Abstract
AIM
To evaluate macular microvasculature changes in eyes after pars plana vitrectomy (PPV) and intraocular silicone oil (SO) tamponade for macula-off rhegmatogenous retinal detachment (RRD) using optical coherence tomography angiography (OCTA).
METHODS
Totally 19 eyes (19 patients) with macula-off RRD who underwent PPV and intraocular SO tamponade were retrospectively reviewed. The parafoveal superficial capillary plexus (SCP) vessel density (VD), deep capillary plexus (DCP) VD, choriocapillaris plexus (CCP) VD, and foveal macular thickness were evaluated using OCTA throughout 16wk postoperatively. The values of healthy fellow eyes were used as control.
RESULTS
The parafoveal SCP, DCP, and CCP VDs were significant increased over time in RRD eyes during the 12wk postoperatively, then decreased at 16wk postoperatively (all P<0.01). The ratios of RRD eyes and fellow healthy eyes (r/f ratios) of the SCP and DCP VDs were lower than those of the CCP VD postoperatively (all P<0.05). There were not significant differences in the r/f ratios between SCP and DCP VDs postoperatively (all P>0.05).
CONCLUSION
The parafoveal SCP, DCP, and CCP VDs gradually recover over time after PPV surgery with SO tamponade. Long-time SO tamponade might decrease postoperative macular VDs. Compared to parafoveal CCP VD, the parafoveal SCP and DCP VDs were more vulnerable in RRD eyes postoperatively.
Keywords: rhegmatogenous retinal detachment, vessel density, silicone oil, vitrectomy, optical coherence tomography angiography
INTRODUCTION
Rhegmatogenous retinal detachment (RRD) is one of the main causes of permanent vision loss, especially in cases of macular involvement[1]. Surgical methods include scleral buckling, pars plana vitrectomy (PPV), or pneumatic retinopexy, which can reattach the detached retina well[2]. However, macula-off RRD patients may have suboptimal visual recovery for various reasons[3]. In previous studies using optical coherence tomography (OCT), the ocular macular microstructures of RRD patients were related to best-corrected visual acuity (BCVA) after RRD repair[4]–[6]. Recently, optical coherence tomography angiography (OCTA) has been recommended as a noninvasive method for visualizing retinochoroidal microvasculature with high reproducibility[7]–[9]. Currently, there are only a few studies on macular vessel density (VD) changes in RRD eyes after surgery[10]–[18], especially intraocular silicone oil (SO) tamponade[16],[18]. Until now, it was still unclear that the effect of SO tamponade to macular VDs.
This study evaluated macular microvasculature and retinal thickness changes in macula-off RRD eyes within 4mo after PPV with SO tamponade using OCTA.
SUBJECTS AND METHODS
Ethical Approval
This retrospective study was approved by the Ethics Committee of Taiyuan Aier Eye Hospital and adhered to the tenets of the Declaration of Helsinki (EYETYYY-20190507-01). Written informed consent was obtained from all participants.
Experimental Design
Nineteen unilateral macula-off RRDs were diagnosed by fundoscopy from June 2019 to September 2019. All patient eyes were successfully repaired using PPV with SO tamponade, and retinal redetachment never occurred during the ensuing months (at least 4mo) prior to SO removal.
The exclusion criteria were as follows: 1) duration of detachment >15d; 2) prior ocular surgery; 3) ocular diseases e.g., retinal vascular disease, age-related macular degeneration, uveitis, glaucoma, macular hole, or epiretinal membrane in either eye; 4) postoperative complications e.g., vitreous hemorrhage, proliferative vitreoretinopathy, endophthalmitis, or subretinal fluid; 5) axial length ≥26.5 mm, or axial length difference of more than 0.3 mm between bilateral eyes; and 6) poor image quality.
Standard 25 G PPV was performed after retrobulbar anesthesia by a single experienced retinal surgeon (Jiang J) using the Alcon Constellation system (Alcon Laboratories, Inc., Fort Worth, TX, USA). The vitreous was completely removed with the help of triamcinolone acetonide. After drainage of subretinal fluid and endophotocoagulation, SO (Oxane 5700; Bausch & Lomb, Inc., NY) was injected into the vitreous cavity. All patients were advised to remain in the face-down position for two weeks postoperatively. The follow-up time was at least 4mo after surgery, and retinal attachment was confirmed in the eyes by a comprehensive ophthalmic examination. All 19 participants underwent examinations preoperatively and 2, 4, 8, 12, and 16wk postoperatively from June 2019 to January 2020, which included BCVA, intraocular pressure (IOP), slit-lamp biomicroscopy examination, fundus examination, and OCTA.
The OCTA images were obtained using RTVue XR Avanti AngioVue (Optovue Inc., Fremont, CA, USA) with AngioVue OCTA software (version 2018.1.0.43). A scanning area of 6×6 mm2 was captured automatically centered on the fovea. The circular region included three circles: a central circle (diameter of 1 mm), a middle circle (diameter of 3 mm), and an outer circle (diameter of 6 mm). The foveal region and parafoveal region were defined within the central circle and the ring between the central and middle circles, respectively. The boundary of superficial capillary plexus (SCP) was defined from 3 µm below the internal limiting membrane to 15 µm below the inner plexiform layer. The boundary of deep capillary plexus (DCP) was defined from 15 to 70 µm below the inner plexiform layer. and the boundary of choriocapillaris plexus (CCP) were defined in a 30 µm thick layer posterior to the retinal pigment epithelium-Bruch membrane complex. In DCP and CCP OCTA image analysis, a projection removal method (three-dimensional projection artifacts removal algorithm) was used to retain the real blood flow signals. OCTA images with scan quality >6 were included for the analysis.
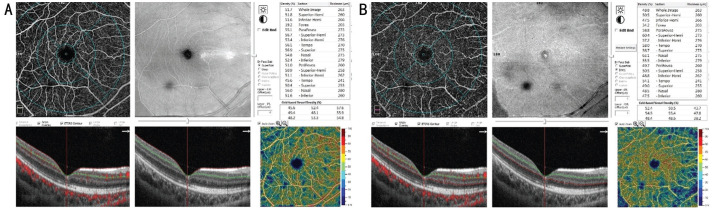
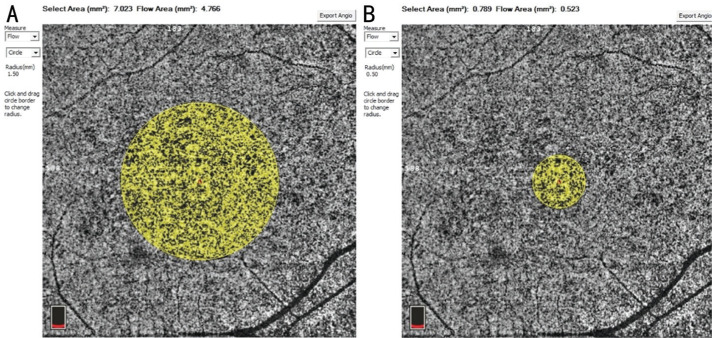
The SCP and DCP VDs in the parafoveal region and foveal macular thickness (FMT) in the foveal region were automatically evaluated (Figure 1). Using the flow areas of the CCP vessel in parafoveal regions, we calculated the parafoveal CCP VD (flow area/select area; Figure 2). The ratios of RRD eyes and fellow healthy eyes (r/f ratios) in postoperative parafoveal VDs were counted to evaluate changes. The values of healthy fellow eyes were used as control data.
Figure 1. Optical coherence tomography angiography images of a 35-year-old male patient with macula-off RRD showing superficial capillary plexus (SCP) and deep capillary plexus (DCP) of the RRD eye at week 12 postoperatively.
A: Measurements for SCP vessel density (VD): OCTA en-face image and B-scan image (red line: internal limiting membrane; green line: inner plexiform layer); B: Measurements for DCP VD: OCTA en-face image and B-scan image (green line: inner plexiform layer; red line: outer plexiform layer).
Figure 2. Optical coherence tomography angiography images of a 35-year-old male patient with macula-off RRD showing choriocapillaris plexus (CCP) of the RRD eye at week 12 postoperatively.
A: Radius of yellow circle is 1.5 mm; B: Radius of yellow circle is 0.5 mm. The parafoveal flow area=flow area of A yellow circle−flow area of B yellow circle. The parafoveal area=selected area of A yellow circle−selected area of B yellow circle. CCP VD=parafoveal flow area/parafoveal area.
Statistical Analysis
SPSS 25.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used to analyze all study results. The postoperative values between the RRD eyes and the fellow healthy eyes and the differences among the r/f ratios of postoperative macular VDs in the RRD eyes at different time points were compared using a paired t-test. The changes in the parafoveal VDs and FMT in the follow-up period were evaluated by one-way ANOVA. P<0.05 was considered as statistically significant.
RESULTS
Table 1 provides the demographics and ocular characteristics of the participants. All 19 eyes underwent PPV with SO tamponade. Lens extraction was not performed, and perfluorocarbon was avoided in all 19 eyes successfully. None of the eyes had redetachments during the follow-up period. Four eyes (21.1%) experienced transient increased IOP within 1mo after surgery, and topical antiglaucoma medication was used to control IOP in those eyes.
Table 1. Demographics and baseline characteristics of the patients in the study.
| Variable | Study eyes (n=19) |
| Age, y (range) | 49.6±15.3 (18-69) |
| Sex (male/female) | 11/8 |
| RRD eye (right/left) | 10/9 |
| Duration of detachment, d (range) | 8.6±4.7 (1-15) |
| Axial length, mm (range) | 24.70±1.21 (22.32-26.45) |
| Duration of SO tamponade, mo (range) | 4.9±0.9 (4.0-7.0) |
| Operation time, min (range) | 59.7±5.9 (50-70) |
RRD: Rhegmatogenous retinal detachment; SO: Silicone oil.
Significant increases were observed in the parafoveal SCP, DCP, and CCP VDs in the RRD eyes within 12wk, then decreased at 16wk postoperatively, respectively (F=6.737, P<0.001; F=19.665, P<0.001; F=5.413, P=0.001; Table 2). The parafoveal SCP and DCP VDs in the RRD eyes were close to, but still lower than those of the fellow healthy eyes at 12wk after surgery (t=-0.993, P=0.334; t=-1.196, P=0.247); however, the differences became statistically significant again at 16wk postoperatively, respectively (t=-3.341, P=0.004; t=-3.021, P=0.007). The parafoveal CCP VD was higher in the RRD eyes than in the fellow eyes at 12wk postoperatively (t=3.760, P=0.001); however, the difference between RRD eyes and fellow eyes became non-significant at 16wk postoperatively (t=1.718, P=0.103). There were no significant differences in the parafoveal SCP, DCP, and CCP VDs in the fellow eyes at different postoperative time points (F=0.019, P=0.999; F=0.011, P>0.999; F=0.045, P=0.996; Table 2).
Table 2. Postoperative macular parameters in RRD eyes and fellow eyes (control) at specific time points.
| Time points | Groups | SCP VD (%) | DCP VD (%) | CCP VD (%) | FMT (µm) |
| 2wk | RRD | 38.52±6.36 | 41.76±5.51 | 61.99±3.33 | 233.84±41.40 |
| Control | 48.94±4.74 | 54.13±3.17 | 64.78±2.79 | 250.74±26.37 | |
| P | <0.001 | <0.001 | 0.001 | 0.107 | |
| 4wk | RRD | 41.54±6.83 | 46.94±4.46 | 63.95±3.23 | 223.42±38.52 |
| Control | 48.91±4.73 | 54.17±3.15 | 64.81±2.75 | 250.53±26.22 | |
| P | <0.001 | <0.001 | 0.171 | 0.020 | |
| 8wk | RRD | 44.00±6.26 | 47.42±3.94 | 64.61±2.74 | 214.68±40.95 |
| Control | 48.91±4.74 | 54.22±3.28 | 64.76±2.77 | 251.00±26.57 | |
| P | <0.001 | <0.001 | 0.790 | 0.003 | |
| 12wk | RRD | 48.52±6.08 | 53.54±4.27 | 66.78±3.70 | 209.74±36.28 |
| Control | 49.25±4.44 | 54.33±3.38 | 64.48±2.97 | 250.58±25.74 | |
| P | 0.334 | 0.247 | 0.001 | 0.001 | |
| 16wk | RRD | 45.95±6.93 | 51.49±4.04 | 65.97±4.25 | 210.37±43.07 |
| Control | 48.93±4.75 | 54.28±3.32 | 64.80±2.76 | 250.16±26.13 | |
| P | 0.004 | 0.007 | 0.103 | 0.001 |
RRD: Rhegmatogenous retinal detachment; SCP: Superficial capillary plexus; DCP: Deep capillary plexus; CCP: Choriocapillaris plexus; VD: Vessel density; FMT: Foveal macular thickness. P: RRD vs control by paired t-test.
FMTs became thinner over time in the RRD eyes postoperatively (F=1.231, P=0.303; Table 2). The differences in the FMTs between RRD and fellow eyes became statistically significant since 4wk postoperatively (t=-2.557, P=0.020). There were no significant changes in the FMT over time in the fellow eyes (F=0.003, P>0.999; Table 2).
Significant increases were observed in the mean r/f ratios of the parafoveal SCP, DCP, and CCP VDs from 2 to 12wk postoperatively, then decreased at 16wk postoperatively (F=15.404, P<0.001; F=18.895, P<0.001; F=9.277, P<0.001; Figure 3). The mean r/f ratios of the parafoveal SCP and DCP VDs were lower than those of the parafoveal CCP VD at all time-points postoperatively (all P<0.05). There were nonsignificant differences in the r/f ratios between SCP and DCP VDs at all time-points postoperatively (all P>0.05).
Figure 3. The curve of mean r/f ratio of parafoveal VDs.
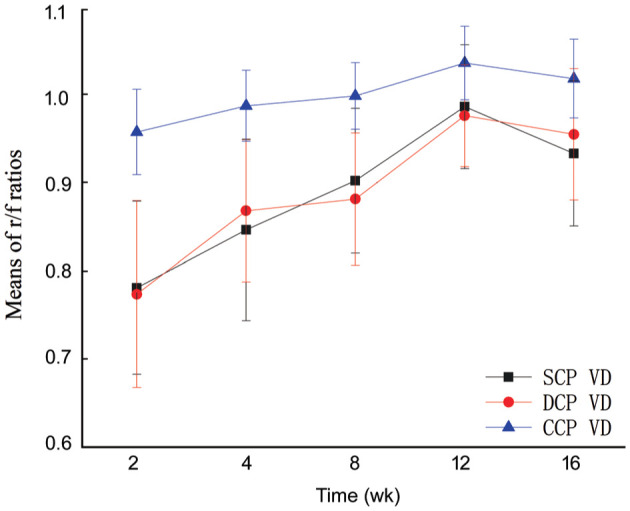
The mean r/f ratios of the parafoveal SCP, DCP, and CCP VDs increased within 12wk postoperatively, then decreased at the 16wk postoperatively.
DISCUSSION
The retinal microcirculation system includes retinal and choroidal capillary networks. The SCP and the DCP, which are predominantly located in the ganglion cell layer and the inner nuclear layer respectively, provide nourishment to the inner retinal layers. The outer retinal layers, including photoreceptors, are avascular regions supplied with nutrients and oxygen by choriocapillaris. In the past, many techniques were applied to detect retinal blood flow in RRD eyes. Nowadays, OCTA has been applied to analyze the macular VD per layer in macula-off RRD eyes[11],[13]–[18], which was a rapid, non-invasive manner without dye injection.
Wang et al[13] reported that the SCP and DCP VDs were significantly lower in the RRD eyes than in the fellow healthy eyes at 2wk postoperatively after successful PPV intraocular gas tamponade using OCTA. Our study showed that the parafoveal SCP and DCP VDs in RRD eyes after PPV with SO tamponade were lower than in the fellow eyes in the early postoperative period, consistent with gas tamponade. Previous study proved that the retinal blood flow on the optic nerve head (ONH) by laser speckle flowgraph is reduced in RRD eyes preoperatively, but there were no significant differences in the mean blur rate (MBR)-vessel on the ONH in eyes with an epiretinal membrane (ERM) or a cataract following the PPV surgery[19]. Therefore, we thought that RRD itself resulted in the decrease of macular VD in the early period postoperatively.
Wang et al[13] reported that significant increases were observed in the parafoveal SCP and DCP VDs over time postoperatively in the RRD eyes, and both values were not significantly different from those in fellow healthy eyes at 12wk postoperatively, which was consistent with our study results. Hong et al[17] reported that the SCP and DCP VDs in the RRD eyes did not differ significantly from those in the fellow eyes 6mo postoperatively. Lee et al[16] reported that SCP VD in the treated RRD eye was not significantly different from the normal contralateral eye at 3mo after SO removal. Therefore, we thought that the SCP and DCP VDs in the SO-filled RRD eyes might rehabilitate over time in the early postoperative period, but never reached the level of healthy eyes.
The safety of SO has been controversial, although it is a valuable tool for the treatment of retinal detachment. Until now, whether SO has side effects on macular VD remains unclear. In the previous study of 3-month follow-up, the parafoveal retinal VDs in SO-filled eyes increased but were still lower than those of healthy fellow eyes[18]. Our study showed that the macular VDs rehabilitated over time after vitrectomy with SO tamponade within 12wk after surgery. But the parafoveal SCP and DCP VDs decreased unexpectedly at 16wk postoperatively. Lee et al[16] revealed that the duration of SO tamponade was significantly correlated with DCP VD. We cautiously speculated that macular VDs decreased at a specific time-point after surgery because of the harmful effect of SO on retinal tissues and harmful substances in the removed SO[20]–[21].
Previous studies showed that CCP VD decreased in macula-off RRD eyes[13],[17] and increased over time after surgery[13], consistent with our study. But our study proved that the rehabilitation curve of CCP VD was earlier than that of gas tamponade and decreased at 16wk postoperatively. The difference might come from SO tamponade instead of gas tamponade. The mechanisms of impairment or restoration of the choriocapillaris before and after surgery remain unclear. Teng et al[22] reported an increase in CCP VD after PPV in a patient with an idiopathic macular hole (IMH) using OCTA. Ahn et al[23] believed that the possible restoration of the choriocapillaris was associated with the restoration of the retinal structure. The choroid has intrinsic neurons, mostly behind the central retina, which may modulate choroidal blood flow as well[24]. Obviously, RRD itself and retinal repair are the incentives to choroidal blood flow, which might be the reason why the CCP VD increased over time within 12wk and decreased at 16wk postoperatively, consistent with the result of the retinal repair.
Figure 3 shows that the rehabilitation curves of parafoveal SCP and DCP VDs were similar postoperatively. The difference of mean r/f ratios between parafoveal SCP and DCP VDs was not statistically significant at all time-points postoperatively. A previous study showed that the SCP and DCP interconnect via a perpendicular vessel and nourish the inner retinal layers[25]. Therefore, we speculated that the changes in parafoveal SCP and DCP VDs were consistent in RRD eyes after retinal repair. In our study, the mean r/f ratios of parafoveal SCP and DCP VDs were lower than those of parafoveal CCP VD at all time-points postoperatively. Then, all the mean r/f ratios of parafoveal VDs increased over time within 12wk postoperatively. We suspected that the parafoveal SCP and DCP VDs were more susceptible to RRD itself and retinal repair than the parafoveal CCP VD.
Hong et al[17] reported that FMT decreased in the macula-off RRD eyes treated with PPV and gas tamponade 6mo after surgery compared with fellow healthy eyes (P=0.009), but FMT in macula-on RRD eyes was thicker than in fellow eyes (P=0.130). This result was consistent with that of the study by Bonfiglio et al[15] at a minimum of 12mo postoperatively. Therefore, macular involvement itself might possibly be one factor of the FMT decrease in the treated RRD eyes. Previous studies proved that retinal thickness increased overtime after PPV with gas tamponade[6],[26]. In the present study, the FMTs decreased over time postoperatively in the RRD eyes. Therefore, we speculated that mechanical stress to the macula and potential hazards coming from the SO might be one of the main reasons for retinal thinning observed in our study. Several studies have revealed a significant reduction in macular thickness in eyes operated with SO tamponade[27]–[29]. Another study showed that SO removal alone can achieve resolution of these structural changes when faced with changes in macular thinning during tamponade[30].
Our study has several limitations. First, a retrospective study and all participants from a single hospital might have caused a selection bias. Second, this study had a relatively small sample size, and we only evaluated the macular VD changes throughout the postoperative 16wk. A larger sample size should be performed to confirm our findings, and there might be a different result after a longer investigation period. Third, the OCTA findings in this present study are possibly due to RRD itself or surgical factors cannot be excluded. Finally, retinal reattachment was defined in the state of SO tamponade.
In conclusion, our study showed that the postoperative macular VDs decreased and gradually improved over time in the macular-off RRD eyes treated with PPV and SO tamponade. The postoperative macular VDs decreased again in SO-filled eyes beyond 3mo. The recovery of parafoveal SCP and DCP VDs was consistent in RRD eyes after retinal repair. The parafoveal SCP and DCP VDs were more vulnerable to surrounding changes than the parafoveal CCP VD in the RRD eyes postoperatively.
Acknowledgments
The authors thank Prof. Ming-Lin Liu for his helpful scientific discussions on this work.
Conflicts of Interest: Jiang J, None; Chen S, None; Jia YD, None; Li R, None; Zhou JX, None; Li RM, None.
REFERENCES
- 1.Machemer R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. XLI Edward Jackson Memorial Lecture. Am J Ophthalmol. 1984;98(6):681–693. doi: 10.1016/0002-9394(84)90682-2. [DOI] [PubMed] [Google Scholar]
- 2.Hatef E, Sena DF, Fallano KA, Crews J, Do DV. Pneumatic retinopexy versus scleral buckle for repairing simple rhegmatogenous retinal detachments. Cochrane Database Syst Rev. 2015(5):CD008350. doi: 10.1002/14651858.CD008350.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bussel EM, van der Valk R, Bijlsma WR, La Heij EC. Impact of duration of macula-off retinal detachment on visual outcome: a systematic review and meta-analysis of literature. Retina. 2014;34(10):1917–1925. doi: 10.1097/IAE.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y, Chen S, Gu ZH, Zhang YL, Li LY, Yang N. Natural history of persistent subretinal fluid following the successful repair of rhegmatogenous retinal detachment. Int J Ophthalmol. 2020;13(10):1621–1628. doi: 10.18240/ijo.2020.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park DH, Choi KS, Sun HJ, Lee SJ. Factors associated with visual outcome after macula-off rhegmatogenous retinal detachment surgery. Retina. 2018;38(1):137–147. doi: 10.1097/IAE.0000000000001512. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Iwase T, Yamamoto K, Ra E, Murotani K, Matsui S, Terasaki H. Association between photoreceptor regeneration and visual acuity following surgery for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2016;57(3):889–898. doi: 10.1167/iovs.15-18403. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(8):3907–3913. doi: 10.1167/iovs.16-19570. [DOI] [PubMed] [Google Scholar]
- 8.Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M, Coscas G, Souied EH. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016;161:160–171.e1-2. doi: 10.1016/j.ajo.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Farecki ML, Gutfleisch M, Faatz H, Rothaus K, Heimes B, Spital G, Lommatzsch A, Pauleikhoff D. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-Angiography. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):913–921. doi: 10.1007/s00417-017-3588-y. [DOI] [PubMed] [Google Scholar]
- 10.Woo JM, Yoon YS, Woo JE, Min JK. Foveal avascular zone area changes analyzed using OCT angiography after successful rhegmatogenous retinal detachment repair. Curr Eye Res. 2018;43(5):674–678. doi: 10.1080/02713683.2018.1437922. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Aggarwal K, Akella M, Agrawal R, Khandelwal N, Bansal R, Singh R, Gupta V, OCTA Study Group Fractal dimension and optical coherence tomography angiography features of the central macula after repair of rhegmatogenous retinal detachments. Retina. 2019;39(11):2167–2177. doi: 10.1097/IAE.0000000000002276. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa Y, Shoji T, Kanno J, Ibuki H, Ozaki K, Ishii H, Ichikawa Y, Kimura I, Shinoda K. Evaluation of microvascular changes in the macular area of eyes with rhegmatogenous retinal detachment without macular involvement using swept-source optical coherence tomography angiography. Clin Ophthalmol. 2018;12:2059–2067. doi: 10.2147/OPTH.S177933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Xu X, Sun XD, Ma YY, Sun T. Macular perfusion changes assessed with optical coherence tomography angiography after vitrectomy for rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2019;257(4):733–740. doi: 10.1007/s00417-019-04273-7. [DOI] [PubMed] [Google Scholar]
- 14.Tsen CL, Sheu SJ, Chen SC, Wu TT. Imaging analysis with optical coherence tomography angiography after primary repair of macula-off rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1847–1855. doi: 10.1007/s00417-019-04381-4. [DOI] [PubMed] [Google Scholar]
- 15.Bonfiglio V, Ortisi E, Scollo D, Reibaldi M, Russo A, Pizzo A, Faro G, Macchi I, Fallico M, Toro MD, Rejdak R, Nowomiejska K, Toto L, Rinaldi M, Cillino S, Avitabile T, Longo A. Vascular changes after vitrectomy for rhegmatogenous retinal detachment: optical coherence tomography angiography study. Acta Ophthalmol. 2020;98(5):e563–e569. doi: 10.1111/aos.14315. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Kim JY, Lee SY, Jeong JH, Lee EK. Foveal microvascular structures in eyes with silicone oil tamponade for rhegmatogenous retinal detachment: a swept-source optical coherence tomography angiography study. Sci Rep. 2020;10(1):2555. doi: 10.1038/s41598-020-59504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong EH, Cho H, Kim DR, Kang MH, Shin YU, Seong M. Changes in retinal vessel and retinal layer thickness after vitrectomy in retinal detachment via swept-source OCT angiography. Invest Ophthalmol Vis Sci. 2020;61(2):35. doi: 10.1167/iovs.61.2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roohipoor R, Tayebi F, Riazi-Esfahani H, Khodabandeh A, Karkhaneh R, Davoudi S, Khurshid GS, Momenaei B, Ebrahimiadib N, Modjtahedi BS. Optical coherence tomography angiography changes in macula-off rhegmatogenous retinal detachments repaired with silicone oil. Int Ophthalmol. 2020;40(12):3295–3302. doi: 10.1007/s10792-020-01516-z. [DOI] [PubMed] [Google Scholar]
- 19.Iwase T, Kobayashi M, Yamamoto K, Yanagida K, Ra E, Terasaki H. Changes in blood flow on optic nerve head after vitrectomy for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2016;57(14):6223–6233. doi: 10.1167/iovs.16-20577. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Iriyama A, Kadonosono K, Tamaki Y, Yanagi Y. Effects of perfluorocarbon liquids and silicone oil on human retinal pigment epithelial cells and retinal ganglion cells. Retina. 2009;29(5):677–681. doi: 10.1097/IAE.0b013e318196fca1. [DOI] [PubMed] [Google Scholar]
- 21.Bambas B, Eckardt C, Vowinkel E, Kruse H. Toxic substances with silicone oil after intraocular injections. Ophthalmologe. 1995;92(5):663–667. [PubMed] [Google Scholar]
- 22.Teng YF, Yu M, Wang Y, Liu XX, You QS, Liu W. OCT angiography quantifying choriocapillary circulation in idiopathic macular hole before and after surgery. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):893–902. doi: 10.1007/s00417-017-3586-0. [DOI] [PubMed] [Google Scholar]
- 23.Ahn J, Yoo G, Kim JT, Kim SW, Oh J. Choriocapillaris layer imaging with swept-source optical coherence tomography angiography in lamellar and full-thickness macular hole. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):11–21. doi: 10.1007/s00417-017-3814-7. [DOI] [PubMed] [Google Scholar]
- 24.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis) J Neurosci. 1992;12(4):1169–1193. doi: 10.1523/JNEUROSCI.12-04-01169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.dell'Omo R, Viggiano D, Giorgio D, Filippelli M, di Iorio R, Calo' R, Cardone M, Rinaldi M, dell'Omo E, Costagliola C. Restoration of foveal thickness and architecture after macula-off retinal detachment repair. Invest Ophthalmol Vis Sci. 2015;56(2):1040–1050. doi: 10.1167/iovs.14-15633. [DOI] [PubMed] [Google Scholar]
- 27.Christensen UC, la Cour M. Visual loss after use of intraocular silicone oil associated with thinning of inner retinal layers. Acta Ophthalmol. 2012;90(8):733–737. doi: 10.1111/j.1755-3768.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 28.Caramoy A, Droege KM, Kirchhof B, Fauser S. Retinal layers measurements in healthy eyes and in eyes receiving silicone oil-based endotamponade. Acta Ophthalmol. 2014;92(4):e292–e297. doi: 10.1111/aos.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH, Han JW, Byeon SH, Kim SS, Koh HJ, Lee SC, Kim M. Retinal layer segmentation after silicone oil or gas tamponade for macula-on retinal detachment using optical coherence tomography. Retina. 2018;38(2):310–319. doi: 10.1097/IAE.0000000000001533. [DOI] [PubMed] [Google Scholar]
- 30.Lo DM, Flaxel CJ, Fawzi AA. Macular effects of silicone oil tamponade: optical coherence tomography findings during and after silicone oil removal. Curr Eye Res. 2017;42(1):98–103. doi: 10.3109/02713683.2016.1146776. [DOI] [PubMed] [Google Scholar]