Abstract
Background
Ischemic diabetic foot ulcer is one of the terminal complications of diabetes. The high amputation rate, recurrence rate, and treatment cost have caused a huge burden on patients and society. This study designed the modified tibial transverse transport (mTTT) technology to treat diabetic ischemic diabetic foot ulcers in patients with type 2 diabetes and investigated the effectiveness and safety of this technique.
Methods
This was a retrospective analysis of patients with type 2 diabetes and ischemic diabetic foot ulcers at two hospitals during January 2016–October 2019. These patients underwent mTTT surgery combined with wound debridement and vacuum sealing drainage negative pressure drainage treatment. In-hospital follow-up was performed at 1 month after the operation, while outpatient follow-up was performed at 3, 6, and 12 months after the operation. The ulcer healing time, recurrence rate, major amputation rate, and complications were analysed.
Results
A total of 201 patients were enrolled in this study, including 107 males and 94 females (mean age: 68.3 ± 7.1 years). The wounds of all patients healed completely (mean healing time: 4.6 ± 1.6 months). There was no occurrence of major amputation, recurrence, and treatment-related complications in the patients.
Conclusion
mTTT can effectively and safely treat ischemic diabetic foot ulcers in patients with type 2 diabetes. This technology is an important part of the ischemic diabetic foot ulcer treatment system and warrants further research.
The Translational Potential of this Article
This study introduced a new method to treat the ischemic diabetic foot ulcer which was called modified tibial transverse transport. The promising outcomes of patients indicated that this surgical method had great potential for clinical application and was worthy of further clinical research with high evidence level.
Keywords: Tibia transverse transport, Ischemic diabetic foot ulcer, Type 2 diabetes, Cohort study
1. Introduction
The concept of diabetic foot was first proposed by Oakley [1] and is one of the important complications that cause disability and death in patients with diabetes [2]. Epidemiological studies have shown that approximately 15% of people with diabetes will develop diabetic foot [3]. The incidence of diabetic foot reaches approximately 2% per year, and the recurrence rate of ulcers in the first year after healing is 30–40% [4]. Of the patients, 85% eventually have to undergo amputation [5] and the 5-year survival rate of patients following amputation is only 50% [6].
The formation mechanism of diabetic foot is complicated. Co-infection and ischemia, as well as hypoxia caused by micro artery injury will further aggravate the disease. However, approximately 40% of patients cannot undergo vascular surgery due to complete occlusion of small blood vessels in the lower extremities. Based on the stress-tension principle of the Ilizarov [7], many Chinese researchers and doctores invented a technique called tibial transverse transport (TTT) to restablised the microcirculation of lower limb to cure the DFU which could improve the ulcer cure rate up to 96% [[8], [9], [10]].
However, TTT surgery was done with a relative large incision on the tibial bone and skin which might result in the complications with the wound in the early period without proper guideline. The authors modified the traditional TTT to minimize the incision and simplify the procedure. We describe here how to perform modified TTT (mTTT) surgery and share the results of this technique with relative long follow up.
2. Methods
2.1. Patients
The study included patients with DFUs treated at Peking University People's Hospital and Beijing Longfu Hospital who received the surgery by the same surgeon from January 2016 to October 2019.
2.2. Inclusion criteria
-
1.
Diagnosis of diabetic foot of Texas University grades 2C, 2D, 3C, and 3D.
-
2.
Patency of the popliteal artery >50%, diagnosed through doppler ultrasound examination.
2.3. Exclusion criteria
-
1.
Mental illness that did not allow the patients to cooperate with the treatment.
-
2.
Diagnosis (by an endocrinologist) of other uncontrollable serious complications of diabetes.
-
3.
Inability to tolerate anaesthesia due to cardiovascular complications or renal failure.
-
4.
Presence of non-diabetic ulcers.
-
5.
Calf skin condition not meeting the surgical requirements.
-
6.
Presence of active Charcot foot.
-
7.
A history of cerebral infarction, myocardial infarction, heart failure, cancer, or renal failure in the past 3 months.
-
8.
Treatment with corticosteroids, immunosuppressive drugs, and/or chemotherapy.
2.4. General treatments
The patients underwent routine preoperative examinations, and consulted the department of endocrinology and vascular surgery to control and monitor the blood glucose levels. The vascular condition of the limbs and blood glucose fluctuations were evaluated and adjusted (target blood glucose control standard: fast blood glucose before meals <7 mmol/L; fast 2 h after meals blood sugar <11 mmol/L). All patients received standard foot care.
2.5. Pre-operation
The wound secretions of patients with foot ulcers and infections were obtained for bacterial culture and drug susceptibility testing. According to the results of the drug susceptibility analysis, antibiotics to which the patients were sensitive were selected for intravenous administration. All patients immediately underwent complete debridement. The infected bones were surgically removed, and the patients were treated with antibiotics. At the same time, continuous closed negative pressure drainage treatment and antibiotic bone cement implantation were performed to control the infection.
2.6. Surgery
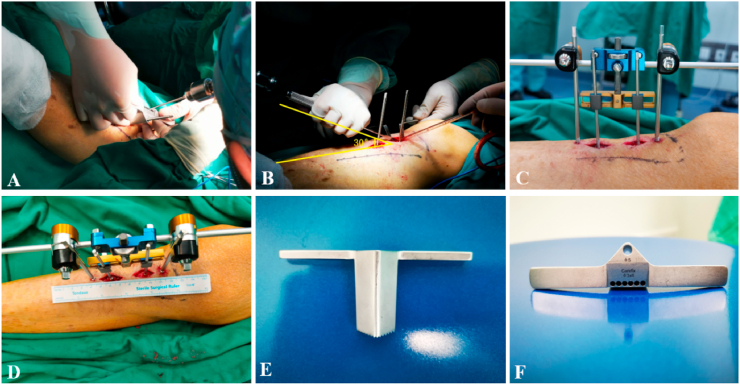
Following nerve block anaesthesia, the patient was placed in the supine position, and the affected limb was routinely disinfected. In the anteromedial area of the proximal tibia of the affected limb, the external fixation frame was compared with the area along the midline of the medial longitudinal axis of the proximal tibia. Subsequently, two 3.0 Steinmann pins were inserted through the single layer of cortical bone. The skin was cut along the long axis with the 3.0 Steinmann pin as the centre, and the subcutaneous tissue was separate bluntly to expose the periosteum. The Steinmann pins were used as the centre point for the drilling on four sides with a 2.0 drill bit and use of a rapid osteotomy device; the length of each side was 2.5 cm. Subperiosteal osteotomy was performed with a 5-mm narrow bone knife at an angle of 15°–30° to the bone surface. The surgeons should pay attention to protect the blood supply of the periosteum during this procedure. The external fixators were fixed with 4.0 Steinmann pins at the distal and proximal ends. The subcutaneous tissue and skin were sutured. The result of the osteotomy was reviewed using anteroposterior and lateral radiography after operation (Fig. 1).
Fig. 1.
(a) Drilling along the edge of the rapid osteotomy device. (b) After fixing the Steinmann pin on the two tilted bone flaps, osteotomy was performed at a 30° angle. (c, d) Installation of the external fixing frame; the distance between the two Steinmann pins is approximately 4 cm. (e, f) The rapid osteotomy device designed by the researchers. The lower end of the osteotomy is serrated fixed to the bone surface, and the middle is a pilot hole for drilling.
2.7. Post-operation
After the operation, the treating physicians in the endocrinology department should continue to monitor and strictly control the blood glucose levels. The blood glucose levels pre meal and 2 h after a meal were <7 mmol/L and <11 mmol/L, respectively. Antibiotics to which the patients were sensitive were selected for intravenous infusion based on the results of preoperative wound secretions and intraoperative deep tissue biopsy for drug sensitivity and bacterial culture identification. The dressing was changed according to the exudation of the wound, and the nail passage opening was maintained clean and dry. The nail passage opening was cleansed with 75% ethanol thrice daily.
Transverse bone transportation was initiated 3 days after operation for a total of 14 days. The 1 mm daily transportation was performed in 4 times per day. Subsequently, 2 mm reverse transportation of the bones was performed four times daily for a total of 7 days. In case of intolerable pain experienced by the patient, darkening of the local skin, or deterioration of the blood supply, the outward movement should be terminated and replaced by gradual inward movement to avoid skin flap necrosis. The plan was changed to carry 1 mm per 2 days and 1 mm daily following significant pain relief. The external fixator was removed and replaced by the brace after resetting the bone block. The X-rays of the tibia were reviewed once monthly to observe the healing of the bone.
The early ulcer may continue to have secondary necrosis after the first debridement, which may involve skin, fascia, muscle, tendon, bone tissue, etc. To promote wound healing, debridement and dressing changes or vacuum sealing drainage (VSD) negative pressure drainage was continued in patients with wounds that had not healed.
2.8. Follow-up
The patients were followed up in the hospital at 1 month after the operation and in the outpatient clinic at 3, 6, and 12 months following the operation. The follow-up included wound healing, limb salvage rate (no major amputation, i.e., no amputation above the ankle joint), amputation, complications, ankle skin temperature (T), transcutaneous oxygen pressure (TCPO) of ankle skin, ankle brachial index (ABI), and ulcer recurrence rate. The ulcer was considered healed when complete epithelialization was observed without drainage and maintained for at least 2 weeks [11].
All the procedures had been approved by the ethic committee of Peking University People's Hospital (No.2020PHB100-01).
2.9. Statistics
The Shapiro–Wilk test was used to test the normality of demographic and clinical data. Continuous variables are represented by the mean ± standard deviation, and categorical variables are expressed as numbers and percentages. The median (P50) and 95% confidence interval (95% CI) was used if the data did not obey the normal distribution. A paired design of rank sum test (Wilcoxon paired method) was used to compare TCPO, T and ABI. The inspection level a = 0.05. Two-sided test P < 0.05 indicates that the difference is statistically significant.
3. Results
3.1. General information
A total of 201 patients were enrolled in this study, including 107 males and 94 females (mean age: 68.3 ± 7.1 years). Among them, 139, 36, and 26 cases had diabetic foot of Texas University grades 2C, 2D, and 3D, respectively. All patients were followed up for 1 year after surgery (Table 1).
Table 1.
Patient demographics.
| Characteristics | Value |
|---|---|
| Sex | |
| Male (N) | 107 |
| Female (N) | 94 |
| Age, P50 with 95% CI (years) | 68, 95%CI [67.32,69.30] |
| BMI | 23.53, 95%CI [23.07,23.89] |
| Course of diabetes (months) | 93, 95%CI [91.65,95.45] |
| Course of diabetic foot (months) | 1, 95%CI [1.34,1.54] |
| HbA1C (%) | 10.00, 95%CI [10.01,10.44] |
| Ulcer area (cm2) | 2.00, 95%CI [1.87,2.16] |
| Texas classification (N) | |
| 2C | 139 |
| 2D | 36 |
| 3C | 0 |
| 3D | 26 |
BMI, body mass index; HbA1C, haemoglobin A1c
The DFU wounds of all the patients included in this study healed completely (Table 2, Fig. 2). The mean and longest healing time was 4.6 ± 1.6 and 8 months, respectively. There was no recurrence of ulcer, amputation after bone removal, incision infection, nail channel infection, osteomyelitis, and fractures at the bone transport site (Table 2).
Table 2.
Outcomes.
| Item | Number of patients |
|---|---|
| Healing | 201 |
| Time to heal, P50 with 95%CI (months) | 4, 95% CI [4.36,4.80] |
| Recurrence | 0 |
| Major amputation | 0 |
| Fractures at bone transport site | 0 |
| Incision infection | 0 |
| Nail channel Infection | 0 |
| Osteomyelitis | 0 |
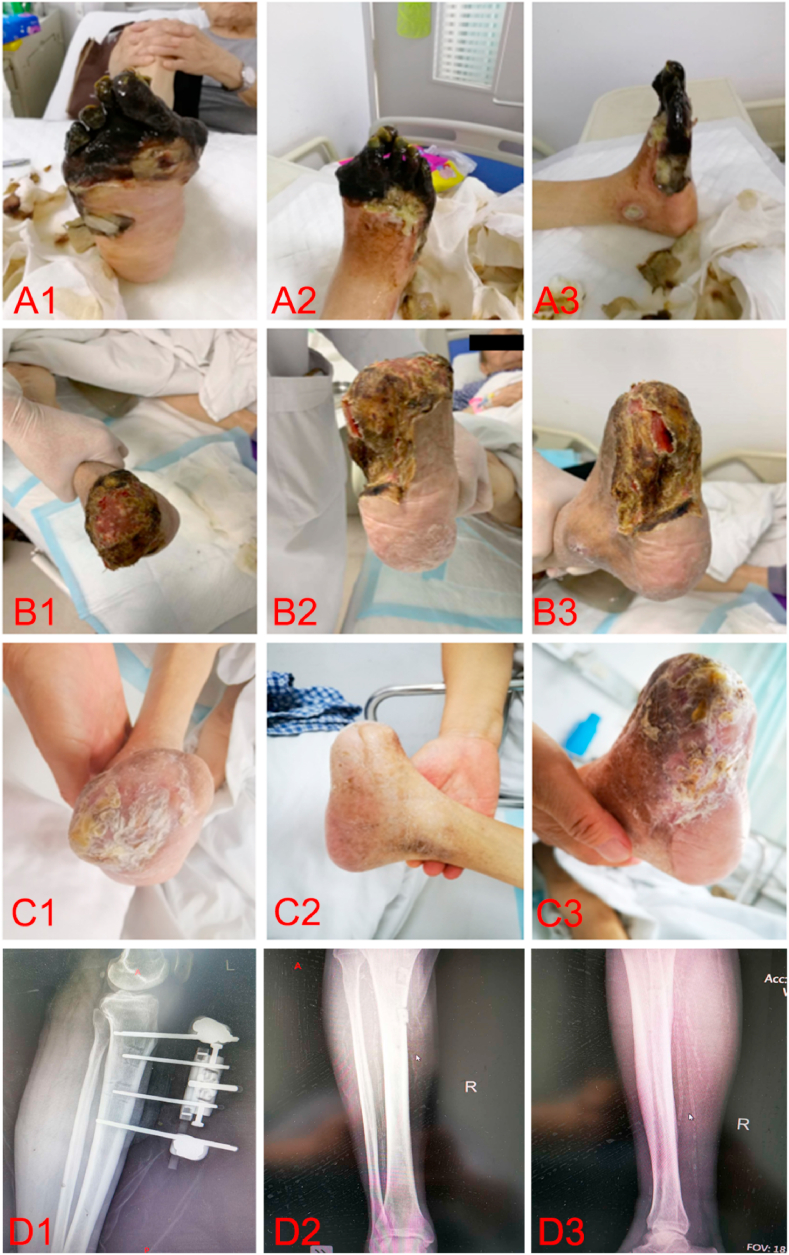
Fig. 2.
A typical case of mTTT. A 98-year-old male with 32-year type 2 diabetes mellitus. Ulcers, gangrene, and tissue necrosis were found on the right foot of the patient for more than 2 months before he coming to our hospital. During this period, debridement and conservative treatment were ineffective after dressing changes. He was diagnosed with DFU in 4D stage of TEXAS classification. The skin temperature of the dorsum of the foot, ankle-brachial index, transcutaneous oxygen partial pressure were 28.6 °C, 0.36 and 30 mmHg respectively before mTTT. After removal of necrotic tissue and mTTT treatment, the wound healed completely 3 months after the operation. The skin temperature on the dorsum of the foot was 34.8 °C, ankle-brachial index was 0.92, and the transcutaneous oxygen partial pressure was 46 mmHg at 3 months after operation. (a) The appearance of foot ulcers prior to the operation. The entire forefoot had gangrened with black skin. All five toes were amputated after debridement. (b) The appearance of the foot at 10 weeks after mTTT combined with antibiotic bone cement implantation and vacuum-assisted closure (VAC) drainage. Black crust was observed on the amputation wound. (c) The appearance of the foot at 16 weeks after mTTT. The wound had basically healed. D1 is the tibia X-ray image captured 1 day after the mTTT. D2–D3 are the tibial X-ray images captured at 2 and 6 weeks after the removal of the tibial outer frame. It was observed that the transport bone zone had basically healed with callus covering.
In this study, the healing time of patients who continued to smoke and those who did not smoke was 4.1 ± 1.9 and 4.7 ± 1.5 months, respectively. People who did not drink alcohol required longer time to heal than those who drunk alcohol. Moreover, the healing time of hypertensive patients was similar to that of non-hypertensive patients. The healing time of ulcers in patients with coronary heart disease was longer than that noted in patients with non-coronary heart disease (Table 3).
Table 3.
Risk factors.
| Characteristic | Number of patients | Time to heal (months) |
|---|---|---|
| Smoking history | ||
| Smoked prior to surgery | 11 | 4.1 ± 1.9 |
| Quit smoking | 55 | 4.3 ± 1.6 |
| Do not smoke | 135 | 4.7 ± 1.5 |
| Alcohol consumption | ||
| Drank prior to surgery | 14 | 4.2 ± 1.9 |
| Quit drinking | 92 | 4.3 ± 1.5 |
| Do not drink | 95 | 4.9 ± 1.5 |
| Hypertension | ||
| Yes | 70 | 4.6 ± 1.4 |
| No | 131 | 4.6 ± 1.6 |
| Coronary artery disease | ||
| Yes | 32 | 4.8 ± 1.5 |
| No | 169 | 4.5 ± 1.6 |
The ankle skin temperature, TCPO and ABI all improved significantly at 12 months after surgery (Table 4).
Table 4.
T, TCPO and ABI of patients.
| Test | Pre-op | 12 months post-op | Z value | P value |
|---|---|---|---|---|
| T (°C) | 29.50 [29.56,29.76] | 35.50 [35.52,35.58] | −13.36 | <0.001 |
| TCPO (mmHg) | 31.00 [30.31,30.93] | 43.00 [42.60,43.00] | −12.40 | <0.001 |
| ABI | 0.20 [0.19,0.21] | 0.80 [0.79,0.81] | −14.18 | <0.001 |
Pre-op: pre-operation; Post-op: post-operation.
4. Discussion
In 1989, Ilizarov conducted a traction osteogenesis experiment on dog legs. They found that the regeneration of the microvascular network had begun and was very active prior to the occurrence of osteogenesis in the gap between the traction area of the dog bone [[12], [13], [14]]. Through angiography of the dog leg, Atkins et al. confirmed that reconstruction of the microcirculation existed in the traction area [15]. Based on this phenomenon, scholars in China and abroad proposed a new theory of tissue regeneration. This theory suggested that application of a suitable external compressive stress to the bones could stimulate the natural repair ability of the tissues in the body. This effect could lead to the regeneration of blood vessels, nerves, muscles, bones, and other tissues, eventually resulting in the natural reconstruction of the microcirculation of the damaged tissue [16].
Recent studies observed a large number of tiny holes (diameter: ~10 μm) in human long bones. In addition, blood vessels run through these tiny holes, forming a dense closed blood circulation of the transcortical vessel system (60% arteries and 40% veins) [17]. The modified tibial transverse transport (mTTT) technology used in this study did not damage the periosteum and protected the blood supply of the soft tissue around the bone flap to the greatest extent possible. Therefore, the transcortical vessel system may be the anatomical basis for the periodic transport of tibial bone flaps to induce the formation of a microvascular network.
Control of blood glucose levels is the basis for the treatment of DFUs. Studies have confirmed that strengthening of foot care for patients with diabetes can prevent 80% of DFUs [18] and effectively prevent amputation caused by foot ulcers [19]. Patients received corresponding medical hypoglycaemic treatments according to the type of diabetes and foot care in this study, which could help improve the efficacy of mTTT in treating DFUs and reduce the pain experienced by patients.
All patients in this study underwent thorough debridement surgery and VSD negative pressure drainage prior to surgery. Steed et al. found that debridement was beneficial to the healing of ulcer wounds and patients with a higher frequency of debridement had a higher wound healing rate [20]. VSD negative pressure drainage used an externally connected negative pressure suction device to continuously or intermittently suction and drain the wound. This approach discharged tissue exudate in time, reduced infection and tissue oedema, improved microcirculation, and promoted local blood flow, thereby inducing the growth of granulation tissue [21].
Ilizarov proposed that the transfer speed of 1 mm daily was most suitable for the regeneration of bone tissue after testing through basic experiments and clinical research. The frequency of bone transfer was 1 mm daily and divided into 4–6 sessions [12]. The study was carried out on 3 days after the operation, and 1 mm was carried daily. It was completed in four sessions, which accorded with the optimal tissue repair speed under the stress-tension principle.
In this study, the foot ulcers of all patients healed completely and did not require major amputation following mTTT. This, indicated that mTTT can effectively treat chronic ischemic DFUs in patients with type 2 diabetes. There was no occurrence of treatment-related complications in any of the patients, unlike in the study conducted by Hua et al. [8] This is probably because double-bone block handling and double-longitudinal incision exert relatively limited stress on the skin.
In the subgroup analysis, the wound healing time of patients who drank alcohol and smoked was shorter than that recorded for patients who did not drink or smoke. This may be due to the lower number of patients in the subgroup of drinkers and smokers prior to surgery, resulting in larger statistical bias.
The significant improvements of ankle skin temperature, TCPO and ABI indirectly showed that mTTT could improve the microcirculation of the ankle which was consistent with the research results of Hua et al. [8].
Compared with the traditional TTT surgery [8,22], the mTTT used two 2 cm-longitudinal incision parallel to the long axis of the tibia instead of an arc-shaped long incision. Another major difference is that we periodically carried two 2.5 cm∗2.5 cm bone blocks instead of one rectangular bone block. A self-developed bone-handling rapid osteotomy device was used to assist in the positioning of the osteotomy and shorten the operation time. The outer frame was easier to carry, which reduced the postoperative handling time. In terms of inclusion criteria, mTTT alone was only used in patients with a popliteal artery patency rate >50%, ensuring the blood supply formed by the collateral circulation around the surgical area.
The clinical data was from a retrospective study with certain information bias; however, the retention of objective data (e.g., imaging examinations) could reduce the occurrence of bias. This study lacked lower extremity arteriography and other indicators of vascular reconstruction, because most of the patients included in this investigations experienced renal insufficiency due to diabetic foot (an end-stage complication of diabetes); renal insufficiency is a relative contraindication for computed tomography imaging. Hence, arteriography was not included in the observation indicators of this study. Hua et al. suggested the presence of a new capillary network around the transferred bone flap, which corroborated the conclusion of this study despite the lack of imaging evidence regarding the blood vessels in this study.[24] This was a single-arm cohort study and lacked comparative research data. However, it summarized the clinical usefulness of mTTT in the treatment of ischemic DFUs in patients with type 2 diabetes, providing the basis and favourable support for the design and implementation of high-quality clinical controlled trials.
5. Conclusion
In summary, mTTT can effectively treat DFUs, reduce the rate of major amputation and recurrence caused by DFUs, and improve patient acceptance. Although there is a risk of fractures and skin necrosis, the incidence of complications caused by mTTT is low under standard medical procedures. Of course the current protocol is still subject to further refinement and improvement with continuous endeavor and enthusiasm from the colleagues in the field, to promote TTT surgery for the benefit of diabetic and other patients worldwide.
Declaration of competing interest
The author(s) declared no potential conflicts of interest withrespect to the research, authorship, and/or publication of this article.
Acknowledgments
This study was supported by the Capital's Funds for Health Improvement and Research (Grant No. 2020-2-4086); Beijing Municipal Science and Technology Project (Grant No. Z181100001718159); Pilot project on clinical collaboration of major and difficult diseases of Chinese and Western medicine (Grant No. 201803190106); Health Science and Technology Project of Beijing Dongcheng District Health Commission (Grant No. [2018]-13); Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education (Grant No. BMU2019XY007-01); and Ministry of Education Innovation Program of China (Grant No. IRT_16R01).
Contributor Information
Yuanli Wang, Email: 13797757@qq.com.
Hailin Xu, Email: xuhailinfa@163.com.
References
- 1.Apelqvist J., Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16(Suppl 1):S75–S83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Dekker R.G., 2nd, Qin C., Ho B.S., Kadakia A.R. The effect of cumulative glycemic burden on the incidence of diabetic foot disease. J Orthop Surg Res. 2016;11(1):143. doi: 10.1186/s13018-016-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 4.Hinchliffe R.J., Brownrigg J.R., Apelqvist J., Boyko E.J., Fitridge R., Mills J.L. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):37–44. doi: 10.1002/dmrr.2698. [DOI] [PubMed] [Google Scholar]
- 5.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. J Am Med Assoc. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 6.Humphries M.D., Brunson A., Li C.S., Melnikow J., Romano P.S. Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J Vasc Surg. 2016;64(6):1747–17455 e3. doi: 10.1016/j.jvs.2016.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker K.L., Lamb S.E., Simpson A.H. Functional recovery in patients with nonunion treated with the Ilizarov technique. J Bone Joint Surg Br. 2004;86(1):81–85. [PubMed] [Google Scholar]
- 8.Chen Y., Kuang X., Zhou J., Zhen P., Zeng Z., Lin Z. Proximal tibial cortex transverse distraction facilitating healing and limb salvage in severe and recalcitrant diabetic foot ulcers. Clin Orthop Relat Res. 2020;478(4):836–851. doi: 10.1097/CORR.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua Q., Zhang Y., Wan C., Zhang D., Xie Q., Zhu Y. Chinese association of orthopaedic surgeons (CAOS) clinical guideline for the treatment of diabetic foot ulcers using tibial cortex transverse transport technique (version 2020) J Orthop Translat. 2020;25:11–16. [Google Scholar]
- 10.Liu G., Li S., Kuang X., Zhou J., Zhong Z., Ding Y. The emerging role of tibial cortex transverse transport in the treatment of chronic limb ischemic diseases. J Ortho Transl. 2020;25:17–24. [Google Scholar]
- 11.van Netten J.J., Bus S.A., Apelqvist J., Lipsky B.A., Hinchliffe R.J., Game F. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3268. doi: 10.1002/dmrr.3268. [DOI] [PubMed] [Google Scholar]
- 12.Ilizarov G.A. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 13.Ilizarov G.A. The principles of the Ilizarov method. 1988. Bull Hosp Jt Dis. 1997;56(1):49–53. [PubMed] [Google Scholar]
- 14.Robert Rozbruch S., Weitzman A.M., Tracey Watson J., Freudigman P., Katz H.V., Ilizarov S. Simultaneous treatment of tibial bone and soft-tissue defects with the Ilizarov method. J Orthop Trauma. 2006;20(3):197–205. doi: 10.1097/00005131-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lovisetti G., Agus M.A., Pace F., Capitani D., Sala F. Management of distal tibial intra-articular fractures with circular external fixation. Strategies Trauma Limb Reconstr. 2009;4(1):1–6. doi: 10.1007/s11751-009-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson J.Y., Sutherland M., Pandit H.G., McNally M. The rate of symptomatic venous thromboembolism in patients undergoing elective Ilizarov surgery and the cost of chemical prophylaxis. Bone Joint Lett J. 2014;96-B(3):426–430. doi: 10.1302/0301-620X.96B3.32939. [DOI] [PubMed] [Google Scholar]
- 17.Gruneboom A., Hawwari I., Weidner D., Culemann S., Muller S., Henneberg S. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab. 2019;1(2):236–250. doi: 10.1038/s42255-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavitha K.V., Tiwari S., Purandare V.B., Khedkar S., Bhosale S.S., Unnikrishnan A.G. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546–556. doi: 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford F., Inkster M., Kleijnen J., Fahey T. Predicting foot ulcers in patients with diabetes: a systematic review and meta-analysis. QJM. 2007;100(2):65–86. doi: 10.1093/qjmed/hcl140. [DOI] [PubMed] [Google Scholar]
- 20.Steed D.L., Donohoe D., Webster M.W., Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183(1):61–64. [PubMed] [Google Scholar]
- 21.Wang R., Feng Y., Di B. Comparisons of negative pressure wound therapy and ultrasonic debridement for diabetic foot ulcers: a network meta-analysis. Int J Clin Exp Med. 2015;8(8):12548–12556. [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Ling S.K.K., Li H.A., Zhang Y.T., Hu J. How to perform minimally invasive tibial cortex transverse transport surgery. J Orthop Translat. 2020;25:28–32. [Google Scholar]