Highlights
-
•
Time to treatment initiation for cervical cancer in Zambia is long.
-
•
Public hospitals have longer diagnostic and referral turnaround time.
-
•
Cervical cancer patients indicated for surgery have the longest delays to treatment initiation.
-
•
Further study into TT's impact on survival of cervical cancer patients is needed.
Keywords: Cancer Diseases Hospital, Cervical cancer, Turnaround time, Screening, Public health facility
Abstract
Expedited diagnostic processes for all suspected cervical cancer cases remain essential in the effort to improve clinical outcomes of the disease. However, in some developing countries like Zambia, there is paucity of data that assesses factors influencing diagnostic and treatment turnaround time (TAT) and other metrics vital for quality cancer care. We conducted a retrospective hospital-based study at the Cancer Diseases Hospital (CDH) for cervical cancer cases presenting to the facility between January 2014 and December 2018. Descriptive statistics were used to summarize demographic characteristics while a generalized linear model of the negative binomial was used to assess determinants of overall TAT. Our study included 2121 patient case files. The median age was 49 years (IQR: ±17) and most patients (n = 634, 31%) were aged between 41 and 50 years. The International Federation of Gynaecology and Obstetrics (FIGO) Cancer stage II (n = 941, 48%) was the most prevalent while stage IV (n = 103, 5.2%) was the least. The average diagnostic TAT in public laboratories was 1.48 (95%CI: 1.21–1.81) times longer than in private laboratories. Furthermore, referral delay was 55 days (IQR: 24–152) and the overall TAT (oTAT) was 110 days (IQR: 62–204). The age of the patient, HIV status, stage of cancer and histological subtype did not influence oTAT while marital status influenced oTAT. The observed longer oTAT may increase irreversible adverse health outcomes among cervical cancer patients. There is a need to improve cancer care in Zambia through improved health expenditure especially in public health facilities.
1. Introduction
Cervical cancer is the fourth most common cancer but the leading cause of mortality due to cancer among women worldwide (Arbyn et al., 2020, Vu et al., 2018). The highest burden of disease (BOD) and mortality from cervical cancer are in low-and middle-income countries (LMICs) (Vu et al., 2018, Sloan and Gelband, 2007, Akinyemiju, 2012) where screening programs for the disease are scarce (Gutnik et al., 2016, Sankaranarayanan et al., 2001). Furthermore, poor access to medical facilities especially in rural areas, low quality of medical care, limitation of treatment facilities and poverty are among the barriers increasing the burden of cervical cancer in developing countries (Dunyo et al., 2018, Sowemimo et al., 2017). Early detection of cervical cancer and timely initiation of treatment optimises disease outcomes. In addition, early detection and accurate diagnosis of cervical cancer in the most affected communities and access to quality health facilities for cancer screening are critical to reducing the BOD (Toliman et al., 2018). Over the years, the rise in the demand for quality health care among cervical cancer patients, coupled with the increasing cost of cancer treatment and shortage of health workers has led to a reduction in the efficiency of cervical cancer clinical care pathways. This may negatively impact patient survival by the resulting delays in diagnosis and initiation of treatment, worsening the morbidity and mortality from the disease.
In recognition of the growing burden of cervical cancer and demand for quality health care (Jedy-Agba et al., 2020, Barsom et al., 2020), understanding the turnaround times within patient treatment pathways and health facility workflow remains key in identifying weaknesses within the health care system and developing strategies to improve health outcomes in patients with cervical cancer (Lenz et al., 2005). Emerging evidence suggests that turnaround time (TAT) from diagnosis to treatment of cancer has been used to guide health policy aimed at improving patients’ quality of life (Nascimento, 2015;49:92.). Earlier studies indicate that poverty, delayed diagnosis of disease, inefficient patient referral patterns, poor access to health facilities and the scarcity of health resources contribute to the high prevalence and mortality from cervical cancer (Akinyemiju, 2012, Kroman et al., 2000, Yagi et al., 2019). Furthermore, studies from several LMICs have reported delays in the diagnosis of cancer (Ross and Rayne, 2017, Lohlun et al., 2015) and the high cost of health care (Arbyn et al., 2020) as factors contributing to the high cancer fatality rates. Despite this, few studies have examined delays to treatment in patients with cervical cancer, especially in countries such as Zambia where the cancer treatment centres are centralized to a few urban areas (Chen et al., 2019).
In Zambia, all cancer patients are treated at the Cancer Diseases Hospital (CDH) based in Lusaka. The hospital, established in 2006, has attracted substantial investment into modern oncology equipment, training of specialised personnel and sustained procurement of medication for the treatment of cancer, all aimed at improving cancer patient outcomes. However, this centralized investment may be ineffective if barriers influencing cervical cancer patient outcomes are not positively modified (Govardhan and Sarkar, 2017). Therefore, to improve clinical outcomes and ensure cervical cancer patient satisfaction, evaluation and continuous improvement of TAT in the disease diagnostic and treatment pathways remain essential. Although there is paucity of data on the TAT from diagnosis to cervical cancer treatment in LMICs, available information suggests that TAT in LMICs is considerably longer than that observed in high-income countries (Benk et al., 2006, Masamba et al., 2017). Therefore, the current study quantified factors influencing the TAT among patients with cervical cancer in Zambia. Also, the study quantified delays in patient treatment by characterizing the clinical pathways utilised before initiation of therapy.
2. Method
2.1. Study site and setting
The study was conducted at the Cancer Diseases Hospital (CDH) in Lusaka, the only specialized cancer treatment centre for the entire Zambian population of 17.8 million (CSO. Population and Demographic Projections, 2013). Patients are filtered through the district and provincial hospitals serving as primary centres for cervical cancer screening countrywide before being referred to CDH for definitive treatment. Treatment at CDH is done according to the nationally and globally developed guidelines (Karjane and Chelmow, 2013). Currently, available treatments include chemotherapy, radiotherapy (RT), chemo-radiotherapy (CRT), surgery and palliation. Curative treatment involves one or more of these treatment methods. Palliative treatment may or may not involve one or more of them.
2.2. Patients selection and study design
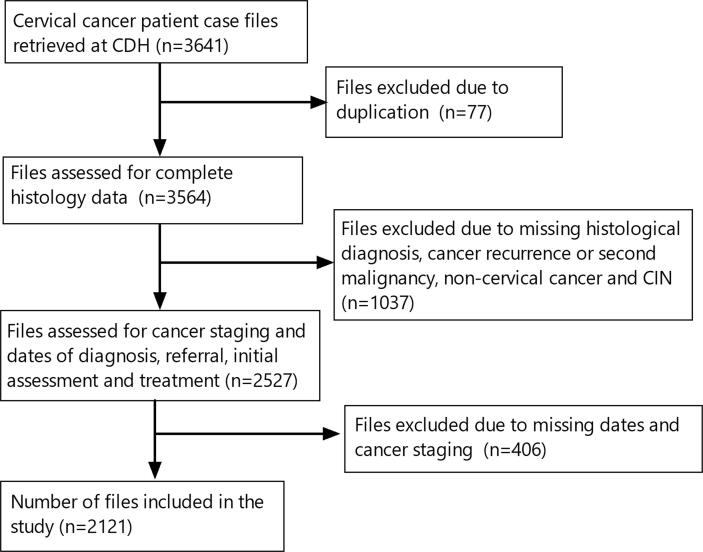
This was a retrospective cross-sectional study that involved screening of case files of all patients presenting to CDH for the first time with a histologically confirmed diagnosis of cervical cancer. We initially used the electronic database records to identify all file numbers for all patients with cervical cancer at CDH. Thereafter, we retrieved the hardcopy medical records of all patients who had been referred to CDH from other facilities between the period 1st January 2014 to 30th December 2018. Initially, we retrieved 3641 patient case files. We employed a 3-phase selection process to identify files for inclusion in the study :
Phase 1: This involved retrieval and identification of files of all patients referred to CDH for assessment and treatment. During this phase, 77 files were dropped due to duplication of medical records.
Phase 2: This phase involved screening of the remaining files for histological diagnoses. During this phase, 1037 files were excluded because the patients had previously been diagnosed and treated before 2014 with cervical cancer (recurrence or second malignancy), had cervical intraepithelial neoplasia (CIN) or other cancers besides cervical cancer. This study focused on the maiden histological diagnosis of cervical cancer.
Phase 3: At this phase, screening for completeness of the information on files was done. The information included the date of biopsy, date of receipt of histopathology results from the laboratory, date of initial patient assessment at CDH, and the date for initiation of treatment at CDH. During this phase, 406 more files were excluded as they were missing two or more of the four dates needed for TAT determination. (Fig. 1).
Fig 1.
Data sampling strategy.
2.3. Outcome measures
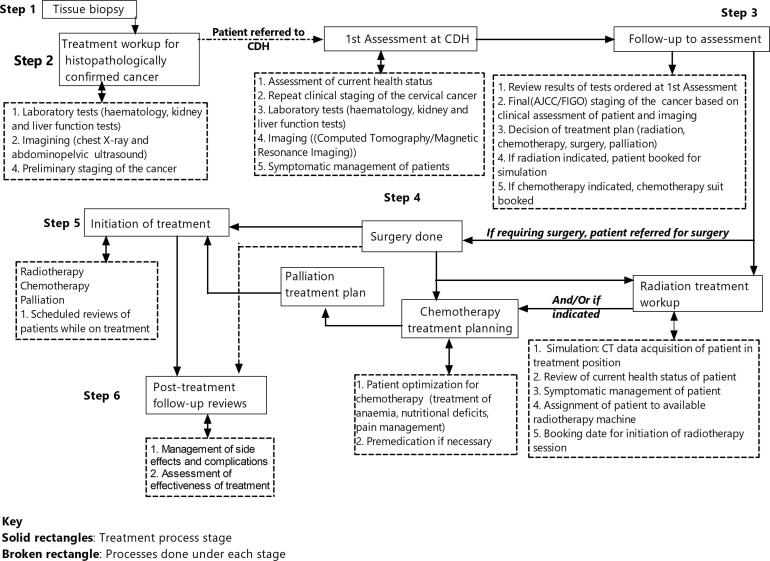
The study sought to determine the turnaround time (TAT) to the definitive treatment of patients with cervical cancer at CDH. Thus, the time interval from histological diagnosis to the initiation of treatment was the main outcome of the study. In the current study, turnaround time was defined as the time elapsed from the date the tissue biopsy results were reported (histological diagnosis) to the date of the initial treatment session of the patient with cervical cancer. To arrive at the initiation of treatment, a clinical workflow pathway (Fig. 2) is followed in the management of cervical cancer. For this study, turnaround time was assessed for cancer diagnostic and treatment procedures at the referring hospitals and those at CDH.
Fig 2.
Cancer clinical workflow pathway stages and stage-processes handled at referral facilities (stage 1–2) and the Cancer Diseases hospital (stages 3–6).
In Zambia, the clinical workflow pathway constitutes six main stages, each embracing different activities. The initial stages (tissue biopsy and treatment workup for histologically confirmed cancer) are done at the referring hospitals in the different provinces across the country. Patients with histopathological results positive for cervical cancer are then referred to CDH where they undergo treatment and follow-up reviews in specialist clinics as necessary (Fig. 2, Supplementary file X1).
2.4. Data collection
Using CDH standardized patient case files, data abstraction and collection was done. Data abstracted was thereafter entered into an Excel sheet. Data collected included patient demographics, information relating to patient social habits, dates of hospital visits, histopathology results, stage of cancer, medical comorbid conditions and information on hospital follow-up visits.
2.5. Ethics
The study was approved by the University of Zambia Biomedical Research Ethics Committee (UNZABREC) [Ref. No. 425–2019] and the National Health Research Authority (NHRA). Written permission to carry out the study was obtained from the management of the Cancer Diseases Hospital [Ref: MH/CDH/101/14/1]. Consent from patients was not necessary. All files were designated a unique study number for use as an identifier and the names of patients remained confidential.
2.6. Data Analysis
Data cleaning and analysis were done using R statistical software version 4.0.3 (RCoreTeam, 2013). Before data was formally analysed, it was pre-processed as follows: Stages of cancer recorded according to the American Joint Committee on Cancer (AJCC) were restaged according to the FIGO staging system. Accordingly, AJCC cancer stages 1A-B were reassigned FIGO stage I, AJCC stages 2A-B FIGO stage II, AJCC stages 3A-C FIGO stage III and AJCC stages 4A-B FIGO stage IV (Bhatla and Denny, 2018). Unstaged cancer according to FIGO was designated ‘missing’. Concerning the location of the laboratory to which the histology sample was submitted for processing, laboratories located outside Zambia were assigned to the ‘Others’ category. These included samples from Eastern, Luapula, Muchinga, Northern, North-Western, Southern and Western Provinces. Considering the HIV status, HIV test results that were not found in patient records were also recorded as ‘missing’.
Descriptive statistics were used to summarize the age (in years), marital status, HIV status, stage of cancer, class of laboratory (private or public) and location of the laboratory to which the biopsy was submitted for histopathology. We determined three TAT; Time 1, the diagnostic turnaround time (dTAT), was determined as the time interval between the tissue biopsy and receipt of histopathology results. Referring hospitals are responsible for biopsying patients and sending the tissue sample to either public or private laboratories for processing. To determine the difference in the dTAT between private laboratories and public laboratories, we used the Wilcoxon rank-sum test. Time 2, herein called referral delay (rTAT), is related to the patient and was determined as the time interval between receipt of the histopathology result by the patient and the patient’s initial assessment at CDH. During rTAT, the pre-CDH referral workup (laboratory tests, basic imaging and preliminary staging of cancer) is done at the referring hospital. Time 3 is the assessment turnaround (aTAT) at CDH where patients go for further examinations and treatment and was calculated as the time interval between the first assessment at CDH and initiation of the definitive cancer treatment. Time 4, the overall turnaround time (oTAT), was the time interval from the receipt of biopsy results at the referring hospitals and initiation of definitive treatment at CDH and therefore involves both referring hospitals and CDH. To determine the differences in TAT and cancer stage, the Kruskal Wallis test was used. Furthermore, we fitted a generalized linear model of the negative binomial family to study the patient and facility-related factors on average oTAT.
3. Results
In this study, we included 2121 patient case files for analysis. The median age of the patients was 49 (IQR: ±17). Most of the patients (n = 634, 31%) were aged between 41 and 50 years while the least number of patients (n = 18, 0.9%) was aged between 81 and 90 years. The majority of patients were married (n = 1092, 57%). The study observed that 1,083 (54%) biopsies were submitted to private laboratories for processing while 937 (46%) were submitted to public laboratories. In addition, the majority of biopsy samples (n = 1381, 69%) were submitted to laboratories in Lusaka province while 153 (7.7%) were submitted to laboratories in other provinces that included Eastern, Luapula, Muchinga, Nothern, North-Western, Southern and Western provinces (Table 1).
Table 1.
Demographic characteristics for cancer patients.
| Variable | Frequency (%) | |
|---|---|---|
| Age | 21–30 | 50 (2.4%) |
| 31–40 | 416 (20%) | |
| 41–50 | 634 (31%) | |
| 51–60 | 516 (25%) | |
| 61–70 | 281 (14%) | |
| 71–80 | 145 (7.0%) | |
| 81–90 | 18 (0.9%) | |
| Marital status | Married | 1,092 (57%) |
| Widowed | 501 (26%) | |
| Single | 220 (11%) | |
| Divorced | 109 (5.7%) | |
| HIV status | No | 1,099 (55%) |
| Yes | 898 (45%) | |
| FIGO Stage of Cancer | I | 162 (8.2%) |
| II | 941 (48%) | |
| III | 771 (39%) | |
| IV | 103 (5.2%) | |
| Laboratory location | Lus | 1,381 (69%) |
| CB | 275 (14%) | |
| CP | 186 (9.3%) | |
| other | 153 (7.7%) | |
| Laboratory type | Public | 937 (46%) |
| Private | 1083 (54%) | |
Note: Laboratory location: CB = Copperbelt Province, CP = Central Province, EP = Eastern Province, Lus = Lusaka Province, Other = Eastern, Luapula, Muchinga, Northern, North-Western, Southern and Western Provinces.
Most of the patients presented with cancer stage II (n = 941, 48%) while the least (n = 103, 5.2%) presented with stage IV disease (Table 2). When the stages of cancer were stratified by the location where patients were referred from, we observed that most of the patients were referred from hospitals from Lusaka Province. Provinces classified as other comprising Eastern, Luapula, Muchinga, Northern, North-Western, Southern and Western provinces referred the least number of patients (Table 2).
Table 2.
Cervical cancer cases reported from different provinces.
| Cancer stage | CB, N = 275 | CP, N = 186 | Lus, N = 1,381 | Other, N = 153 |
|---|---|---|---|---|
| I | 20 (7.8%) | 15 (8.4%) | 108 (8.2%) | 9 (6.2%) |
| II | 142 (55%) | 81 (45%) | 606 (46%) | 73 (50%) |
| III | 91 (36%) | 70 (39%) | 521 (40%) | 57 (39%) |
| IV | 3 (1.2%) | 13 (7.3%) | 76 (5.8%) | 7 (4.8%) |
Note: CB = Copperbelt Province, CP = Central Province, EP = Eastern Province, Lus = Lusaka Province, Other = Eastern, Luapula, Muchinga, Northern, North-Western, Southern and Western Provinces.
Of the available methods used to treat cervical cancer, chemoradiotherapy (n = 1,226; 64%) was the most commonly used at CDH while surgery (n = 17; 0.9%) was the least. Furthermore, histopathology results showed that squamous cell carcinoma (1,916, 92%) was the most common histological subtype of cervical cancer treated at CDH . Other histological subtypes included clear cell carcinoma (n = 1;<0.1%), leiomyosarcoma (n = 1; <0.1%), smooth muscle tumor of uncertain malignant potential (n = 1; <0.1%) and spindle-cell malignancy (n = 1; <0.1%) were also observed (Table 3).
Table 3.
Cervical cancer treatment methods and histopathology subtypes recorded at CDH.
| Variable | Frequency | |
|---|---|---|
| Treatment method | Chemoradiotherapy | 1,226 (64%) |
| Radiotherapy | 587 (30%) | |
| Palliation | 52 (2.7%) | |
| Chemotherapy | 44 (2.3%) | |
| Surgery | 17 (0.9%) | |
| Histopathological subtype | Squamous Cell Carcinoma | 1,916 (92%) |
| Adenocarcinoma | 122 (5.9%) | |
| Undifferentiated carcinoma | 22 (1.1%) | |
| Adenosquamous carcinoma | 8 (0.3%) | |
| Neuroendocrine carcinoma | 9 (0.4%) | |
| Small cell carcinoma | 2 (<0.1%) | |
| Clear cell carcinoma | 1 (<0.1%) | |
| Leiomyosarcoma | 1 (<0.1%) | |
| Smooth muscle tumour of uncertain malignant potential | 1 (<0.1%) | |
| Spindle-shaped malignancy | 1 (<0.1%) | |
Our study showed that the overall average dTAT was 30 days (IQR: ±103). Furthermore, significant differences (p < 0.001) in dTAT in patients whose biopsies were submitted to public laboratories for processing and those submitted to private laboratories were observed. The average dTAT was 1.48 (95%CI: 1.21–1.81) fold longer in patients using public laboratories compared to those using private laboratories for biopsy tissue processing. Furthermore, significant differences in dTAT were observed among the laboratories located in different provinces (p = 0.05). When compared to laboratories based on the Copperbelt province, the patient’s dTAT involving laboratories based in Lusaka province (Coeff: 0.61, 95% 0.39–0.92), Central Province (Coeff 0.60; 95% CI: 0.37–0.93) and other provinces (Coeff 0.48; 95% CI: 0.27–0.90) was shorter.
The median TAT for the different stages of cervical cancer stages are shown in Table 4. No significant differences were observed in dTAT (p = 0.5), rTAT (p = 0.2) and oTAT (p = 0.2) among the various stages of cervical cancer. However, dTAT for patients with stage I (38 days; IQR:9–142), rTAT for those with stage IV (77 days; IQR: 26–195) and oTAT for those with stages I (124 days, IQR 68–204) and IV (124 day; IQR 39–200) were longer. Results of the analysis of aTAT showed significant differences (p < 0.01), with aTAT for those with stage IV cervical cancer being shorter (24 days: IQR5–74) than other stages of cervical cancer (Table 4).
Table 4.
Turnaround time for various stages of cervical cancer.
| Cancer stage classification according to FIGO |
|||||
|---|---|---|---|---|---|
| I, N = 162 | II, N = 941 | III, N = 771 | IV, N = 103 | p-value2 | |
| dTAT | 38 (IQR:9–142) | 26 (8–93) | 31 (8–133) | 36 (9–123) | 0.5 |
| rTAT | 63 (27–140) | 56 (26–147) | 52 (23–153) | 77 (26–195) | 0.2 |
| aTAT | 76 (40–172) | 70 (34–139) | 61 (27–132) | 24 (5–74) | <0.001 |
| oTAT | 124 (68–204) | 116 (67–214) | 106 (57–208) | 124 (39–200) | 0.2 |
The average rTAT was 55 days (IQR: 24–152). Furthermore, rTAT for patients from public hospitals (rTAT: 62 days; IQR: 28–163) was significantly longer (p = 0.007) compared to those from private hospitals (rTAT: 48 days; IQR: 22–36).
Our results also showed that the average aTAT was 62 days (IQR: 27–136) and the average oTAT was 110 days (IQR: 62–204). In addition, significant differences in aTAT were observed among patients undergoing different cervical cancer treatment methods (p < 0.01). Those undergoing palliation had the shortest aTAT (32 days; IQR7–73) while those who were treated with chemotherapy had the longest aTAT (111 days; IQR: 60–167). No significant differences were observed in the oTAT among patients treated with the various treatment (p = 0.3). However, those treated with surgery (204 days; IQR: 128–295) had the longest oTAT while those placed on palliation had the shortest (85 days; IQR: 38–188) oTAT (Table 5).
Table 5.
Assessment and overall turnaround time by different corrective measures offered at CDH.
| TAT | Chemo, N = 44 | CRT, N = 1,226 | Pal, N = 52 | Rad, N = 587 | Surg, N = 17 | p-value |
|---|---|---|---|---|---|---|
| aTAT | 111 (IQR: 60–167) | 62 (33–134) | 32 (7–73) | 62 (26–128) | 96 (40–147) | <0.001 |
| oTAT | 126 (78–216) | 106 (62–202) | 85 (38–188) | 112 (61–209) | 204 (128–295) | 0.3 |
Note: Chemo = Chemotherapy, CRT = Chemoradiotherapy, Pal = Palliation, Rad = Radiotherapy, Surg = Surgery.
It was also observed that HIV status (p = 0.61), stage of cancer (p = 0.41) and histological subtype of cancer (p = 0.54) did not influence oTAT. However, Age (p = 0.015) was observed to be a significant predictor of oTAT. Those aged between 81 and 90 years old were observed to have a shorter oTAT (Coeff: −0.86; 95% CI: −1.5– −0.09) compared to those aged between 21 and 30 years. Furthermore, we observed no significant difference in the oTAT among different indicated treatment methods. However, those indicated for surgery had a longer oTAT (Coeff: 0.06; 95% CI: −0.73–1.0) than the other treatment methods. Marital status (p = 0.037) influenced oTAT. When compared to those who were divorced, widows had a significantly shorter (Coeff: −0.30; 95% CI: −0.55– −0.06) oTAT (Table 6).
Table 6.
Factors influencing oTAT.
| Characteristic | Coeff | 95% CI1 | p-value | |
|---|---|---|---|---|
| Age | 21–30 | Ref | ||
| 31–40 | 0.02 | −0.34 – 0.35 | 0.9 | |
| 41–50 | −0.14 | −0.50 – 0.18 | 0.4 | |
| 51–60 | −0.04 | −0.40 – 0.29 | 0.8 | |
| 61–70 | 0.05 | −0.33 – 0.41 | 0.8 | |
| 71–80 | 0.19 | −0.22 – 0.58 | 0.4 | |
| 81–90 | −0.86 | −1.5 – −0.09 | 0.018 | |
| Marital status | Divorced | Ref | ||
| Married | −0.13 | −0.37 – 0.09 | 0.2 | |
| Single | −0.24 | −0.51 – 0.02 | 0.074 | |
| Widowed | −0.30 | −0.55 – −0.06 | 0.018 | |
| HIV status | No | Ref | ||
| Yes | −0.03 | −0.15 – 0.09 | 0.6 | |
| Stage of cancer | I | Ref | Ref | |
| II | −0.15 | −0.35 – 0.04 | 0.13 | |
| III | −0.12 | −0.33 – 0.07 | 0.2 | |
| IV | −0.04 | −0.33 – 0.25 | 0.8 | |
| Treatment type | Chemo | Ref | ||
| CRT | −0.22 | −0.58 – 0.10 | 0.2 | |
| Palliation | −0.36 | −0.82 – 0.10 | 0.12 | |
| Radiotherapy | −0.22 | −0.59 – 0.10 | 0.2 | |
| Surgery | 0.06 | −0.73 – 1.0 | 0.9 |
4. Discussion
Turnaround time (TAT) is an essential metric in health care delivery and its determination may improve effective management of resources and the implementation of treatment plans by clinicians to ultimately improve patient care (Hawkins, 2007, Storrow et al., 2008). The findings from our study suggest the need for timeliness in the cervical cancer clinical care pathway in Zambia to conform to international standards (Shiferaw and Yismaw, 2019). In this study, we found that dTAT, rTAT and oTAT for cervical cancer are significantly longer compared to standards set by health systems in high-income countries (Benk et al., 2006, Shen et al., 2016, Mike et al., 2017). For instance, in England, Scotland and Wales, the oTAT following referral is 62 days (Mike et al., 2017, Lim, 2020). On the other hand, Zambia and many other low-income countries have longer oTAT, elevating the risk of poor disease outcomes such as low treatment yield and survival rates (Govardhan and Sarkar, 2017, Anorlu, 2008).
In our study, we observed that dTAT was longer than what has been observed in Rwanda (Mpunga et al., 2014), Kenya (Macharia et al., 2015, Mwogi et al., 2020) and Nigeria (Malami and Iliyasu, 2008). Also, the dTAT for patients submitting their biopsy samples to public laboratories for processing was longer compared to those making use of private laboratories, despite private laboratories having facilitated the processing of more biopsy samples. This may be explained in part by the critical shortage of pathologists to read biopsies in the public sector in Zambia, which is one of the country’s main obstacles to quality cancer care (Mudenda et al., 2020). Private laboratories reduce the dTAT by sending some samples to be processed outside the country, in addition to their use of local pathologists. Concerning the treatment method used to treat cancer, aTAT was shortest in the palliation group, likely because the advanced disease results in complications (severe anaemia, debilitating pain, obstruction of viscera, thromboembolic phenomena) requiring urgent attention such as the utilization of shorter radiation therapy regimens (i.e single fraction treatment therefore not requiring a radiation therapy treatment wait). As observed in other studies, widows had shorter oTAT due to social sympathy and support following the loss of their spouses (AL-Baddareen et al., 2020). On the other hand, perceived longer oTAT among the elderly population may be due to multimorbidity in the older population increasing the time for symptomatic management of the patients.
With only 13% of all hospitals in Zambia being private, the majority of the country’s population depends on the more accessible and less costly public hospitals for health care (Lukama et al., 2019). Therefore, the long dTAT in public hospitals may compromise disease outcomes for most women with cervical cancer in Zambia, especially those living in rural areas. Women from urban areas are likely to have more access to accurate and high-quality medical diagnosis and essential medicines compared to those in rural areas due to improved health facilities, and premium health insurance leading to an increased early diagnosis rate (Eggleston et al., 2006, Gong et al., 2014). To attain universal health coverage and meet the United Nations’ third Sustainable Development Goal (SDG), equitable access to accurate, affordable, and high-quality diagnostics remain essential pillars of achievement (Gostin et al., 2019). To reduce barriers to medical care in rural Zambia, the government and its cooperating partners have instituted rural medical outreach programmes where screening for cervical cancer is done (Parham et al., 2010). To optimise the success of these programmes, we suggest that these programmes incorporate health education activities to improve people’s health-seeking behaviour, improve the current cervical cancer screening programs and increase investment in rural primary health care. Studies have shown that increased health expenditure leads to better health outcomes (Robone et al., 2011).
Implementation of appropriate clinical decisions is highly dependent on timely receipt of histopathological diagnosis, early patient referral and specialist assessment. The findings from our study suggest that in Zambia, delays in cervical cancer patient referral for specialist care are longer than those acceptable elsewhere (Coles et al., 2003, Ramey et al., 2018). We believe that the centralization of cancer treatment to CDH and its high costs associated with diagnostics and staging workup negatively influence rTAT. According to Ngoma (Ngoma et al., 2019), increasing gynaecology facilities, upgrading existing health facilities, improving access to these facilities and improving communication and transportation of patients would be vital in improving cervical cancer care. Improving diagnostic facilities and the early management of patient comorbid conditions may reduce rTAT (Mwogi et al., 2020). Owing to the limitation in the diagnostic capability of many health facilities in Zambia, some patients referred to CDH are delayed at the referring institutions while they look for resources to complete their investigations at private facilities. Those who can not afford private facilities have to do the basic investigations at CDH, further delaying their treatment. The delay may upstage their cancer, resulting in the need for further patient evaluation and depressing their prognosis.
The long dTAT and oTAT observed in our study suggest the need for increasing local diagnostics, staging facilities and human resources for improved cervical cancer outcomes. We postulate that the long oTAT observed in our study may in part be due to the high patient to equipment ratio. The available few machines frequently break down owing to their advanced age and sometimes unmet high maintenance costs, leading to lengthy waiting times before treatment can be initiated. Furthermore, the Health Professional Council of Zambia (HPCZ) indicates that Zambia has nine (9) Radiation Oncologists, three (3) Gynaecology Oncologists against a population of 9,033,248 women (CSO. Population and Demographic Projections, 2013). This translates to 0.0996 Oncologists (0.033 Gynaecology Oncologists) per 100,000 women. The scarcity of both Radiation and Gynaecology Oncologists may directly contribute to the poor outcomes of cervical cancer at CDH. Therefore, formulating policy guidelines for the diagnostic and therapeutic management of cervical cancer, improving health care through human resource training and infrastructure development would be vital in improving cervical cancer care outcomes. The high prevalence of cervical cancer in Zambia (Kalima et al., 2007), coupled with the high rate of HIV infection and other co-morbidities against a contracted human resource workforce may further increase oTAT.
Although our findings showed that HIV status did not influence oTAT. HIV infection has been observed to accelerate the progression of cervical cancer (Finocchario-Kessler et al., 2016, Li et al., 2019, Gichangi et al., 2003). Our contrasting findings on HIV status may have been influenced by the small number of cases of HIV infected individuals observed. Our study also showed that squamous cell carcinoma (SCC) was the most common histological subtype of cervical cancer; and this conforms with other studies (Arnold et al., 2015, Vizcaino et al., 2000). In addition, most patients were treated with chemoradiotherapy (64%), accurately reflecting the treatment of choice for most patients with stages II-IV cervical cancer (Eifel, 2006). While only 8.2% of the patients presented with stage I disease (theoretically requiring monotherapy with either surgery or radiotherapy), more patients were treated with radiotherapy (30%) because some patients with early-stage II disease were also correctly treated with radiotherapy alone as guided by literature (Cibula et al., 2018). Since monotherapy with chemotherapy is reserved for those patients not amenable to curative treatment and as neo-adjuvant therapy (chemotherapy given before definitive surgery or radiotherapy) (Cibula et al., 2018, Barra et al., 2017), the 2.3% chemotherapy treatment rate in our study is high in our opinion. This rate may be an indication that a significant proportion of patients are referred to CDH with advanced or incurable stages of cervical cancer. Thus, accelerated efforts to ensure early diagnosis and referral of patients for definitive treatment of cervical squamous cell carcinoma should be prioritised.
Our study highlights the need to assess the effect of diagnostic and treatment delays on the survival of cervical cancer patients in Zambia. We observed that palliation was not indicated to have been done with or without chemotherapy, radiotherapy and surgery. With this omission, we were unable to determine what proportion of the patients on palliation received palliative chemotherapy or palliative surgery. This information is necessary for characterising the care of dying cervical cancer patients. Also, due to the duplication of files and missing vital information on the medical records, 69% were included in the study, 1% shy of the target. Thus, we maximised the value of the research with our exclusion criteria. The poor record-keeping at the National Cancer Registry (NCR) may be due to the understaffing and resource constraints at the institution (Kalima et al., 2007). There is need to improve health data collection at CDH to improve clinical workflow. Furthermore, there is need to move from paper-based data collection methods to web-based electronic data capture (EDC) systems which have become more prevalent. Also, standardization of data collection tools across all health facilities that have common terminologies is needed to enhance data aggregation and encourage interoperability.
5. Conclusion
Despite the challenges in the storage of data at the CDH registry, oTAT cervical cancer patients in Zambia is long and requires improvement to reduce the risk of poor disease outcomes. There is a need to strengthen cervical cancer screening and diagnostic facilities at all levels of health care in Zambia for early detection and referral of suspected cases for specialized treatment. In addition, there is a need to develop cervical cancer treatment guidelines and standardise diagnostic and treatment TAT in line with the International Organization for Standardization (ISO) to improve cervical cancer outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2021.100784.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. The Lancet Global Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu M., Yu J., Awolude O.A., Chuang L. Cervical cancer worldwide. Curr. Probl. Cancer. 2018;42(5):457–465. doi: 10.1016/j.currproblcancer.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Sloan F.A., Gelband H. The cancer burden in low-and middle-income countries and how it is measured. Cancer control opportunities in low-and middle-income countries. National Academies Press (US) 2007 [PubMed] [Google Scholar]
- Akinyemiju T.F. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the World Health Survey. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0048834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnik L.A., Matanje-Mwagomba B., Msosa V., Mzumara S., Khondowe B., Moses A. Breast cancer screening in low-and middle-income countries: a perspective from Malawi. J. Global Oncol. 2016;2(1):4. doi: 10.1200/JGO.2015.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R., Budukh A.M., Rajkumar R. Effective screening programmes for cervical cancer in low-and middle-income developing countries. Bull. World Health Organ. 2001;79:954–962. [PMC free article] [PubMed] [Google Scholar]
- Dunyo P., Effah K., Udofia E.A. Factors associated with late presentation of cervical cancer cases at a district hospital: a retrospective study. BMC Public Health. 2018;18(1):1–10. doi: 10.1186/s12889-018-6065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowemimo O.O., Ojo O.O., Fasubaa O.B. Cervical cancer screening and practice in low resource countries: Nigeria as a case study. Trop. J. Obstetr. Gynaecol. 2017;34(3):170–176. [Google Scholar]
- Toliman P., Kaldor J., Tabrizi S., Vallely A. Innovative approaches to cervical cancer screening in low-and middle-income countries. Climacteric. 2018;21(3):235–238. doi: 10.1080/13697137.2018.1439917. [DOI] [PubMed] [Google Scholar]
- Jedy-Agba E., Joko W.Y., Liu B., Buziba N.G., Borok M., Korir A. Trends in cervical cancer incidence in sub-Saharan Africa. Br. J. Cancer. 2020;123(1):148–154. doi: 10.1038/s41416-020-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsom E.Z., Jansen M., Tanis P.J., van de Ven A.W., van Oud-Alblas M.B., Buskens C.J., Bemelman W.A., Schijven M.P. Video consultation during follow up care: effect on quality of care and patient-and provider attitude in patients with colorectal cancer. Surg. Endosc. 2020;1–10 doi: 10.1007/s00464-020-07499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz R., Buessecker F., Herlofsen H., Hinrichs F., Zeiler T., Kuhn K.A. Demand driven Evolution of IT Systems in Healthcare. Methods Inf Med. 2005;44(1):4–10. [PubMed] [Google Scholar]
- Nascimento M.Id. Waiting time for radiotherapy in women with cervical cancer. Rev. Saude Publica. 2015;49:92. doi: 10.1590/S0034-8910.2015049005953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroman N., Tutt A., Jensen M.-B., Wohlfahrt J., Mouridsen H.T., Andersen P.K., Melbye M., Ross G. Factors influencing the effect of age on prognosis in breast cancer: population based studyCommentary: much still to learn about relations between tumour biology, prognosis, and treatment outcome in early breast cancer. BMJ. 2000;320(7233):474–479. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi A., Ueda Y., Kakuda M., Tanaka Y., Ikeda S., Matsuzaki S. Epidemiologic and clinical analysis of cervical cancer using data from the population-based Osaka Cancer Registry. Cancer Res. 2019;79(6):1252–1259. doi: 10.1158/0008-5472.CAN-18-3109. [DOI] [PubMed] [Google Scholar]
- Ross D.G., Rayne S. An audit of provider delay in newly diagnosed breast cancer in a central referral hospital in Johannesberg, South Africa. S. Afr. J. Surg. 2017;55:44. [Google Scholar]
- Lohlun K., Kotzen J., Lakier R. A prospective study on the impact of waiting times for radiotherapy for cervical cancer at Charlotte Maxeke Johannesburg Academic Hospital, South Africa. South African J. Obstetr. Gynaecol. 2015;21(1):6–9. [Google Scholar]
- Chen C.-P., Kung P.-T., Wang Y.-H., Tsai W.-C. Effect of time interval from diagnosis to treatment for cervical cancer on survival: a nationwide cohort study. PLoS ONE. 2019;14(9) doi: 10.1371/journal.pone.0221946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardhan H., Sarkar N. Pattern of treatment delay in carcinoma cervix patients and effect on survival. Ann. Oncol. 2017;28 [Google Scholar]
- Benk V., Przybysz R., McGowan T., Paszat L. Waiting times for radiation therapy in Ontario. Can. J. Surg. 2006;49(1):16. [PMC free article] [PubMed] [Google Scholar]
- Masamba L.P., Mtonga P.E., Kalilani Phiri L., Bychkovsky B.L. Cancer pathology turnaround time at Queen Elizabeth Central Hospital, the largest referral center in Malawi for oncology patients. J. Global Oncol. 2017;3(6):734–739. doi: 10.1200/JGO.2015.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSO. Population and Demographic Projections, 2011-2035. Lusaka: Central Statistics Office; 2013.
- Karjane N., Chelmow D. New cervical cancer screening guidelines, again. Obstetr. Gynecol. Clinics. 2013;40(2):211–223. doi: 10.1016/j.ogc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Bhatla N., Denny L. FIGO cancer report 2018. Wiley Online. Library. 2018 doi: 10.1002/ijgo.12608. [DOI] [PubMed] [Google Scholar]
- Hawkins R.C. Laboratory turnaround time. Clin. Biochemist Rev. 2007;28(4):179. [PMC free article] [PubMed] [Google Scholar]
- Storrow A.B., Zhou C., Gaddis G., Han J.H., Miller K., Klubert D., Laidig A., Aronsky D. Decreasing lab turnaround time improves emergency department throughput and decreases emergency medical services diversion: a simulation model. Acad. Emerg. Med. 2008;15(11):1130–1135. doi: 10.1111/j.1553-2712.2008.00181.x. [DOI] [PubMed] [Google Scholar]
- Shiferaw, M.B., Yismaw, G., 2019. Magnitude of delayed turnaround time of laboratory results in Amhara Public Health Institute, Bahir Dar, Ethiopia. BMC health services Res. 19, 1, 1–6. [DOI] [PMC free article] [PubMed]
- Shen S.-C., Hung Y.-C., Kung P.-T., Yang W.-H., Wang Y.-H., Tsai W.-C. Factors involved in the delay of treatment initiation for cervical cancer patients: a nationwide population-based study. Medicine. 2016;95(33) doi: 10.1097/MD.0000000000004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike S., David D., Paul M. Waiting Times for Suspected and Diagnosed Cancer Patients 2016–17 Annual Report. NHS England. 2017;25(02):2021. [Google Scholar]
- Lim SC. Waiting times for suspected and diagnosed cancer patients. 2020.
- Anorlu R.I. Cervical cancer: the sub-Saharan African perspective. Reproductive health matters. 2008;16(32):41–49. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- Mpunga T., Tapela N., Hedt-Gauthier B.L., Milner D., Nshimiyimana I., Muvugabigwi G. Diagnosis of cancer in rural Rwanda: Early outcomes of a phased approach to implement anatomic pathology services in resource-limited settings. Am. J. Clin. Pathol. 2014;142(4):541–545. doi: 10.1309/AJCPYPDES6Z8ELEY. [DOI] [PubMed] [Google Scholar]
- Macharia B., Chumba D., Ndiangui F. Eldoret; Kenya: 2015. Evaluation of turnaround time of biopsy and surgical specimens in Moi teaching and referral hospital. [Google Scholar]
- Mwogi T., Mercer T., Tran D.N., Tonui R., Tylleskar T., Were M.C. Therapeutic turnaround times for common laboratory tests in a tertiary hospital in Kenya. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0230858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malami S.A., Iliyasu Y. Local audit of diagnostic surgical pathology as a tool for quality assurance. Nigerian J. Med. 2008;17(2):186–190. doi: 10.4314/njm.v17i2.37381. [DOI] [PubMed] [Google Scholar]
- Mudenda V., Malyangu E., Sayed S., Fleming K. Addressing the shortage of pathologists in Africa: Creation of a MMed Programme in Pathology in Zambia. African J. Laboratory Med. 2020;9(1):1–7. doi: 10.4102/ajlm.v9i1.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Baddareen GS, Al Ali TM, Akour MM, editors. Perceived social support among widowed women in Jordan: An exploratory study. Women's Studies International Forum; 2020: Elsevier.
- Lukama, L., Kalinda, C., Aldous, C., 2019. Africa’s challenged ENT services: highlighting challenges in Zambia. BMC health services Res. 19, 1, 1-9. [DOI] [PMC free article] [PubMed]
- Eggleston, K.S., Coker, A.L., Williams, M., Tortolero-Luna, G., Martin, J.B., Tortolero, S.R., 2006. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995–2001. J. Women's Health. 15, 8, 941–951. [DOI] [PubMed]
- Gong W., Luo S., Hu R., Wang H., Pan J., Fei F., He Q., Yu M. Analysis of survival rate of breast, cervical, and ovarian cancer patients during 2005–2010 in Zhejiang province, China. Zhonghua yu Fang yi xue za zhi [Chin. J. Prev. Med.]. 2014;48(5):366–369. [PubMed] [Google Scholar]
- Gostin, L.O., Abubakar, I., Guerra, R., 2019. The Lancet Commission on diagnostics: advancing equitable access to diagnostics. [DOI] [PubMed]
- Parham, G.P., Mwanahamuntu, M.H., Sahasrabuddhe, V.V., Westfall, A.O., King, K.E., Chibwesha, C., et al., 2010. Implementation of cervical cancer prevention services for HIV-infected women in Zambia: measuring program effectiveness. HIV Therapy. 4, 6, 713–722. [DOI] [PMC free article] [PubMed]
- Robone, S., Rice, N., Smith, P., 2011. Health systems’ responsiveness and its characteristics: A cross-country comparative analysis. Health Serv. Res., 46, 2079–100. [DOI] [PMC free article] [PubMed]
- Coles C., Burgess L., Tan L. An audit of delays before and during radical radiotherapy for cervical cancer–effect on tumour cure probability. Clin. Oncol. 2003;15(2):47–54. doi: 10.1053/clon.2002.0178. [DOI] [PubMed] [Google Scholar]
- Ramey S.J., Asher D., Kwon D., Ahmed A.A., Wolfson A.H., Yechieli R., Portelance L. Delays in definitive cervical cancer treatment: an analysis of disparities and overall survival impact. Gynecol. Oncol. 2018;149(1):53–62. doi: 10.1016/j.ygyno.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Ngoma T., Asiimwe A.R., Mukasa J., Binzen S., Serbanescu F., Henry E.G. Addressing the second delay in saving mothers, giving life districts in Uganda and Zambia: reaching appropriate maternal care in a timely manner. Global Health: Sci. Pract. 2019;7(Supplement 1):S68–S84. doi: 10.9745/GHSP-D-18-00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalima, M., Lishimpi, K., Meza, J.L., Watanabe-Galloway, S., Msadabwe, S.C., Mwaba, C.K., et al., 2015. Observed and expected incidence of cervical cancer in lusaka and the southern and Western provinces of Zambia, 2007 to 2012. Int. J. Gynecol. Cancer. 25, 1. [DOI] [PMC free article] [PubMed]
- Finocchario-Kessler S., Wexler C., Maloba M., Mabachi N., Ndikum-Moffor F., Bukusi E. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Women's Health. 2016;16(1):1–25. doi: 10.1186/s12905-016-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chi X., Li R., Ouyang J., Chen Y. HIV-1-infected cell-derived exosomes promote the growth and progression of cervical cancer. Int. J. Biolog. Sci. 2019;15(11):2438. doi: 10.7150/ijbs.38146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichangi P.B., Bwayo J., Estambale B., De Vuyst H., Ojwang S., Rogo K., Abwao H., Temmerman M. Impact of HIV infection on invasive cervical cancer in Kenyan women. Aids. 2003;17(13):1963–1968. doi: 10.1097/00002030-200309050-00015. [DOI] [PubMed] [Google Scholar]
- Arnold M., Soerjomataram I., Ferlay J., Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- Vizcaino, A.P., Moreno, V., Bosch, F.X., MUNoz, N., Barros‐Dios, X.M., Borras, J., Parkin, D.M., 2000. International trends in incidence of cervical cancer: II. Squamous‐cell carcinoma. Int. J. Cancer. 86, 3, 429–435. [DOI] [PubMed]
- Eifel PJ, editor Chemoradiotherapy in the treatment of cervical cancer. Seminars in radiation oncology; 2006: Elsevier. [DOI] [PubMed]
- Cibula D., Pötter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Haie-Meder C. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch. 2018;472(6):919–936. doi: 10.1007/s00428-018-2362-9. [DOI] [PubMed] [Google Scholar]
- Barra F., Lorusso D., Leone Roberti Maggiore U., Ditto A., Bogani G., Raspagliesi F., Ferrero S. Investigational drugs for the treatment of cervical cancer. Expert Opin. Invest. Drugs. 2017;26(4):389–402. doi: 10.1080/13543784.2017.1302427. [DOI] [PubMed] [Google Scholar]
- RCoreTeam 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.