Abstract
Transureteroureterostomy (TUU) is a urinary reconstructive procedure seldom used but has a role when conventional reconstructive techniques are not possible. However, the concern is whether it places the opposite, non-diseased ureter and kidney at risk. Hence a retrospective study was conducted to evaluate indications, methods, and outcomes of transureteroureterostomy in children. The study included seven children who underwent TUU between January 2011 and December 2015. The mean age of the study group was 4.5 ± 2.9 years. Six (86%) patients were males. Two patients had primary bladder diverticulum, two posterior urethral valves, two cases of vesico-ureteric reflux, and one had a persistent urogenital sinus. All patients presented with recurrent urinary tract infections. Three (43%) patients had bladder outlet obstruction. Four (57%) patients underwent left to right TUU with right ureteric reimplantation. Two (29%) patients underwent an additional procedure. No complications were found. The key to a good outcome in TUU is case selection. Surgical technique plays a very important role in ensuring good long-term outcome without compromising the normal moiety.
Keywords: Bladder diverticulum, posterior urethral valves, transureteroureterostomy, ureteric reimplantation, vesico-ureteric reflux
Introduction
After its introduction by Higgins in 1935 [1], Transureteroureterostomy (TUU) was used to treat a wide range of urinary tract disorders in pediatric and adult age groups [2,3]. But there is a concern on whether it places the opposite, non-diseased ureter and kidney at risk. This is one of the reasons TUU is infrequently done. But it is very useful in patients with tumor involving lower ureter, damaged lower ureter (traumatic or iatrogenic), lower ureteric strictures, failed ureteric reimplantation with an unhealthy ureter, patients with a history of pelvic radiation or previous pelvic surgery that preclude the use of ureteric reimplantation with or without bladder flap or psoas hitch [4]. TUU is a good alternative in patients whose dilated ureters can be used to augment the bladder (Posterior urethral valves, Neurogenic bladder, Large bladder diverticula) and in case of an incontinent bladder (Neurogenic or myogenic bladder) where the lower ureter can be used as a continent catheterizable stoma.
There are a few studies in the literature about TUU in adults, but the study on TUU in children is scarce. The versatility of this procedure lies in the fact that indications constitute a wide spectrum, and technique replicable. Broadly, this can be looked at as a salvage procedure when the bladder is small and reimplantation of ureters is not feasible or if there is an inadequate length of a ureter to reimplant into the bladder. The effectiveness of the procedure can be determined by the follow-up of renal functions and sonography. However, the fear of placing stress on the recipient kidney function has precluded its use. This retrospective clinical study was conducted to evaluate indications, methods, and outcomes of transureteroureterostomy in children. The technical aspects are also discussed, highlighting key surgical steps. Medium-term safety has been shown in this study and long-term safety established in the literature review.
Material and methods
A retrospective study was conducted in the Department of Pediatric Surgery in a tertiary care hospital. All children who underwent TUU between January 2011 and December 2015 were included in the study. Data were obtained from the hospital medical records. The child’s age, gender, clinical presentation, diagnosis, micturating cystourethrogram (MCU) findings, and nuclear imaging findings, type of procedure performed, indications, and additional procedures performed were documented.
Techniques of TUU
All children underwent open TUU after the informed assent and consent. The approach was either through a Pfannenstiel or a lower midline incision. The posterior peritoneum was incised to expose both the ureters. Ureters were mobilized extensively ensuring their vascular supply wasn’t hampered. The ipsilateral ureter was taken to the opposite side through a tunnel in the sigmoid mesentery. The site of anastomosis was selected in such a way that the ureter going across was not under any tension. Also, the recipient ureter was mobilized and reimplanted into the bladder first, and then TUU was done at an appropriate site along its length. To prevent ureteral impingement or angulation, the course of the transected and transposed ureter was always cranial to the inferior mesenteric artery. The length of anastomosis was kept equal to the diameter of the donor ureter. The anastomosis was an end-to-side, spatulated, using absorbable monofilament 6-0 RB interrupted sutures with knots outside (Figure 1). A double J stent was placed across the anastomosis with the lower J in the bladder. Foley’s catheter was used to drain the bladder for 48 hours. Stent removal was done in four to six weeks postoperatively.
Figure 1.
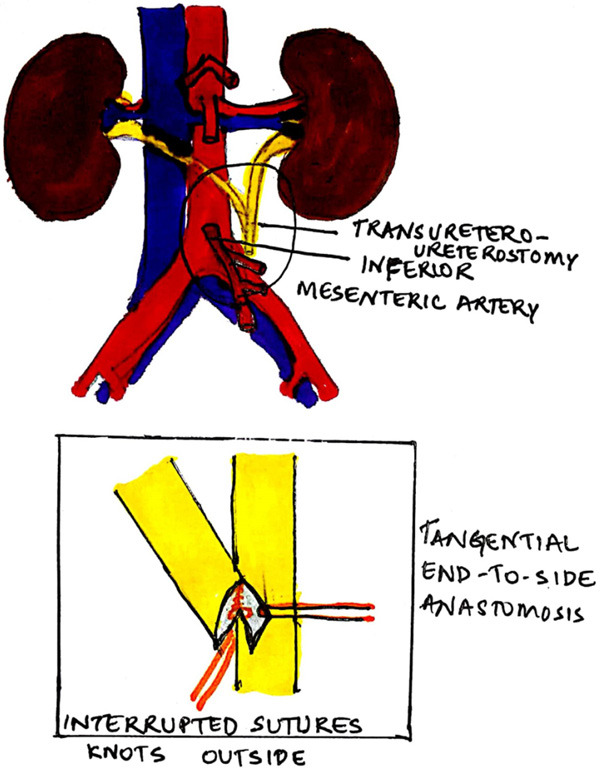
Technique of transureteroureterostomy.
Postoperative follow-up
The children post-TUU were followed up with an ultrasonogram (USG) to check for the status of kidneys and increasing hydronephrosis or hydroureter (indirect evidence of anastomotic stricture). Renal function tests were also done when indicated. Patients with worsening hydronephrosis underwent a diuretic renogram with a focus on the anastomotic site and also the lower end of the recipient ureter to check for any obstruction. A routine DMSA was done six months postoperatively. The outcomes documented were patency of the anastomosis, renal function, urolithiasis, stricture, and need for re-treatment.
Statistical analysis
The categorical variables such as gender, indications, and side of TUU were expressed as numbers and percentages. Continuous variables like age and follow-up were expressed as mean ± standard deviation.
Results
Seven patients underwent TUU during the study period. The mean age was 4.5 ± 2.9 years. The smallest child was a three-month-old boy and the eldest was a ten-year-old boy. Six were boys. All patients presented with recurrent urinary tract infections (UTI). Three (43%) patients had bladder outlet obstruction. All patients except one with unilateral large bladder diverticulum had bilateral hydroureteronephrosis on ultrasonography. All except 2 patients with bladder diverticulum demonstrated high-grade vesicoureteric reflux (VUR) on micturating cystourethrography. All patients with VUR showed bilateral renal scars on DMSA.
Inclusion criteria
The patient selection and inclusion criteria were mainly based on 2 factors, viz, ureteric reimplantation into a small bladder and/or inadequate length of the ureter due to previous surgery or other cause. Patients with a poor functioning recipient ureter’s kidney or unhealthy recipient ureter are best excluded.
Presentation and management
Four (57%) patients underwent left to right TUU with right ureteric reimplantation while three (43%) patients underwent right to left TUU with left ureteric reimplantation. Two patients underwent an additional procedure. One child had a formal bowel augment with appendicular Mitrofanoff and in another; the lower ureter end was brought out as a continent catheterizable stoma for clean intermittent catheterization. Follow-up ranged from five years to nine years with a mean follow-up of 7 ± 1.4 years. None of the patients developed surgical site infection. No urinary leak was found. None developed urinary stricture or urolithiasis. No patient developed renal failure, and none required re-treatment.
The individual presentation and management are as below.
Patient 1: A three-month-old male child presented with recurrent UTI. USG showed bladder diverticulum, MCU showed irregular bladder outline with no evidence of VUR. The Dimercaptosuccinic acid (DMSA) scan was normal. Cystoscopy revealed a para ureteric diverticulum. The ureteric opening was outside the diverticulum. He underwent laparoscopic extravesical bladder diverticulectomy. But he developed a prolonged urinary leak in the postoperative period. On open re-exploration, the terminal portion of the left ureter was unhealthy and that was also the site of the urinary leak. Intraoperatively, as there was an insufficient ureteric length for the reimplantation into the bladder, he underwent left to right TUU (Table 1). The child recovered and was discharged on the fifth postoperative day. His renal functions were preserved on follow-up. He had no further episodes of UTI.
Table 1.
Patient diagnosis and indication
| No. | Diagnosis | Indication |
|---|---|---|
| 1 | Primary large Bladder diverticulum | Iatrogenic ischemic necrosis of lower ureter. |
| 2 | Primary Bladder diverticulum | Small capacity bladder. |
| 3 | Posterior Urethral Valves with failed reimplantation | Failed reimplantation and short ureter. |
| 4 | Persistent urogenital sinus | Small capacity high pressure bladder, ureter used to augment bladder. |
| 5 | Horseshoe kidney with bilateral VURf with the left ectopic ureter | Short ureter. |
| 6 | Right Vesico-Ureteric Junction Obstruction with left Grade 5 VURf | Short ureter. |
| 7 | Posterior Urethral Valves with myogenic failure with VURf | Neurogenic bladder, ureter used as continent bladder stoma. |
Vesicoureteric reflux.
Patient 2: A two-year-old male child presented with recurrent UTI. USG showed bilateral paraureteric bladder diverticula and bilateral hydroureteronephrosis. MCU revealed an irregular bladder outline, two large bladder diverticula, and no vesicoureteric reflux (VUR). DMSA was normal. Cystoscopy confirmed bilateral paraureteric diverticula. Ureteric openings were outside the diverticula. He underwent open excision of the bilateral diverticula by a combined intra and extravesical approach. Bilateral ureteric reimplantation was not feasible due to small bladder hence left to right TUU and right-sided Cohen’s ureteric reimplantation was done (Table 1). He is thriving well on follow-up. Renal functions are preserved. No further episodes of UTI. USG on follow-up showed an adequate bladder volume.
Patient 3: A four-year-old male child presented to us in urosepsis. He was treated with antibiotics and bladder catheterization. He had undergone the fulguration of Posterior Urethral Valves (PUV) during neonatal life. He had also undergone bilateral Politano-Leadbetter ureteric reimplantation elsewhere for bilateral grade-5 VUR and recurrent UTI, six months before admission to our hospital. No further details were available about these procedures. Once the child improved and urine culture was negative, MCU was repeated, which showed bilateral failed ureteric reimplantation (bilateral grade-5 VUR). Dimercaptosuccinic acid (DMSA) scan showed bilateral scars. Cystoscopy revealed a mildly trabeculated bladder, wide-open ureteric orifices, and no bladder outlet obstruction. The child was posted for bilateral open Cohen’s ureteric reimplantation. After mobilizing the ureters, the lower part of the left ureter looked unhealthy, hence underwent left to right TUU and right-sided Cohen’s ureteric reimplantation (Table 1). Follow up was uneventful. No further episodes of UTI. Renal functions are preserved.
Patient 4: A four-year-old female child presented with recurrent UTI and urinary retention. On examination, she had persistent urogenital sinus. The anus was normal. USG showed bilateral hydroureteronephrosis. MCU revealed a small capacity bladder with a left-sided grade-5 VUR, DMSA showed bilateral scars, and cystoscopy found a 1 cm long common channel. She underwent bladder augmentation using the lower part of the grossly dilated left ureter, left to right TUU, right-sided Cohen’s ureteric reimplantation, Mitrofanoff, and partial urogenital mobilization (Table 1). She had no further episodes of UTI. On follow-up, she is having an adequate bladder capacity for the age, no VUR, and no vaginal stenosis.
Patient 5: A four-year-old male child underwent hypospadias repair elsewhere. He developed urinary retention in the immediate postoperative period which was managed by suprapubic cystostomy (SPC). Although urinary retention was relieved by SPC, he started developing recurrent UTI. He presented to us with a history of recurrent episodes of urinary tract infection. USG abdomen showed a horseshoe kidney and bilateral hydronephrosis, MCU detected bilateral grade 5 VUR, and DMSA found bilateral renal scars. Urethrocystoscopy revealed bilateral laterally placed ectopic ureteric orifices. He was posted for open bilateral ureteric reimplantation. After adequate mobilization, the lower part of the right ureter looked unhealthy with a compromised blood supply. Hence he underwent right to left TUU and left-sided Cohen’s ureteric reimplantation (Table 1). SPC was removed seven days after TUU. He had no further episodes of UTI. Renal functions are maintained. DMSA shows no further worsening.
Patient 6: A six-year-old male child presented with recurrent UTI after a robotic extravesical right ureteric reimplantation for right vesicoureteric junction obstruction (VUJO) at another center. USG showed bilateral gross hydronephrosis. MCU showed left-sided grade-5 VUR. The diuretic renogram showed right VUJO and DMSA scan showed bilateral renal scars. He was diagnosed as recurrent right VUJO and left-sided grade-5 VUR. In view of dense adhesions and inadequate length of the right ureter after mobilization, the right to left TUU with left ureteric Cohen’s reimplantation was done. The child had no further episodes of UTI. Hydronephrosis was resolved on follow-up. Renal functions normal. DMSA showed no worsening of scars or function.
Patient 7: A ten-year-old male child presented with recurrent UTI. He was diagnosed with posterior urethral valves (PUV) with bilateral VUR and had undergone the fulguration of PUV at the age of two years. USG showed bilateral hydroureteronephrosis. MCU showed a large capacity bladder with bilateral grade-5 VUR. DMSA showed bilateral scars. A urodynamic study showed a picture of myogenic failure. As the child had a sensate urethra, clean intermittent catheterization (CIC) wasn’t possible. Hence, he underwent right to left TUU with left ureter reimplantation and right lower ureter as a continent catheterizable conduit (Table 1). The child is asymptomatic, his renal functions maintained, and having an uneventful follow-up.
Discussion
Transureteroureterostomy is a procedure used to reconstruct the urinary tract in cases where the ureter cannot be reimplanted into the bladder for any reason or where conventional urological procedures cannot be undertaken [4]. The risk to the contralateral kidney and ureter is a major reason compelling urologists to opt for other reconstructive approaches. This concern seems to be over-emphasized as the studies published on TUU revealed high success and low complication rates [2-7]. No child in our follow up had deteriorating renal function of the recipient kidney or any new scars on DMSA.
TUU was first tried in animals by Sharpe in 1906 [8]. Higgins introduced it to humans in 1935 [1]. The main indications of TUU are when a ureteric pathology precludes its reimplantation into the bladder. This may be seen in conditions such as [4].
● Lower ureteric stricture - A long segment ureteric stricture, post excision may fall short in length for reimplantation into the bladder [9].
● Proximal ureteric stricture and obstructions [10,11].
● Ectopic ureter, iatrogenic injuries, ureteric duplication, and traumatic injuries to the lower ureter [9-17].
● Malignant lesions of the pelvis invading the lower ureter [18].
Jacob et al used TUU to achieve a single ureteric reimplant to drain both kidneys in children with neurogenic bladder and high-grade reflux undergoing bladder augmentation [19]. They reported good results.
Our study demonstrates a few other indications of TUU besides using it as a salvage procedure for short ureters needing reimplant. They are -
● Small capacity bladder (secondary to excision of large diverticulae, posterior urethral valves, gross vesicoureteric reflux) requiring ureteric reimplantation. Here there would be inadequate space to tunnel both ureters in the bladder wall maintaining Paquin’s rule.
● Dilated ureters with non-compliant bladder, where one ureter can be used to augment the bladder as well as function as a catheterizable conduit.
The recipient ureter must be healthy. Hence patient selection is individualized based on the status of the bladder and ureters; and the inability to perform conventional techniques. We found 100% success in our study as no patient had neither intraoperative or postoperative surgical complications nor any adverse impact on recipient kidney at follow-up. A study by Iwaszko et al found more than 95% success [4].
The complications expected following TUU include prolonged ileus, wound infection, urinary leak, deep vein thrombosis, anastomotic stricture, renal failure, urolithiasis, and retreatment [1-4]. We had none of these complications in our series. In a large series by Iwaszko et al 50% of patients with preexisting renal disease developed renal failure after TUU [4]. We did not have children with pre-existing renal failure in this study group. A study by Noble et al also found that none of the patients developed any complication of TUU [2]. We found no worsening hydronephrosis following TUU. Iwaszko et al found three of their 63 patients developed worsening of hydronephrosis [4]. They concluded that it was due to the yo-yo effect of the donor ureter on the recipient ureter.
Pesce et al evaluated the long-term safety of 70 children who underwent TUU. It was mainly performed as a salvage renal saving procedure and they caution that long-term follow-up is very essential in children with neurogenic bladder who undergo this procedure [20]. Recent studies show that this procedure can be done laparoscopically or even robotic-assisted and the procedure is gaining in popularity as a good and safe alternative [9-13,21,22].
Lee et al found that the robotic TUU had a better rate of resolution of hydronephrosis and a shorter length of hospital stay compared to open TUU [9]. The complication rates were comparable in both groups.
Our study had no postoperative complications. Our results are comparable to many other studies (Table 2).
Table 2.
Postoperative complications
| No. | Complication | Our study | Noble et al [2] | Mure et al [3] | Lee et al [9] | Biles et al [12] | Leavitt et al [13] |
|---|---|---|---|---|---|---|---|
| 1 | Prolonged ileus | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | Wound infection | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Urinary leak | 0 | 6% | 1% | 0 | 0 | 0 |
| 4 | Deep venous thrombosis | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | Anastomotic stricture | 0 | 1% | 1% | 0 | 0 | 0 |
| 6 | Renal failure | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | Urolithiasis | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | Retreatment | 0 | 1% | 1% | 0 | 0 | 0 |
| 9 | Death | 0 | 0 | 0 | 0 | 0 | 0 |
The alternate procedures for short ureters include [2-4].
1. Psoas hitch procedure - It’s fixing the bladder to the ipsilateral psoas muscle so that the bladder moves closer and to the direction of the ureter easing the ureteric reimplantation. But it’s contraindicated in a small capacity bladder and it can injure the femoral nerve or genitofemoral nerve during the procedure.
2. Renal mobilization - It’s mobilizing the ipsilateral kidney to shorten the distance between the kidney and bladder.
3. Intestinal interposition - Either ileum or appendix is used to replace the lost length of the ureter. It’s associated with increased chances of obstruction and reflux.
4. Boari flap - Bladder is tubularized and anastomosed to the short ureter end-to-end. It’s associated with VUR in 100% cases. It is contraindicated in a small capacity bladder. It can cause pseudodiverticulum. The other complications include bladder pedicle ischemia, urinary leak, and urinary stricture leading to ureteral obstruction.
5. Autotransplantation - It involves relocating the ipsilateral kidney to the pelvis with anastomosing the renal vessels to iliac vessels and renal pelvis or short ureter to the bladder.
6. Urinary diversion - It involves diverting the ipsilateral urinary tract into the sigmoid colon. It’s is associated with increased chances of urinary tract infections and metabolic complications.
But these techniques are no simpler or less fraught with possible complications and are beyond the purview of this article to compare them with TUU.
Conclusion
In children, TUU may be indicated when bilateral reimplantation is not feasible or the lower end of one ureter is damaged. TUU can give a reliable, safe, and good long-term result without compromising the other renal moiety. Patient selection is key. Meticulous attention to the technique and skill in surgery are also prerequisites for this challenging procedure.
Acknowledgements
Data were obtained from the hospital medical records. The names were coded and used for analysis. No intervention was performed on any subject as part of the study. As the study was retrospective in nature and only data from hospital records were used, the ethical committee approval wasn’t required as per our institution policy.
Patient consent was obtained at the time of surgery for possible publication.
Disclosure of conflict of interest
None.
References
- 1.Higgins C. Transureteroureteral anastomosis: report of a clinical case. J Urol. 1935;34:349. [Google Scholar]
- 2.Noble IG, Lee KT, Mundy AR. Transureteroureterostomy: a review of 253 cases. Br J Urol. 1997;79:20. doi: 10.1046/j.1464-410x.1997.02794.x. [DOI] [PubMed] [Google Scholar]
- 3.Mure PY, Mollard P, Mouriquand P. Transureteroureterostomy in childhood and adolescence: long-term results in 69 cases. J Urol. 2000;163:946. doi: 10.1016/s0022-5347(05)67859-7. [DOI] [PubMed] [Google Scholar]
- 4.Iwaszko MR, Krambeck AE, Chow GK, Gettman MT. Transureteroureterostomy revisited: long-term surgical outcomes. J Urol. 2010;183:1055–1059. doi: 10.1016/j.juro.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Hendren WH, Hensle TW. Transureteroureterostomy: experience with 75 cases. J Urol. 1980;123:826–833. doi: 10.1016/s0022-5347(17)56151-0. [DOI] [PubMed] [Google Scholar]
- 6.Hodges CV, Moore RI, Lehman TH, Benham AM. Clinical experiences with transureteroureterostomy. J Urol. 1963;90:552–556. doi: 10.1016/S0022-5347(17)64453-7. [DOI] [PubMed] [Google Scholar]
- 7.Hodges CV, Barry JM, Fuchs EF, Pears HD, Tank ES. Transureteroureterostomy: 25-year experience with 100 patients. J Urol. 1980;123:834–838. doi: 10.1016/s0022-5347(17)56153-4. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe NW. VIII. Trans-Uretero-Ureteral Anastomosis. I. Intraperitoneal. II. Retroperitoneal: (a) Anterior to Aorta and Vena Cava; (b) Posterior to Aorta and Vena Cava. Ann Surg. 1906;44:687–707. doi: 10.1097/00000658-190611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee NG, Corbett ST, Cobb K, Bailey GC, Burns AS, Peters CA. Bi-institutional comparison of robot-assisted laparoscopic versus open ureteroureterostomy in the pediatric population. J Endourol. 2015;29:1237–1241. doi: 10.1089/end.2015.0223. [DOI] [PubMed] [Google Scholar]
- 10.Passerotti CC, Diamond DA, Borer JG, Eisner BH, Barrisford G, Nguyen HT. Robot-assisted laparoscopic ureteroureterostomy: description of technique. J Endourol. 2008;22:581–586. doi: 10.1089/end.2007.9838. [DOI] [PubMed] [Google Scholar]
- 11.Smith K, Shrivastava D, Ravish I, Nerli R, Shukla A. Robot-assisted laparoscopic ureteroureterostomy for proximal ureteral obstructions in children. J Pediatr Urol. 2009;5:475–479. doi: 10.1016/j.jpurol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Biles MJ, Finkelstein JB, Silva MV, Lambert SM, Casale P. Innovation in robotics and pediatric urology: robotic ureteroureterostomy for duplex systems with ureteral ectopia. J Endourol. 2016;30:1041–1048. doi: 10.1089/end.2015.0645. [DOI] [PubMed] [Google Scholar]
- 13.Leavitt DA, Rambachan A, Haberman K, DeMarco R, Shukla AR. Robot-assisted laparoscopic ipsilateral ureteroureterostomy for ectopic ureters in children: description of technique. J Endourol. 2012;26:1279–1283. doi: 10.1089/end.2012.0041. [DOI] [PubMed] [Google Scholar]
- 14.Chacko JK, Koyle MA, Mingin GC, Furness PD. Ipsilateral ureteroureterostomy in the surgical management of the severely dilated ureter in ureteral duplication. J Urol. 2007;178:1689–1692. doi: 10.1016/j.juro.2007.05.098. [DOI] [PubMed] [Google Scholar]
- 15.Rushton HG, Parrott TS, Woodard JR. The expanded role of transureteroureterostomy in pediatric urology. J Urol. 1987;138:357. doi: 10.1016/s0022-5347(17)43145-4. [DOI] [PubMed] [Google Scholar]
- 16.Strup SE, Sindelar WF, Walther MM. The use of transureteroureterostomy in the management of complex ureteral problems. J Urol. 1996;155:1572. [PubMed] [Google Scholar]
- 17.Halpern GN, King LR, Belman AB. Transureteroureterostomy in children. J Urol. 1973;109:504. doi: 10.1016/s0022-5347(17)60464-6. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker PH, Gutman M, Verghese M. Transureteroureterostomy: an adjunct to the management of advanced primary and recurrent pelvic malignancy. Int J Colorectal Dis. 2003;18:40–44. doi: 10.1007/s00384-002-0399-2. [DOI] [PubMed] [Google Scholar]
- 19.Jacob TJK, James Sam C, Jacob Kurian J, Karl IS, Kisku SMC, Sen S. Transureteroureterostomy as an adjunctive antireflux procedure in children undergoing bladder augmentation for neurogenic bladder with major reflux. J Pediatr Urol. 2020;16:190.e1–190.e6. doi: 10.1016/j.jpurol.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Pesce C, Costa L, Campobasso P, Fabbro MA, Musi L. Successful use of transureteroureterostomy in children: a clinical study. Eur J Pediatr Surg. 2001;11:395–398. doi: 10.1055/s-2001-19730. [DOI] [PubMed] [Google Scholar]
- 21.Piaggio LA, González R. Laparoscopic transureteroureterostomy: a novel approach. J Urol. 2007;177:2311–2314. doi: 10.1016/j.juro.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Ellison JS, Lendvay TS. Robot-assisted ureteroureterostomy in pediatric patients: current perspectives. Robot Surg. 2017;4:45–55. doi: 10.2147/RSRR.S99536. [DOI] [PMC free article] [PubMed] [Google Scholar]


