Abstract
Introduction/Background:
Patients with rheumatoid arthritis (RA) have a high risk of infections that may require intensive care unit (ICU) admission in case of resulting sepsis. Data regarding the mortality of these patients are very limited. This study investigated clinical characteristics and outcomes of patients with RA admitted to the ICU for sepsis and compared the results to a control cohort without RA.
Methods:
All patients with RA as well as sex-, age-, and admission year-matched controls admitted to the ICU of a university hospital for sepsis between 2006 and 2019 were retrospectively analyzed. Mortality was calculated for both the groups, and multivariate logistic regression was used to determine independent risk factors for sepsis mortality. The positive predictive value of common ICU scores was also investigated.
Results:
The study included 49 patients with RA (mean age 67.2 ± 9.0 years, 63.3% females) and 51 matched controls (mean age 67.4 ± 9.5 years, 64.7% females). Among the patients with RA, 42.9% (n = 21) were treated with conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) and 30.6% (n = 15) received glucocorticoids only. Seven (14.3%) patients received biologic (b) DMARDs. The hospital mortality was higher among patients with RA (42.9% vs 15.7%, P = .0016). Rheumatoid arthritis was independently associated with mortality in multivariate logistic regression (P = .001). In patients with RA, renal replacement therapy (P = .024), renal failure (P = .027), and diabetes mellitus (P = .028) were independently associated with mortality. Acute Physiology and Chronic Health Evaluation II (APACHE II), Simplified Acute Physiology Score II (SAPS II), and Sequential Organ Failure Assessment (SOFA) scores were good predictors of sepsis mortality in patients with RA (APACHE II area under the curve [AUC]: 0.78, P = .001; SAPS II AUC: 0.78, P < .001; SOFA AUC 0.78, P < .001), but their predictive power was higher among controls.
Conclusions:
Hospital sepsis mortality was higher in patients with RA than in controls. Rheumatoid arthritis itself is independently associated with an increased sepsis mortality. Renal replacement therapy, renal failure, and diabetes were associated with an increased mortality. Common ICU scores were less well predictors of sepsis mortality in patients with RA compared to non-RA controls.
Keywords: rheumatoid arthritis, sepsis, mortality, intensive care unit
Introduction
Rheumatic diseases are associated with an increased risk of infectious complications. Patients with rheumatoid arthritis (RA) are particularly susceptible to serious infectious diseases such as septic arthritis, osteomyelitis, and infections of the skin but also pneumonia and genitourinary infections.1 Reasons for a higher susceptibility to infections might be a high disease activity,2–4 a prematurely aged immune system (immunosenescence),5–9 a potentially impaired humoral immunity to some pathogens,10 or the often required immunosuppressive medication including corticosteroids.1,2,11,12 Compared to the general population, mortality risk of infections is increased up to 2- to 6-fold.13–16 Nevertheless, despite national and international recommendations,17,18 vaccination rates for common preventable pathogens such as Streptococcus pneumoniae are low among patients with RA.19 Furthermore, population-based studies show an increased risk for admission to an intensive care unit (ICU) in patients with RA compared to the general population (hazard ratio [HR]: 1.65, 95% confidence interval [CI]: 1.50-1.83).20 The second most common reason for ICU admission in patients with RA (after ischemic cardiovascular diseases) are infections.20 Hospitalization rates for sepsis more than tripled in patients with RA between 1993 and 2013.21 However, knowledge of sepsis mortality in patients with RA in an intensive care setting is limited.22 Investigations on RA and intensive care treatment focus on particular sepsis entities such as Staphylococcus aureus sepsis23 or intensive care treatment of patients with RA in general.24,25
We therefore aimed to retrospectively investigate the in-hospital mortality of sepsis requiring ICU treatment in patients with RA in comparison to an age-, sex-, and admission year-matched control group in a single university center in Germany.
Methods and Study Design
We retrospectively analyzed all cases of patients with RA (≥ 18 years) admitted to the ICU of our University Hospital for sepsis between February 2006 and January 2019. To acquire a realistic sample of patients, no further restrictions to comorbidities, gender, or age have been applied. All data were extracted from the patient’s electronic record. We searched the hospital records using the International Classification of Diseases-10 (ICD-10)-German modification 2019 codes of patients admitted to the medical ICU (A39.x-A41.x for sepsis and M05.xx-M06.xx for rheumatoid arthritis [excluding M06.1x for adult-onset Still’s disease]). The integrity of both diagnoses was double-checked independently by 2 authors (Marco Krasselt and Christoph Baerwald) reviewing the medical records and discharge letters. All diagnoses of the identified and included patients were found to be appropriate. To compare the epidemiological data and clinical outcome with non-RA patients, we randomly selected patients having sepsis without RA or any other autoinflammatory disease from the ICU, matched for age, sex, and year of ICU treatment.
Sepsis severity is assessed and electronically documented generally at admission to our medical ICU using Sequential Organ Failure Assessment (SOFA) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II). These scores are helpful to determine the degree of organ dysfunction, disease severity, and therefore estimation of sepsis mortality. Data regarding patient demographics (eg, gender and ongoing medication) and clinical as well as laboratory parameters were obtained from the electronic patient charts and laboratory records at the time of ICU admission. The ethics committee of the Medical Faculty of the University of Leipzig has approved the design of the study (Reg-No. 352/19-ek).
Biostatistical Analysis
Continuous data were described using either mean and standard deviation or median and interquartile range. Categorical data were described using absolute or relative frequencies. Fisher exact test was performed to compare frequencies of categorical variables. To compare continuous data, student t test or Mann-Whitney U test, as appropriate, was used after performing the Kolmogorov-Smirnov normality test. Logistic regression, adjusted for age and sex, was performed to identify independent risk factors for nonsurvival. Variables with P < .1 in bivariate analysis were entered into the final regression model. To further investigate the power of ICU scores (SOFA, APACHE II, and SAPS II) in predicting mortality, receiver–operating characteristic (ROC) analysis was conducted. A significant statistical difference was assumed when the P value was below .05. Analyses were conducted using IBM SPSS Statistics version 24.0 for Macintosh (IBM, Chicago, Illinois).
Results
A total of 49 patients with RA admitted to the ICU for sepsis (mean age at admission 67.2 ± 9.0 years, 63.3% female) were enrolled. Prior to ICU admission, 42.9% (n = 21) were treated with conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs, mostly methotrexate [MTX], n = 17) and 30.6% (n = 15) received glucocorticoids only. Biologic (b) DMARDs were used in 14.3% (n = 7) of the patients with 28.6% (n = 2) of them being tumor necrosis factor (TNF) inhibiting agents and 42.9% (n = 3) rituximab (Table 1). The control group included 51 patients (age 67.4 ± 9.5 years, 64.7% females). Median ICU length of stay was 6.0 (2.0-10.0) days for patients with RA and 4.0 (2.0-8.0) days for the controls (P = .260). There was no significant difference for APACHE II (21.4 ± 7.1 vs 21.2 ± 7.5), SAPS II (44.8 ± 15.7 vs 43.3 ± 16.0), and SOFA scores (7.3 ± 3.6 vs 6.7 ± 4.1) between patients with RA and controls.
Table 1.
Characteristics and Antirheumatic Medication Among Patients With Sepsis Having RA.
| Characteristics | Result |
|---|---|
| ACPA positivity (%) | 19 (38.8) |
| RF positivity (%) | 32 (65.3) |
| ANA positivity (%) | 6 (12.2) |
| Medication, n (%) | |
| csDMARDs | 21 (42.9) |
| bDMARD | 7 (14.3) |
| Glucocorticoid monotherapy | 15 (30.6) |
| csDMARDs, n (%) | |
| Methotrexate | 17 (34.7) |
| Leflunomide | 1 (2.0) |
| Sulfasalazine | 1 (2.0) |
| Hydroxychloroquine | 2 (4.1) |
| bDMARDs, n (%) | |
| Adalimumab | 1 (14.3) |
| Etanercept | 1 (14.3) |
| Abatacept | 1 (14.3) |
| Rituximab | 3 (42.9) |
| Tocilizumab | 1 (14.3) |
Abbreviations: ACPA, anticitrullinated proteins/peptides antibodies; ANA, antinuclear antibodies; bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; RF, rheumatoid factor; TNF, tumor necrosis factor; RA, rheumatoid arthritis.
During ICU treatment, patients with RA required mechanic ventilation in 69.4% (n = 34), vasopressor support in 77.6% (n = 38), and renal replacement therapy (RRT) in 24.5% (n = 12) cases. Among controls, mechanic ventilation was considered necessary in 62.8% (n = 32), vasopressor support in 51.0% (n = 26), and RRT in 35.3% (n = 18). While there was no difference regarding mechanic ventilation and RRT, vasopressor support was significantly more often required in patients with RA (P = .007). Clinical data are presented in Table 2. For further autoantibody status of patient with RA and medication details, see Table 1. Comparing the epidemiological data of patients with RA and controls, we saw some differences (Table 2): glucocorticoids have been used more often in patients with RA (P < .0001). Furthermore, while not reaching statistical significance, there was a trend for a higher frequency of hypertension and heart failure with preserved ejection fraction (HFpEF) among the controls (P = .067 and .066, respectively). Heart failure with reduced ejection fraction (HFrEF) was significantly more prevalent in patients with RA (P = .0035). Regarding other comorbidities, there was no between-group difference for atrial fibrillation, chronic obstructive pulmonary disease (COPD), coronary heart disease, and type 2 diabetes. Moreover, no difference for neither infections site nor pathogens was observed (Table 2).
Table 2.
Clinical and Laboratory Data of Patients Having Sepsis With Versus Without Rheumatoid Arthritis.a
| Characteristics | RA | Non-RA | P |
|---|---|---|---|
| Mean age, years | 67.2 ± 9.04 | 67.4 ± 9.54 | .9538 |
| Female, n (%) | 31 (63.3) | 33 (64.7) | >.9999 |
| Median lactate, mmol/L | 3.0 (1.9-4.475) | 2.5 (1.4-3.3) | .035 |
| Median WBC, cells/μL | 14.5 (7.1-23.45) | 14.1 (10.10-21.4) | .955 |
| Median PLT, cells/μL | 158 (66.5-301) | 165 (86-240) | .969 |
| Median IL-6, pg/mL | 1000 (297.8-5738) | 426.5 (93.61-1704) | .051 |
| Median PCT, ng/mL | 2.18 (0.89-25.42) | 8.715 (2.28-34.56) | .014 |
| Mean CRP, mg/L | 208.7 ± 128.2 | 219.7 ± 136.4 | .689 |
| Mean APACHE IIb | 21.44 ± 7.119 | 21.18 ± 7.469 | .859 |
| Mean SOFAb | 7.25 ± 3.558 | 6.745 ± 4.132 | .517 |
| Mean SAPS IIb | 44.83 ± 15.66 | 43.29 ± 16.0 | .630 |
| Glucocorticoids, n (%) | |||
| All users | 32 (65.3) | 3 (5.9) | <.0001 |
| Low-dosec users | 18 (36.7) | 3 (5.9) | .270 |
| Median dosed | 5 (5.0-11.25) | 5 (5.0-5.0) | .283 |
| Infection sites, n (%) | |||
| Lung | 17 (34.7) | 23 (45.1) | .314 |
| Urinary tract | 8 (16.3) | 12 (23.5) | .456 |
| Peritoneum | 2 (4.1) | 1 (2.0) | .613 |
| Skin | 5 (10.2) | 2 (3.9) | .264 |
| GI tract | 3 (6.1) | 4 (7.8) | >.999 |
| Bone/joint | 6 (12.2) | 2 (3.9) | .156 |
| Cardiac | 2 (4.1) | 2 (3.9) | >.999 |
| Other | 2 (4.1) | 4 (7.8) | .678 |
| Unknown | 4 (8.2) | 1 (2.0) | .200 |
| Frequent pathogens, n (%) | |||
| MSSA | 8 (16.3) | 5 (9.8) | .384 |
| MRSA | 2 (4.1) | 2 (3.9) | >.999 |
| Escherichia coli | 4 (8.2) | 11 (21.6) | .092 |
| Enterococcus faecalis | 1 (2.0) | 6 (11.8) | .112 |
| Pseudomonas aeruginosa | 1 (2.0) | 2 (3.9) | >.999 |
| Aspergillus fumigatus | 2 (4.08) | 0 (0) | .238 |
| Streptococcus pneumoniae | 0 (0) | 3 (5.9) | .343 |
| Frequent comorbidities, n (%) | |||
| Atrial fibrillation | 16 (32.7) | 13 (25.5) | .511 |
| COPD | 7 (14.3) | 8 (15.7) | >.999 |
| Coronary heart disease | 12 (24.5) | 6 (11.8) | .122 |
| HFpEF | 14 (28.6) | 24 (47.1) | .066 |
| HFrEF | 10 (20.4) | 1 (2.0) | .0035 |
| Arterial hypertension | 24 (49.0) | 35 (68.6) | .067 |
| Type 2 diabetes | 8 (16.3) | 10 (19.6) | .796 |
| ICU treatment, n (%) | |||
| Acute renal failuree | 26 (53.1) | 32 (62.8) | .418 |
| Mechanic ventilation | 34 (69.4) | 32 (62.8) | .531 |
| Median admission days | 6.0 (2.0-10.0) | 4.0 (2.0-8.0) | .260 |
| Positive culture | 35 (71.4) | 35 (68.6) | .829 |
| RRT | 12 (24.5) | 18 (35.3) | .279 |
| Septic shock (SEPSIS-3) | 32 (65.3) | 15 (29.4) | .0006 |
| Vasopressor use | 38 (77.6) | 26 (51.0) | .007 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; GI tract, gastrointestinal tract; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICU, intensive care unit; IL-6, interleukin-6; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PCT, procalcitonin; PLT, platelets; RRT, renal replacement therapy; RA, rheumatoid arthritis; SAPS II, Simplified Acute Physiology Score II; SEPSIS-3, septic shock according to the Third International Consensus Definition for Sepsis and Septic Shock28; SOFA, Sequential Organ Failure Assessment; WBC, white blood cells.
a Given are numbers and % in brackets, mean ± standard deviation or median and interquartile range in brackets, respectively.
b Calculated after ICU admission.
c The dose was considered being low when not exceeding 7.5 mg prednisolone equivalent.26
d In mg prednisolone equivalent.
e At least acute kidney injury (AKI) stage 1, following the definition of the Guidelines for AKI of the Kidney Disease: Improving Global Outcomes (KDIGO).27
Septic shock was more often diagnosed among patients with RA, 65.3% versus 29.4%, P = .0006. This finding is in line with the more frequent use of vasopressors (P = .007) as well as the higher lactate levels in the RA group (P = .035). In bivariate analysis, septic shock was significantly more frequent in nonsurviving patients with RA (P = .001; see Table 3); this was also true for vasopressor use (P = .007).
Table 3.
Bivariate Analysis of Potential Risk Factors for Mortality in Patients With RA.
| Characteristics | Survivor (n = 26) | Nonsurvivor (n = 23) | P |
|---|---|---|---|
| Admission days | 6.0 (3.0-10.0) | 4.0 (1.0-14.0) | .451 |
| AF | 5 (20.0) | 11 (47.8) | .041 |
| Age | 66.27 ± 7.49 | 68.35 ± 10.59 | .428 |
| APACHE II | 18.12 ± 5.689 | 25.04 ± 6.846 | .0001 |
| bDMARD therapy | 4 (15.4) | 3 (13.0) | .815 |
| COPD | 2 (7.7) | 5 (21.7) | .161 |
| Coronary heart disease | 7 (26.9) | 5 (21.7) | .674 |
| CRP | 212.02 ± 104.81 | 244.04 ± 135.48 | .382 |
| csDMARD therapy (any) | 11 (42.3) | 10 (43.5) | .934 |
| Female gender | 16 (61.5) | 15 (65.2) | .790 |
| Glucocorticoid therapy | 19 (73.1) | 13 (56.6) | .224 |
| Heart failure | 13 (54.2) | 11 (45.8) | .773 |
| HFpEF | 9 (36.0) | 5 (23.8) | .371 |
| HFrEF | 4 (16.0) | 6 (28.6) | .303 |
| Hypertension | 16 (64.0) | 8 (34.8) | .043 |
| IL-6 | 388.6 (191.0-3752.50) | 757.5 (341.575-1000.0) | .527 |
| Lactate | 2.644 ± 1.4672 | 4.752 ± 3.1181 | .004 |
| Methotrexate | 10 (38.5) | 7 (30.4) | .556 |
| PCT | 1.345 (0.38-17.3975) | 8.08 (1.53-47.9825) | .115 |
| PLT | 247.44 ± 144.296 | 145.91 ± 139.133 | .017 |
| Renal failurea | 8 (32.0) | 19 (82.6) | .0001 |
| RRT | 1 (3.8) | 11 (47.8) | .0001 |
| SAPS II | 37.56 ± 13.301 | 52.74 ± 14.344 | .0001 |
| Septic shock (SEPSIS-3) | 11 (44.0) | 21 (91.3) | .001 |
| SOFA | 5.52 ± 2.220 | 9.13 ± 3.817 | .0001 |
| T2DM | 2 (8.0) | 6 (26.1) | .093 |
| Vasopressor use | 16 (64.0) | 22 (95.7) | .007 |
| Ventilation | 15 (57.7) | 19 (82.6) | .059 |
| WBC | 11.2 (7.875-16.025) | 15.85 (2.375-24.875) | .600 |
Abbreviations: AF, atrial fibrillation; bDMARD, biological disease-modifying antirheumatic drug; APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; csDMARD, conventional synthetic disease-modifying antirheumatic drug; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; heart failure, HFrEF+HFpEF; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IL-6, interleukin-6; PCT, procalcitonin; PLT, platelets; RRT, renal replacement therapy; SAPS II, Simplified Acute Physiology Score II; septic shock (SEPSIS-3), septic shock according to the Third International Consensus Definition for Sepsis and Septic Shock28; SOFA, Sequential Organ Failure Assessment; T2DM, type 2 diabetes; WBC, white blood cells.
a At least acute kidney injury (AKI) stage 1, following the definition of the Guidelines for AKI of the Kidney Disease: Improving Global Outcomes (KDIGO).27
The in-hospital mortality was significantly higher in patients with RA than among controls (42.9% vs 15.7%, P = .0016). Regarding the individual RA medication (including daily glucocorticoid dose) or antibody status, no difference in mortality was found (Table 3). The same was true for the site of infection and the cultured pathogen in the RA cohort (data not shown).
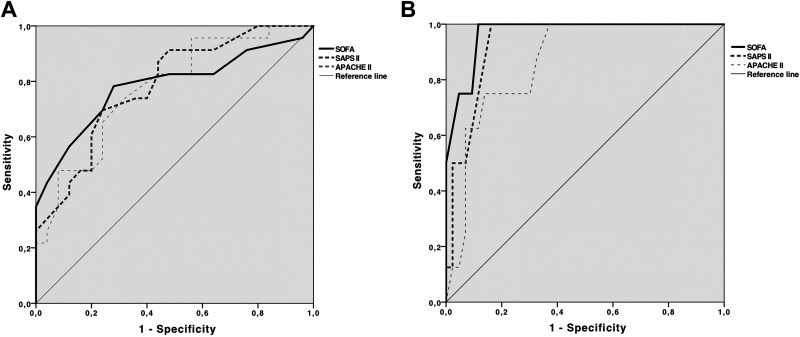
The performed ROC analysis (Table 4 and Figure 1) revealed that the area under the curve (AUC) for SOFA, SAPS II, and APACHE II was lower among patients with RA, reflecting a less well-predictive power of the scores for mortality in this group (Figure 1). A subset analysis of the RA cohort revealed all scores being significantly higher in nonsurvivors (Table 3); 70% of RA nonsurvivors had an SOFA and SAPS II above median, and 65% had an APACHE II above median (data not shown).
Table 4.
Receiver–Operating Characteristic (ROC) Analysis of the Value of the SOFA, SAPS II, and APACHE II Score in Predicting Mortality.a
| Patients With RA, n = 49 | Non-RA Patients, n = 51 | |
|---|---|---|
| SOFA | AUC: 0.784 ± 0.07, 95% CI: 0.648-0.921, P < .001 | AUC: 0.968 ± 0.0226, 95% CI: 0.924-1.000, P < .0001 |
| SAPS II | AUC: 0.784 ± 0.065, 95% CI: 0.656-0.912, P < .001 | AUC: 0.933 ± 0.0346, 95% CI: 0.936-1.000, P = .0001 |
| APACHE II | AUC: 0.775 ± 0.067, 95% CI: 0.644-0.906, P = .001 | AUC: 0.866 ± 0.056, 95% CI: 0.756-0.977, P = .001 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; AUC, area under the curve; CI, confidence interval; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; RA, rheumatoid arthritis.
a Area under the curve is given with standard error (±SE), 95% confidence interval and P value.
Figure 1.
Receiver–operating characteristic (ROC) analysis of the value of the SOFA, SAPS II, and APACHE II score in predicting in-hospital mortality among RA (A) and non-RA (B) patients. See Table 4 for numbers. APACHE II indicates Acute Physiology and Chronic Health Evaluation II; RA, rheumatoid arthritis; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment.
In a multivariate analysis, RRT (odds ratio [OR]: 24.202, 95% CI: 1.528-383.219, P = .024), renal failure (OR: 7.958, 95% CI 1.270-49.875, P = .027), and type 2 diabetes mellitus (OR: 12.063, 95% CI: 1.314-110.78, P = .028) were independently associated with nonsurvival in patients with sepsis having RA (Table 5). Rheumatoid arthritis itself (OR: 32.644, 95% CI: 4.462-238.844, P = .001) was found to be the most important independent risk factor for sepsis mortality.
Table 5.
Multivariate Analysis Using Logistic Regression, Adjusted for Age and Gender, of the Relationship Between Mortality of Patients With RA and the Given Parameters.
| Coefficient | SE | P | ORs | 95% CI | |
|---|---|---|---|---|---|
| Renal failurea | 2.074 | 0.936 | .027 | 7.958 | 1.270-49.875 |
| RRT | 3.186 | 1.409 | .024 | 24.202 | 1.528-383.219 |
| T2DM | 2.490 | 1.131 | .028 | 12.063 | 1.314-110.78 |
a At least acute kidney injury (AKI) stage 1, following the definition of the Guidelines for AKI of the Kidney Disease: Improving Global Outcomes (KDIGO).27
Abbreviations: CI, confidence interval; OR, odds ratio; RRT, renal replacement therapy; SE, standard error; T2DM, type 2 diabetes.
Discussion
In this retrospective analysis, in-hospital mortality was found to be significantly higher among patients with RA compared to controls. One reason most likely is the higher incidence of septic shock in the RA group. Although multivariate analysis did not reveal septic shock to be independently associated with nonsurvival in our logistic regression model (maybe caused by the strong association of septic shock with other variables, eg, SOFA and vasopressor use), septic shock is surely highly associated with mortality.29 Another reason for the high mortality might be the tertiary character of our hospital predominantly admitting severely ill, immunocompromised patients with RA. Furthermore, HFrEF was more prevalent the RA cohort than among non-RA patients. This finding was also reported by others30 and could contribute to an increased mortality.
Interpreting our results in the light of the existing literature, we found one study that included 50 patients with various inflammatory rheumatic diseases, showing a mortality rate among the RA subgroup similar to that of our cohort.25 Colleagues from Israel also reported a rather comparable mortality of 34.9% in 43 patients with RA under ICU treatment. Almost three-fourth of these patients were admitted to ICU for infection, 55.8% for septic shock. 24 In contrast, an investigation from Austria found a very low mortality rate of 10.8% in 74 patients with RA who have been admitted to an ICU for infection.31 However, that study only included patients that needed parenteral antibiotics for infection and were admitted to an ICU, and sepsis was not specifically discussed. Another investigation from the United States exclusively focusing on S aureus sepsis in patients with RA found a low mortality of 22.9%.23 While this low mortality rate could be a consequence of the distinct S aureus sepsis entity (mortality declined from 2007 to 2015 to 18%),32 the authors do not specify whether the patients needed ICU treatment or have been in septic shock23; the results of this study are therefore barely comparable to the results of our study. Barrett et al found a sepsis mortality rate of >50% in 124 patients with RA, which exceeds the mortality in our investigation.22 The authors explained this high mortality with a low ratio of ICU-to-general ward beds (3%) in the included hospitals which in turn would lead to admission of severely ill patients only. Furthermore, the population’s mean age of 71.0 ± 11.5 years (our population: 67.2 ± 9.0 years) was believed to negatively influence mortality.22
An interesting cohort study from the United States demonstrated a lower sepsis mortality among patients with autoimmune diseases compared to nonautoimmune controls (26.6% vs 34.6%, P < .001).33 The variety of included diseases was wide, ranging from Crohn’s disease, multiple sclerosis, systemic lupus erythematosus (SLE), and inflammatory myopathies to RA.33 Patients with RA accounted for 26% of the patients, limiting the value of this investigation for individual diseases such as RA. Moreover, mortality was not related to preadmission DMARD or glucocorticoid use.33 Brünnler et al reported a mortality rate of 16% only, but the studied cohort consisted of patients with different rheumatic diseases (also including spondyloarthritis and connective tissue diseases) and was not restricted to septic conditions.34 Rheumatoid arthritis accounted for 44%, with the main reasons for admission being cardiovascular complications and infections (20% and 31%, respectively). The median APACHE II score was low with 12 (2-33), emphasizing the exceeding severity and worse prognosis in our cohort. Another investigation by Faguer et al found a similar mortality of only 16%.35 Again, patients with different rheumatic diseases were included, and most of the patients (63%) had SLE. Besides, main reason for ICU admission was flare up of the rheumatic disease (48%) followed by infection (47%). Due to their mixed cohorts of different rheumatic diseases and lack of restriction to patients with sepsis, the results of Brünnler and Faguer et al are difficult to compare to our findings.
Of particular interest of our study is the finding that no kind of disease-modifying or immunosuppressive medication (including bDMARDs, csDMARDs and glucocorticoids) had any influence on sepsis mortality in the patients with RA. This is partly in line with the findings of Sams et al who showed that S aureus sepsis mortality in patients with RA was not associated with the use of bDMARDs.23 An analysis of the German RABBIT register (Rheumatoid Arthritis: Observation of Biologic Therapy) including 947 patients with RA concluded that the use of TNF inhibitors (TNFi) was associated with a reduced sepsis mortality (OR: 0.28, 95% CI: 0.12-0.63).36 There was no significant risk reduction for non-TNFi bDMARDs such as rituximab or tocilizumab though. Congruently, a report from a large UK registry (>15 000 patients with RA) showed an OR of 0.5 (95% CI: 0.3-0.8) for sepsis mortality under TNFi therapy.37 While these findings explain our results at least partly, the number of patients with bDMARDs in our study is too small to make a meaningful conclusion in this regard. As for glucocorticoids and csDMARDs users, susceptibility to infections is well known to be increased, particularly for glucocorticoids and cyclophosphamide over azathioprine and MTX.11 Nevertheless, even low-dose MTX is known to possibly cause myelosuppression with pancytopenia and sepsis.38 Data on the impact of glucocorticoids and csDMARDs on sepsis and sepsis mortality is scarce, since most investigations focus on bDMARDs.36,37 Although we were not able to show an impact of DMARDs on sepsis mortality, the immunosuppressive effect most likely negatively affects sepsis course. As we know from anti-neutrophil cytoplasmatic antibodies (ANCA) associated vasculitis, infections during immunosuppressive therapy (including glucocorticoids) greatly contribute to mortality.39 Tumor necrosis factor inhibitors therapy in RA might be the exclusion.
Multivariate analysis revealed RA itself being the most important independent risk factor for sepsis mortality. Other risk factors for nonsurvival were the need for RRT, renal failure, and type 2 diabetes. Diabetes mellitus was earlier identified as a strong predictor of infection in patients with RA40 and has also been associated with sepsis mortality in patients with RA before (HR: 1.78, 95% CI: 1.19-3.52, P = .005).22 Renal failure in patients with sepsis was also positively correlated to mortality (OR: 2.7, 95% CI: 1.2-6.2, P = .021).41 The requirement of RRT in patients with sepsis in general is known to be associated with a very high mortality of 60% to 70%, independent of the RRT timing.42,43 This explains the high association with mortality we found. Furthermore, the need for RRT usually reflects a higher degree of renal failure,27 emphasizing the impact of acute kidney injury on sepsis mortality.
As demonstrated using ROC analysis, APACHE II44, SAPS II,45 and SOFA46 show a good power in predicting sepsis mortality, but the association clearly seems to be stronger in the control group than among the patients with RA. This finding is remarkable and raises the question for the underlying cause(s). The literature on the association of ICU scores and mortality in patients with rheumatic diseases is scarce and mostly focuses on vasculitis and SLE.47,48 While SOFA was associated with mortality and showed a good predictive power in vasculitis patients, APACHE II was not associated with mortality.48 In patients with SLE, APACHE II was unable to accurately predict mortality.47 The results of our RA cohort are in line with the findings of Haviv-Yadid et al who showed an association between mortality and APACHE II as well as SOFA.24 None of these studies compared the score’s power with a control group though. Different reasons could be causative for our finding of an impaired power in predicting mortality of patients with sepsis having RA. Since RA is not seldom associated with thrombocytosis,49,50 platelet count could have been overestimated. This would particularly effect SOFA score, which shows the largest AUC deviation between patients with RA and non-RA controls in our study. Since overestimated platelets have no impact on APACHE II or SAPS II, there also have to be other reasons for the observed AUC deviation. Although both APACHE II and SAPS II account for chronic disease, they might not be ideal for RA: APACHE II only considers “chronic health problems” including a variety of diagnoses and therapies (liver cirrhosis, New York Hearth Association Class 4 heart failure, severe COPD, pulmonary hypertension, regular dialysis or immunocompromising conditions such as immunosuppression, chemotherapy, radiation, long-term or high-dose steroids, leukemia, lymphoma or AIDS) as 1 single parameter. If a patient with RA has no immunosuppression, then the score would not rise, despite the fact that RA itself impairs the immune system5–9 and increases the risk of infections.1,3,4 The SAPS II also only accounts for metastatic cancer, hematologic malignancy, and AIDS under the term “chronic disease.” Consequently, both scores could underestimate sepsis severity which might explain the observed AUC deviation. We therefore speculate that the RA-related impairment of the immune system (“immunosenescence”), a potentially reduced response to some pathogens, thrombocytosis, and disease activity itself lead to a reduced reliability of ICU scores in terms of mortality prediction.
Our study has several limitations. The sample size is relatively small. Nevertheless, the data using a comparable non-RA septic cohort allow a good understanding of the scenario. Since the study is retrospective, there may be the risk of misclassification due to incorrect coding (ICD-10). However, we have reduced this risk by double-checking the diagnoses using the medical records. As a single-center study, there may also be a bias in disease severity. However, we believe that the results may contribute to further our understanding of patients with rheumatoid arthritis suffering from sepsis.
Conclusions
Mortality in patients with RA has been demonstrated to be substantially higher than in controls. Independent risk factors for sepsis mortality in patients with RA were RRT, renal failure, and diabetes. Rheumatoid arthritis itself was identified as being strongly related to sepsis mortality. The commonly used APACHE II, SAPS II, and SOFA scores demonstrated reduced power in predicting sepsis mortality in patients with RA compared to non-RA patients.
Footnotes
Authors’ Note: All procedures performed in this survey were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Data obtained in this study did not interfere with the course of treatment for patients included.
Marco Krasselt conceived the project, collected and interpreted the data, performed the statistical analysis, and drafted the manuscript. Christoph Baerwald contributed to data collection, interpretation, and was involved in manuscript drafting. Sirak Petros was responsible for ICU patient care and contributed important intellectual content to the manuscript. Olga Seifert was involved in the statistical analysis as well as manuscript preparation. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor Baerwald received lecture fees from Merck, MSD, Mundipharma, and Pfizer. Doctores Krasselt, Petros and Seifert have nothing to declare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marco Krasselt, MD  https://orcid.org/0000-0002-1842-0754
https://orcid.org/0000-0002-1842-0754
References
- 1. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–2293. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhary NS, Donnelly JP, Moore JX, Baddley JW, Safford MM, Wang HE. Association of baseline steroid use with long-term rates of infection and sepsis in the REGARDS cohort. Crit Care. 2017;21(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Au K, Reed G, Curtis JR. et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–791. [DOI] [PubMed] [Google Scholar]
- 4. Mehta B, Pedro S, Ozen G. et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US National Cohort Study. RMD Open. 2019;5(1):e000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(suppl 5):S422–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):583–588. [DOI] [PubMed] [Google Scholar]
- 7. Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28- T cells in the aging immune system. Mech Ageing Dev. 1998;102(2-3):131–147. [DOI] [PubMed] [Google Scholar]
- 8. Krasselt ML, Wagner U. Ageing and immunity. Osteology. 2014;23(3):195–201. [Google Scholar]
- 9. Krasselt M, Baerwald C, Wagner U, Rossol M. CD56+ monocytes have a dysregulated cytokine response to lipopolysaccharide and accumulate in rheumatoid arthritis and immunosenescence. Arth Res Therap. 2013;15(5): R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krasselt M, Baerwald C, Liebert UG, Seifert O. Humoral immunity to varicella zoster virus is altered in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38(9):2493–2500. [DOI] [PubMed] [Google Scholar]
- 11. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford). 2007;46(7):1157–1160. [DOI] [PubMed] [Google Scholar]
- 12. Ramiro S, Sepriano A, Chatzidionysiou K. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1101–1136. [DOI] [PubMed] [Google Scholar]
- 13. Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol. 2003;30(5):958–965. [PubMed] [Google Scholar]
- 14. Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33(4):221–227. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez A, Icen M, Kremers HM. et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35(6):1009–1014. [PMC free article] [PubMed] [Google Scholar]
- 16. van den Hoek J, Boshuizen HC, Roorda LD. et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int. 2017;37(4):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furer V, Rondaan C, Heijstek MW. et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. [DOI] [PubMed] [Google Scholar]
- 18. Robert Koch Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut. Berlin, Germany: Epidemiologisches Bulletin 34/2015; 2015:327–362. [Google Scholar]
- 19. Krasselt M, Ivanov JP, Baerwald C, Seifert O. Low vaccination rates among patients with rheumatoid arthritis in a German outpatient clinic. Rheumatol Int. 2017;37(2):229–237. [DOI] [PubMed] [Google Scholar]
- 20. Peschken CA, Hitchon CA, Garland A. et al. A population-based study of intensive care unit admissions in rheumatoid arthritis. J Rheumatol. 2016;43(1):26–33. [DOI] [PubMed] [Google Scholar]
- 21. Jinno S, Lu N, Jafarzadeh SR, Dubreuil M. Trends in hospitalizations for serious infections in patients with rheumatoid arthritis in the US between 1993 and 2013. Arthritis Care Res (Hoboken). 2018;70(4):652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrett O, Abramovich E, Dreiher J, Novack V, Abu-Shakra M. Short- and long-term mortality due to sepsis in patients with rheumatoid arthritis. Rheumatol Int. 2017;37(6):1021–1026. [DOI] [PubMed] [Google Scholar]
- 23. Sams M, Olsen MA, Joshi R, Ranganathan P. Staphylococcus aureus sepsis in rheumatoid arthritis. Rheumatol Int. 2015;35(9):1503–1510. [DOI] [PubMed] [Google Scholar]
- 24. Haviv-Yadid Y, Segal Y, Dagan A. et al. Mortality of patients with rheumatoid arthritis requiring intensive care: a single-center retrospective study. Clin Rheumatol. 2019:9. [DOI] [PubMed] [Google Scholar]
- 25. Rutter LA, Rutter S, Winkler M, Keysser G . Outcome of intensive medical care for inflammatory rheumatic diseases [in German]. Z Rheumatol. 2017;76(9):780–787. [DOI] [PubMed] [Google Scholar]
- 26. Krasselt M, Baerwald C. The current relevance and use of prednisone in rheumatoid arthritis. Expert Rev Clin Immunol. 2014;10(5):557–571. [DOI] [PubMed] [Google Scholar]
- 27. Khwaja A. KDIGO Clinical practice guidelines for acute kidney injury. Neph Clin Practice. 2012;120(4):c179–c184. [DOI] [PubMed] [Google Scholar]
- 28. Singer M, Deutschman CS, Seymour CW. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleischmann C, Thomas-Rueddel DO, Hartmann M. et al. Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int. 2016;113(10):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicola PJ, Maradit-Kremers H, Roger VL. et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. [DOI] [PubMed] [Google Scholar]
- 31. Pieringer H, Hintenberger R, Pohanka E. et al. RABBIT risk score and ICU admission due to infection in patients with rheumatoid arthritis. Clin Rheumatol. 2017;36(11):2439–2445. [DOI] [PubMed] [Google Scholar]
- 32. Austin ED, Sullivan SS, Macesic N. et al. Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007-2015. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheth M, Benedum CM, Celi LA, Mark RG, Markuzon N. The association between autoimmune disease and 30-day mortality among sepsis ICU patients: a cohort study. Crit Care. 2019;23(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brünnler T, Susewind M, Hoffmann U, Rockmann F, Ehrenstein B, Fleck M. Outcomes and prognostic factors in patients with rheumatologic diseases admitted to the ICU. Intern Med. 2015;54(16):1981–1987. [DOI] [PubMed] [Google Scholar]
- 35. Faguer S, Ciroldi M, Mariotte E. et al. Prognostic contributions of the underlying inflammatory disease and acute organ dysfunction in critically ill patients with systemic rheumatic diseases. Eur J Intern Med. 2013;24(3): e40–44. [DOI] [PubMed] [Google Scholar]
- 36. Richter A, Listing J, Schneider M. et al. Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galloway JB, Hyrich KL, Mercer LK. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford). 2011;50(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kivity S, Zafrir Y, Loebstein R, Pauzner R, Mouallem M, Mayan H. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13(11):1109–1113. [DOI] [PubMed] [Google Scholar]
- 39. Yang L, Xie H, Liu Z. et al. Risk factors for infectious complications of ANCA-associated vasculitis: a cohort study. BMC Nephrol. 2018;19(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294–2300. [DOI] [PubMed] [Google Scholar]
- 41. Pereira M, Rodrigues N, Godinho I. et al. Acute kidney injury in patients with severe sepsis or septic shock: a comparison between the ‘Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease’ (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications. Clin Kidney J. 2017;10(3):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chou YH, Huang TM, Wu VC. et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care. 2011;15(3):R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barbar SD, Clere-Jehl R, Bourredjem A. et al . Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–1442. [DOI] [PubMed] [Google Scholar]
- 44. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 45. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. [DOI] [PubMed] [Google Scholar]
- 46. Vincent JL, de Mendonca A, Cantraine F. et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. [DOI] [PubMed] [Google Scholar]
- 47. Namendys-Silva SA, Baltazar-Torres JA, Rivero-Sigarroa E, Fonseca-Lazcano JA, Montiel-Lopez L, Dominguez-Cherit G. Prognostic factors in patients with systemic lupus erythematosus admitted to the intensive care unit. Lupus. 2009;18(14):1252–1258. [DOI] [PubMed] [Google Scholar]
- 48. Haviv Y, Shovman O, Bragazzi NL. et al. Patients with vasculitides admitted to the intensive care unit: implications from a single-center retrospective study. J Intensive Care Med. 2019;34(10):828–834. [DOI] [PubMed] [Google Scholar]
- 49. Masson C. Rheumatoid anemia. Joint Bone Spine. 2011;78(2):131–137. [DOI] [PubMed] [Google Scholar]
- 50. Kiraz S, Ertenli I, Ozturk MA. et al. Bloodstream thrombopoietin in rheumatoid arthritis with thrombocytosis. Clin Rheumatol. 2002;21(6):453–456. [DOI] [PubMed] [Google Scholar]