Abstract
Anti-quorum sensing (QS) or quorum quenching (QQ) is known as a new anti-bacterial strategy to combat bacterial infection. One of the best candidates for this strategy is a natural plant or traditional herbal medicine. This review aimed to summarize and introduce Iranian medicinal plants with anti-QS properties. Biomedical databases (PubMed, Scopus, Google Scholar and Web of sciences) were investigated to retrieve all related manuscripts published in English and Persian. Out of 65 documents, 47 papers were published during 2010–2020. We categorized and summarized 19 papers that particularly presented the anti-QS activity of Iranian medicinal plants. Based on our results, different studies have been completed on the QQ effects of medicinal plants. We identified 106 plant species with different properties in medicine that have been evaluated for anti-QS activities in Iran. The QQ effects of herbal extracts were identified through different in vitro examinations on biosensor and clinical bacterial strains. Only 35 medicinal plants have shown these effects at sub-MICs. Our review summarizes Iranian medicinal plants with anti-QS properties. Some of these herbal extracts showed anti-QS activity against biosensors, standard and clinical bacterial strains. This result is very important because QS systems can be considered as a new target for the development of new remedial strategies and it is a good opportunity to perform QQ studies to effectively combat bacterial infections in the future.
Keywords: Anti-quorum sensing, bacterial signaling, in-vitro assays, Iran, medicinal plants, new remedial, quorum quenching
Introduction
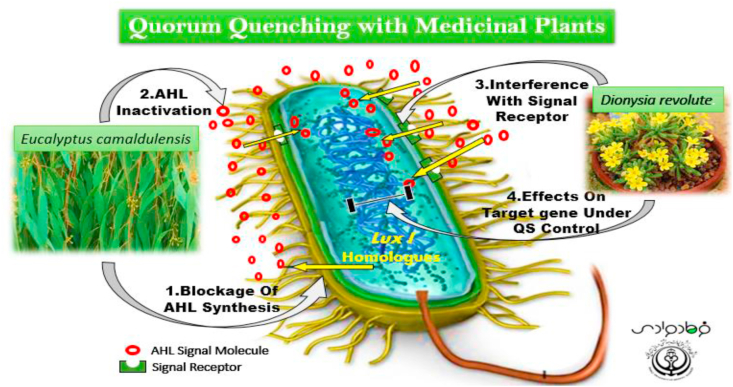
Controlling vital functions for different types of bacterial communication, such as biofilm formation or expression of virulence factor genes, relies on the quorum-sensing (QS) signalling system. It would be attractive to determine or design new powerful agents to interfere with these signalling systems to develop antibacterial drugs and new antimicrobial therapies to control bacterial infections [1]. This novel inhibition in bacterial pathogens is popularly known as quorum quenching (QQ) and the agents with this action are named quorum quenchers. This process is a hot topic in microbiology because the quorum quenchers are not related to expansion of bacterial resistance and they inhibit the vital activity of bacterial cells in a unique population. Quorum quencher compounds have been isolated from various natural sources, such as many eukaryotes, different microorganisms, fruits, vegetables, and natural plants [1,2]. One of the best candidates to combat bacterial pathogens, especially through inhibiting the QS system, is traditional herbal medicine or natural plants. The QS systems in Gram-negative or Gram-positive bacterial populations produce and diffuse signal molecules such as acyl-homoserine lactones around the bacterial cells; identification of these signals through specific receptors occurs at high concentrations during population growth, and increases the transcription of target gene under QS system control. So, the medicinal plant compounds act as an active quorum quencher because they disrupt these pathways by interfering with signal molecule synthesis, inactivating signals by destroying them, interfering with signal receptors in bacterial cells, and blocking target genes under QS controls (Fig. 1) [3,4]. This new approach is also appropriate to combat bacterial infections with high resistance to chemical antibiotics and it may help to design new drug, based on herbal medicines. More than 4500 species of plants have been grown from all over the world that are used for medicinal purposes. Research in various countries shows the QQ activity of the extracts of many traditional medicinal plants with numerous secondary metabolites (e.g. essential oils, alkaloids, saponins, flavonoids, tannins) and antimicrobial compounds (e.g. phenolics, quinones, flavanones and alkaloids) [[1], [2], [3], [4]]. Traditionally, medicinal plants and their extracts have been the main source of medicines and in some countries they are used in pharmaceutical industries, to increase the safety of food systems, in veterinary medicine and in the treatment of different illnesses to promote the health of human society. Many people in different countries still rely on traditional herbal medicines for the treatment of many infectious diseases, so changing the focus from antibacterial studies to evaluation of the anti-QS activity of medicinal plants could expose new QQ agents to control many bacterial infections [3,5,6]. Because traditional medicinal plants with antibacterial properties have played an important role in therapeutic purposes and treating bacterial infections in the Middle East, especially in Iran, we decided to perform this brief review to provide a current overview of the traditional and endemic medicinal plants with anti-QS properties in Iran. As the medicinal plant extracts with anti-QS activities could be considered as a novel anti-pathogenic drug, several studies have been completed in Iran to identify the QQ activity of different traditional medicine plant species with antibacterial properties. In this review, we summarize this information about herbal extracts that act as a quorum quenchers in Iran.
Fig. 1.
Schematic of quorum-quenching strategies in a bacterial cell through traditional herbal medicines.
Materials and methods
Search strategy
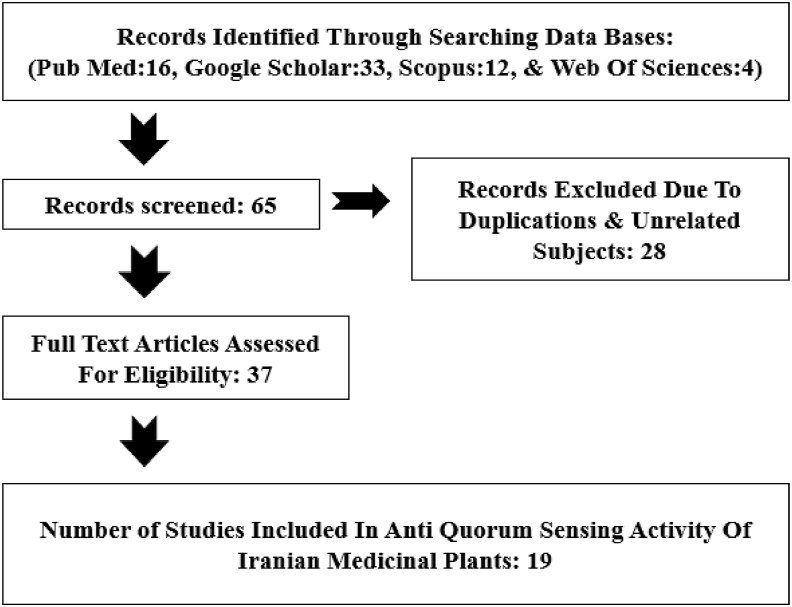
Biomedical databases (PubMed, Scopus, Google Scholar and Web of sciences) were searched to retrieve all related manuscripts published in English and Persian from January 2010 to January 2021. We used the terms ‘Quorum sensing AND medicine plant AND Iran’, ‘anti-quorum sensing AND traditionally herbal medicine AND Iran’, ‘Quorum Quenching AND medicine plant AND Iran’, ‘medicine plant OR herbal medicine AND quorum sensing AND Iran’, ‘anti-quorum sensing OR Quorum Quenching AND plant products AND Iran’. Also, references cited within these articles were implemented to find additional relevant articles. Out of 65 papers, 47 papers were identified that were published between 2010 and 2021 (Fig 2).
Fig. 2.
Flow chart of study.
Inclusion and exclusion criteria
We selected published literature about Iranian medicinal plants with anti-QS properties in the years between 2010 and 2020. Medicinal plants with QQ effects that have grown endemic or non-endemic in a different region of Iran were selected. We considered all of the herbal extracts with phenotypic or molecular QQ effects on the QS system of different bacterial biosensor strains, or bacterial pathogens isolated from clinical specimens in Iran. For this reason, we collected studies that were related to the anti-QS system activity of herbal extracts, for example, anti-biofilm activity. Articles that only provided other effects of Iranian medicine plants such as antioxidant, anticancer, anti-inflammation and some others were excluded. Also, studies on QQ effects of other agents, such as a different form of vitamins, metal complexes (e.g. copper, iron, curcumin, zinc), many microorganisms (quorum quenchers isolated from bacterial strains) and herbal medicine in other countries, as weel as duplicate documents were excluded.
Data extraction and analyses
We extracted and categorized all of the information about medicinal plants evaluated for QQ activity in Iran including scientific name, part of plant used for anti-QS examination, type of extraction and products used (ethanol extract, methanol extract, essential oil), bacterial strains for QQ assays (bacterial biosensor strain or isolated from clinical specimens), plant species with positive anti-QS actions or other effects related to QQ such as antibiofilm activity, type of QQ effect on different strain (phenotypic or molecular), the purpose of studies and application of herbal extracts that authors recommended. Statistical analyses were completed, using Microsoft excel 2019 for numerical calculations.
Results
Based on our results, different studies have been performed on the QQ effects of medicinal plants in Iran. We identified 106 plant species with different effects in medicine, which have been evaluated for anti-QS properties. These medicinal plants were commonly used for different treatment purposes in Iran. Only 35 species of these medicinal plants have shown QQ effects at sub-MICs. These effects are acceptable because at this concentration, the microbial population is stable, bacterial species are alive, and they carry out signal exchange using QS systems. These studies evaluated the QQ activity of herbal extracts on biosensor strains (e.g. Chromobacterium violaceum wild type or CV026, Pectobacterium carotovorum, Agrobacterium tumefaciens NTL/PZLR4, Pseudomonas aeruginosa PAO1 wild type or PAO1 (MH873), and Aeromonas veronii Sobria strain BC88) or different bacterial species isolated from clinical specimens such as Streptococcus pneumoniae, P. aeruginosa, Staphylococcus aureus, Salmonella typhi and Salmonella Typhimurium. These anti-QS effects have been studied phenotypically (e.g. repress violacein production in C. violaceum, prevent biofilm formation by P. aeruginosa or Staphylococcus aureus, reduce pyocyanin, protease and elastase production in P. aeruginosa) and molecularly such as down-regulation of QS genes (sdiA and luxS in Salmonella Typhimurium, LuxS and pfs in Streptococcus pneumoniae, or lasI and lasR in P. aeruginosa through different methods. For example, real-time PCR method for gene expression assay, microtitre plate assay for anti-biofilm activity, different bacteriological or innovative methods (e.g. use of soft top agar contained Luria–Bertani (LB) agar and semi-solid LB with biosensor strains, melted LB agar with biosensor strains, moulten semi-solid LB agar and signal molecules, and LB soft agar with herbal extracts) for in vitro QQ assay of herbal extracts. Plant materials were obtained from natural areas in different regions of Iran or purchased from reputable companies, for instance Bonyan Salamat Kasra Co. (Tehran, Iran). Herbal medicines were extracted using polar and non-polar solvents (e.g. acetone, methanol, ethanol, n-hexane) or preparing essential oils by different methods (includeing Soxhlet, maceration, digestion and superficial extraction). Some of the studies reported the QQ activity of some herbal extracts at high concentrations and researchers used different solvents to maximally extract polar and dipolar compounds from these medicinal plants instead of using alcohol or water extraction, which just extracts some of the bioactive compounds. It may for this reason that medicinal plants consist of many different compounds. A few studies used different chromatography methods, such as thin-layer and gas chromatography, to identify the bioactive compounds of herbal medicines. In some studies, some of the plant extracts with antibacterial properties showed QQ effects. Researchers have new applications for these herbal extracts with anti-QS properties. For example, treatment of chronic infections caused by bacterial pathogens by permitting the human immune system to eradicate the pathogens, designing the new anti-biofilm agents based on herbal medicine compounds, using these plant compounds as alternatives to antibiotics for control and treatment of infections caused by multidrug-resistant bacterial strains, and developing herbal drugs such as mouthwashes against dental bacterial infection. All of this information is provided in Table1.
Table 1.
Anti-quorum sensing studies of traditional medicinal plants in Iran
| First authors, publication year [reference] | Plant species evaluated (part tested) | Products | Strains tested | Plant species with anti-quorum-sensing properties | Quorum-quenching effects | Other effects and authors comments |
|---|---|---|---|---|---|---|
| Mahmoudi E et al., 2014 [16] | Pelargonium hortorum, Triticum aestivum, Lycopersicum esculentum, Hordeum vulgare, Cucumis sativus, Oryza sativa, Mentha spp., Allium sativum, Zea mays, Onobrychis sativa, Coriandrum sativum, Capsicum annuum, Allium porrum, Foeniculum vulgare, Beta vulgare, Olea europaea, Sage Salvia officinalis, Petroselinum crispum, Phaseolus vulgare, Lentis sativa, Cicer arietinum, Pisum sativum, Ocimum basilicum, Vicia radiata, Raphanus sativus, Phaseolus vulgare, Allium ampeloprasum, Anethum graveolens, Althea officinalis, Abelmoschus esculentus, Foeniculum vulgare, Solanum melongena, Medicago sativa, Capsicum annuum, Amaranthus blitum, Zataria multiflora, Artemisia dracunculus, Apium graveolens, Trifolium repens, Satureja hortensis, Cucumis melo, Thymus sp., Solanum tuberosum | Methanolic extract of 44 medicine plant species |
Chromobacterium violaceum CV026, Pectobacterium carotovorum subsp. Carotovorum |
Althea officinali, Artemisia dracunculus, Raphanus sativus | Repress violacein production in CV026, inhibit QS-regulated virulence in Pectobacterium on potato tubers, contains AHL-mimicking molecules that can activate QS function in Trifolium repens | Plants have quorum sensing-mimicking signals that could potentially be used for disrupting quorum sensing |
| Makhfian M et al., 2015 [5] | Artemisia dracunculus, Coriandrum sativum, Trigonella foenum, Satureja hortensis, Tagetes minuta, Ocimum basilicum, Descurainia sophi, Spinacia oleracea, Cicer arietinum, Medicago sativa, Cardaria draba, Equisetum arvense, Anethum graveolens, Sophora secundiflora, Trifolium spp., Hordeum morinum,Solanum tuberosum, Cyclamen hederifolium, Lactuca sativa, Phleum spp, Zea mays, Centaurea cyanus, Allium ampeloprasum, Triticum spp, Daucus carota, Allium sativum, Capsicum frutescens, Apium graveolens, Abelmoschus esculentus, Phasaeolus vulgaris, Mentha piperata | Ethanol extracts of 31 medicine plant species |
C. violaceum CV026, P. carotovorum subsp. carotovorum strain 116B |
Trigonella foenum, Cardaria draba, Equisetum arvense, Anethum graveolens, Phleum spp., Allium ampeloprasum, Capsicum frutescens | Inhibition of violacein pigmentation in C. violaceum CV026 and reduction in tissue maceration in test plants | These plants can mimic quorum-sensing signals (especially Eugenol as major component in Anethum graveolens) |
| Sepahi E et al., 2015 [17] | Ferula (Ferula asafoetida), Dorema (Dorema aucheri) | Essential oils | Pseudomonas aeruginosa PA01, C. violaceum CV026 | Ferula asafetida, Dorema aucheri. | Reduced the violacein in C. violaceum, Pyocyanin, pyoverdine, elastase and biofilm production and decrease QS-dependent genes in P. aeruginosa. But Dorema oil could not show anti-biofilm effects | These plants as novel QS and virulence inhibitors. |
| Korkorian N et al., 2017 [18] | Rumex alveolatus (leaves and roots) | Aqueous and methanol extracts | P. aeruginosa, Staphylococcus aureus | Rumex alveolatus | Prevented biofilm formation by P. aeruginosa and S. aureus, reduced pyocyanin production in P. aeruginosa. Main phenolic compound was 1,2-benzene dicarboxylic acid | Aqueous extracts of R. alveolates had not antibacterial and anti-QS activity. |
| Kordbacheh H et al., 2017 [19] | Pistacia atlantica (leaf) | Methanolic extract | P. aeruginosa PAO1 | Pistacia atlantica | Inhibition of biofilm and pyocyanin and LasR protein with active compounds (myricetin, 3-O-rutinoside, kaempferol-3-O-rutinoside) | Treatment of infections caused by P. aeruginosa |
| Mohabi S et al., 2017 [1] | Quercus infectori (galls) | Methanol extract | Five strains of P. aeruginosa (ATCC 27853, PAO1 wild, PAO1 (MH873), MS.PS.50/35, and PDO 300 (mucA2e, a hyper alginate producer)), C. violaceum CV026 |
Quercus infectori | Decreasing the biofilm formation, level of protease LasA, LasB, swarming and twitching motility & lasR gene expression in P. aeruginosa strains. Inhibited the QS in C. violaceum CV026 |
Antibacterial effects & best candidate for alternative treatment of pseudomonad infections in future. |
| Karbasizade V et al., 2017 [20] | Quercus infectoria, Zataria multiflora, Trachyspermum copticum | Acetone extract of Q. infectoria and methanol extracts of Z. multiflora and T. copticum | P. aeruginosa strain (PTCC 1430) | Quercus infectoria, Zataria multiflora, Trachyspermum copticum | Inhibition of pyocyanin, protease, elastase production and biofilm formation | These herbs can be used as antipathogenic drugs |
| Sharifi A et al., 2018 [21] | Thymus daenensis, Satureja hortensis, Origanum vulgare. | Essential oils | S. pneumoniae | Thymus daenensis, Satureja hortensis, Origanum vulgare. | Anti-biofilm activity, down-regulated LuxS and pfs (QS genes) by thymol, carvacrol, p-cymene, pulegone and 1,8-cineole | Introduced as new anti-biofilm and QS inhibitor agents |
| Jamalifar H et al., 2019 [22] | Green coffee beans powder (Coffee arabica L.) | Dissolved in boiling distilled water |
P. aeruginosa (ATCC 15449), P. aeruginosa strains were isolated from clinical samples |
(Coffee arabica L.) | Pathogenesis-related genes, lasI and lasR in P. aeruginosa are down-regulated through chlorogenic acid | Could be useful as an adjuvant & effective inhibition and eradication of P. aeruginosa |
| Pishgar E et al., 2019 [23] | Rubus ulmifolius (Raspberry blossom), Artemisia dracunculus (leaves), Centaurea cyanu (flower), Descurainia sophia (leaves and flower) | Ethanol extract |
Agrobacterium tumefaciens NTL/PZLR4. S. aureus were isolated from patients with dental implant infection |
Rubus ulmifolius, | Showed the QQ effects on biosensor strain by producing the blue colony and without hydrolysis of X-gal, anti-biofilm effects by microtitre plate (MTP) assay | Rubus ulmifolius can be considered as a mouthwash against dental bacterial infection, other plant species just show Antibacterial effects |
| Arjmandi A et al., 2020 [24] | Citrus limon (L.) (lemon peel) | Essential oil | P. aeruginosa Biosensor strain | Citrus limon (L.) | Inhibition of biofilm with essential oil ligands geranyl acetate, α-terpineol and β-bisabolene | It can be considered as a source of anti-biofilm and antimicrobial formulation. |
| Sharchi R et al., 2020 [25] | Satureja sahendica (flower) | Hydroalcoholic extract | Salmonella Typhimurium isolated from avian | Satureja sahendica | Decrease the expression of QS-associated gene (sdiA) | Antibacterial effects and it can be used to control the expression of virulence genes in S. Typhimurium. |
| Moradi F et al., 2020 [2] | Syzygium aromaticum (Stem), Dionysia revoluta (whole plant), Eucalyptus camaldulensis (leaves) | n-hexane, methanol, 96% ethanol mixed solvent |
C. violaceum CV026, Aeromonas veronii bv. Sobria strain BC88, P. aeruginosa isolated from a patient with cystic fibrosis |
Syzygium aromaticum Dionysia revoluta Eucalyptus camaldulensis |
Anti-QS activities by reducing the violacein formation and depletion of QS signals produced in A. veronii and P. aeruginosa | Antibacterial effects, and depleting the signalling system in bacterial clonality, thus permitting the immune system to eradicate the infection |
| Ghaderi L et al., 2020 [9] | Lavandula angustifolia, Rosmarinus officinalis, Satureja khuzistanica | Essential oil nanoemulsions, carvacrol and 1,8-cineol) | P. aeruginosa PAO1 | Satureja khuzistanica, carvacrol and 1,8-cineol | Eradication of PAO1 biofilm and decrease pyocyanin production | Nanoemulsions could improve the antibacterial and antibiofilm activity of essential oil |
| Hakimi Alni R et al., 2020 [26] |
Allium sativum Cuminum cyminum |
Essential oils | Salmonella Typhimurium isolates |
Allium sativum Cuminum cyminum |
Down-regulated of QS (sdiA and luxS) and cellulose synthesis (csgD and adrA) genes and reduced the S. Typhimurium biofilm. | Antibiofilm effects without cytotoxic effect on the eukaryotic Vero cells with terpineol, carene and pinene in C. cyminum and sulfur in A. sativum |
| Fekrirad Z et al., 2020 [27] | Eugenol (the major clove extract) | Prepared by adding dimethyl sulfoxide | Serratia marcescens ATCC 13880 and S. marcescens Sm2 (isolates from clinical sample) | Eugenol (4-allyl-2 methoxyphenol) | Decrease the biofilm formation, haemolysin, protease, swarming, motility pigment formation and expression of genes involved in motility (flhD), attachment (fimC), biofilm formation (bsmB, bsmA), and QS regulatory (swrR) in S. marcescens | Anti-QS and anti-biofilm effects against S. marcescens strains. |
| Tanhay Mangoudehi H et al., 2020 [28] | Curcuma longa | Curcumin, component | Aeromonas hydrophila strains isolated from fish | Curcuma longa | Attenuate QS regulating genes (ahyI/R) and several QS-associated phenotypes (biofilm, swarming, proteolytic, haemolytic activity) | It can be used as an anti-QS agent, to be used in aquaculture. |
| Hosseinzadeh S et al., 2020 [29] | Licochalcone A, epigallocatechin-3-gallate | Stock solution was made in dimethyl sulphoxide | S. Typhimurium RITCC1730, 23 clinical isolates of S. typhimurium from poultry flocks |
Licochalcone A, epigallocatechin-3-gallate | Anti-QS activity with down-regulation of both sdiA and luxS genes in S. typhimurium | Use of these plant for anti-QS based prophylactic/therapeutic against salmonellosis |
| Mohammadi Pelarti S et al., 2021 [30] | Artemisia dracunculus | Essential oil | Salmonella enterica serovar Typhimurium, Staphylococcus aureus | Artemisia dracunculus | Anti-biofilm activity and down-regulation of luxS, pfs and hld genes by estragole as a main compound | Use of this plant compounds as alternatives to antibiotics. |
Abbreviations: QQ, quorum-sensing; QS, quorum-quenching.
Discussion
Today, an effective treatment system and development of new antibacterial drugs to control bacterial infections are very important, because of the appearance of new resistance genes in many pathogenic bacteria and the spread of antibiotic resistance are increasing. Interfering with the bacterial communication systems and inhibition of signal exchange in microbial populations through QQ activity of medicinal plants can be recommended as a new treatment in microbiology. Following this inhibition, the ability of bacterial species to control and express virulence genes for invasive actions decreases.
Some of the studies documented the adverse effects of herbal products caused by toxic constituents (e.g. allergens, pollen, spores, toxins or carcinogenic compounds), which can produce negative effects such as allergic reactions, rashes, asthma, headaches, nausea, vomiting, irritability, increased blood pressure, heart rhythm disorders and diarrhoea. Nephrotoxicity from aristolochic acid and other components of herbal medicine can cause adverse renal effects and pyrrolizidine-alkaloid-containing medicine plants can be hepatotoxic. Most serious adverse effects originate from overuse or misuse of such herbal medicines [7,8].
Although these studies described adverse effects for medicinal plants, they also reported that traditional herbal medicine can act as a powerful quorum quencher by inhibiting the QS pathways in bacterial cells. Unlike other chemical and synthetic antibiotics, bacterial species do not show resistance to these natural compounds and they will lose their resistance to the human immune system. Resistance is increasing in different parts of the world and the use of these herbal medicines may help to decrease the production and consumption of chemical drugs, resulting in the human body being protected from the adverse effects of chemical drugs.
Based on the different climates present, a wide variety of medicinal plants are grown in Middle Eastern countries, including Iran. Iran is an ancient country with 7500–8000 plant species that have been used as medicinal plants in medicine and the treatment of microbial infections. Many of these medicinal plants are endemic in various natural places, including mountainous areas. Some species are not specific to Iran and they also grow in other countries [7,9].
Numerous studies have show the medical application of Iranian medicinal plants. For example, more than 20 species of Scutellaria (skullcaps) are used as an anti-arteriosclerosis, anti-allergy, antimicrobial and antiviral drug and 13 species of Stachys (Lamiaceae) are endemic and are used for their antitoxic, anti-anoxic and antiseptic effects. Other herbal medicines such as Achillea kellalensis Bioss (for treatment of wounds and skin infections), Tanacetum polycephalum Schultz or Artemisia kermanensis (as anti-Candida agents) and Zataria multiflora ((Latiatae family; used for pain-relief) are used in Iranian medicines [[10], [11], [12]].
Since QS and interfering with these communication systems in bacterial species were introduced as a hot topic in microbiology, many researchers in Iran have been attracted to investigate this field and have studied the anti-QS effects of various Iranian medicinal plants. These medicinal plants with active compounds were used as antioxidant, antimicrobial and antifungal agents to develop new antimicrobial drugs, for the treatment of various diseases, and for the inhibition of pathogenic bacteria with antibiotic resistance [[13], [14], [15]]. In this review, we have tried to summarize and introduce Iranian medicinal plants with anti-QS properties. These medicinal plants have been used for treatment of infectious disease in Iran. Some of these herbal extracts showed anti-QS activity against biosensors, standard and clinical bacterial strains. This result is very important because the QS systems can be considered a new target for the development of new remedial strategies and it is a good opportunity to develop QQ studies to effectively fight bacterial infections in the future.
In several types of research on the QQ activity of medicinal plants, researchers have made different suggestions for future QQ studies; for example, improving QQ studies to recognize all medicinal plants with anti-QS properties and evaluation of these studies in both in vitro and in vivo conditions. Additionally, because the effective compounds of some medicinal plants with QQ activity have not been identified, it is essential to identify these bioactive agents to expand and develop the anti-QS research. Many anti-QS examinations were completed on biosensor strains or standard species and it is better to develop this research on different bacterial species isolated from clinical specimens or polymicrobial biofilms. Furthermore, defining minimal QS inhibitory concentration (MQSIC) and the MQSIC: MIC ratio for different herbal extracts would be useful when discussing the respective efficacy of these plant species.
In conclusion, empirical methods in QS studies to discover new quorum quenchers and their use as a novel treatment agent in medical microbiology should be developed, especially for traditional herbal extracts worldwide.
Author contributions
Both authors were responsible for conceptualization, methodology, data curation, writing, reviewing and editing.
Conflict of interest
The authors declare that there are no conflicts of interest.
Data availability
We thanks Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences (SUMS) for scientific assistance in carrying out this study.
References
- 1.Mohabi S., Kalantar-Neyestanaki D., Mansouri S. Inhibition of quorum sensing–controlled virulence factor production in Pseudomonas aeruginosa by Quercus infectoria gall extracts. Iran J Microbiol. 2017;9:26. [PMC free article] [PubMed] [Google Scholar]
- 2.Moradi F., Hadi N., Bazargani A. Evaluation of quorum-sensing inhibitory effects of extracts of three traditional medicine plants with known antibacterial properties. New Microbe New Infect. 2020;38:100769. doi: 10.1016/j.nmni.2020.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aparna Y., Sarada J. Quorum quenchers – past, present and future of this novel therapeutics world. J Pharm Life Sci. 2015;1:108–128. [Google Scholar]
- 4.Bouyahya A., Dakka N., Et-Touys A., Abrini J., Bakri Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac J Trop Med. 2017;10:729–743. doi: 10.1016/j.apjtm.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Makhfian M., Hassanzadeh N., Mahmoudi E., Zandyavari N. Anti-quorum Sensing effects of ethanolic crude extract of Anethum graveolens L. J Essent Oil-Bearing Plants. 2015;18:687–696. [Google Scholar]
- 6.Sharafzadeh S., Alizadeh O. Some medicinal plants cultivated in Iran. J Appl Pharm Sci. 2012;2(1):134–137. [Google Scholar]
- 7.George P. Concerns regarding the safety and toxicity of medicinal plants – an overview. J Appl Pharm Sci. 2011;1(6):40–44. [Google Scholar]
- 8.Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012;1:44. doi: 10.4103/2277-9175.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaderi L., Aliahmadi A., Nejad Ebrahimi S., Rafati H. Effective Inhibition and eradication of Pseudomonas aeruginosa biofilms by Satureja khuzistanica essential oil nanoemulsion. J Drug Deliv Sci Technol. 2020;102260 doi: 10.1016/j.jddst.2020.102260. [DOI] [Google Scholar]
- 10.Rezazadeh Sh, Pirali-Hamedani M., Hadjiakhondi A., Ajani Y., Yarigar-Ravesh M., Shafiee A. Chemical composition of the essential oils of Stachys atherocalyx and S. sylvatica from Iran. Chem Nat Compounds. 2009;45:742–745. [Google Scholar]
- 11.Ghannadi A., Mehregan I. Essential oil of one of the Iranian skullcaps. Z Naturforsch. 2003;58c:316–318. doi: 10.1515/znc-2003-5-604. [DOI] [PubMed] [Google Scholar]
- 12.Ghasemi Pirbalouti A., Bahmani M., Avijgan M. Anti-Candida activity of some of the Iranian medicinal plants. Electron. J Biol. 2009;5:85–88. [Google Scholar]
- 13.Nikbakht A., Kafi M., Haghighi M. The abilities and potentials of medicinal plants production and herbal medicine in Iran. Acta Horticult. 2008;790:259–262. [Google Scholar]
- 14.Fallah-Hoseini H., Fakhrzadeh H., Larijani B., Shikhsamani A. Review of anti-diabetic medicinal plant used in traditional medicine. J Med Plant. 2006;5:1–8. [Google Scholar]
- 15.Azizi M.H. Gondishapur School of Medicine: the most important medical center in antiquity. Arch Iranian Med. 2008;11:116–119. [PubMed] [Google Scholar]
- 16.Mahmoudi E., Tarzaban S., Khodaygan P. Dual behaviour of plants against bacterial quorum sensing: inhibition or excitation. J Plant Pathol. 2014;96:295–301. [Google Scholar]
- 17.Sepahi E., Tarighi S., Ahmadi F.S., Bagheri A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J Microbiol. 2015;53:176–180. doi: 10.1007/s12275-015-4203-8. [DOI] [PubMed] [Google Scholar]
- 18.Korkorian N., Mohammadi-Sichani M. Anti-quorum sensing and antibacterial activity of Rumex alveolatus. Zahedan J Res Med Sci. 2017;19 [Google Scholar]
- 19.Kordbacheh H., Eftekhar F., Ebrahimi S.N. Anti-quorum sensing activity of Pistacia atlantica against Pseudomonas aeruginosa PAO1 and identification of its bioactive compounds. Microb Pathog. 2017;110:390–398. doi: 10.1016/j.micpath.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Karbasizade V., Dehghan P., Sichani M.M., Shahanipoor K., Jafari R., Yousefian R. Evaluation of three plant extracts against biofilm formation and expression of quorum sensing regulated virulence factors in Pseudomonas aeruginosa. Pak J Pharm Sci. 2017;30(2 Suppl. l):585–589. [PubMed] [Google Scholar]
- 21.Sharifi A., Ahmadi A., Mohammadzadeh A. Streptococcus pneumoniae quorum sensing and biofilm formation are affected by Thymus daenensis, Satureja hortensis, and Origanum vulgare essential oils. Acta Microbiol Immunol Hung. 2018;65:345–359. doi: 10.1556/030.65.2018.013. [DOI] [PubMed] [Google Scholar]
- 22.Jamalifar H., Samadi S., Jamileh Nowroozi J., Dezfulian M., Fazeli M.R. Down-regulatory effects of green coffee extract on las I and las R virulence-associated genes in Pseudomonas aeruginosa. DARU J Pharm Sci. 2019;27:35–42. doi: 10.1007/s40199-018-0234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pishgar E., Makhfian M. The evaluation of five plant extracts inhibitory potential against bacterial quorum sensing of Staphylococcus aureus. J Arak Uni Med Sci. 2019;22:11–21. [Google Scholar]
- 24.Arjmandi A., Chehri K., Karimi I. Effects of Citrus limon (L.) essential oil on Pseudomonas aeruginosa: complemented with a computational approach; focus on quorum sensing. Int J Mol Clin Microbiol. 2020;10:1258–1274. [Google Scholar]
- 25.Sharchi R., Shayegh J., Hosseinzadeh S. Anti-quorum sensing and antibacterial activities of Satureja sahendica hydroalcoholic extract against avian isolate of Salmonella Typhimurium. Iran J Vet Sci Technol. 2020;1(22) doi: 10.22067/veterinary.v12i1.85433. [DOI] [Google Scholar]
- 26.Hakimi Alni R., Ghorban K., Dadmanesh M. Combined effects of Allium sativum and Cuminum cyminum essential oils on planktonic and biofilm forms of Salmonella typhimurium isolates. 3 Biotech. 2020;10:315. doi: 10.1007/s13205-020-02286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fekrirad Z., Gattali B., Kashef N. Quorum sensing-regulated functions of Serratia marcescens are reduced by eugenol. Iran J Microbiol. 2020;12:451–459. doi: 10.18502/ijm.v12i5.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanhay Mangoudehi H., Zamani H., Shahangian S.S., Mirzanejad L. Effect of curcumin on the expression of ahyI/R quorum sensing genes and some associated phenotypes in pathogenic Aeromonas hydrophila fish isolates. World J Microbiol Biotechnol. 2020;36:70. doi: 10.1007/s11274-020-02846-x. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh S., Habib Dastmalchi Saei H., Ahmadi M., Zahraei-Salehi T. Anti-quorum sensing effects of licochalcone A and epigallocatechin-3-gallate against Salmonella Typhimurium isolates from poultry sources. Vet Res Forum. 2020;11:273–279. doi: 10.30466/vrf.2019.95102.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi Pelarti S., Karimi Zarehshuran L., Babaeekhou L., Ghane M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): a study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch Microbiol. 2021 doi: 10.1007/s00203-020-02138-w. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We thanks Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences (SUMS) for scientific assistance in carrying out this study.