Abstract
Significance: Prolonged inflammation and impaired angiogenesis are the two principal factors that prevent successful wound healing, which is exacerbated in people with diabetes. There is a significant need for new wound healing treatments that target both these factors simultaneously. This review discusses the emerging evidence that high-density lipoproteins (HDL) have pleiotropic wound healing benefits.
Recent Advances: Numerous in vitro and in vivo studies have demonstrated the anti-inflammatory and proangiogenic effects of HDL. In endothelial cells, HDL mediate these effects through interaction with the scavenger receptor SR-BI, which activates the PI3K/Akt pathway, causing a decrease in inflammatory protein production and an increase in proangiogenic growth factors. In macrophages, HDL inhibit inflammation through suppression of the nuclear factor kappa B activation pathway. This review details the molecular disturbances that cause impaired wound healing in diabetes with a particular focus on inflammation and angiogenesis and the pathways in which HDL provide benefit.
Critical Issues: Diabetic foot ulcers (DFUs) impose a major public health challenge worldwide. It is estimated that 20% patients with DFUs require amputation, which is accompanied by a significant social and economic burden. To date, there are no therapeutic agents with pleiotropic effects that actively improve wound healing, highlighting a therapeutic void for this complex disease.
Keywords: high-density lipoproteins, diabetes, anti-inflammatory, angiogenesis, wounds, ulcers

Christina Bursill, PhD
SCOPE AND SIGNIFICANCE
Diabetes is one of the most common metabolic disorders,1,2 with the World Health Organization reporting 382 million diabetic people in 2013, with 592 million predicted by 2035.1 Patients with diabetes have a predisposition for the development of peripheral arterial disease and peripheral neuropathy, both of which are precursors for the development of diabetic foot ulcers (DFUs).3,4 It is estimated that 20% of patients with DFUs require amputation.3 The risk of death in patients with an existing DFU is 2.5 times higher than diabetic patients without.3,5 Wound healing is a complex physiological process and this process is complicated further by diabetes.6
TRANSLATIONAL RELEVANCE
There are currently very few therapies that actively improve wound healing and exhibit pleiotropic effects.7 Emerging evidence suggests high-density lipoproteins (HDL) possess significant wound healing properties through regulation of multiple important wound healing mechanisms. HDL have traditionally been viewed as a cardioprotective protein since the discovery of a significant inverse relationship between plasma HDL cholesterol levels and the risk of myocardial infarction.8 More recently, HDL have been reported to exhibit anti-inflammatory, endothelial protective, and proangiogenic effects in response to ischemia.9,10 As a result of these multiple benefits, HDL promise to be a highly effective wound healing agent.
CLINICAL RELEVANCE
This article will discuss the molecular mechanisms involved in diabetic wound healing, focusing on the inflammatory disturbances and impaired angiogenesis. We will compare the successes and limitations of current clinical strategies being tested to improve healing. Finally, this article will highlight the accumulating evidence supporting the role of HDL in diabetic wound healing, and why it may have advantages over other therapeutic approaches that will facilitate its translation into the clinical setting.
BACKGROUND
Acute and chronic wounds
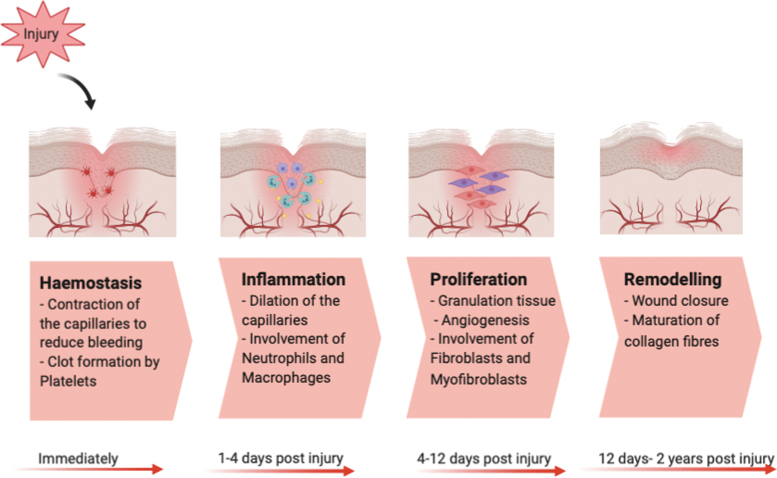
Wound healing is a dynamic process consisting of four continuous, yet overlapping, phases characterized by hemostasis, inflammation, proliferation, and remodeling (Fig. 1).11 During optimal wound healing, these phases occur in a precise and timely manner.12
Figure 1.
Different Stages of healthy wound healing in humans consisting of hemostasis, inflammation, proliferation, and remodeling, which usually occur immediately, 1–2 days, 4–12 days, and 12 days-2 years postinjury, respectively. Color images are available online.
Some flexibility exists in the wound healing process to support imbalances within each step and sequence; however, if this not carefully regulated and system is disrupted too much, it can lead to the development of a chronic nonhealing wound. Diabetes causes substantial imbalances in each stage of the wound healing process, and if a wound fails to heal within the first 8 weeks in a diabetic patient, this is classified as “chronic.”6
Diabetes is a condition characterized by chronically elevated blood glucose levels. In many people with diabetes, this can be controlled with insulin therapy and/or other antidiabetic drugs. Despite this, the risk of impaired wound healing is significantly elevated in people living with diabetes and is associated with persistent, nonresolving inflammation and defective tissue repair responses.13,14 For example, in hyperglycemia, neutrophils produce more superoxide and proinflammatory cytokines.15 Neutrophils from diabetic patients are also found to be in a hyperactive state, indicated by increased expression of activation marker CD11b.16 In terms of the adaptive immune system, the study by Moura et al. reported decreased diversity of blood T cell T-cell receptor-β in diabetes, particularly in DFUs, despite the accumulation of T cells in the wound tissues of diabetic patients.17
Diabetes also increases the levels of circulating inflammatory protein C-reactive protein in foot wounds18 and wounds have increased total numbers of inflammatory cells, paralleled by decreased concentrations of growth factors.19 This hinders the migration of the epidermis layer over the wound, which is essential for healing.19 Diabetes also causes alterations in wound structure and the remodeling process, causing a loss of heparan sulfate proteoglycans such as syndecan-420 and increased degradation of the extracellular matrix.21 In addition, diabetes significantly impacts angiogenesis, which is the formation of new vessels from existing ones. Diabetic wounds have poor angiogenic responses resulting in decreased capillary density. This is due to the negative effect of hyperglycemia on different range of transcription factors, signaling molecules, and growth factors such as reactive oxygen species (ROS) and vascular endothelial growth factor (VEGF).
Limitations of current and previous topical wound healing therapies
Based on the evidence-based guidelines published by the International Working Group on Diabetic Foot (IWGDF), the most common treatment options for DFUs are limited to debridement of hypertrophic tissue surrounding the wound, application of appropriate dressings, and orthotic devices to offload the pressure from the ulcer area.22 In some cases, vascular reconstruction is required if macrovascular ischemia is contributing to chronic wound development.22
A recent comprehensive review, part of the IWGDF process, identified that sharp debridement was the only intervention studied in a controlled manner and with adequate evidence demonstrating benefit.23 For topical interventions, a sucrose octasulfate dressing was found to be the most effective in a recent well-controlled randomized multicenter clinical trial across France, Spain, Italy, Germany, and United Kingdom.24 The mechanism of action is believed to be through inhibition of matrix metalloproteases, which are found in higher concentrations in chronic wounds, increasing inflammation and breaking down the remodeling process.24
Negative pressure wound therapy (NPWT) (only when used after surgery), has demonstrated promising benefits in clinical trials.25 However, for chronic wounds, there is not enough evidence to establish whether NPWT has any benefit over standard care. NPWT is predominantly recommended for acute and larger postsurgical wounds, which have excess exudate that impacts wound healing.
Growth factors are essential for wound healing, particularly for promoting angiogenesis.23 Numerous clinical trials have tested the application of growth factors, including fibroblast growth factor (FGF), epidermal growth factor (EGF), combined FGF-EGF, and other growth factors, on diabetic wounds. Topical application of basal FGF in a spray format onto DFUs caused a 75% reduction in ulcer area; however, the analysis for this study was as per protocol analysis leading to bias.26 Furthermore, a recent Phase III multicenter clinical trial used a spray form of recombinant human EGF that significantly improved wound healing in 167 patients with DFUs.27 Despite their promise, a major confounder for the translation of growth factors is their lack of cost-effectiveness, with an additional significant consideration being that they only target a single mechanistic pathway of healing.
In summary, despite numerous clinical trials that have tested a wide variety of interventions, there is still no universally approved or effective therapy for diabetic wound healing, which has been implemented to support the best standard of care. Most clinical interventions target only a single mechanism of action in the wound healing process. This may be insufficient to effectively heal mechanistically complex diabetic wounds. Agents that offer pleiotropic actions on the wound healing process are therefore far more likely to be effective.
DISCUSSION
HDL: the “Good Cholesterol”
HDL is primarily composed of an outer layer of phospholipids and proteins (known as apolipoproteins, predominantly apoA-I) and a hydrophobic core of cholesterol (esterified and nonesterified) and triglycerides.28 Circulating plasma HDL comprise a highly heterogeneous family of lipoprotein particles varying in density, size, surface charge, and lipid/protein composition.29 HDL interact with three key cell surface proteins. These are the cholesterol transporters ABCA1 and ABCG1 and the scavenger receptor SR-BI.30 A vast number of in vitro, in vivo, and epidemiological studies have demonstrated that HDL exhibit atheroprotective, antithrombotic, antioxidant, antiapoptotic, anti-inflammatory, antiproteolytic, and proangiogenic properties.29 HDL are characterized into several subtypes based on variations in size and composition; these subtypes include HDL2 and HDL3.28 HDL3 in particular is believed to be a subpopulation of HDL with superior anti-inflammatory effects.31 The majority of the beneficial effects of HDL are conducive to improving wound repair.
Epidemiological evidence of a role for HDL in wound healing
A large body of evidence supports a relationship between low circulating HDL cholesterol levels and the risk of a cardiovascular event.32 More recently, epidemiological studies have shown an inverse relationship between HDL levels and the risk of developing diabetes. For instance, levels of HDL2 were found to be inversely proportional with the incidence of type 2 diabetes.33 Interestingly, lower levels of HDL have been associated with an increased risk of developing diabetic neuropathy, an important contributing factor for DFU formation, in both type 1 and type 2 diabetes.34 While pharmacological elevation of circulating HDL cholesterol levels using cholesterol ester transfer protein (CETP) inhibitors was not effective in reducing cardiovascular events, interestingly, it did delay the onset of T2DM, indicating an antidiabetic effect.33
Only one clinical study has specifically evaluated the relationship between endogenous HDL cholesterol levels and diabetic wound healing outcome.35 In 163 patients with an existing DFU in a single hospital in Japan, this study found that there was an inverse correlation between circulating HDL cholesterol levels and three endpoints: (1) minor amputation, or below the ankle amputation, (2) major amputation or amputation above the ankle, and (3) wound-related death, defined as a death with unhealed ulcer. This independently suggested that endogenous HDL cholesterol is a predictor for lower extremity amputation.
Inflammation in diabetic wound healing and the role of HDL
Inflammation is important in the early stages of wound healing to combat infection, however, when prolonged and inappropriate inflammation negatively affect wound healing at every step in the repair process. Diabetes, in particular, exacerbates inflammation and substantially slows its resolution, causing multiple complications in wound biology and healing. Agents that suppress inflammation are therefore likely to have significant wound healing benefits. It is well established that HDL have anti-inflammatory effects that include (1) the suppression of endothelial cell inflammation and improved endothelial function and (2) the reduction of monocyte/macrophage activation and recruitment to sites of inflammation (Table 1).28 These properties of HDL all have benefits on the wound healing process and the prevention of chronic wound development.
Table 1.
Anti-inflammatory effects of high-density lipoproteins
| Study | Mechanism of Action for HDL's Anti-inflammatory Properties |
|---|---|
| Kimura et al.36 | Inhibition of key cell adhesion molecules, including VCAM-1 and ICAM-1 |
| Yuhanna et al.64 | Stimulating eNOS and production of nitric oxide |
| Bursill et al.37 | Suppressing endothelial cell chemokine expression (CCL2, CCL5, and CX3CL1) |
| Murphy et al.10 | Decreasing the expression of monocyte CD11b in a dose-dependent manner |
| Sanson et al.43 | Promoting the transition of M1-like macrophages to M2-like macrophages |
| Liu et al.44 | Inhibition of inflammatory cytokine production from monocytes |
eNOS, endothelial nitric oxide synthase; HDL, high-density lipoproteins.
Endothelial protective effects
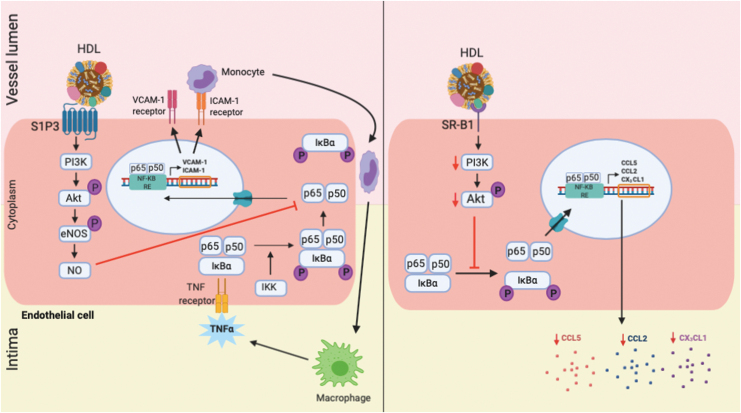
Endothelial cells play a key role in wound healing. They are the building blocks of wound neovessels that are essential for repair. Through their expression of adhesion molecules, chemokines, and cytokines, endothelial cells mediate the delivery of monocytes and macrophages to the wound site. Controlled endothelial cell inflammation is therefore critical for successful healing, but can lead to chronic wound development if inappropriately prolonged or elevated. HDL exhibit potent inhibitory effects on endothelial cell inflammation (Fig. 2). For example, HDL have been shown to reduce the in vitro and in vivo expression of key adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin.31,36 One of the mechanisms for the anti-inflammatory effects of HDL on endothelial cells starts with the interaction between HDL and the sphingosine-1-phosphate 3 (S1P3) receptor. This results in the phosphorylation and activation of the PI3K/Akt pathway, which leads to the activation of endothelial nitric oxide synthase (eNOS) and the production of nitric oxide (NO). While NO plays an important role in the promotion of wound angiogenesis and maintenance of endothelial function, it also exhibits anti-inflammatory effects. It has been demonstrated that NO can inhibit the tumor necrosis factor (TNF)-α activation pathway through the suppression of nuclear factor kappa B (NF-κB); the pivotal inflammatory transcription factor comprised subunits p65 and p50 that normally promotes VCAM-1, ICAM-1, and chemokine expression.31 Reconstituted HDL (rHDL, apoA-I complexed with phospholipid) also suppress endothelial cell chemokine expression, including CCL2, CCL5, and CX3CL1. This is mediated through interaction with SR-B1, inhibition of downstream kinase signaling, and suppression of NF-κB activation.37
Figure 2.
Mechanisms for the anti-inflammatory effects of HDL in endothelial cells. Left panel: HDL increase formation of bioactive nitric oxide (NO) through interaction with the S1P3 receptor, which activates PI3K/Akt, leading to inhibition in action of NF-κB. This prevents the translocation of the NF-κB subunits p65/p50 to the nucleus, where it binds to the NF-κB response elements (RE) in the promoter region of inflammatory genes, including vascular cell adhesion molecule (VCAM)-1 and intercellular cell adhesion molecule (ICAM)-1. Right panel: in an alternative pathway, HDL reduce the production of inflammatory cytokines CCL2, CCL5, and CX3CL1 through interaction with scavenger receptor (SR)-B1 that leads to suppression of downstream kinase signaling (PI3K/Akt) and inhibition in the activation of NF-κB pathway. NF-κB, nuclear factor kappa B; S1P3, sphingosine-1-phosphate 3. Color images are available online.
Preclinical models of vascular inflammation have also demonstrated the anti-inflammatory effects of HDL. For example, in a rabbit model of carotid occlusion,38 systemic infusion of rHDL inhibited neutrophil infiltration and the expression of VCAM-1, ICAM-1, and the chemokine CCL2.38 HDL have also been reported to increase the anti-inflammatory enzyme platelet-activating factor acetyl hydrolase, which inactivates platelet-activating factor and promotes inflammation.39
Macrophages
Macrophages are an important cell type contributing to both the initiation and resolution of the inflammatory phase of wound healing.40 Macrophages are highly heterogeneous and can express a wide range of markers with an adaptable and plastic nature.41 The two prominent macrophage populations that are active at the wound site during the wound healing process are tissue-resident macrophages and monocyte-derived macrophages. Under normal uninjured conditions, the population of tissue-resident macrophages is higher than monocyte-derived macrophages. However, upon injury, an influx of monocyte-derived macrophages results in a twofold increase in their number and an acute reduction in tissue-resident macrophages.40 Shortly after injury and hemostasis, monocyte-derived macrophages enter the wound site and differentiate into proinflammatory (M1) macrophages releasing inflammatory cytokines, including interleukin (IL)-1β, TNF-α, and IL-6.42 This is followed by the subsequent healing stages through polarization into the anti-inflammatory (M2) reparative macrophages, which release anti-inflammatory cytokines such as transforming growth factor (TGF)-β and IL-10 and support tissue remodeling and fibrosis.42 In pathological conditions such as diabetes, the transition from the M1 to the M2 phenotype is delayed, causing an increase in wound inflammation through excess M1-derived chemokine/cytokine expression. This persistent inflammation is likely to result in the development of a chronic wound.40
Multiple studies have demonstrated that HDL have anti-inflammatory effects on human monocytes and macrophages, which is likely to have substantial benefits on the wound healing process. For example, HDL prevent the activation of primary human monocytes by significantly decreasing the expression of CD11b.10 In an in vitro study,10 HDL inhibited phorbol 12-myristate-13-acetate (PMA)-induced activation of monocyte CD11b in a dose-dependent manner.10 Furthermore, when monocytes were pretreated with HDL followed by stimulation with PMA, there was a significant reduction in CD11b expression.10
HDL have also been shown to promote a shift from M1-like macrophages to M2-like macrophages. In a key study by Sanson et al.,43 HDL suppressed both basal and interferon gamma-induced expression of M1 inflammatory markers such as inducible nitric oxide synthase, IL-6, and TNF-α. Furthermore, HDL caused a 10-fold increase in the expression of M2-like markers Arg-1 and Fizz-1.43 It is important to note that both Arg-1 and Fizz-1 are likely to contribute to tissue remodeling in wound healing as they mediate the deposition of extracellular matrix in animal models.44 These findings further highlight the biological plausibility of utilizing the anti-inflammatory properties of HDL for wound healing.
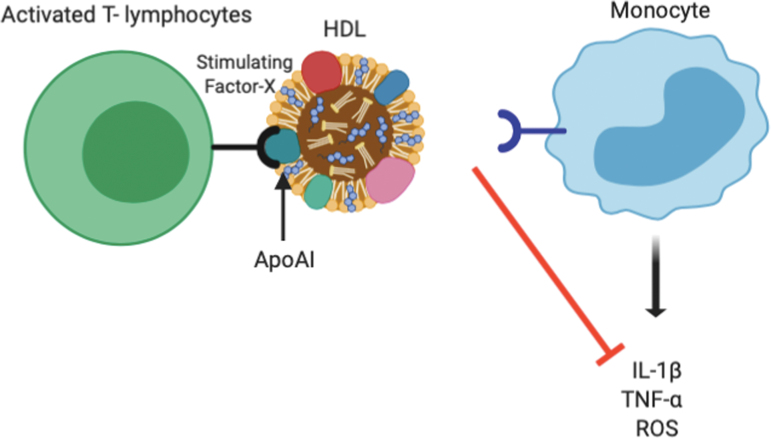
HDL also inhibit the production of inflammatory cytokines from monocytes. One mechanism by which HDL does this is through binding of the apoA-I component of HDL to the “stimulating factor” on circulating stimulated T lymphocytes. This blocks the normal interaction between monocytes and stimulated T lymphocytes, thereby preventing cytokine release (e.g., IL-1β and TNF-α) from monocytes at sites of inflammation (Fig. 3).45 ApoA-I has also been shown to inhibit the production of inflammatory cytokines such as IL-1β, TNF-α, and intercellular ROS by blocking the interaction of T cell ligands with the leukocyte β2-integrin subunit.31 Consistent with these findings, rHDL reduces the secretion of chemokines CCL2, CCL5, and CX3CL1 from monocytes and the expression of chemokine receptors CCR2 and CX3CR1.37
Figure 3.
Anti-inflammatory effects of HDL on monocytes/macrophages. HDL block the interaction between stimulated T lymphocytes and monocytes by interacting with “stimulating factor X” on T lymphocytes. This reduces the interaction between stimulated T lymphocytes and monocytes, which in turn reduces monocyte secretion of inflammatory cytokines and ROS. ROS, reactive oxygen species. Color images are available online.
HDL also suppress Toll-like receptor (TLR)-induced expression of proinflammatory cytokines from macrophages.46 For example, pretreatment of bone marrow-derived macrophages with HDL reduced IL-6 and TNF-α secretion in response to a variety of TLR ligands. The mechanism for these effects of HDL was found to be through regulation of the transcription factor ATF3 that is downstream of the TLR. ATF3 is induced through TLR stimulation and acts through a negative feedback system to inhibit excessive production of proinflammatory cytokines. Interestingly, infusion of HDL into mice increased the level of ATF3 mRNA expression in the Kupffer cells (macrophages) of the liver. In the same study, HDL demonstrated an endothelial protective effect by increasing reendothelialization of carotid arteries after carotid injury, an effect that was not observed in Atf-3-deficient mice.46
Diabetes significantly prolongs wound inflammation and halts the normal sequence of the wound repair process. This includes disruption to wound macrophage regulation. In a study done by Mirza et al.,13 macrophages isolated from chronic wounds of patients with type 2 diabetes prominently exhibited a proinflammatory M1-like phenotype, expressing high levels of inflammatory cytokines, including IL-1β, matrix metalloproteinase (MMP)-9, and TNF-α.13 Furthermore, wound macrophages obtained from diabetic mice persisted as proinflammatory M1-like macrophages and failed to transition to the reparative M2-like phenotype.13 The phenotypic switch from M1-like macrophages to M2-like macrophages is also believed to be controlled by factors such as IL-4/IL13, IL-1, TLR activation, and optimal activity of stress-associated MAPK, P38.47 Suppression of inflammation can, however, overcome these issues. The use of an IL-1β blocking antibody was found to improve wound healing, reduce M1-like macrophages, and increase the presence of M2-like macrophages in wounds.13 This highlights the importance of controlling inflammation to ensure successful wound healing in diabetes. Despite this, very few wound healing agents target inflammation. While HDL have potent anti-inflammatory properties, there is no published literature to date specifically reporting the anti-inflammatory effects of HDL in diabetic wounds. Our laboratory recently found that topical application of rHDL in a diabetic wound healing model was able to reduce the gene expression of inflammatory markers such as CCL2, IL-6, TGF-β, and relA in wound tissues, 3 days postwounding and treatment. This provides promising support for an anti-inflammatory effect of HDL in the context of diabetic wound healing.
Angiogenesis in wound healing and the role of HDL
One of the most important contributors to wound healing is angiogenesis.48 Angiogenesis is the formation of new blood vessels from pre-existing vessels and is critical in the proliferation phase of wound healing.
Proangiogenic effects of HDL
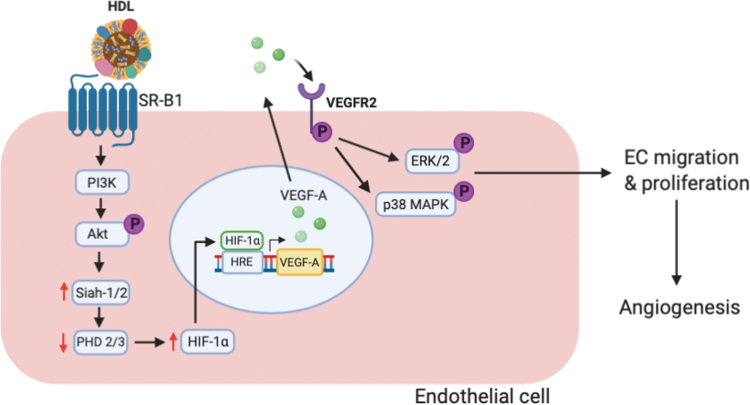
A number of studies demonstrate a proangiogenic effect of HDL in vitro in response to hypoxia and in preclinical murine models of hind limb ischemia and wound healing (Table 2).49 In vitro, HDL have been shown to significantly induce proliferation, migration, and tubule formation of endothelial cells, which are critical for angiogenesis. Investigation of the intracellular mechanisms has revealed that these changes in endothelial cell function occur through Src family kinases PI3K and MAPK with the involvement of SR-BI.50 SR-BI has also been implicated as the receptor that mediates the augmentation of ischemia-driven angiogenesis in in vivo murine models of hindlimb ischemia and wound healing.51 This study delineated that HDL interaction with SR-B1 activates the PI3K/Akt pathway leading to increased levels of HIF-1α and the transcription and production of VEGF-A (Fig. 4). Further investigation of the mechanisms of action revealed that in endothelial cells, rHDL is able to stabilize HIF-1α in high glucose.52 It was found to do this through an increase in the ubiquitin ligases Siah-1 and Siah-2, which subsequently suppress the prolyl hydroxylase domain (PHD) proteins PHD-2 and PHD-3 that normally target HIF-1α for degradation. There is also emerging evidence that rHDL stabilizes HIF-1α and improves angiogenesis in diabetes by correction of impaired metabolic reprogramming responses to hypoxia.53
Table 2.
Proangiogenic effects of high-density lipoproteins
| Study | Mechanism of Action for HDL's Proangiogenic Properties |
|---|---|
| Tan et al.52 | Inducing proliferation, migration, and tubule formation of endothelial cells |
| Cannizo et al.54 | |
| Primer et al.53 | |
| Tan et al.52 | Activation of PI3K/Akt pathway leading to increased VEGF-A production |
| Cannizo et al.54 | Augmenting VEGFR2 phosphorylation and downstream activation of critical signaling proteins involved in endothelial cell proliferation and migration |
| Tan et al.52 | Stabilization of HIF-1 α, through increasing Siah 1/2 and decreasing PHD-1 |
| Jin et al.55 | Activation of VEGFR2 through S1P3 signaling pathway |
| Yuhanna et al.64 | Stimulating eNOS and production of nitric oxide |
PHD, prolyl hydroxylase domain; S1P3, sphingosine-1-phosphate 3; VEGFR2, vascular endothelial growth factor receptor 2.
Figure 4.
The proangiogenic effects of HDL in endothelial cells. Following interaction with scavenger receptor (SR)-B1, HDL activates downstream PI3K/Akt signaling. This increases HIF-1α stabilization through an increase in the ubiquitin ligases Siah-1 and Siah-1, which inhibit the PHD proteins PHD-2 and PHD-4 that normally target HIF-1α for degradation. With increased HIF-1α stabilization, it allows for elevated levels of HIF-1α translocation to the nucleus. In the nucleus, HIF-1α binds to HIF response elements (HRE) in the promoter regions of proangiogenic genes, including VEGF-A, which increases their expression. This increases the interaction between VEGF-A and its receptor VEGFR2, leading to enhanced activation of ERK1/2 and p38 MAPK that promote endothelial cell migration and proliferation, respectively, and results in increased angiogenesis. PHD, prolyl hydroxylase domain; VEGFR2, vascular endothelial growth factor receptor 2. Color images are available online.
VEGF-A is a potent proangiogenic growth factor that is under the control of HIF-1α. It activates numerous angiogenesis cellular activities once bound to its receptor, vascular endothelial growth factor receptor 2 (VEGFR2), on endothelial cells. Following binding, activation of the ERK1/2 and p38MAPK downstream signaling pathways occurs, which are important for cell proliferation and cell migration, respectively (Fig. 4). In a recent in vitro study, rHDL was shown to augment VEGFR2 phosphorylation and cause activation of downstream ERK1/2 and p38MAPK proteins in endothelial cells under hypoxic conditions, adding another piece to the puzzle of how rHDL promotes angiogenesis.54
S1P is a bioactive lipid mediator that regulates angiogenesis, vascular stability, and permeability.55 HDL transports 60% of the S1P in the plasma making it the main acceptor and carrier of S1P. It has been reported that HDL-mediated angiogenesis involves activation of VEGFR2 also through the S1P3 signaling pathway.55 This occurs through binding of the HDL/S1P to S1P3 receptor, which then phosphorylates (activates) VEGFR2 and promotes angiogenesis functions, including proliferation, migration, and tubule formation in endothelial cells.55 The Ras-activated MAP kinase pathway was the intracellular mechanism of action for these HDL-S1P/S1P3 receptor-driven proangiogenic effects of HDL.56
Impaired angiogenesis and wound healing in diabetes
Diabetes leads to a significant impairment in angiogenesis as a result of a number of mechanisms. First, endothelial cells become dysfunctional when exposed to elevated systemic glucose concentrations, leading to a loss in their integrity and increased apoptosis.4 Second, high glucose causes imbalances in cytokine/chemokine and growth factor levels that disturb normal angiogenesis.
Studies have shown that the ability of VEGF-A to simulate the migration of monocytes from diabetic patients is impaired, when compared to monocytes from healthy individuals. This was despite higher levels of VEGF-A in the plasma of patients with diabetes indicating this is due to a deficiency in the activation of VEGFR2.57 Furthermore, in diabetic mice wounds, the mRNA and protein levels of VEGF-A are significantly reduced, compared to healthy controls.58 Higher levels of the antiangiogenic growth factor pigment epithelium-derived factor59 and low levels of FGF-2 cell surface receptors syndecan-4 and glypican-120 in diabetic patients also lead to impaired angiogenesis in diabetes. Furthermore, increases in antiangiogenic molecules that cause proteolysis of VEGF-A have been observed in chronic venous ulcers.60,61 Another vascular maturation pathway relevant to angiogenesis in diabetic wound is the Ang1/Ang2/Tie2 complex. In diabetic mice, the ratio of Ang1 to Ang2 has been decreased, which leads to reduced maturation of nascently formed blood vessels.4
Another reason for the impairment of angiogenesis in diabetes is that high glucose impairs angiogenesis responses to wound ischemia, a result of a low oxygen supply due to tissue injury or peripheral artery occlusion.49 Central to this is the high-glucose-induced destabilization of HIF-1α, the key transcription factor that is activated in response to hypoxia/ischemia to promote VEGF-A and angiogenesis.49 Related to this is a reduction in the recruitment of endothelial progenitor cells (EPCs) to the site of diabetic wounds. EPCs make significant contributions to wound vascularization in response to ischemia. In diabetes, the production of the chemokine stromal cell-derived factor (SDF)-1α in the wound is reduced. SDF-1α normally facilitates the migration and recruitment of EPCs to the wound and thus a reduction in its concentration at the wound site leads to less EPC recruitment and neovascularization. In summary, diabetes leads to a multitude of events that cause a reduction in angiogenesis, which is essential for wound repair. An agent that remains proangiogenic in hyperglycemia would therefore be immensely valuable for improving healing in DFUs.
An emerging role for HDL in wound repair
Accumulating evidence demonstrates that HDL display significant wound healing benefits.
For example, dropwise topical application of rHDL to nondiabetic and diabetic wounds in a murine model of wound healing improved the rate of wound closure.51,62 Using Laser Doppler imaging, topical HDL was also found to increase wound angiogenesis in both nondiabetic and diabetic mice. This effect of rHDL was attenuated in SR-BI−/− mice, demonstrating that SR-BI, at least in part, mediates improved wound healing and wound angiogenesis by rHDL.51 Daily topical application of HDL formulated in a 20% Pluronic F-127 gel (PH 7.2) was also found to increase wound healing in atherosclerosis-prone apolipoprotein (apo)E−/− mice through an increase in granulation tissue formation and reepithelialization.63 Furthermore, a study in 24-month-old mice also found that topical rHDL promoted the rate of wound healing and wound angiogenesis.62
Overall, HDL improve wound healing in the context of diabetes, high cholesterol, and aging. The pathways for this are likely to be through an improvement in angiogenesis and an accelerated resolution of inflammation that together assist with wound tissue granulation and reepithelialization.
FUTURE DIRECTIONS
This review provides a comprehensive insight into the currently known evidence on the effects of HDL on inflammation and angiogenesis and highlights its promise as an effective wound healing agent. Future research investigating the efficacy of topical HDL on wound healing in diabetes using in vitro, preclinical and clinical testing will facilitate its translation.
SUMMARY
Despite the significant clinical need for effective wound healing therapies with pleiotropic actions, there is still no commonly used therapeutic agent that actively improves healing in chronic wounds such as DFUs. The prevalence of diabetes and the associated complications of diabetes such as DFUs are rising. As discussed in detail, HDL exhibit valuable properties such as anti-inflammatory and proangiogenic effects, making it a highly promising therapeutic treatment for wound healing. Future clinical trials that test the effect of topical HDL on acute and chronic wounds are required and eagerly awaited.
TAKE-HOME MESSAGES
Appropriate inflammation and angiogenesis are essential for optimal wound healing. These processes are impaired in chronic conditions such as diabetes, resulting in nonhealing wounds.
Currently, there is no effective treatment that actively improves wound healing in diabetes, highlighting a significant unmet clinical need.
HDL exhibit anti-inflammatory effects in endothelial cells and macrophages, while also promoting proangiogenic functions in endothelial cells. HDL therefore presents as a highly promising therapeutic option for chronic wound healing.
Preclinical models show that topical HDL can improve wound healing in the contexts of diabetes, atherosclerosis, and aging.
Acknowledgments and Funding Sources
No external funding sources were used in the preparation of this article
Abbreviations and Acronyms
- CETP
cholesterol ester transfer protein
- DFU
diabetic foot ulcer
- EGF
epithelial growth factor
- eNOS
endothelial nitric oxide synthase
- FGF
fibroblast growth factor
- HDL
high-density lipoprotein
- ICAM
intercellular cell adhesion molecule
- IGF
insulin-like growth factor
- IL-1β
interleukin beta 1
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor kappa B
- NGF
nerve growth factor
- NO
nitric oxide
- PDGF
platelet-derived growth factor
- PHD
prolyl hydroxylase domain
- PMA
phorbol 12-myristate-13-acetate
- ROS
reactive oxygen species
- S1P3
sphinogase-1 phosphate receptor 3
- SDF
stromal cell-derived factor
- TGF-β
transforming growth factor-β
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
Author Disclosure and Ghostwriting
All authors confirm no conflict of interest and no ghost writers were used to write this article
About the Authors
Zahra Lotfollahi is a PhD student and qualified podiatrist based at the South Australian Health and Medical Research Institute (SAHMRI) and the University of Adelaide. Her project is focusing on characterizing the anti-inflammatory properties of HDL in wound healing. Joseph Dawson, MBBS, ChM, MD, MRCS, FRCS, FRACS, is a consultant vascular surgeon at the Royal Adelaide Hospital (RAH) and senior lecturer at the University of Adelaide with vast experience in the management of diabetic foot disease. Robert Fitridge, MBBS, MS, FRACS, is a consultant vascular surgeon at the RAH and is Professor of Vascular Surgery at the University of Adelaide. Professor Fitridge is a member of the International Working Group for the Diabetic Foot. Christina Bursill, PhD, co-directs the Vascular Research Centre at SAHMRI and has expertise in vascular inflammation, angiogenesis, wound healing mechanisms, and diabetes.
References
- 1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–149 [DOI] [PubMed] [Google Scholar]
- 2. Pena G, Cowled P, Dawson J, Johnson B, Fitridge R. Diabetic foot and lower limb amputations: underestimated problem with a cost to health system and to the patient. ANZ J Surg 2018;88:666–667 [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 4. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci 2017;18:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leese GP, Morris AD. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: response to Boyko et al. Diabetes Care 2006;29:2562–2563 [DOI] [PubMed] [Google Scholar]
- 6. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608 [DOI] [PubMed] [Google Scholar]
- 7. Game FL, Apelqvist J, Attinger C, et al. . Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metabol Res Rev 2016;32(S1):154–168 [DOI] [PubMed] [Google Scholar]
- 8. Castelli WP. Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can J Cardiol 1988;4 Suppl A:5A–10A [PubMed] [Google Scholar]
- 9. Prosser HC, Tan JT, Dunn LL, et al. . Multifunctional regulation of angiogenesis by high-density lipoproteins. Cardiovasc Res 2014;101:145–154 [DOI] [PubMed] [Google Scholar]
- 10. Murphy AJ, Woollard KJ, Hoang A, et al. . High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 2008;28:2071–2077 [DOI] [PubMed] [Google Scholar]
- 11. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing—a literature review. An Bras Dermatol 2016;91:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong SL, Demers M, Martinod K, et al. . Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21:815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karima M, Kantarci A, Ohira T, et al. . Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol 2005;78:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delamaire M, Maugendre D, Moreno M, Le Goff M-C, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 1997;14:29–34 [DOI] [PubMed] [Google Scholar]
- 17. Moura J, Rodrigues J, Goncalves M, Amaral C, Lima M, Carvalho E. Impaired T-cell differentiation in diabetic foot ulceration. Cell Mol Immunol 2017;14:758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu CO, Leung KS, Fung KP, et al. . The characterization of a full-thickness excision open foot wound model in n5-streptozotocin (STZ)-induced type 2 diabetic rats that mimics diabetic foot ulcer in terms of reduced blood circulation, higher C-reactive protein, elevated inflammation, and reduced cell proliferation. Exp Anim 2017;66:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferguson MW, Herrick SE, Spencer MJ, Shaw JE, Boulton AJ, Sloan P. The histology of diabetic foot ulcers. Diabet Med 1996;13 Suppl 1:S30–S33 [PubMed] [Google Scholar]
- 20. Das S, Singh G, Majid M, et al. . Syndesome therapeutics for enhancing diabetic wound healing. Adv Healthc Mater 2016;5:2248–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 2008;25:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinchliffe RJ, Forsythe RO, Apelqvist J, et al. . Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metabol Res Rev 2020;36 Suppl 1:e3276. [DOI] [PubMed] [Google Scholar]
- 23. Vas P, Rayman G, Dhatariya K, et al. . Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes Metabol Res Rev 2020;36 Suppl 1:e3284. [DOI] [PubMed] [Google Scholar]
- 24. Edmonds M, Lazaro-Martinez JL, Alfayate-Garcia JM, et al. . Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol 2018;6:186–196 [DOI] [PubMed] [Google Scholar]
- 25. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–1710 [DOI] [PubMed] [Google Scholar]
- 26. Uchi H, Igarashi A, Urabe K, et al. . Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009;19:461–468 [DOI] [PubMed] [Google Scholar]
- 27. Park KH, Han SH, Hong JP, et al. . Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: a phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Pract 2018;142:335–344 [DOI] [PubMed] [Google Scholar]
- 28. Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol 2005;46:1792–1798 [DOI] [PubMed] [Google Scholar]
- 29. Calabresi L, Gomaraschi M, Simonelli S, Bernini F, Franceschini G. HDL and atherosclerosis: insights from inherited HDL disorders. Biochim Biophys Acta 2015;1851:13–18 [DOI] [PubMed] [Google Scholar]
- 30. Prosser HC, Ng MK, Bursill CA. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr Opin Lipidol 2012;23:182–189 [DOI] [PubMed] [Google Scholar]
- 31. Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol 2010;37:710–718 [DOI] [PubMed] [Google Scholar]
- 32. Arca M, Montali A, Valiante S, et al. . Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol 2007;100:1511–1516 [DOI] [PubMed] [Google Scholar]
- 33. Femlak M, Gluba-Brzozka A, Cialkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis 2017;16:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Zhi Y, Li C, et al. . HDL cholesterol and risk of diabetic nephropathy in patient with type 1 diabetes: a meta-analysis of cohort studies. Diabetes Res Clin Pract 2016;122:84–91 [DOI] [PubMed] [Google Scholar]
- 35. Ikura K, Hanai K, Shinjyo T, Uchigata Y. HDL cholesterol as a predictor for the incidence of lower extremity amputation and wound-related death in patients with diabetic foot ulcers. Atherosclerosis 2015;239:465–469 [DOI] [PubMed] [Google Scholar]
- 36. Kimura T, Tomura H, Mogi C, et al. . Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem 2006;281:37457–37467 [DOI] [PubMed] [Google Scholar]
- 37. Bursill CA, Castro ML, Beattie DT, et al. . High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler Thromb Vasc Biol 2010;30:1773–1778 [DOI] [PubMed] [Google Scholar]
- 38. Nicholls SJ, Dusting GJ, Cutri B, et al. . Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005;111:1543–1550 [DOI] [PubMed] [Google Scholar]
- 39. Van Lenten BJ, Navab M, Shih D, Fogelman AM, Lusis AJ. The role of high-density lipoproteins in oxidation and inflammation. Trends Cardiovasc Med 2001;11:155–161 [DOI] [PubMed] [Google Scholar]
- 40. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol 2017;199:17–24 [DOI] [PubMed] [Google Scholar]
- 41. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010;10:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dal-Secco D, Wang J, Zeng Z, et al. . A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med 2015;212:447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanson M, Distel E, Fisher EA. HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process. PLoS One 2013;8:e74676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu T, Dhanasekaran SM, Jin H, et al. . FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol 2004;164:1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmun Rev 2002;1:111–117 [DOI] [PubMed] [Google Scholar]
- 46. De Nardo D, Labzin LI, Kono H, et al. . High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014;15:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care (New Rochelle) 2016;5:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther 1991;52:407–422 [DOI] [PubMed] [Google Scholar]
- 49. Tan JTM, Ng MKC, Bursill CA. The role of high-density lipoproteins in the regulation of angiogenesis. Cardiovasc Res 2015;106:184–193 [DOI] [PubMed] [Google Scholar]
- 50. Seetharam D, Mineo C, Gormley AK, et al. . High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res 2006;98:63–72 [DOI] [PubMed] [Google Scholar]
- 51. Tan JT, Prosser HC, Dunn LL, et al. . High-density lipoproteins rescue diabetes-impaired angiogenesis via scavenger receptor class B type I. Diabetes 2016;65:3091–3103 [DOI] [PubMed] [Google Scholar]
- 52. Tan JT, Prosser HC, Vanags LZ, Monger SA, Ng MK, Bursill CA. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha. FASEB J 2014;28:206–217 [DOI] [PubMed] [Google Scholar]
- 53. Primer KR, Psaltis PJ, Tan JTM, Bursill CA. The role of high-density lipoproteins in endothelial cell metabolism and diabetes-impaired angiogenesis. Int J Mol Sci 2020;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cannizzo CM, Adonopulos AA, Solly EL, et al. . VEGFR2 is activated by high-density lipoproteins and plays a key role in the proangiogenic action of HDL in ischemia. FASEB J 2018;32:2911–2922 [DOI] [PubMed] [Google Scholar]
- 55. Jin F, Hagemann N, Sun L, et al. . High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis 2018;21:381–394 [DOI] [PubMed] [Google Scholar]
- 56. Miura S, Fujino M, Matsuo Y, et al. . High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 2003;23:802–808 [DOI] [PubMed] [Google Scholar]
- 57. Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation 2000;102:185–190 [DOI] [PubMed] [Google Scholar]
- 58. Seitz O, Schurmann C, Hermes N, et al. . Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res 2010;2010:476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Qi W, Yang C, Dai Z, et al. . High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes 2015;64:1407–1419 [DOI] [PubMed] [Google Scholar]
- 60. Eming SA, Koch M, Krieger A, et al. . Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res 2010;9:4758–4766 [DOI] [PubMed] [Google Scholar]
- 61. Eming SA, Lauer G, Cole M, et al. . Increased levels of the soluble variant of the vascular endothelial growth factor receptor VEGFR-1 are associated with a poor prognosis in wound healing. J Invest Dermatol 2004;123:799–802 [DOI] [PubMed] [Google Scholar]
- 62. Tsatralis T, Ridiandries A, Robertson S, et al. . Reconstituted high-density lipoproteins promote wound repair and blood flow recovery in response to ischemia in aged mice. Lipids Health Dis 2016;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gordts SC, Muthuramu I, Amin R, Jacobs F, De Geest B. The impact of lipoproteins on wound healing: topical HDL therapy corrects delayed wound healing in apolipoprotein E deficient mice. Pharmaceuticals (Basel) 2014;7:419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yuhanna IS, Zhu Y, Cox BE, et al. . High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001;7:853–857 [DOI] [PubMed] [Google Scholar]