Abstract
Background
Gestational syphilis is underdiagnosed and undertreated, leading to stillbirth, prematurity, low birthweight, neonatal death, and congenital syphilis. Most patients who label as allergic to penicillin are misdiagnosed.
Objective
To assess the efficacy and safety of an algorithm to guide re-exposure to penicillin in pregnant women with syphilis and reporting allergy to the antibiotic.
Methods
We performed a prospective study assessing pregnant women with syphilis and labeled as allergic to penicillin. Based on clinical history, patients were divided in two groups: high-risk and low-risk to penicillin allergy. Low-risk patients with negative skin testing and negative serum specific IgE to penicillin underwent drug provocation test. The remaining patients underwent desensitization.
Results
Ninety-one patients were enrolled. Allergy to penicillin was confirmed in 7.69% of pregnant women with syphilis and clinical history of allergy to penicillin; in all cases the diagnosis was made through intradermal testing, which predicted 100% of the breakthrough reactions observed during rapid drug desensitization (p < 0.001). Risk stratification based on the initial clinical reaction and skin testing to guide penicillin re-introduction through drug challenge or desensitization was safe (97.8%) and effective (97.8%).
Conclusion
We developed and showed the efficacy and safety of an algorithm to guide re-exposure to penicillin in pregnant women with syphilis and labeled as allergic to this drug. Intradermal test is an excellent biomarker in the diagnosis of immediate hypersensitivity reaction to penicillin and to predict breakthrough reaction during rapid drug desensitization. Further studies may confirm the greater safety of the intravenous protocol compared to the oral protocol.
Keywords: Gestational syphilis, Congenital syphilis, Immediate hypersensitivity reactions, Anaphylaxis, Desensitization, Benzathine penicillin, Algorithm, Pregnancy
Introduction
Syphilis is a systemic bacterial infection that is exclusively human. After the improvement achieved in the treatment of HIV-infection and the consequent decrease in awareness for its prevention, the prevalence of sexually transmitted diseases, including syphilis, has been increasing.1 The main route of infection is sexual, followed by vertical contamination, which can lead to fetus injury, including death, in 30–50% of the cases. A simple and inexpensive rapid test followed by treatment with benzathine penicillin helps to overcome this catastrophic scenario2, 3, 4
Treatment should be started immediately after a positive diagnostic test for syphilis in pregnant women, unless the patient has recently been appropriately treated, which must have been documented in writing. The only effective treatment for gestational syphilis is benzathine penicillin, because it is the only drug that has been proven to cross the placenta and which has bioavailability for the fetus.5,6 Nevertheless, penicillin allergy, both truly and falsely diagnosed, associated with unfamiliarity with the procedure of desensitization has hampered the proper care of these patients. Studies addressing this prevalent and relevant topic in public health are missing.7
Anaphylaxis is a medical emergency with increasing incidence and prevalence that may cause death.8,9 Drugs are the most frequent cause of fatal anaphylaxis world-wide, and penicillin and other beta-lactam antibiotics are highlighted as a major cause.9, 10, 11, 12 The clinical incidence of immediate hypersensitivity reactions (IHR) to beta-lactam antibiotics is 0.04–0.2% and the fatality rate is 0.001%.13
Anaphylaxis in pregnancy is a rare event with an estimated incidence of 1–3 cases per 100 000 maternities.14,15 An anaphylactic reaction may cause placental hypoperfusion and fetal distress, including fetal cerebral hypoperfusion. Newborns may suffer from severe brain damage, even with mild maternal anaphylactic reactions, and in spite of increased levels of the enzyme diamine oxidase during pregnancy, which is produced by the placenta and degrades histamine.15,16 A prospective population-based study observed severe maternal morbidities in 19% of the reactions and a case fatality of 5%, besides a neonatal intensive care unit admission rate of 41%.15 Another study estimated the maternal mortality rate as a result of anaphylaxis in 1 per 1 million maternities.17 A major cause of anaphylaxis during pregnancy is penicillin-based antibiotics, which are used prophylactic before caesarean delivery and intrapartum for group B streptococcal carriage to prevent neonatal transmission.15
Women with gestational syphilis should be treated with benzathine penicillin to insure the treatment of both mother and fetus. Patients with confirmed diagnosis of allergy to penicillin or with high risk to IHR should undergo desensitization.5,7 The procedure is safe, with controlled risks and low failure rate. The present study evaluates an algorithm to manage penicillin reintroduction in pregnant women with syphilis and labeled as allergic to this drug. Efficacy and safety of oral and intravenous desensitization protocols, as well as its potential biomarkers such as immediate skin testing and serum specific IgE (sIgE), are also assessed.
Methods
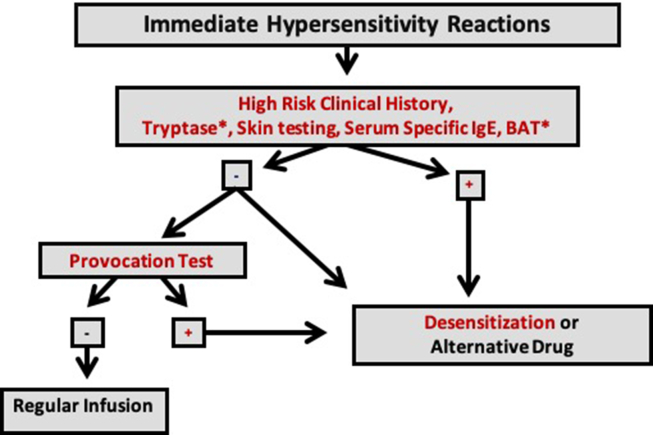
We performed a prospective study to assess the efficacy and safety of an algorithm to guide re-exposure to penicillin in pregnant women with syphilis and reporting allergy to the antibiotic (Fig. 1).18 The study was approved by the Hospital Institutional Review Board, and we conducted the study from January 2016 to December 2019 in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participants provided written informed consent.
Fig. 1.
Algorithm for evaluation and management of beta-lactam allergy in pregnant women with syphilis. Footnote: ∗ Tests not performed in the present study. sIgE: serum specific IgE BAT: basophil activation test. Adapted from Giavina-Bianchi P et al. J Allergy Clin Immunol Pract. 2017; 5(3):593-9(18)
All included patients were pregnant, had documented diagnosis of untreated syphilis, and clinical history of an IHR to penicillins. There were no exclusion criteria. The Brazilian Ministry of Health recommends that pregnant women with history of allergy to penicillin and gestational syphilis should be referred to a specialized and tertiary service to be treated. The Clinical Immunology and Allergy Division of the University of São Paulo School of Medicine is a reference center to manage patients with drug-induced IHR. We developed an algorithm, which includes risk stratification, to guide patients’ re-exposure to penicillin either through Rapid Drug Desensitization (RDD) or Drug Provocation Test (DPT) (Fig. 1). Efficacy of this algorithm was assessed in this study as the percentage of patients whose syphilis could be treated with penicillin, and safety as the percentage of treatments administered without the occurrence of new moderate/severe hypersensitivity reactions. The study also aimed to compare an intravenous with an oral desensitization protocol. As secondary endpoints, skin testing and serum sIgE were assessed as potential biomarkers of penicillin provocation test and desensitization.
First, a detailed and complete clinical history was taken, covering the pregnancy, the Treponema pallidum infection, and, with particular attention, the initial patients’ adverse reaction to penicillin. The level of serum tryptase, if measured during the initial reaction, was recorded. Based on clinical history, patients were divided in 2 groups: high- and low-risk clinical history to penicillin allergy. The high-risk clinical history group had clinical histories that fulfilled at least 2 of the following 3 criteria: drug-induced IHR in the last 10 years; initial reaction compatible with IHR such as pruritus, urticaria, angioedema, acute hoarseness, rash, flushing, bronchospasm, hypotension, dizziness, blurred vision and anaphylaxis; no history of tolerated re-exposure to penicillins after the initial reaction. Patients with documented elevated serum tryptase (>11.5 mcg/L) during the initial reaction and/or belonging to the high-risk clinical history group were considered allergic to penicillin and underwent rapid drug desensitization, regardless of other in vivo and/or in vitro tests.
All patients underwent immediate reading skin tests, prick and intradermal tests, and had their blood drawn to measure sIgE to penicillin G and penicillin V. For skin prick testing, a drop of penicillin (10,000 UI/ml) was applied to the volar surface of the forearm. For intradermal injection, 0.03 ml of penicillin (10,000 UI/ml) was injected in the volar surface of the forearm. Skin prick or intradermal tests showing a wheal with an average diameter 3 mm larger than that of the negative control were considered positive. Serum sIgEs to penicillin G and V were measured by the ImmunoCap System, Thermo Fisher Scientific. According to our algorithm, a high-risk patient was the one who had at least one of the following alternatives:
-
-
high-risk clinical history
-
-
elevated serum tryptase level at the initial reaction
-
-
positive skin testing
-
-
positive specific serum IgE
Low-risk patients, with low-risk clinical history, without elevated serum tryptase during the initial reaction, with negative skin testing and negative serum sIgE underwent penicillin provocation test. The challenge protocol involved the administration of 5 000 000 IU crystalline penicillin in 3 steps: 1%, 9%, and 90% of the total dose. If the drug provocation test was negative, drug allergy was excluded, and the patient was treated with regular infusion of penicillin. If the test was positive, allergy to penicillin was confirmed, and desensitization was indicated. Patients belonging to the high-risk group underwent penicillin desensitization.
At the study beginning, the patients were randomized to undergo desensitization through an oral (Fig.S1 in the Supplementary Appendix)19 or an intravenous protocol (Fig.S2 in the Supplementary Appendix),20 the last one adapted by us for the present study. After some severe breakthrough reactions were observed during the oral desensitization procedures, in an interim analysis, we decided to stop randomization and apply only the intravenous desensitization protocol thereafter.
Statistical analysis
All continuous variables were expressed as means and their ranges. Comparisons between 2 groups were made with the unpaired sample t-test. Categorical variables were presented as numbers and percentages, being analyzed by Fisher exact test. Patients with high- and low-risk clinical history to penicillin allergy were compared. Patients with positive and negative intradermal tests were compared. Desensitizations with and without breakthrough reactions were compared, as well as the oral and intravenous protocol. Efficacy of the algorithm to guide re-exposure to penicillin was calculated by the rate of patients that could be treated with this antibiotic related to all patients; safety was shown by the percentage of patients re-exposured to penicillin without new moderate/severe breakthrough reactions. Analysis was performed with GraphPad Prism software (version 6.0a) and P values of less than 0.05 were considered significant.
Results
The study included 91 pregnant women with latent syphilis confirmed by laboratory tests and a history of IHR to penicillin. Penicillin was indicated to treat all these patients, since they lack a documented history of being previously treated for syphilis. Patients’ mean age and gestational age at the time of evaluation were 25.1 years (range, 14 to 42) and 19.8 weeks (range, 5 to 38), respectively (Table 1).
Table 1.
Characteristics of pregnant women with syphilis and reporting allergy to penicillin.
| Characteristic | Total Population | High-risk Clinical History | Low-risk Clinical History | Statistical Difference |
|---|---|---|---|---|
| Mean age (year, range) | 25.1 (14–42) | 25.2 (15–41) | 25 (14–42) | NS |
| Mean gestational age (weeks, range) | 19.8 (5–38) | 18.4 (6–33) | 21.6 (5–38) | NS |
| ≥2 criteria for high-risk clinical-history | 56% | 100% | 0% | Definition criteria |
| First criterion high-risk clinical-history | 67% | 74.5% | 17.5% | p < 0.01 |
| Second criterion high-risk clinical-history | 49.5% | 100% | 25% | p < 0.01 |
| Third criterion high-risk clinical-history | 47.3% | 70.6% | 17.5% | p < 0.01 |
| Positive Intradermal Test | 7.7% | 13.72% | 0% | p = 0.02 |
| Anaphylaxis as the initial IHR | 46.2% | 56.9% | 17.5% | p < 0.01 |
IHR: Immediate hypersensitivity reaction.
NS: not significant.
First criterion: IHR to penicillin in the last 10 years.
Second criterion: Clinical manifestation compatible with IHR.
Third criterion: No history of tolerated re-exposure to penicillins after the initial IHR.
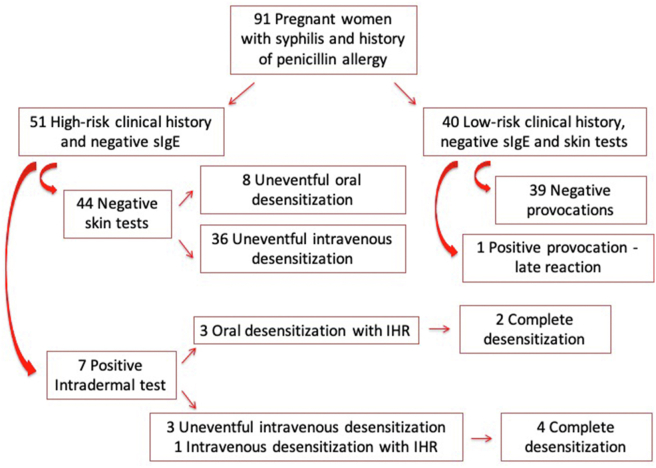
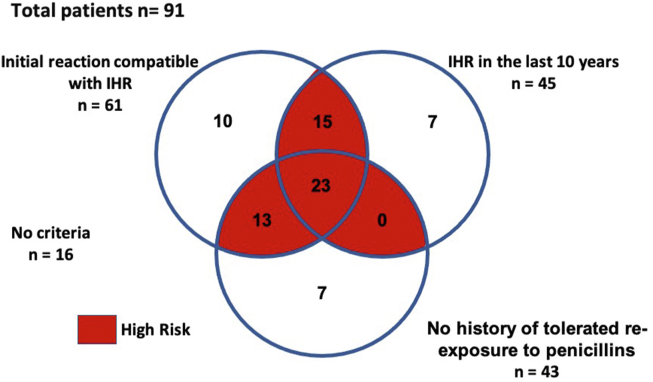
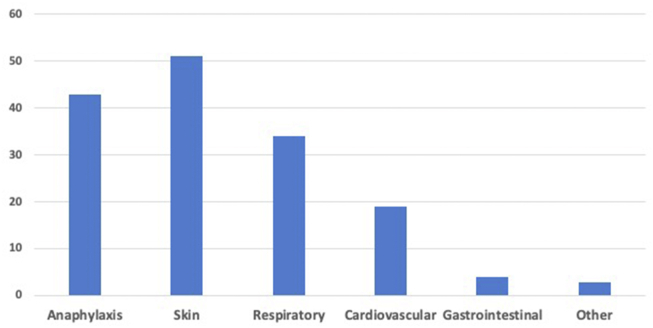
We stratified our patients according to the risk of having a new IHR during penicillin re-exposure (Fig. 2). Those having 2 of the 3 criteria (IHR to penicillin in the last 10 years; clinical manifestation compatible with IHR; no history of tolerated re-exposure to penicillins after the initial reaction) were considered high-risk clinical history and underwent desensitization, regardless of the auxiliary tests. Patients reported the initial IHR, on average, 13.8 years (range, 1 to 40) before their enrolment in the study, 49.5% having the reaction in the last 10 years (Fig. 3). In 61 of the 91 patients (67.0%), the initial IHRs were compatible with anaphylactic type reactions (Fig. 4), and 47.3% of these patients had not been re-exposed to a penicillin antibiotic (Fig. 3). After assessing all criteria, we identified 44.0% (40/91) low-risk clinical history and 56.0% (51/91) high-risk clinical history patients (Fig. 2). There were no statistical differences in age and gestational age between the 2 groups. Serum tryptase measurement was not done during the initial IHR in any of our patients.
Fig. 2.
Risk stratification, procedures and outcomes. Footnote: sIgE: serum specific IgE IHR: immediate hypersensitivity reaction
Fig. 3.
Number of patients according criteria for high-risk to penicillin allergy. Footnote: IHR: immediate hypersensitivity reaction
Fig. 4.
Clinical manifestations of the initial immediate hypersensitivity reaction
All low-risk clinical history pregnant women had both serum sIgE and immediate reading skin testing (prick and intradermal) to penicillin negatives (Fig. 2). They underwent our intravenous challenge protocol with 5 000 000 IU crystalline penicillin administered in 3 steps: 1%, 9%, and 90% of the total dose. All patients completed the penicillin provocation test without having IHR. Only 1 pregnant woman had a delayed reaction, 24 h after the end of the challenge, characterized by rash on the face and trunk. She was treated with systemic corticosteroid and the reaction resolved in 48 h. This patient received alternative treatment for gestational syphilis with doxycycline, her baby was born uneventfully, and neonatal syphilis was excluded. The other patients completed the treatment for gestational syphilis with benzatine penicillin and did not have any reaction.
All high-risk clinical history patients were desensitized. They had both prick test and serum sIgE measurement negatives. Of the 51 high-risk clinical history patients, 44 (86.27%) had negative intradermal tests (Fig. 2). Eight (18.18%) underwent the oral desensitization protocol, and 36 (81.81%) the intravenous desensitization protocol. All patients completed desensitization without reactions.
However, 7 out the 51 high-risk clinical history patients (13.72%) had positive intradermal tests (Fig. 2). Three of them underwent the oral desensitization protocol and reacted during the procedure. Two patients completed the desensitization, but 1 patient had a severe breakthrough anaphylactic reaction, which included uterine contractions, and required intramuscular epinephrine administration. She did not finish the procedure and received alternative treatment for gestational syphilis with doxycycline. The remaining 4 intradermal positive patients underwent the intravenous desensitization protocol, and only 1 reacted during the procedure, experiencing a bronchospasm, which resolved with bronchodilator. This patient had non-controlled asthma, and although the bronchospasm could be an asthma attack unrelated to the desensitization procedure, it was considered a breakthrough reaction. All patients submitted to the intravenous desensitization completed the procedure. There was a statistically significant association between positive intradermal test and breakthrough reaction during desensitization (p < 0.001). Within the high-risk clinical history and intradermal positive patients, there were a higher incidence of breakthrough reaction with the oral desensitization protocol in comparison to the intravenous protocol, but the difference was not statistically significant.
The algorithm analyzed in the present study was 97.8% effective and 97.8% safe, since 89 of 91 pregnant women with syphilis could be treated with penicillin. Two patients did not complete the treatment due to moderate/severe reactions, one had a severe IHR during oral desensitization and another developed a moderate delayed reaction after drug challenge.
Discussion
According to the World Health Organization, in 2018, 1% or more of antenatal care attendees were diagnosed with gestational syphilis in half of the reporting countries. Syphilis in pregnancy is the second leading cause of stillbirth globally, and also results in prematurity, low birthweight, neonatal death, and congenital syphilis.21 The infection has to be treated as soon as possible, and the infant does not need to be treated if the mother's treatment is completed at least 30 days before delivery.6 A pregnant woman with infection by Treponema pallidum and clinical history of allergy to penicillin must be referred from basic health care to a tertiary health care center to be assisted by a doctor specialized in allergy and clinical immunology.
In Brazil, congenital syphilis incidence is higher than gestational syphilis detection rate, showing a lack of diagnosis and inadequate treatment. In a study, 17.2% of pregnant women with syphilis did not attend to prenatal care. Although the adequacy of prenatal care of pregnant women with syphilis in the Brazilian state capitals, measured by the modified Kotelchuck index, was 82.4% on average, merely 27.3% of the primary health care units reported “always” having the syphilis rapid test available, and only 67.7% have benzathine penicillin to syphilis treatment. The proportion of congenital syphilis causing fetal death ranged from 6.2% to 17.2%. These results showed the need of a closer and personalized approach on this population, in order to compensate its vulnerability.6
In the present study, 91 patients came to us at a gestational age of 19.8 weeks on average, allowing the conclusion of the treatment up to 4 weeks before delivery, but representing a delay in medical care and an unwanted prolonged fetal exposure to the infection. Efforts should be made to ensure that after the diagnosis of gestational syphilis is confirmed, a patient with suspicious of penicillin allergy gets fast access to specialized medical care to allow the best treatment for the mother and the fetus as soon as possible.
The diagnosis of allergy to penicillin was confirmed in only 8.79% of our patients who had been previously labeled as allergic, 7 patients with IgE-mediated allergy confirmed by positive immediate intradermal tests, and 1 who had a delayed reaction during a drug provocation test. It is not possible to be sure whether that last patient had a delayed hypersensitivity reaction or a Herxheimer reaction.
Drug-induced IHRs are undertreated and may be underdiagnosed as observed with non-steroidal anti-inflammatory drugs, or misdiagnosed as is the case with penicillin allergy.22 Although about 10% of the in-hospital patients are labeled as allergic to penicillin, only nearly 5% of them will have the diagnosis confirmed in a drug provocation test.23 This misleading label is harmful, since patients wrongly designated as allergic to penicillin have longer hospitalizations, with higher morbidity and higher cost.12,24,25 Most patients labeled with penicillin allergy do not undergo any evaluation to determine the accuracy or persistence of this diagnosis.24 Formal penicillin allergy evaluation has been recommended by several entities, including the Centers for Disease Control and Prevention; the American Academy of Allergy, Asthma, and Immunology; and the Infectious Disease Society of America, among others. In this scenario, there has been increasing awareness and implementation of programs to delabel patients who were mistakenly diagnosed as penicillin allergic, and several studies have shown the importance and effectiveness of these programs.26,27
The present study stratified the risks of 91 pregnant women with latent syphilis and history of IHR to penicillin through an algorithm in order to guide drug re-exposure (Fig. 1). IHR to penicillin is a real IgE-mediated allergy and clinical history is still crucial to the diagnosis. How best to define a high-risk clinical history of allergy to penicillin is uncertain, and a practical tool to classify patients in high- and low-risk clinical history is needed. We developed a specific questionnaire, which included 3 criteria. The first criterion was to verify if the IHR was clinically consistent with a reaction induced by mast cell/basophil degranulation. Many patients have another cause for a clinical manifestation they think represent a penicillin allergic reaction, such as a concomitant viral infection.28 The second criterion was occurrence of the penicillin reaction in the last 10 years. About 80% of patients with demonstrable IgE-mediated hypersensitivity will lose their allergy altogether within 10 years of avoiding the drug class.29 The last criterion was lack of a tolerated re-exposition to penicillins after the initial reaction. Our patients met the first, second and third criterion in 67.03%, 49.45%, and 47.25% of the times, respectively. Fifty-one of the 91 patients (56.04%) had 2 or the 3 criteria and, therefore, were designated as high-risk clinical history and underwent desensitization, regardless of the other tests performed in the diagnostic investigation. The remaining 40 patients with one or none criterion were considered low-risk clinical history, completed the investigation with immediate skin testing and sIgE measurement, which were negatives, and were challenged. None of them had new IHRs.
Unfortunately, serum tryptase measurement was not done during the initial IHR in any of our cases. Although the diagnosis of anaphylaxis is based on clinical criteria, knowing the serum tryptase level might have helped in the diagnoses of difficult and doubtful cases. In Brazil, as well as in many countries, physicians are not aware of the tryptase measurement and are not used to asking for it; furthermore, the test is not available in most public and private services, creating a vicious circle. Another barrier for the diagnosis of allergy to penicillin is the low sensitive of the sIgE tests for penicillin V, penicillin G, and amoxicillin. Sensitivity varies according to the study in the literature, ranging from 0 to 45%, and the positive and negative predictive values of sIgE to penicillins are not well established.30,31 None of the patients evaluated in the present study had detectable serum sIgE to penicillin. These findings are not just due to intrinsic test failures, but also because a specific IgE response to penicillin is often a transient phenomenon. The majority of patients with IgE sensitization at the time of reaction has converted to IgE undetectable if the test is performed more than a year after the incident took place.32 Therefore, the way sIgE test has been measured is not adding too much to the algorithms to manage penicillin allergy. This reinforces the need for prompt and adequate evaluation of all patients with IHRs to penicillin, with tryptase measurement 30 min to 2 h after the onset of the reaction, and sIgE at least after 4–6 weeks, but not exceeding 1 year.
Intradermal skin testing with benzylpenicillin was positive in 7.69% of all patients and 13.73% of those with a high-risk clinical history, confirming the diagnosis of allergy to penicillin in these patients. The test was an excellent biomarker to identify patients prone to react during RDD, since there were no reactions in the participants with negative tests (p < 0.001). IHRs to penicillins involve IgE response to the beta lactam ring or side chains of the antibiotic molecule, or to the drug metabolites, which are classified into major and minor antigenic determinant. The debate continues on the necessity or not of carrying out skin testing with a complete diagnostic kit constituted by penicillin G, benzylpenicilloyl-poly-l-lysine, and minor determinate mixture (MDM).33 In the present study, only penicillin G was available, but the inclusion of other antigenic determinants in the skin testing would not have made a difference in predicting patients who would react during the desensitization.
There are 2 goals in RDD: 1) To administer the full dose of the drug involved in the initial IHR; 2) To complete the RDD without a new breakthrough IHR, ie, to perform a silent RDD. We complete RDD in 97.8% of our pregnant patients with syphilis, with silent desensitization in 92.2% of them. There are 2 possibilities in patients who had silent desensitization: either they were not allergic to penicillin or the desensitizations were very successful in achieving both of their goals. After delivery, the pregnant women will be invited to undergo provocation with penicillin and complete the diagnostic investigation.
We observed more IHRs during the oral desensitization to penicillin than in the intravenous procedure. There is no study comparing an oral and an IV protocol for rapid desensitization to penicillin. Although an IHR may be triggered faster in IV desensitization, the physician is able to stop allergen exposure immediately. During an oral desensitization, a dose from a previous step may be sufficient to induce a reaction, but since it may take longer to occur, the patient may have already taken the next dose. The increment of doses in IV desensitization is more precise.
Our study has some limitations. As pregnancy may be considered an isolated criterion for defining a patient as high-risk,34 we use strict criteria to define the 51 high-risk clinical history patients that underwent desensitization, including 44 women with negative skin testing. It is probable that some of them were not truly allergic, making our safety results overestimated. Anyway, the urgent need to complete penicillin treatment was reached in 97.8% of our patients and it will be possible to perform penicillin provocation tests after their delivery. Only the 40 women with zero or 1 clinical criterion of high-risk clinical history and negative skin test were challenged.
Immediate hypersensitivity reaction to penicillin was diagnosed in 7.69% of pregnant women with syphilis and clinical history of allergy to penicillin; in all cases the diagnosis was made through intradermal testing, which predicted 100% of the breakthrough reactions observed during rapid drug desensitization. Risk stratification based on clinical history and skin testing to guide penicillin re-introduction through drug challenge or desensitization was safe (97.8%) and effective (97.8%). Further studies may prove the greater safety of the intravenous protocol compared to the oral protocol.
Abbreviations
Immediate hypersensitivity reactions, IHR; Rapid Drug Desensitization, RDD; Serum specific IgE, sIgE; Drug Provocation Test, DPT; Minor determinant mixture, MDM
Ethics Approval
The study was done at the Clinical Immunology and Allergy Division of the University of São Paulo School of Medicine, Brazil, from 2016 to 2019, and it was approved by the Hospital Institutional Review Board (CAPPesq, protocol 28897219.0.0000.0068).
The patients' medical records and the results of the procedures are filed electronically in our Department, and the data and materials are available.
Declaration of competing interest
The authors have no financial or conflicts of interest to disclosure. All authors participated in the design of the present study and in the analysis of its results. Juliana F. B. Garcia and Pedro Giavina-Bianchi collected the data and wrote the manuscript. All authors read, approved and consented to the publication of this manuscript.
Footnotes
Full list of author information is available at the end of the article https://doi.org/10.1016/j.waojou.2021.100549
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100549.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
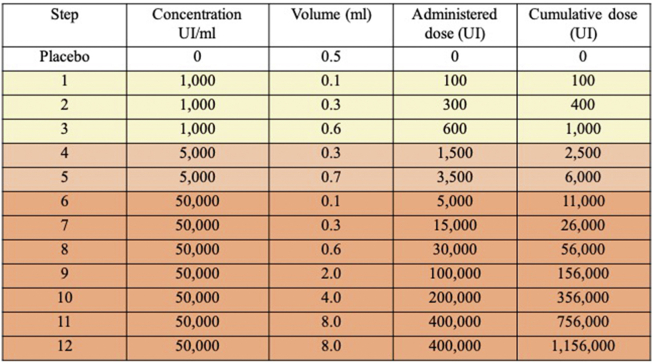
Fig. S1.
Oral desensitization protocol. Footnote: Adapted from the protocol described by Wendel GD Jr et al. N Engl J Med 1985; 312: 1229-32(19).
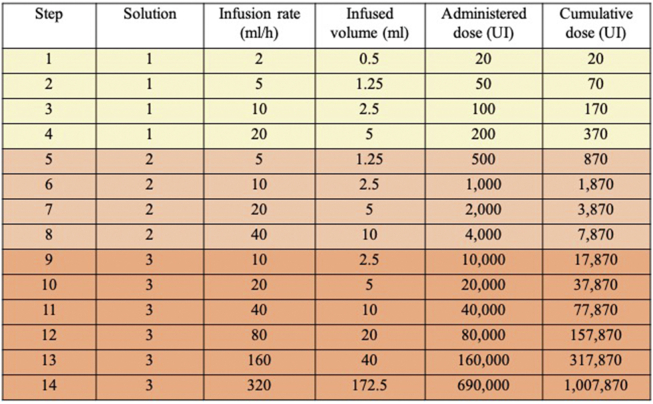
Fig. S2.
Intravenous desensitization protocol. Division of Clinical Immunology and Allergy. Footnote: Adapted from the protocol described by Castells MC et al. J Allergy Clin Immunol 2008; 122: 574-80(20).
References
- 1.Nelson R. Congenital syphilis and other STIs rise in the USA. Lancet Infect Dis. 2018;18(11):1186–1187. doi: 10.1016/S1473-3099(18)30618-2. [DOI] [PubMed] [Google Scholar]
- 2.Tsai S., Sun M.Y., Kuller J.A., Rhee E.H.J., Dotters-Katz S. Syphilis in pregnancy. Obstet Gynecol Surv. 2019;74(9):557–564. doi: 10.1097/OGX.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 3.Lin J.S., Eder M.L., Bean S.I. Screening for syphilis infection in pregnant women: updated evidence report and systematic review for the US preventive services task force. J Am Med Assoc. 2018;320(9):918–925. doi: 10.1001/jama.2018.7769. [DOI] [PubMed] [Google Scholar]
- 4.Storey A., Seghers F., Pyne-Mercier L., Peeling R.W., Owiredu M.N., Taylor M.M. Syphilis diagnosis and treatment during antenatal care: the potential catalytic impact of the dual HIV and syphilis rapid diagnostic test. Lancet Glob Health. 2019;7(8):e1006–e1008. doi: 10.1016/S2214-109X(19)30248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinh T., Leal A.F., Mello M.B. Syphilis management in pregnancy: a review of guideline recommendations from countries around the world. Sex Reprod Health Matters. 2019;27(1):69–82. doi: 10.1080/26410397.2019.1691897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzaken A.S., Pereira G.F.M., Cunha A.R.C.D., Souza F.M.A., Saraceni V. Adequacy of prenatal care, diagnosis and treatment of syphilis in pregnancy: a study with open data from Brazilian state capitals. Cad Saúde Pública. 2019;36(1) doi: 10.1590/0102-311X00057219. [DOI] [PubMed] [Google Scholar]
- 7.Furness A., Kalicinsky C., Rosenfield L., Barber C., Poliquin V. Penicillin skin testing, challenge, and desensitization in pregnancy: a systematic review. J Obstet Gynaecol Can. 2020;42(10):1254–1261.e3. doi: 10.1016/j.jogc.2019.11.067. [DOI] [PubMed] [Google Scholar]
- 8.Simons F.E., Ebisawa M., Sanchez-Borges M. Update of the evidence base: world Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32. doi: 10.1186/s40413-015-0080-1. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullins R.J., Wainstein B.K., Barnes E.H., Liew W.K., Campbell D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46(8):1099–1110. doi: 10.1111/cea.12748. [DOI] [PubMed] [Google Scholar]
- 10.Castells M., Khan D.A., Phillips E.J. Penicillin allergy. N Engl J Med. 2019;381(24):2338–2351. doi: 10.1056/NEJMra1807761. [DOI] [PubMed] [Google Scholar]
- 11.Jerschow E., Lin R.Y., Scaperotti M.M., McGinn A.P. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134(6):1318–1328. doi: 10.1016/j.jaci.2014.08.018. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy E.S., Macy E., Rowe T., Blumenthal K.G. Evaluation and management of penicillin allergy: a review. J Am Med Assoc. 2019;321(2):188–199. doi: 10.1001/jama.2018.19283. [DOI] [PubMed] [Google Scholar]
- 13.Idsoe O., Guthe T., Willcox R.R., de Weck A.L. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38(2):159–188. [PMC free article] [PubMed] [Google Scholar]
- 14.Mulla Z.D., Ebrahim M.S., Gonzalez J.L. Anaphylaxis in the obstetric patient: analysis of a statewide hospital discharge database. Ann Allergy Asthma Immunol. 2010;104(1):55–59. doi: 10.1016/j.anai.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 15.McCall S.J., Bunch K.J., Brocklehurst P. The incidence, characteristics, management and outcomes of anaphylaxis in pregnancy: a population-based descriptive study. BJOG. 2018;125(8):965–971. doi: 10.1111/1471-0528.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maintz L., Schwarzer V., Bieber T., van der Ven K., Novak N. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update. 2008;14(5):485–495. doi: 10.1093/humupd/dmn014. [DOI] [PubMed] [Google Scholar]
- 17.Knight Me, Nair Me, Tuffnell De, Shakespeare Je, Kenyon Se, Kurinczuk JJe. Saving Lives, Improving Mothers' Care : Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013-15.
- 18.Giavina-Bianchi P., Patil S.U., Banerji A. Immediate hypersensitivity reaction to chemotherapeutic agents. J Allergy Clin Immunol Pract. 2017;5(3):593–599. doi: 10.1016/j.jaip.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Wendel G.D., Stark B.J., Jamison R.B., Molina R.D., Sullivan T.J. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985;312(19):1229–1232. doi: 10.1056/NEJM198505093121905. [DOI] [PubMed] [Google Scholar]
- 20.Castells M.C., Tennant N.M., Sloane D.E. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 21.World Heath Organization. www.who.int/gho/sti/en/. Last accessed 21 of October of 2020.
- 22.Aun M.V., Blanca M., Garro L.S. Nonsteroidal anti-inflammatory drugs are major causes of drug-induced anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(4):414–420. doi: 10.1016/j.jaip.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Sacco K.A., Bates A., Brigham T.J., Imam J.S., Burton M.C. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72(9):1288–1296. doi: 10.1111/all.13168. [DOI] [PubMed] [Google Scholar]
- 24.Macy E., Contreras R. Health care use and serious infection prevalence associated with penicillin "allergy" in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 25.MacFadden D.R., LaDelfa A., Leen J. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis. 2016;63(7):904–910. doi: 10.1093/cid/ciw462. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal K.G., Shenoy E.S., Wolfson A.R. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616–625. doi: 10.1016/j.jaip.2017.02.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed O.E., Beck S., Huissoon A. A retrospective critical analysis and risk stratification of penicillin allergy delabeling in a UK specialist regional allergy service. J Allergy Clin Immunol Pract. 2019;7(1):251–258. doi: 10.1016/j.jaip.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Chovel-Sella A., Ben Tov A., Lahav E. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424–e1427. doi: 10.1542/peds.2012-1575. [DOI] [PubMed] [Google Scholar]
- 29.Trubiano J.A., Adkinson N.F., Phillips E.J. Penicillin allergy is not necessarily forever. J Am Med Assoc. 2017;318(1):82–83. doi: 10.1001/jama.2017.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macy E., Goldberg B., Poon K.Y. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol. 2010;105(2):136–141. doi: 10.1016/j.anai.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Fontaine C., Mayorga C., Bousquet P.J. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy. 2007;62(1):47–52. doi: 10.1111/j.1398-9995.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 32.Hjortlund J., Mortz C.G., Stage T.B., Skov P.S., Dahl R., Bindslev-Jensen C. Positive serum specific IgE has a short half-life in patients with penicillin allergy and reversal does not always indicate tolerance. Clin Transl Allergy. 2014;4:34. doi: 10.1186/2045-7022-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adkinson N.F., Mendelson L.M., Ressler C., Keogh J.C. Penicillin minor determinants: history and relevance for current diagnosis. Ann Allergy Asthma Immunol. 2018;121(5):537–544. doi: 10.1016/j.anai.2018.09.459. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal K.G., Shenoy E.S. Penicillin allergy in pregnancy. J Am Med Assoc. 2020;323(12):1216. doi: 10.1001/jama.2019.19809. [DOI] [PubMed] [Google Scholar]