Abstract
Calcium (Ca) and phosphorus (P) transporters are responsible for their absorption and transport in small intestine and kidney, contributing to eggshell formation. The light-dark cycle is a primary cue in the reproduction of laying hen. In this study, we investigated the effect of different light-dark programs on eggshell quality and the expression of genes related to Ca and P transportation in laying hens. Seventy-two 56-week-old laying hens were randomly divided into two groups and reared at 16-h light and 8-h dark (control) or 9-h light and 15-h dark regime (long dark phase, LDP). The expressions of calcium transporter calbindin-D28k (CaBP-D28k), plasma membrane Ca ATPase 1b (PMCA1b), and phosphorus transporter NaPi-IIb (NPt2b) and NaPi-IIa (NPt2a) were measured in the small intestine, kidney, and eggshell gland. The results showed that feed intake (P < 0.001) and egg weight (P = 0.05) were decreased by LDP treatment. Compared with control, the eggshell hardness was increased (P = 0.011) by LDP treatment, but the eggshell thickness and the percentage of eggshell were not changed. The Ca and P contents in eggshell were increased by LDP treatment. During the scotophase, LDP-hens showed higher serum Ca (P = 0.0056) and P levels (P = 0.079) but lower alkaline phosphatase (ALP) activity than that of control hens. In the duodenum, the relative higher expression of CaBP-D28k and PMCA1b in scotophase compared to photophase was masked by LDP treatment. The expression of CaBP-D28k and osteopontin (OPN) in the eggshell gland were increased by LDP treatment, compared to control hens. In the jejunum, the protein expression levels of CaBP-D28k and PMCA1b decreased during photophase in LDP-hens. The result indicates that the increased blood Ca and P concentration during scotophase by LPD treatment is beneficial to the deposition of Ca and P in the eggshell. The result offers an alternative strategy for managing laying hens with poor eggshell quality.
Key words: light regime, eggshell, calcium, phosphorus, transporter, laying hens
INTRODUCTION
In commercial egg industry, poor eggshell quality causes tremendous economic losses. Eggshell quality is essential for the laying hen in persistent laying cycle and a good layer can produce 500 eggs within 100 wk of age (Bain et al., 2016). When laying hen ages, the eggs laid by the hens before 39 wk of age are markedly different from the eggs laid afterward according to the physical and quality characteristics of the eggs (Sirri et al., 2018). During the process of eggshell formation, insufficient calcium (Ca) deposition is one of the root causes of poor eggshell quality. The challenges in providing an adequate Ca supply are the limited feeding during scotophase (Stonerock et al., 1975) and bone loss in older laying hens (Bustany and Elwinger, 1987; Fleming et al., 1998; Bolukbasi et al., 2005; Jiang et al., 2014).
The light perception and photoperiod are the primary cues in the reproduction of laying hens (Bédécarrats, 2015). Light source (Baxter and Bédécarrats, 2019), light spectrum (Rozenboim et al., 1998; Archer, 2019), light intensity (Renema et al., 2001), and the uniformity of light distribution (Barros et al., 2020) all have an influence on laying performance of hens. When the illumination time increases to 24 h, eggshell quality is improved (Makled and Charles, 1987). Light supplement at night improves the eggshell quality during the late laying stage without significantly reducing egg production (grizzle et al., 1992). In contrast, with a 27-h of light cycle (14 h of light and 13 h of dark), the eggshell weight is elevated by 10%, due to the increased time the egg spent in the eggshell gland during egg formation (Melek et al., 1973).
In avian species, calcium binding protein (CaBP-D28k) has a high affinity for Ca. It plays a key role in the transmission of the calcium signaling system, regulating physiological metabolism and gene expression, controlling the normal growth and development of cells, and playing an important role in calcium transport in the small intestine, kidney and eggshell gland (Wasserman and Taylor, 1966; Corradino et al., 1976; Bar and Hurwitz, 1979; Bar and Hurwitz, 1984; Pasteels et al., 1987). Plasma membrane calcium ATPase (PMCA) is involved in the transport of Ca and transfer Ca through the membrane to the extracellular space. PMCA is mainly expressed in the form of plasma membrane ca-atpase-1b (PMCA1b) in the small intestine, kidney, and eggshell gland of poultry (Melancon and De, 1970; Davis et al., 1987; Qin and Klandorf, 1993; Quinn et al., 2007; Parker et al., 2008). The NaPi-IIb (NPt2b) cotransporter is the main phosphorus transporter in the small intestine of poultry while NaPi-IIa (NPt2a) cotransporter is mainly expressed in the apical membrane of proximal tubular epithelial cells (Murer et al., 2004; Biber et al., 2009). NPt2b is highly expressed in the duodenum of chicken and altered by dietary P levels (Li et al., 2018). Hence, we hypothesized that light regime has an influence on eggshell formation via altering the expression of genes relevant to Ca and P transportation.
In this study, 56-wk-old ISA hens with similar body weights were randomly selected to study the effects of a prolonged dark time on production performance, egg quality and the expression of the Ca and P transporters in the small intestine, kidney, and eggshell gland under a 24 h photo-schedule.
MATERIALS AND METHODS
Animals
Seventy-two 56-week-old ISA hens with similar body weights (2.18 ± 0.15 kg) and laying rates (88.8±1.0%) were selected. The laying hens were randomly divided into two groups and each group had 6 replicates of 6 hens. The two groups of hens were reared in two separate environment-controlled rooms, in which all the rearing facilities were kept the same. The hens were reared in cages with 2 hens per cage (45 × 35 × 35 cm, length × width × height). In each cage, there was a nipple drinker and a feeder (45 cm). The room temperature was controlled at 20 ± 1°C. The basal diet was formulated according to the recommendations of the National Research Council standard (NRC, 1994; Sun et al., 2020). Before the experiment began, all the hens were given a light-dark cycle of 16 h light and 8 h dark and the incandescent lights were used to produce a light intensity of 20 lx at feeder level. When the experiment began, the two groups of laying hens were subjected to one of the two light-dark cycles: a 16 h light and 8 h dark (16 L: 8 D, control group) or a 9 h light and 15 h dark (9 L: 15 D, long dark phase, LDP). The experiment lasted for 8 wks. Throughout the trial, the hens had free access to feed and water. During the experiment, the feed intake, egg number, and egg weight were recorded daily. The laying rate and egg production was calculated.
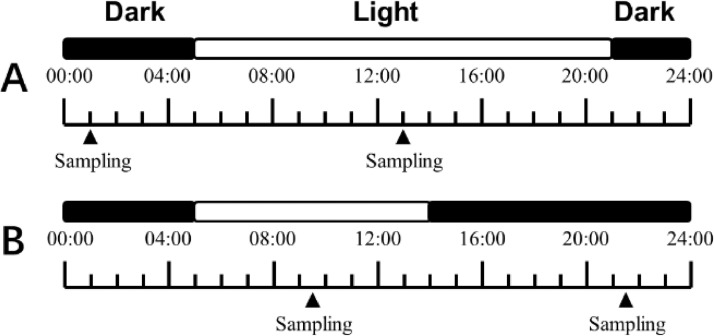
At Week 8, 16 hens were randomly selected from each treatment. One half of the hens were sampled at the midpoint of photophase (control, 13:00; LDP, 09:30) and the other half of hens were sampled at the midpoint of scotophase (Control, 01:00; LDP, 21:30; Fig 1). After a blood sample was obtained from a wing vein, the hens were sacrificed by cervical dislocation (Close et al., 1997; Huang et al., 2015). The tissue samples of eggshell gland, kidney, and mucosa of duodenum, jejunum, and ileum were obtained. The tissue samples were immediately snap-frozen in liquid nitrogen and stored at -80°C for RNA or protein extraction. The serum was separated and stored at -20°C for further measurement. All experimental protocols used in this study were approved by the Animal Care Committee of Shandong Agricultural University.
Figure 1.
The schematic graph of the light regime. 16 L:8 D, control (A), 9 L:15 D, experimental group (B).
Egg Quality
At Week 2, 4, 6, and 8, eggs were collected for three consecutive days in the two treatments. Eggshell thickness was measured with an eggshell Thickness Meter (EFG-0503, ROBOTMATION, Japan) in three regions of the egg: the equator, the sharp pole, and blunted pole, and the mean value was taken as the eggshell thickness. The egg shape index was calculated by long diameter/short diameter. The eggshell strength was measured with an eggshell strength tester (EFG-0503, ROBOTMATION, Japan). The yolk color, albumen height, and Haugh unit were detected by a multifunctional egg detector (EMT-5200, ROBOTMATION, Japan). The percentage of yolk and eggshell was respectively calculated as the yolk weight and eggshell weight/egg weight. The eggs collected at Week 8 were further used for the measurement of Ca and P contents in eggshell. Eggshell Ca and P outputs were calculated as egg mass times eggshell percentage times eggshell Ca and P contents.
Determination of Ca and P in the Diet and Eggshell
Ca content in the feed and eggshells was measured by potassium permanganate titration (Rhee, 1972; Song et al., 2020). The P content in the feed and eggshell was measured by a spectrophotometer at 420 nm (UV-2450; Shimadzu Corp., Kyoto, Japan).
Serum Ca, P, and ALP Analysis
The serum contents of Ca and P and activity of ALP were detected by commercial kits (Sichuan Mike Biotechnology Co., Ltd., China, Wang et al., 2018) with an automatic biochemical analyzer (7020 Clinical Analyzer: Hitachi High-Tech GLOBAL, Japan).
Total RNA Extraction and Real-Time PCR Analyses
The expression of NPt2a, NPt2b, CaBP-D28k, PMCA1b, and OPN were measured with a reverse -transcription PCR (real-time RT-PCR). The total RNA was extracted from the eggshell gland, kidney, duodenum, jejunum and ileum with the TRIzol reagent (TransGen Biotech, China), and then reverse transcription PCR and real-time quantitative PCR were performed (Wang et al., 2020; Song et al., 2020). The mRNA values were normalized to the expression of chicken β-actin mRNA. The relative expression level of the mRNA was calculated by the 2−ΔΔCT method (Chen et al., 2018; Uerlings et al., 2018; Tang et al., 2019). The primer sequences for the real-time PCR were designed using Primer 5.0 software and are synthesized by Sangon Bioteach (Shanghai, China) and shown in Table 1.
Table 1.
Gene-specific primer of related genes.
| Gene | Sequences (5’→3’) | Accession NO. | Product size (bp) |
|---|---|---|---|
| CaBP-D28 | F: TGTTATGGAGTGCAGGATGG | NM_205513 | 131 |
| R: TAGAGCGAACAAGCAGGTGA | |||
| PMCA1b | F: TTCAGGTACTCATGTGATGGAAGG | XM_015277056 | 98 |
| R: CAGCCCCAAGCAAGGTAAAG | |||
| NPt2a | F: CCAAACTGCACGGCTTCT | XM_015293846 | 249 |
| R: TGGGAGGTCAGT GTTGATGA | |||
| NPt2b | F: ACTGGCTTGCTGTGTTTGC | NM_204474 | 113 |
| R: AGGGGCATCTTCACCACTTT | |||
| β-actin | F: CTGGCACCTAGCACAATGAA | NM_205518 | 123 |
| R: CTGCTTGCTGATCCACATCT |
Western Blot Analysis
The tissue samples of eggshell gland, kidney, duodenum, jejunum, and ileum were put into 1 mL of RIPA buffer (Beyotime, China), and homogenized in ice bath. Then, it was centrifuged at 4°C, 12000 g for 10 min. The protein concentration was detected by a BCA protein assay kit (Beyotime, China). The protein samples were separated by 10% SDS–PAGE (Bio-Rad, Richmond, 246 CA) and then transferred to PVDF membranes (Millipore, Boston, MA), at 200 mA and 2 h at 4°C. After blocking with western blocking buffer (Beyotime, China) for 1 h at room temperature, the membranes were incubated with anti-Calbindin-d28k (Sigma, Boston, MA), anti-PMCA (Thermo, Waltham, MA), anti-β-actin and anti-β-tubulin (Beyotime, Jiangsu, China) overnight at 4°C. The membranes were then washed in 1 × TBST buffer and probed with the secondary antibody (HRP-conjugated anti-rabbit or anti-mouse IgG, Beyotime) diluted at 1:1000, at 4°C for 4 h. The bands were then visualized with Hyperfilm ECL reagent (Beyotime, Jiangsu, China) using BioSpectrum 810 (UVP LLC, Jena, Germany) (Uerlings et al., 2018; Wang et al., 2020).
Statistical Analyses
Prior to the analysis, all data were examined for their homogeneity and normal distribution plots of variances among the treatments by using the UNIVARIATE procedure. As lack of normality, the percentage data were first subjected to square root arcsine transformation before analysis. The main effect of lighting treatment was estimated with one-way ANOVA by using SAS software (Version 8e, SAS Institute, Cary, NC). P < 0.05 was considered statistically significant. Trends were reported where 0.05 < P ≤ 0.10.
RESULTS
Laying Performance
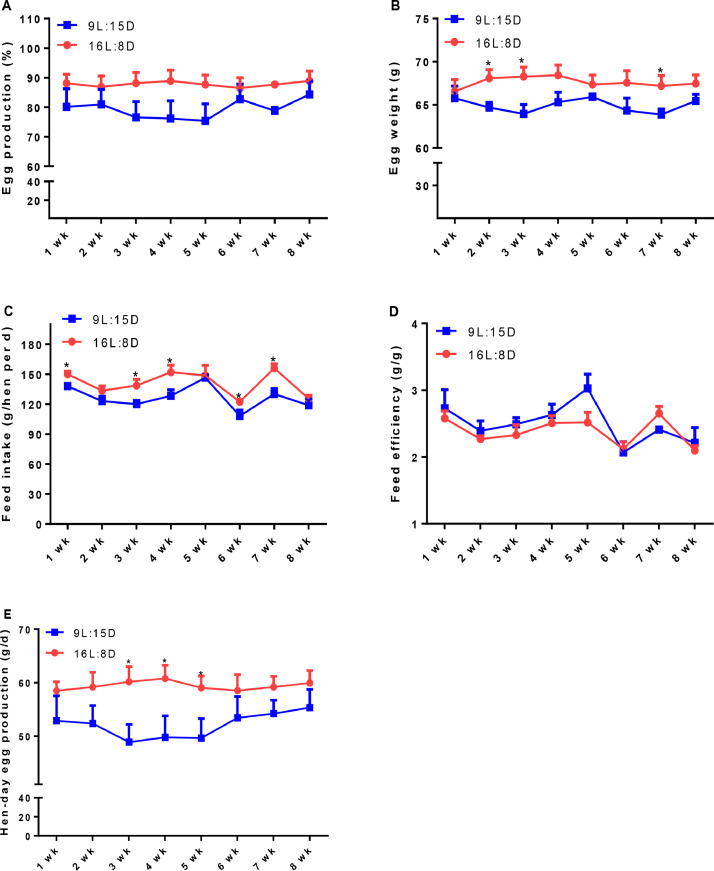
Compared with the control group, feed intake was significant decreased (P < 0.001), while egg weight (P = 0.05) and egg production showed a trend to be decreased by LDP treatment (Table 2). Within the 8-wks period, however, the reduced feed intake was observed at Week 1 (P = 0.0139), 3 (P = 0.0267), 4 (P = 0.0258), 6 (P = 0.0469), and 7 (P = 0.0039), the decreased egg weight was detected at week 2 (P = 0.0164), 3 (P = 0.0185), and 7 (P = 0.0341), while the decreased hen-day egg production was observed at week 3 (P = 0.0269), 4 (P = 0.0426), and 5 (P = 0.0209; Figure 2B, C, E). In contrast, the laying rate and feed efficiency was not changed by LDP treatment (Table 2).
Table 2.
Effects of different light regime on laying performance of hens.
| 16 L:8 D | 9 L:15 D | P value | |
|---|---|---|---|
| Laying rate, % | 87.9 ± 2.44 | 80.1 ± 4.2 | 0.142 |
| Egg weight, g | 67.6 ± 1.02a | 64.9 ± 0.67a | 0.050 |
| Feed intake, g/hen per d | 140.9 ± 1.25a | 126.7 ± 2.32a | < 0.001 |
| Hen-day egg production, g/d | 59.4 ± 1.98 | 52.1 ± 2.9 | 0.063 |
| Feed efficiency, g/g | 2.38 ± 0.07 | 2.5 ± 0.13 | 0.466 |
| Egg broken rate, % | 2.60 ± 0.78 | 1.65 ± 0.81 | 0.395 |
Data were presented as mean ± SE (n = 6).
Means with different superscripts differ significantly (P < 0.05).
Figure 2.
Effect of different light regimes on the performance of laying hens. Egg production (A), egg weight (B), feed intake (C), feed efficiency (D), and hen-day egg production (E). Data represent the mean ± SE (n = 6); ∗P < 0.05.
Egg Quality
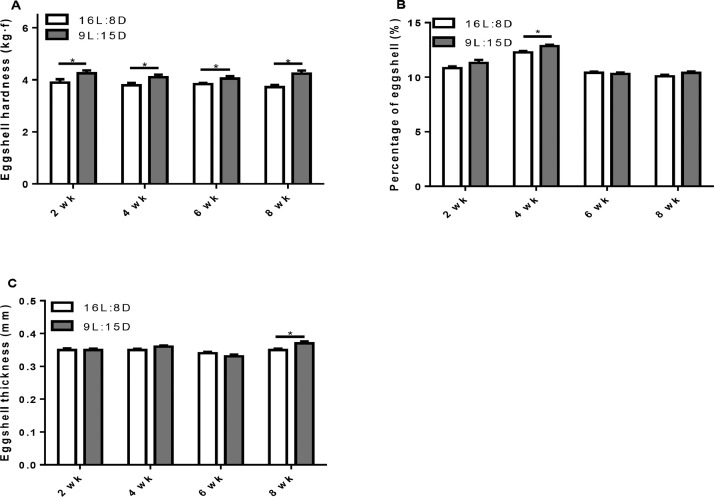
Compared to control, eggshell hardness was significantly increased by LDP treatment (Table 3) and the elevated eggshell hardness by LDP (P = 0.011) was detected at all the measuring times (Figure 3A). LDP treatment, however, did not results in any significant difference in eggshell thickness, egg shape index, albumen height, yolk color, Haugh units, and the proportions of yolk and shell (Table 3). In contrast, eggshell percentage and eggshell thickness were increased by LDP at week 4 (P = 0.0011) and 8 (P = 0.0066, Figure 3B, C).
Table 3.
Effects of different light regime on egg quality of laying hens.
| 16 L:8 D | 9 L:15 D | P value | |
|---|---|---|---|
| Eggshell thickness, mm | 0.346 ± 0.003 | 0.352 ± 0.004 | 0.277 |
| Egg shape index | 1.29 ± 0.01 | 1.3 ± 0.01 | 0.508 |
| Eggshell hardness, kg. f | 3.81 ± 0.05a | 4.14 ± 0.1a | 0.011 |
| Albumen height, mm | 4.4 ± 0.13 | 4.57 ± 0.14 | 0.377 |
| Yolk color | 7.67 ± 0.06 | 7.61 ± 0.1 | 0.617 |
| Haugh units | 60.0 ± 0.8 | 62.3 ± 1.5 | 0.214 |
| Percentage of yolk, % | 28.5 ± 0.5 | 28.8 ± 0.6 | 0.698 |
| Percentage of eggshell, % | 10.9 ± 0.1 | 11.2 ± 0.2 | 0.141 |
Data were presented as mean ± SE (n = 6).
Means with different superscripts differ significantly (P < 0.05).
Figure 3.
Effect of different light regimes on egg quality. Eggshell hardness (A), percentage of eggshell (B) and eggshell thickness (C). Data represent the mean ± SE (n = 6); ∗P < 0.05.
Ca and P Contents in the Eggshells and Dietary
Compared to control, the eggshell contents of Ca (P = 0.0270) and P (P = 0.0099) were increased by LDP treatment (Table 4). The LDP-hens had less Ca (P = 0.0164) and P (P = 0.0164) intake compare with control hens (Table 4). The eggshell output of Ca (P = 0.0361) and P (P < 0.0001) were higher in LDP treatment than that in control (Table 4).
Table 4.
Effect of lighting regime on calcium (Ca) and phosphorus (P) intake and contents in eggshell (Week 8).
| 16 L:8 D | 9 L:15 D | P value | |
|---|---|---|---|
| Dietary Ca intake, g/d | 6.11 ± 0.08a | 5.79 ± 0.08a | 0.016 |
| Dietary P intake, g/d | 0.72 ± 0.01a | 0.68 ± 0.01a | 0.016 |
| Ca content in the eggshell, % | 32.89 ± 1.13a | 39.69 ± 1.79a | 0.027 |
| P content in the eggshell, % | 0.08 ± 0.005a | 0.14 ± 0.02a | 0.010 |
| Ca output in eggshell, g/d | 1.99 ± 0.08a | 2.29 ± 0.10a | 0.036 |
| P output in eggshell, g/d | 0.005 ± 0.0002a | 0.008 ± 0.0003a | <0.001 |
Data were presented as mean ± SE (n = 6).
Means with different superscripts differ significantly (P < 0.05).
Serum Parameters
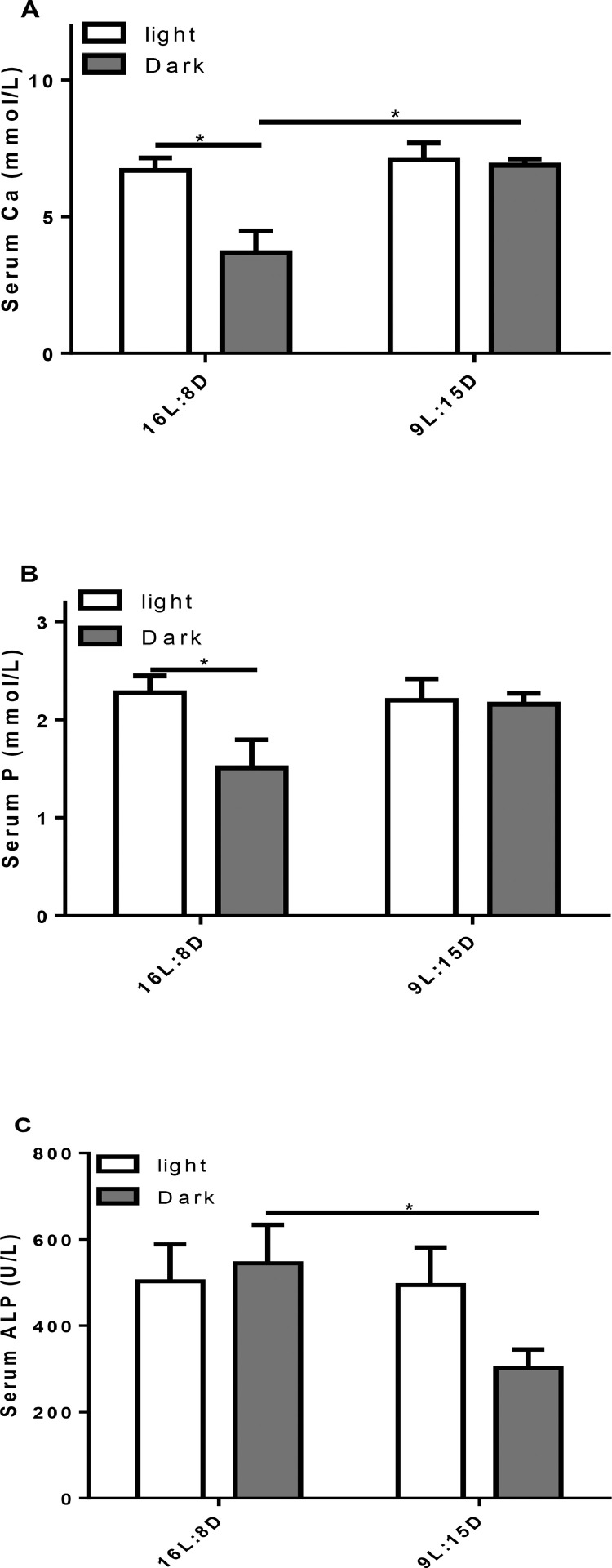
In the control group, the serum Ca (P = 0.0056) and P (P = 0.0346) concentrations of hens in the photophase were higher than that in the scotophase. In LDP treatment, however, the serum Ca and P concentrations had no significant difference between photoperiod and scotophase (Figure 4A, B). In addition, the serum Ca level of LDP-hens was higher in the scotophase than that in control group (P = 0.0056, Figure 4A). In photophase, there was no significant difference in ALP activity between the control group and LDP treatment, whereas LDP-hen had lower ALP activity in scotophase (P = 0.0259) compared to control (Figure 4C).
Figure 4.
Effects of different light regimes on serum calcium (Ca; A), phosphorus (P; B), and alkaline phosphatase activity (ALP; C). Data represent the mean ± SD (n = 8); ∗P < 0.05.
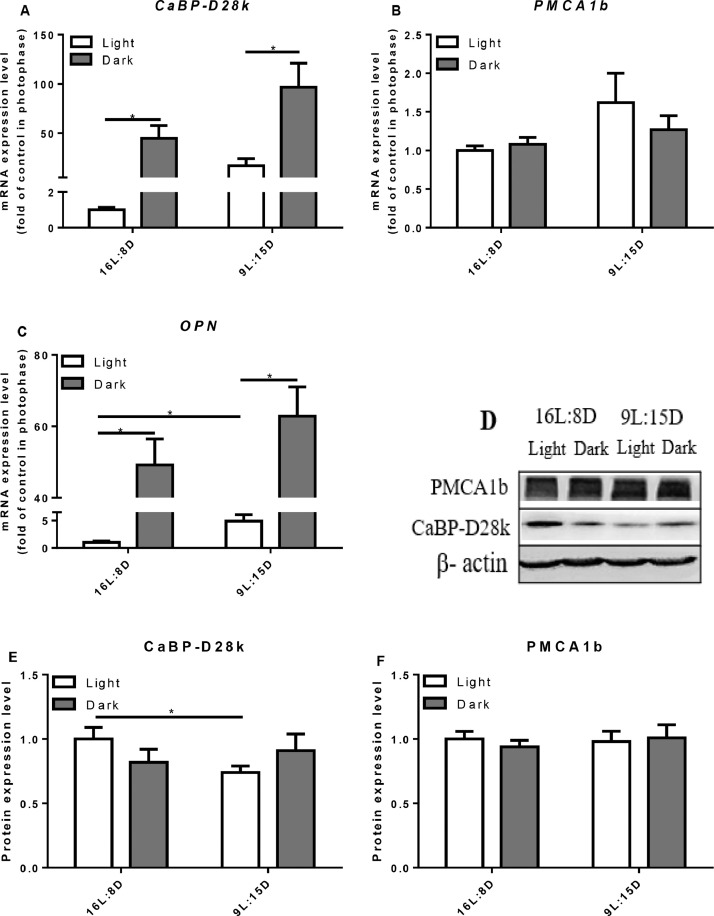
The Expression of Calcium Transporters
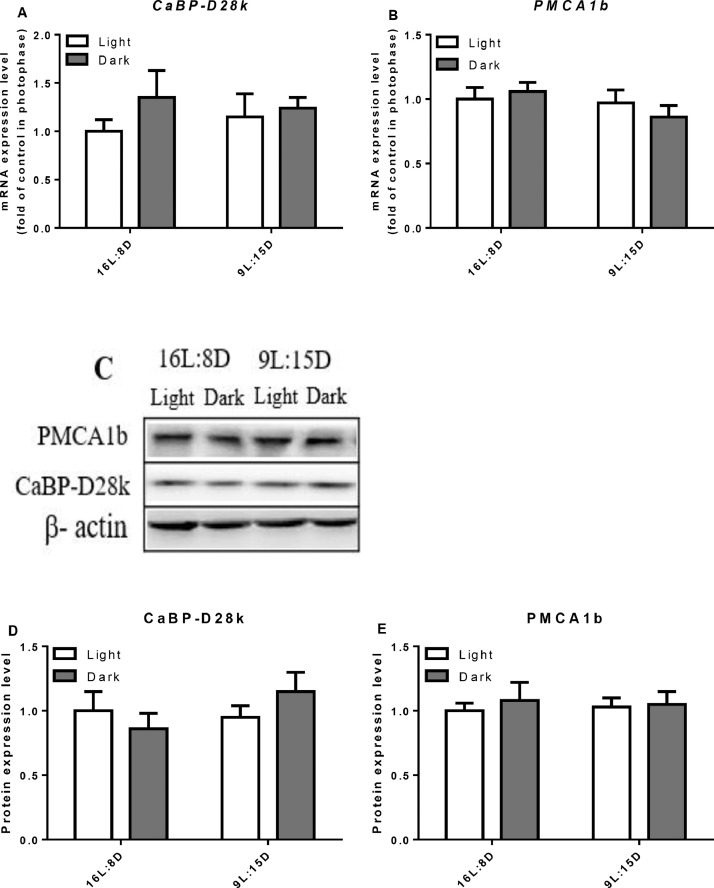
In eggshell gland, the expressions of CaBP-D28k (P = 0.0038, Figure 5A) and OPN (P = 0.0122, Figure 5C) were significantly higher in scotophase than that in photophase. In contrast, there was no difference in CaBP-D28k protein levels between the photophase and scotophase (Figure 5E). LDP treatment significantly decreased the protein level of CaBP-D28k (P = 0.0245, Figure 5E), LDP had no influence on the mRNA and protein levels of PMCA1b (Figure 5B, D, F).
Figure 5.
Effect of different light regimes on the mRNA expression levels of CaBP-D28k, PMCA1b and OPN and the protein expression level of CaBP-D28k and PMCA1b in the eggshell gland. Relative mRNA expression level of CaBP-D28k (A), PMCA1b (B) and OPN (C) were evaluated by RT-PCR. Western blot analysis for detection of CaBP-D28k and PMCA1b (D, E, F). Data represent the mean ± SD (n = 8); ∗P < 0.05.
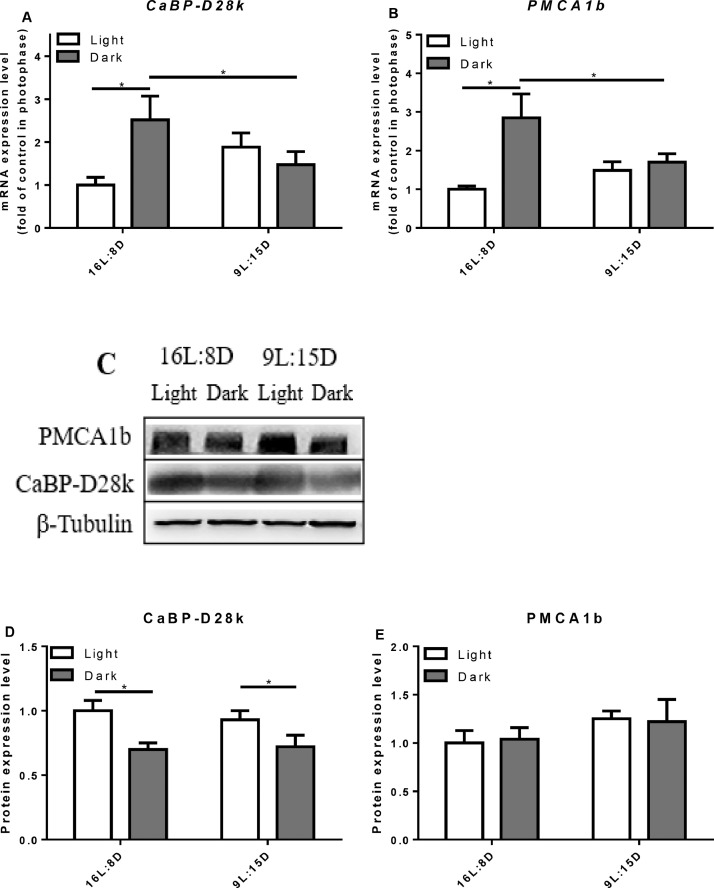
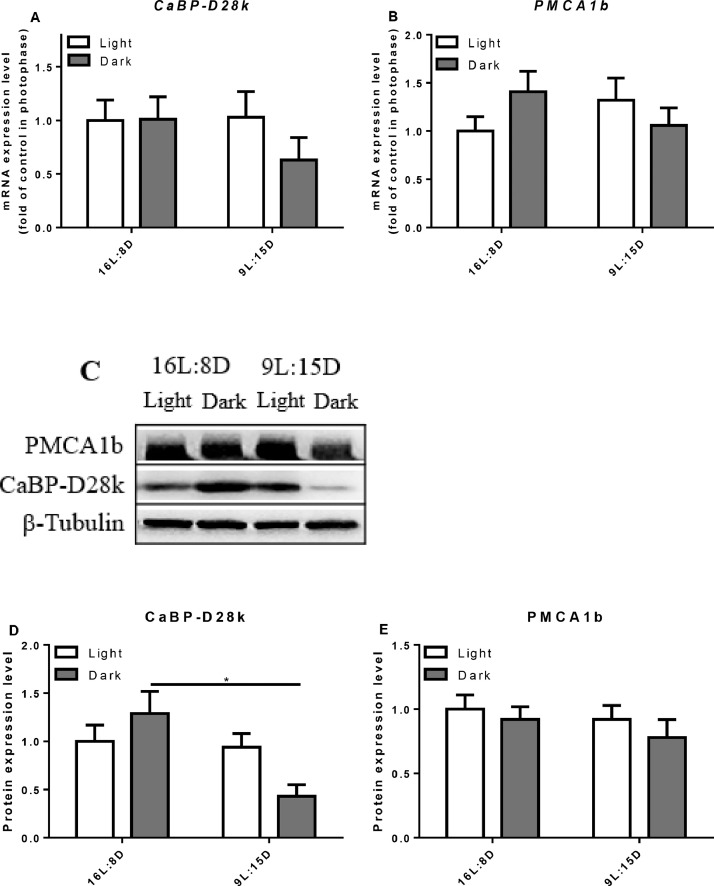
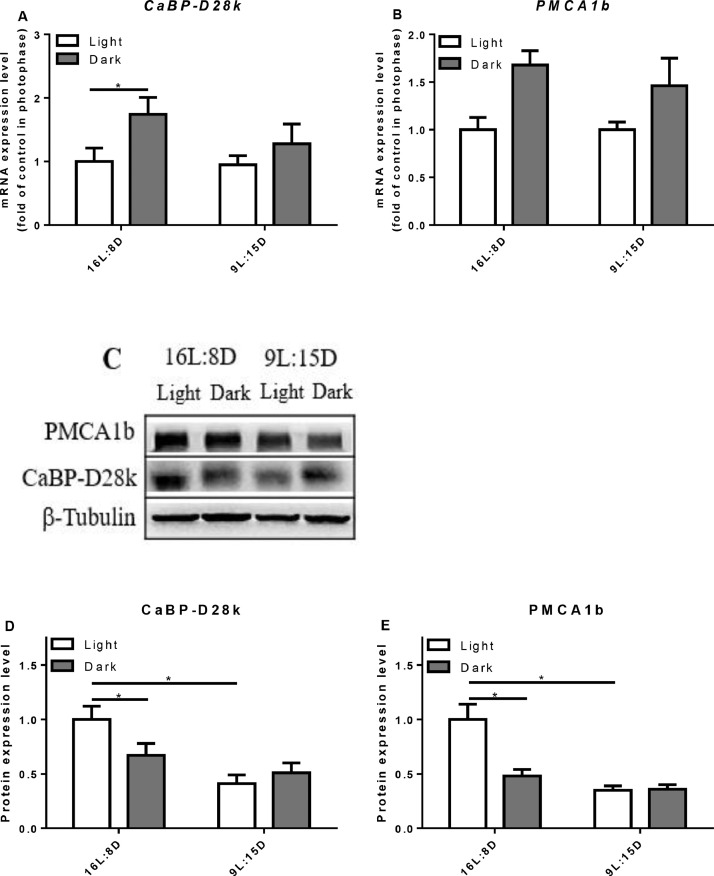
In the duodenum and jejunum of control group, the relative mRNA expression of CaBP-D28k was higher in the scotophase (P = 0.0382) but not in the ileum (Figures 6–8). LDP treatment did not change the mRNA expression level of CaBP-D28k. In the control group, the mRNA level of PMCA1b in the duodenum increased in the scotophase (P = 0.0045), but there was no significant change in the jejunum and ileum. Similarly, LDP treatment did not change the expression of PMCA1b in the photophase and scotophase. However, in the jejunum, the protein expression of PMCA1b was lowered in LDP treatment (P < 0.0001) compared to control group (Figure 7). In the kidney, LDP treatment did not change the mRNA and protein levels of the CaBP-D28k and PMCA1b (Figure 9).
Figure 6.
Effect of different light regimes on the mRNA and protein expression level of CaBP-D28k and PMCA1b in the duodenum. The mRNA and protein levels of CaBP-D28k (A, C, D); the mRNA and protein expression levels of PMCA1b (B, C, E). The values are presented as the means ± SD (n = 8); ∗P < 0.05.
Figure 8.
Effect of different light regimes on the mRNA and protein expression level of CaBP-D28k and PMCA1b in the ileum. The mRNA and protein levels of CaBP-D28k (A, C, D); the mRNA and protein expression levels of PMCA1b (B, C, E). The values are presented as the means ± SD (n = 8); ∗P < 0.05.
Figure 7.
Effect of different light regimes on the mRNA and protein expression level of CaBP-D28k and PMCA1b in the jejunum. The mRNA and protein levels of CaBP-D28k (A, C, D); the mRNA and protein expression levels of PMCA1b (B, C, E). The values are presented as the means ± SD (n = 8); ∗P < 0.05.
Figure 9.
Effect of different light regimes on the mRNA and protein expression levels of CaBP-D28k and PMCA1b in the kidney. Relative mRNA expression level of CaBP-D28k (A) and PMCA1b (B) were evaluated by RT-PCR. Western blot analysis for the detection of CaBP-D28k and PMCA1b (C, D, E). Data represent the mean ± SD (n = 8); ∗P < 0.05.
The Expression of Phosphorus Transporters
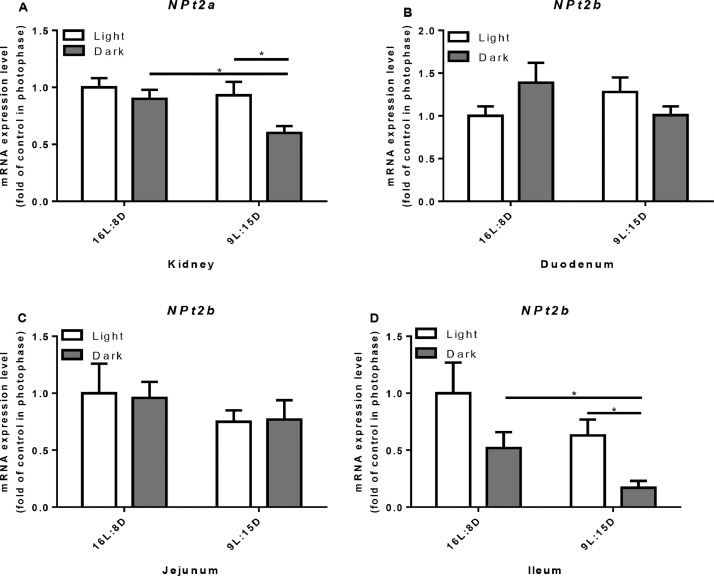
In the control group, there was no difference in the mRNA level of NPt2a in the kidney between photo- and scotophase (Figure 10A). In LDP group, however, the expression level of NPt2a was higher in the photophase than that in the scotophase (P = 0.0174, Figure 10A). Photoperiod treatment had no effect on the mRNA expression level of NPt2b in the duodenum and jejunum (Figure 10B, C). In ileum of LDP treatment, however, the mRNA expression level of NPt2b was higher (P = 0.0247) in photophase compared with scotophase (Figure 10D). Compared to control, LDP treatment significantly decreased the mRNA expression level of NPt2b in the scotophase (P = 0.0133).
Figure 10.
Effect of different light regimes on the mRNA expression level of NPt2a and NPt2b in kidney and intestine. Kidney (A); duodenum (B); jejunum (C); ileum (E). Data represent the mean ± SD (n = 8); ∗P < 0.05.
DISCUSSION
In general, the present result showed a trend that prolonged scotophase in the 24 h light-schedule reduces the daily feed intake, hen-day egg production, and egg weight, but had no adverse effect on feed efficiency. The contents of Ca and P in the eggshell were increased, and the eggshell hardness was improved by prolonged scotophase. The result suggests that the increased serum Ca and P concentration during the scotophase is beneficial for the improvement of eggshell quality. This study provides an effective method to ameliorate eggshell quality in the latter stages of the laying period.
The laying performance of hens are influenced by illumination time, light source, and light intensity (Petersen and Mennicken, 1999; Wang et al., 2015). In this study, under a 24 h photo-schedule, prolonging scotophase reduced feed intake and egg production, but not the feed to egg ratio. In the light-schedule of 16 L:8 D, broilers did not eat during the scotophase (Rodrigues and Choct, 2019). Under a 0.25 L: 0.75 D illumination system, laying hens consume less feed as a result of decreased activity time (Morris and Butler, 1995). Therefore, the reduced feed intake in LDP-hens is suggested to be a result of shortened photophase or prolonged scotophase. This speculation, however, should be explained with a caution as the influence of short-photophase induced reproductive regression cannot be excluded.
Compared to control, the increased eggshell strength in the LDP group was consistent with the decreased broken egg rate (Control, 2.60% vs. LDP, 1.65%). Eggshell strength and thickness can be improved by providing a diet with high Ca content (Kang et al., 2008). The increased eggshell hardness, however, was not in line with the elevated eggshell thickness. This result was in accordance with the previous works by Cufadar et al. (2011) and Xiao et al. (2019), who reported that there is no necessary linkage between eggshell thickness and eggshell hardness. In the present study, the increase eggshell hardness in LDP-hens in accompanied with the elevated Ca content in the eggshell, implying the possible that LDP improves eggshell quality by enhanced Ca deposition. Recently, it is proved that the nanostructure of mineralization of eggshell contributes to the eggshell hardness (Athanasiadou et al., 2018). Hence, the underlying mechanism needs to be investigated further.
The content of serum Ca in laying hens varies with the photophase and scotophase, showing a circadian rhythm (Pablos et al., 1995). In control hens, the higher contents of serum Ca and P in photophase compared to scotophase indicated that there is a circadian rhythm. Ren et al. (2019) reported that blood P and Ca levels gradually increased after the first oviposition, peaked in 6 h, and then gradually decreased until the second oviposition. In layer pullets, serum Ca content was higher in the scotophase and lower in the photophase (Parsons and Combs, 1981). It is interesting to note that the rhythm of serum Ca and P in LDP-hens was not as evident as that in the control, suggesting prolonged scotophase masks the rhythm of circulating Ca and P. The elevated blood Ca is a result of an extra release of bone Ca during night (Frost et al., 1991). In this study, serum ALP activity maintained relatively low level in LDP-hens during scotophase, indicating that Ca mobilization in bone was lower in scotophase. As the hen-day egg production tended to be decreased by LDP treatment, the relative higher blood Ca and lower ALP activity during scotophase in LDP-hens may be a result of reduced Ca requirement for eggshell formation. The possible influence of sampling time, however, cannot be ruled out as the blood was sampled at 8 h after light off in LDP-hens, which was 4-h later compared to control. Moreover, in the laying hen, blood Ca level is affected by the ovulatory cycle (Parsons and Combs, 1981; Ren et al., 2019). Hence, the effect of changed timing of oviposition cannot be excluded. Collectively, the result suggests that prolonged scotophase is beneficial for Ca deposition and eggshell formation via extended eggshell formation time and elevated blood Ca content in scotophase.
In laying hens, the expression of CaBP-D28k is highest in the duodenum, followed by the jejunum and ileum (Taylor and Wasserman, 1967; Ebeid et al., 2012; Li et al., 2018). Ca intake from the diet is mainly absorbed by the vascular system of the duodenum and upper jejunum with the participation of CaBP-D28k (Craviso et al., 1987). In duodenum and jejunum of control hens, the protein expression level of CaBP-D28k was contrary to the trend of mRNA expression level, higher in photophase and lower in scotophase. This result was in line with the feeding activity in photophase. This phenomenon, however, was changed in jejunum of LDP treatment but not in duodenum and ileum, suggesting that prolonged scotophase has a little influence on the expression of CaBP-D28k. PMCA mediates Ca extrusion from cells (Carafoli, 1991; Zylińska et al., 2002; Fleet and Schoch, 2010). The different trends in the expression PMCA1b between LDP and control groups were also observed in jejunum, where PMCA1b was highly expressed in control hens during photophase. The changed expression of Ca transporters may be a result of reduced Ca intake of the LDP hens (89.9% of the control group).
OPN is a glycosylated protein secreted by the epithelial cells in eggshell glandular cavity (Fernandez et al., 2003; Chien et al., 2008). As a component of the organic matrix of eggshell, OPN plays an important role in eggshell calcification. The present result indicated that OPN was highly expressed in scotophase and lowly expressed in photophase, in line with the previous work by Pines et al. (1995), who reported that the gene expression of OPN in the eggshell gland shows a circadian rhythm, lower expressed in the light compared to that in the dark. Generally, the key period of eggshell formation is at night. When the egg enters the uterus, uterine wall pressure is exerted, which is involved in the expression of OPN (Lavelin et al., 1998). Hence, the result implies that the upregulated OPN in scotophase is associated with eggshell formation. Moreover, in vitro study indicates that the hardness of calcite containing OPN is higher than that of calcite without OPN (Athanasiadou et al., 2018). Compared with control, LDP increased the expression of OPN in either the photophase (4.87 fold) or the scotophase (1.28 fold), suggesting that the upregulation of OPN expression is involved in the improved eggshell strength by LDP treatment.
In the eggshell gland, the mRNA level of CaBP-D28k was higher in scotophase in both treatments, which is overlapping with the eggshell formation period. As the protein levels of CaBP-28k and PMCA1b were not altered by LDP treatment during the scotophase, indicating that prolonging the scotophase had no effect on the Ca secretion of eggshell gland. The protein expression level of CaBP-D28k and PMCA1b in the kidney was not changed by photoperiod treatment, indicating that the reabsorption of Ca by the kidney is not changed by treatment.
There are two families of Na/Pi cotransporters, Na/Pi-II and NaPi-III. NPt2b mainly regulates the absorption of P by the small intestine (Werner and Kinne, 2001; Marks et al., 2006; Yan et al., 2007; Sabbagh et al., 2009; Ikuta et al., 2018). In poultry, the main expression site of the NPt2b gene is the duodenum (Yan et al., 2007; Li et al., 2018). The mRNA expression level of NPt2b in the duodenum and jejunum was not affected by light regime or light-dark cycle, indicating that the absorption of P by the small intestine was not affected by the light regimen. In the kidney, the main type of phosphorus transporter is NPt2a. P homeostasis in laying hens depends on phosphorus transporters in the kidney (Huber et al., 2006). In the scotophase, the mRNA expression level of NPT2a in LDP laying hens was relatively low, which is consistent with their high P level in the serum. Li et al. (2018) found that dietary high available P decreased the mRNA expression level of NPT2a in the kidney of laying hens, which is consistent with our results.
In conclusion, prolonged scotophase from 8 h to 15 h reduced the laying performance of laying hens. The eggshell strength and the contents of Ca and P in the eggshell were increased by the prolonged scotophase. The result suggests that the increased circulating Ca and P concentrations during the scotophase are beneficial for the deposition of Ca and P in the eggshell and in turn the eggshell quality. This result offers an alternative strategy for managing laying hens with poor eggshell quality.
ACKNOWLEDGMENTS
This work was supported by the National Key Research Program of China (grant number 2016YFD0500510), the China Agriculture Research System (CARS-40-K09), and National Natural Science Foundation of China (31772619). The authors declare that they have no conflicts of interest. We thank the reviewers for their valuable comments and suggestions on this paper.
DISCLOSURES
The authors declare that they have no conflicts of interest. We greatly thank the reviewers for their valuable comments and suggestions on the paper.
REFERENCES
- Archer G.S. How does red light affect layer production, fear, and stress? Poult. Sci. 2019;98:3–8. doi: 10.3382/ps/pey302. [DOI] [PubMed] [Google Scholar]
- Athanasiadou D., Jiang W., Goldbaum D., Saleem A., Basu K., Pacella M.S., Böhm C.F., Chromik R.R., Hincke M.T., Rodríguez-Navarro A.B., Vali H., Wolf S.E., Gray J.J., Bui K.H., McKee M.D. Nanostructure, osteopontin, and mechanical properties of calcitic avian eggshell. Sci. Adv. 2018;4:eaar3219. doi: 10.1126/sciadv.aar3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A., Hurwitz. S. The interaction between dietary calcium and gonadal hormones in their effect on plasma calcium, bone, 25-hydroxycholecalciferol- 1-hydroxylase, and duodenal calcium-binding protein, measured by a radioimmunoassay in chicks. Endocrinology. 1979;104:1455–1460. doi: 10.1210/endo-104-5-1455. [DOI] [PubMed] [Google Scholar]
- Bar A., Hurwitz. S. Egg shell quality, medullary bone ash, intestinal calcium and phosphorus absorption, and calcium-binding protein in phosphorus-deficient hens. Poult. Sci. 1984;63:1975–1979. doi: 10.3382/ps.0631975. [DOI] [PubMed] [Google Scholar]
- Barros J.S.G., Barros T.A.D.S., Sartor K., Raimundo J.A., Rossi L.A. The effect of linear lighting systems on the productive performance and egg quality of laying hens. Poult. Sci. 2020;99:1369–1378. doi: 10.1016/j.psj.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M., Bédécarrats. G.Y. Evaluation of the impact of light source on reproductive parameters in laying hens housed in individual cages. Poult. Sci. 2019;56:148–158. doi: 10.2141/jpsa.0180054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédécarrats G.Y. Control of the reproductive axis: balancing act between stimulatory and inhibitory inputs. Poult. Sci. 2015;94:810–815. doi: 10.3382/ps/peu042. [DOI] [PubMed] [Google Scholar]
- Biber J., Hernando N., Forster I., Murer H. Regulation of phosphate transport in proximal tubules. Pflugers. Arch. 2009;458:39–52. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- Bolukbasi S.C., Celebi S., Utlu N. The effects of calcium and vitamin D3 in diet on plasma calcium and phosphorus, eggshell calcium and phosphorus levels of laying hens in late laying production period. Int. J. Poult. Sci. 2005;4:246–247. [Google Scholar]
- Bustany Z.A., Elwinger. K. Shell and interior quality and chemical composition of eggs from hens of different strains and ages fed different dietary lysine levels. Acta. Agric. Scand. 1987;37:175–187. [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol. Rev. 1991;71:129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang H., Jiao H.C., Wang X.J., Zhao J.P., Lin H. Feed habituation alleviates decreased feed intake after feed replacement in broilers. Poult. Sci. 2018;97:733–742. doi: 10.3382/ps/pex358. [DOI] [PubMed] [Google Scholar]
- Chien Y.C., Hincke M.T., Vali H., McKee M.D. Ultrastructural matrix–mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J. Struct. Biol. 2008;163:84–99. doi: 10.1016/j.jsb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Close B., Banister K., Baumans V., Bernoth E.M., Bromage N., Bunyan J., Erhardt W., Flecknell P., Gregory N., Hackbarth H., Morton D., Warwick C. Recommendations for euthanasia of experimental animals: part 2. DGXT of the European Commission. Lab. Anim. 1997;31:1–32. doi: 10.1258/002367797780600297. [DOI] [PubMed] [Google Scholar]
- Corradino R.A., Fullmer C.S., Wasserman R.H. Embryonic chick intestine in organ culture: stimulation of calcium transport by exogenous vitamin d-induced calcium-binding protein. Arch. Biochem. Biophys. 1976;174:738–743. doi: 10.1016/0003-9861(76)90404-5. [DOI] [PubMed] [Google Scholar]
- Craviso G.L., Garrett K.P., Clemens T.L. 1,25-Dihydroxyvitamin D3 induces the synthesis of vitamin D-dependent calcium-binding protein in cultured chick kidney cells. Endocrinology. 1987;120:894–902. doi: 10.1210/endo-120-3-894. [DOI] [PubMed] [Google Scholar]
- Cufadar Y., Olgun O., Yildiz A.Ö. The effect of dietary calcium concentration and particle size on performance, eggshell quality, bone mechanical properties and tibia mineral contents in moulted laying hens. Br. Poult. Sci. 2011;52:761–768. doi: 10.1080/00071668.2011.641502. [DOI] [PubMed] [Google Scholar]
- Davis W.L., Jones R.G., Farmer G.R., Matthews J.L., Martin J.H., Bridges G. Electron microscopic cytochemical localization of a basolateral calcium adenosine triphosphatase in vitamin D replete chick enterocytes. Anat. Rec. 1987;219:384–393. doi: 10.1002/ar.1092190409. [DOI] [PubMed] [Google Scholar]
- Ebeid T.A., Suzuki T., Sugiyama T. High ambient temperature influences eggshell quality and calbindin-d28k localization of eggshell gland and all intestinal segments of laying hens. Poult. Sci. 2012;91:2282–2287. doi: 10.3382/ps.2011-01898. [DOI] [PubMed] [Google Scholar]
- Fernandez M.S., Escobar C., Lavelin I., Pines M., Arias J.L. Localization of osteopontin in oviduct tissue and eggshell during different stages of the avian egg laying cycle. J. Struct. Biol. 2003;143:171–180. doi: 10.1016/j.jsb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Fleet J.C., Schoch. R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010;47:181–195. doi: 10.3109/10408363.2010.536429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., Whitehead C.C. Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. Br. Poult. Sci. 1998;39:434–440. doi: 10.1080/00071669889024. [DOI] [PubMed] [Google Scholar]
- Frost T.J., Roland D.A., Sr, Marple D.N. The effects of various dietary phosphorus levels on the circadian patterns of plasma 1,25-dihydroxycholecalciferol, total calcium, ionized calcium, and phosphorus in laying hens. Poult. Sci. 1991;70:1564–1570. doi: 10.3382/ps.0701564. [DOI] [PubMed] [Google Scholar]
- Grizzle J., Iheanacho M., Saxton A., Broaden J. Nutritional and environmental factors involved in egg shell quality of laying hens. Br. Poult. Sci. 1992;33:781–794. doi: 10.1080/00071669208417520. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Huber K., Hempel R., Rodehutscord M. Adaptation of epithelial sodium-dependent phosphate transport in jejunum and kidney of hens to variations in dietary phosphorus intake. Poult. Sci. 2006;85:1980–1986. doi: 10.1093/ps/85.11.1980. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Segawa H., Sasaki S., Hanazaki A., Fujii T., Kushi A., Kaneko I. Effect of Npt2b deletion on intestinal and renal inorganic phosphate (Pi) handling. Clin. Exp. Nephrol. 2018;22:517–528. doi: 10.1007/s10157-017-1497-3. [DOI] [PubMed] [Google Scholar]
- Jiang S., Cui L.Y., Hou J.F., Shi C., Ke X., Yang L.C., Ma X.P. Effects of age and dietary soybean oil level on eggshell quality, bone strength and blood biochemistry in laying hens. Br. Poult. Sci. 2014;55:653–661. doi: 10.1080/00071668.2014.949624. [DOI] [PubMed] [Google Scholar]
- Kang H.K., Kang G.H., Kim D.W., Na J.C., Yu D.J., Lee S.J., Kim S.H. Effects of feeding high and low Ca additive on laying performance and egg quality in laying hens. J. Anim. Sci. Technol. 2008;50:799–806. [Google Scholar]
- Lavelin I., Yarden N., Ben-Bassat S., Bar A., Pines M. Regulation of osteopontin gene expression during egg shell formation in the laying hen by mechanical strain. Matrix. Biol. 1998;17:615–623. doi: 10.1016/s0945-053x(98)90112-3. [DOI] [PubMed] [Google Scholar]
- Li P., Wang R., Jiao H., Wang X., Zhao J., Lin H. Effects of Dietary Phosphorus Level on the Expression of Calcium and Phosphorus Transporters in Laying Hens. Front. Physiol. 2018;9:627. doi: 10.3389/fphys.2018.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makled M.N., Charles. O.W. Eggshell quality as influenced by sodium bicarbonate, calcium source, and photoperiod. Poult. Sci. 1987;66:705–712. doi: 10.3382/ps.0660705. [DOI] [PubMed] [Google Scholar]
- Marks J., Srai S.K., Biber J., Murer H., Unwin R.J., Debnam E.S. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp. Physiol. 2006;91:531–537. doi: 10.1113/expphysiol.2005.032516. [DOI] [PubMed] [Google Scholar]
- Melek O., Morris T.R., Jennings R.C. The time factor in egg formation for hens exposed to ahemeral light-dark cycles. Br. Poult. Sci. 1973;14:493–498. doi: 10.1080/00071667308416056. [DOI] [PubMed] [Google Scholar]
- Melancon M.J., Jr., DeLuca. H.F. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry. 1970;9:1658–1664. doi: 10.1021/bi00810a002. [DOI] [PubMed] [Google Scholar]
- Morris T.R., Butler. E.A. New intermittent lighting programme (the Reading system) for laying pullets. Br. Poult. Sci. 1995;36:531–535. doi: 10.1080/00071669508417799. [DOI] [PubMed] [Google Scholar]
- Murer H., Forster I., Biber J. The sodium phosphate cotransporter family SLC34. Pflugers. Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. National Academy Press; Washington DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pablos M.I., Perezgallardo L., Agapito M.T., Recio J.M. Circadian oscillations of calcium, sodium and potassium in chick serum, ultrafiltered serum and pineal gland. Comp. Biochem. Phys. A. 1995;112:339–345. [Google Scholar]
- Parker S.L., Lindsay L.A., Herbert J.F., Murphy C.R., Thompson M.B. Expression and localization of Ca2+-atpase in the uterus during the reproductive cycle of king quail (coturnix chinensis) and zebra finch (poephila guttata) Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;149:30–35. doi: 10.1016/j.cbpa.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Parsons A.H., Combs G.F., Jr. Blood ionized calcium cycles in the chicken. Poult. Sci. 1981;60:1520–1524. doi: 10.3382/ps.0601520. [DOI] [PubMed] [Google Scholar]
- Pasteels B., Parmentier M., Lawson E.M., Verstappen A., Pochet R. Calcium-binding protein immunoreactivity in pigeon retina. Invest. Ophthalmol. Vis. Sci. 1987;28:658–664. [PubMed] [Google Scholar]
- Petersen J., Mennicken. L. Effects of asymmetrical intermittent lighting in the rearing and laying period on bodyweight development, performance and egg quality traits in laying hens. Arch. Geflugelk. 1999;63:100–110. [Google Scholar]
- Pines M., Knopov V., Bar A. Involvement of osteopontin in egg shell formation in the laying chicken. Matrix. Biol. 1995;14:765–771. doi: 10.1016/s0945-053x(05)80019-8. [DOI] [PubMed] [Google Scholar]
- Qin X., Klandorf. H. Effect of estrogen in relation to dietary vitamin D3 and calcium on activity of intestinal alkaline phosphatase and Ca-ATPase in immature chicks. Gen. Comp. Endocrinol. 1993;90:318–327. doi: 10.1006/gcen.1993.1087. [DOI] [PubMed] [Google Scholar]
- Quinn S.J., Kifor O., Kifor I., Butters R.R., Brown E.M. Role of the cytoskeleton in extracellular calcium-regulated PTH release. Biochem. Biophys. Res. Commun. 2007;354:8–13. doi: 10.1016/j.bbrc.2006.12.160. [DOI] [PubMed] [Google Scholar]
- Ren Z., Sun W., Liu Y., Li Z., Han D., Cheng X., Yan J., Yang X. Dynamics of serum phosphorus, calcium, and hormones during egg laying cycle in Hy-Line Brown laying hens. Poult. Sci. 2019;98:2193–2200. doi: 10.3382/ps/pey572. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E., Oosterhoff H.H., Feddes J.J.R., Wilson J.L. Effects of photostimulatory light intensity on ovarian morphology and carcass traits at sexual maturity in modern and antique egg-type pullets. Poult. Sci. 2001;80:47–56. doi: 10.1093/ps/80.1.47. [DOI] [PubMed] [Google Scholar]
- Rhee S. A study on the calcium and iron content of the undaria pinnatifida suringar. J. Korean. Soc. Food. Sci. Nutr. 1972;1:25–31. [Google Scholar]
- Rodrigues I., Choct. M. Feed intake pattern of broiler chickens under intermittent lighting: do birds eat in the dark? Anim. Nutr. 2019;5:174–178. doi: 10.1016/j.aninu.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenboim I., Zilberman E., Gvaryahu G. New monochromatic light source for laying hens. Poult. Sci. 1998;77:1695–1698. doi: 10.1093/ps/77.11.1695. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y., O"Brien S.P., Song W., Boulanger J.H., Stockmann A., Arbeeny C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009;20:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri F., Zampiga M., Berardinelli A., Meluzzi A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018;97:1818–1823. doi: 10.3382/ps/pex456. [DOI] [PubMed] [Google Scholar]
- Song M., Lin X., Zhao J.P., Wang X., Jiao H., Li H., Lin H. High frequency vaccination-induced immune stress reduces bone strength with the involvement of activated osteoclastogenesis in layer pullets. Poult. Sci. 2020;99:734–743. doi: 10.1016/j.psj.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonerock R.H., Roland D.A., Sr., Voitle R.A. Status of the digestive system and tibiae of cropectomized hens at night. Poult. Sci. 1975;54:466–472. doi: 10.3382/ps.0540466. [DOI] [PubMed] [Google Scholar]
- Sun M., Zhao J., Wang X., Jiao H., Lin H. Use of encapsulated L-lysine-HCl and DL-methionine improves postprandial amino acid balance in laying hens. J. Anim. Sci. 2020;98:1–12. doi: 10.1093/jas/skaa315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wu J., Jiao H., Wang X., Zhao J.P., Lin H. The development of antioxidant system in the intestinal tract of broiler chickens. Poult. Sci. 2019;98:664–678. doi: 10.3382/ps/pey415. [DOI] [PubMed] [Google Scholar]
- Taylor A.N., Wasserman. R.H. Vitamin D3-induced calcium-binding protein: partial purification, electrophoretic visualization, and tissue distribution. Arch. Biochem. Biophys. 1967;119:536–540. doi: 10.1016/0003-9861(67)90488-2. [DOI] [PubMed] [Google Scholar]
- Uerlings J., Song Z., Hu X., Wang S., Lin H., Buyse J., Everaert N. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult. Sci. 2018;97:3681–3690. doi: 10.3382/ps/pey229. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang X., Zhao J., Jiao H., Lin H. Low protein diet supplemented with crystalline amino acids suppressing appetite and apo-lipoprotein synthesis in laying hens. Anim. Feed. Sci. Tech. 2020:266. [Google Scholar]
- Wang R.M., Zhao J.P., Wang X.J., Jiao H.C., Wu J.M., Lin H. Fibroblast growth factor 23 mRNA expression profile in chicken and its response to dietary phosphorus. Poult. Sci. 2018;97:2258–2266. doi: 10.3382/ps/pey092. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ding J.T., Yang H.M., Cao W., Li Y.B. The effect of new monochromatic light regimes on egg production and expression of the circadian gene BMAL1 in pigeons. Poult. Sci. 2015;94:836–840. doi: 10.3382/ps/pev057. [DOI] [PubMed] [Google Scholar]
- Wasserman R.H., Taylor. A.N. Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966;152:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Werner A., Kinne. R.K.H. Evolution of the Na-Pi cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R301. doi: 10.1152/ajpregu.2001.280.2.R301. [DOI] [PubMed] [Google Scholar]
- Xiao Y.Q., Shao D., Sheng Z.W., Wang Q., Shi S.R. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult. Sci. 2019;98:3298–3303. doi: 10.3382/ps/pez178. [DOI] [PubMed] [Google Scholar]
- Yan F., Angel R., Ashwell C.M. Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult. Sci. 2007;86:67–76. doi: 10.1093/ps/86.1.67. [DOI] [PubMed] [Google Scholar]
- Zylińska L., Kawecka I., Lachowicz L., Szemraj J. The isoform- and location-dependence of the functioning of the plasma membrane calcium pump. Cell. Mol. Biol. Lett. 2002;7:1037–1045. [PubMed] [Google Scholar]