbs have been reported. The type of alkaloids is characterized by the absence of ring E, a common aromatic ring D, and piperidine ring F. Hydroxide radical (β-OH) at the C3 position is the common characteristic and with a trans-configuration between A and B rings, similar to cevanine-type those. Most of these alkaloids types have trans configuration between B and C rings except for certain components without hydrogen at the C8 position, such as puqienine C 37 and puqienine E 39 isolated from the bulbs of F. puqiensis [36]. Impranine 31 and dihydroimpranine 32, as new C-nor-D-homo alkaloids, were first and individually authenticated from F. imperialis, which is used for various ailments in Turkish folklore [37]. Puqienine F 36 is a novel veratramine alkaloid with a 12,16-epoxy ring, was authenticated from F. puqiensis and elucidated by X-ray crystallographic analyses [38]. Suchengbeisine 71 is the only veratramine alkaloid containing an epoxide ring adjacent to ring D; it was elucidated as (22R,25R)-13α,21-epoxy-5,6,12,13-tetrahydro-3β-hydroxy-5α-veratraman-6-one from F. shuchengensis in Anhui Province, China by intensive spectroscopic methods [34]. The type of alkaloids is species-dependent, and a specific constituent is found in a certain species.
Jervine-type alkaloids were isolated and identified from nineteen Fritillaria species with four botanical parts. Peimisine 44 and hupehenisine 50 were discovered from the aerial parts of F. ussuriensis and F. hupehensis, respectively. Other constituents of this alkaloid type were authenticated from the bulbs. The alkaloid types consist of hexacyclic components and a furan ring between C17 and C22, which form a bridge for the piperidine ring F and a six-membered ring D. Hydroxide radical commonly exists at the C3 position of ring A forming a trans the configuration between rings A, B, and C, which is similar to other isosteroidal alkaloids. Cycloposine 47 and peimisine-3-O-β-d-glucopyranoside 66 were isolated from F. pallidiflora [32], F. unibracteata [39], and F. yuminensis [40], in which the hydroxide radical was connected with glucopyranosyl. Yibeinone A 79 is a rare jervine alkaloid with 12α,13α-epoxy ring from F. pallidiflora authenticated as 22,26-imino-17,23-oxido-5α-jerv-6-oxo-3β,14β-diol-12α,13α-epoxy, [41]. Walujewine A 91 was isolated from the bulbs of F. walujewii, which is distributed in Xinjiang Province, China, it contains one olefinic carbon at the C18 position, which extremely differs from that of other jervine alkaloids [27].
Steroidal alkaloids
Verazine alkaloids have been extracted from six Fritillaria species and characterized as the steroidal skeleton combined with 22/23,26-epiminocholestane heterocyclic skeleton. Puqietinedione 40 [36], as a new steroidal alkaloid, was first isolated from the bulbs of F. puqiensis, whereas three other similar components, including puqietinone 61 [42], N-demethylpuqietinone 62, and puqietinonoside 63 [43], were extracted from the same species. Hapepunine 94 and its glycosidal form (hapepunine-3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranoside 95) are newly discovered natural compounds that were isolated together from the aerial parts of F. thunbergii [44]. Similar to the extraction parts, pingbeinine 59 was discovered in the stems and leaves of F. pallidiflora, it has an α-orientation hydroxy at C27 and β-OH at C15 [45]. Delavidine 60 [46], ferisinine 64 [37], and michainine 108 [22] exist in F. delavayi, F. imperialis, and F. michailovskyi, respectively. The latter two species are endemic species in Turkey.
Solanidine has a hexacyclic carbon framework comprising of a base steroidal skeleton and an indolizidine ring. Eight solanidine alkaloids were discovered from five Fritillaria species. Solanidine 57 and its glycoalkaloids (92 and 93) were discovered in F. thunbergii, F. yuminensis, and F. cirrhosa, which are three native species of China. Component 58 were the initial solanidine alkaloids with the 22-S configuration discovered in F. anhuiensis, isolated from natural materials [47].
High percentage of these chemical components were isolated from the bulbs part of nearly 30 species summarized in the review. An interesting finding showed that peimine, as an index component, is distributed in four botanical parts of F. ussuriensis, which implies that the non-medicinal parts may be a potential resource for industrial extraction [26, 45, 48, 49]. There is possible relationship between commercial potential and research frequency focusing on chemical components. Eleven Fritillaria species have been recorded in the Chinese Pharmacopeia, in which 9/11 (except for F. przewalskii and F. taipaiensis) were subjected to isolation and authentication of chemical components. The crude herbs, including Zhe Bei Mu and Yi Bei Mu are the most popular research objectives in the study of Bei Mu. The research focuses on the collection of both Bei Mu materials from certain Himalayan medicinal species or other high-altitude species, several of which are registered as proprietary medicines with a cross-border trade among China, Nepal, Australia, Canada, and Singapore [19]. Moreover, the narrow altitude range of several species is a restraining factor resulting in high-profit Bei Mu materials and expensive herbs that are popular research objectives [50].
Amide alkaloids
The current analytical strategy confirmed the presence of five amide alkaloids (fritenolide A–E 125–129), and their occurrence was reported for the first time in Fritillaria species. The target species, F. unibracteata, is one of type of Chuan Bei Mu, and which enriched the types of alkaloids as unusual α-amino butenolides only recorded in marine organisms and fungus before [51].
Terpenoids
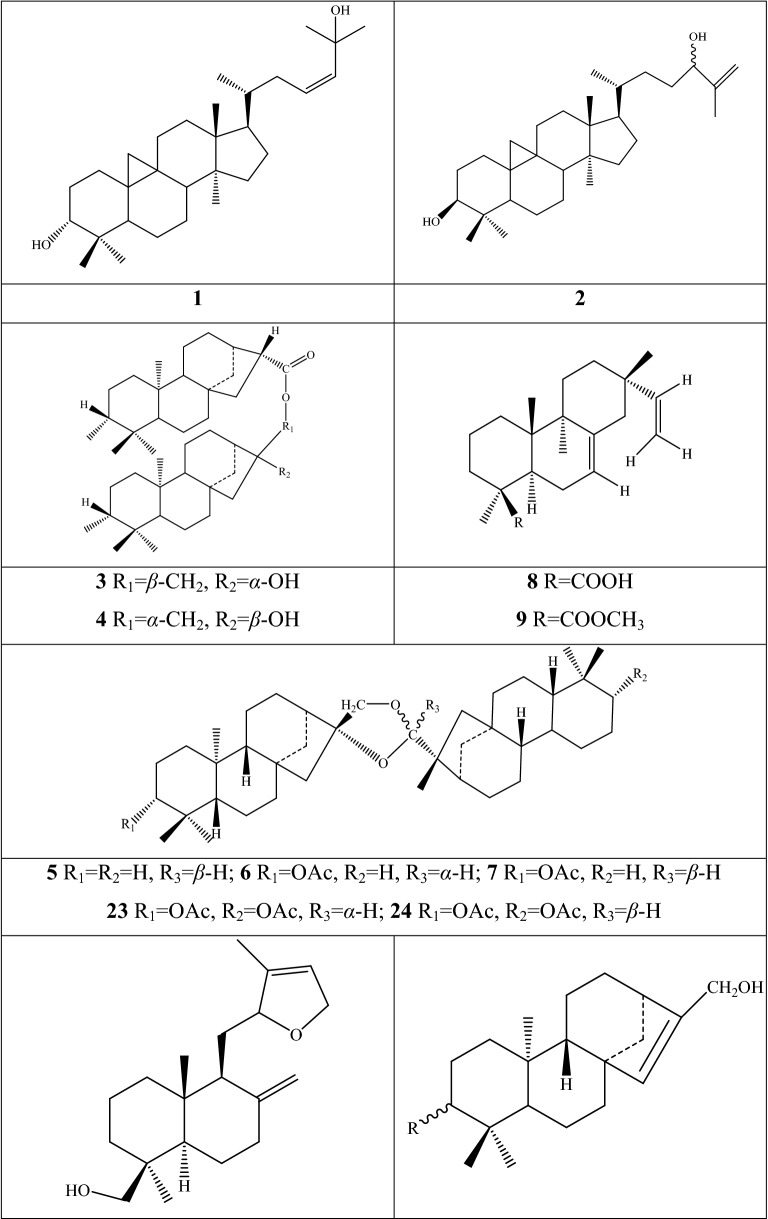
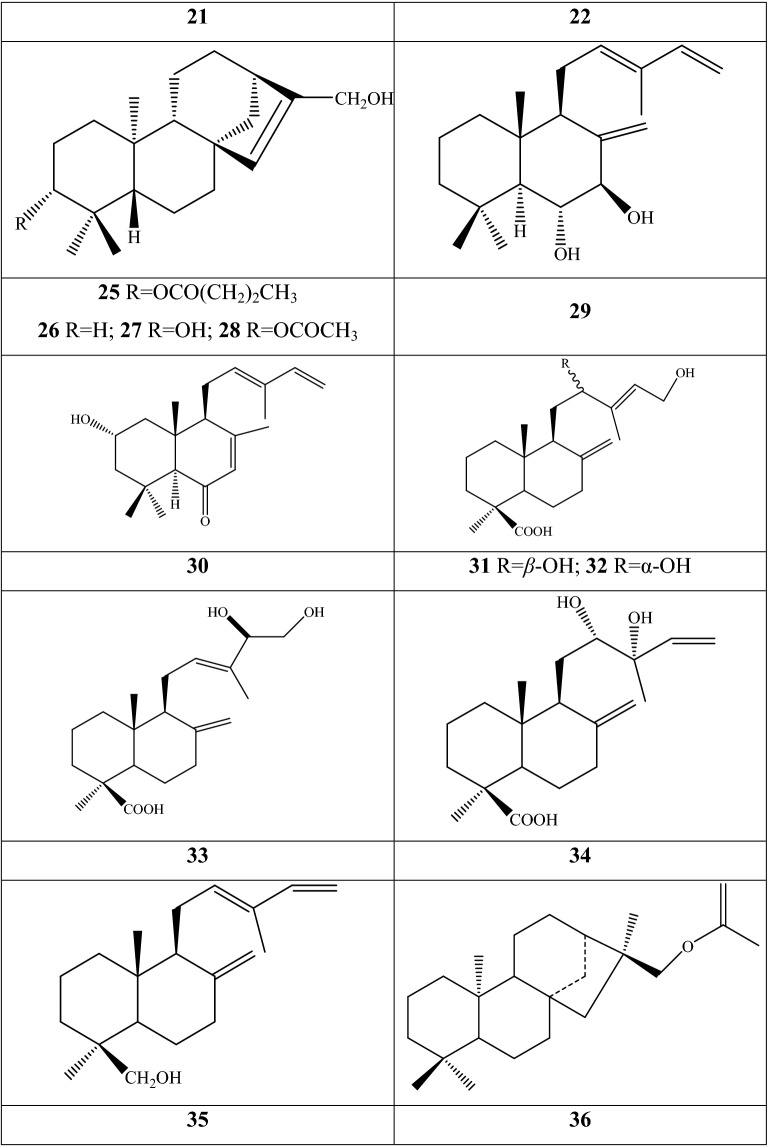
40,000 terpenoids compounds have been authenticated and characterized as the most abundant plant-specialized metabolites in the plant [52]. 49 terpenoids have been discovered from seven Fritillaria species. The occurrence of the types of metabolites in each species and botanical part are showed in Additional file 1: Table S4 and Fig. 4. Two new cycloartane triterpenoids (1 and 2) were isolated from the leaves and stems of F. hupehensis by chemical and spectroscopic techniques; compound 2 was the firstly found in the genus [53]. Another two similar triterpenoids were further discovered from the same botanical parts of the species, and their structure were elucidated as 25-hydroxyl-9,19-cycloart-22-ene-3-one 18 and cycloeucalenol 19 [54]. The bulbs contain other terpenoids constituents, several of which are dominantly characterized as diterpene type. Tetracyclic diterpenoids account for high percentage, with 22 components (11–14, 17, 20, 22, 25–28, 36–39, 40–44, and 46–49) obtained from six species, encompassing F. thunbergii, F. ebeiensis, F. ebeiensis var. purpurea, F. anhuiensis, F. hupehensis, and F. monanth, which are native in China. Component 46 is a novel ent-kauran diterpenoid with chloro-substituted structure established as ent-kauran-16β-hydroxy-chloride from F. ebeiensis var. purpurea by spectral methods. F. ebeiensis was discovered the presence of nine diterpenoid dimers: fritillebin A 15, fritillebin B 16, fritillebin C 3, fritillebin D 4, fritillebinide A 5, fritillebinide B 6, fritillebinide C 7, fritillebinide D 23, and fritillebinide E 24. Herein, component 6 was also described in a variety of F. ebeiensis, that is, F. ebeiensis var. purpurea, which is mainly distributed in the northwest region of Hubei Province, China. Two tricyclic diterpenoids (8 and 9) were detected in the ethanolic extract of F. imperialis, whereas one (45) was identified from the methanol extract of F. thunbergii; their structures were elucidated as isopimara-7,15-dien-19-oic acid 8, isopimara-7,15-dien-19-methyl ester 9, and isopimara-7,15-dien 45, respectively. Nine bicyclic diterpenoids (10, 21, 29, 30–32, 33–35) have been reported in two Chinese species, namely, F. anhuiensis, and F. ebeiensis.
Fig. 4.
Structures of terpenoid in Fritillaria species
Steroidal saponins
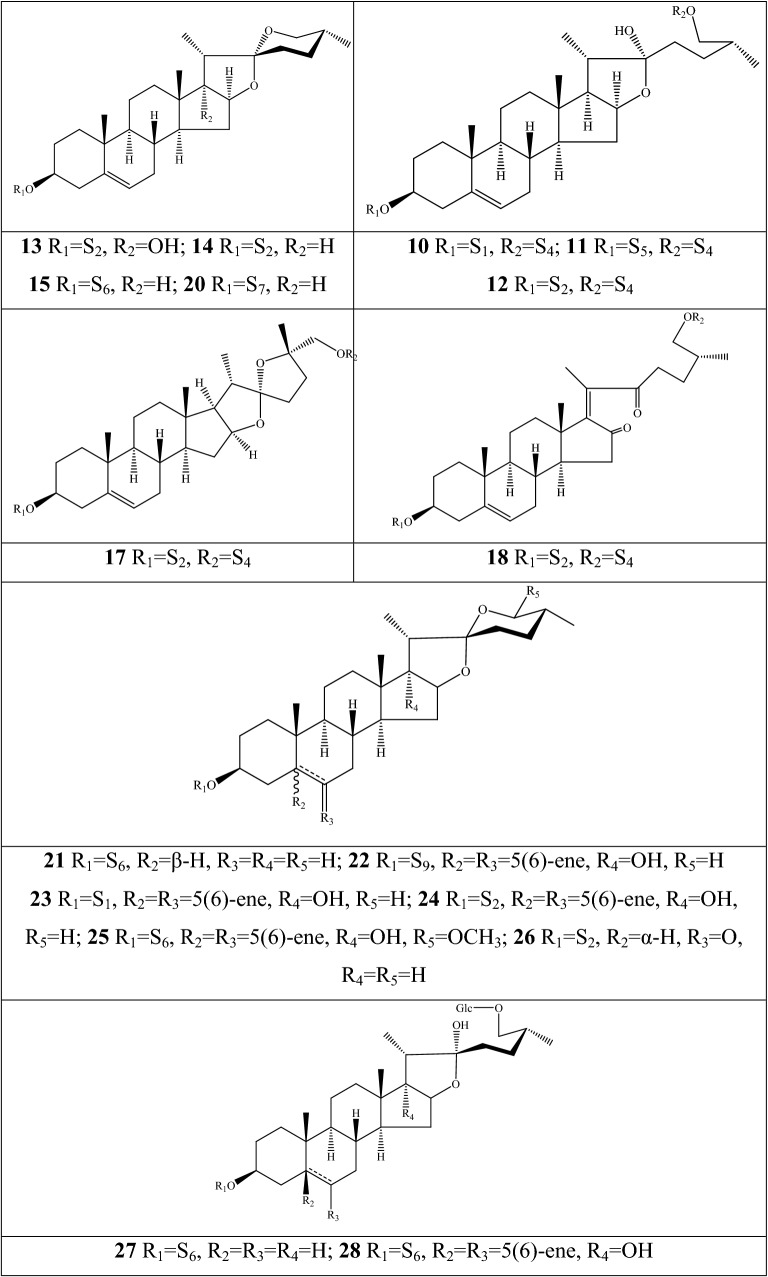
An impressive diversity of steroidal saponins have been discovered in the dried bulbs of F. pallidiflora and F. meleagris; no study reported obtaining these components from aerial parts (Additional file 1: Table S5 and Fig. 5). Herein, F. pallidiflora is a genuine-geographical Bei Mu species in Xinjiang Province of China, whereas F. meleagris extensively grows in Asian and northwestern Europe [55]. The early isolation and structural elucidation of steroidal saponins was dated from 2011, when three new constituents were identified as 26-O-β-d-glucopyranosyl-(25R)-furost-5,20(22)-dien-3β,26-diol-3-O-β-d-xylopyranosyl(1→4)-[α-l-rhamnopyranosyl(1→2)]-β-d-glucopyranoside, (25R)-spirost-5-ene-3β,17α-diol-3-O-β-d-glucopyranosyl(1→4)-β-d-galactopyranoside, and 26-O-β-d-glucopyranosyl-3β,26-dihydroxyl-20,22-seco-25(R)-furost-5-en-20,22-dione-3-O-α-l-rhamnopyranosyl(1→2)-β-d-glucopyranoside from F. pallidiflora, which were named as pallidifloside A 1, pallidifloside B 2, and pallidifloside C 3, respectively [56]. Thereafter, pallidifloside D 4, pallidifloside E 5, pallidifloside G 8, pallidifloside H 9, and pallidifloside I 10 were obtained from the same species by the same research team [57]. 7 components (6, and 11–16) were first reported in Fritillaria; especially the polyphyllin V and parispseudoside B, the main chemical components in Paris, and spongipregnoloside A was detected in nearly 10 Paris species [58]. The same constituents of Fritillaria and Paris can be interpreted as important clades among the 60 genera of Liliaceae. (25R)-∆5(6)-Isospirost-17α,3β-diol-3-O-β-d-glucopyranosyl-(1→3)-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranoside 39 is a novel compound that was first authenticated in F. pallidiflora [59], whereas constituents 17–20 were reported in one of the original plants of Yi Bei Mu. Matsuo and her co-authors [55] conducted a comprehensive investigation in terms of the extraction techniques of steroidal glycosides and their cytotoxic activities in F. meleagris. A total of eighteen steroidal saponins (21–38), with 10 compounds authenticated as new structures, were extracted from hot by methyl alcohol.
Fig. 5.
Structures of steroidal saponins in Fritillaria species
Phenylpropanoids
The term phenylpropanoid describes a broad conception of herbal medicine possessing one or several C6–C3 skeletons; this group includes simple phenylpropanoids, lignans, coumarins, lignins, and flavonoids. Amongst, lignans, coumarins, and flavonoids have been isolated in F. pallidiflora and F. thunbergii, which were reported to yield 13 phenylpropanoid constituents from their hypogeal and aerial parts (Additional file 1: Table S6 and Fig. 6). Three bisepoxylignans were detected in the bulbs (syringaresinol 1 and pinoresinol 3 in F. pallidiflora), aerial parts (syringaresinol 1 in F. thunbergii), and flowers (clemaphenol A 2 in F. pallidiflora), and they were characterized by two tetrahydrofuran rings formed by two phenylpropanoid molecules. A single bisepoxylignan, that is, zhebeiresinol 6, was described in the aerial parts of F. thunbergii. Two coumarins (murrayone 4 and 2′-methoxyseselin 5) were extracted and autenticated from the bulbs of F. pallidiflora. Seven flavonol compounds (7–13) belonging to flavonoids exist in the bulbs and flowers of F. pallidiflora and flowers of F. thunbergii, in which flavonol 8–10 are glycosides connected with rhamnose or glucose at the C3 position.
Fig. 6.
Structures of phenylpropanoids in Fritillaria species
Fatty acids
Five species were isolated and authenticated, and six fatty acids were reported to originate from the bulbs of Fritillaria (Additional file 1: Table S7). Stearic acid was isolated from F. michailovskyi for the first time [22]. Shi and her co-authors isolated for the first time linoleic acid from F. walujewii, which also was the earliest isolation conducted on the genus [60]. Lignoceric acid and azelaic acid discovered in the bulbs of F. hupehensis, without records in other species of the genus until now [61]. Palmitic acid was detected in two species (F. hupehensis and F. michailovskyi) [22, 61], whereas laurostearic acid was reported in F. pallidiflora [59].
Sterides
Nine species of the genus have been reported that their bulbs or aerial yielded β-sitosterol, which is widely distributed in the plant domain and an important components of plant cells (Additional file 1: Table S8). This plant-specialized component has been reported in the bulbs/aerials parts of F. thunbergii [62, 63], flowers of F. pallidiflora [64], and bulbs of F. anhuiensis [29], F. unibracteata [51], F. michailovskyi [22], F. hupehensis [65], F. thunbergii var. chekiangensis [66], F. monantha [67], and F. walujewii [60]. The C3-OH is connected to a glucose-forming daucostenine (β-sitosterol-glucoside), which was identified from bulbs of F. anhuiensis [29] and F. michailovskyi [22].
Other metabolites
Apart from the six types of metabolites mentioned above, several other compounds have been identified in Fritillaria taxa, in which chemical components (1–59) were encompassed in four botanical parts of 11 species (Additional file 1: Table S9). Several common constituents related to the plant physiology have been isolated and identified since 2012, when a cyclic peptide was extracted from the bulbs of F. anhuiensis and whose structure was elucidated as cyclo-(Leu–Val) based on spectroscopic analysis [29]. The presence of this component was reported in Liliaceae for the first time. Recently, l-pyroglutamic acid, Cyclo (l-Pro-l-Ala) and Cyclo-(Phe-Val), as two new cyclic peptides, were obtained from the medicinal bulbs of F. pallidiflora in 2016 [64] and 2019 [68], respectively. Choline was first discovered in the bulbs of F. walujewii, which was also the earliest discovery in the genus [60]. Several nitrogenous bases, their glycosides, and nucleoside compounds were also discovered in certain species of Fritillaria, such as uridine, uracil, adenosine, thymidine, thymine, adenine, and guanosine, which take part in genetic evolution and other physiological activities. Followed by small-molecule alcohols (13–22), components 15–17 were separated by water steam distillation from volatile oil and authenticated by gas chromatography–mass spectrometry in the bulbs of F. cirrhosa [69]. Some esters (23–37) were also obtained from the bulbs or flowers. Certain acid components, such as cinnamic acid (38 and 40) [59, 68] and 4-(β-d-glucopyranosyloxy) benzoic acid [70], rather than fatty acid were extracted in certain Fritillaria species, such as the bulbs of F. pallidiflora. Ketones occur with four compounds and, comprised of 42–45, were present in the flowers of F. thunbergii [71] and the bulbs of F. cirrhosa [69] and F. pallidiflora [68]. Hydrocarbons identified as 1-dodecene 46, 4-octadecene 47, and pentatriacontane 48 were detected in F. cirrhosa [69] and F. michailovskyi [22]. Other chemical components are displayed in Additional file 1: Table S9, in which gastrodin 49 is the index component for the quality control of Gastrodia elata Bl, whereas icariside D2 50 (4-O-β-d-glucoside of tyrosol) is an effective constituent that was first isolated from Epimedium diphyllum [72]. An acidic water-soluble heteropolysaccharide, which is a macromolecule with good antioxidant activity and DNA protection effect, was extracted and purified from F. unibracteata var. wabuensis, which implies that the small-molecule compound is not the only active component in Fritillaria species [73]. In addition, the herbal medicine made from Fritillaria is rich in chemical components that were not characterized nor investigated in the study.
Alkaloids (isosteroidal, steroidal, and amide alkaloids) are certainly the main chemical constituents of Fritillaria. The portions of terpenoids and steroidal saponins account for a high percentage of these constituents and should be given attention in-deep investigation of the genus. Although vast literatures focused on the isolation and authentication of chemical components, limited studies reported the systematic protocol of individual compounds with the help of molecular or genetic methods. Sun et al. found those alkaloid biosynthesis genes in F. cirrhosa with the help of two common database, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes databases [74]. In addition, de novo transcriptomics is regarded as a useful approach in interpreting genes of complex biochemical pathways to identify steroid alkaloid biosynthesis in F. imperialis [75]. In the future, researchers may pay attention to the investigation and validation of these biosynthesis genes of the main chemical components of Fritillaria species with the aid of genomics, proteomics, biochemical, and computational analyses. The biochemical pathways will decrease the production cost because of the long cultivation period of Fritillaria species. What’s more, the extraction of chemical components from the aerial parts would provide reference for the resource utilization of these species of the genus, because the aerial parts have been discarded after the bulbs were harvested.
Pharmacological activities
The dried bulbs of Fritillaria species have been used as an anti-tussive drug and other respiratory diseases, such as expectoration and asthma, in traditional folk medicine since Han Dynasty of China (around AD 220). Thus, several studies focused on the respiratory diseases in vivo and in vitro. Aside from studies on the respiratory system, the increasing pharmacological research indicates that the chemical components or extracts from herbal medicines have potential antineoplastic, anti-inflammatory, antihypertensive, bacteriostasis, and anti-tumor effects (Additional file 1: Table S10).
Respiratory diseases
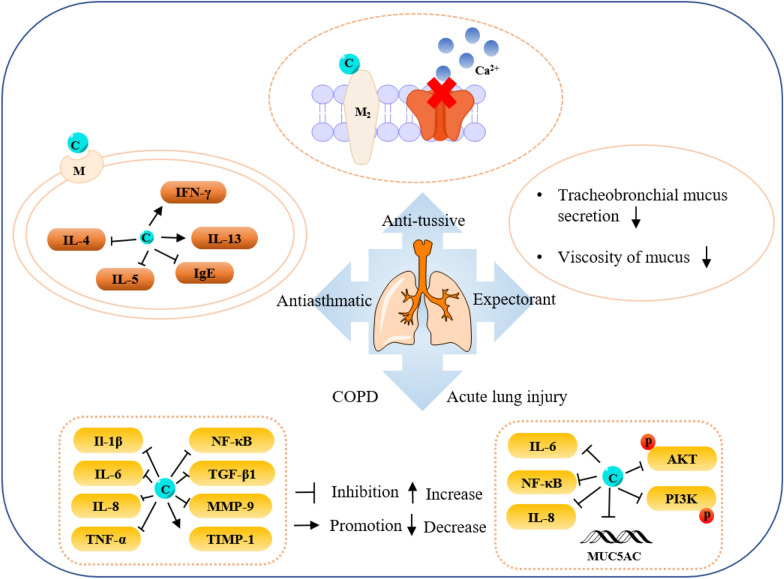
Respiratory diseases seriously affect the physical and mental well-being of patients with symptoms of sneezing, cough, and difficulty in breathing, which are leading causes of mortality and morbidity [76]. The natural botanical materials from Fritillaria have significant pharmacological effects on the respiratory system, including the alleviation of cough, phlegm, asthma, COPD, and acute lung injury (ALI) (Fig. 7).
Fig. 7.
The action mechanism of respiratory diseases using chemical components from Fritillaria species
Anti-tussive effect
The anti-tussive efficacy is consistent pharmacological activity between the traditional clinical usage and modern utilization in the daily life. Anti-tussive pharmacological comparison of 11 commercial Fritillaria species indicated that the total alkaloid of 11 Fritillaria species had significant or extremely significant effect on ammonia-induced cough in mice. The ethanol extracts of 9 Fritillaria species, except for F. delavayi and F. pallidiflora, also have significant anti-tussive effect [77]. Further studies confirmed that steroidal alkaloids are the main effective components in these species, and they play vital roles in anti-tussive activity. Individual investigation of four new steroidal alkaloids (33, 34, 62, and 63) isolated from the bulbs of F. puqiensis, was performed their anti-tussive activity on mouse induced with ammonia liquor. The results indicated that four compounds reduced the coughing times and prolonged the latent period [43]. Other study obtained consistent performance with the induction studies of ammonia liquor [78–81]. Ammonia-induced cough experiment showed that four steroidal alkaloids (2, 8, 11, and 13) from F. cirrhosa and three components (4, 20, and 21) from F. wabuensis, significantly inhibited cough frequency and increased the latent period of in mice [78, 79].
Ebeinone 26 of F. imperialis strongly exhibited a higher affinity for muscarinic M2 receptors than for M3 receptors in a study on guinea pigs in 1997, and it interacted allosterically with M2 receptors during a rat trail [82]. The same results were described for five alkaloids (2, 11, 13, 88, and 61), demonstrating significant elevation of the cAMP concentration in the human embryonic kidney cells transfected with muscarinic M2 receptor plasmid [83]. The action mechanism of imperialine 11 and sinpeinine A 6 was interpreted as their selective inhibitory on muscarinic M2 receptors, whereas the mechanism of 3β-acetyl-imperialine was performed by its selective antagonism of muscarinic M3 receptor [84]. Chan and co-authors compared the relaxant effects of five major steroidal alkaloids (2, 11, 13, 99, and 61); the former four components have a cevanine-type structure, whereas puqietinone 61 is a verazine alkaloid, as investigated by rat-isolated tracheal and bronchial preparations pre-contracted with carbachol. The results demonstrated the potential mechanisms of these constituents were competitive antagonism of muscarinic pathway and the inhibition of Ca2+ influx [85].
Expectorant effect
The expectorant effect can be observed in the mixed preparations of fresh pear and dried powders, which are normally cooked by the elderly for their therapeutic effects. The total alkaloids and saponins are the prominent compounds contributing to the expectorant effect. Compounds 2, 11, and 13 from F. cirrhosa and 4, 11, 20, and 21 from F. wabuensis enhanced the phenol red output from a mouse tracheal [78, 79]. Expectorant effects are generally related to the relaxation of smooth muscles. The sputum volume was increased after the drug was fed in rats without a vagus nerve, thus which confirming that the bulbs of Fritillaria are a non–evil expectorant [86].
Anti-asthmatic effect
Asthma is an allergic disease caused by broad bronchial obstruction and exhalation dyspnea is the main symptom. The main inducements of bronchial obstruction are interpreted by three aspects, including bronchial smooth muscle contraction, excessive mucus secretion, and adhesion to the bronchial wall. The main anti-asthmatic mechanisms comprise the relaxation of bronchial smooth muscles, relief of trachea and bronchus spasm, and improvement of ventilation status. Current scientific research shows that the anti-asthmatic effect of Fritillaria is related to the antagonism of the tracheal M receptor [82, 83]. The water extract of F. cirrhosa exhibits obvious inhibitory effects on airway inflammation by several pathways, which includes the suppression of helper T cell-2 cytokines and immunoglobulin-E, histamine production, reduction of eosinophilic accumulation, and increase in interferon-γ (IFN-γ) production [87]. Yibeinones B–D (80–82) and imperialine (11) isolated from the bulbs of F. pallidiflora showed an evident concentration-dependent relaxation effect on the isolated tracheal preparation, whereas components 11 and 81 exhibited significant effects with pA2 values of 6.19 ± 0.02 and 8.41 ± 0.10, respectively [41].
Other respiratory diseases
The effects of bulbs from Fritillaria species have been extended to other respiratory diseases, such as COPD and ALI. Wang et al. investigated the performance of imperialine 11 on pulmonary function and inflammation in a COPD-like rat model, of which the model was established by the exposure to cigarette smoke and intratracheal administration using lipopolysaccharide (LPS). The obtained results displayed that the alkaloid mitigated pulmonary impairment and suppressed the inflammatory response by mediating the expression of related cytokines in lung tissues [88]. The therapeutic effect of peiminine 13 implied that the component can reduce the wet-to-dry ratio and the myeloperoxidase activity on LPS-induced ALI. IL-6 was inhibited after peiminine treatment. Peiminine also showed a significant inhibition trait for the LPS-induced IL-8 production in human lung adenocarcinoma cells (A549). Meanwhile, Western blot analysis displayed that peiminine remarkably suppressed the activity of the NF-κB pathway. In addition, peiminine disrupted lipid raft formation by attenuating the cholesterol content [89]. The extracts of F. thunbergii have been used as mucoregulators for controlling airway inflammatory diseases, which is related to the expression of mucin 5 subtype AC (MUC5AC). Three isosteroidal alkaloids (2, 72, and 99) inhibit the gene expression of MUC5AC by directly acting on airway epithelial cells [90].
Antineoplastic effect
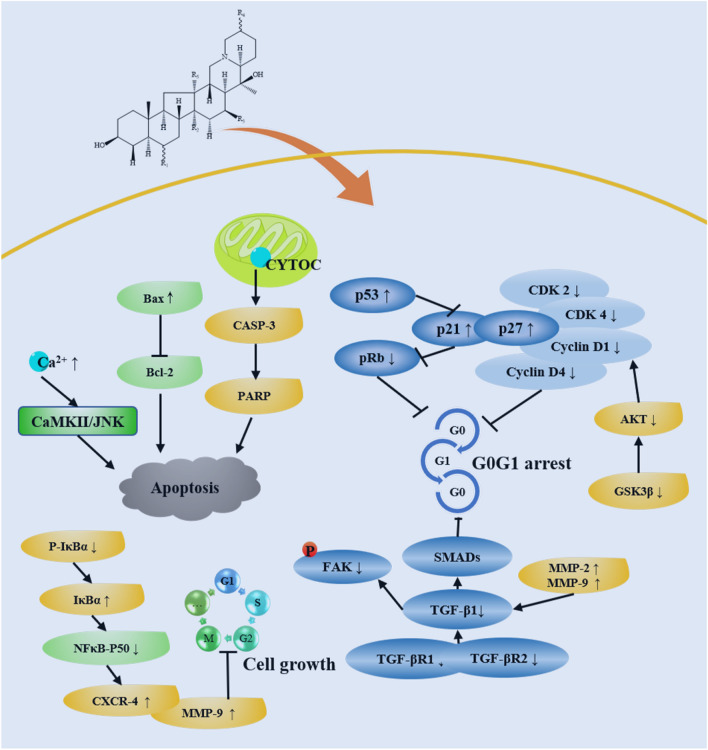
Plant-derived agents may became a potential resource with a vital role in antineoplastic treatment; the therapeutic properties of several species have been discovered in a variety of cancers or cancer cells, such as human promyelocytic leukemia cells (HL-60) [55, 91], A549 cells [55], oral keratinocytes [92], ovarian and endometrial cancer cell lines [93, 94], S180 sarcoma and Lewis lung carcinoma [88, 95], HeLa cells [96], human colorectal carcinoma cells [97], glioblastoma (GBM) cells [98], non-small cell lung cancer (NSCLC) [99], and prostate cancer [100] (Fig. 8). These modern pharmacological results were consistent with the ancient efficacy showing that Bei Mu herbs can remove stasis and tumor. The antineoplastic effect is mainly performed by the proliferation inhibition of cancer cells. In recent years, several studies have been conducted to explore the potential mechanisms of anticancer activity using chemical components or extracts from Fritillaria herbs; These mechanisms include promotion of cell differentiation [91], induction of apoptosis and G0G1 cell cycle arrest [95] through a caspase pathway [88, 92], apoptosis induction without affecting the caspase-3 activity level [55], activation inhibition of NF-κB [93, 99], downregulation of TGF-β/SMAD signaling pathways [94], inhibition of tumor angiogenesis [88], induction of cellular stress and activating several anti- and pro-survival pathways [96], induction of autophagic cell death via activating autophagy-related signaling pathway AMPK-mTOR-ULK by promoting SQSTM1 (P62) [97, 98], and disruption of intracellular calcium homeostasis through Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) pathway [100]. Peimine, as a common component of 12 Fritillaria species, has showed reversal of the multidrug resistance of tumor cells in vitro and reversed tumor cells through in vitro multidrug resistance activity. Peimine was the first steroidal alkaloid reversing drug resistance of tumor cells, and its mechanism may be interpreted that of increasing concentration in drug-resistant cells and the expression inhibition of P-glycoprotein protein in drug-resistant cells [101].
Fig. 8.
Antineoplastic effect of chemical components and extract in Fritillaria species
The water extract of F. cirrhosa can significantly decreased cell growth and the invasive potential of ovarian and endometrial cancer cell lines. The mechanism was interpreted to be caused by the in activation of caspase-3, G0/G1 phase cell cycle arrest, downregulation of cyclins D1 and D3, induction of p27, decreased NF-κB DNA binding, expression reduction of phosphorylated IkBa, abrogated NF-κB activation, and downregulation of NF-κB-regulated metastasis-promoting proteins [93]. Investigation of the antitumor activity of different extraction fractions and isosteroidal alkaloids from F. ussuriensis showed that the chloroform extract and purified alkaloids exhibited stronger cytotoxic activity. Four main steroidal alkaloids (2, 11, 44, and 13) were isolated and showed significant cytotoxicity; peimisine 44 can induce G0/G1 phase arrest and promote apoptosis [94]. Four cevanine-type steroidal alkaloids (4, 8, 20, and 21) isolated from the total alkaloids of F. pallidiflora displayed significant cytotoxicity, with chuanbeinone 8 showing the highest activity against Lewis lung carcinoma cells. The results displayed that the antitumor activity was exhibited the compound in vivo, in which the increased expression of caspase-3 generated the performance of tumor angiogenesis and induced apoptosis [88].
Verticinone 13, which exists in approximately 13 Fritillaria species, inhibited the growth of HL-60 cells by inducing their differentiate into granulocytes [91] and existed apoptosis induction via a caspase pathway [92]. Component 21, (25R)-5β-Spirostan-3β-ylO-β-d-glucopyranosyl-(1→4)-O-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranoside, was authenticated as a steroidal saponin from the dried bulbs of F. meleagris; it induces the apoptotic cell death of HL-60 cells through several mechanisms. Component 32, (22S,25S)-Spirosol-5-en-3β-ylO-β-d-glucopyranosyl-(1→4)-O-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranoside, can selectively induced apoptosis in A549 cells which can’t affect the activity level of caspase-3 [55]. Isopimara-7,15-Dien-19-oic acid, a terpenoid isolated and authenticated from the dried bulbs of F. imperialis; it induced cellular stress and activated several anti- and pro-survival pathways in HeLa cells [96]. Peiminine suggest a new strategy for GBM therapy, which induction of autophagic cell death is performed by activating the autophagy-related signaling pathway AMPK-mTOR-ULK and promoting SQSTM1 (P62) [97] as well as inhibiting GMB in vitro and in vivo via arresting the cell cycle and blocking autophagic flux [98]. Imperialine exerted anti-cancer effects against NSCLC, which potential mechanism was related to the NF-κB centered inflammation-cancer feedback loop [99]. In addition, the toxicity assays revealed that imperialine treatments did not significantly disturb the blood cell counts in mice nor exert any significant damage to the main organs, indicating a robust systemic safety [99]. Peimine can generated inhibition performance of the growth and motility of prostate cancer cells and is interpreted as the disruption of intracellular calcium homeostasis through the Ca2+/CaMKII/JNK pathway [100].
Anti-inflammatory effect
The bulbs of Fritillaria have a certain therapeutic effect on COPD and ALI as mentioned above, and this phenomenon is closely related to their anti-inflammatory effect (Fig. 9). The ethanol extracts of two commercial species (F. cirrhosa and F. pallidiflora) inhibited the development of ear edema [81]. Chuanbeinone 8 and imperialine 11 have the same inhibition performance in ear edema of an anti-inflammatory assessment depending on a dose-dependent manner [78]. Four isosteroidal alkaloids (4, 11, 20, and 21) obtained the same anti-inflammatory performance [79]. The alkaloid fraction of F. cirrhosa showed several anti-inflammatory activities (inhibition of acetic acid-induced capillary permeability accentuation, carrageenan-induced paw edema, and cotton pellet-induced granuloma formation; suppressing recruitment of inflammatory cell and production of cytokine in the bronchoalveolar lavage fluid from ALI mice) [102]. 12,15-Sulfonyl-8(17),13-labdadien-19-oic acid (terpenoid component 21) isolated from F. anhuiensis obviously attenuated the NO production in a macrophage cell line of RAW264.7 cells stimulated with IFN-γ [103].
Fig. 9.
Anti-inflammatory effect of chemical components in Fritillaria species
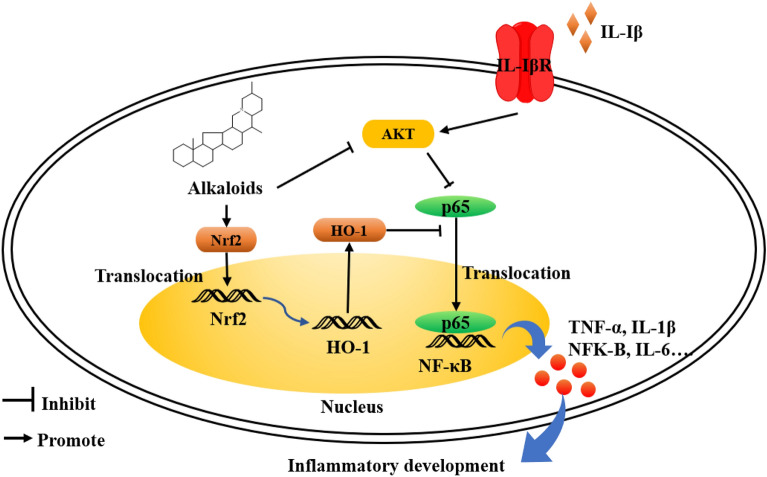
In addition, the anti-inflammatory mechanism of Fritillaria bulbs is interpreted as closely related to various signal pathways (the inhibition of NF-κB [104, 105], expression reduction of inflammatory cytokines [102, 104, 106, 107], inhibition of the mitogen-activated protein kinase (MAPK) pathway [108, 109].) Two main components (verticinone 13 and imperialine 11) can suppress the production of pro-inflammatory cytokines with a dose-dependent manner. The crude polysaccharide, extracted from F. hupehensis by hot water, also displayed obviously inhibition performance to mouse ear swelling induced by xylene and the toe swelling induced by egg white, in which the inhibition performance was interpreted that the secretion of inflammatory cytokines, meanwhile dynamic balance between pro-inflammatory and anti-inflammatory factors [106]. Marker component (peimine 2) suppressed IL-1β-induced inflammation in mouse chondrocytes and inhibited he LPS-induced RAW264.7 macrophages via inhibition of the MAPK pathway [108, 109]. What’s more, the chemical component can ameliorate murine osteoarthritis via inhibition of the AKT phosphorylation, nuclear transfer of NF-κB and activation of nuclear factor 2 (Nrf2)/heme oxygenase-1 in an osteoarthritis model [107].
Antihypertensive effect
Antihypertensive effect is a novel pharmacological activity that is different from the traditional ethnopharmacological properties of Fritillaria species. The water extract of F. ussuriensis can prevent the increase of systolic blood pressure in NG-nitro-l-arginine methylester-induced hypertension and interpreted as the enhanced generation of vascular NO and amelioration of renal functions [110]. The comparison of different extracts of F. ussuriensis showed that intravenous injection of water extract can decrease the mean arterial pressure of anesthetized rats. The angiotensin converting enzyme (ACE) activities were significantly inhibited by ethylacetate and butanol extracts (half maximal inhibitory concentrations were 292 and 320 mg ml−1, respectively). The results showed that the extracts had a hypotensive effect via the ACE inhibition and release of NO/cyclic guanosine 3ʹ,5ʹ-monophosphate in the vascular tissue on rats [111]. Alkaloids components 2, 13, and 44, which were isolated from the dried bulbs of F. ussuriensis, have ACE inhibition activity in a dose-dependent manner (50% inhibitory concentration values of 165.0, 312.8, and 526.5 μM, respectively) [112]. Three veratramine-type isosteroidal alkaloids (34, 35, and 39) isolated from F. puqiensis exhibited an inhibitory activity (inhibition ratios of 70.2% ± 0.5%, 24.7% ± 0.5% and 20.4% ± 2.8%, respectively, at the concentration of 200 µM) [42].
Anti-cholinesterase (cholinomimetic) activity
Acetylcholinesterase (AchE) inhibition is considered a promising strategy for the treatment of Alzheimer’s disease [113]. Herein, the dichloromethane fraction of F. michailovskyi shows a positive butyrylcholinesterase (BchE) inhibitory activity [22]. Zhou compared the antimuscarinic effects of five Fritillaria species (F. puqiensis, F. hupehensis, F. ebeiensis, F. pallidiflora, and F. delavayi). The results showed that the methanol extracts of F. unibracteata generated the best antimuscarinic effects with the help of several steroidal alkaloids [105]. Five steroidal alkaloids (11, 15, 16, 18, and 22) isolated from the bulbs of F. imperialis, of which forticine 15, persicanidine A 16, and impericine 18 were isolated from the species, showed anti-AchE and anti-BchE inhibitory activity [114]. Moreover, delavine 22 exists in F. delavayi, and imperialine 11 is a common constituent of the genus; N-demethylpuqietinone 62 is present in F. puqiensis, hupeheninoside 25 in F. lichuanensis, ebeiedinone 9 in F. ebeiensis or other species, and yibeinoside A 6 in F. pallidiflora (or other species). Chuanbeinone 8 from F. delavayi (or other species) has anti-red blood cell AchE and anti-plasma BchE activities [115].
Antibacterial and antiviral activities
Pharmacological research on herbs all over the world for the selection of bioactive constituents or plant extracts against fungi and bacteria may be an alternative protocol to combat antibiotic resistance [116]. Antibacterial investigation of the ethanol and aqueous extracts of F. thunbergii indicated remarkably inhibitory against six Helicobacter pylori strains (minimum inhibitory concentrations is close to 60.0 μg ml−1) [117]. β-Sitosterol-3-O-glucopyranoside (steride component 2) exhibits antibacterial activity against three bacterial including Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus [118], that the MIC values are 50, 200, and 400 μg ml−1, respectively. In addition, the water extract of F. thunbergii exerts antiviral effects against influenza H1N1 virus without inducing toxicity in vitro, in ovo, or in vivo [119].
Antioxidant effect
The antioxidant effect occurs as species- and fraction-dependent fluctuation in Fritillaria species. The antioxidant activity of F. ussuriensis extracts decreases with different polarity (crude flavonoid extract > crude saponin extract > ethanol extract) [120]. Three isosteroidal alkaloids (peimisine, peimine, and peiminine) that are abundantly found in Fritillaria bulbs exist as acidic water-soluble heteropolysaccharides (average molecular weight: ~ 7.44 kDa) in F. unibracteata var. wabuensis; the acidic fraction FPSP-H2-1 from F. pallidiflora process a strong antioxidant effect against 1,1-diphenyl-2-picrylhydrazyl and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) free radicals [73, 121, 122]. The comparison analysis of six isosteroidal alkaloids (2, 11, 13, 22, 44, and 88) with different chemical structures from F. cirrhosa supported the results, showing that five components (2, 13, 22, 44, and 88) exhibited more potent effect against cigarette smoke extract-induced oxidative stress than imperialine 11 by activating the Nrf2-mediated antioxidant pathway [123].
Antinociceptive effect
Records of traditional medicine applications show that the bulbs of the Fritillaria can be used for the treatment of chest heat and pain in Mongolian national minorities. Verticinone 13, which has been extracted and authenticated from approximately 13 Fritillaria species, can exert a good antinociceptive performance on inflammatory and cancer-related neuropathic pains probably via peripheral and central mechanisms [124]. Fritillaria herbs may be used as potential pain reliever, because peimine 2 not only blocks the Nav1.7 ion channel but also inhibits the Kv1.3 ion channel [125].
Anti-allergy effect
F. ussuriensis significantly inhibited the passive cutaneous anaphylaxis reaction and the release of histamine from rat peritoneal mast cells after extracted by ethanol. The activity was conducted the mechanism and interpreted as the inhibition of IL-6, IL-8, and TNF-α and the MAPKs phosphorylation [126]. The investigation of individual compounds showed peiminine 13 as the active agent [127].
Neuroprotective effect
Kaurane diterpene and two labdane diterpenes were isolated from dried bulbs of F. ebeiensis, a native species in China, prevented MPP+-induced neuronal cell death and displaying significant neuroprotective effect on human dopaminergic neuroblastoma SH-SY5Y cells [128, 129]. Peimisine-3-O-β-d-glucopyranoside 66 from F. unibracteata and F. yuminensis displayed moderate protective effect on the neurotoxicity of PC12 cell lines which suffer from rotenone [39]. LPS-induced Parkinson’s disease rat model has shown that peiminine inhibited the loss of dopaminergic neurons and microglial activation and obviously attenuated the behavioral dysfunction. In BV-2 cells, peiminine 13 significantly decreased the LPS-induced expression of pro-inflammatory mediators TNF-α, IL-6 and IL-1β, cyclooxygenase-2 and inducible nitric oxide synthase with the inhibition of the phosphorylation of extracellular signal-regulated kinase 1/2, AKT and NF-κB p65 [130].
Anti-diabetic effect
The diabetes is a major concern worldwide caused by high-sugar intake or other induction factors. The relevant investigation of verticinone 13 demonstrated the component exhibited hypoglycemic effects by increasing insulin secretion and glucose uptake. What’s more, the component can inhibit of carbohydrate-hydrolyzing enzymes on β-TC6 pancreatic and C2C12 skeletal muscle cells [131].
In general, these summarized modern pharmacological activities are consistent with the traditional records of efficacy, which showed that the dried bulbs are mainly used for respiratory diseases including their anti-tussive, expectorant, and anti-asthmatic effects. The crude materials were gradually developed to exhibit broader activities, such as antinociceptive, anti-allergy, neuroprotective, and anti-diabetic effects, with a promising utilization. However, the detailed interpretation is unclear, and modern scientific methods should be used to further study the pharmacological action to clarify the multi-target and multi-channel mechanism of Bei Mu materials. It is necessary for researchers to conduct pharmacokinetics and pharmacodynamics, which will be beneficial for studying the action mechanism and developing the medicinal value of Bei Mu. A limited number of studies focused on pharmacokinetics and pharmacodynamics, such as the pharmacokinetics of ebeiedinone in mouse blood [132] and pharmacokinetic study of delavinone in mice [133].
Toxicity
The dried bulbs of Fritillaria exhibit an extremely low toxicity. Li et al. [134] have summarized the toxicity properties of F. thunbergii, one of main medicinal herbs of Fritillaria, which indicated the dosage recorded in Pharmacopeia of China is safe and tremor and reduction of spontaneous motor activities were found in the 3 mg/kg group. The estimated median lethal dosage (LD50) of the water extract of F. thunbergii was 52.2 mg kg−1 body weight of mice in the acute toxicity tests, while a dose of 1 mg kg−1 body weight was no toxicity in the sub-chronic toxicity tests. The main adverse symptom was observed in body or head tremor and spontaneous motor activity reduction when the dosage is above the 1 mg kg−1 dose in male rats. There was no significant fluctuation observed in hematology, blood biochemistry, organ weight and organ histology [135]. The comparison analysis of acute toxicity between F. cirrhosa and F. pallidiflora indicated that the LD50 of the latter was 213.57 g kg−1 body weight, whereas the maximum feasible dose of the former was 452.14 g kg−1 in the studied mice. A histopathological analysis was performed, and the results reflect that inflammatory cell infiltration and cell edema in the liver, multinucleated giant cell proliferation in spleen, perivascular exudate and hemorrhage in lungs, and glomerulus atrophy in the kidney of mice after oral administrations of F. pallidiflora extracts. Only liver cell edema was observed in the F. cirrhosa group [80].
In addition, Fritillaria species have certain cytotoxicity to various tumor cells. The aqueous extract of F. cirrhosa induced mitotic aberrations and chromosomal instability in human colon epithelial NCM460 cells via the dysfunction pathway of mitotic checkpoint and cytokinesis failure [136, 137]. The aqueous and ethanolic extracts of F. imperialis presented cytotoxic, cytostatic, and pro-apoptotic activities against human liver cancer cells and breast cancer cells. The ethanolic extract was more potent than the aqueous extract [138].
Analytical techniques for chemical evaluation of Fritillaria species
Gas chromatography
Gas chromatography (GC) and its combination approach with mass spectrometry is a powerful tool for phytochemical analysis because of their high separation efficiency and short analysis time. The isosteroidal alkaloids in bulbs of Fritillaria were identified and determined by GC [81, 139] and GC–MS with pre-column derivatization [140]. However, given the polarity of isosteroidal alkaloids, they cannot be eluted from conventional GC columns, and derivatization processes are required prior to GC analysis. The constituents of volatile oil of F. cirrhosa were identified by GC–MS [69]. The metabolomic multivariable analysis using GC–MS dataset, including principle component analysis, partial least square discriminate analysis (PLS-DA), orthogonal PLS-DA, and heat map analysis were applied to assess the quality, distinguishing bulbs, stems, leaves, and flowers of F. thunbergii [141]. Moreover, 3-methyl-2-butene-1-thiol leading to the foxy odor in F. imperialis was identified using dynamic headspace gas chromatography–olfactometry and GC–MS [142].
Liquid chromatography
High performance liquid chromatography (HPLC) equipped with various detector is a conventional technique applied to determine the isosteroidal alkaloids in the dried bulbs of Fritillaria, in addition to evaporative light scattering detection (ELSD) [143–149] and charged aerosol detector [150] used for the sulfur-fumigation process. Nucleosides and nucleobases in Fritillaria bulbs have been mentioned in the above chapters and these components in different Fritillaria species were simultaneously determined by a HPLC diode-array detector (DAD) [151–153]. In addition, HPLC coupled with an ELSD and DAD was utilized for the quantitative analysis of four alkaloids and nine nucleosides and nucleobases from F. taipaiensis that had been cultivated in the same field for 2–6 years [154]. The overall quality evaluation was established via the combination strategy between HPLC fingerprint and chemometrics [155]. What’s more, there are both HPLC fingerprints and DNA barcoding combined to analyze Fritillaria species [156]. In general, ELSD is not a commonly utilized detector, whereas the qualitative and quantitative analyses of isosteroidal alkaloids by conventional LC with DAD detector is difficult given the lack of chromophore groups. Isosteroidal alkaloid derivatives are suitable for ultraviolet and fluorescent determination, showing a high detection sensitivity and meeting the requirements of analysis. Therefore, the development of derivatization reagents is a key to the quality control of isosteroidal alkaloids of Fritillaria.
Liquid chromatography with several mass spectrometry [triple quadrupole (QQQ), ion trap (IT), and time-of-flight (TOF)] has been extensively used in the authentication and isolation of chemical components of herbal medicine because of its high efficiency and accurate identification. The combination technique can be comprehensively applied to analyze those components with different polarity, equipped with atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI). Although LC–MS can be used to analyze trace components, good sample pretreatment, including extraction and enrichment, is the key to discover trace active components. The steroidal alkaloids in Fritillaria species were identified and characterized using LC/ESI-TOF-MS [157–159], HPLC–MS method [158, 160], LC-ESI-QQQ-MS [159, 161], RRLC-Q-TOF-MS coupled with multivariate statistical analysis [162], and UPLC-QTOF-MS [163, 164] with metabolomic strategy, which were used to determine potential markers for quality control at different growth stages [165]. In addition, the major alkaloids in F. hupehensis were determined by HPLC-ELSD and HPLC-ESI-MSn [166]. A new ionization technique, that is, wooden-tip ESI/MS has been applied to the chemical investigation of Fritillaria species. Wooden-tip ESI/MS [167], UPLC-Q-TOF/MS, and UPLC-TQ/MS [168], and matrix-assisted laser desorption/ionization mass spectrometry [169] combined with multivariate statistical analysis had been applied to discriminate officinal species of Fritillariae Bulbus and adulterated samples [167]. Alkaloids and flavonoids in five botanical parts (flowers, flower buds, stems, leaves, and bulbs) of F. thunbergii and isosteroidal alkaloids in vitro propagation of F. cirrhosa bulbs were identified by LC-LTQ-Orbitrap MSn [170] and LC-LTQ MSn [171], respectively. Other kinds of nucleosides and nucleobases were also authenticated and determined by HPLC–ESI–MS [172].
Pharmacokinetic investigations by LC–MS focused on isosteroidal alkaloids (such as peimine, peiminine, verticine, verticinone and isoverticine, puqietinone, imperialine, delavinone, and ebeiedinone) in rat [173–177], mouse [132, 133], and beagle dog [178]. Studies had also been conducted to investigate the tissue distribution and excretion of two components (peimine and peiminine) in rats on the basis of pharmacokinetics [179].
Apart from the analytical techniques mentioned above, a spectroscopy analytical method reflecting integrated metabolic information was used for the chemical assessment of Fritillaria species. The two-dimensional correlation analysis of Fourier transform-near infrared spectroscopy (NIR) has been successfully applied to quality control and assessment of Fritillariae Bulbus combined with scientific chemometrics [180]. NIR hyperspectral imaging is also a rapid and non-destructive technique to detect SO2 residual in the dried bulbs of F. thunbergii. Target analysis using chromatography is a quantitative analytical method, whereas non-deductive spectroscopy is a promising qualitative approach for further chemical assessment of Fritillaria.
Conclusion and future perspective
Geographical distribution has shown that Fritillaria species are naturally distributed in Asian and Europe, with huge species diversity (Fig. 1). Immature cultivation techniques cannot meet the entire market demand, especially those for F. cirrhosa and F. roylei, even though there has been a large cultivated scale of other species. Therefore, comprehensive physiological and ecological research should focus on artificial breeding, which will also protect the wild resources from excessive excavation. Modern molecular assisted breeding may become an important topic for overcoming the cultivation obstacle of several high-profit species avoid the long growth period of their perennial counterparts.
There is no doubt that alkaloids are the prominent constituents in the medicinal parts of Fritillaria species, and they have become the evaluation index for ensuring whether the target objective can be used as a substitution for the endangered medicinal Bei Mu. However, natural herbal medicines are inherently complex mixtures, with varying constituents depending on species origins or botanical parts, and different from their pharmaceutical counterparts. In addition, the quantities and identities of the authenticated components are unclear. Moreover, pharmacological activity may be synergetic variation with these factors. It is worthwhile to note that some degradation and combination reactions occur during boiling and metabolism in the body and are therefore promising for investigation in the future experimental or clinical studies. Other non-alkaloids are also important constituents for medicinal purposes. The chemically modified components or gold nanoparticles, such as polysaccharide-zinc complex of F. ussuriensis [181] and F. cirrhosa gold nanoparticles [182], show a promising tendency, that the amount of substitution zinc determined the antioxidant activity. There are little research focusing the structure modification of chemical components isolated from Fritillaria, which should raise more attention in the further analytical study. From a serious perspective, the way in which the original plants are classified and authenticated is more important than the relative products from which they are prepared, and much of the research investigated in the further should be paid more attention, regardless of their similar chemical components.
The extensive pharmacological activity and clinical application value show the development and utilization potential of the bulbs Fritillaria. However, their mechanisms of action are still unclear. Therefore, modern scientific methods should be used to further study the pharmacological actions to clarify the multi-target and multi-channel mechanism of Bei Mu materials. The pharmacological investigation should focus on the potential metabolism of the separated components in future, which have been conducted the detailed research in animals, and explore the relationship between their pharmacokinetics and pharmacodynamics, in addition, new dosage forms, administration methods, such as the research and development of nano-preparations and inhalation preparations, and Fritillaria materials should be developed and applied.
Supplementary Information
Additional file 1: Table S1. Traditional usage in national minorities of China. Table S2. Traditional usage of Fritillaria species in different countries. Table S3. Distribution information of alkaloids in Fritillaria species. Table S4. Distribution information of terpenoids in Fritillaria species. Table S5. Distribution information of steroidal saponins in Fritillaria species. Table S6. Distribution information of phenylpropanoids in Fritillaria species. Table S7. Distribution information of fatty acids in Fritillaria species. Table S8. Distribution information of sterides in Fritillaria species. Table S9. Distribution information of other components in Fritillaria species. Table S10. Pharmacological activities of Fritillaria species.
Acknowledgements
Not applicable.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
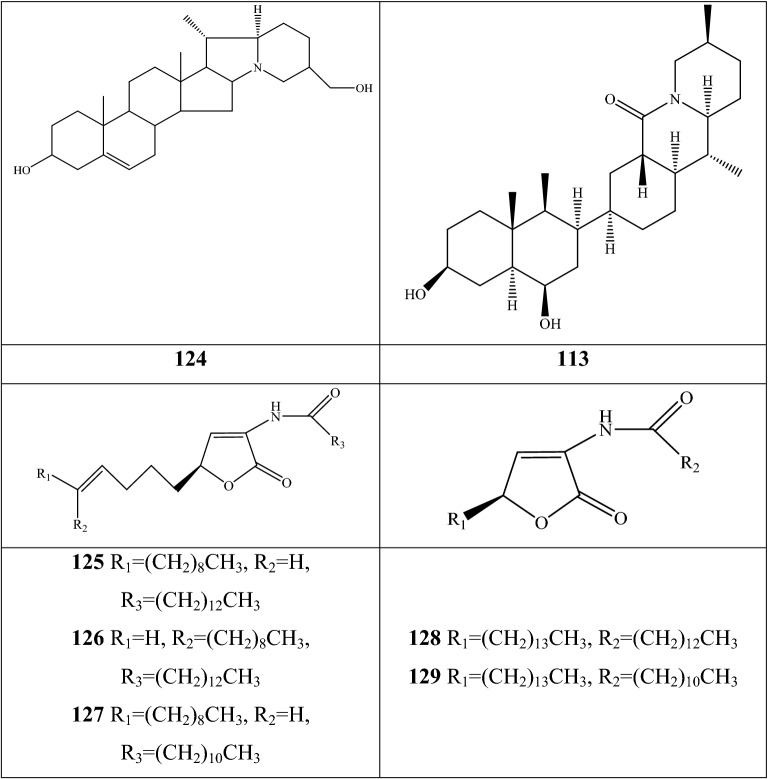
- COVID-19
Coronavirus disease 19
- BC
Before Christ
- AD
Anno Domini
- ALI
Acute lung injury
- LPS
Lipopolysaccharide
- MUC5AC
Mucin 5 subtype AC
- GBM
Glioblastoma
- NSCLC
Non-small cell lung cancer
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- JNK
C-Jun N-terminal kinase
- Nrf2
Nuclear factor 2
- ACE
Angiotensin converting enzyme
- AchE
Acetylcholinesterase
- BchE
Butyrylcholinesterase
- LD50
Lethal dosage
- GC
Gas chromatography
- MS
Mass spectra
- HPLC
High performance liquid chromatography
- ELSD
Evaporative light scattering detection
- DAD
Diode-array detector
- QQQ
Triple quadrupole
- IT
Ion trap
- TOF
Time-of-flight
- APCI
Atmospheric pressure chemical ionization
- ESI
Electrospray ionization
- PLS-DA
Partial least square discriminate analysis
- NIR
Near infrared spectroscopy
Authors’ contributions
YW, HH, QR and HYH contributed to the writing of the manuscript; TY and XL are responsible for the language and framework. All authors read and approved the final manuscript.
Funding
The study was supported by the National Key Research and Development Program (2019YFC1710601), Independent project of Institute of Chinese Materia Medica, Academy of Chinese Medical Sciences (ZXKT17021) and National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2019ZX09201005-006-001; 2019ZX09201005).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ye Wang and Hongping Hou—Co-first author
References
- 1.Liu Q, Gao Y, Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid Med Cell Longev. 2019;2019:1–17. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W, Zheng X, Chung KF, Zhong N. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. The Lancet. 2016;388:1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 3.Collaborators GCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Data Platform. World health data platform, vol. 2021.
- 5.Xian Y, Zhang J, Bian Z, Zhou H, Zhang Z, Lin Z, et al. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Jiang J, Wang C, Fitzgerald M, Hu W, Zhou Y, et al. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm Sin B. 2020;10:1192–1204. doi: 10.1016/j.apsb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Cao F, Wang Y, Xu X, Sun Y, Li J, et al. The efficacy and safety of Jinhua Qinggan granule (JHQG) in the treatment of coronavirus disease 2019 (COVID-19) Medicine. 2020;99:e20531. doi: 10.1097/MD.0000000000020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y, Yin Z, Dai J, Yang Z, Ye B, Ma Y, et al. Evidence-based complementary and alternative medicine exploring active components and mechanism of Jinhua Qinggan Granules in treatment of COVID-19 based on virus-host interaction. Nat Prod Commun. 2020;15:1–11. [Google Scholar]
- 9.Hao D, Gu X, Xiao P, Peng Y. Phytochemical and biological research of Fritillaria medicine resources. Chin J Nat Med. 2013;11:330–344. doi: 10.3724/SP.J.1009.2013.00330. [DOI] [PubMed] [Google Scholar]
- 10.Majnooni MB, Fakhri S, Shokoohinia Y, Kiyani N, Stage K, Mohammadi P, et al. Phytochemicals: potential therapeutic interventions against coronavirus-associated lung injury. Front Pharmacol. 2020;11:1–22. doi: 10.3389/fphar.2020.588467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Marco R, Accordini S, Cerveri I, Corsico A, Antó JM, Künzli N, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Jiang Y, Li P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat Prod Rep. 2006;23:735. doi: 10.1039/b609306j. [DOI] [PubMed] [Google Scholar]
- 13.Olsen CS, Larsen HO. Alpine medicinal plant trade and Himalayan mountain livelihood strategies. Geogr J. 2003;169:243–254. doi: 10.1111/1475-4959.00088. [DOI] [Google Scholar]
- 14.Li X, Liu L, Gu X, Xiang J. Heavy collecting induces smaller and deeper Fritillariae Cirrhosae Bulbus in the wild. Plant Divers. 2017;39:208–213. doi: 10.1016/j.pld.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Guo S, Guan Y, Li M, An Y, Liu H. The research progress of medicinal plants Fritillaria. Mol Plant Breed. 2019;17:6198–6206. [Google Scholar]
- 16.Hu Z, Xiong Q, Xiao X. Research progress on the breeding technology of Fritillaria cirrhosa D. Don Anhui Agric Sci Bull. 2017;23:133–135. [Google Scholar]
- 17.Wang S, Gao W, Yu L, Xiao P. Progress of taxonomic study on Fritillaria (Liliaceae) medicinal plant. China J Chin Mater Med. 2007;32:1609–1614. [PubMed] [Google Scholar]
- 18.Xiao P, Jiang Y, Li P, Luo Y, Liu Y. The botanical origin and pharmacophylogenetic treatment of Chinese materia medica Beimu. Acta Phytotax Sin. 2007;45:473–487. doi: 10.1360/aps06113. [DOI] [Google Scholar]
- 19.Cunningham AB, Brinckmann JA, Pei SJ, Luo P, Schippmann U, Long X, et al. High altitude species, high profits: can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J Ethnopharmacol. 2018;223:142–151. doi: 10.1016/j.jep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Strategy for Plant Conservation. (2021). Strategy for plant conservation, vol. 2021.
- 21.Wei M, Zhao J, Zhao X, Jin Y, Zhang W, Peng H, et al. Textual research on Fritillaria Bulbus in Chinese classical prescription. Mod Chin Med. 2020;22:1201–1213. [Google Scholar]
- 22.Wang Y, Aamer M, Aslay M, Sener B, Khan F, Wahab A, et al. A new steroidal alkaloid from Fritillaria michailovskyi Fomin. Nat Prod Res. 2020 doi: 10.1080/14786419.2020.1786828. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wang H, Chen C, Pi H, Raun H, Zhang P, et al. Addictive evaluation of cholic acid-verticinone ester, a potential cough therapeutic agent with agonist action of opioid receptor. Acta Pharmacol Sin. 2009;30:559–566. doi: 10.1038/aps.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura Y, Kaneko K, Shiro M, Chen Y, Hsh H, Lee P, et al. Tortifoline, a novel (20S, 22R)-5α-cevanine alkaloid from Fritillaria tortifolia. Chem Pharm Bull. 1989;37:1514–1516. doi: 10.1248/cpb.37.1514. [DOI] [Google Scholar]
- 25.Hu Z, Zong J, Yili A, Yu M, Aisa HA, Hou A. Isosteroidal alkaloids from the bulbs of Fritillaria tortifolia. Fitoterapia. 2018;131:112–118. doi: 10.1016/j.fitote.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Duan D. A new alkaloid from Fritillaria ussuriensis Maxim. Fitoterapia. 2012;83:137–141. doi: 10.1016/j.fitote.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Feng Y, Lu X, Nie J, Li W, Wang L, et al. Isosteroidal alkaloids as potent dual-binding site inhibitors of both acetylcholinesterase and butyrylcholinesterase from the bulbs of Fritillaria walujewii. Eur J Med Chem. 2017;137:280–291. doi: 10.1016/j.ejmech.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Yang X, Zhang P, Zhou X, Ruan H, Pi H, et al. Cytotoxic alkaloids from the bulbs of Fritillaria hupehensis. C&B. 2008;5:259–266. doi: 10.1002/cbdv.200890023. [DOI] [PubMed] [Google Scholar]
- 29.Shou Q, Wohlmuth H, He X, Liu L, Shen Z. Chemical constituents from Fritillaria anhuiensis. Biochem Syst Ecol. 2012;45:16–19. doi: 10.1016/j.bse.2012.07.002. [DOI] [Google Scholar]
- 30.Zhang J, Lao A, Xu R. Studies on chemical constituents of Fritillaria yuminensis. Acta Botanica Sin. 1993;35:963–967. [Google Scholar]
- 31.Wu J, Wang Y, Ling D. Study on chemical constituent of hubeibeimu (Fritillaria hupehensis Hsiao et K.C. Hsia) V isolation and identification of hupehensine. Acta Pharm Sin. 1986;21:546–550. [PubMed] [Google Scholar]
- 32.Xu D, Huang E, Wang S, Wen X, Wu X. Studies on the chemical constituents of Fritillaria palidiflora Schrenk. Acta Botanica Sin. 1990;32:789–793. [Google Scholar]
- 33.Chen Q, Zhu L, Xu Y, Fan J. A new steroidal alkaloid from the bulbs of Fritillaria wabuensia. Acta Pharm Sin. 2004;39:348–350. [PubMed] [Google Scholar]
- 34.Huang S, Zhou X, Wen J, Wang C, Wang H, Shan L, et al. A novel steroidal alkaloid from Fritillaria shuchengensis. J Nat Med. 2013;67:647–651. doi: 10.1007/s11418-012-0702-7. [DOI] [PubMed] [Google Scholar]
- 35.Pi H, Ruan H, Zhang Y, Niu L, Wu J. Steroidal alkaloids from bulbs of Fritillaria lichuanensis. J Asian Nat Prod Res. 2006;8:133–136. doi: 10.1080/10286020500530797. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Li P, Li H, Yu H. New steroidal alkaloids from the bulbs of Fritillaria puqiensis. Steroids. 2006;71:843–848. doi: 10.1016/j.steroids.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar MN, Atta-ur-Rahman, Choudhary MI, Sener B, Erdogan I, Tsuda Y. New class of steroidal alkaloids from Fritillaria imperialis. Phytochemistry. 2003;63:115–122. doi: 10.1016/S0031-9422(02)00569-1. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Jiang Y, Li P, Ye W, Puqienine F. a novel veratramine alkaloid from the bulbs of Fritillaria puqiensis. Chem Pharm Bull. 2006;54:722–724. doi: 10.1248/cpb.54.722. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Zheng Z, Yu D. Steroidal alkaloids from the bulbs of Fritillaria unibracteata. J Asian Nat Prod Res. 2011;13:1098–1103. doi: 10.1080/10286020.2011.619980. [DOI] [PubMed] [Google Scholar]
- 40.Jian-fa Z, Zhu H, Aisa H, Chun L, Yili A, Ai-jun H. Studies on alkaloid constituents of Fritillaria yuminensis. China J Chin Materia Med. 2019;44:495–499. doi: 10.19540/j.cnki.cjcmm.20181121.005. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Yili A, Li J, Muhamat A, Aisa HA. New isosteroidal alkaloids with tracheal relaxant effect from the bulbs of Fritillaria pallidiflora Schrenk. Bioorg Med Chem Lett. 2016;26:1983–1987. doi: 10.1016/j.bmcl.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 42.An J, Zhou J, Li H, Jiang Y, Li P. Puqienine E: an angiotensin converting enzyme inhibitory steroidal alkaloid from Fritillaria puqiensis. Fitoterapia. 2010;81:149–152. doi: 10.1016/j.fitote.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Li H, Li P, Cai Z, Ye W. Steroidal alkaloids from the bulbs of Fritillaria puqiensis. J Nat Prod. 2005;68:264–267. doi: 10.1021/np0497649. [DOI] [PubMed] [Google Scholar]
- 44.Kitajima J, Komori T, Kawasaki T, Schulten H. Basic steroid saponins from aerial parts of Fritillaria thunbergii. Phytochemistry. 1982;21:187–192. doi: 10.1016/0031-9422(82)80040-X. [DOI] [Google Scholar]
- 45.Xu D, Wang S, Huang E, Xu M. New steroidal saponin of stem and leaf of Fritillaria ussuriensis Maxim. Acta Botanica Sin. 1989;31:285–288. [Google Scholar]
- 46.Cao X, Chen S, Li J, Xiao P, Chen S. Steroidal alkaloids from the bulbs of Fritillaria delavayi Franch. (Liliaceae) Biochem Syst Ecol. 2008;36:665–668. doi: 10.1016/j.bse.2008.05.002. [DOI] [Google Scholar]
- 47.Shou QY, Tan Q, Wu SZ. Two 22S-solanidine-type steroidal alkaloids from Fritillaria anhuiensis. Fitoterapia. 2010;81:81–84. doi: 10.1016/j.fitote.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Xu M, Xu D, Huang E, Zheng W. Alkaloids research of Fritillaria ussuriensis Maxim flower. Bull Chin Mater Med. 1988;13:32–33. [Google Scholar]
- 49.Xu D, Huang E, Xu M, Zheng W, Sun Z. Chemical components of stems and leaves in Fritillaria ussuriensis. Trad Chin Med J. 1986;11:40–41. [Google Scholar]
- 50.Lakey DK. Ecological status of high altitude medicinal plants and their sustainability: Lingshi. Bhutan BMC Ecol. 2016;16:45. doi: 10.1186/s12898-016-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Peng C, He C, Liu J, He Y, Guo L, et al. New amino butenolides from the bulbs of Fritillaria unibracteata. Fitoterapia. 2014;98:53–58. doi: 10.1016/j.fitote.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Abbas F, Ke Y, Yu R, Yue Y, Amanullah S, Jahangir MM, et al. Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta. 2017;246:803–816. doi: 10.1007/s00425-017-2749-x. [DOI] [PubMed] [Google Scholar]
- 53.Pi H, Zhang P, Zhu T, Ruan H, Zhang Y, Sun H, et al. A new cycloartane triterpenoid from the leaves and stems of Fritillaria hupehensis. Chin Chem Lett. 2007;18:418–420. doi: 10.1016/j.cclet.2007.02.005. [DOI] [Google Scholar]
- 54.Pi H, Zhang P, Ruan H, Zhang Y, Sun H, Wu J. Two new triterpenoids from the leaves and stems of Fritillaria hupehensis. J Asian Nat Prod Res. 2009;11:779–782. doi: 10.1080/10286020903071050. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo Y, Shinoda D, Nakamaru A, Mimaki Y. Steroidal glycosides from the bulbs of Fritillaria meleagris and their cytotoxic activities. Steroids. 2013;78:670–682. doi: 10.1016/j.steroids.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Shen S, Chen C, Bu R, Ga L, Li G, Tan Y, et al. Three new steroidal saponins from Fritillaria pallidiflora. J Asian Nat Prod Res. 2011;13:1014–1022. doi: 10.1080/10286020.2011.619979. [DOI] [PubMed] [Google Scholar]
- 57.Shen S, Li G, Huang J, Chen C, Ren B, Lu G, et al. Steroidal saponins from Fritillaria pallidiflora Schrenk. Fitoterapia. 2012;83:785–794. doi: 10.1016/j.fitote.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Zhang J, Zhang J, Wang Y. Research progress in chemical constituents in plants of Paris L. and their pharmacological effects. Chin Trad Herbal Drugs. 2018;47:3301–3322. [Google Scholar]
- 59.Li W, Bu R, Chen C, Yu T, Wang J. Isolation and identification of non-alkaloid constituents from Fritillaria pallidiflora Schrenk. Mod Chin Med. 2013;15:175–177. [Google Scholar]
- 60.Shi L, Lu X, Meng L, Lv W, Liu Q, Liu Y. Chemical constituents from the bulbs of Fritillaria walujewii. J Chin Med Mater. 2017;40:2098–2100. [Google Scholar]
- 61.Ruan H, Zhang Y, Wu J, Deng S, Sun H, Fujita T. Structure of a novel diterpenoid ester, fritillahupehin from bulbs of Fritillaria hupehensis Hsiao and K.C. Hsia. Fitoterapia. 2002;73:288–291. doi: 10.1016/S0367-326X(02)00085-0. [DOI] [PubMed] [Google Scholar]
- 62.Yan M, Jin X, Xu D. Studies on the chemical constituents of the stems and leaves of Thunberg Fritillary (Fritillaria thunbergii) Chin Trad Herbal Drugs. 1994;25:344–346. [Google Scholar]
- 63.Zhang J, Lao A, Xu R. Studies on the chemical constituents of fresh bulbs of Fritillaria thunbergii Miq. China J Chin Mater Med. 1993;18:354–355. [PubMed] [Google Scholar]
- 64.Ying-Ma, Gao T, Wang J, Tian L, Yun-Zhu, Cheng S, et al. Chemical constituents of the flowers of Fritillaria pallidiflora. Chem Nat Compd. 2016;52:309–310. doi: 10.1007/s10600-016-1624-5. [DOI] [Google Scholar]
- 65.Wu J, Pu Q, Jiang H, Jin B. Research of chemical components of Fritillaria genus plants VII. Isolation and authentication of non-alkaloid components. Chin Trad Herbal Drugs. 1989;20:4–6. [Google Scholar]
- 66.Zhang J, Lao A, Chen Q, Xu R. Studies on the chemcial constituents of Dongbeimu (Fritillaria thunbergii var. chekiangensis) (I) Chin Trad Herbal Drugs. 1993;24:341–342. [Google Scholar]
- 67.Zhang Z, Fan C. Research of chemical components of Jiangxi Fritillaria monantha Migo (I). Chin Trad Herbal Drugs. 1994;48.
- 68.Yue-xian J, Wei-qin P, Hong-yan W, Guo-xu M, Lei-ling S, Jing Z. Chemical constituents from plant of Fritillaria palldiflora. Chin Trad Herbal Drugs. 2019;50:2534–2538. [Google Scholar]
- 69.Wang X, Li Y. Analysis of volatile oil of Fritillaria cirrhosa D. Don by GC-MS Asian J Chem. 2013;25:3252–3254. [Google Scholar]
- 70.Shen S, Li G, Huang J, Tan Y, Chen C, Ren B, et al. Chemical constituents from Fritillaria pallidiflora Schrenk. Biochem Syst Ecol. 2012;45:183–187. doi: 10.1016/j.bse.2012.07.034. [DOI] [Google Scholar]
- 71.Wei P, Ting H, Qing-Chun L, Lu-Ping Q. Chemical constituents of the flower of Fritillaria thunbergii. Chem Nat Compd. 2012;48:491–492. doi: 10.1007/s10600-012-0286-1. [DOI] [Google Scholar]
- 72.Liu X, Li L, Liu J, Qiao J, Zhao G. Metabolic engineering Escherichia coli for efficient production of icariside D2. Biotechnol Biofuels. 2019;12:1–12. doi: 10.1186/s13068-018-1346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan F, Su T, Liu Y, Hou K, Chen C, Wu W. Extraction, purification and antioxidation of a polysaccharide from Fritillaria unibracteata var. wabuensis. Int J Biol Macromol. 2018;112:1073–1083. doi: 10.1016/j.ijbiomac.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 74.Sun C, Sun Y, Song J, Li C, Li X, Zhang X, et al. Discovery of genes related to steroidal alkaloid biosynthesis in Fritillaria cirrhosa by generating and mining a dataset of expressed sequence tags (ESTs) J Med Plants Res. 2011;5:5307–5314. [Google Scholar]
- 75.Eshaghi M, Shiran B, Fallahi H, Ravash R, Đeri BB. Identification of genes involved in steroid alkaloid biosynthesis in Fritillaria imperialis via de novo transcriptomics. Genomics. 2019;111:1360–1372. doi: 10.1016/j.ygeno.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Bai Y, Liu Y, Su Z, Ma Y, Ren C, Zhao R, et al. Gene editing as a promising approach for respiratory diseases. J Med Genet. 2018;55:143–149. doi: 10.1136/jmedgenet-2017-104960. [DOI] [PubMed] [Google Scholar]
- 77.Li P, Ji H, Xu G, Xu L. Study on the antitussive and expectorant effects of Fritillary herbs. J China Pharm Univ. 1993;24:360–362. [Google Scholar]
- 78.Wang D, Zhu J, Wang S, Wang X, Ou Y, Wei D, et al. Antitussive, expectorant and anti-inflammatory alkaloids from bulbus Fritillariae cirrhosae. Fitoterapia. 2011;82:1290–1294. doi: 10.1016/j.fitote.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Wang D, Wang S, Chen X, Xu X, Zhu J, Nie L, et al. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from bulbus of Fritillaria wabuensis. J Ethnopharmacol. 2012;139:189–193. doi: 10.1016/j.jep.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 80.Xu Y, Ming TW, Gaun TKW, Wang S, Ye B. A comparative assessment of acute oral toxicity and traditional pharmacological activities between extracts of Fritillaria cirrhosae Bulbus and Fritillaria pallidiflora Bulbus. J Ethnopharmacol. 2019;238:111853. doi: 10.1016/j.jep.2019.111853. [DOI] [PubMed] [Google Scholar]
- 81.Wu X, Chan S, Ma J, Li P, Shaw P, Lin G. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J Ethnopharmacol. 2018;210:39–46. doi: 10.1016/j.jep.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 82.Gilani AH, Shaheen F, Christopoulos A, Mitchelson F. Interaction of ebeinone, an alkaloid from Fritillaria imperialis, at two muscarinic acetylcholine receptor subtypes. Life Sci. 1997;60:535–544. doi: 10.1016/S0024-3205(96)00691-1. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y, Ji H, Lin B, Jiang Y, Li P. The effects of five alkaloids from bulbus Fritillariae on the concentration of cAMP in HEK cells transfected with muscarinic M2 receptor plasmid. Am J Chin Med. 2006;34:901–910. doi: 10.1142/S0192415X06004375. [DOI] [PubMed] [Google Scholar]
- 84.Lin B, Ji H, Li P, Jiang Y, Fang W. Selective antagonism activity of alkaloids from bulbs Fritillariae at muscarinic receptors: functional studies. Eur J Pharmacol. 2006;551:125–130. doi: 10.1016/j.ejphar.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Li P, Kwan Y, Lin G. In vitro tracheobronchial relaxation of Fritillaria alkaloids. Chin J Nat Med. 2011;9:345–353. [Google Scholar]
- 86.Wang L, Han C, Wang P. Comparison of antitussive and expectorant effects of Wanbei, Chuanbei and Zhebei. Anhui Med J. 1993;14:57–58. [Google Scholar]
- 87.Yeum H, Lee Y, Kim S, Roh S, Lee J, Seo Y. Fritillaria cirrhosa, Anemarrhena asphodeloides, Lee-Mo-Tang and cyclosporine a inhibit ovalbumin-induced eosinophil accumulation and Th2-mediated bronchial hyperresponsiveness in a murine model of asthma. Basic Clin Pharmacol Toxicol. 2007;100:205–213. doi: 10.1111/j.1742-7843.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang D, Du Q, Li H, Wang S. The isosteroid alkaloid imperialine from bulbs of Fritillaria cirrhosa mitigates pulmonary functional and structural impairment and suppresses inflammatory response in a COPD-like rat model. Mediat Inflamm. 2016;2016:1–17. doi: 10.1155/2016/4192483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du B, Cao L, Wang K, Miu J, Yao L, Xu Z, et al. Peiminine attenuates acute lung injury induced by LPS through inhibiting lipid rafts formation. Inflammation. 2020;43:1110–1119. doi: 10.1007/s10753-020-01198-w. [DOI] [PubMed] [Google Scholar]
- 90.Kim EJ, Yoon YP, Woo KW, Kim J, Min SY, Lee HJ, et al. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine. 2016;23:95–104. doi: 10.1016/j.phymed.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 91.Pae H, Oh H, Choi B, Oh G, Paik S, Jeong S, et al. Differentiation-inducing effects of verticinone, an isosteroidal alkaloid isolated from the bulbus of Fritillaria ussuriensis, on human promyelocytic leukemia HL-60 cells. Biol Pharm Bull. 2002;25:1409–1411. doi: 10.1248/bpb.25.1409. [DOI] [PubMed] [Google Scholar]
- 92.Yun YG, Jeon BH, Lee JH, Lee SK, Lee HJ, Jung KH, et al. Verticinone induces cell cycle arrest and apoptosis in immortalized and malignant human oral keratinocytes. Phytother Res. 2008;22:416–423. doi: 10.1002/ptr.2345. [DOI] [PubMed] [Google Scholar]
- 93.Kavandi L, Lee LR, Bokhari AA, Pirog JE, Jiang Y, Ahmad KA, et al. The Chinese herbs Scutellaria baicalensis and Fritillaria cirrhosa target NFκB to inhibit proliferation of ovarian and endometrial cancer cells. Mol Carcinog. 2015;54:368–378. doi: 10.1002/mc.22107. [DOI] [PubMed] [Google Scholar]
- 94.Bokhari AA, Syed V. Inhibition of transforming growth factor-β (TGF-β) signaling by Scutellaria baicalensis and Fritillaria cirrhosa extracts in endometrial cancer. J Cell Biochem. 2015;116:1797–1805. doi: 10.1002/jcb.25138. [DOI] [PubMed] [Google Scholar]
- 95.Wang D, Jiang Y, Wu K, Wang S, Wang Y. Evaluation of antitumor property of extracts and steroidal alkaloids from the cultivated Bulbus Fritillariae ussuriensis and preliminary investigation of its mechanism of action. BMC Complement Altern Med. 2015;15:29. doi: 10.1186/s12906-015-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abu N, Yeap SK, Pauzi AZM, Akhtar MN, Zamberi NR, Ismail J, et al. Dual regulation of cell death and cell survival upon induction of cellular stress by isopimara-7,15-dien-19-oic Acid in cervical cancer, heLa cells in vitro. Front Pharmacol. 2016;7:89. doi: 10.3389/fphar.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhi Z, Qinsi H, Liting X, Wenhao C, Hua B, Zhe Z, et al. The peiminine stimulating autophagy in human colorectal carcinoma cells via AMPK pathway by SQSTM1. Open Life Sci. 2016;11:358–366. doi: 10.1515/biol-2016-0047. [DOI] [Google Scholar]
- 98.Zhao B, Shen C, Zheng Z, Wang X, Zhao W, Chen X, et al. Peiminine inhibits glioblastoma in vitro and in vivo through cell cycle arrest and autophagic flux blocking. Cell Physiol Biochem. 2018;51:1566–1583. doi: 10.1159/000495646. [DOI] [PubMed] [Google Scholar]
- 99.Lin Q, Qu M, Patra HK, He S, Wang L, Hu X, et al. Mechanistic and therapeutic study of novel anti-tumor function of natural compound imperialine for treating non-small cell lung cancer. J Ethnopharmacol. 2020;247:112283. doi: 10.1016/j.jep.2019.112283. [DOI] [PubMed] [Google Scholar]
- 100.Tan H, Zhang G, Yang X, Jing T, Shen D, Wang X. Peimine inhibits the growth and motility of prostate cancer cells and induces apoptosis by disruption of intracellular calcium homeostasis through Ca2+ /CaMKII/JNK pathway. J Cell Biochem. 2019;121:81–92. doi: 10.1002/jcb.28870. [DOI] [PubMed] [Google Scholar]
- 101.Hu K, Zheng H, Qi J, Hou L, Zuo M, Chen X, et al. Study on the reversal of multidrug resistance of Fritillaria thunbergii in leukemia cells. Chin J Hematol. 1999;20:650–651. [Google Scholar]
- 102.Wang D, Yang J, Du Q, Li H, Wang S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J Ethnopharmacol. 2016;193:150–158. doi: 10.1016/j.jep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Shou Q, Tan Q, Shen Z. A novel sulfur-containing diterpenoid from Fritillaria anhuiensis. Tetrahedron Lett. 2009;50:4185–4187. doi: 10.1016/j.tetlet.2009.05.002. [DOI] [Google Scholar]
- 104.Wu K, Mo C, Xiao H, Jiang Y, Ye B, Wang S. Imperialine and verticinone from bulbs of Fritillaria wabuensis inhibit pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Planta Med. 2015;81:821–829. doi: 10.1055/s-0035-1546170. [DOI] [PubMed] [Google Scholar]
- 105.Zhou M, Ma X, Ding G, Wang Z, Liu D, Tong Y, et al. Comparison and evaluation of antimuscarinic and anti-inflammatory effects of five Bulbus Fritillariae species based on UPLC-Q/TOF integrated dual-luciferase reporter assay, PCA and ANN analysis. J Chromatogr B. 2017;1041–1042:60–69. doi: 10.1016/j.jchromb.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Fan B, Li T, Xu S, Chen L, Wei G, Qian C. Efficient, accurate and comprehensive evaluation of polysaccharides from Fritillaria and their inhibitory responses to mouse inflammation. Food Funct. 2019;10:7913–7925. doi: 10.1039/C9FO02209K. [DOI] [PubMed] [Google Scholar]
- 107.Luo Z, Zheng B, Jiang B, Xue X, Xue E, Zhou Y. Peiminine inhibits the IL-1β induced inflammatory response in mouse articular chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2019;10:2198–2208. doi: 10.1039/C9FO00307J. [DOI] [PubMed] [Google Scholar]
- 108.Chen K, Lv ZT, Zhou CH, Liang S, Huang W, Wang ZG, et al. Peimine suppresses interleukin-1β-induced inflammation via MAPK downregulation in chondrocytes. Int J Mol Med. 2019;43:2241–2251. doi: 10.3892/ijmm.2019.4141. [DOI] [PubMed] [Google Scholar]
- 109.Liu S, Yang T, Ming TW, Gaun TKW, Zhou T, Wang S, et al. Isosteroid alkaloids with different chemical structures from Fritillariae Cirrhosae Bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. Int Immunopharmacol. 2020;78:106047. doi: 10.1016/j.intimp.2019.106047. [DOI] [PubMed] [Google Scholar]
- 110.Kang DG, Sohn EJ, Lee YM, Lee AS, Han JH, Kim TY, et al. Effects of bulbus Fritillaria water extract on blood pressure and renal functions in the L-NAME-induced hypertensive rats. J Ethnopharmacol. 2004;91:51–56. doi: 10.1016/j.jep.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 111.Kang DG, Oh H, Cho DK, Kwon EK, Han JH, Lee HS. Effects of bulb of Fritillaria ussuriensis Maxim. on angiotensin converting enzyme and vascular release of NO/cGMP in rats. J Ethnopharmacol. 2002;81:49–55. doi: 10.1016/S0378-8741(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 112.Oh H, Kang DG, Lee S, Lee Y, Lee HS. Angiotensin converting enzyme (ACE) inhibitory alkaloids from Fritillaria ussuriensis. Planta Med. 2003;69:564–565. doi: 10.1055/s-2003-40659. [DOI] [PubMed] [Google Scholar]
- 113.Adewusi EA, Moodley N, Steenkamp V. Medicinal plants with cholinesterase inhibitory activity a review. Afr J Biotech. 2010;49:8257–8276. [Google Scholar]
- 114.Atta-Ur-Rahman, Akhtar MN, Choudhary MI, Tsuda Y, Sener B, Khalid A, et al. New steroidal alkaloids from Fritillaria imperialis and their cholinesterase inhibiting activities. Chem Pharm Bull. 2002;50:1013–1016. doi: 10.1248/cpb.50.1013. [DOI] [PubMed] [Google Scholar]
- 115.Lin B, Ji H, Li P, Fang W, Jiang Y. Inhibitors of acetylcholine esterasein vitro-screening of steroidal alkaloids from Fritillaria species. Planta Med. 2006;72:814–818. doi: 10.1055/s-2006-947168. [DOI] [PubMed] [Google Scholar]
- 116.Abdalla MA, Zidorn C. The genus Tragopogon (Asteraceae): a review of its traditional uses, phytochemistry, and pharmacological properties. J Ethnopharmacol. 2020;250:112466. doi: 10.1016/j.jep.2019.112466. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-gelicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98:329–333. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 118.Kim S, Shin D, Oh M, Chung S, Lee J, Chang I, et al. Inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by β-itosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci Biotechnol Biochem. 2003;67:2477–2479. doi: 10.1271/bbb.67.2477. [DOI] [PubMed] [Google Scholar]
- 119.Kim M, Nguyen D, Heo Y, Park KH, Paik H, Kim YB. Antiviral activity of Fritillaria thunbergii extract against human influenza virus H1N1 (PR8) in vitro, in ovo and in vivo. J Microbiol Biotechnol. 2020;30:172–177. doi: 10.4014/jmb.1908.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X, Gao W, Huang L, Huang L, Liu C. In vitro antioxidant and in vivo anti-inflammatory potential of crude non-alkaloid fractions from Fritillaria ussuriensis Maxim. Lat Am J Pharm. 2010;8:1328–1335. [Google Scholar]
- 121.Ruan X, Yang L, Cui W, Zhang M, Li Z, Liu B, et al. Optimization of supercritical fluid extraction of total alkaloids, peimisine, peimine and peiminine from the bulb of Fritillaria thunbergii Miq, and evaluation of antioxidant activities of the extracts. Materials. 2016;9:524. doi: 10.3390/ma9070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rozi P, Abuduwaili A, Mutailifu P, Gao Y, Rakhmanberdieva R, Aisa HA, et al. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int J Biol Macromol. 2019;131:97–106. doi: 10.1016/j.ijbiomac.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Liu S, Yang T, Ming TW, Gaun TKW, Zhou T, Wang S, et al. Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia. 2020;140:104434. doi: 10.1016/j.fitote.2019.104434. [DOI] [PubMed] [Google Scholar]
- 124.Xu F, Xu S, Wang L, Chen C, Zhou X, Lu Y, et al. Antinociceptive efficacy of verticinone in murine models of inflammatory pain and paclitaxel induced neuropathic pain. Biol Pharm Bull. 2011;34:1377–1382. doi: 10.1248/bpb.34.1377. [DOI] [PubMed] [Google Scholar]
- 125.Xu J, Zhao W, Pan L, Zhang A, Chen Q, Xu K, et al. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia. 2016;111:1–6. doi: 10.1016/j.fitote.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 126.Cho I, Lee MJ, Kim J, Han NY, Shin KW, Sohn Y, et al. Fritillaria ussuriensis extract inhibits the production of inflammatory cytokine and MAPKs in mast cells. Biosci Biotechnol Biochem. 2011;75:1440–1445. doi: 10.1271/bbb.110076. [DOI] [PubMed] [Google Scholar]
- 127.Lee B, Kim E, Kim J, Min J, Jeong D, Jun J, et al. Antiallergic effects of peiminine through the regulation of inflammatory mediators in HMC-1 cells. Immunopharm Immunot. 2015;37:351–358. doi: 10.3109/08923973.2015.1059441. [DOI] [PubMed] [Google Scholar]
- 128.Xu J, Liu C, Guo P, Guo Y, Jin D, Song X, et al. Neuroprotective labdane diterpenes from Fritillaria ebeiensis. Fitoterapia. 2011;82:772. doi: 10.1016/j.fitote.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 129.Xu J, Guo P, Liu C, Sun Z, Gui L, Guo Y, et al. Neuroprotective kaurane diterpenes from Fritillaria ebeiensis. Biosci Biotechnol Biochem. 2011;75:1386–1388. doi: 10.1271/bbb.110146. [DOI] [PubMed] [Google Scholar]
- 130.Chen G, Liu J, Jiang L, Ran X, He D, Li Y, et al. Peiminine protects dopaminergic neurons from inflammation-induced cell death by inhibiting the ERK1/2 and NF-κB signalling pathways. IJMS. 2018;19:821. doi: 10.3390/ijms19030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boojar FMA, Aghaei R, Mashhadi Akbar Boojar M. Data on possible in vitro anti-diabetic effects of verticinone on β-TC6 pancreatic and C2C12 skeletal muscle cells. Data Brief. 2020;28:1828. doi: 10.1016/j.dib.2019.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin Y, Chen Y, Liu J, Bao X, Zhi Y, Wen C, et al. Pharmacokinetics of ebeiedinone in mouse blood by UPLC–MS/MS. Acta Chromatogr. 2020;32:194–198. doi: 10.1556/1326.2019.00680. [DOI] [Google Scholar]
- 133.Wang S, Zhang Z, Yu Z, Han C, Wang X. Pharmacokinetic study of delavinone in mice after intravenous and oral administration by UPLC-MS/MS. Biomed Res Int. 2019;2019:1–6. doi: 10.1155/2019/3163218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li H, Hung A, Li M, Yang A. Fritillariae Thunbergii Bulbus: traditional Uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. IJMS. 2019;20:1667. doi: 10.3390/ijms20071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Z, Qiao Y, Li J, An C, Hu K, Tang M. Acute and sub-chronic toxicity studies of the extract of Thunberg Fritillary Bulb. Regul Toxicol Pharm. 2014;68:370–377. doi: 10.1016/j.yrtph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Guo X, Ni J, Xue J, Wang X. Extract of bulbus Fritillaria cirrhosa perturbs spindle assembly checkpoint, induces mitotic aberrations and genomic instability in human colon epithelial cell line. Exp Toxicol Pathol. 2017;69:163–171. doi: 10.1016/j.etp.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 137.Guo X, Wu X, Ni J, Zhang L, Xue J, Wang X. Aqueous extract of bulbus Fritillaria cirrhosa induces cytokinesis failure by blocking furrow ingression in human colon epithelial NCM460 cells. Mutat Res Gen Tox En. 2020;850–851:503147. doi: 10.1016/j.mrgentox.2020.503147. [DOI] [PubMed] [Google Scholar]
- 138.Zarei O, Yaghoobi MM. Cytotoxic effects of Fritillaria imperialis L. extracts on human liver cancer cells, breast cancer cells and fibroblast-like cells. Biomed Pharmacother. 2017;94:598–604. doi: 10.1016/j.biopha.2017.07.127. [DOI] [PubMed] [Google Scholar]
- 139.Li S, Li P, Lin G, Chan S, Ho Y. Simultaneous determination of seven major isosteroidal alkaloids in bulbs of Fritillaria by gas chromatography. J Chromatogr A. 2000;873:221–228. doi: 10.1016/S0021-9673(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 140.Kiani M, Sefidkon F, Babaei A, Naghavi MR. Phytochemical profiling of medicinal isosteroidal alkaloids of Iranian Fritillaria spp. (Liliaceae) Ind Crop Prod. 2015;70:451–458. doi: 10.1016/j.indcrop.2015.03.064. [DOI] [Google Scholar]
- 141.Cui M, Chen S, Wang H, Li Z, Chen H, Chen Y, et al. Metabolic profiling investigation of Fritillaria thunbergii Miq. by gas chromatography–mass spectrometry. J Food Drug Anal. 2018;26:337–347. doi: 10.1016/j.jfda.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Helsper JPFG, Bücking M, Muresan S, Blaas J, Wietsma WA. Identification of the volatile component(s) causing the characteristic foxy odor in various cultivars of Fritillaria imperialis L. (Liliaceae) J Agric Food Chem. 2006;54:5087–5091. doi: 10.1021/jf0605594. [DOI] [PubMed] [Google Scholar]
- 143.Li S, Lin G, Chan S, Li P. Determination of the major isosteroidal alkaloids in bulbs of Fritillaria by high-performance liquid chromatography coupled with evaporative light scattering detection. J Chromatogr A. 2001;909:207–214. doi: 10.1016/S0021-9673(00)01083-9. [DOI] [PubMed] [Google Scholar]
- 144.Li P, Zeng L, Li S, Lin G. The extraction of imperialine and imperialine-3β-glucoside from Fritillaria pallidiflora Schrenk and quantitative determination by HPLC-evaporative light scattering detection. Phytochem Anal. 2002;13:158–161. doi: 10.1002/pca.635. [DOI] [PubMed] [Google Scholar]
- 145.Li P, Zeng LJ, Li SL, Bi ZM, Lin G. Simultaneous determination of the major isosteroidal alkaloids and their glucosides in the bulbs of Fritillaria by high performance liquid chromatography coupled with evaporative light scattering detection. Yao xue xue bao Acta Pharm Sin. 2004;39:56–59. [PubMed] [Google Scholar]
- 146.Yu H, Jiang Y, Li P, Li S, Wang Y. Study on analytical method for alkaloids in Bulbus Fritillariae Cirrhosae. China J Chin Mater Med. 2005;30:572–575. [PubMed] [Google Scholar]
- 147.Zhang P, Li J, Zhang G, Pi H, Zhang Y, Ruan H, et al. Analysis of hupehenine in the total alkaloids from Fritillaria hupehensis by HPLC-ELSD. J Huazhong Univ Sci Technol [Med Sci] 2008;28:118–120. doi: 10.1007/s11596-008-0130-9. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Ma P, Peng R. Quantitative determination of peimisin and total alkaloids in Fritillaria taipaiensis of different growing stage. J Chin Med Mater. 2011;34:1034–1037. [PubMed] [Google Scholar]
- 149.Duan B, Huang L, Chen S. Study on the destructive effect to inherent quality of Fritillaria thunbergii Miq. (Zhebeimu) by sulfur-fumigated process using chromatographic fingerprinting analysis. Phytomedicine. 2012;19:562–568. doi: 10.1016/j.phymed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 150.Long Z, Guo Z, Acworth IN, Liu X, Jin Y, Liu X, et al. A non-derivative method for the quantitative analysis of isosteroidal alkaloids from Fritillaria by high performance liquid chromatography combined with charged aerosol detection. Talanta. 2016;151:239–244. doi: 10.1016/j.talanta.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 151.Cao X, Li J, Chen S, Li X, Xiao P, Chen S, et al. Simultaneous determination of nine nucleosides and nucleobases in different Fritillaria species by HPLC-diode array detector. J Sep Sci. 2010;33:1587–1594. doi: 10.1002/jssc.200900866. [DOI] [PubMed] [Google Scholar]
- 152.Duan B, Huang L, Chen S. Chemical fingerprint analysis of Fritillaria delavayi Franch. by high-performance liquid chromatography. J Sep Sci. 2012;35:513–518. doi: 10.1002/jssc.201100373. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Duan B, Wang Z, Huang L, Chen S. Quality control of Fritillaria unibracteata by UPLC-PAD fingerprint combined with hierarchical clustering analysis. J Liq Chromatogr R T. 2012;35:2381–2395. doi: 10.1080/10826076.2011.631265. [DOI] [Google Scholar]
- 154.Peng R, Ma P, Mo R, Sun N. Analysis of the bioactive components from different growth stages of Fritillaria taipaiensis P.Y. Li. Acta Pharm Sin B. 2013;3:167–173. doi: 10.1016/j.apsb.2013.04.006. [DOI] [Google Scholar]
- 155.Zhang C, Sun L, Chen R, Su JH, Zhang HF, Gu BR, et al. Multiple analytical methods for identification and quality evaluation of Fritillariae Thunbergii Bulbus based on biological single molecules by high-performance liquid chromatography. J Sep Sci. 2016;39:3536–3543. doi: 10.1002/jssc.201600356. [DOI] [PubMed] [Google Scholar]
- 156.Zhong Y, Wang H, Wei Q, Cao R, Zhang H, He Y, et al. Combining DNA barcoding and HPLC fingerprints to trace species of an important traditional Chinese medicine Fritillariae bulbus. Molecules. 2019;24:1–12. doi: 10.3390/molecules24183269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou J, Li P, Li H, Jiang Y, Ren M, Liu Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J Chromatogr A. 2008;1177:126–137. doi: 10.1016/j.chroma.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 158.Zhou J, Xin G, Shi Z, Ren M, Qi L, Li H, et al. Characterization and identification of steroidal alkaloids in Fritillaria species using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A. 2010;1217:7109–7122. doi: 10.1016/j.chroma.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 159.Du Y, Zheng Z, Yu Y, Wu Z, Liang D, Li P, et al. Rapid discovery of cyclopamine analogs from Fritillaria and Veratrum plants using LC-Q-TOF-MS and LC-QqQ-MS. J Pharmaceut Biomed. 2017;142:201–209. doi: 10.1016/j.jpba.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 160.Kumar P, Partap M, Ashrita, Rana D, Kumar P, Warghat AR. Metabolite and expression profiling of steroidal alkaloids in wild tissues compared to bulb derived in vitro cultures of Fritillaria roylei—high value critically endangered Himalayan medicinal herb. Ind Crop Prod. 2019;145:1–11. [Google Scholar]
- 161.Wang L, Yao ZP, Li P, Chen S, So P, Shi Z, et al. Global detection and semi-quantification of Fritillaria alkaloids in Fritillariae Ussuriensis Bulbus by a non-targeted multiple reaction monitoring approach. J Sep Science. 2016;39:287–295. doi: 10.1002/jssc.201500880. [DOI] [PubMed] [Google Scholar]
- 162.Li Y, Zhang L, Wu H, Wu X, Ju L, Zhang Y. Metabolomic study to discriminate the different Bulbus fritillariae species using rapid resolution liquid chromatography-quadrupole time-of-flight mass spectrometry coupled with multivariate statistical analysis. Anal Methods. 2014;6:2247–2259. doi: 10.1039/c3ay41928b. [DOI] [Google Scholar]
- 163.Mohammat A, Yili A, Aisa HA. Rapid quantification and quantitation of alkaloids in Xinjiang Fritillaria by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Molecules. 2017;22:1–17. doi: 10.3390/molecules22050719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu F, Jiang Y, Li P, Liu Y, Yao Z, Xin G, et al. Untargeted metabolomics coupled with chemometric analysis reveals species-specific steroidal alkaloids for the authentication of medicinal Fritillariae Bulbus and relevant products. J Chromatogr A. 2020;1612:1–8. doi: 10.1016/j.chroma.2019.460630. [DOI] [PubMed] [Google Scholar]
- 165.Geng Z, Liu Y, Gou Y, Zhou Q, He C, Guo L, et al. Metabolomics study of cultivated Bulbus Fritillariae Cirrhosae at different growth stages using UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem Anal. 2018;29:290–299. doi: 10.1002/pca.2742. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Z, Lu L, Liu Y. Comparing major alkaloids of Fritillariae Hupehensis Bulbs (FHB) and congeneric plants by HPLC-ELSD and HPLC-ESI-MSn. Nat Prod Res. 2014;28:1171–1175. doi: 10.1080/14786419.2014.921917. [DOI] [PubMed] [Google Scholar]
- 167.Xin G, Hu B, Shi Z, Lam YC, Dong TT, Li P, et al. Rapid identification of plant materials by wooden-tip electrospray ionization mass spectrometry and a strategy to differentiate the bulbs of Fritillaria. Anal Chim Acta. 2014;820:84–91. doi: 10.1016/j.aca.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 168.Wang L, Liu L, Wang J, Shi Z, Chang W, Chen M, et al. A strategy to identify and quantify closely related adulterant herbal materials by mass spectrometry-based partial least squares regression. Anal Chim Acta. 2017;977:28–35. doi: 10.1016/j.aca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 169.Wang Z, Xie H, Ren J, Chen Y, Li X, Chen X, et al. Metabolomic approach for rapid differentiation of Fritillaria bulbs by matrix-assisted laser desorption/ionization mass spectrometry and multivariate statistical analysis. J Pharm Biomed. 2020;185:1–5. doi: 10.1016/j.jpba.2020.113177. [DOI] [PubMed] [Google Scholar]
- 170.Ming-Chao C, Jia-Yu Z, Shao-Jun C, Hai-Long J, Hai-Bin Z, Qing-Zhi L. Identification of alkaloids and flavonoids in all parts of Fritillaria thunbergii using LC-LTQ-Orbitrap MSn. China J Chin Mater Med. 2016;41:2124–2130. doi: 10.4268/cjcmm20161124. [DOI] [PubMed] [Google Scholar]
- 171.Chang H, Xie H, Lee M, Lin C, Yip M, Agrawal DC, et al. In vitro propagation of bulblets and LC–MS/MS analysis of isosteroidal alkaloids in tissue culture derived materials of Chinese medicinal herb Fritillaria cirrhosa D. Don Bot Stud. 2020;61:1–9. doi: 10.1186/s40529-019-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duan B, Wang L, Dai X, Huang L, Yang M, Chen S. Identification and quantitative analysis of nucleosides and nucleobases in aqueous extracts of Fritillaria cirrhosa D. Don. using HPLC–DAD and HPLC-ESI-MS. Anal Lett. 2011;44:2491–2502. doi: 10.1080/00032719.2011.551856. [DOI] [Google Scholar]
- 173.Wu X, Chen J, Pan Y. Simultaneous determination of peimine and peiminine in rat plasma by LC-ESI-MS employing solid-phase extraction. Biomed Chromatogr. 2010;33:324–335. doi: 10.1002/bmc.1384. [DOI] [PubMed] [Google Scholar]
- 174.Wu X, Chen H, Sun J, Peng Y, Liang Y, Wang G, et al. Development and validation of a liquid chromatography–mass spectrometry method for the determination of verticinone in rat plasma and its application to pharmacokinetic study. J Chromatogr B. 2010;878:2067–2071. doi: 10.1016/j.jchromb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 175.Xin G, Zhou J, Qi L, Li C, Liu P, Li H, et al. Turbulent-flow chromatography coupled on-line to fast high-performance liquid chromatography and mass spectrometry for simultaneous determination of verticine, verticinone and isoverticine in rat plasma. J Chromatogr B. 2010;878:435–441. doi: 10.1016/j.jchromb.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 176.Xin G, Cao L, Shi Z, Li H, Wen X, Chen J, et al. Direct pharmacokinetic analysis of puqietinone by in vivo microdialysis sampling and turbulent-flow chromatography coupled with liquid chromatography–mass spectrometry. J Chromatogr B. 2012;899:127–134. doi: 10.1016/j.jchromb.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 177.Lin Q, Zhang Q, Song X, Gong T, Sun X, Zhang Z. Novel LC–MS/MS method for analyzing imperialine in rat plasma: development, validation, and application to pharmacokinetics. J Chromatogr B. 2013;938:51–59. doi: 10.1016/j.jchromb.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 178.Wang Z, Cao F, Chen Y, Tang Z, Wang Z. Simultaneous determination and pharmacokinetics of peimine and peiminine in beagle dog plasma by UPLC-MS/MS after the oral administration of Fritillariae ussuriensis Maxim and Fritillariae thunbergii Miq powder. Molecules. 2018;23:1–14. doi: 10.3390/molecules23071573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen L, Zhang H, Guan Z, Zhu W, Yi W, Guan Y, et al. Sex dependent pharmacokinetics, tissue distribution and excretion of peimine and peiminine in rats assessed by liquid chromatography–tandem mass spectrometry. J Ethnopharmacol. 2013;145:77–84. doi: 10.1016/j.jep.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 180.Chen J, Wang Y, Liu A, Rong L, Wang J. Two-dimensional correlation spectroscopy reveals the underlying compositions for FT-NIR identification of the medicinal bulbs of the genus Fritillaria. J Mol Struct. 2018;1155:681–686. doi: 10.1016/j.molstruc.2017.11.013. [DOI] [Google Scholar]
- 181.Zhang M, Zhao H, Shen Y, Wang Y, Zhao Z, Zhang Y. Preparation, characterization and antioxidant activity evaluation in vitro of Fritillaria ussuriensis polysaccharide-zinc complex. Int J Biol Macromol. 2020;146:462–474. doi: 10.1016/j.ijbiomac.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 182.Guo Y, Jiang N, Zhang L, Yin M. Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats. Arab J Chem. 2020;13:5096–5106. doi: 10.1016/j.arabjc.2020.02.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Traditional usage in national minorities of China. Table S2. Traditional usage of Fritillaria species in different countries. Table S3. Distribution information of alkaloids in Fritillaria species. Table S4. Distribution information of terpenoids in Fritillaria species. Table S5. Distribution information of steroidal saponins in Fritillaria species. Table S6. Distribution information of phenylpropanoids in Fritillaria species. Table S7. Distribution information of fatty acids in Fritillaria species. Table S8. Distribution information of sterides in Fritillaria species. Table S9. Distribution information of other components in Fritillaria species. Table S10. Pharmacological activities of Fritillaria species.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.