Abstract
Gulf war illness (GWI), is a chronic multi-symptom illness that has impacted approximately one-third of the veterans who served in the 1990 to 1991 Gulf War. GWI symptoms include cognitive impairments (eg, memory and concentration problems), headaches, migraines, fatigue, gastrointestinal and respiratory issues, as well as emotional deficits. The exposure to neurological chemicals such as the anti-nerve gas drug, pyridostigmine bromide (PB), and the insecticide permethrin (PER), may contribute to the etiologically related factors of GWI. Various studies utilizing mouse models of GWI have reported the interplay of these chemical agents in increasing neuroinflammation and cognitive dysfunction. Astrocytes are involved in the secretion of neuroinflammatory cytokines and chemokines in pathological conditions and have been implicated in GWI symptomology. We hypothesized that exposure to PB and PER causes lasting changes to hippocampal astrocytes, concurrent with chronic cognitive deficits that can be reversed by cervical vagus nerve stimulation (VNS). GWI was induced in CD1 mice by injecting the mixture of PER (200 mg/kg) and PB (2 mg/kg), i.p. for 10 consecutive days. VNS stimulators were implanted at 33 weeks after GWI induction. The results show age-related cognitive alterations at approximately 9 months after exposure to PB and PER. The results also showed an increased number of GFAP-labeled astrocytes in the hippocampus and dentate gyrus that was ameliorated by VNS.
Keywords: Gulf war illness, veterans, pyridostigmine bromide, permethrin, symptomology, vagus nerve stimulation
Introduction
Gulf war illness (GWI) is a chronic multi-symptom disorder that has impacted approximately one-third of the veterans involved with Operation Desert Shield and Desert Storm in the 1990 to 1991 Gulf War.1 GWI symptoms include cognitive and motor impairments, mood deficits, headaches, migraines, fatigue, gastrointestinal and respiratory issues.2-4 Gulf war illness is described in the widely accepted Kansas criteria, by the chronic symptom domains such as fatigue/sleep problems, pain symptoms, cognitive/mood symptoms, gastrointestinal symptoms, respiratory symptoms, and skin symptoms.5
Although the precise etiology of GWI is unknown, the exposure to chemicals, such as anti-nerve gas pills, pyridostigmine bromide (PB), and the insecticide permethrin (PER), may have contributed to the etiologically related factors of GWI.6,7 Soldiers were given PER to prevent vector-borne disease and PB as a prophylactic to protect from nerve gas agent.8 PB, a reversible acetyl cholinesterase and butyryl cholinesterase inhibitor does not readily cross the blood-brain-barrier. That PB is correlated with working and long-term memory deficits in preclinical models suggests that PB alters neural networks directly or indirectly.9-13 PER, a synthetic pyrethroid, is a widely used insecticide14 that can cause pathological sustained opening of voltage-gated sodium channels,15 and cell death in humans.16 Exposure of these chemicals in preclinical models was reported to cause symptoms including neuroinflammation,17 similar to those observed in GWI.18-21 These mechanisms may underlie the chronic functional disorders that characterize GWI.22-25 The chemical exposure hypothesis is supported by the report of the Research advisory committee (RAC) on GWI indicating that the overall prevalence of GWI is greater in veterans who were exposed to higher amounts of pesticides than veterans who had limited exposure to pesticides during the Gulf war.25,26
Brain structural and functional abnormalities have also been observed in Gulf war veterans. These include reduced hippocampal volume,27-29 astrocytosis,30 neuroinflammation,31-33 and increased diffusivity in white matter connections.3,6,34 In addition to the human data, several established animal models are available to study the influence of Gulf war illness-related (GWIR) chemical exposure.9,17 Among these, the model in which PB and PER are administered intraperitoneally (i.p.) to mice for 10 consecutive days results in cognitive deficits, altered pain thresholds, long-term memory impairment, changes to the proteomic profiles and neuroinflammation.7,11,23,35,36 Therefore, this animal model enables essential basic research needed to understand GWI exposure factors, potential disease mechanisms, and to identify therapeutic targets.
Multiple studies have reported that GWIR chemical exposure is associated with astrocyte activation.11,37–46 Cognitive impairments are reported with increased reactive astrocytes in the cerebral cortex in the GWI model.17 GWIR chemical exposure is also associated with decreased neurogenesis and hypertrophy of astrocytes.9 Astrocytes, a type of neuroglial cell, meditate chemical exchange in synaptic transmission, and are involved in the secretion of neuroinflammatory cytokines and chemokines during pathological conditions.47 Neural injury and inflammatory conditions induce elevated glial fibrillary acidic protein (GFAP) expression in astrocytes,48 and GFAP-expressing astrocytes can be used to assess the astrocytic population. Astrocyte dysfunction can lead to impaired synaptic glutamate clearance and dysregulated neuronal activity.3 Behavioral impairments have also been linked to the presence of reactive astrocytes along with increased proinflammatory cytokine markers,17 and such changes are associated with hypertrophied astrocytes in the hippocampus after GWI chemical exposure.9 Rayhan et al.3 reported that astrocyte dysfunction was associated with altered brain function in GWI veterans. Therefore, treatments that target astrocyte changes in GWI might improve outcome measures. Specifically, hippocampal astrocytes may be important because the hippocampus is involved in learning and memory, emotion, affect, and many other cognitive functions.
Vagus nerve stimulation (VNS) has been shown to directly influence activated astrocytes, neurogenesis and plasticity in the hippocampus.49 VNS is FDA-approved to treat refractory seizures in epilepsy patients, depression, and has been used to treat numerous other maladies. These include, obesity, chronic refractory headaches, migraines, rapid-cycling bipolar disorder, treatment resistant anxiety disorders, and Alzheimer’s disease. Many of these disorders, as well as GWI, are known or thought to have a chronic inflammatory disease component. VNS has been previously shown to be anti-inflammatory50-53 and anti-neuroinflammatory54-57 among its possible mechanisms of therapeutic efficacy. We hypothesized that exposure to PB and PER in mice causes lasting changes to GFAP-labeled astrocytes in the hippocampus and dentate gyrus that can be reversed by stimulation of the cervical vagus nerve. We further hypothesized that VNS in a mouse model of GWI would reduce the long-term behavioral and cognitive deficits.
To test this hypothesis, this study incorporated the mouse model of GWI in which PB and PER are injected once daily for 10 days, after which vagus nerve stimulators were implanted at 33 weeks post-exposure. Chronic cognitive-behavioral performance was examined, as was astrocytosis in the hippocampus.
Methods
Animals
All animal experiments were approved by the Texas A&M Health Science Center and Baylor Scott and White Institutional Animal Care and Use Committee and adhere to Federal and State Guidelines. Animals were housed in individually ventilated cages under controlled environment with a 12-hour light dark cycle (light on 6:00 and light off 18:00). All animals had continuous access to food and water, and maintained on a standard diet (Envigo #8604). Mice were randomly assigned to either the chemical exposure group or the control group. CD1 mice (male, age 10 weeks) were purchased from Jackson Laboratories and were allowed to acclimate for 1 week in the animal facility. GWI was induced by giving a single i.p. injection of PER and PB, for 10 consecutive days, as previously described7,23,35 Briefly, the mixture consisted of 200 mg/kg of PER and 2 mg/kg of PB in volume of 50 µl (1:600 drug cocktail ratio of volume/weight) dimethyl sulfoxide (DMSO) or the same volume of DMSO alone for vehicle control animals. In total, we had 5 groups: Naïve (litter matched, no manipulations), DMSO (as vehicle for GWI chemicals), GWI, GWI + sham VNS, and GWI + VNS.
Vagus nerve implantation and stimulation
A total of 17 mice were implanted with the stimulators (n = 8 GWI + sham VNS and n = 9 GWI + VNS). Vagus nerve stimulation (VNS) was achieved by implanting stimulators at 33 weeks after induction of GWI. Under anesthesia, a midline incision was made at the level of the front neck by placing the animal on its back. Most commonly, vagus nerve stimulators are implanted around the left vagus nerve for effective stimulation and programmable microprocessors allow precise control of the stimulation paradigm. The bipolar stimulator coil electrodes were positioned around the carotid sheath on the left side of the vagus. The stimulator was placed subcutaneously below the right axilla. Once implanted, the mice were allowed 4 days of recovery, after which the stimulators were activated and kept on continuously for 2 weeks. VNS frequency was set at 5 Hz with 1 ms pulse width, which cycled continuously for 30 seconds and then turned off for 4 minutes and 30 seconds. Vagus nerve stimulators were regularly monitored by an emittance of audible tone from stimulators at 660 kHz and received via an AM radio. Stimulators were set to “OFF” position (toggled with a magnet and confirmed with the AM radio) at the end of the Vagus nerve stimulation. The post-VNS assessment was performed following VNS de-activation. GWI + Sham VNS group mice received the GWI injections and the VNS stimulators were implanted, but not activated.
Behavioral analyses
Previous studies reported that GWI animals exhibited cognitive impairment up to 4 months after the induction of the GWI model.9,10,37,58-60 To extend the timing of GWI models to better align with the almost 30 years since the 1990 to 1991 Gulf War, we examined mice using the object location test (OLT) to examine memory deficits at almost 9 months after GWI-induction. OLT evaluates spatial learning related to hippocampal activity.61 For behavioral testing, the number of animals used for each group were as follows: OLT: Naïve (n = 10); DMSO (n = 14); GWI (n = 12); GWI + VNS Sham (n = 7); GWI + VNS ON (n = 8).
Object location test (OLT)
This test consists of 3 successive trials.62,63 The first trial (habituation trial) comprised placing the mice in the center of the open field box and allowing the mice to freely explore the box for 5 minutes with an inter-trial interval of 1 hour. In the second trial (sample trial), mice were allowed to explore 2 familiar objects (FO1 and FO2) placed on opposite sides of the box for 5 minutes. Following the 1-hour inter-trial interval, the third trial (test trial) was performed for 5 minutes. In this trial, the mice were allowed to explore the same objects in the open field box with one of the familiar objects (FO1) remaining in the previous location while the other object (FO2) moved to a new location in the box. The mice were continuously video recorded using NOLDUS Ethovision automated movement tracking software. The box was cleaned with 70% alcohol and air-dried prior to and between tests for every mouse. A mouse is considered to be exploring an object when its nose was within 1 cm of the respective area. Data were collected as the time spent in exploring the object moved to a new location or the object remaining in the familiar location and the total time spent in object exploration. Times spent in exploring the novel place object, familiar place object, and total object exploration time were measured. Furthermore, the place discrimination index was calculated by using the formula, time spent with novel place object/total time spent in exploring the novel place object and familiar place object ×100. Then, the percentage of object exploration time spent with a novel place object and familiar place object were compared within each group. The novel place discrimination index was also directly compared between naïve and GWI mice. The total distance moved, the velocity of movement and the total exploration times were also measured for test trial and compared across groups to determine whether testing in OLT was influenced by locomotion.
Tissue preparation
After behavioral testing, mice were deeply anesthetized with Euthasol (390 mg pentobarbital sodium and 50 mg phenytoin sodium i.p.) and transcardially perfused with 0.9% saline until blood ran clear, followed by 4% paraformaldehyde.64,65 The brains were allowed to postfix for 24 hours in the skull, after which they were extracted and fixed for an additional 24 hours in 4% paraformaldehyde and subsequently cut into 50-µm thick serial coronal sections with a vibratome and stored in tissue cryoprotectant at −20°C until further use. For GFAP analysis, a subset of animals from each group were randomly selected, as follows: Naïve mice (n = 7); DMSO (n = 7); GWI (n = 6); GWI + sham VNS (n = 7); GWI + VNS (n = 5). Other animals from this study underwent fresh dissections for analyses that were not included in this study.
Glial fibrillary acidic protein (GFAP) Immunohistochemistry
Astrocytes can be identified by immunohistochemical staining with anti-GFAP, which labels intermediate filaments in the astrocytic cytoplasm. The size, shape and number of astrocytes can be indicative of their activation state and can provide information on neuroinflammation. Thus, the use of anti-GFAP allows for the quantitative and morphological analysis of GFAP-labeled astrocytes in the hippocampus. GFAP-immunohistochemistry was performed to label astrocytes in coronal sections containing hippocampi.64,66-68 Five tissue sections per mouse were incubated in CY3 fluorescently tagged GFAP antibody (1:1000, Sigma) for 24 hours at room temperature in the dark. These sections were then washed with 0.01 M PBS for 3 times. Slides were mounted and coverslips were applied using Vectashield hard set (Vector labs, Burlingame, CA). To eliminate potential rater bias, slides were coded and all image capture and analysis was performed by raters blind to the condition of the mouse. Sections were visualized on an Olympus IX81 (Olympus Inc.) laser-scanning confocal microscope.
Astrocyte quantification
Quantitative analysis of GFAP-labeled astrocytes was performed using a modified optical dissector method by raters blind to the condition of the mice, as previously described.64,66-68 Briefly, slides were coded and overlain with a numbered grid. Regions of analysis within each numbered grid were selected via a random number generator. The dentate gyrus hilus and molecular layers, as well as stratum radiatum of hippocampal areas CA1 and CA3 were delineated in 5 sections per animal, ranging from bregma −1.45 mm to −2.46 mm.69 The sections were equally sampled within this range to ensure equal representation of the counting areas between the groups. A counting frame of 40 μm × 40 μm was randomly positioned in a lattice of 150 μm × 150 μm. Results were statistically analyzed using ANOVA with Bonferroni’s multiple comparison post hoc tests. Radial glial like astrocytes were quantified in the infra and supra pyramidal blades of the dentate gyrus, as previously described.67
Statistical Analysis
We employed one-way ANOVA with Dunnett’s multiple comparison post hoc tests for comparison of behavior data across all groups. Immunohistochemistry data were statistically analyzed using one-way ANOVA with Bonferroni’s multiple comparison post hoc tests. All data are presented as mean ± SEM. All statistical analysis was performed using Graph Pad Prism. The statistical difference was considered significant for P values <.05.
Results
Deficits in the object location task are improved by VNS
Maintenance of cognitive function depends on the hippocampal trisynaptic pathway.70 OLT is based on the role of the hippocampus in providing spatial memory information between objects and places in the environment.71 Accordingly, the relocation of an object in OLT assesses the animal’s capability to recognize changes in the object location within its immediate environment. Animals in the GWI (P = .02) and GWI + VNS sham (P = .01) groups displayed significantly reduced total novel object location exploration time (Fig. 1a). Examination of the frequency of visits to the object in the novel location was also significantly impaired in the GWI group relative to the naïve group (P = .02, Figure 1b). The GWI + VNS sham group showed a trend (P = .07, NS) toward a reduced frequency of visits to the novel place object. In both the total exploration time and the frequency, the GWI + VNS group did not show the impairment that was observed in the GWI and the GWI + VNS sham group, indicating that the VNS improved the outcome of this behavioral test. The total distance traveled, and mean velocity were also analyzed in the test phase and there were no significant differences between the groups (P > .05, Figure 1c and d). These results suggest that the VNS improved the performance of the hippocampal-dependent spatial learning task.
Figure 1.
GWI chemical exposure induces deficits in the object location task that are improved by VNS. In (a), the total time (in seconds) spent exploring the object in the novel location is significantly decreased in the GWI mice and GWI + VNS sham mice, relative to the naïve mice. No such deficit is observed in the DMSO or GWI + VNS mice. In (b), the frequency of visits to the object in the novel location is significantly decreased in the GWI mice relative to the naïve mice, and there was a trend toward a significant decrease in the GWI + VNS sham mice. No such deficit was observed for the DMSO or the GWI + VNS mice. Analysis of the total distance traveled (c), and the mean velocity (d) revealed no significant differences between the groups. Data are represented as mean ± SEM.
*P < .05. **P < .005.
Chronic astrocytosis in GWI model is ameliorated by VNS
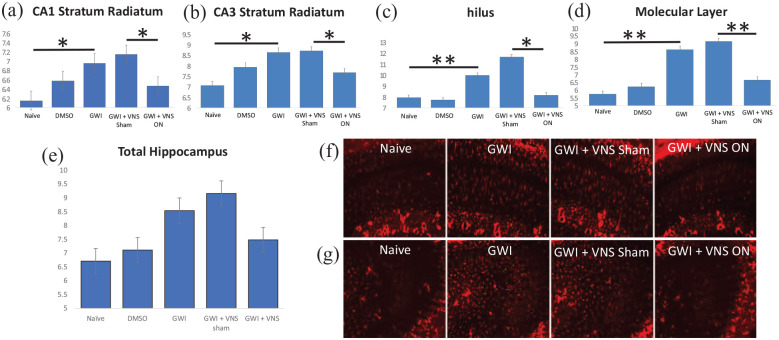
To examine the glial response following GWI in mice, we performed immunohistochemistry for astrocytes in the hippocampus using fluorescent-tagged anti-GFAP. The results show that at approximately 9 months after exposure to the Gulf War chemicals PB and PER, there is an increased number of GFAP-labeled astrocytes in stratum radiatum of hippocampal areas CA1 and CA3, and in the hilus and molecular layer of the dentate gyrus (Figure 2a–d). Mice in the GWI + VNS sham group also showed these increased astrocytes in the hippocampal CA1 (P = .05), CA3 (P = .05), dentate gyrus hilus (P = .01), and molecular layer (P = .005), suggesting that GWI chemical exposure results in a chronic increase in hippocampal astrocytes. Examination of the effects of VNS revealed that it significantly reduced the number astrocytes in the hippocampal CA1 (P = .05), CA3 (P = .05), dentate gyrus hilus (P = .01), and molecular layer (P = .005). This is consistent with previous studies in other disease models in which VNS reduces astrocytes.56,72
Figure 2.
GWI chemical exposure induces an increase in hippocampal and dentate gyrus astrocytes that is ameliorated by VNS. In a mouse model of GWI, GFAP-labeled astrocytes are increased by PB + PER in the hippocampus at approximately 9 months after PB + PER administration. There is more GFAP labeling in the GWI mouse and the GWI + VNS Sham mouse, relative to the naïve mouse stratum radiatum of hippocampal CA1 (a) and CA3 (b), and in the dentate gyrus hilus (c) and molecular layer (d). In these regions, VNS significantly ameliorates the significant astrocytic increase after GWI chemical exposure, so that it appears more like a naïve mouse. The mean number of total astrocytes per 1600 µm2 of hippocampus (e) is also shown. The representative images from CA1 (f) and CA3 (g) depict the increased astrocyte numbers in GWI mice that is reversed by VNS. Data are represented as mean ± SEM.
*P < .05. **P < .005.
Discussion
This study expands on the available evidence showing that exposure to PB + PER chemicals for 10 days is sufficient to induce chronic cognitive deficits and shows a potentially beneficial effect of VNS. In the current study, deficits were observed at approximately 9 months after GWIR chemical exposure that is accompanied by a concomitant hippocampal astrocytosis. It is pertinent to note that the VNS seemed to be effective in improving OLT performance, suggesting that VNS may target hippocampal-dependent tasks. This result is supported by the reduction in hippocampal astrocytosis in response to the VNS and further supports a role for astrocytosis in the cognitive deficits observed in GWI and GWI models. The behavioral test performed in the current study is associated with intact hippocampal function and persisted until approximately 9 months after GWI-related chemical exposure. This finding is analogous to some of the cognitive deficits that are observed in veterans with chronic GWI.73,74 Previous studies have estimated that 8 to 9 mouse months is roughly equivalent to 30 to 40 human years,75-78 and these findings are extremely important because as of writing, it has been approximately 30 years since the Gulf War. Therefore, this model of GWI reliably reproduces the chronic cognitive deficits that are often observed in GWI patients, in addition to chronic hippocampal astrocytosis.
Neuroinflammation was shown in GWI patients and in GWI models.73,74,79-84 A recent study offers supporting evidence for the presence of neuroinflammation by production of autoantibodies against astrocytes, oligodendrocytes, and neurons in GWI patients.30 Therefore, it is possible that chronic neuroinflammation, including astrocyte activation in the hippocampus, might contribute to the cognitive deficits observed in many GWI patients. In this study, astrocytes in the hippocampus and dentate gyrus were examined. The results show that these astrocytes are significantly affected by GWI-related chemical exposure. The fact that VNS in this study significantly reduced the astrocytosis in the hippocampus and hippocampal dentate gyrus suggests a possible beneficial effect in the hippocampus. The hippocampus contributes to brain networks that enable engagement in successful and adaptive behavior.85 A study reported smaller hippocampi and reduced gray matter volumes in frontal, parietal, and occipital cortices in 1991 Gulf War veterans.27,86-88 The findings in the current study are consistent with previous studies that used VNS in other disease models and reported beneficial effects in the hippocampus.49,89-93 Taken together, these findings suggest that reducing hippocampal astrocyte activation might be one of the therapeutic mechanisms of VNS.
The observation that VNS may improve cognitive and neuroinflammatory outcomes is supported by a vast experimental and clinical literature, in a number of disorders. Studies demonstrate the beneficial effects of VNS in epilepsy patients by decreasing the seizure frequency and reducing the symptoms of depression.94 Another study showed reduced inflammation after VNS in cerebral ischemic rats by reducing glial activation and increasing anti-inflammatory molecules.75 Several other studies have demonstrated neuroprotective effects and improved behavior after VNS.95,96 The long-term beneficial effect of VNS in recurrent treatment-resistant depression has also been reported.97-100 Wu et al.,101 reported that transcutaneous auricular vagus nerve stimulation is a safe and effective method to treat major depressive disorder and it alleviates the depressive symptoms, demonstrating that non-invasive VNS is also available and shows therapeutic efficacy. Therefore, VNS is widely used in clinical populations, is well-established as a safe and effective treatment, and may be worth exploring as a viable treatment option for GWI patients. The beneficial effects of chronic VNS were also observed in patients with migraines, another symptom domain seen in some GWI patients. VNS reduced the frequency and severity of migraine headaches and antinociceptive effects were also observed.100,102,103 In a previous study, we reported that VNS treatment lowered the nociceptive threshold in this GWI model.35
VNS administered by a surgically implanted stimulator has also been shown to be an effective tool in the treatment of human inflammatory diseases such as rheumatoid arthritis104 and inflammatory bowel disease,105 the latter of which is also observed in some GWI patients. Therefore, both invasive and non- invasive VNS have a demonstrated efficacy in treating numerous clinical disorders that include neuroinflammation, astrogliosis, and symptom domains exhibited by GWI patients.
Conclusion
Our results show that administration of Gulf War agents can induce chronic neurobehavioral deficits and hippocampal astrocytosis at approximately 9 months after gulf war chemical exposures. These results support that notion that the chronic inflammation that is often observed in GWI Veterans, could involve astrocyte activation in the brain. This study is also the first to show that VNS can improve cognitive outcomes and reduce astrocytosis in a model of GWI. These findings are consistent with previous studies that have observed beneficial effects of VNS in other disorders with a neuroinflammatory component. Considering the urgent need for therapeutic strategies to treat the Veterans who served in the Gulf War, the well-established safety of VNS in humans and its demonstrated efficacy at targeting symptom domains associated with GWI, it is tempting to consider this therapeutic avenue. The fact that more recent, minimally invasive, transcutaneous VNS devices are available for clinical use further supports the testing of this type of therapy in this patient population. It will be extremely interesting to learn the results of the recently completed clinical trial using transcutaneous VNS in 27 GWI patients to treat pain and migraines. Regardless of those results, the current study provides evidence that VNS might warrant further examination to treat other symptom domains in GWI and future studies are needed to further assess this potential.
Acknowledgments
The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army Position, policy, or decision unless so designated by other documentation. Special thanks to Dr. Ashok K. Shetty, Professor, Department of Molecular and Cellular Medicine, Texas A & M Health Science Center, College Station, TX, for his thoughtful input in designing these experiments. We are also very grateful for Harald Stauss for providing the VNS stimulators and providing expertise on the proper insertion and operation of these devices.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work is supported by DoD GWI grant #W81XWH-15-1-0340.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Lee A Shapiro conceived, planned and supervised the study including the manuscript; Damir Nizamutdinov performed the experiments; Jaclyn Jenkins performed astrocyte analysis; Lavanya Venkatasamy analysed the results and wrote the manuscript with Lee A Shapiro.
ORCID iD: Lavanya Venkatasamy  https://orcid.org/0000-0002-5360-3943
https://orcid.org/0000-0002-5360-3943
References
- 1. Binns J, Golomb B, Graves J, Haley R, Know M, Meggs W. Scientific Progress in Understanding Gulf War Veterans’ Illnesses: Report and Recommendations, Research Advisory Committee on Gulf War Veterans’ Illnesses. US Department of Veterans Affairs; 2004. [Google Scholar]
- 2. Smith BN, Wang JM, Vogt D, Vickers K, King DW, King LA. Gulf war illness: symptomatology among veterans 10 years after deployment. J Occup Environ Med. 2013;55(1):104-110. [DOI] [PubMed] [Google Scholar]
- 3. Rayhan RU, Raksit MP, Timbol CR, Adewuyi O, VanMeter JW, Baraniuk JN. Prefrontal lactate predicts exercise-induced cognitive dysfunction in Gulf War Illness. Am J Transl Res. 2013;5(2):212. [PMC free article] [PubMed] [Google Scholar]
- 4. Kang HK, Mahan CM, Lee KY, Magee CA, Murphy FM. Illnesses among united states veterans of the gulf war: a population-based survey of 30,000 veterans. J Occup Environ Med. 2000;42(5):491-501. [DOI] [PubMed] [Google Scholar]
- 5. Steele L. Prevalence and patterns of gulf war illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol. 2000;152(10):992-1002. [DOI] [PubMed] [Google Scholar]
- 6. White RF, Steele L, O’Callaghan JP, et al. Recent research on gulf war illness and other health problems in veterans of the 1991 gulf war: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdullah L, Evans JE, Montague H, et al. Chronic elevation of phosphocholine containing lipids in mice exposed to gulf war agents pyridostigmine bromide and permethrin. Neurotoxicol Teratol. 2013;40:74-84. [DOI] [PubMed] [Google Scholar]
- 8. Sox HC, Liverman CT, Fulco CE. Gulf War and Health: Volume 1: Depleted Uranium, Sarin, Pyridostigmine Bromide, and Vaccines. Vol. 1. National Academies Press; 2000. [PubMed] [Google Scholar]
- 9. Parihar VK, Hattiangady B, Shuai B, Shetty AK. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 2013;38(12):2348-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci. 2014;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zakirova Z, Tweed M, Crynen G, et al. Gulf war agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of gulf war illness. PLoS One. 2015;10(3): e0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamproglou I, Barbier L, Diserbo M, Fauvelle F, Fauquette W, Amourette C. Repeated stress in combination with pyridostigmine: part I: long-term behavioural consequences. Behav Brain Res. 2009;197(2):301-310. [DOI] [PubMed] [Google Scholar]
- 13. Macht V, Woodruff J, Burzynski H, Grillo C, Reagan L, Fadel J. Interactions between pyridostigmine bromide and stress on glutamatergic neurochemistry: insights from a rat model of gulf war illness. Neurobiol Stress. 2020;12:100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vijverberg HP, vanden Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol. 1990;21(2):105-126. [DOI] [PubMed] [Google Scholar]
- 15. Narahashi T. Nerve membrane ionic channels as the primary target of pyrethroids. Neurotoxicology.1985;6(2):3-22. [PubMed] [Google Scholar]
- 16. Paz-Trejo C, Gómez-Arroyo S. Genotoxic evaluation of common commercial pesticides in human peripheral blood lymphocytes. Toxicol Ind Health. 2017;33(12):938-945. [DOI] [PubMed] [Google Scholar]
- 17. Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK. Neuroinflammation in gulf war illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immunity. 2019;81:430-443. [DOI] [PubMed] [Google Scholar]
- 18. Kamel F, Tanner C, Umbach D, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364-374. [DOI] [PubMed] [Google Scholar]
- 19. Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev Environ Health. 2007;22(1):57-74. [DOI] [PubMed] [Google Scholar]
- 20. Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Persp. 2004;112(9):950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies D, Mrcpsych M, Ahmed G, Freer T. Chronic organophosphate induced neuropsychiatric disorder (COPIND): results of two postal questionnaire surveys. J Nutr Environ Med. 1999;9(2):123-134. [Google Scholar]
- 22. Steele L, Sastre A, Gerkovich MM, Cook MR. Complex factors in the etiology of Gulf war illness: wartime exposures and risk factors in veteran subgroups. Environ Health Persp. 2012;120(1):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdullah L, Crynen G, Reed J, et al. Proteomic CNS profile of delayed cognitive impairment in mice exposed to gulf war agents. Neuromol Med. 2011;13(4):275-288. [DOI] [PubMed] [Google Scholar]
- 24. Torres-Altoro MI, Mathur BN, Drerup JM, Thomas R, Lovinger DM, O’Callaghan JP, Bibb JA. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. J Neurochem. 2011;119(2):303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binns JH, Barlow C, Bloom FE, et al. Gulf war illness and the health of Gulf war veterans: scientific findings and recommendations, Research Advisory Committee Report on Gulf War Veterans’ Illnesses. US Department of Veterans Affairs. US Government Printing Office; 2008;1-465. [Google Scholar]
- 26. Binns JH, Bloom FE, Bunker JA, et al. Gulf war illness and the health of Gulf war veterans: research update and recommendations, 2009-2013. Research Advisory Committee on Gulf War Veterans’ Illnesses. US Department of Veterans Affairs. US Government Printing Office; 2014;1-123. [Google Scholar]
- 27. Li X, Spence JS, Buhner DM, et al. Hippocampal dysfunction in gulf war veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology. 2011;261(1):218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apfel BA, Ross J, Hlavin J, et al. Hippocampal volume differences in gulf war veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69(6):541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31(5):493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with gulf war illness. Neurotoxicol Teratol. 2017;61:36-46. [DOI] [PubMed] [Google Scholar]
- 31. Alshelh Z, Albrecht DS, Bergan C, et al. In-vivo imaging of neuroinflammation in veterans with gulf war illness. Brain Behav Immunity. 2020;87:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Blood biomarkers of chronic inflammation in gulf war illness. PLoS One. 2016;11(6):e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toomey R, Alpern R, Vasterling JJ, et al. Neuropsychological functioning of US Gulf War veterans 10 years after the war. J Int Neuropsychol Soc. 2009;15(5):717-729. [DOI] [PubMed] [Google Scholar]
- 35. Nizamutdinov D, Mukherjee S, Deng C, Stauss HM, Shapiro LA. Gulf War agents pyridostigmine bromide and permethrin cause hypersensitive nociception that is restored after vagus nerve stimulation. Neurotoxicology. 2018;69:93-96. [DOI] [PubMed] [Google Scholar]
- 36. Emmerich T, Zakirova Z, Klimas N, et al. Phospholipid profiling of plasma from GW veterans and rodent models to identify potential biomarkers of gulf war illness. PLoS One. 2017;12(4):e0176634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ojo JO, Abdullah L, Evans J, et al. Exposure to an organophosphate pesticide, individually or in combination with other gulf war agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of gulf war agent exposure. Neuropathology. 2014;34(2):109-127. [DOI] [PubMed] [Google Scholar]
- 38. Abdel-Rahman A, Shetty AK, Abou-Donia MB. Subchronic dermal application of N,N- diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Exp Neurol. 2001;172(1):153-171. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Rahman A, Shetty A, Abou-Donia M. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: dose–response relationships. Neuroscience. 2002;113(3):721-741. [DOI] [PubMed] [Google Scholar]
- 40. Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood–brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol Dis. 2002;10(3):306-326. [DOI] [PubMed] [Google Scholar]
- 41. Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, et al. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health A. 2004;67(4):331-356. [DOI] [PubMed] [Google Scholar]
- 42. Abdel-Rahman A, Abou-Donia S, El-Masry E, Shetty A, Abou-Donia M. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J Toxicol Environ Health A. 2004;67(2):163-192. [DOI] [PubMed] [Google Scholar]
- 43. Abou-Donia MB, Goldstein LB, Jones KH, et al. Locomotor and sensorimotor performance deficit in rats following exposure to pyridostigmine bromide, DEET, and permethrin, alone and in combination. Toxicol Sci. 2001;60(2):305-314. [DOI] [PubMed] [Google Scholar]
- 44. Abou-Donia M, Goldstein L, Dechovskaia A, Bullman S, Jones K, Herrick E, Abdel-Rahman A, Khan W. Effects of daily dermal application of DEET and permethrin, alone and in combination, on sensorimotor performance, blood-brain barrier, and blood-testis barrier in rats. J Toxicol Environ Health Part A. 2001;62(7):523-541. [DOI] [PubMed] [Google Scholar]
- 45. Abou-Donia MB, Dechkovskaia AM, Goldstein LB, Bullman SL, Khan WA. Sensorimotor deficit and cholinergic changes following coexposure with pyridostigmine bromide and sarin in rats. Toxicol Sci. 2002;66(1):148-158. [DOI] [PubMed] [Google Scholar]
- 46. Abou-Donia MB, Dechkovskaia AM, Goldstein LB, Abdel-Rahman A, Bullman SL, Khan WA. Co-exposure to pyridostigmine bromide, DEET, and/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol Biochem Behav. 2004;77(2):253-262. [DOI] [PubMed] [Google Scholar]
- 47. Arranz AM, De Strooper B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 2019;18(4):406-414. [DOI] [PubMed] [Google Scholar]
- 48. Rempe DA, Nedergaard M. Targeting glia for treatment of neurological disease. Neurotherapeutics. 2010;7(4):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, Mocchetti I, Follesa P. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12(9):1209-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lei W, Duan Z. Advances in the treatment of cholinergic anti-inflammatory pathways in gastrointestinal diseases by electrical stimulation of vagus nerve. Digestion. 2019;2019:1-11. [DOI] [PubMed] [Google Scholar]
- 51. Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Therap. 2017;179:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwan H, Garzoni L, Liu HL, et al. Vagus nerve stimulation for treatment of inflammation: systematic review of animal models and clinical studies. Bioelectron Med. 2016;3(1):1-6. [PMC free article] [PubMed] [Google Scholar]
- 53. Koopman F, Schuurman P, Vervoordeldonk M, Tak P. Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract Res Clin Rheumatol. 2014;28(4):625-635. [DOI] [PubMed] [Google Scholar]
- 54. Liu C-H, Yang M-H, Zhang G-Z, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflamm. 2020;17(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. 2019;12(1):19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen X, He X, Luo S, et al. Vagus nerve stimulation attenuates cerebral microinfarct and colitis-induced cerebral microinfarct aggravation in mice. Front Neurol. 2018;9:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meneses G, Bautista M, Florentino A, et al. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm. 2016;13(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shetty AK, Attaluri S, Kodali M, et al. Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro-and systemic inflammation in a model of gulf war illness. Redox Biol. 2020;28:101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kodali M, Hattiangady B, Shetty G, Bates A, Shuai B, Shetty A. Curcumin treatment leads to better cognitive and mood function in a model of gulf war illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immunity. 2018;69:499-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abdullah L, Evans JE, Bishop A, et al. Lipidomic profiling of phosphocholine containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromol Med. 2012;14(4):349-361. [DOI] [PubMed] [Google Scholar]
- 61. Denninger JK, Smith BM, Kirby ED. Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp 2018;2018:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Long Q, Upadhya D, Hattiangady B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci. 2017;114 (17):E3536-E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hattiangady B, Shetty AK. Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl Med. 2012;1(9):696-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mukherjee S, Zeitouni S, Cavarsan CF, Shapiro LA. Increased seizure susceptibility in mice 30 days after fluid percussion injury. Front Neurol. 2013;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tobin RP, Mukherjee S, Kain JM, et al. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol Commun. 2014;2(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Newell-Rogers MK, Rogers SK, Tobin RP, Mukherjee S, Shapiro LA. Antagonism of macrophage migration inhibitory factory (MIF) after traumatic brain injury ameliorates astrocytosis and peripheral lymphocyte activation and expansion. Int J Mol Sci. 2020;21(20):7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Robinson C, Apgar C, Shapiro LA. Astrocyte hypertrophy contributes to aberrant neurogenesis after traumatic brain injury. Neural Plast. 2016;2016:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040(1-2):81-91. [DOI] [PubMed] [Google Scholar]
- 69. Paxinos G, Franklin KB. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Academic Press; 2019. [Google Scholar]
- 70. Warburton EC, Barker GR, Brown MW. Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology. 2013;74:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Memory. 2009;16(10):616-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Farrand AQ, Helke KL, Gregory RA, Gooz M, Hinson VK, Boger HA. Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimulat. 2017;10(6):1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cooper CM, Briggs RW, Farris EA, Bartlett J, Haley RW, Odegard TN. Memory and functional brain differences in a national sample of US veterans with gulf war illness. Psychiatry Res Neuroimaging. 2016;250:33-41. [DOI] [PubMed] [Google Scholar]
- 74. Odegard TN, Cooper CM, Farris EA, Arduengo J, Bartlett J, Haley R. Memory impairment exhibited by veterans with gulf war illness. Neurocase. 2013;19(4):316-327. [DOI] [PubMed] [Google Scholar]
- 75. Budworth H, Harris FR, Williams P, et al. Suppression of somatic expansion delays the onset of pathophysiology in a mouse model of Huntington’s disease. PLoS Genet. 2015;11(8):e1005267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sengupta P. The laboratory rat: relating its age with human’s. Int J Prevent Med. 2013;4(6):624. [PMC free article] [PubMed] [Google Scholar]
- 77. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153(8):3571-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Flurkey KC, Harrison DE. Mouse models in aging research. Fac Res. 2007;2000:20091685. [Google Scholar]
- 79. O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J Neurochem. 2015;133(5):708-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Phillips KF, Deshpande LS. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology. 2016;52:127-133. [DOI] [PubMed] [Google Scholar]
- 81. Koo B-B, Michalovicz LT, Calderazzo S, et al. Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of gulf war illness. Brain Behav Immunity. 2018;67:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miller JV, LeBouf RF, Kelly KA, et al. The neuroinflammatory phenotype in a mouse model of Gulf War Illness is unrelated to brain regional levels of acetylcholine as measured by quantitative HILIC-UPLC-MS/MS. Toxicol Sci 2018;165(2):302-313. [DOI] [PubMed] [Google Scholar]
- 83. Joshi U, Evans JE, Joseph R, et al. Oleoylethanolamide treatment reduces neurobehavioral deficits and brain pathology in a mouse model of Gulf War Illness. Sci Rep. 2018;8(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Michalovicz LT, Locker AR, Kelly KA, et al. Corticosterone and pyridostigmine/DEET exposure attenuate peripheral cytokine expression: Supporting a dominant role for neuroinflammation in a mouse model of gulf war illness. Neurotoxicology. 2019;70:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. 2014;8:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chao LL, Kriger S, Buckley S, Ng P, Mueller SG. Effects of low-level sarin and cyclosarin exposure on hippocampal subfields in Gulf War Veterans. Neurotoxicology. 2014;44:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chao LL, Zhang Y, Buckley S. Effects of low-level sarin and cyclosarin exposure on white matter integrity in Gulf War Veterans. Neurotoxicology. 2015;48:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chao LL, Zhang Y. Effects of low-level sarin and cyclosarin exposure on hippocampal microstructure in gulf war Veterans. Neurotoxicol Teratol. 2018;68:36-46. [DOI] [PubMed] [Google Scholar]
- 89. Shin HC, Jo BG, Lee CY, Lee KW, Namgung U. Hippocampal activation of 5-HT1B receptors and BDNF production by vagus nerve stimulation in rats under chronic restraint stress. Eur J Neurosci. 2019;50(1):1820-1830. [DOI] [PubMed] [Google Scholar]
- 90. Perini GI, Toffanin T, Pigato G, et al. Hippocampal gray volumes increase in treatment-resistant depression responding to vagus nerve stimulation. J ECT. 2017;33(3):160-166. [DOI] [PubMed] [Google Scholar]
- 91. Raedt R, Clinckers R, Mollet L, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117(3):461-469. [DOI] [PubMed] [Google Scholar]
- 92. Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214(2):259-265. [DOI] [PubMed] [Google Scholar]
- 93. Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119(1):124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Spindler P, Bohlmann K, Straub HB, Vajkoczy P, Schneider UC. Effects of vagus nerve stimulation on symptoms of depression in patients with difficult-to-treat epilepsy. Seizure. 2019;69:77-79. [DOI] [PubMed] [Google Scholar]
- 95. Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C. PPARγ upregulation induced by vagus nerve stimulation exerts anti-inflammatory effect in cerebral ischemia/reperfusion rats. Medical Sci Monit. 2015;21:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu B, Hong J-S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Therap. 2003;304(1):1-7. [DOI] [PubMed] [Google Scholar]
- 97. Nahas Z, Marangell LB, Husain MM, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66(9):1097-1104. [DOI] [PubMed] [Google Scholar]
- 98. George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58(5):364-373. [DOI] [PubMed] [Google Scholar]
- 99. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 2005;58(5):347-354. [DOI] [PubMed] [Google Scholar]
- 100. Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain 2003;4(9):530-534. [DOI] [PubMed] [Google Scholar]
- 101. Wu C, Liu P, Fu H, Chen W, Cui S, Lu L, Tang C. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: a systematic review and meta-analysis. Medicine. 2018;97(52):e13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 2015;16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lai YH, Huang YC, Huang LT, Chen RM, Chen C. Cervical noninvasive vagus nerve stimulation for migraine and cluster headache: a systematic review and meta-analysis. Neuromodulation. 2020;23(6):721-731. [DOI] [PubMed] [Google Scholar]
- 104. Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci 2016;113(29):8284-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bonaz B, Sinniger V, Hoffmann D, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motility 2016;28(6):948-953. [DOI] [PubMed] [Google Scholar]