Introduction
Ventricular tachycardia (VT) is a potentially life-threatening arrhythmia characterized by electrical re-entry within patches of heterogeneous myocardial fibrosis leading to sustained consecutive ventricular beats at a rate >100/min.1 In patients with monomorphic VT, implantable cardioverter-defibrillators (ICD) have become the cornerstone of therapy in decreasing mortality through the prevention of sudden death.2 However, ICDs have no effect on the arrhythmogenic substrate and address symptom-control only. Patients often receive recurrent and debilitating shocks associated with an increase in mortality.3 Currently, catheter ablation (CA) for VT is used as an adjunctive therapy for those refractory to medical therapy.4 A recent systematic review and meta-analysis shows that CA is superior to medical therapy for scar-related VT with respect to VT recurrence and life-threatening VT storm.2 Despite this, there is still a high incidence of VT recurrence in both medically treated (48%) and ablation-treated (39%) patients suggesting the current treatment paradigm is suboptimal.
In 2015, Loo et al5 were the first to use stereotactic ablative radiation therapy (StAR) for VT in humans. In 2017, Cuculich et al6 built on this technique, identifying arrhythmogenic scar regions by noninvasive cardiac mapping. More recently, Robinson et al7 demonstrated positive results in the Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia (ENCORE-VT) trial using a similar StAR technique for refractory VT. These works report a marked reduction in VT burden,6,7 a decrease in antiarrhythmic drug use,7 and improvement in quality of life.7 Since then, several centers have reported their early experience with this technique8 with both positive9, 10, 11 and negative12 results. In this report, we add to the limited evidence detailing our experience with 2 patients for StAR for VT at the McGill University Health Centre. Written informed consent for StAR-VT and publication of the treatment process was obtained from both patients. All images are published under agreement of the patients with anonymity.
In addition, we add to the very limited data on cardiac substructure countouring and complementary dose volume histograms (DVH; Table 1).
Table 1.
Mean and max radiation dose to cardiac structures
| Case 1 |
Case 2 |
Knutson et al14 | |||
|---|---|---|---|---|---|
| Structure | DMax (Gy) | DMedian (Gy) | DMax (Gy) | DMedian (Gy) | DMedian (Gy) |
| Heart (total) | 33.8 | 5.9 | 32.8 | 2.5 | Not reported |
| Right atrium | 14.1 | 4.3 | 3.4 | 1.8 | 2.9 |
| Right ventricle | 14.1 | 4.9 | 32.8 | 4.8 | 8.3 |
| Left atrium | 31.5 | 4.6 | 3.3 | 1.3 | 3.6 |
| Left ventricle | 33.8 | 9.1 | 32.5 | 5.1 | 11.3 |
| Left coronary artery | 1.0 | 0.9 | 0.4 | 0.3 | 1.3 |
| Right coronary artery | 28.6 | 6.4 | Not visualized | Not visualized | 3.2 |
| Left anterior descending | 10.2 | 0.9 | 31.0 | 25.6 | 10.1 |
| Circumflex artery | 31.3 | 21.6 | 3.6 | 2.5 | 9.2 |
| Pulmonary artery | 0.7 | 0.4 | 17.0 | 0.3 | 0.66 |
| Aorta | 26.5 | 0.5 | 5.1 | 0.2 | 1.6 |
| Superior vena cava | 0.4 | 0.3 | 1.9 | 0.1 | 0.9 |
| Inferior vena cava | 11.7 | 5.8 | 2.6 | 1.8 | 2.7 |
| Interventricular septum | 11.2 | 4.8 | 30.5 | 6.5 | Not reported |
| Aortic valve | Not visualized | Not visualized | 5.0 | 3.0 | 3.5 |
| Pulmonic valve | Not visualized | Not visualized | 1.3 | 0.6 | 1.8 |
| Mitral valve | Not visualized | Not visualized | 2.9 | 1.7 | Not reported |
| Tricuspid valve | Not visualized | Not visualized | 3.6 | 2.5 | Not reported |
Case Descriptions
Case 1
A 65-year-old man known with recurrent VT secondary to ischemic cardiomyopathy presenting to the emergency department (ED) in VT storm requiring multiple ICD shocks. Reversible precipitating factors including ischemia, electrolyte imbalances, and drug interactions were ruled out in the ED. The patient had a significantly reduced left ventricular ejection fraction 30%, and multiple comorbidities, including coronary artery disease, type 2 diabetes mellitus, and chronic kidney disease. The patient underwent quintuple coronary artery bypass graft surgery (in 2004) after a myocardial infarction, and in 2013, a prophylactic ICD was placed. The patient was on maximum tolerable amiodarone (400 mg daily). Ablation was attempted in 2017 targeting a scar on the lateral wall of the LV. Reviewing the ICD suggested multiple VT circuits and cycle lengths consistent with the previous electrophysiologists (EP) study. The likelihood of clinical success with a repeat CA was deemed low given the large number of morphologies and complex substrate underlying the arrhythmias.
Case 2
A 65-year-old man with recurrent VT secondary to myocardial infarction (in 2002) presented to the ED in VT storm requiring multiple ICD shocks. Reversible precipitating factors including ischemia, electrolyte imbalances, and drug interactions were ruled out in the ED. The patient's left ventricular ejection fraction was 35%, and he had an apical aneurysm containing a chronic, prominent LV thrombus, making him ineligible for CA.13 An ICD was placed for sudden cardiac death prophylaxis in 2019 after an out of hospital infarct, otherwise the patient's past medical history is unremarkable. He was on maximum dose amiodarone (400 mg daily).
After detailed consultation, the decision to perform StAR was reached. Both patients were considered for the experimental treatment as they had no further options for either medical or surgical treatment. They were treated on a compassionate basis after multiple conversations about the risks with both the cardiology and radiation oncology teams.
Treatment
For treatment planning, 4-dimensional computed tomography (4D-CT), cardiac mapping (case 1), cardiac ultrasound, electrocardiogram (ECG), and technetium-99m perfusion scan (case 1) were acquired for target localization.
Cardiac mapping was performed using the Carto system and merged with intracardiac ECG. A single transseptal approach was used for voltage mapping. A window of 0.5 to 1.5 mV was used to differentiate between scar and healthy tissue. More than 7 different VT morphologies were induced, all poorly tolerated hemodynamically, making the mapping procedure extremely challenging. Enough information was obtained to delineate the area of scar and get tracings of VT morphologies to localize the exit sites of the circuits. In case 2, owing to the chronic and prominent LV thrombus, the patient was considered unsafe for electrophysiology study.13
Target delineation is done through an iterative process with medical physicists, EP, and radiation oncologists (RO). The patient's clinical information and imaging is reviewed. The EP translates mapping/scar location to the tomographic equivalent of the heart using the 17-section cardiac model.14 The RO contours these segments with real-time feedback from the EP. The internal target volume (ITV) contains components of the respiratory and cardiac motion and are not decoupled. The ITV was created by contouring the clinical target volume (CTV) on all respiratory phases of the 4D-CT. Finally, an isotropic margin of 3 mm was added to create the planning target volume (PTV).
Before creating a radiation treatment plan, organs-at-risk, including the spinal cord, stomach, liver, lungs, esophagus, and heart substructures were contoured. Normal cardiac structure and substructures were contoured based on the atlas by Duane et al15 by the RO and reviewed by the EP (Table 1). The PTVs were prescribed 25 Gy in 1 fraction with the plan normalized so that 95% of the PTV was covered by the 25 Gy isodose. Pretreatment quality assurance plan review was performed according to internal department guidelines for stereotactic body radiation therapy. Radiation was delivered with a TrueBeam STx Linear Accelerator (Varian Medical Systems) using a 6-MV flattening-filter-free beam and a volumetric modulated arc therapy technique. The treatments were delivered with 3 coplanar partial arcs. Although the heart can be considered central, in both cases, the target's centroid was near the middle of the left lung. Angles were chosen to avoid unnecessary irradiation of the right lung. The use of abdominal compression was evaluated, but it did not provide ITV margin reductions, brought the stomach closer to the PTV, and thus was not used. Respiratory gating was not performed to minimize treatment time and complexity because it had minimal ITV margin gains. Pretreatment position was verified with kV-kV x-rays and cone beam CT. Treatment was delivered under cardiac monitoring, with the ICD in service-mode, as per recommendation by the cardiology team, and emergency team stand-by.
Case 1
CT-imaging was performed without contrast as the patient's creatinine was >300 μmol/L (estimated glomerular filtration rate = 41) and was unwilling to consider dialysis as part of the therapy. Treatment planning imaging and EP mapping demonstrated a targetable substrate localized to the lateral and inferior walls of the LV. Owing to the proximity of the esophagus to the target, 4 mL of the substrate directly adjacent to this organ was not included in the PTV to prioritize safety over target coverage.
Case 2
A targetable substrate in the apical aneurysm was localized through contrast-enhanced 4D-CT, echocardiogram, and ECG.
Results
Dose quality information as per The International Commission on Radiation Units and Measurements report 91,16 and single-fraction stereotactic body radiation therapy dose constraints17,18 are summarized in Table 2 for both cases. In case 1, the only unmet organs-at-risk constraint was Dmax of the stomach (21.3 Gy). All recommended dose constraints were met in case 2. For both cases, the ICDs received <0.1 Gy.
Table 2.
Treatment plan quality information
| Case 1 | Case 2 | |
|---|---|---|
| Dose quality information | ||
| No. of arcs (angle coverage) | 3 (330-180) | 3 (315-145) |
| Treatment time | 24 min (8 min, 0 seconds beam-on) | 20 min (7 min 20 seconds beam-on) |
| CTV (mL) | 41.9 | 17.4 |
| PTV (mL) | 102.9 | 66.4 |
| Target covered, % | 95 by Rx dose (25 Gy) | 95 by Rx dose (25 Gy) |
| Prescription isodose volume/tumor volume (CI) | 1.03 | 0.95 |
| Max dose/prescribed dose (HI) | 1.35 | 1.31 |
| PTV | ||
| D98% | 24.2 Gy | 24.5 Gy |
| D50% | 28.3 Gy | 28.0 Gy |
| D2% | 32.2 Gy | 30.1 Gy |
| Dmean | 28.3 Gy | 27.7 Gy |
| V20 Gy (%) ≧95% | V20 Gy (%) = 99.5% | V20 Gy (%) = 100% |
| V19 Gy (%) ≧99% | V19 Gy (%) = 99.6% | V19 Gy (%) = 100% |
| Esophagus | ||
| Esophagus V11.9 Gy (mL) <5 mL | V11.9 Gy (mL) = 0.3 mL | V11.9 Gy (mL) = 0.0 mL |
| Esophagus D0.035 mL (Gy) <16 Gy | D0.035 mL (Gy) = 11.7 Gy | D0.035 mL (Gy) = 1.4 Gy |
| Esophagus Dmax <19 Gy | Dmax = 16.3 Gy | Dmax = 1.7 Gy |
| Stomach | ||
| Stomach V13 Gy (mL) <10 mL | V13 Gy (mL) = 4.3 mL | V13 Gy (mL) = 0.08 mL |
| Stomach D0.035 mL (Gy) <16 Gy | D0.035 mL (Gy) = 19.8 Gy | D0.035 mL (Gy) = 13.6 Gy |
| Stomach Dmax <16 Gy | Dmax = 21.3 Gy | Dmax = 15.2 Gy |
| Lungs | ||
| V7 Gy (mL) <1,500 mL (L) | V7 Gy (mL) = 376 mL | V7 Gy (mL) = 182 mL |
| V7 Gy (mL) <1,500 mL (R) | V7 Gy (mL) = 0.1 mL | V7 Gy (mL) = 3.2 mL |
Abbreviations: CI = confidence interval; CTV = clinical target volume; HI = homogeneity index; PTV = planning target volume; Rx = prescription.
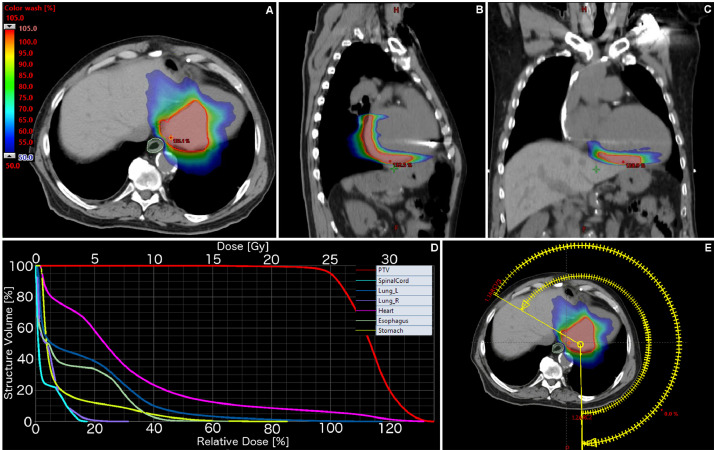
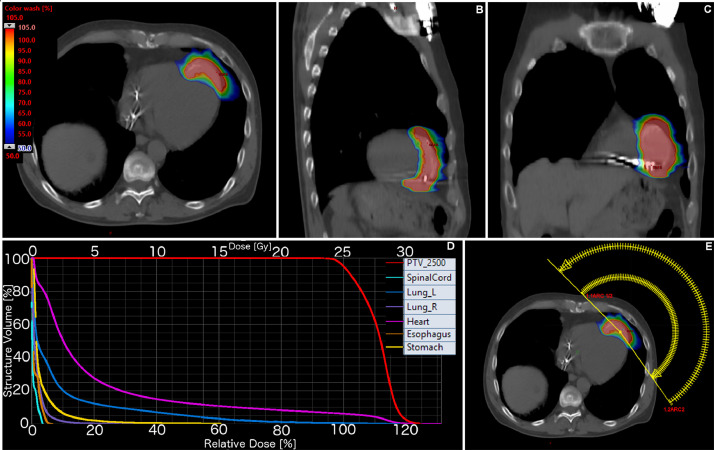
Figure 1, Figure 2 summarize the treatment volumes, DVH and treatment arcs for case 1 and case 2, respectively. DMax and DMedian to the different cardiac substructures is summarized in Table 1. Figure 3 highlights an illustrative case of the cardiac contours and associated DVH for case 1.
Figure 1.
Case 1: Planning computed tomography with overlaid dose distribution in axial (A), sagittal (B), and coronal (C) planes. Dose volume histograms of the treatment plan with relevant organs-at-risk (D). Treatment arcs (E). PTV = planning target volume.
Figure 2.
Case 2: Planning computed tomography with overlaid dose distributions in axial (A), sagittal (B), and coronal (C) planes. Dose volume histograms of the treatment plan with relevant organs-at-risk (D). Treatment arcs (E). PTV = planning target volume.
Figure 3.
Contoured cardiac substructures in the axial (A), coronal (B), and sagittal (C) planes, and dose volume histograms (D). CA = coronary artery; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; RA = right atrium; RCA = right coronary artery; RV = right ventricle; SVC = superior vena cava.
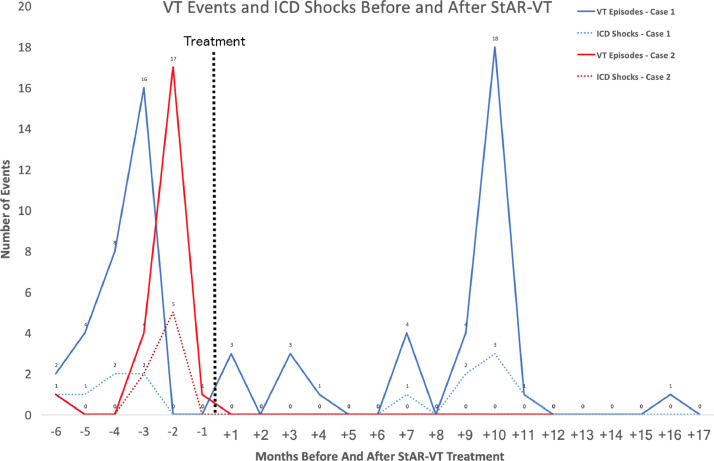
Neither patient has experienced any lasting radiation-related side effects. Both patients demonstrated an initial decrease in both VT burden and ICD shocks. Figure 4 highlight the incidence of VT events and ICD shocks before and after treatment for both patients. Both patients have routine follow-up in cardiology and radiation oncology for toxicity surveillance. The complete follow-up schedule is included as supplemental material.
Figure 4.
Ventricular tachycardia events for 6 months before treatment and up to 15 months after. Abbreviation: ICD = implantable cardioverter-defibrillator; StAR-VT = stereotactic arrhythmia radioablation for ventricular tachycardia; VT = ventricular tachycardia.
Case 1
After treatment, this patient experienced mild esophagitis, which was treated with pantoprazole and was self-limited. Two days after treatment the patient had 3 episodes of asymptomatic VT with additional intermittent asymptomatic episodes until 7 months posttreatment when an ICD shock was delivered. Ten months after treatment, the patient had VT storm and was admitted to the hospital for an acute pneumonia which was successfully treated. There has been one recorded VT event since and no other ICD shocks. He remains on the same dose of amiodarone.
Case 2
After treatment, this patient experienced mild self-limited presyncopal symptoms for 1 hour and has no documented episodes of VT since treatment. His amiodarone is currently weaned to 100-mg/d.
Discussion
We present our experiences with 2 patients treated with StAR-VT at the McGill University Health Centre. Cases 1 and 2 have been followed-up for 17 months and 12 months, respectively since treatment.
Case 2 remains VT free and has been weaned to 100 mg/d of amiodarone. Case 1 had a relapsing event 10-months after treatment thought to be provoked by an acute pneumonia. In case 1, part of the relevant substrate was not irradiated. Given the large PTV and esophagus safety concerns, the relapsing event could be related to a “geographic miss” of this region. The consequence of suboptimal coverage and subsequent relapse has also been reported by Gianni et al12 in 2 out of 5 patients treated. Despite suboptimal coverage with the prescription dose the minimum threshold dose for response has not been established, and positive results have been reported with lower doses than what was prescribed here.8 Neither patient has exhibited any significant acute or late radiation-related side effects.
Conclusions
The effects of extensive radiation to cardiac substructures is not well understood, and there are no well-established constraints for most. In our present experience, we have yet to encounter any radiation-related side effects to these substructures; however, it is early, with only 12-month follow-up to make any strong conclusions about this.
Despite increasing experience in the literature, there are no established criteria to predict success for this treatment, making it difficult to identify optimal selection criteria for StAR-VT, a treatment that can only be delivered once. The mechanism of action for success remains unclear as the marked clinical improvement seems to occur before the formation of new fibrotic tissue in the area of irradiation.19 The present limited evidence suggests that StAR-VT is effective for treatment-resistant VT and often provides an immediate reduction in disease burden. Safety remains a prominent question despite mild adverse events and rare severe (gastropericardial fistula, bleeding) adverse events8,20 (10.5% rate of severe events including pericardial effusion and CHF exacerbations19). Owing to the stochastic nature of radiation-induced damage, and limited safety and efficacy data, it is not possible to assume StAR-VT is completely safe and it should not be performed outside of tailored clinical studies for treatment-resistant disease.
Footnotes
Sources of support: The authors received no financial support for the research, authorship, or publication of this article.
Disclosures: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or nonfinancial interest in the subject matter or materials discussed in this manuscript.
All data requests should be submitted to the corresponding author for consideration. Access to anonymized data may be granted after review.
References
- 1.AlKalbani A, AlRawahi N. Management of monomorphic ventricular tachycardia electrical storm in structural heart disease. J Saudi Heart Assoc. 2019;31:135–144. doi: 10.1016/j.jsha.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RD, Ariyarathna N, Lee G. Catheter ablation versus medical therapy for treatment of ventricular tachycardia associated with structural heart disease: Systematic review and meta-analysis of randomized controlled trials and comparison with observational studies. Heart Rhythm. 2019;16:1484–1491. doi: 10.1016/j.hrthm.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Hohnloser SH, Kuck KH, Dorian P. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 4.Aziz Z, Tung R. Novel mapping strategies for ventricular tachycardia ablation. Curr Treat Options Cardiovasc Med. 2018;20:34. doi: 10.1007/s11936-018-0615-1. [DOI] [PubMed] [Google Scholar]
- 5.Loo BW, Soltys SG, Wang L. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–750. doi: 10.1161/CIRCEP.115.002765. [DOI] [PubMed] [Google Scholar]
- 6.Cuculich PS, Schill MR, Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:24. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson CG, Samson PP, Moore KMS. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. doi: 10.1161/CIRCULATIONAHA.118.038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Ree MH, Blanck O, Limpens J. Cardiac radioablation: A systematic review. Heart Rhythm. 2020;17:1381–1392. doi: 10.1016/j.hrthm.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Krug D, Blanck O, Demming T. Stereotactic body radiotherapy for ventricular tachycardia (cardiac radiosurgery): First-in-patient treatment in Germany. Strahlenther Onkol. 2020;196:23–30. doi: 10.1007/s00066-019-01530-w. [DOI] [PubMed] [Google Scholar]
- 10.Mayinger M, Kovacs B, Tanadini-Lang S. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother Oncol. 2020;152:203–207. doi: 10.1016/j.radonc.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Choi Y. Stereotactic cardiac radiation to control ventricular tachycardia and fibrillation storm in a patient with apical hypertrophic cardiomyopathy at burnout stage: Case report. J Korean Med Sci. 2020;35:27. doi: 10.3346/jkms.2020.35.e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianni C, Rivera D, Burkhardt JD. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020:1241–1248. doi: 10.1016/j.hrthm.2020.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Sapp JL, Wells GA, Parkash R. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 15.Duane F, Aznar MC, Bartlett F. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122:416–422. doi: 10.1016/j.radonc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilke L, Andratschke N, Blanck O. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams: Statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther Onkol. 2019;195:193–198. doi: 10.1007/s00066-018-1416-x. [DOI] [PubMed] [Google Scholar]
- 17.Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12:267–292. doi: 10.1120/jacmp.v12i2.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna GG, Murray L, Patel R. UK Consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol (R Coll Radiol) 2018;30:5–14. doi: 10.1016/j.clon.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Elliott J, Linder K, Nolan MW. Feasibility study evaluating arrhythmogenesis and cardiac damage after heart-base irradiation in mice: A brief communication. Vet Med Sci. 2020;6:1009–1016. doi: 10.1002/vms3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson C. Longer term results from a phase I/II study of EP-guided Noninvasive Cardiac Radioablation for Treatment of Ventricular Tachycardia (ENCORE-VT) Int J Radiat Oncol Biol Phys. 2019;105:682. [Google Scholar]