Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l‐histidine monohydrochloride (HCl) monohydrate produced by fermentation with Escherichia coli (NITE SD 00268) when used as a nutritional additive or as a feed flavouring compound in feed for all animal species. The active substance of the additive is l‐histidine. The production strain has been modified by conventional mutagenesis and it does not raise safety concerns. The additive under assessment is safe for the target species when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet and the status of some essential trace elements. This conclusion would cover its use as flavouring compound. l‐Histidine HCl monohydrate produced by E. coli NITE SD 00268, when used at the proposed conditions of use, is safe for the consumer and for the environment. l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 is not a skin irritant. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or a skin sensitiser. The additive l‐histidine HCl monohydrate is regarded as an effective source of the amino acid l‐histidine when used as a nutritional additive. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen. l‐Histidine is efficacious as a flavouring compound.
Keywords: nutritional additive, amino acid, flavouring compound, l‐histidine monohydrochloride monohydrate, Escherichia coli NITE SD 00268, Safety
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Kyowa Hakko Europe GmbH2 for authorisation of the product l‐histidine monohydrochloride monohydrate produced using Escherichia coli NITE SD 00268, when used as a feed additive for all animal species (categories: nutritional additives and sensory additives; functional groups: amino acids and flavouring compounds).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 20 November 2020.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐histidine monohydrochloride monohydrate minimum 98% produced using Escherichia coli NITE SD 00268, when used under the proposed conditions of use (see Section 3.1.3).
1.2. Additional information
The additive under assessment is authorised in the European Union for all fin fish.3 The current application is for an extension of the authorisation of l‐histidine monohydrochloride (HCl) monohydrate minimum 98% produced by E. coli NITE SD 00268 for its use as a nutritional additive for all animal species, and as a flavouring compound.
l‐Histidine is authorised for use in food,4 cosmetics5 and as a veterinary medicinal product.6,7
l‐Histidine HCl monohydrate is described in a monograph of the European Pharmacopoeia (PhEur 10th edition, 2021): monograph 01/2005:0910.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier8 in support of the authorisation request for the use of l‐histidine HCl monohydrate minimum 98% produced by E. coli NITE SD 00268 as a feed additive for all animal species.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in the previous assessment regarding the methods used for the control of the l‐histidine HCl monohydrate in animal feed are valid and applicable for the current application.9
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐histidine HCl monohydrate minimum 98% produced by E. coli NITE SD 00268 is in line with the principles laid down in Regulation (EC) No 429/200810 and the relevant guidance documents: Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019a) and the Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018b).
3. Assessment
The additive l‐histidine HCl monohydrate (minimum 98%) produced by a non‐genetically modified strain of E. coli (NITE SD 00268) is authorised as a nutritional additive for use in all finfish.11 This assessment regards the request to extend the use of the additive as a nutritional additive (functional group: amino acids, their salts and analogues) and its use as a sensory additive (functional group: flavouring compounds) in feed for all animal species.
3.1. Characterisation
The additive was characterised in the previous opinion (EFSA FEEDAP Panel, 2020), including the production strain (modified by conventional mutagenesis) and the manufacturing process. The applicant has provided new data on the characterisation of the additive which are reported below.
3.1.1. Characterisation of the active substance/additive
l‐Histidine monohydrochloride monohydrate (International Union of Pure and Applied Chemistry (IUPAC) name: (2S)‐2‐amino‐3‐(1H‐imidazol‐5‐yl)propanoic acid hydrate hydrochloride), is a compound identified with the Chemical Abstract Service (CAS) No 5934‐29‐2, and the European Inventory of Existing Commercial Chemical Substances (EINECS) No 211‐438‐9. l‐Histidine is used in food as a flavouring compound [17.008]. It has a molecular weight of 209.63 Da. The chemical formula of l‐histidine monohydrochloride monohydrate is C6H9N3O2.HCl.H2O. The structural formula is given in Figure 1.
Figure 1.
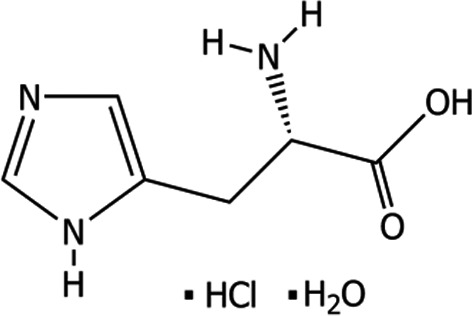
Structural formula of l‐histidine monohydrochloride monohydrate
The additive is a powder specified to contain a minimum of 98% l‐histidine HCl monohydrate and not more than 1% loss on drying.
The batch‐to‐batch variation of 5 batches of the additive showed an average histidine content of 74.3% (range 72.8–75.2%) on as is basis,12 and a loss on drying of 0.1% (range 0–0.1%). The histidine content is equivalent to approximately 100% l‐histidine HCl monohydrate (the proportion of HCl and water by stoichiometric calculation in l‐histidine HCl monohydrate is of 26%). The amount of identified material on a dry matter basis was > 99%.
Impurities
The analyses of three batches of the additive for heavy metals (lead, mercury and cadmium) and arsenic showed values below the limits of quantification (LOQs).13 Mycotoxins (aflatoxins B1, B2, G1, G2; deoxynivalenol, zearalenone, T2 toxin, HT2 toxin, nivalenol and fumonisins B1 and B2) were below the LOQ.14
Microbial contamination of three batches of the additive showed that total plate counts at 30°C were below the LOQ except in one batch in which it was 10 CFU/g. Coliforms at 30°C were below the LOQ. Salmonella spp. was not detected in 25 g samples, and Enterobacteriaceae, yeasts and filamentous fungi were not detected in 1 g samples.15
3.1.2. Stability and homogeneity
New information on the stability of the additive in premixtures and feedingstuffs, as well as on its capacity to distribute homogeneously in feed for chickens for fattening was provided and is described here.16
The stability of the additive (one batch) in a vitamin/mineral premixture containing 46,100 mg choline chloride/kg was studied when supplemented with 10.3% histidine and stored at 25°C in sealed polyethylene pots for 6 months. At the end of the storage period, a loss of 9% free histidine was observed.
The stability of the additive (one batch) in a complete feed for chickens for fattening (meal and pelleted) was studied when supplemented with 0.14% histidine HCl monohydrate (corresponding to 0.1% supplemented histidine) and stored at 25°C in sealed polyethylene pots for 3 months. The basal diet consisted of wheat, barley and rapeseed meal, had a background histidine of 0.37%, and the additive was directly added to the complete feed. Pelleting was performed at 80°C and the pelleting process caused no histidine loss. No losses were observed at the end of the storage period in meal or pelleted feed.
The stability of the additive (one batch) in a complete feed for pigs for fattening (mash and pelleted) was studied when supplemented with 0.14% histidine HCl monohydrate (corresponding to 0.1% histidine) and stored at 25°C in sealed polyethylene pots for 3 months. The basal diet consisted of wheat, soybean meal and maize, had a background histidine of 0.54%, and the additive was directly added to the complete feed. Pelleting was performed at 69°C and the pelleting process caused a loss of 8% histidine. At the end of the storage period, an additional loss of 8% was observed in meal feed and of 9% in pelleted feed.
The stability of the additive (one batch) in a complete feed for ruminants (mash and pelleted) was studied when supplemented with 0.14% histidine HCl monohydrate (corresponding to 0.1% histidine) and stored at 25°C in sealed polyethylene pots for 3 months. The basal diet consisted of rapeseed meal, soybean hulls and wheat bran, had a background histidine of 0.45%, and the additive was directly added to the complete feed. Pelleting was performed at 67°C and the pelleting process caused no loss of histidine. No losses were observed at the end of the storage period in meal or pelleted feed.
The capacity of the additive to distribute homogeneously in feed for chickens for fattening was studied measuring free histidine in the meal and pelleted feed described above. A total of 20 subsamples of each form were analysed.17 The coefficient of variation was 8% in the meal feed and 6% in the pelleted feed.
3.1.3. Conditions of use
The additive is currently authorised for finfish as nutritional, and the applicant asks for the extension of the authorisation for all species. The additive can be added directly in compound feed, through complementary feed or through premixtures and is aimed for all animal species. No proposed inclusion levels are provided, as the optimal daily allowance in quantitative terms depends on the species, the physiological state of the animal, the performance level and the environmental conditions, in particular on the amino acid composition of the unsupplemented diet.
l‐Histidine monochloride monohydrate is proposed to be used also as a feed flavouring in feed for all animal species, at a typical inclusion rate of 5 mg/kg, being the maximum recommended level of inclusion 25 mg/kg.
3.2. Safety
3.2.1. Safety for the target species
The essentiality of the amino acid histidine, its content in feedingstuffs, the requirements for the different target species, normal use levels, absorption, distribution, metabolism and excretion of histidine were discussed in a previous opinion of the FEEDAP Panel (EFSA FEEDAP Panel, 2020).
The additive is highly purified, containing > 98% l‐histidine HCl monohydrate, and the amount of unidentified material is < 1% on a dry matter basis. Concerns from the use of the additive would not derive from the amino acid l‐histidine, which is considered safe, but may arise from residues of the fermentation process/production strain remaining in the final product. The production microorganism is a non‐genetically modified strain ■■■■■ and is considered safe (EFSA FEEDAP Panel, 2020). No cells or DNA of the production strain were found in the final product. Endotoxin activity of the product (< 100 IU/g) is considered low and of no concern for the target species (Wallace et al., 2016).18 The FEEDAP Panel considers that no safety concerns would derive from the fermentation process.
l‐Histidine chelates divalent metal ions and it is necessary for the regulation and catabolism of trace elements such as zinc, copper, iron, manganese and molybdenum. High levels of histidine could therefore theoretically cause deficiencies of the free forms of these metal ions due to increased excretion (Aoyama et al., 1992; Aoyama and Cato, 2000; EFSA, 2005; VKM, 2016). This interaction of histidine with trace elements should be considered when formulating the animal diets.
The proposed use of l‐histidine monohydrochloride monohydrate as flavouring (up to 25 mg/kg complete feed) is substantially lower than the animal requirements (range from 1,500 to 6,000 mg/kg feed) and/or the supplementation of histidine in complete feeds which varies from 500 mg/kg (laying hens and gestating sows) to 3,500 mg/kg feed (chickens for fattening and early weaned piglets) (EFSA FEEDAP Panel, 2019b). Therefore, the FEEDAP Panel considers the additive is safe when used as a flavouring compound at recommended levels.
3.2.1.1. Conclusions on safety for the target species
The use of l‐histidine monohydrochloride monohydrate produced by fermentation using E. coli NITE SD 00268 is safe for the target species when used as nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet. However, the Panel notes that the interaction of histidine with trace elements should be considered in the formulation of the animal diets. These conclusions would also cover the proposed use as a flavouring compound according to the proposed conditions of use.
3.2.2. Safety for the consumer, the user and the environment
The safety of the product under assessment was assessed in a previous opinion and it was concluded that the use of l‐histidine in feed for finfish does not pose a risk for the safety of the consumers (EFSA FEEDAP Panel, 2020). To the knowledge of the FEEDAP Panel, there are no records of histamine poisoning associated with raw mammal or poultry edible tissues and products. Therefore, the FEEDAP Panel considers it unlikely that supplementation of feed with histidine to cover the requirements of animals other than finfish will increase the risk of histamine poisoning.
The safety for the user of the additive under assessment was assessed in a previous opinion and it was concluded that the additive is not a skin irritant and it is not hazardous by inhalation due to exposure to endotoxins for people handling the additive. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or a skin sensitiser (EFSA FEEDAP Panel, 2020). No additional information was provided in the dossier that would justify a change of the previous conclusions.19
In a previous opinion it was concluded that the use of l‐histidine HCl monohydrate produced by E. coli NITE SD 00268 in animal nutrition is not expected to represent a risk to the environment (EFSA FEEDAP Panel, 2020).
3.2.2.1. Conclusions on the safety for the consumer, the user and the environment
The FEEDAP Panel considers that the proposed extension of uses (to all animal species and use as flavouring compound) would not introduce concerns not already considered in the previous assessments. l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268, when used at the proposed conditions of use, is safe for the consumer and the environment.
As regards the safety for the user, l‐histidine HCl monohydrate produced using E. coli NITE SD 00268 is not a skin irritant. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or to be a skin sensitiser.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐histidine monohydrochloride monohydrate is well established in the scientific literature (NRC, 1994, 1998, 2011, 2012).
In general, the product l‐histidine monohydrochloride monohydrate is considered as efficacious source of the essential amino acid l‐histidine for non‐ruminant animal species. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
l‐Histidine is used in food as a flavouring compound [17.008] and it is expected to have a similar function in feed. Therefore, the FEEDAP Panel considers that no further data is necessary to demonstrate its flavouring properties.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation20 and Good Manufacturing Practice.
4. Conclusions
The use of l‐histidine HCl monohydrate produced by fermentation using E. coli NITE SD 00268 is safe for the target species when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet and the status of some essential trace elements. This conclusion would cover its use as a flavouring compound.
l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 when used at the proposed conditions of use is safe for the consumer and for the environment.
l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 is not a skin irritant. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or to be a skin sensitiser.
l‐Histidine HCl monohydrate is considered an efficacious source of the essential amino acid l‐histidine for non‐ruminant animal species. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen. l‐Histidine is efficacious as a flavouring compound.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 13/07/2020 | Dossier received by EFSA. L‐Histidine monohydrochloride monohydrate for all animal species. Kyowa Hakko Europe GmbH. |
| 11/09/2020 | Reception mandate from the European Commission |
| 20/11/2020 | Application validated by EFSA – Start of the scientific assessment |
| 22/02/2021 | Comments received from Member States |
| 03/03/2021 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the additive, characterization of the production strain, safety for the user. |
| 10/03/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 05/05/2021 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- NITE
Japanese National Institute of Technology and Evaluation
- NRC
National Research Council
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Herman L, Anguita M, Galobart J, Pettenati E and Tarrés‐Call J, 2021. Scientific Opinion on the safety and efficacy of a feed additive consisting of l‐histidine monohydrochloride monohydrate produced using Escherichia coli NITE SD 00268 for all animal species (Kyowa Hakko Europe GmbH). EFSA Journal 2021;19(5):6622, 10 pp. 10.2903/j.efsa.2021.6622
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00604
Panel members: Giovanna Azimonti, Vasileios Bampidis Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa, and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel wishes to acknowledge the contribution of Davide Guerra, Orsolya Holczknecht Paola Manini, Fabiola Pizzo to this opinion.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © ECDC
Adopted: 5 May 2021
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Kyowa Hakko Europe GmbH, Am Wehrhain 50, D‐40211 Dűsseldorf, Germany.
Commission Implementing regulation (EU) 2020/2116 of 16 December 2020 concerning the renewal of the authorisation of l‐histidine monohydrochloride monohydrate produced by Escherichia coli ATCC 9637 as a feed additive for salmonids and its extension of use to other finfish, and repealing Regulation (EC) No 244/2007. OJ L 426, 17.12.2020, p 7.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009, OJ L 181, 29.6.2013, p. 35.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OL L 152, 16.6.2009, p. 11.
FEED dossier reference: FAD‐2020‐0056.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2006-0022.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Commission implementing Regulation (EU) 2020/2116 of 16 December 2020 concerning the renewal of the authorisation of l‐histidine monohydrochloride monohydrate produced by Escherichia coli ATCC 9637 as a feed additive for salmonids and its extension of use to other finfish, and repealing Regulation (EC) No 244/2007. OJ L 426/8, 17.12.2020, p. 3.
Technical dossier/Section II/Annex II.1. Histidine was analysed using the European Official method as described in Commission Regulation EC 152/2009.
Technical dossier/Section II/Annex II.1. LOQ in µg/kg was 5 for mercury, 10 for cadmium, 50 for lead and 100 for mercury.
Technical dossier/Section II/Annex II.1. LOQ in µg/kg was 0.1 for each aflatoxin; 10 for zearalenone, T2 toxin and HT2 toxin; 20 for deoxynivalenol and nivalenol; and 200 for each fumonisin.
Technical dossier/Section II/Annex II.1 and supplementary information March 2021/Sin reply KHB. LOQ in CFU/g was 10 for average plate count, and 1 for coliforms.
Technical dossier/Section II/Annexes II.47 and II.48.
Technical dossier/Section II/Annex II.48.
Normal feedingstuffs may be contaminated with varying concentrations of endotoxins, with values of 1,000 IU/mg feed not being unusual.
Technical dossier/Supplementary information March 2021/Sin reply KHB.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Aoyama Y and Cato C, 2000. Suppressive effect of excess dietary histidine on the expression of hepatic metallothionein‐1 in rats. Bioscience, Biotechnology and Biochemistry, 64, 588–591. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Mori M, Hitomi‐Ohmura E and Yoshida A, 1992. Effects of dietary excess histidine and varying levels of copper on the metabolism of lipids and minerals in rats. Bioscience, Biotechnology and Biochemistry, 56, 335–337. [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on the safety and the bioavailability of product L‐Histidine monohydrochloride monohydrate for salmonids. EFSA Journal 2005;3(4):195, 10 pp. 10.2903/j.efsa.2005.195 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019a. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Flachowsky G, Glandorf B, Herman L, Mantovani A, Saarela M, Svensson K, Tosti L, Wallace RJ, Anguita M, Tarrés‐Call J and Ramos F, 2019b. Safety and efficacy of L‐histidine monohydrochloride monohydrate produced using Corynebacterium glutamicum KCCM 80172 for all animal species. EFSA Journal 2019;17(7):5783, 20 pp. 10.2903/j.efsa.2019.5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Glandorf B, Herman L, Mantovani A, Saarela M, Svensson K, Tosti L, Anguita M, Pettenati E, Tarrés‐Call J and Ramos F, 2020. Scientific Opinion on the assessment of the application for renewal of authorisation of l‐histidine monohydrochloride monohydrate produced with Escherichia coli NITE SD 00268 for salmonids and its extension of use to other fin fish. EFSA Journal 2020;18(4):6072, 23 pp. 10.2903/j.efsa.2020.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council), 1994. Nutrient Requirements of Poultry, 9th revised Edition. The National Academies Press, Washington, DC, 176 pp. [Google Scholar]
- NRC (National Research Council), 1998. Nutrient Requirements of Swine. 10th Revised Edition. Washington, DC, The National Academies Press, 212 pp. 10.17226/6016 [DOI] [Google Scholar]
- NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington, DC, USA, 376 pp. [Google Scholar]
- NRC (National Research Council), 2012. Nutrient Requirement of Swine. 11th revised Edition. The National Academies Press, Washington, DC. 10.17226/13298 [DOI] [Google Scholar]
- PhEur (European Pharmacopoeia), 10th Edition, 2021. European Directorate for the Quality of Medicines and Health, Monograph 01/2017:0910. [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety), 2016. Risk assessment of “other substances” – l‐histidine. Opinion of the Panel on Nutrition, dietetic products, Novel Food an Allergy of the Norwegian Scientific Committee for Food Safety. ISBN: 978‐82‐8259‐214‐7, Oslo, Norway. Available online: https://vkm.no/download/18.645b840415d03a2fe8f2600f/1502800356013/Risk%20assessment%20of%20%22other%20substances%22%20%E2%80%93%20L-histidine.pdf
- Wallace RJ, Gropp J, Dierick N, Costa LG, Martelli G, Brantom PG, Bampidis V, Renshaw DW and Leng L, 2016. Risks associated with endotoxins in feed additives produced by fermentation. Environmental Health, 15, 5. 10.1186/s12940-016-0087-2. PMID: 26768246; PMCID: PMC4714429. [DOI] [PMC free article] [PubMed] [Google Scholar]


