Abstract
Introduction
Numerous studies have described aberrant patterns of rhythmic neural activity in patients along the Alzheimer's disease (AD) spectrum, yet the relationships between these pathological features and cognitive decline are uncertain.
Methods
We acquired magnetoencephalography (MEG) data from 38 amyloid‐PET biomarker‐confirmed patients on the AD spectrum and a comparison group of biomarker‐negative cognitively normal (CN) healthy adults, alongside an extensive neuropsychological battery.
Results
By modeling whole‐brain rhythmic neural activity with an extensive neuropsychological profile in patients on the AD spectrum, we show that the spectral and spatial features of deviations from healthy adults in neural population‐level activity inform their relevance to domain‐specific neurocognitive declines.
Discussion
Regional oscillatory activity represents a sensitive metric of neuronal pathology in patients on the AD spectrum. By considering not only the spatial, but also the spectral, definitions of cortical neuronal activity, we show that domain‐specific cognitive declines can be better modeled in these individuals.
Keywords: neural oscillations, neuropsychology, resting‐state magnetoencephalography
1. BACKGROUND
Alzheimer's disease (AD) is increasingly recognized as a spectrum or continuum of progressive, multi‐domain cognitive declines. 1 , 2 Although the hallmark neuropsychological deficit in patients with AD is amnestic memory impairment, attention, executive function, learning, verbal fluency, and processing speed have also been found to decline as a function of AD severity. 3 , 4 Clinical measures of these domains have also been found to predict conversion from preclinical to dementia phases of the disease. 5 Mirroring these neuropsychological deficits is the early accumulation of pathological levels of amyloid beta (Aβ) plaques in frontal, temporal, and parietal cortices, followed by a build‐up of tau‐rich neurofibrillary tangles and aberrant functioning of neuronal populations in the hippocampus and across the neocortex. 6 As scientists researching AD have shifted toward viewing it as a disease without strict clinical stages, objective and continuous measures of direct relevance to cognitive/functional impairments are sorely needed.
Among functional neuroimaging studies of patients on the AD spectrum, spectrally defined patterns of rhythmic population‐level neural activity have emerged as a particularly sensitive method for differentiating patients with probable AD from cognitively normal (CN) older adults. 7 , 8 , 9 In particular, electro‐ and magneto‐encephalography (MEG) studies of patients with AD have consistently shown increased spontaneous neural activity in the slower delta (2 to 4 Hz) and theta (5 to 7 Hz) frequency bands, as well as decreased activity in the faster alpha (8 to 12 Hz) and beta (15 to 30 Hz) bands. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Though widely replicated, the relationship between these findings and domain‐specific cognitive function has not been extensively investigated. Because of this shortcoming, it is not clear which, if any, of these deviations from healthy rhythmic neural activity covary meaningfully with cognitive decline in patients on the AD spectrum. Identifying the relationships between rhythmic neural activity and cognitive decline in AD is critical, as the current knowledge gap in this area limits our understanding of the clinical implications of these findings. More specifically, knowledge of the direction of these relationships would indicate whether altered rhythmic neuronal activity represents pathological or compensatory processes, and thus could be useful in bolstering diagnosis and informing intervention. Additionally, previous studies of oscillatory aberrations in patients with AD have often been conducted at a coarse spectral and/or spatial resolution, and the vast majority were performed in patient groups who were not biomarker‐confirmed.
In this study, we examine MEG data collected from 38 patients on the AD spectrum, all of whom were assessed as being biomarker‐positive using 18F florbetapir positron emission tomography (PET), and 20 demographically matched CNs (19 biomarker‐negative) using methods that provide remarkably high spatial and spectral resolution. By combining these data with an extensive neuropsychological battery, we show that spectrally and spatially defined neural oscillations significantly predict domain‐specific neuropsychological function and instrumental activities of daily living in ways that are clinically informative. Further, we find that the spatial and spectral properties of the relationships with cognitive and functional impairments only partially overlap with commonly reported group differences from CNs in the amplitude of oscillations. This demonstrates that not all neural oscillatory differences in patients on the AD spectrum necessarily reflect pathology that is directly relevant to cognitive and functional impairments, while also providing key new pathways for research aimed at improving patient outcomes.
RESEARCH IN CONTEXT
Systematic review: Previous literature was reviewed through a search of online databases (e.g., PubMed). Despite numerous studies on the effects of Alzheimer's disease (AD) on neural oscillations, little is known regarding the relationship of these deviations and cognitive/functional impairments.
Interpretation: This study links rhythmic patterns of cortical neural activity to cognitive and functional declines in patients on the AD spectrum. While the nature of many of these relationships indicated pathology, others unexpectedly indicated compensation.
Future directions: This study paves the way for two new avenues of research. First, the finding of a compensatory role for commonly reported increases in theta‐frequency neural activity should be used to better appreciate the nuanced role of neural oscillations in AD. Second, the combination of high spatial and spectral resolution should be leveraged by emerging studies of non‐invasive, frequency‐modulated neurostimulation. Using these findings, directional hypotheses can be generated that are both spatially and spectrally informed.
2. METHODS
2.1. Participants
Forty‐four patients with amnestic mild cognitive impairment (aMCI) or mild probable AD, as determined by a fellowship‐trained neurologist specializing in memory disorders, were enrolled in this study. One participant disenrolled from the study due to COVID‐19–related safety concerns, one was excluded due to a major incidental finding, and four were excluded after whole‐brain PET imaging with florbetapir 18F indicated amyloid‐negativity. The remaining 38 amyloid‐PET biomarker‐confirmed patients on the AD spectrum were compared to a control group of 20 older adults with normal cognition (19 amyloid‐negative and one without PET). The CN participant without biomarker confirmation was included after careful examination of their neuropsychological profile, which indicated that they were well above the 50th percentile for every cognitive domain, relative to healthy adults of the same age (Mini‐Mental State Examination [MMSE]: 30; attention composite z‐score: + 1.39; learning: + 1.17; memory: + 0.98; verbal: + 0.90; processing speed: + 0.78). Group neuropsychological profiles and demographics can be found in Table 1. The groups were matched on key demographics except age, which was included as a nuisance covariate in all statistical modeling. Exclusion criteria included any medical illness affecting central nervous system function, any neurological disorder (other than AD/aMCI), history of head trauma, moderate or severe depression (Geriatric Depression Scale ≥ 10), and current substance abuse.
TABLE 1.
Participant demographics and neuropsychological profiles
| Age (years) | Sex (% female) | Handedness (# left) | Education (years) | ||||
|---|---|---|---|---|---|---|---|
| CN | 72.70 (4.73) | 60 | 1 | 16.60 (2.87) | |||
| ADS | 69.21 (6.91) | 47 | 3 | 15.50 (2.72) | |||
| P | .028* | .360 | .679 | .166 | |||
| MoCA | Processing | ||||||
| (n = 52) | MMSE | Learning | Memory | Attention | Verbal Fluency | Speed | |
| CN | 27.43 (1.99) | 29.20 (1.06) | 0.60 (0.76) | 0.33 (0.56) | 0.53 (0.60) | 0.18 (0.76) | 0.66 (0.83) |
| ADS | 19.13 (4.76) | 23.66 (4.15) | –2.04 (0.88) | –2.28 (0.70) | –0.77 (1.06) | –1.04 (1.01) | –0.90 (1.42) |
| P | < .001* | < .001* | < .001* | < .001* | < .001* | < .001* | < .001* |
Abbreviations: ADS: Alzheimer's disease spectrum; CN: cognitively normal; MMSE, Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment.
*P < .05.
The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Written informed consent was obtained from each participant after detailed description of the study. Patient informants were also required to be present for each participant on the AD spectrum to ensure their comfort over the course of the study, as well as to complete certain questionnaires (e.g., the Functional Activities Questionnaire), and as such informed consent was obtained from each informant as well. To ensure that the interests of all patients were represented appropriately, for patients whose capacity to consent was questionable, informed assent was obtained from the research participant, in addition to informed consent from a legally authorized representative.
2.2. Neuropsychological testing
After screening and informed consent, participants underwent a battery of neuropsychological tests, with raw scores for each participant being converted to demographically adjusted z‐scores based on published normative data. 19 , 20 , 21 , 22 This battery was developed in collaboration with a clinical neuropsychologist specializing in memory disorders, and focused on five cognitive domains generally impacted in patients with AD: verbal memory (Wechsler Memory Scale Fourth Edition [WMS‐IV] Logical Memory II Delayed Recall and Recognition; 23 Hopkins Verbal Learning Test‐Revised [HVLT‐R] Delayed Recall and Recognition Discriminability Index 24 ), learning (WMS‐IV Logical Memory I Recall; 23 HVLT‐R Learning Trials 1‐3 24 ), attention and executive function (Wechsler Adult Intelligence Scale Fourth Edition [WAIS‐IV] Digit Span Forward, Backward, and Sequencing; 22 Trail Making Test Part B 20 ), language (Boston Naming Test; 20 Controlled Oral Word Association Test/Phonemic Verbal Fluency; 20 Animals/Semantic Verbal Fluency 20 ), and processing speed (WAIS‐IV Digit Symbol Coding; 22 Trail Making Test Part A 20 ). Demographically corrected z‐scores based on test‐specific normative data were averaged to create composite cognitive domain z‐scores by participant. These domain composite scores were corroborated within the CN group by calculating a ratio of z‐scores representing the average of all correlations among intra‐domain tests, divided by the average of all correlations with inter‐domain tests. All domains had a ratio of zintra/zinter > 1.50, and on average zintra/zinter = 3.46 (standard deviation [SD] = 1.85), indicating that these domains were ≈250% more internally than externally related. In addition, instrumental activities of daily living (IADLs) were measured (with an informant for patients on the AD spectrum) using the Functional Activities Questionnaire (FAQ), 25 and general cognitive status was measured using the Montreal Cognitive Assessment (MoCA) 26 and the MMSE. 27
2.3. Florbetapir 18F positron emission tomography
Combined PET/computed tomography (CT) data using 18F‐florbetapir (Amyvid, Eli Lilly) and a GE Discovery MI digital scanner were collected following the standard procedures described by the Society of Nuclear Medicine and Molecular Imaging (3D acquisition; single intravenous slow‐bolus < 10 mL; dose = 370 MBq; waiting period = 30–50 minutes; acquisition = 10 minutes). 28 Images were attenuation corrected using the CT data, reconstructed in MIMneuro (slice thickness = 2 mm), 29 converted to voxel standardized uptake values (SUV) based on body weight, and normalized into Montreal Neurological Institute space. Each scan was read by a fellowship‐trained neuroradiologist blinded to group assignment and assessed as being “amyloid‐positive” or “amyloid‐negative” using established clinical criteria. 29 At this stage, patients who were amyloid‐negative were excluded from the AD spectrum group. Images were then normalized to the crus of the cerebellum (SUIT template) 30 to generate voxel‐wise maps of SUV ratios (SUVr), 31 and back‐transformed into each patient's native magnetic resonance imaging (MRI) space using their FreeSurfer‐processed T1 data. The PET data overlapping with each individual's cortical gray‐matter ribbon was then projected onto a tessellated FSAverage template surface using mri_vol2surf (maximum value; projection fraction = 1; steps of 2). 32
2.4. Magnetoencephalography recording and preprocessing
Our MEG recording and preprocessing pipeline has been extensively described in previous papers. 33 , 34 , 35 , 36 Eight minutes of seated eyes‐closed resting state MEG data were collected from each participant using a 306‐sensor Elekta/MEGIN system at 1 kHz (bandwidth 0.1 to 330 Hz). Continuous head position indicator coils were used to measure slight movements during each recording, and the position of these coils was digitized, along with each participant's fiducials and scalp surface, using a 3D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences). Head motion correction and signal space separation with a temporal extension 37 were implemented to reduce noise, and only the data from the 204 gradiometers were used for analysis. Using the digitized head points, each participant's MEG data were co‐registered with their own high‐resolution structural T1‐weighted MRI data (Siemens Prisma 3T; 64‐channel head coil; TR: 2.3 seconds; TE: 2.98 ms; flip angle: 9°; FOV: 256 mm; slice thickness: 1 mm; voxel size: 1 mm3) using an iterative closest‐point rigid‐body registration in Brainstorm (September 3, 2020 distribution) 38 and, after visual inspection, these fits were manually corrected. Triangulated cortical surfaces were computed from the T1 MRI data using FreeSurfer recon_all 32 and imported into Brainstorm. Individual cortical surfaces (including the cerebellum) were down‐sampled to ≈17,000 vertices for computation of the forward model for use in MEG source imaging.
2.5. Magnetoencephalography analysis
MEG data were bandpass filtered between 1 and 200 Hz and notch filtered at 60, 120, and 180 Hz, and ocular and cardiac artifacts were identified using an automated identification algorithm, supplemented by visual inspection of their temporal and spatial topography. From these topographies, signal‐space projectors (SSPs) were generated and reviewed for each type of artifact, and those accounting for ocular and cardiac components were removed from the gradiometer data. Artifact‐reduced MEG data were then epoched into non‐overlapping blocks of 4 seconds and down‐sampled to 500 Hz. Epochs still containing major artifacts (e.g., SQUID jumps) were excluded within each participant using the ∪ of standardized thresholds of ± 2.5 median absolute deviations from the median for signal amplitude and gradient. After exclusions, a mean of 98.90 (SD: 8.56) and 96.89 (SD: 7.93) epochs were included for further analysis for the CN and AD spectrum groups, respectively. Importantly, there was no significant difference in the amount of data used between the two groups (P = .377). Empty‐room recordings of ≥ 2 minutes, collected around each individual scanning session, were processed using an identical pipeline to the one described above (with the exception of artifact SSPs), to empirically model the magnetically‐shielded room noise statistics for source analysis.
Source analysis of neuromagnetic fields used an overlapping‐spheres forward model, unconstrained to the cortical surface. This approach models a single sphere per each sensor, which in this study included the 204 gradiometers (minus any channels previously marked as “bad”) for each participant. A linearly constrained minimum variance beamformer implemented in Brainstorm was used to spatially filter the epoched data based on the data covariance computed from the resting‐state recording and the noise covariance computed from recordings of the empty room. These source‐level time series data were then transformed into the frequency‐domain using Welch's method for estimating power spectral density (PSD; window = 1 second; 50% overlap), grouped into canonical frequency bands (delta: 2 to 4 Hz; theta: 5 to 7 Hz; alpha: 8 to 12 Hz; beta: 15 to 29 Hz), and these spectral maps were normalized to the total power across the frequency spectrum. The norm of the three unconstrained orientations per location and map were then projected onto a common FSAverage template surface (including the cerebellum) for statistical modeling.
2.6. Statistical analysis and visualization
Statistical comparisons were performed, accounting for the effects of age, using SPM12. Initial tests using parametric general linear models investigated the effects of group (i.e., CN vs. AD spectrum; unpaired t‐test; unequal variance) and cognitive function (i.e., neuropsychological domain z‐scores on functional neural maps; regression). Regression analyses relating neural activity and neuropsychological domain z‐scores were corrected for multiple comparisons at this stage using a set‐level correction of pBonferroni < .05 (P = .05/[4 spectral maps × 5 cognitive domains] = .0025). Tests that survived this initial threshold were examined further. To account for non‐uniform spatial autocorrelation in the data, avoid assumptions of parametric modeling, and avoid selecting arbitrary cluster‐forming thresholds, threshold‐free cluster enhancement (TFCE; E = 1.0, H = 2.0; 5000 permutations) 39 was performed, with multiple comparisons correction set to cluster‐wise P FWE < .05. Clusters surviving at this threshold were used to create logical masks that were applied to the original statistical contrasts (i.e., vertex‐wise F‐values) for visualization in Brainstorm. Peak‐vertex data from these clusters were extracted and plotted using ggplot2 40 for interpretation of directional effects. Labels for peak‐vertex data were derived from the Desikan‐Killiany atlas. 41 Aβ SUVr data were also extracted from the same peak‐vertices of the surface‐based florbetapir PET images, for use in secondary analyses in the statistical software R. 42 Mediations were tested using a hierarchical regression approach. 43
3. RESULTS
To examine the spatially and spectrally specific relationships between altered patterns of neuronal oscillations and cognitive/functional impairments in patients on the AD spectrum, we analyzed MEG, neuropsychological, and PET data from a final sample of 38 confirmed Aβ‐positive patients and 20 (19 confirmed Aβ‐negative) CN older adults (Figure 1). The cortical surface MEG maps exhibited the expected spatio‐spectral activity patterns both between and across groups (Figure S1 in supporting information).
FIGURE 1.
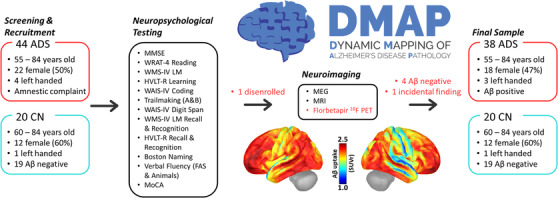
Dynamic mapping of Alzheimer's pathology (DMAP) study flowchart. After initial screening and recruitment, participants performed an extensive series of neuropsychological tests designed to tap five cognitive domains: attention, memory, verbal function, processing speed, and learning. This testing visit was followed by another, neuroimaging‐focused visit, during which participants underwent functional and structural neuroimaging with MEG and MRI. Participants in the patient group returned for a third visit, in which they underwent a quantitative PET/CT scan with florbetapir 18F, and were excluded from further analysis at this stage if they were biomarker‐negative. Previous PET data was available for 19 CN adults. The cortical surface maps (bottom center) represent the grand average of these PET scans across all patients in the final (biomarker‐positive) AD spectrum group. Red text indicates protocols that only pertain to the AD spectrum group. Aβ, amyloid beta; ADS, Alzheimer's disease spectrum; CN, cognitively normal; HVLT‐R, Hopkins Verbal Learning Test‐Revised; MEG, magnetoencephalography; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; PET, positron emission tomography; WAIS‐IV, Wechsler Adult Intelligence Scale Fourth Edition; WMS‐IV LM, Wechsler Memory Scale Fourth Edition Logical Memory; WRAT‐4, Wide Range Achievement Test 4
3.1. Pathological patterns of rhythmic neural activity in patients on the AD spectrum
Replicating an extensive literature, we observed a robust increase in theta‐frequency (5 to 7 Hz) activity in patients on the AD spectrum, as well as decreases in neuronal activity in the alpha (8 to 12 Hz) and beta (15 to 29 Hz) bands (Figure 2). In the theta range, these differences were strongest in the left (tpeak[55] = 4.43; P < .001) and right (tpeak[55] = 4.08; P < .001) inferior parietal cortices (IPC), and the left (tpeak[55] = 3.92; P < .001) and right (tpeak[55] = 3.47; P = .001) cerebellum. In the alpha band, patients on the AD spectrum exhibited decreases in neuronal activity in the left (tpeak[55] = 3.47; P = .001) and right (tpeak[55] = 3.13; P = .003) middle temporal cortices (MTC). Beta frequency activity was mainly reduced in these patients in the left IPC (tpeak[55] = 3.39; P = .001). No significant differences were found in the delta range.
FIGURE 2.
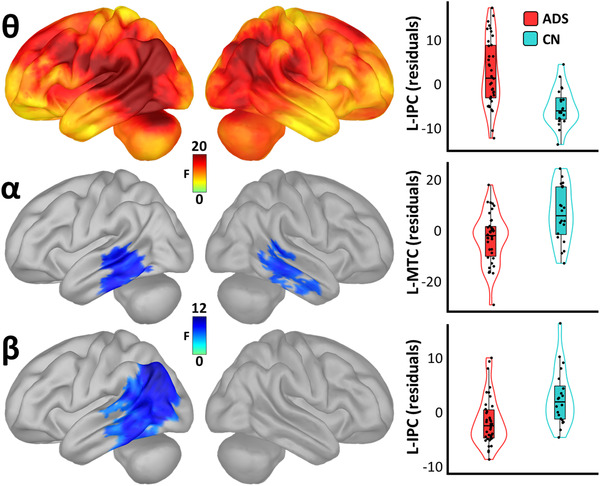
Spatio‐spectral group differences in cortical neural oscillatory amplitude. Surface maps to the left indicate significant statistical differences in oscillatory amplitude between patients (Alzheimer's disease spectrum [ADS]) and cognitively normal older controls (CN), beyond the effects of age, and corrected for multiple comparisons using a stringent threshold‐free cluster enhancement approach (pFWE = .05). Theta maps are shown at the top, with alpha maps in the middle and beta maps at the bottom. Plots to the right of each map indicate the direction and nature of these effects at the vertices where they were most pronounced. Box plots represent conditional means, first and third quartiles, and minima and maxima, and violin plots show the probability density. L‐IPC, left inferior parietal cortex; L‐MTC, left middle temporal cortex
3.2. Spatio‐spectral neural dynamics predict cognitive decline along the Alzheimer's disease spectrum
To investigate the relevance of these spectrally specific neural deviations to cognitive decline in patients on the AD spectrum, we next regressed these neural maps on each of the five neuropsychological composite scores representing cognitive domains impacted by AD: memory, learning, attention and executive function, verbal fluency, and processing speed. Importantly, because the direction of these differences from CNs was frequency‐dependent (i.e., a decrease for the faster alpha and beta band frequencies, and an increase for the slower delta and theta bands), the results of this analysis must be interpreted in light of this fact. For example, more positive values in the theta band indicate greater deviations from normal aging, while more positive beta values indicate the opposite effect.
These relationships were largely non‐overlapping in regard to their spatial, spectral, and cognitive definitions (Figure 3). In the delta band, pathologically higher levels of neural activity in the right temporal pole (rpeak[35] = –.54, P < .001) and left superior temporal cortex (STC; rpeak[35] = –.46, P = .004) predicted declines in processing speed. In contrast, greater right STC (rpeak[35] = .45, P = .005) and supramarginal gyrus (SMG; rpeak[35] = .45, P = .005) deviations from CNs in the theta band predicted better memory performance, indicating that these commonly reported differences are likely compensatory in nature. Pathological beta‐frequency deviations from CNs in the right (rpeak[35] = .45, P = .005) and left (rpeak[35]) = .36, P = .027) IPC predicted declines in attention function, as well as reduced processing speed in the left MTC (rpeak[35] = .37, P = .023). Notably, despite robust differences in alpha‐frequency amplitude between patients on the AD spectrum and CNs, replicating many past studies, no significant relationships were found between cognitive function and alpha‐frequency cortical activity.
FIGURE 3.
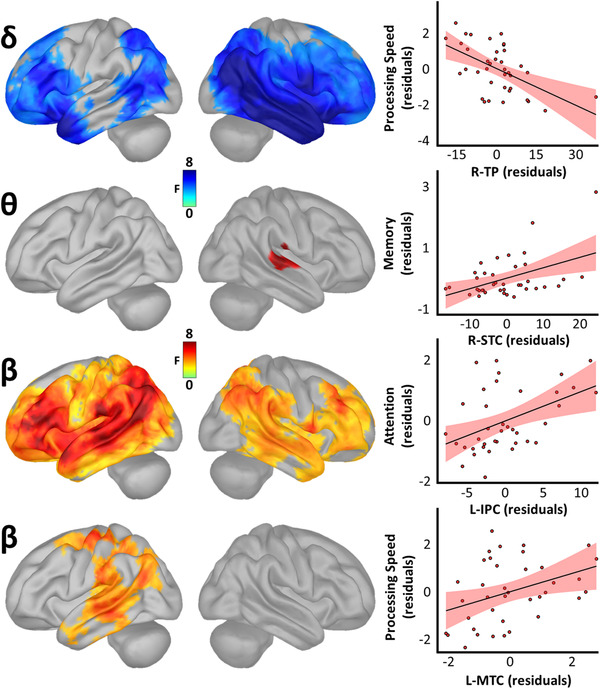
Spatio‐spectral neural oscillations predict cognitive decline along the Alzheimer's disease (AD) spectrum. Surface maps to the left indicate significant statistical outputs of whole‐brain models relating oscillatory amplitude and domain‐specific cognitive function in patients on the AD spectrum, beyond the effects of age, and corrected for multiple comparisons using a stringent threshold‐free cluster enhancement approach (pFWE = .05). Plots to the right of each map indicate the direction and nature of these effects at the vertices where they were most pronounced, with lines‐of‐best‐fit and corresponding confidence intervals overlaid. L‐IPC, left inferior parietal cortex; L‐MTC, left middle temporal cortex; R‐STC, right superior temporal cortex; R‐TP, right temporal pole
3.3. Spatio‐spectral neurocognitive relationships are not mediated by regional Aβ accumulation
To examine whether regional Aβ uptake was responsible for any of these effects, we extracted SUVrs from each patient's florbetapir 18F PET images for each of the vertices that exhibited a significant relationship between neuronal oscillatory activity and cognitive impairment in patients on the AD spectrum. We found no evidence for a mediation of any of these spatio‐spectral neurocognitive relationships by Aβ uptake. In addition, Bayesian analysis of these models revealed evidence that Aβ uptake provides no additional predictive information regarding cognitive status for any relationship in the theta (all BF01 > 2.30) or delta (all BF01 > 2.40) band, nor for the right IPC‐attention relationship in the beta band (BF01 = 2.30). In contrast, Aβ did not exhibit robust evidence for or against the null hypothesis of no relationship to attention in the left IPC (BF01 = 1.02), and predicted processing speed in the left MTC, above and beyond the effects of beta‐frequency neural activity in the same region (r[34] = –.34, P = .044; BF10 = 2.07). However, this relationship should be interpreted cautiously, as it did not survive correction for multiple comparisons.
3.4. Occipito‐parietal theta oscillations predict functional independence in patients on the AD spectrum
Finally, to establish which, if any, of these spectro‐spatial neuronal maps related to patient functional independence, we regressed each spectrally specific map on IADLs (measured with the FAQ) in the patient group. No significant relationships between neuronal activity and functional independence were found in the delta, alpha, or beta bands; however, a robust relationship was observed in the theta band in right lateral occipital cortex (LOC; rpeak[35] = –.46, P = .004; Figure 4). This effect again suggested compensation, such that stronger theta activity in this region predicted increased functional independence (i.e., lower FAQ scores).
FIGURE 4.
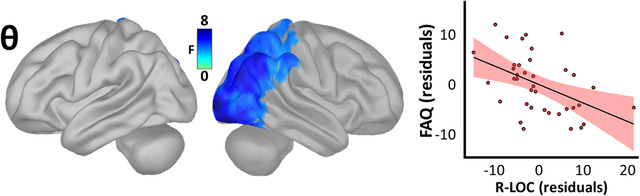
Spatio‐spectral neural oscillations predict functional independence along the Alzheimer's disease spectrum. The surface maps to the left indicate the significant statistical output of a whole‐brain model relating oscillatory amplitude and functional independence (instrumental activities of daily living) in patients on the AD spectrum, beyond the effects of age, and corrected for multiple comparisons using a stringent threshold‐free cluster enhancement approach (pFWE = .05). The plot to the right indicates the direction and nature of this effect at the vertex where it was most pronounced, with the line‐of‐best‐fit and corresponding confidence interval overlaid. FAQ, Functional Activities Questionnaire; R‐LOC, right lateral occipital cortex
4. DISCUSSION
Decades of research have found pathological population‐level neuronal activity in patients on the AD spectrum, and this study provides key new knowledge in this established area by comprehensively associating these neural changes with domain‐specific cognitive declines. By doing so, we find support for the traditional conceptualization of delta and beta frequency deviations from healthy aging as signaling pathological changes, and show these changes are regionally specific and linked to cognitive decline in specific domains. Conversely, we find no such cognitive correlate of the commonly found decreases in alpha oscillatory amplitude. Unexpectedly, we also find that the often‐reported perturbations in theta amplitude in patients on the AD spectrum appear to predict better cognition and functional outcomes.
We first replicated previous findings of increased low‐frequency neural activity and decreased high‐frequency activity in patients on the AD spectrum. Importantly, by leveraging a powerful source imaging approach, our findings provide enhanced spatial resolution compared to otherwise comparable investigations conducted previously. This increased sensitivity revealed that commonly observed decreases in alpha and beta frequency activity are strongest in the bilateral MTC and left IPC, respectively, while increases in the theta band occurred in a widespread network encompassing the bilateral IPC, left MTC, and left dorsolateral prefrontal cortex. By significantly enhancing previous knowledge regarding the spatial foci of such oscillatory deviations in AD, these results provide exciting new avenues for future study, particularly in regard to emerging noninvasive electrical stimulation therapies. Although interesting, these spatio‐spectral neural deviations from healthy aging alone do not inform us regarding their relevance to clinical outcomes.
To understand the nature of such relationships between oscillatory neural deviations and cognitive impairments, we regressed spectrally specific maps of cortical neural activity on five cognitive domains, measured with a thorough neuropsychological battery. As expected, many of these neural deviations from healthy aging predicted declines in cognitive performance, indicating pathology. Increased delta amplitude across a bilateral network of inferior parietal, anterior and posterior temporal, and dorsolateral prefrontal regions predicted worse processing speed, which aligns well with previous research indicating the importance of delta rhythms for temporal expectation and reaction time. 44 Decreased beta‐frequency amplitude similarly predicted declines in processing speed. However, beta's relationship to processing speed was conspicuously spatially non‐overlapping with that of delta, and instead included a left‐lateralized network of middle temporal, supramarginal, and somato‐motor cortices. Emerging work has suggested an overarching role for human beta oscillations as a “top‐down,” bi‐directional regulator of cognitive flexibility and prediction. 45 , 46 Thus, these complementary relationships between delta and beta oscillatory amplitude and processing speed point to a multi‐spectral, distributed pathology affecting both “early” temporal readiness and “late” temporal prediction signals in patients on the AD spectrum. Supporting this conceptualization further, pathologically low levels of beta, but not delta, oscillations also predicted declines in attention across a bilateral network of inferior parietal, middle temporal, and prefrontal regions.
In contrast to the relationships observed in the delta and beta frequencies, deviations from healthy aging in theta oscillatory amplitude predicted better cognitive function. Specifically, increases in theta amplitude in right superior temporal and supramarginal cortices were related to better memory function, while similar increases in right parieto‐occipital cortices signaled greater functional independence. This was unexpected, and in fact, many previous studies have combined theta and delta frequency information into one “low‐frequency” metric for simplicity. This compensatory theta effect suggests that theta and delta frequency deviations from healthy aging in patients on the AD spectrum are functionally distinct, both in terms of their relationships to differing cognitive domains, as well as in the ultimate direction of such relationships. The spatial definitions of the theta‐frequency effects are also of interest, as better memory performance was predicted by neuronal deviations in the superior temporal cortex, while better daily functioning (i.e., IADLs) was predicted by similar deviations in parieto‐occipital cortices. This signals that, while compensatory theta deviations in key association cortices may robustly predict alterations in memory function, those deviations in “lower‐order” regions typically associated with visuospatial and visual attention function appear to be more directly relevant to the challenges to independence that these patients face.
Absent from these neuro‐cognitive relationships was alpha activity, which was conspicuous as oscillations in this range are likely the most consistently reported to differ in the AD spectrum relative to healthy aging. Although this null finding is certainly far from conclusive, it was not due to power or a lack of sensitivity in the alpha band, as we observed robust amplitude differences in the alpha range between CNs and patients. It remains possible that these deviations from healthy aging in the alpha band do not represent functional insults until later in the course of the disease, or alternatively could represent other psychiatric or situational comorbidities not examined here. Also absent were any relationships between oscillatory amplitude and regional Aβ accumulation, but this was less surprising. A growing literature has shown that regional tau, and not Aβ, pathology better predicts cognitive and functional outcomes in clinical AD. 47 , 48 , 49 , 50 , 51 Accordingly, while these data allowed us to confirm that all patients in our AD spectrum group were Aβ‐positive, they appear to yield little information regarding functional neural or cognitive declines, at least at the stage of the disease that we study herein.
CONFLICTS OF INTEREST
The authors declare no competing conflicts of interest, financial or otherwise.
Supporting information
Supporting information
ACKNOWLEDGMENTS
First and foremost, we would like to acknowledge the efforts of our research participants. Without their selflessness and kind demeanor, none of this work would have been possible. We would also like to thank the research and clinical staff who sustained patient recruitment and data collection for this study, Dr. Clifford Jack for helpful input regarding normalization of our PET data, as well as our funding sources for their support. Finally, we would like to extend our sincerest gratitude to the late Mr. Marvin Welstead, who selflessly dedicated so much of his time to raising funds for Alzheimer's disease research and education initiatives. This research was supported by grants R01‐MH116782 (TWW), R01‐MH118013 (TWW), R01‐DA047828 (TWW), RF1‐MH117032 (TWW), F31‐AG055332 (AIW), and F32‐NS119375 (AIW) from the National Institutes of Health, as well as by a grant from the Fremont Area Alzheimer's Fund (FAAF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Wiesman AI, Murman DL, May PE, et al. Spatio‐spectral relationships between pathological neural dynamics and cognitive impairment along the Alzheimer's disease spectrum. Alzheimer's Dement. 2021;13:e12200. 10.1002/dad2.12200
REFERENCES
- 1. Jones DT, Knopman DS, Gunter JL, et al. Cascading network failure across the Alzheimer's disease spectrum. Brain. 2016;139(2):547‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neurosci Biobehav Rev. 2000;24(3):365‐374. [DOI] [PubMed] [Google Scholar]
- 4. Haworth J, Phillips M, Newson M, Rogers PJ, Torrens‐Burton A, Tales A. Measuring information processing speed in mild cognitive impairment: clinical versus research dichotomy. J Alzheimers Dis. 2016;51(1):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33(7):1203‐1214.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiner MW, Veitch DP, Aisen PS, et al. Recent publications from the Alzheimer's Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13(4):e1‐e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandal PK, Banerjee A, Tripathi M, Sharma A. A comprehensive review of magnetoencephalography (MEG) studies for brain functionality in healthy aging and Alzheimer's disease (AD). Front Comput Neurosci. 2018;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jafari Z, Kolb BE, Mohajerani MH. Neural oscillations and brain stimulation in Alzheimer's disease. Prog Neurobiol. 2020;194:101878. [DOI] [PubMed] [Google Scholar]
- 9. Engels M, van Der Flier W, Stam C, Hillebrand A, Scheltens P, van Straaten E. Alzheimer's disease: the state of the art in resting‐state magnetoencephalography. Clin Neurophysiol. 2017;128(8):1426‐1437. [DOI] [PubMed] [Google Scholar]
- 10. Osipova D, Ahveninen J, Jensen O, Ylikoski A, Pekkonen E. Altered generation of spontaneous oscillations in Alzheimer's disease. Neuroimage. 2005;27(4):835‐841. [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Wahlund L‐O, Dierks T, Julin P, Winblad B, Jelic V. Discrimination of Alzheimer's disease and mild cognitive impairment by equivalent EEG sources: a cross‐sectional and longitudinal study. Clin Neurophysiol. 2000;111(11):1961‐1967. [DOI] [PubMed] [Google Scholar]
- 12. Penttilä M, Partanen JV, Soininen H, Riekkinen P. Quantitative analysis of occipital EEG in different stages of Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1985;60(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 13. Schreiter‐Gasser U, Gasser T, Ziegler P. Quantitative EEG analysis in early onset Alzheimer's disease: a controlled study. Electroencephalogr Clin Neurophysiol. 1993;86(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 14. Berendse H, Verbunt J, Scheltens P, Van Dijk B, Jonkman E. Magnetoencephalographic analysis of cortical activity in Alzheimer's disease: a pilot study. Clin Neurophysiol. 2000;111(4):604‐612. [DOI] [PubMed] [Google Scholar]
- 15. Fernández A, Maestú F, Amo C, et al. Focal temporoparietal slow activity in Alzheimer's disease revealed by magnetoencephalography. Biol Psychiatry. 2002;52(7):764‐770. [DOI] [PubMed] [Google Scholar]
- 16. Montez T, Poil S‐S, Jones BF, et al. Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early‐stage Alzheimer disease. Proc Natl Acad Sci. 2009;106(5):1614‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández A, Hornero R, Mayo A, Poza J, Gil‐Gregorio P, Ortiz T. MEG spectral profile in Alzheimer's disease and mild cognitive impairment. Clin Neurophysiol. 2006;117(2):306‐314. [DOI] [PubMed] [Google Scholar]
- 18. de Haan W, Stam CJ, Jones BF, Zuiderwijk IM, van Dijk BW, Scheltens P. Resting‐state oscillatory brain dynamics in Alzheimer disease. J Clin Neurophysiol. 2008;25(4):187‐193. [DOI] [PubMed] [Google Scholar]
- 19. Wechsler D. Advanced Clinical Solutions For The WAIS‐IV And WMS‐IV. San Antonio, TX: The Psychological Corporation; 2009. [Google Scholar]
- 20. Heaton R, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for An Expanded Halstead‐Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources. 2004. [Google Scholar]
- 21. Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: normative data and analysis of inter‐form and test‐retest reliability. Clin Neuropsychol. 1998;12(1):43‐55. [Google Scholar]
- 22. Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson. 2008;22(498):1. [Google Scholar]
- 23. Wechsler D. WMS‐IV: Wechsler Memory Scale. 4th ed. New York, NY: The Psychological Corporation; 2009. [Google Scholar]
- 24. Brandt J, Benedict RH. Hopkins Verbal Learning Test–Revised: Professional Manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 25. Pfeffer RI, Kurosaki TT, Harrah Jr C, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 28. Minoshima S, Drzezga AE, Barthel H, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57(8):1316‐1322. [DOI] [PubMed] [Google Scholar]
- 29. Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378‐384. [DOI] [PubMed] [Google Scholar]
- 30. Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33(1):127‐138. [DOI] [PubMed] [Google Scholar]
- 31. Jack Jr CR, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13(3):205‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiesman AI, O'Neill J, Mills MS, et al. Aberrant Occipital Dynamics Differentiate HIV‐infected Patients With and Without Cognitive Impairment. Brain. 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groff BR, Wiesman AI, Rezich MT, et al. Age‐related visual dynamics in HIV‐infected adults with cognitive impairment. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lew BJ, McDermott TJ, Wiesman AI, et al. Neural dynamics of selective attention deficits in HIV‐associated neurocognitive disorder. Neurology. 2018;91(20):e1860‐e1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiesman AI, Heinrichs‐Graham E, McDermott TJ, Santamaria PM, Gendelman HE, Wilson TW. Quiet connections: reduced fronto‐temporal connectivity in nondemented Parkinson's disease during working memory encoding. Hum Brain Mapp. 2016;37(9):3224‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. Apr 7 2006;51(7):1759‐1768. [DOI] [PubMed] [Google Scholar]
- 38. Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user‐friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83‐98. [DOI] [PubMed] [Google Scholar]
- 40. Wickham H. ggplot2: elegant graphics for data analysis. Springer; 2016. [Google Scholar]
- 41. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968‐980. [DOI] [PubMed] [Google Scholar]
- 42. Team RC, R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 43. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173‐1182. [DOI] [PubMed] [Google Scholar]
- 44. Stefanics G, Hangya B, Hernádi I, Winkler I, Lakatos P, Ulbert I. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci. 2010;30(41):13578‐13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engel AK, Fries P. Beta‐band oscillations—signalling the status quo?. Curr Opin Neurobiol. 2010;20(2):156‐165. [DOI] [PubMed] [Google Scholar]
- 46. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci. 2017;20(3):327‐339. [DOI] [PubMed] [Google Scholar]
- 47. Pontecorvo MJ, Devous Sr MD, Navitsky M, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140(3):748‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(5):1551‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ossenkoppele R, Smith R, Ohlsson T, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92(6):e601‐e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Kant R, Goldstein LS, Ossenkoppele R. Amyloid‐β‐independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2019:1‐15. [DOI] [PubMed] [Google Scholar]
- 51. Mattsson N, Insel PS, Donohue M, et al. Predicting diagnosis and cognition with 18F‐AV‐1451 tau PET and structural MRI in Alzheimer's disease. Alzheimers Dement. 2019;15(4):570‐580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


