Abstract
Cancer patients are at markedly increased risk for venous thromboembolism (VTE). Early detection of VTE may decrease morbidity and mortality in this population. We conducted this study to evaluate the ability of FDG-PET/CT to detect thrombosis in cancer patients. This retrospective study included 131 cancer patients with a history of deep vein thrombosis (DVT) or pulmonary embolism (PE) referred for 2-deoxy-2-[18F]-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT). All subjects underwent PET/CT imaging 60 minutes after FDG injection. Images were visually assessed for increased FDG uptake within the venous lumen. For positive cases, clinical follow-up and Doppler ultrasonography and/or contrast-enhanced CT scans were reviewed. FDG-PET/CT revealed abnormal uptake in the venous system of 26 (19.8%) patients. Eighteen (69.2%) had a history of DVT, and 13 (50%) had a history of PE. The most common site of thrombosis was the inferior vena cava (IVC) (n=14, 53.8%), followed by lower extremities veins (n=9, 34.6%), jugular veins (n=2, 7.7%), and superior vena cava (n=1, 3.8%). The presence of thrombi was confirmed by reviewing clinical follow-up in 6 (23.1%) patients. Among this group, thrombosis was detected in lower extremity veins (n=4, 15.8%), jugular veins (n=1, 3.8%), and IVC (n=1, 3.8%). Our study demonstrates that thrombi prior to their clinical manifestation can be detected by FDG-PET/CT in cancer patients. Moving forward, physicians must carefully consider the venous system when reporting FDG-PET/CT for cancer patients.
Keywords: Venous thromboembolism, cancer, positron-emission tomography, computed tomography, FDG
Introduction
Venous thromboembolism (VTE), which can manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE), represents a major source of morbidity and mortality in cancer patients. Malignancy is associated with a several-fold increased risk of developing thromboembolic complications [1,2]. Post-mortem analysis of cancer patients has demonstrated an incidence of VTE as high as 50%, and the overall survival rate among these patients plummets from 90% to 20% after the diagnosis of VTE [3]. Many DVT progress to PE within 3 months [4]. Therefore, the early detection of VTE may have the potential to decrease the occurrence of adverse events in cancer patients.
Structural imaging techniques such as venography, Doppler ultrasonography, and contrast-enhanced computed tomography (CECT) are commonly used to diagnose VTE. However, these modalities typically can only detect VTE in the late stages of the disease [5]. In contrast, molecular imaging via 2-deoxy-2-[18F]-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) may identify signs of thromboembolic disease at an earlier stage and with greater sensitivity and specificity across the body. FDG has previously been widely used in the detection of intravascular inflammatory lesions [6,7]. On the cellular level, thrombus formation begins with the accumulation of inflammatory infiltrates, leading to focally increased FDG uptake; therefore, FDG-PET/CT may be feasible in the detection of venous thrombi [8,9]. Recent studies have aimed to answer this question, with varying success [10-12].
Molecular imaging is widely used for staging, prognosis, radiation therapy planning, and treatment response assessment in cancer patients [13-17]. We postulate that routine scans of cancer patients may also demonstrate molecular evidence of VTE prior to its clinical manifestations. Therefore, we conducted this retrospective study to evaluate the ability of FDG-PET/CT to detect incidental venous thrombosis in individuals with cancer.
Materials and methods
Subject selection
We cross-referenced the list of individuals who underwent clinical FDG-PET/CT at the Hospital of the University of Pennsylvania from December 2012 to December 2014 with the Abramson Cancer Center database of patients diagnosed with cancer. Patient characteristics are detailed in Table 1. Inclusion criteria were as follows: a history of cancer, a history of DVT or PE, completed FDG-PET/CT with an available clinical report, and lab results available within 2 months from the PET/CT imaging date. Exclusion criteria included unavailable imaging or clinical data. After applying inclusion and exclusion criteria, we enrolled 131 subjects in our study. Our protocol study was approved by the institutional review board. It was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Table 1.
Characteristics of patients (n=26) with thrombosis identified on FDG-PET/CT
| Age (years) | |
| Mean | 61.9 |
| Range | 24-82 |
| Gender | |
| Female | 11 (42.3%) |
| Male | 15 (57.7%) |
| Race | |
| White | 16 (61.6%) |
| Black | 5 (19.2%) |
| Asian | 2 (7.7%) |
| Hispanic | 1 (3.8%) |
| Unknown | 2 (7.7%) |
| Patients status | |
| Deceased | 19 (73.1%) |
| Alive | 7 (26.9%) |
| BMI (kg/m2) | |
| Mean | 25.4 |
| Range | 18.6-43.5 |
| BMI between 18.5-24.5 | 15 (57.7%) |
| BMI between 25-29.9 | 5 (19.2%) |
| BMI between 30-39.9 | 4 (15.4%) |
| BMI more than 40 | 1 (3.8%) |
| Unknown | 1 (3.8%) |
| History of thromboembolic events | |
| DVT | 18 (69.2%) |
| PE | 13 (50.0%) |
| Thrombosis site | |
| IVC | 14 (53.8%) |
| SVC | 1 (3.8%) |
| Lower extremity vein | 9 (34.6%) |
| Jugular vein | 2 (7.7%) |
| Confirmation with other imaging modality | |
| Yes | 6 (23.1%) |
| No | 20 (76.9%) |
| Underlying malignancy | |
| Lung cancer | 7 (26.9%) |
| Lymphoma | 6 (23.1%) |
| Gastrointestinal cancers | 3 (11.6%) |
| Gynecological cancer | 3 (11.6%) |
| Breast cancer | 1 (3.8%) |
| Bladder cancer | 1 (3.8%) |
| Melanoma | 1 (3.8%) |
| Thymoma | 1 (3.8%) |
| Head and neck | 1 (3.8%) |
| Unknown | 2 (7.7%) |
| Histological subtype of the cancer | |
| Adenocarcinoma | 9 (34.6%) |
| SCC | 3 (11.5%) |
| Diffuse large B-cell lymphoma | 3 (11.5%) |
| Hodgkin’s disease | 2 (7.7%) |
| Non-small cell lung cancer | 2 (7.7%) |
| Unknown primary | 1 (3.8%) |
| Others | 6 (23.1%) |
| Stage of the malignancy | |
| Stage 1 | 1 (3.8%) |
| Stage 2 | 1 (3.8%) |
| Stage 3 | 6 (23.1%) |
| Stage 4 | 14 (53.8%) |
| Unknown | 4 (15.4%) |
| Treatment of underlying malignancy | |
| Chemotherapy | 12 (46.2%) |
| Radiotherapy | 7 (26.9%) |
| Surgery | 9 (34.6%) |
| Presence of metastasis | |
| At the time of diagnosis | 21 (80.8%) |
| At the time of PET/CT scan | 23 (88.5%) |
| Underlying condition | |
| Pulmonary disease | 5 (19.2%) |
| Renal disease | 9 (34.6%) |
| History of infection prior PET/CT | 11 (42.3%) |
| Presence of indwelling catheter | 17 (65.4%) |
| Anemia | 20 (76.9%) |
| Leukocytosis | 1 (3.8%) |
| Leukopenia | 2 (7.7%) |
| Thrombocytosis | 4 (15.4%) |
| Thrombocytopenia | 2 (7.7%) |
| Complete blood count (mean, mg/dl) | |
| WBC | 8.2 |
| Hgb | 10.9 |
| Platelet | 313.6 |
BMI = body mass index, DVT = deep vein thrombosis, PE = pulmonary embolism, IVC = inferior vena cava, SVC = superior vena cava, SCC = squamous cell carcinoma, WBC = white blood cells, Hgb = hemoglobin.
Image acquisition
Each patient underwent FDG-PET/CT acquisition, either from the top of the head to the toes (total-body) or from the base of the skull to the mid-thigh. All imaging was performed using hybrid PET/CT scanners (Siemens Biograph 64 mCT, Siemens Healthineers AG, Chicago, IL, USA, and Philips Gemini TF, Philips medical system) 60 ± 10 minutes after intravenous injection of 5 MBq/kg of FDG. Three acquisition protocols were used for Gemini TF, Biograph mCT, and Ingenuity TF: one for BMI under 30, another for BMI between 30 and 35, and the third BMI over 35; the CT settings were 50, 100, and 150 mAs, respectively, and all at 120 kVp. For the PET acquisitions, the time per bed was 1.5, 2, and 3 minutes, respectively.
All scans were acquired in the cranial-to-caudal direction. For total-body scans, after scanning the entire torso, the time-per-bed was halved across the patient’s legs. The reconstruction protocol for the Gemini TF PET/CT scanner was BLOB-OS-TF, 3 iterations with 33 subsets, and for the Siemens Biograph PET/CT scanner was OP-OSEM with corrections for point-spread-function and time-of-flight, 2 iterations with 21 subsets, and Gaussian postfilter with full-width-at-half-maximum 3.0 mm. Model-based scatter corrections and delayed coincidence random correction were used for both. The pixel size for the Gemini TF PET scanner was 4.0 mm × 4.0 mm, while for the Siemens Biograph was 4.07 mm × 4.07 mm. The slices were contiguous for the Gemini TF with a 4 mm slice thickness. On the Siemens Biograph, they overlapped by 1 mm, with a slice thickness of 4 mm.
Image and statistical analysis
Every patient’s FDG-PET/CT scan was examined for evidence of venous thrombosis. Scans were independently assessed by two physicians trained in nuclear medicine. The investigators assessed all axial, sagittal, and coronal slices to qualitatively identify abnormal venous FDG uptake. FDG-PET interpretation was blinded to clinical data. Each patient was recorded as either “positive” or “negative” based on the focal or linear FDG uptake within the venous lumen. For positive cases, the location of the lesions was recorded, and then Doppler ultrasonography and/or CECT radiology reports were reviewed to confirm the presence of the thrombosis. Additionally, clinical data were evaluated for the presence of VTE risk factors. Descriptive statistics were calculated and compiled using Microsoft Excel Version 16.29.1 (Microsoft, Redmond, WA).
Results
We retrospectively evaluated 131 cancer patients who underwent at least one FDG-PET/CT imaging for their malignancy who were diagnosed with either DVT or PE at any point. Our visual assessment revealed abnormal venous FDG uptake within 26 (19.8%) of the 131 patients. Characteristics and lab values of these 26 subjects are detailed in Table 1. The mean age was 61.9 years, and the mean BMI was 25.4 kg/m2. Eighteen (69.2%) had a history of VTE diagnosed by venous ultrasound, and 13 (50%) had a history of PE diagnosed by CT pulmonary angiography. The most common thrombosis site was the inferior vena cava (IVC), found in 14 (53.8%) patients. Other thrombosis sites included the lower extremity veins (n=9, 34.6%), jugular veins (n=2, 7.7%), and superior vena cava (SVC) (n=1, 3.8%). The presence of venous thrombosis using CECT and/or Doppler ultrasonography was confirmed in 6 (23.1%) patients, per attending radiologist reports. Among this group, thrombosis was detected in lower extremity veins (n=4, 15.8%, Figures 1 and 2), jugular veins (n=1, 3.8%), and IVC (n=1, 3.8%, Figure 3). The presence of thrombosis was not mentioned in the FDG-PET/CT report of any of these patients.
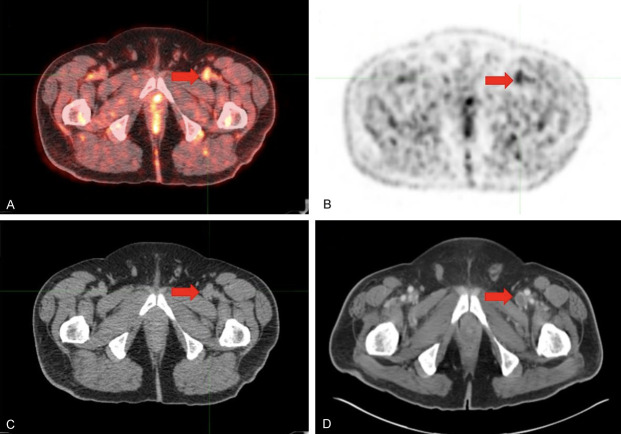
Figure 1.
Venous thrombosis in the left common femoral vein of a 65-year old male with a history of metastatic melanoma. One day prior to FDG-PET/CT imaging, the patient had +1 edema of the left calf and foot. (A) Axial FDG-PET/CT, (B) axial FDG-PET, and (C) axial low-dose CT confirm high metabolic activity in the dilated lumen of the left common femoral vein, consistent with venous thrombosis. (D) Six-month follow-up, abdominopelvic contrast-enhanced CT scan showed a filling defect in the same location, suggestive of venous thrombosis.
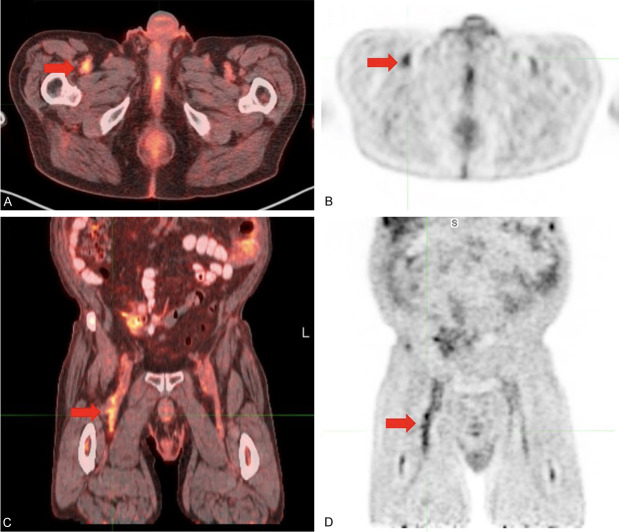
Figure 2.
Venous thrombosis in the right common femoral vein, diagnosed prior to the ultrasound. (A) Axial FDG-PET/CT, (B) axial FDG-PET, (C) coronal FDG-PET/CT, and (D) coronal FDG-PET demonstrated high metabolic activity in the lumen of the right common femoral vein, consistent with venous thrombosis. Ultrasound performed two weeks later confirmed non-occlusive thrombus in the right common femoral vein.
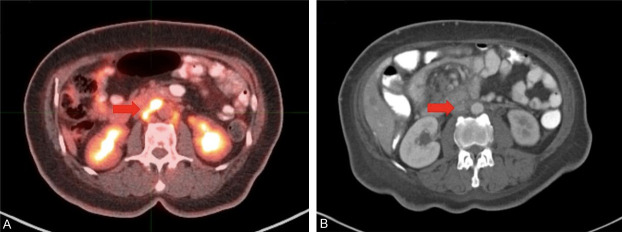
Figure 3.
Venous thrombosis in the inferior vena cava (IVC) of a 70-year old female with a history of uterine carcinosarcoma. Patient underwent FDG-PET/CT for staging. (A) Axial FDG-PET/CT demonstrated increased intra-luminal radiotracer uptake in the IVC. (B) At 2-month follow-up, abdominopelvic contrast-enhanced CT scan showed filling in the IVC, suggestive of chronic thrombosis.
Discussion
In the present study, we demonstrated that venous thrombi may present as incidental findings on routine FDG-PET/CT imaging for cancer patients. In our cohort, the most common site for thrombosis was the IVC, followed by the lower extremities, jugular veins, and SVC. Thus, only a third of the lesions we identified on FDG-PET/CT could realistically have been detected by conventional imaging [18]. In addition, none of the venous thrombi we identified were reported in the available PET/CT reports; rather, most of them were interpreted as lymphadenopathy.
Several case reports have utilized FDG-PET/CT to identify incidental venous thrombosis in cancer patients [19-25]. However, only a handful of studies have reported on multiple subjects. Rondina et al. conducted a case series of 12 patients to evaluate the accuracy of FDG-PET/CT for the detection and evaluation of DVTs [26]. They reported that FDG uptake in affected vessels was visually higher than in unaffected vessels, and they noted a significantly increased maximum standardized uptake value (SUVmax) within affected veins. They also identified a significant negative correlation between DVT onset and FDG uptake. Similarly, Houshmand et al. presented results for metabolically active volume, total lesion glycolysis, and SUVmax relative ratios (sensitivity 84%, specificity 100%) [27]. Hara et al. utilized FDG-PET/CT to identify neutrophil-dependent thrombus inflammation in mice, and they determined that FDG accumulation decreases with time in the identification of experimental DVT [28]. Their findings suggest that PET imaging may have the potential to distinguish between newer and more mature incidences of VTE, which could help to determine if a patient will benefit from anticoagulation therapy [29]. Le Roux et al. also observed significantly higher FDG uptake within thrombosed-vessels in comparison to the contralateral non-affected vessels [30]. However, they did not identify a specific cut-off for SUVmax to differentiate between affected and non-affected vessels, limiting the use of this measurement in routine clinical practice. Finally, Miceli et al. observed an increased FDG uptake in vessels affected by septic thrombosis, but not within DVT-thrombosed vessels, in 11 acute and 16 scans of DVT patients [31]. The present study examined a larger patient cohort than any of these aforementioned studies, further suggesting a role for FDG-PET/CT in VTE.
Focal FDG uptake may be a molecular marker of VTE prior to the onset of clinical symptoms [32]. We confirmed the presence of venous thrombosis in 6 of 26 positive patients using additional imaging reports present in the patients’ charts. Many unconfirmed patients did not undergo structural imaging to screen for VTE around the time of FDG-PET/CT imaging. Meanwhile, in subjects who did undergo structural imaging, these modalities might not have detected the lesions (e.g. IVC thrombosis, our most common finding). Thus, in cases with positive molecular findings but negative structural findings, we cannot differentiate between false-positive PET results and false-negative structural results.
Our study must be interpreted in the context of its limitations. Namely, the retrospective study design prevented us from confirming our findings by other imaging modalities for every case. Also, as this was a descriptive study, it did not include a control group, and the patients included had a history of cancer, DVT, or PE, which may have led to selection bias. Furthermore, some patients were on anticoagulant therapies, which may have resolved thrombi prior to confirmatory structural imaging. The prevalence of venous FDG signal in cancer patients without known VTE is largely understudied. As such, the relatively low uptake of individual thrombi, in addition to the lower quality of images utilized in the study, may have led to false-negative PET findings [30]. This may account for the small proportion of cases with documented venous thrombi. As such, patients with suspected venous thrombi as reflected through FDG signal may benefit from additional confirmatory imaging in conjunction with FDG-PET/CT, such as CT venography/arteriography or ultrasonography to guide anticoagulation therapy [18]. Moreover, the proximity of thrombi to other lesions exhibiting high metabolic activity, such as primary tumors or adjacent lymph nodes, may hamper the ability to visualize the former by the partial volume effect [33-35].
The introduction of total-body PET instruments will play a major role in assessing cancer patients with a predisposition to VTE [36,37]. Oncologic PET/CT scans are routinely performed from the base of the skull to the mid-thigh [18]. However, the lower extremity is a common site for venous thrombosis, so many cases of VTE may be overlooked by the standard PET/CT protocol. In our cohort, we detected thrombosis of the lower leg veins within a melanoma patient who underwent full-body FDG-PET/CT imaging. Additionally, this approach may be combined with NaF-PET/CT for a stronger impression of the overall plaque burden in these patients [38-40]. As such, adopting total-body PET imaging may be of great importance to clinicians moving forward for the management of individuals with cancer.
Conclusion
Our study demonstrates that venous thrombosis can be detected using FDG-PET/CT imaging. Venous thrombosis is a common complication of cancer and chemotherapy. Early detection and management of VTE by PET imaging may alleviate a major source of morbidity and mortality in these patients. However, interpreting physicians rarely report suspicious venous lesions on FDG-PET/CT as VTE. In the future, physicians should carefully consider this life-threatening pathology in their differential diagnosis and treatment approach.
Acknowledgements
Dr. Damrauer is supported by the Veterans Administration [IK2-CX001780]. This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Disclosure of conflict of interest
None.
References
- 1.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 2.Wilbur J, Shian B. Deep venous thrombosis and pulmonary embolism: current therapy. Am Fam Physician. 2017;95:295–302. [PubMed] [Google Scholar]
- 3.Donnellan E, Kevane B, Bird BR, Ainle FN. Cancer and venous thromboembolic disease: from molecular mechanisms to clinical management. Curr Oncol. 2014;21:134–43. doi: 10.3747/co.21.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess S, Frary EC, Gerke O, Werner T, Alavi A, Høilund-Carlsen PF. FDG-PET/CT in venous thromboembolism: a systematic review. Clin Transl Imaging. 2018;6:369–78. [Google Scholar]
- 5.Kaghazchi F, Borja A, Rojulpote C, Seraj SM, Zadeh MZ, Werner T, Revheim ME, Alavi A. Role of FDG-PET/CT in the detection and management of venous thromboembolism. J Nucl Med. 2020;61:1147–1147. [Google Scholar]
- 6.Borja AJ, Hancin EC, Dreyfuss AD, Zhang V, Mathew T, Rojulpote C, Werner TJ, Patil S, Gonuguntla K, Lin A, Feigenberg SJ, Swisher-McClure S, Alavi A, Revheim ME. 18F-FDG-PET/CT in the quantification of photon radiation therapy-induced vasculitis. Am J Nucl Med Mol Imaging. 2020;10:66–73. [PMC free article] [PubMed] [Google Scholar]
- 7.Rojulpote C, Patil S, Gonuguntla K, Borja A, Hancin E, Kaghazchi F, Ghorpade R, Revheim ME, Werner T, Alavi A. Role of nuclear imaging in detecting and assessing chemotherapy induced cardiotoxicity. J Nucl Med. 2020;61:1367–1367. [Google Scholar]
- 8.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–12. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer M, Borja AJ, Hancin EC, Auslander T, Revheim ME, Moghbel MC, Werner TJ, Alavi A, Rajapakse CS. Imaging atherosclerosis by PET, with emphasis on the role of FDG and NaF as potential biomarkers for this disorder. Front Physiol. 2020;11:511391. doi: 10.3389/fphys.2020.511391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sydow BD, Srinivas SM, Newberg A, Alavi A. Deep venous thrombosis on F-18 FDG PET/CT imaging. Clin Nucl Med. 2006;31:403–4. doi: 10.1097/01.rlu.0000222950.98284.94. [DOI] [PubMed] [Google Scholar]
- 11.Kaghazchi F, Borja A, Seraj SM, Hancin E, Rojulpote C, Zadeh MZ, Hess S, Werner T, Alavi A, Revheim ME. Venous thromboembolism detected by FDG PET/CT in cancer patients: a life-threatening, yet commonly missed observation. J Nucl Med. 2020;61:1600–1600. [Google Scholar]
- 12.Gupta P, Kramer EL, Ponzo F. FDG uptake in tumor thrombus in inferior vena cava from rectal cancer on positron emission tomography. Clin Nucl Med. 2005;30:342–3. doi: 10.1097/01.rlu.0000159683.54083.17. [DOI] [PubMed] [Google Scholar]
- 13.Aly M, Borja A, Asadollahi S, Rojulpote C, Werner T, Alavi A, Hunt S. FDG PET as a prognostic tool for treatment response in cholangiocarcinoma. J Nucl Med. 2020;61:1193–1193. [Google Scholar]
- 14.Borja A, Aly M, Seraj SM, Zadeh MZ, Werner T, Alavi A, Hunt S. Role of FDG in the management of metastatic hepatic tumors treated with chemoembolization. J Nucl Med. 2020;61:1181–1181. [Google Scholar]
- 15.Karam MB, Doroudinia A, Goodarzi SB, Kaghazchi F, Koma AY, Mehrian P, Alizadeh N. Prognostic value of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography scan volumetric parameters in head-and-neck cancer patients after treatment. Biomed Biotechnol Res J. 2018;2:196. [Google Scholar]
- 16.Zadeh MZ, Seraj SM, Østergaard B, Mimms S, Raynor WY, Aly M, Borja AJ, Aranni LS, Gerke O, Werner TJ, Zhuang H, Revheim ME, Abildgaard N, Hoilund-Carlsen PF, Alavi A. Prognostic significance of 18F-sodium fluoride in newly diagnosed multiple myeloma patients. Am J Nucl Med Mol Imaging. 2020;10:151–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Zirakchian Zadeh M, Raynor WY, Østergaard B, Hess S, Yellanki DP, Ayubcha C, Mehdizadeh Seraj S, Acosta-Montenegro O, Borja AJ, Werner TJ, Zhuanh H, Revheim ME, Abildgaard N, Hoilund-Carlsen PF, Alavi A. Correlation of whole-bone marrow dual-time-point 18F-FDG, as measured by a CT-based method of PET/CT quantification, with response to treatment in newly diagnosed multiple myeloma patients. Am J Nucl Med Mol Imaging. 2020;10:257–64. [PMC free article] [PubMed] [Google Scholar]
- 18.Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc Bayl Univ Med Cent. 2005;18:321–30. doi: 10.1080/08998280.2005.11928089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang KJ, Zhuang H, Alavi A. Detection of chronic recurrent lower extremity deep venous thrombosis on fluorine-18 fluorodeoxyglucose positron emission tomography. Clin Nucl Med. 2000;25:838–9. doi: 10.1097/00003072-200010000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Capete-Sanchez FM, Gandhi M, Evans TL, Alavi A, Torigian DA. Detection by 18F-FDG-PET/CT of upper extremity acute deep venous thrombosis. Hell J Nucl Med. 2011;14:81–2. [PubMed] [Google Scholar]
- 21.Kaida H, Ishibashi M, Kurata S, Uchida M, Hayabuchi N. Tumor thrombus in the inferior vena cava from colon cancer detected by 18F-FDG-PET. Ann Nucl Med. 2007;21:185–8. doi: 10.1007/s12149-007-0003-5. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen BD. Positron emission tomography imaging of renal vein and inferior vena cava tumor thrombus from renal cell carcinoma. Clin Nucl Med. 2005;30:107–9. doi: 10.1097/00003072-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Strobel K, Steinert HC, Bhure U, Koma AY, Gassmann N, Stöckli SJ. Tumour thrombus in the superior vena cava from anaplastic carcinoma of the thyroid: FDG-PET/CT imaging findings. Eur J Nucl Med Mol Imaging. 2007;34:813–813. doi: 10.1007/s00259-006-0349-2. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi M, Yamamoto E, Shiomi Y, Nakamoto Y, Fujiwara K, Watanabe F, Shinohara S. Internal and external jugular vein thrombosis with marked accumulation of FDG. Br J Radiol. 2004;77:888–90. doi: 10.1259/bjr/32956594. [DOI] [PubMed] [Google Scholar]
- 25.Do B, Mari C, Biswal S, Kalinyak J, Quon A, Gambhir SS. Diagnosis of aseptic deep venous thrombosis of the upper extremity in a cancer patient using fluorine-18 fluorodeoxyglucose positron emission tomography/computerized tomography (FDG PET/CT) Ann Nucl Med. 2006;20:151–5. doi: 10.1007/BF02985628. [DOI] [PubMed] [Google Scholar]
- 26.Rondina MT, Lam UT, Pendleton RC, Kraiss LW, Wanner N, Zimmerman GA, Hoffman JM, Hanrahan C, Boucher K, Christian PE, Butterfield RI, Morton KA. (18)F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med. 2012;37:1139–45. doi: 10.1097/RLU.0b013e3182638934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houshmand S, Salavati A, Hess S, Høilund-Carlsen P, Alavi A. Detection of deep vein thrombosis using FDG-PET/CT volumetric parameters: a diagnostic performance study. J Nucl Med. 2015;56:1724–1724. [Google Scholar]
- 28.Hara T, Truelove J, Tawakol A, Wojtkiewicz GR, Hucker WJ, MacNabb MH, Brownell AL, Jokivarsi K, Kissinger CW, Jaff MR, Hennke PK, Weissleder R, Jaffer FA. FDG-PET/CT enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation. 2014;130:1044–52. doi: 10.1161/CIRCULATIONAHA.114.008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura M, Yamada N, Ito M. Novel anticoagulant therapy of venous thromboembolism: current status and future directions. Ann Vasc Dis. 2017;10:92–8. doi: 10.3400/avd.ra.17-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Roux PY, Robin P, Delluc A, Tardy B, Abgral R, Couturaud F, Reffad A, Le Gal G, Salaun PY. Performance of 18F fluoro-2-désoxy-D-glucose positron emission tomography/computed tomography for the diagnosis of venous thromboembolism. Thromb Res. 2015;135:31–5. doi: 10.1016/j.thromres.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Miceli M, Atoui R, Walker R, Mahfouz T, Mirza N, Diaz J, Tricot G, Barlogie B, Anaissie E. Diagnosis of deep septic thrombophlebitis in cancer patients by fluorine-18 fluorodeoxyglucose positron emission tomography scanning: a preliminary report. J. Clin. Oncol. 2004;22:1949–56. doi: 10.1200/JCO.2004.10.160. [DOI] [PubMed] [Google Scholar]
- 32.Houshmand S, Salavati A, Hess S, Ravina M, Alavi A. The role of molecular imaging in diagnosis of deep vein thrombosis. Am J Nucl Med Mol Imaging. 2014;4:406–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Cysouw MCF, Kramer GM, Schoonmade LJ, Boellaard R, De Vet HCW, Hoekstra OS. Impact of partial-volume correction in oncological PET studies: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2017;44:2105–16. doi: 10.1007/s00259-017-3775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofman MS, Hicks RJ. How we read oncologic FDG PET/CT. Cancer Imaging. 2016;16:35. doi: 10.1186/s40644-016-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimiento JM, Davis-Yadley AH, Kim RD, Chen DT, Eikman EA, Berman CG, Malafa MP. Metabolic activity by (18)F-FDG-PET/CT is prognostic for stage I and II pancreatic cancer. Clin Nucl Med. 2016;41:177–81. doi: 10.1097/RLU.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, Ding Y, Spencer BA, Nardo L, Liu W, Bao J, Jones T, Li H, Cherry SR. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60:299–303. doi: 10.2967/jnumed.119.226498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borja AJ, Rojulpote C, Hancin EC, Høilund-Carlsen PF, Alavi A. An update on the role of total-body PET imaging in the evaluation of atherosclerosis. PET Clin. 2020;15:477–85. doi: 10.1016/j.cpet.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Raynor WY, Borja AJ, Rojulpote C, Høilund-Carlsen PF, Alavi A. 18F-sodium fluoride: an emerging tracer to assess active vascular microcalcification. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02138-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Rojulpote C, Aly M, Zhang V, Patil S, Gonuguntla K, Borja A, Koa B, Kaghazchi F, Narayanareddy P, Revheim ME, Werner T, Gerke O, Hoilund-Carlsen PF, Alavi A. Role of NaF PET-CT in quantifying total body atherosclerotic burden in major arteries. J Nucl Med. 2020;61:1610–1610. [Google Scholar]
- 40.Asadollahi S, Rojulpote C, Bhattaru A, Patil S, Gonuguntla K, Karambelkar P, Borja AJ, Vuthaluru K, Seraj SJ, Zhang V, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Comparison of atherosclerotic burden in non-lower extremity arteries in patients with and without peripheral artery disease using 18F-NaF-PET/CT imaging. Am J Nucl Med Mol Imaging. 2020;10:272–8. [PMC free article] [PubMed] [Google Scholar]