Abstract
Avapritinib is a protein kinase inhibitor designed to selectively inhibit oncogenic KIT and platelet-derived growth factor receptor alpha (PDGFRA) mutants by targeting the active conformation of the kinase. On 24 September 2020, a marketing authorisation valid through the European Union was issued for avapritinib as treatment of adult patients with unresectable or metastatic gastrointestinal stromal tumours (GIST) harbouring the PDGFRA D842V mutation. The drug was evaluated in an open-label, phase I, first-in-human, dose-escalation, open-label study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of avapritinib in adults with unresectable or metastatic GIST. The benefit of avapritinib was observed in patients with GIST harbouring the PDGFRA D842V mutation. The overall response rate was 95% (95% confidence interval 82.3%-99.4%), with a median duration of response of 22.1 months (95% confidence interval 14.1-not estimable months). The most common adverse events were nausea, fatigue, anaemia, periorbital and face oedema, hyperbilirubinaemia, diarrhoea, vomiting, increased lacrimation, and decreased appetite. Most of the reported cognitive effects were mild memory impairment. Rarer events were cases of severe encephalopathy and intracranial or gastrointestinal bleeding. The aim of this manuscript is to summarise the scientific review of the application leading to regulatory approval in the European Union.
Key words: EMA, GIST, avapritinib, PDGFRA, D842V
Highlights
-
•
Avapritinib is a protein kinase inhibitor designed to inhibit oncogenic KIT and PDGFRA mutants.
-
•
A marketing authorisation was issued for avapritinib as treatment of patients with GIST harbouring the PDGFRA D842V mutation.
-
•
The overall response rate was 95% (95% CI 82.3-99.4), with a median duration of response of 22.1 months (95% CI 14.1-NE).
-
•
The most common adverse events were nausea, fatigue, anaemia, periorbital and face oedema, hyperbilirubinaemia and diarrhoea.
Introduction
Gastrointestinal stromal tumours (GIST) are rare sarcomas arising from Cajal's interstitial cells across the entire gastrointestinal (GI) tract,1 representing approximately 0.1%-3% of all GI malignancies.2,3 More than 85% of patients with GIST harbour an oncogenic KIT (∼75% of cases) or platelet-derived growth factor receptor alpha (PDGFRA, ∼10% of cases) mutation that drives tumour growth.4
For localised, potentially resectable disease, initial treatment includes surgery followed by adjuvant therapy with imatinib for patients with increased risk of recurrence (i.e. high mitotic index or large tumour size).5 Since non-targeted chemotherapy and radiation are largely ineffective, the current recommendation for advanced GIST involves the sequential administration of the tyrosine kinase inhibitors (TKIs) imatinib, sunitinib, and regorafenib.4,5 First-line treatment with imatinib is associated with a 60% response rate and median progression-free survival (PFS) of 18-24 months.6 Subsequent treatment with sunitinib and regorafenib achieve response rates of 5%-7%, and a median PFS of 5-6 months.7
Avapritinib (AYVAKYT®) is a type 1 TKI designed to inhibit both KIT and PDGFRA mutants,8 including activation loop mutants that escape approved therapies.9, 10, 11 Avapritinib showed marked selectivity for KIT and PDGFRA in a comprehensive kinome screening. The medicinal product was designated as an orphan medicinal product on 17 July 2017 for the treatment of GIST. To qualify for orphan designation, a medicine must be intended for the treatment, prevention, or diagnosis of a life-threatening or chronically debilitating disease, the prevalence of the condition in the European Union (EU) must not be >5 in 10 000, and the medicine must be of significant benefit to those affected by the condition.
The final indication approved by the Committee for Medicinal Products for Human Use (CHMP) was ‘avapritinib is indicated as monotherapy for the treatment of adult patients with unresectable or metastatic GIST harbouring the PDGFRA D842V mutation.’ The review was conducted by CHMP and a positive opinion was issued on 23 July 2020.
Nonclinical aspects and clinical pharmacology
Avapritinib showed broad inhibitory activity against a panel of GIST relevant KIT and PDGFRA mutant enzymes, including the KIT exon 11, 17 and 18 mutants, and all PDGFRA exon 18 mutants tested. In contrast, the medicinal product demonstrated a lower binding affinity for wild-type KIT, wild-type PDGFRA, PDGFRB, CSF1R and FLT3.8 In vivo studies showed significant activity in several tumour models harbouring KIT mutations, with inhibition of KIT mutant activity and downstream signalling markers.12 Although inhibition of PDGFRA exon 18 mutants was demonstrated, the effect of avapritinib in PDGFRA-driven in vivo tumour models was not examined.
Avapritinib is a CYP3A substrate, and concomitant use of strong or moderate CYP3A inhibitors should be avoided. Based on in vitro data, avapritinib has been shown to autoinhibit and induce CYP3A enzymes. Population pharmacokinetic (PK) analysis indicated that age, race, sex, body weight, albumin concentration, mild hepatic impairment, or mild or moderate renal impairment had no clinically meaningful effect on the PK of avapritinib.
Avapritinib had a large interpatient PK variability, with an 8- to 10-fold range in exposure at the 300 mg dose level. Across this exposure range, there was no apparent exposure-efficacy relationship. By contrast, relationships were observed between increasing avapritinib exposure and safety endpoints such as cognitive effects, increased bilirubin and decreased haemoglobin. A relationship was also observed between steady-state exposure at day 15 and time to dose interruption or reduction. All patients within the lowest quartile of exposure withstood the 300 mg dose for 2 months, while patients in the 2nd to 4th quartile required dose reductions after 1 month. Still, the currently approved dosing strategy is starting all patients with the 300 mg dose and lowering the dose in reaction to occurring adverse effects.
Trial design
A single pivotal study (BLU-285-1101, NAVIGATOR study) was presented to support the claimed indication.13,14 This was a phase I, first-in-human, dose-escalation, open-label study to evaluate the safety, tolerability, PK, pharmacodynamics, and efficacy of avapritinib in adults with unresectable GIST. The population included in the initial proposed indication was composed by two different patient subsets: (i) patients with GIST who had received at least three lines of prior therapy (4L+ population, n = 121); and (ii) patients with GIST carrying the PDGFRA D842V mutation, regardless of number of prior therapies (n = 38). The primary endpoint was overall response rate (ORR), defined as the rate of centrally confirmed complete or partial response by modified RECIST (mRECIST) (version 1.1). Key secondary endpoints were duration of response (DOR), PFS, and clinical benefit rate as per mRECIST version 1.1. Overall survival (OS) was included as an exploratory efficacy endpoint.
Supportive studies were BLU-285-1303 and BLU-285-1105. BLU-285-1303 (VOYAGER) was a global, open-label, randomised, phase III trial designed to evaluate the efficacy and safety of avapritinib versus regorafenib in patients with GIST who had received two or more lines of therapy. Patients were randomised (1 : 1) to receive either avapritinib (300 mg daily dose) or regorafenib (160 mg daily dose for 3 weeks followed by 1 week off medication). The primary endpoint was PFS by blinded, independent central radiology review, based on mRECIST version 1.1 criteria. BLU-285-1105 was an open-label, multicentre, phase I/II study designed to evaluate the safety, PK and clinical efficacy of avapritinib in Chinese patients with unresectable or metastatic GIST.
In all trials, avapritinib was administered in continuous 28-day courses until unacceptable toxicity or progressive disease. Mutational PDGFRA D842V status was centrally assessed. During the evaluation, the applicant withdrew the fourth-line indication request from the marketing authorisation application.
Clinical efficacy
The dose finding phase was conducted following a 3 + 3 design in which dose-limiting toxicities (DLT) were assessed in continuous 28-day courses. The optimal biologically active dose was unknown, and the maximum tolerated dose was initially set to 400 mg daily due to DLT observed at the 600 mg daily dose. After initiation of the study extension, the starting dose was reduced to 300 mg daily in view of common treatment interruptions and dose reductions due to adverse events (AEs).
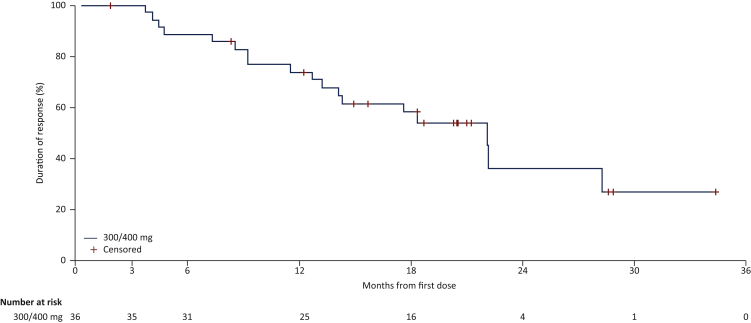
The number of patients with unresectable or metastatic PDGFRA D842V-mutant GIST who received 300 or 400 mg daily recruited into the BLU-285-1101 (NAVIGATOR) trial was 38. The regulatory decision was based on results from the cut-off date (COD) of 17 April 2020 (median follow-up of 26 months). In this patient population, 36/38 (95%) responses were observed [95% confidence interval (CI) 82.3% to 99.4%], including 5 (13%) complete responses, with a median DOR of 22.1 months [95% CI 14.1-not estimable (NE) months, Figure 1] and a median PFS of 24 months (95% CI 18.4-NE months). At the same COD, the median OS had not been reached yet, but the estimated OS at 36 months was 70.6% (95% CI 55.2-86.0 months) (Table 1).
Figure 1.
Duration of response by central radiology review (mRECIST 1.1) in patients with PDGFRA D842V mutation only (BLU-285-1101 study).
Note: Duration of response was defined as the time from first documented response (complete or partial response) to the date of first documented disease progression or death due to any cause, whichever came first. Patients without confirmed response were excluded from this analysis. Patients who were still in response at time of data cut-off were censored at their last valid assessment. The product-limit method was used to obtain Kaplan–Meier estimates of survival.
mRECIST, modified RECIST; PDGFRA, platelet-derived growth factor receptor alpha.
Table 1.
Effects table for avapritinib in studies BLU-285-1101 [cut-off date (COD) for efficacy: 17 April 2020, COD for safety: 9 March 2020] and study BLU-285-1303 (COD: 9 March 2020)
| Effect | Unit | Results | Uncertainties/Strength of evidence | ||
|---|---|---|---|---|---|
| PDGFRA D842V-mutated population in BLU-285-1101 study (n = 38) | ORR Median DOR Median PFS Median OS |
% (95% CI) Months (95% CI) Months (95% CI) Months (95% CI) |
95 (82.3-99.4) 22.1 (14.1-NE) 24 (18.4-NE) Not reached [70.6% (55.2-86.0) at 36 months] |
||
| PDGFRA D842V-mutated population in BLU-285-1303 study (n = 13) | ORR Median PFS |
% Months (95% CI) |
Avapritinib (n = 7) | Regorafenib (n = 6) | These data are preliminary and limited as the study is ongoing |
| 43 NE (9.7-NE) |
0 4.5 (1.7-NE) |
||||
| All AEs | Treatment-related AEs | ||||
| General safety profile | AEs overall Grade ≥3 AEs/SAEs AEs leading to death AEs leading to treatment discontinuation |
% | 98.4 75.1/49.8 8.0 18.0 |
93.8 55.5/23.1 0.4a 10.9 |
aIncludes reports of disease progression |
| Gastrointestinal AEs | Overall Grade ≥3 AEs/SAEs Nausea Vomiting Diarrhoea Abdominal pain |
% | 61.5 5.5/3.1 45.1 24.2 26.4 10.9 |
SAEs include gastrointestinal bleeding | |
| Cytopenia AEs | Overall Grade ≥3 AEs/SAEs Grade ≥3 anaemia Grade ≥3 neutropenia |
% | 44.5 27.1/6.4 20.0 8.9 |
||
| Fluid retention AEs | Peripheral oedema Facial oedema Periorbital oedema Pleural effusion |
% | 22.5 26.5 32.9 6 |
||
| Cognitive AEs | Overall Grade ≥3 AEs/SAEs Memory impairment Cognitive disorders Confusional status Encephalopathy |
% | 33.1 2.2/1.3 20.2 11.8 4.7 0.9 |
||
| Haemorrhage | Overall Grade ≥3 AEs/SAEs Intracranial |
% | 3.8 2.0/2.0 1.6 |
||
AEs, adverse events; CI, confidence interval; DOR, duration of response; NE, not estimable; ORR, overall response rate; OS, overall survival; PDGFRA, platelet-derived growth factor receptor alpha; PFS, progression-free survival; SAEs, serious adverse events.
In the BLU-285-1303 (VOYAGER) study, at the COD of 9 March 2020, the ORR were 42.9% (all partial responses) versus 0% in the subset of patients with PDGFRA D842V mutations receiving avapritinib (n = 7) versus regorafenib (n = 6), respectively. The median PFS was not estimable (95% CI 9.7-NE months) versus 4.5 months (95% CI 1.7-NE months) for patients receiving avapritinib and regorafenib, respectively. Results from study BLU-285-1105, although limited (n = 8), are considered supportive of the benefit–risk of avapritinib in the target patient population: five patients achieved a partial response (62.5% ORR) and three had stable disease.
Clinical safety
The safety profile of avapritinib was characterised in 585 patients with GIST (all doses), of whom 550 received avapritinib at a starting dose of 300 or 400 mg, including 250 patients from BLU-285-1101 and 335 patients from BLU-285-1303 (239 initially allocated to avapritinib and 96 initially allocated to regorafenib but crossed over to avapritinib due to disease progression) (Table 1). The median relative dose intensity was 90%, and the median treatment duration was 23.2 months for patients included in the BLU-285-1101 study and 8.9 months for patients included in the BLU-285-1303 study. An updated safety database with a COD of 9 March 2020 was submitted during the procedure.
Safety data from BLU-285-1101 showed that all patients except one (>99%) reported at least one AE, where 80% had grade ≥3 AEs (13% fatal), 65% had serious AEs (SAEs), and 27% had events that led to permanent drug discontinuation. The most common fatal AEs were disease progression (15/32), physical deterioration (6/32), sepsis (3/32), and tumour haemorrhage (2/32).
Cognitive AEs were reported by 46% (115/250) of the patients (90% grade 1-2 and 10% grade 3), with a median time to onset of 8.3 weeks. Of note, the median treatment duration was 12.4 months in patients who experienced cognitive AEs and 3.8 months in those patients who did not. The most common cognitive AEs were memory impairment, followed by cognitive disorders, confusional state, and encephalopathy. Intracranial haemorrhage was reported in 3% (7/250) of patients. No fatal cognitive or intracranial bleeding AEs were reported.
The safety data in the subset of 56 patients with GIST and PDGFRA D842V mutations were consistent with the overall study population: 100% experienced AEs (80% ≥grade 3), 57% experienced SAEs, and 21% experienced AEs leading to permanent treatment discontinuation.
The safety analysis from the ongoing BLU-285-1303 study was also consistent with the results reported in the BLU-285-1101 study. Based on the pooled analysis of studies BLU-285-1101 and BLU-285-1303, the most frequently reported treatment-related AEs were nausea (45%), fatigue (40%), anaemia (39%), periorbital oedema (33%), hyperbilirubinaemia (27%), face oedema (27%), diarrhoea (26%), vomiting (24%), increased bilirubin (24%), peripheral oedema (23%), increased lacrimation (22%), decreased appetite (21%) and memory impairment (20%). The most common SAEs were anaemia (6%) and pleural effusion (1%), whereas the most common AEs leading to permanent treatment discontinuation were fatigue, encephalopathy and intracranial haemorrhage.
Benefit–risk assessment
The efficacy of avapritinib was evaluated in 59 patients with GIST harbouring a PDGFRA D842V mutation from the NAVIGATOR, VOYAGER, and BLU-285-1105 studies. The pivotal evidence supporting the indication comes from a small population (n = 38) selected from the single-arm NAVIGATOR study that used ORR as a primary endpoint. The results were, however, unprecedented with a 95% ORR and a median DOR of 22.1 months. Data on TKI-naive patients appeared consistent with those of the overall PDGFRA D842V-mutant group, but were even more limited (n = 5). However, it was agreed that results would not be expected to be of a lesser magnitude in the first-line setting and the benefit was considered demonstrated regardless of the line of treatment. Supportive data from the phase III, controlled study and the phase I study in Chinese patients also showed favourable and clinically relevant results, although of smaller magnitude. Overall, the reported results are considered clinically meaningful in the context of a patient population with limited treatment options and poor response to approved TKI agents.
The safety database was substantially extended throughout the assessment procedure, with a median treatment duration of 23.2 months in the BLU-285-1101 study and 8.9 months in the BLU-285-1303 study. The safety profile was largely consistent with that reported for other TKIs. Intracranial bleeding and GI/tumour haemorrhage have been reported and remain AEs of concern. Cognitive disorders were very frequently reported, ranging from common occurrences of mild memory impairment events to rare cases of severe encephalopathy. Both intracranial haemorrhage and cognitive effects were identified as important risks in the risk management plan, including recommendations for dose modification in case of grade 1-3 events and permanent discontinuation in case of grade 4 events. Updated data revealed that the frequency of fatal AEs, SAEs, and AEs leading to treatment discontinuation were lower compared with those reported in the phase I study, and like those observed in the regorafenib control arm. Also, less cognitive AEs and episodes of intracranial bleeding were reported in the phase III trial. Overall, toxicity was manageable with appropriate risk minimisation measures, including close monitoring of AEs. Finally, longer-term follow-up is still needed as uncertainties remain on the safety profile of avapritinib, particularly in patients treated in the first-line setting.
As comprehensive clinical data on the safety and efficacy of avapritinib were not available, a conditional marketing authorisation (CMA) was requested by the applicant in the initial submission. The following requirements were met:
-
•
Positive risk–benefit ratio: The applicant presented high and durable tumour responses that were unprecedented in this patient population with a manageable safety profile.
-
•
Likelihood that comprehensive clinical data will be provided: Specific obligations were imposed by CHMP. Final clinical study reports from studies BLU-285-1101 and BLU-285-1303 will include all available data on the safety and efficacy of avapritinib in patients with PDGFRA D842V-mutant GIST (n = 51). A new study (BLU-285-1406) will contribute with long-term (minimum 2 years) safety and efficacy data in approximately 50 previously untreated patients with GIST harbouring the PDGFRA D842V mutation. Overall, the proposed studies will contribute comprehensive clinical data in approximately 100 patients with PDGFRA D842V-mutant GIST.
-
•
Unmet medical need: The activity of currently available drugs is almost negligible in GIST patients with the PDGFRA D842V mutation.
-
•
The benefit to public health of immediate availability outweighs the risk that additional data are still required: The outstanding activity in a population with an unmet medical need renders the limited evidence acceptable for grant approval.
As a result, CHMP decided to grant a CMA to avapritinib ‘as monotherapy for the treatment of adult patients with unresectable or metastatic GIST harbouring the PDGFRA D842V mutation.’
Acknowledgements
The scientific assessment as summarised in this report is based on the marketing authorisation application submitted by the applicant company and on important contributions from, among others, the rapporteur assessment team, CHMP members, and additional experts.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Miettinen M., Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B., Bumming P., Meis-Kindblom J.M. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era – a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Soreide K., Sandvik O.M., Soreide J.A., Giljaca V., Jureckova A., Bulusu V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Antonescu C.R. The GIST paradigm: lessons for other kinase-driven cancers. J Pathol. 2011;223:251–261. doi: 10.1002/path.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casali P.G., Abecassis N., Aro H.T. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68–iv78. doi: 10.1093/annonc/mdy095. [DOI] [PubMed] [Google Scholar]
- 6.Blanke C.D., Rankin C., Demetri G.D. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 7.von Mehren M., Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. 2018;36:136–143. doi: 10.1200/JCO.2017.74.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans E.K., Gardino A.K., Kim J.L. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;9:eaao1690. doi: 10.1126/scitranslmed.aao1690. [DOI] [PubMed] [Google Scholar]
- 9.Cassier P.A., Fumagalli E., Rutkowski P. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18:4458–4464. doi: 10.1158/1078-0432.CCR-11-3025. [DOI] [PubMed] [Google Scholar]
- 10.Grellety T., Kind M., Coindre J.M., Italiano A. Clinical activity of regorafenib in PDGFRA-mutated gastrointestinal stromal tumor. Future Sci OA. 2015;1:FSO33. doi: 10.4155/fso.15.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichardt P., Demetri G.D., Gelderblom H. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer. 2016;16:22. doi: 10.1186/s12885-016-2051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebreyohannes Y.K., Wozniak A., Zhai M.E. Robust activity of avapritinib, potent and highly selective inhibitor of mutated KIT, in patient-derived xenograft models of gastrointestinal stromal tumors. Clin Cancer Res. 2019;25:609–618. doi: 10.1158/1078-0432.CCR-18-1858. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich M.C., Jones R.L., von Mehren M. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21:935–946. doi: 10.1016/S1470-2045(20)30269-2. [DOI] [PubMed] [Google Scholar]
- 14.Jones R.L., Serrano C., von Mehren M. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur J Cancer. 2021;145:132–142. doi: 10.1016/j.ejca.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]