Abstract
Contact tracing is an important tool for allowing countries to ease lockdown policies introduced to combat SARS-CoV-2. For contact tracing to be effective, those with symptoms must self-report themselves while their contacts must self-isolate when asked. However, policies such as legal enforcement of self-isolation can create trade-offs by dissuading individuals from self-reporting. We use an existing branching process model to examine which aspects of contact tracing adherence should be prioritized. We consider an inverse relationship between self-isolation adherence and self-reporting engagement, assuming that increasingly strict self-isolation policies will result in fewer individuals self-reporting to the programme. We find that policies which increase the average duration of self-isolation, or that increase the probability that people self-isolate at all, at the expense of reduced self-reporting rate, will not decrease the risk of a large outbreak and may increase the risk, depending on the strength of the trade-off. These results suggest that policies to increase self-isolation adherence should be implemented carefully. Policies that increase self-isolation adherence at the cost of self-reporting rates should be avoided.
This article is part of the theme issue ‘Modelling that shaped the early COVID-19 pandemic response in the UK’.
Keywords: COVID-19, contact tracing, SARS-CoV-2, adherence, case isolation, quarantine
1. Background
Since the first cases of SARS-CoV-2 in China in late 2019 [1], the virus has spread globally, resulting in over 600 000 confirmed deaths by August 2020 [2]. Lockdown in the UK began in March 2020 [3] and reduced R0 below 1 while also triggering unprecedented reductions in economic activity [4]. As lockdown restrictions are relaxed, both in the UK and in other countries, other methods for keeping R0 below 1 are needed. Large-scale contact tracing is one of the potential methods for keeping virus spread under control [5–7].
During the current SARS-CoV-2 outbreak, contact tracing has been used to great effect in a number of countries, including Vietnam and South Korea [8,9]. Two broad classes of contact tracing include manual tracing and digital tracing using a smartphone app [10]. Manual contract tracing is the only system currently running in the UK though it is expected that a contact tracing app will be launched soon [11]. In manual contact tracing, trained public health staff ask a case for the names and contact details of people they have recently been in close proximity with, as well as asking for information on which public areas the infected person has visited. The tracers will then identify as many contacts as possible and ask them to self-isolate for a period. Adherence to the contact tracing system is an important determinant of its efficacy [6,10,12].
Adherence applies to a number of different aspects of contact tracing [13] as shown in figure 1. Untraced individuals with symptoms must get themselves tested (the process of which automatically reports them to the contact tracing system) and they and their household should self-isolate. If they test positive they are contacted by the test and trace team and must give identifying information about the people they have been in close proximity with. Then, both the index case, and the traced contacts, must self-isolate for a period [14,15]. If the contact tracing system uses home swab tests, the swabs must be taken carefully [16,17]. Adherence to each of these steps will be imperfect.
Figure 1.
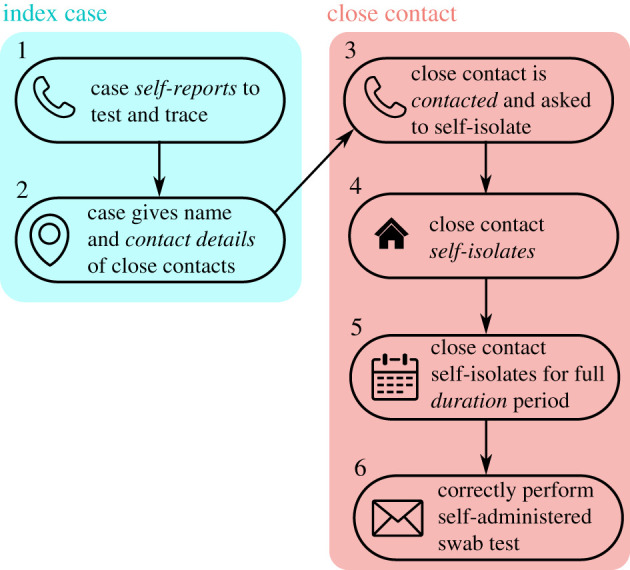
Overview of adherence in test and trace. An untraced individual must self-report and give the name and details of close contacts. The contact tracing team must then manage to contact the close contacts. The close contacts must self-isolate when asked and remain in self-isolation for the full isolation period (14 days in the UK). In some systems, the isolated individual is given a self-administered swab test which must be administered correctly. There is imperfect adherence or performance at each of these stages. In this paper, we focus on trade-offs between self-report rate (stage 1) and self-isolation adherence (stages 4 and 5). In our model, stages 2 and 3 are incorporated into a parameter which we call control effectiveness.
Although there are many unobserved variables involved, we can start to examine some of these adherence rates using public statistics from the UK tracing system [18]. For example, of the 6923 people who were referred to the contact tracing system between the 11 and 17 June, 70% were reached. However, these 6923 cases certainly do not represent 100% of the new cases in the country that week. Of the 6923, 74% gave details of at least one contact though it is not possible to tell how many of the remaining 26% actually had no contacts. Of those that gave details of at least one contact, it is unknown what proportion gave details of all their close contacts. Someone might not give contact information of a close contact deliberately, for reasons of privacy, embarrassment or to save a contact from being asked to self-isolate, or accidentally through not remembering that they were in contact with someone or not knowing any details about the close contact. Eighty-two per cent of close contacts were reached and asked to self-isolate.
However, these adherence rates are not fixed parameters and can be influenced by policy. For example, economic support for those missing work [15,19], daily phonecalls to monitor adherence [20] or legal ramifications for breaking self-isolation, such as those implemented in Singapore and Taiwan [20], might be expected to increase self-isolation rates [19]. In particular, this work was originally undertaken in response to a question from policy makers asking whether legally mandating self-isolation for close-contacts would reduce transmission rates. Furthermore, there are likely to be trade-offs and dependencies between parameters. In particular, contact tracing relies on self-reporting of symptoms in order to initially identify a chain of transmission but introducing penalties for non-compliance to self-isolation might be expected to decrease the proportion of people that report themselves to the system in the first instance. In general, there are few direct, individual benefits to self-reporting oneself to a contact tracing system; instead the benefits are communal and the drivers for self-reporting are likely to be altruism or social norms [21,22]. However, there are direct costs both to the individual that self-reports and to their close contacts. Self-isolation is mentally difficult [23] and will come with economic costs for many [15,21,24–26]. Legally enforcing self-isolation exacerbates these costs.
The exact form that these trade-offs would take are difficult to know. Adherence to self-isolation requirements might largely be binary with people complying for the full 14 days (as requested in the UK) or not adhering at all. In this case, legal enforcement would be expected to increase the proportion of people that self-isolate. Alternatively, it is possible that self-isolation adherence is more continuous with people adhering for a few days instead of the full 14 days. Similarly, legal enforcement might be expected to increase the duration of isolation. Finally, if swab tests are being self-administered, people might be less careful or less willing to endure discomfort if the consequences of a positive test are more severe (though this might change as saliva tests are produced [27,28]). While it is difficult to know the functional effects of different levels of compliance, it is even more difficult to quantify the strengths of the trade-offs. Legal enforcement might have a weak effect on improving self-isolation adherence [23] but a strong deterrent effect on self-reporting. Alternatively, perhaps legal mandation has a strong effect on self-isolation adherence without being a strong deterrent to self-reporting rates. Furthermore, the shapes of these trade-offs are likely to differ in different countries and social groups based on culture, trust in the government and other factors. Careful quantitative and qualitative studies will need to be conducted to quantify these effects.
Here we use a previously published branching process model [6,12] to examine the effects of these trade-offs on the risk of a large outbreak of SARS-CoV-2. We examine trade-offs between self-isolation duration and self-isolation probability with self-reporting rates, contact information reporting probabilities and sensitivity of home swab tests. It is important to note that we do not consider the societal costs [29] of legal enforcement of self-isolation; we aim to quantify the benefits of these policies without considering the costs noting that the costs are not easy to directly compare to the benefits.
2. Methods
In this paper, we extend a previous model of SARS-CoV-2 transmission [12]. An overview of the model is given in the electronic supplementary material, figure S1 while parameter values and references are given in table 1. At the individual-level, the number of potential secondary contacts are modelled by a negative binomial distribution while the exposure times of these new infections are modelled as a gamma distribution. Self-isolating individuals are assumed to be unable to transmit the disease (assuming isolation within households) and therefore potential secondary cases are avoided if the gamma-distributed exposure time occurs during self-isolation of the primary case. The timing of self-isolation depends on whether the case was traced as a potential contact or not and a number of factors affecting adherence as described in detail below. The model proceeds as a branching process with each simulation being seeded with 20 untraced, infected individuals.
Table 1.
Model parameters values/ranges. (Parameters taken from the literature are fixed and for other parameters a range of values are explored.)
| parameter | values | refs |
|---|---|---|
| self-isolation probability | 10–70% | [30] |
| self-reporting probability | 10–70% | |
| test sensitivity | 35–65% | [31–33] |
| minimum isolation duration | 1–14 days | |
| maximum isolation duration | 7, 14 days | |
| contact tracing coverage (%) | 40–80% | |
| number of initial cases | 20 | |
| symptomatic RS under physical distancing | 1.3 | |
| asymptomatic RS under physical distancing | 0.65 | |
| dispersion of RS, k | 0.16 | [12,34] |
| proportion asymptomatic | 50% | [35,36] |
| delay: onset to isolation | 1 day | |
| incubation period (lognormal) | mean log: 1.43, s.d. log: 0.66 | [37] |
| infection time (gamma) | shape: 2.12, rate: 0.69 d−1 | [37] |
| infection time shift | 12.98 days | [37] |
| time to trace contacts (days) | 1 day | |
| delay: isolate to test result | 1 days |
(a). Secondary case distribution
The heterogeneity in the number of potential secondary cases caused by an individual is modelled as a negative binomial distribution. For symptomatic cases, we use a mean value of 1.3 secondary cases while asymptomatic cases are given a 50% lower infection rate. This relates to a scenario where strong social distancing and good hygiene is still being observed. Earlier work [6,7] and preliminary analyses indicated that contact tracing is unable to keep the risk of an outbreak low without being paired with social distancing so this is the scenario we focus on. Estimates for the dispersion parameter, k, for SARS-CoV-2 range from k = 0.1 (0.05–0.2) for pre-lockdown UK [38] to k = 0.25 (0.13–0.88) for Tianjin, China during lockdown measures [39]. Given this range, we have kept the parameter as used in [12,34] setting k = 0.16. This value of k yields a strongly skewed distribution with most individuals causing zero potential secondary cases.
(b). Infection profile
Individuals are labelled as symptomatic or asymptomatic with a probability of 50% [35,36]. The onset time of symptoms is modelled as a lognormal distribution with mean 1.43 days and s.d. of 0.66 [40]. All individuals, whether symptomatic or asymptomatic are given a symptom onset time as the exposure time of secondary cases is calculated relative to this time. The exposure time for each new potential case is drawn from a gamma distribution with shape parameter of 17.77 and a rate of . This distribution is centred 12.98 days before the onset of symptoms. If this randomly sampled value yields a negative generation interval (i.e. the secondary case being infected by the primary case before the primary case is infected) the value is resampled. The parameters for this gamma distribution were estimated by fitting to the data in He et al. [37] in a maximum likelihood framework that accounts for this resampling process. The model fitting does not ignore any data as discussed by Ashcroft et al. [41]. The estimated distributions are qualitatively similar to the original fitted models (electronic supplementary material, figure S2). If the exposure time of a potential secondary case occurs during the primary case’s self-isolation, the infection event does not occur and the potential secondary case does not become a case.
(c). Contact tracing
The first stage in the contact tracing system is an untraced, symptomatic individual self-reporting themselves by seeking a test. We define the control effectiveness of the contact tracing system as the proportion of secondarily infected people that are contacted by the contact tracers. In practice, this variable is never observed, but it can be broken down to a number of processes. For infections transmitted on surfaces, the primary case will rarely know who else touched the same surface. For face-to-face contact in small groups, the primary contact must remember that they were in contact with the secondary case, know their name and chose to divulge this information to the contact tracing team. In the UK the definition of a close contact is being within 2 m for more than 15 min, which probably encompasses most small group infections. For transmission in larger groups, such as at restaurants and bars, contact tracing effectiveness depends on how well the venue recorded who visited. The control effectiveness parameter in the model encodes all of these processes and is varied between 40 and 80%. If contact tracing is successful, the traced individual is asked to self-isolate. We assume it takes one day to contact a contact. If a traced contact subsequently shows symptoms or returns a positive test the next round of contact tracing is initiated. That is, the contacts of the traced contact are then traced.
(d). Testing
As a baseline we assume that tests have a sensitivity of 65% and that it takes 1 day for results to be returned. This reflects the sensitivity of tests observed in the community [31,32]. Given a positive test result contact tracing for the tested individual is initiated. A negative test allows the tested individual to be immediately released from quarantine. Any contacts of a negative-testing case that were successfully identified prior to receiving the test result are still isolated and tested. This process is different to the UK contact tracing system in which the trace team only ask for contact details if a positive test has been returned. In a branching process model, only infected individuals are modelled. Therefore we do not track the number of uninfected people that are unnecessarily asked to quarantine. Test specificity affects the number of uninfected people asked to quarantine but does not directly affect the spread of the disease and therefore we do not define a test specificity. In this study, we are concerned with quantifying the benefits of contact tracing and do not attempt to weigh the epidemiological benefits against the sociological costs.
(e). Adherence trade-offs
We consider three main trade-offs. As we do not have good data to define the shapes of these trade-offs, we run simulations for all combinations of parameters.
First, we assume that without policies to encourage self-isolation most people attempt some self-isolation but the lack of adherence is with respect to the duration of self-isolation that decreases. We keep the probability of self-isolation constant at 70%. We assume that each person that does self-isolate isolates for an amount of time taken from a uniform distribution between a minimum and maximum value. For the maximum values, we use either the full 14 days currently recommended in the UK or a shorter 7 day maximum isolation. We vary the minimum duration of self-isolation from 1 day to being equal to the maximum duration.
Second, we examine the trade-off between self-report probability and self-isolation probability. We expect that policies which increase self-isolation probability will reduce self-report probability. We use values of self-isolation from 10 to 70% in increments of 20% and examine all combinations with self-report probabilities from 10 to 70%, also in increments of 20%. The upper bound for self-isolation here is certainly above the rate of self-isolation currently being achieved in the UK. However, it is below the target rate for other national contact tracing programmes [30]. Furthermore, the very strict restrictions applied to travellers entering countries such as Singapore could also be considered an upper bound on feasible policies. Many of the policies used in these areas, such as enforced isolation in government run hotels, GPS ankle bracelets, and daily video calls, would be considered draconion if applied to the population at large but could be reasonably expected to produce self-isolation rates of 90%. In contrast to the first trade-off, we assume that everyone who does self-isolate does so for the full maximum value of either 7 or 14 days.
Finally, we assume that policies which increase self-isolation probability will decrease test sensitivity. This scenario applies to the case of home administered tests. With strong incentives to test negative, people will be less likely to perform swabs correctly. We therefore examine a range of test sensitivities from a baseline of 65% down to 35% in increments of 10%.
(f). Simulation process
Results presented are the combined output of 15 000 simulations for each parameter combination, or scenario, considered. We define a simulation as leading to a large outbreak if it has more than 2000 cumulative cases or if there are still infected cases after 300 days. The threshold of 2000 cases was chosen by running simulations with a maximum of 5000 cases and noting that of the simulated epidemics that went extinct, 99% of extinction events occurred before reaching 2000 cases. Nearly all simulations either went extinct or reached 2000 cases with very few simulations lasting longer than 300 days. These simulations were then used to calculate the probability of a large outbreak given a certain set of conditions. Here, 95% Clopper–Pearson exact confidence intervals were also calculated. To test the sensitivity of our results to the relative asymptomatic transmission rate and the delay between being contacted by the contact tracers and starting self-isolation, we ran simulations at 60% control effectiveness and varied each parameter in turn. The model was written in R and the code and testing suite [42] is publicly available on GitHub (https://github.com/timcdlucas/ringbp/tree/adherence_tradeoff_runs).
3. Results
(a). Trade-off between self-isolation duration against self-report probability
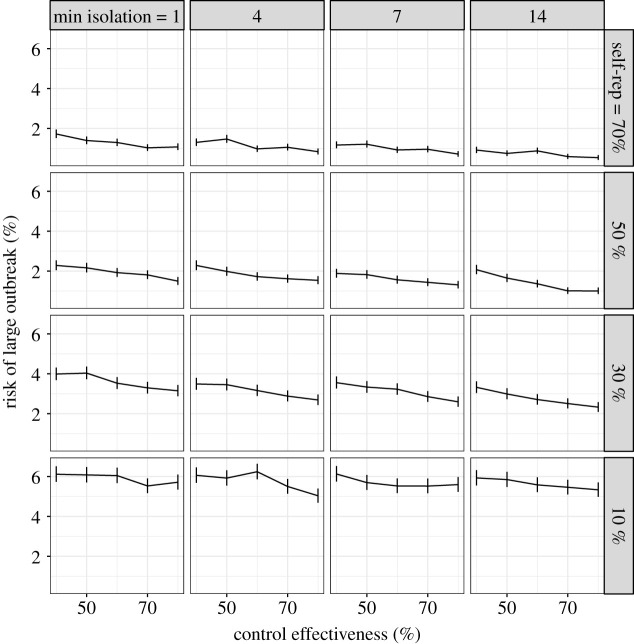
In our first comparison we assumed that increasing the duration of self-isolation would reduce the self-reporting probability. We found that increasing the duration of self-isolation increases the risk of a large outbreak in the presence of reductions in self-reporting rates. The probability of a large outbreak for all combinations of self-isolation duration and self-report rates are shown in figure 2. If we assume that we are currently in the top left panel (high self-report rates but isolation taken uniformly between 1 and 14 days), policies that move us down and right generally increase the risk of a large outbreak. For example, if we consider a control effectiveness of 60%, with a self-isolation duration of between 1 and 14 days and a self-report rate of 70%, the risk of a large outbreak is 1%. If we increase the self-isolation duration to always be 14 days but reduce the self-report rate to 10%, the probability of a large outbreak increases from 1 to 6%. If the trade-off is very weak, such that increasing self-isolation duration to always be 14 days only decreases self-report rates to 50%, we see no change in the probability of an outbreak.
Figure 2.
Trade-off between self isolation time (columns) and self-report rate (rows) with error bars denoting 95% confidence intervals. Individuals self isolate for a randomly selected duration between min isolation and 14 days. Untraced, symptomatic individuals self-report with a probability that varies across the rows. The proportion of close contacts that are divulged and asked to self-isolate varies across the x-axis of each subplot. The y-axis shows the risk of a large outbreak (greater than 2000 cases) over 15 000 simulations. The probability that an individual self-isolates at all is fixed at 70%. If we assume we are currently near the top left we expect that introducing legal ramifications for breaking self isolation would move us down and right. This generally increases risk.
If we assume a more pessimistic starting scenario of a self-isolation duration of between 1 and 14 days and self-reporting rates of 10% and given a control effectiveness of 60% we have a 6% risk of a large outbreak (figure 3a, red line). We find that increasing self-report rates gives a larger reduction in risk. Increasing self-report rates from 10 to 70% reduces risk from 6 to 1% (moving right along the x-axis in figure 3a). By contrast, increasing the duration of isolation to always being 14 days does not change the risk of a large outbreak (purple line in figure 3a). We find that reducing the maximum isolation duration from 14 days to 7 days consistently increases the risk of a large outbreak (electronic supplementary material, figures S3–S5). Altering the relative asymptomatic transmission rate has a strong effect on the overall risk of a large outbreak, but the effects of minimum isolation length and self-report probability remain similar (electronic supplementary material, figure S6). Furthermore, these results are qualitatively robust to changes in the delay between being asked to self-isolate and doing so (electronic supplementary material, figure S7).
Figure 3.
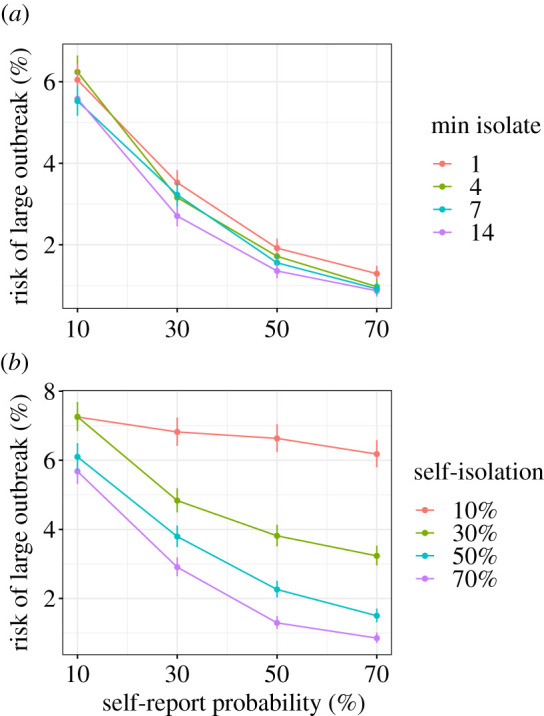
Trade-off between (a) minimum self isolation time and self-report probability, and (b) self-isolation probability and self-report probability. The control effectiveness is held constant at 60%. The results are a subset of those in figures 2 and 4, with each line being a slice through a column of those plots. The y-axis shows the risk of a large outbreak (greater than 2000 cases) over 15 000 simulations. Error bars show the 95% confidence intervals. In (a) if we optimistically assume we currently have 70% self-report probability but 1 day minimum isolation (red), legally mandating isolation would be expected to move us to the left and to the purple line which gives an increased risk of an outbreak. In (b) if we optimistically assume we currently have 70% self-report probability but 10% self isolation probability (red), legally mandating isolation would be expected to move us to the left and to the purple line which gives a marginal decrease in risk of an outbreak. (Online version in colour.)
(b). Trade-off between self-isolation probability against self-report probability
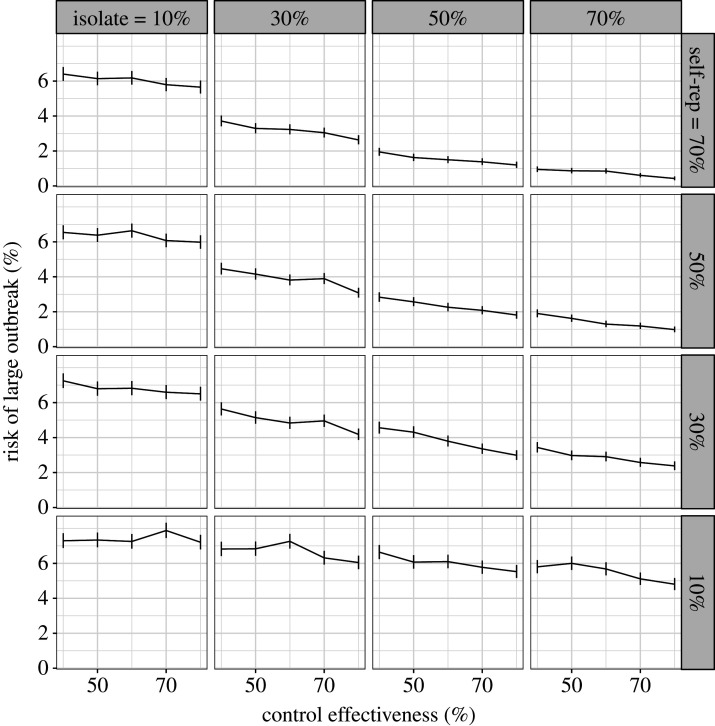
In our second comparison, we assumed that increasing the probability of self-isolation will decrease the self-report probability. We find that increasing self-isolation probability while decreasing self-report probability does not strongly alter the probability of a large outbreak. The probability of a large outbreak for all combinations of self-isolation rates and self-report rates are shown in figure 4. If we assume that we are currently in the top left panel (high self-report rates but low self-isolation rates), policies that increase self-isolation rates but decrease self-report rates would move us down and right. However, whether this decreases the risk of an outbreak depends on the strength of the trade-off. For example, if we consider a control effectiveness of 60%, with a self-isolation rate of 10% and a self-report rate of 70% the risk of a large outbreak is 6%. If we increase the self-isolation rate to 70% and equivalently reduce the self-report rate to 10%, the probability of a large outbreak is still 6%. If the trade-off is weak, such that increasing self-isolation from 10 to 70% only incurs a reduction in self-report rate to 50%, the reduction in risk of a large outbreak is substantial, reducing from 6 to 1.5%. However, if the trade-off is strong, such that increasing self-isolation from 10 to 30% causes a reduction in self-reporting rate from 70 to 10%, the risk of an outbreak instead marginally increases from 6 to 7%.
Figure 4.
Trade-off between self-isolation probability (columns) and self-report probability (rows) with error bars denoting 95% confidence intervals. The y-axis shows the risk of a large outbreak (greater than 2000 cases) over 15 000 simulations. If we assume we are currently near the top left we expect that introducing legal ramifications for breaking self isolation to move us down and right. Whether this decreases risk depends on the strength of the trade-off. If the trade-off is weak, such that as we move from the top left to isolation probability of 70% and self-report probability of 50%, risk is reduced. By contrast, if increasing isolation probability from 10% to 30% incurs a drop in self-reporting from 70 to 10%, risk does not change.
We could instead assume a more pessimistic starting scenario of self-isolation rates of 10% and self-reporting rates of 10%. Given a control effectiveness of 60% we have a 7% risk of a large outbreak (figure 3b, red line). However, from this scenario we can consider whether it is better to increase self-isolation or to increase self-reporting. Increasing self isolation probability to 70% reduces risk to 6% (move right along the x-axis) and increasing self-report probability to 70% also reduces risk to 6% (purple line). Increasing both to 30% reduces risk to 5%. Overall, these two parameters are relatively evenly balanced. The overall risk of a large outbreak changes with relative asymptomatic transmission rate, but the effects of self-isolation probability and self-report probability remain similar (electronic supplementary material, figure S8). The results are similar with different values for the delay between being asked to self-isolate and doing so (electronic supplementary material, figures S9).
(c). Trade-off between self-isolation duration against test sensitivity
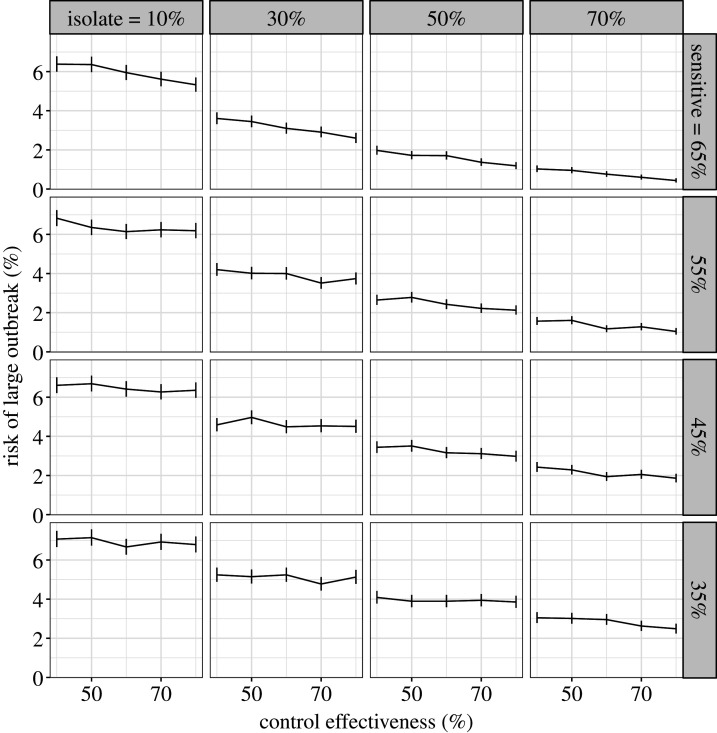
In our final comparison, we assumed that increasing self-isolation probabilities would decrease the probability of careful administration of home swab tests and therefore decrease the test sensitivity. We found that increasing self-isolation rates decreases the risk of a large outbreak even if this occurs in combination with reductions in test sensitivity. The probability of a large outbreak for all combinations of self-isolation rate and test sensitivity are shown in figure 5. If we assume that we are currently in the top left panel (relatively high test sensitivity but low self-isolation rates), policies that increase self-isolation rates but decrease test sensitivity would move us down and right and this in general yields reduced risks of a large outbreak. For example, if we consider a control effectiveness of 60%, with a self-isolation rate of 10% and a test sensitivity of 65%, the risk of a large outbreak is 6%. If we increase the self-isolation rate to 70% while reducing the test sensitivity to 35%, the probability of a large outbreak reduces from 6 to 3%.
Figure 5.
Trade-off between self isolation probability (columns) and test sensitivity (rows) with error bars denoting 95% confidence intervals. Untraced, symptomatic individuals self-report with a probability that varies across the rows. The proportion of close contacts that are divulged and asked to self-isolate varies across the x-axis of each subplot. If we assume we are currently near the top left, introducing legal ramifications for breaking self isolation might move us down and right. This generally decreases risk unless the trade off is very strong such that a small increase in isolation probability incurs a large decrease in test sensitivity.
4. Discussion
Overall we have found that policies which increase self-isolation rates at the expense of self-report rates are unlikely to improve the effectiveness of contact tracing systems. If the primary trade-off is between the duration of self-isolation and the probability of self-reporting, we find that policies which increase self-isolation and reduce self-report rates will cause either an increase or no change in the probability of a large outbreak depending on the strength of the trade-off. When the primary trade-off was instead between the probability of self-isolation and the rate of self-report, policies which increase self-isolation rates and reduce self-report rates can increase or marginally decrease the probability of a large outbreak depending on the strength of the trade-off. Overall this implies that policies such as fines, and police enforcement of self-isolation will have either little benefit or a negative effect. Broadly, policies that improve self-report rates, even at the expense of self-isolation rates should be used. This might include publicity that encourages people to self-report by reminding them that there are no legal consequences to them or their contacts for doing so.
Policies that improve self-report rates or self-isolation rates without an associated trade-off will also improve contact tracing efficacy. For example, economic support and employment protection for individuals that self-isolate would be expected to improve self-isolation rates [15,19,26] without decreasing self-report rates. Similarly, efforts to communicate the reasons why people should self-report and self-isolate may improve both of these rates simultaneously [19,26].
One of the core assumptions to this work is that legal consequences for breaking self-isolation would improve self-isolation rates. However, the evidence for this is not strong and there is evidence that feelings of shame do not promote adherence [22,26]. By contrast, there is good evidence that other factors such as income and boredom [43] do affect self-isolation rates. How effectively legal consequences for breaking self-isolation can increase self-isolation rates is a complex question that will depend on cultural norms, perceived enforcability, and the strength of economic and psychological consequences for self-isolation. An important consequence of this is that self-isolation rates and the effectiveness of policies aimed to improve these rates will be strongly correlated, such that individuals who are most likely to infect each other are also likely to have similar self-isolation rates. This is not included in our model but has the potential to strongly reduce contact tracing efficacy in certain groups and locations.
With regards to test sensitivity, our results are relevant only to self-administered swab-tests. Swab-tests may be replaced with reliable paper-based tests. Given that we found that optimizing self-isolation rates over test-sensitivity minimizes risk, other considerations such as test timing and access are probably more important. Furthermore, currently in the UK, traced contacts are not allowed out of quarantine after a negative test so the system is more robust to low test sensitivity than in our simulations.
Here we have focused solely on the probability of a large outbreak as a consequence of policy change. However, there are other costs and benefits to changing values of self-report rates and self-isolation rates. High self-report rates not only improves contact tracing efficacy directly, it also creates a more effective system for measuring the incidence of SARS-CoV-2 in the community. This gives better early warning for when an outbreak is beginning in an area or group and allows for health care resources to be deployed more efficiently. By contrast, self-isolation comes with many economic and social costs both for the individual and the community. These costs are not the same for all people; the monetary costs to someone who is self-employed or working on very short-term contracts is much higher than for someone who is working at home anyway. Avoiding strict penalties for breaking self-isolation allows those most affected by these costs to self-isolate less (i.e. for a 7 instead of 14 days) and may increase buy-in to the system as a whole. Furthermore, enforcement of self-isolation policies are an infringement on a basic liberty. While we have not tried to compare these costs to the epidemiological benefits, they must always be taken into account when implementing policy.
5. In context
This paper was taken into consideration by the Department for Health and Social Care when deciding whether to impose a legal duty to self-isolate and was referenced in a recent SPI-B report [44]. On the 28th September 2020, the UK government introduced fines for breaching self-isolation rules either after testing positive for SARS-CoV-2 or after being contacted by NHS Test and Trace [45]. The core assumption in our analysis was that introducing penalties for not self-isolating would drive down self-report rates. Our results suggest that increasing self-isolation rates at the expense of reduced self-report rates would make SARS-CoV-2 outbreaks harder to control. However, given that many other restrictions are changing simultaneously, it is unlikely that we will be able tell whether the results from our analysis are borne out after this change. The introduction of legal penalties for breaking isolation also changes the important policy question. The original policy question was whether self-isolation should be legally mandated. Now the more relevant question is when should these restrictions be lifted.
Since the first submission of this paper, results from a large study of adherence in the UK have been released [46]. The study contains self-reported behaviour (rather than intentions) of 42 thousand people between March and August 2020. While the sample was not random, quotas based on age, gender and region were used. Of those with COVID-19 symptoms in the previous seven days, 12% (95% CI 10–14%) requested a test (this measurement corresponds to the self-report parameter used in our analysis) which places the UK in the bottom row of figures 2 and 4. Of those contacted by the track and trace system, 11% (95% CI 8–14%) self-reported as having not left home at all in the following 14 days. This corresponds to the self-isolation probability parameter used in our analysis and places the UK in the left-hand column of figure 4. These adherence rates did not change between March and August. Based on this study, the baseline assumptions made in our analysis were broadly correct. However, further understanding of adherence as a multifaceted continuous variable, rather than a binary variable is required. Measurements of aspects of adherence such as minimum isolation time, as used in our analysis, are still needed.
Acknowledgements
E.L.D., T.C.D.L., A.B., D.A., L.P., G.F.M. and T.D.H. gratefully acknowledge funding of the NTD Modelling Consortium by the Bill & Melinda Gates Foundation (BMGF) (grant number OPP1184344). The following funding sources are acknowledged as providing funding for the named authors. Views, opinions, assumptions or any other information set out in this article should not be attributed to BMGF or any person connected with them.
Data accessibility
All the code used for the simulations are available at https://github.com/timcdlucas/ringbp/tree/adherence_tradeoff_runs.
Authors' contributions
Conceptualization: all authors; formal analysis: E.L.D. and T.C.D.L.; funding acquisition: T.D.H.; investigation: E.L.D., T.C.D.L., A.B., L.P., D.A., T.C. and L.Y.; methodology: E.L.D. and T.C.D.L.; software: E.L.D. and T.C.D.L.; visualization: E.L.D., T.C.D.L. and L.Y.; writing—original draft: E.L.D. and T.C.D.L.; writing—review and editing: all authors.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research was partly funded by the Bill & Melinda Gates Foundation (NTD Modelling Consortium OPP1184344: G.F.M.). This project has received funding from the European Union’s Horizon 2020 research and innovation programme — project EpiPose (101003688: P.K.). Royal Society (RP/EA/180004: P.K.). Wellcome Trust (210758/Z/18/Z: J.H. and S.A.). T.C. is funded by a Sir Henry Wellcome Fellowship from the Wellcome Trust (reference 215919/Z/19/Z). L.Y. is an NIHR Senior Investigator and her research programme is partly supported by NIHR Applied Research Collaboration (ARC)-West, NIHR Health Protection Research Unit (HPRU) for Behavioural Science and Evaluation, and the NIHR Southampton Biomedical Research Centre (BRC) All funders had no role in the study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the manuscript for publication. This work was undertaken as a contribution to the Rapid Assistance in Modelling the Pandemic (RAMP) initiative, coordinated by the Royal Society.
References
- 1.South China Morning Post, Coronavirus: China’s first confirmed COVID-19 case traced back to November 17. See https://www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-case-traced-back (accessed 2 June 2020).
- 2.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. 2020. Coronavirus pandemic (COVID-19). See https://ourworldindata.org/covid-deaths (accessed 10 August 2020).
- 3.Coronavirus: Strict new curbs on life in UK announced by PM. See https://www.bbc.co.uk/news/uk-52012432 (accessed 27 June 2020).
- 4.GDP monthly estimate, UK: April 2020. See https://www.ons.gov.uk/economy/grossdomesticproductgdp/bulletins/gdpmonthlyestimateuk/april2020 (accessed 27 June 2020).
- 5.Aleta A et al. 2020. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 4, 1-8. ( 10.1038/s41562-020-0931-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis EL et al. 2020. An imperfect tool: COVID-19’ test & trace’ success relies on minimising the impact of false negatives and continuation of physical distancing. medRxiv. ( 10.1101/2020.06.09.20124008) [DOI] [Google Scholar]
- 7.Kretzschmar ME, Rozhnova G, Bootsma MC, van Boven M, van de Wijgert JH, Bonten MJ. 2020. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. The Lancet Public Health 5, e452-e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le SM. 2020. Containing the coronavirus (COVID-19): lessons from Vietnam. See https://blogs.worldbank.org/health/containing-coronavirus-covid-19-lessons-vietnam (accessed 3 June 2020).
- 9.Park S, Choi GJ, Ko H. 2020. Information technology–based tracing strategy in response to COVID-19 in South Korea–privacy controversies. Jama 323, 2129-2130. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, Parker M, Bonsall D, Fraser C. 2020. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 368, eabb6936. ( 10.1126/science.abb6936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coronavirus: NHS virus-tracing app downloaded 55, 000 times. See https://www.bbc.co.uk/news/uk-england-hampshire-52617236 (accessed 3 June 2020).
- 12.Hellewell J et al. 2020. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. The Lancet Global Health 8, e488-e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beidas RS et al. 2020. Optimizing and implementing contact tracing through behavioral economics. NEJM Catalyst Innovations in Care Delivery. ( 10.1056/Cat.20.0317) [DOI] [Google Scholar]
- 14.Smith LE, Amlôt R, Lambert H, Oliver I, Robin C, Yardley L, Rubin GJ. 2020. Factors associated with adherence to self-isolation and lockdown measures in the UK; a cross-sectional survey. Pub. Health 187, 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodas M, Peleg K. 2020. Self-isolation compliance in the COVID-19 era influenced by compensation: Findings from a recent survey in Israel: public attitudes toward the COVID-19 outbreak and self-isolation: a cross sectional study of the adult population of Israel. Health Affairs 39, 936-941. ( 10.1377/hlthaff.2020.00382) [DOI] [PubMed] [Google Scholar]
- 16.Seaman CP, Tran LTT, Cowling BJ, Sullivan SG. 2019. Self-collected compared with professional-collected swabbing in the diagnosis of influenza in symptomatic individuals: a meta-analysis and assessment of validity. J. Clin. Virol. 118, 28-35. ( 10.1016/j.jcv.2019.07.010) [DOI] [PubMed] [Google Scholar]
- 17.Dhiman N et al. 2012. Effectiveness of patient-collected swabs for influenza testing. Mayo Clinic Proc. 87, 548–554. [Google Scholar]
- 18.Weekly NHS Test and Trace bulletin, England: 11 to 17 June 2020. https://www.gov.uk/government/publications/nhs-test-and-trace-statistics-england-11-june-to-17-june-2020/weekly-nhs-test-and-trace-bulletin-england-11-to-17-june-2020#fn:1 (accessed 27 June 2020).
- 19.Michie S, West R, Rogers MB, Bonell C, Rubin GJ, Amlôt R. 2020. Reducing SARS-CoV-2 transmission in the UK: a behavioural science approach to identifying options for increasing adherence to social distancing and shielding vulnerable people. Br. J. Health Psychol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung S-C, Marlow S, Tobias N, Alogna I, Alogna A, You S-L. 2020. A rapid systematic review and case study on test, contact tracing, testing, and isolation policies for COVID-19 prevention and control. medRxiv. ( 10.1101/2020.06.04.20122614) [DOI] [Google Scholar]
- 21.Baum NM, Jacobson PD, Goold SD. 2009. ‘listen to the people’: public deliberation about social distancing measures in a pandemic. Am. J. Bioethics 9, 4-14. ( 10.1080/15265160903197531) [DOI] [PubMed] [Google Scholar]
- 22.Travaglino GA, Moon C. 2020. Explaining compliance with social distancing norms during the COVID-19 pandemic: the roles of cultural orientations, trust and self-conscious emotions in the US, Italy, and South Korea. ( 10.31234/osf.io/8yn5b) [DOI]
- 23.DiGiovanni C, Conley J, Chiu D, Zaborski J. 2004. Factors influencing compliance with quarantine in Toronto during the 2003 sars outbreak. Biosecur. Bioterror 2, 265-272. ( 10.1089/bsp.2004.2.265) [DOI] [PubMed] [Google Scholar]
- 24.Blake KD, Blendon RJ, Viswanath K. 2010. Employment and compliance with pandemic influenza mitigation recommendations. Emerg. Infect. Dis. 16, 212. ( 10.3201/eid1602.090638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horney JA, Moore Z, Davis M, MacDonald PD. 2010. Intent to receive pandemic influenza a (H1N1) vaccine, compliance with social distancing and sources of information in NC, 2009. PLoS ONE 5, e11226. ( 10.1371/journal.pone.0011226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baggaley RF et al. 2020. Recommendations for augmenting contact tracing in the UK: learning from other diseases. Isaac Newton Institute for Mathematical Sciences (accessed 18 August 2020).
- 27.Wyllie AL et al. 2020. Saliva or nasopharyngeal swab specimens for detection of SarS-CoV-2. New Eng. J. Med. 383, 1283-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogels CB et al. 2020. Salivadirect: simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance. medRxiv. ( 10.1101/2020.08.03.20167791) [DOI] [Google Scholar]
- 29.Schuklenk U, Gartland KM. 2006. Confronting an influenza pandemic: ethical and scientific issues. Biochem. Soc. Trans. 34, 1151-1154. ( 10.1042/BST0341151) [DOI] [PubMed] [Google Scholar]
- 30.Verrall A. 2020. Rapid audit of contact tracing for COVID-19 in New Zealand. Ministry of Health 310. [Google Scholar]
- 31.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. 2020. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med 173, 262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menni C et al. 2020. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 26, 1037-1040. ( 10.1038/s41591-020-0916-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kasteren P, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, Molenkamp R, Reusken C, Meijer A. 2020. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 128, 104412. ( 10.1016/j.jcv.2020.104412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355-359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizumoto K, Kagaya K, Zarebski A, Chowell G. 2020. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 25, 2000180. ( 10.2807/1560-7917.ES.2020.25.10.2000180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavezzo E et al. 2020. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. medRxiv. ( 10.1101/2020.04.17.20053157) [DOI] [Google Scholar]
- 37.He X et al. 2020. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 1-4. [DOI] [PubMed] [Google Scholar]
- 38.Endo A, Abbott S, Kucharski AJ, Funk S. 2020. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Research 5, 67. ( 10.12688/wellcomeopenres.15842.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Li Y, Wang L, Li M, Zhou X. 2020. Evaluating transmission heterogeneity and super-spreading event of COVID-19 in a metropolis of China. Int. J. Environ. Res. Public Health 3705. ( 10.3390/ijerph17103705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q et al. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New Engl. J. Med. 382, 1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashcroft P, Huisman JS, Lehtinen S, Bouman JA, Althaus CL, Regoes RR, Bonhoeffer S. 2020. COVID-19 infectivity profile correction. (http://arxiv.org/abs/2007.06602). [DOI] [PubMed]
- 42.Lucas TCD, Pollington TM, Davis EL, Hollingsworth TD. 2020. Responsible modelling: unit testing for infectious disease epidemiology. Epidemics 33, 100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff W, Martarelli CS, Schüler J, Bieleke M. 2020. High boredom proneness and low trait self-control impair adherence to social distancing guidelines during the covid-19 pandemic. Int. J. Environ. Res. Public Health 17, 5420. ( 10.3390/ijerph17155420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SPI-B. The impact of financial and other targeted support on rates of self-isolation or quarantine. 2020 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/925133/S0759_SPI-B__The_impact_of_financial_and_other_targeted_support_on_rates_of_self-isolation_or_quarantine_.pdf (accessed 19 October 2020).
- 45.Department of Health and Social Care. 2020 New legal duty to self-isolate comes into force today. https://www.gov.uk/government/news/new-legal-duty-to-self-isolate-comes-into-force-today (accessed 14 October 2020).
- 46. Smith LE, Potts HWW, Amlot R, Fear NT, Michie S, Rubin J. 2020 Adherence to the test, trace and isolate system: results from a time series of 21 nationally representative surveys in the UK (the COVID-19 rapid survey of adherence to interventions and responses [CORSAIR] study). medRxiv.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the code used for the simulations are available at https://github.com/timcdlucas/ringbp/tree/adherence_tradeoff_runs.