Abstract
Objective
To investigate whether protein regulator of cytokinesis 1 (PRC1), which is involved in the regulation of human carcinogenesis, contributes to poor prognosis in patients with cholangiocarcinoma (CCA).
Methods
Data and tissues from patients with CCA were retrospectively studied. Immunohistochemical staining and western blotting were used to evaluate and contrast the PRC1 expression profile at the protein level in CCA tumour and pericarcinomatous tissues from the same study population. Relationships between clinical characteristics and patient survival were observed using univariate and multivariate analyses. Correlations between PRC1 expression and clinical characteristics were analysed by logistic regression.
Results
A total of 45 patients were included. PRC1 expression was found to be upregulated in CCA cancer tissues versus pericarcinomatous tissues. Overexpression of PRC1 was shown to be related to tumour differentiation, tumour node metastasis staging and lymph node metastasis, and was also revealed to be an independent marker of poor CCA prognosis.
Conclusions
The present results suggest that PRC1 may be a prognostic and therapeutic biomarker for patients with CCA.
Keywords: Protein regulator of cytokinesis 1, PRC1, CCA, prognosis
Introduction
Cholangiocarcinoma (CCA) is a heterogeneous hepatobiliary malignancy arising from bile duct epithelial cells.1 The overall prevalence of CCA has increased progressively over the past 40 years, and CCA has become the second most common hepatic malignancy after hepatocellular carcinoma (HCC).2,3 CCAs are epithelial tumours with markers of biliary cell differentiation, and according to their anatomical location, CCAs are classified into three subtypes, including intrahepatic, perihilar and distal.4 There are many clinical methods for early cancer diagnosis, however, the low specificity of tumour markers in diagnosing early stage CCA, and the diversity of clinical CCA, makes diagnosis difficult and results in poor prognosis in patients with CCA.5,6
Several studies have shown that gene and protein changes are associated with the development of CCA. For example, downregulation of protein tyrosine phosphatase non-receptor 3 (PTPN3) is significantly related to the occurrence and prognosis of CCA, and upregulation of PTPN3 has been shown to inhibit the proliferation of CCA cells via inhibition of phosphoinositide 3-kinase/AKT serine/threonine kinase 1 signalling.7 The endocannabinoid anandamide has been shown to exert an anti-proliferative effect on HCC through a cannabinoid G-protein coupled receptor, and by stabilizing lipid rafts.8 In addition, lncRNA- maternally expressed gene 3 (MEG3) inhibits cell proliferation and invasion by modulating B lymphoma Mo-MLV insertion region 1/RING finger protein 2 (Bmi1/RNF2) in CCA.9 Difficulty surrounding diagnosis and treatment of CCA has been increased by the molecular and genetic heterogeneity of these tumours.
Protein regulator of cytokinesis 1 (PRC1) is abnormally expressed in a variety of tumours, such as prostate cancer, liver cancer and breast cancer.10–13 Chen et al.13 determined that PRC1 is a new Wnt target, which can enhance Wnt signalling through positive feedback, thereby promoting the recurrence of early liver cancer, and Lou et al.10 found that aberrant PRC1 expression may predict biochemical recurrence in men with prostate cancer. In the study by Shimo et al.,12 PRC1 was also found to be involved in the occurrence and progression of breast cancer, and plays a role by affecting the cell cycle. However, its role in cholangiocarcinoma remains unclear.
Transcriptome analysis is the analysis of a collection of all transcripts in a cell. Next-generation sequencing can provide a more accurate digital signal, higher detection throughput and wider range of detection versus microarray technology, and may reduce the complexity of diagnosis to some extent.14 Previous analysis of the GSE76297 and GSE26566 datasets using bioinformatics has revealed that the PRC1 protein is likely to be a causative factor in CCA.15,16 To examine the potential role of PRC1 in the metastasis of CCA, patient databases have been examined, and analysis of survival in relation to PRC1 in CCA using The Cancer Genome Atlas (TCGA) database has revealed that PRC1 expression is negatively correlated with survival time in patients with CCA.16,17 PRC1 proteins are the key regulators of cell division and play a vital role in cytokinesis. Moreover, PRC1 has been shown to act as both a tumour suppressor and oncogene.15 However, the role of PRC1 protein CCA remains unknown. The aim of the present study was to analyse changes in PRC1 protein levels in CCA, and to explore the relationship between PRC1 and prognosis in patients with CCA.
Patients and methods
Study population
This retrospective observational study included consecutive patients who were diagnosed with CCA and underwent surgery at the Qingpu Branch of Zhongshan Hospital, Fudan University, between June 2019 and October 2019. The Qingpu Branch of Zhongshan Hospital database, that accrued information on all patients who underwent treatment for CCA at the hospital, was used to screen and extract patient data. All patients with a follow-up of more than 6 months were included in the study. Clinical variables were studied from patient medical records including sex, age, carbohydrate antigen 19-9 (CA 19-9), γ-glutamyl transferase (γ-GGT), alkaline phosphatase (ALP), number, size and differentiation of the tumour, tumour node metastasis (TNM) stage, lymph node metastasis, and tissue PRC1 levels.
Levels of serum CA 19-9 were defined as more or less than 37 ng/ml (normal CA 19-9 range, 0–37 ng/ml). Levels of serum γ-GGT were defined as more or less than 50 U/l (normal γ-GGT range, 0–50 U/l). Tumour number was described as single or multiple. The dividing line of the tumour size was 5 cm, and the degree of tumour differentiation was categorized into three grades, comprising well, moderate and poor. The TNM stage was classified as stage I–II and stage III–IV, and lymph node metastasis was determined as either positive or negative. PRC1 expression in tumour versus pericarcinomatous tissue was determined as positive or negative and high or low levels. The follow-up period was obtained from the date of diagnosis and the time of the last clinical visit or death.
The research protocol was reviewed and approved by the Ethics Committee and Institutional Review Board of the Qingpu Branch of Zhongshan Hospital, Fudan University, and written informed consent for study inclusion and publication was obtained from each patient included in the study.
Immunohistochemistry
Archived tissue samples from all included patients, that were collected during biopsy or surgical treatment, were retrospectively analysed for this study. With imaging assistance, the stump of the hepatic bile duct and surrounding soft tissue without residual cancer cells was surgically removed. If cancer cells were still found in the stump of the bile duct, surgery could only be used as a palliative resection. The CCA tumour samples were fixed in 4% polysorbate solution for 48 h. The fixed samples were dehydrated step by step using increasing concentrations of ethanol, embedded in paraffin wax using standard techniques, and sliced to produce 4–7 µm tissue sections. After dewaxing with xylene and hydration with decreasing concentrations of ethanol, antigen retrieval was performed under high pressure in sodium citrate buffer solution for 20–30 min. Non-specific binding was blocked using bovine serum albumin (BSA) then the samples were incubated with mouse anti-human PRC1 primary antibody (1: 500 dilution; ab119338; Abcam, Cambridge, MA, USA) for 12 h at 4°C. Slides were washed three times with phosphate buffered saline (PBS), then incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1: 1000 dilution; ab205719; Abcam) for 30 min at room temperature. After washing with PBS three times, the slide was observed and photographed under microscopy using the streptavidin-biotin complex method. Analysis of positively stained areas was performed using Image J software (National Institutes of Health). To differentiate between low and high PRC1 expression levels, values of 0–1.3 represented low PRC1 levels and 1.3–2.7 represented high PRC1 levels.
Western blot analysis
Archived tumour and pericarcinomatous tissues from all patients were collected and cut into fragments on ice. Lysis buffer (150–250 μl) was then added per 20 mg of tissue, and the fragments were ground using a homogenizer to aid lysis. The lysed sample was centrifuged at 12 000 g for 15 min at 4°C, and the supernatant was collected for protein quantification using the BCA method. Samples were stored at –80°C prior to use. Protein samples (30 mg) with loading buffer were then added into each well of a polyacrylamide gel to separate the proteins using sodium dodecyl sulphate-polyacrylamide gel electrophoresis that was run at 80 mV for 2 h. Separated proteins were then transferred to PVDF membranes. Membranes were blocked with 5% BSA for 30 min, followed by incubation with mouse anti-human PRC1 primary antibody (1: 500 dilution; ab119338; Abcam) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1: 500 dilution; ab9484; Abcam) as the internal control, overnight at 4°C. After washing with PBS, membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1: 1 000 dilution; ab205719; Abcam) for 30 min at room temperature. Membranes were again washed with PBS and immunoreactive signal was visualised using DAB colour developing solution. Membranes were photographed and positive staining was analysed using Image J software. PRC1 protein level below 0.6 was considered to be low expression level, and levels 0.6–1.0 were considered to represent high expression level.
Statistical Analyses
GraphPad Prism 6 software (GraphPad, La Jolla, CA, USA) was used for statistical analyses and data are presented as mean ± SD. Independent samples t-test was used to analyse normally distributed data. Mann–Whitney U-test was used to compare data that were not normally distributed. Categorical data were analysed by χ2-test. Patient survival was analysed using the Kaplan-Meier method and Cox regression. Univariate analysis was used to reveal statistically significant variables (defined as P < 0.05) that were then included in multivariable analysis; variables that were not statistically significant in univariate analysis were also included in the multivariable analysis. For identification of prognostic factors, hazard ratio (HR), 95% confidence intervals (CI), and P values were obtained. A P value < 0.05 was considered statistically significant. Assays were performed at least three times independently.
Results
A total of 53 consecutive patients with CCA underwent surgery between June 2019 and October 2019. After medical database screening, a total of 45 patients had follow-up data for more than 6 months and were included for analysis. All patients were free of liver fluke.
Clinical and demographic characteristics of the patients
Characteristics of 45 patients (23 male, 22 female) diagnosed with CCA between June 2019 and October 2019 are summarized in Table 1. There were no statistically significant differences between male and female subgroups in any of the demographic or clinical characteristics presented. Most patients with CCA (18 males and 17 females) showed moderate tumour differentiation (Table 1). Most patients were TNM stage I–II (69.6% of males and 72.7% of females). All patients were positive for lymph node metastasis and PRC1 in tumour tissue.
Table 1.
Summary of baseline demographic and clinical characteristics of all patients with cholangiocarcinoma included in the study.
| Characteristic |
Study subgroup |
|
|---|---|---|
| Sex | Male (n = 23) | Female (n = 22) |
| Age, years | 58.5 ± 2.8 | 57.7 ± 3.1 |
| Aged >58 years | 12 (52.17) | 11 (50) |
| Aged ≤58 years | 11 (47.83) | 11 (50) |
| CA 19-9, ng/ml | 52.2 ± 3.6 | 51.8 ± 4.2 |
| γ-GGT, U/l | 46.8 ± 2.2 | 47.1 ± 2.7 |
| ALP, U/l | 119.9 ± 14.5 | 121.1 ± 15.8 |
| Tumour number | 2.5 ± 0.8 | 2.8 ± 0.7 |
| Tumour size, cm | 4.4 ± 0.9 | 4.6 ± 0.7 |
| Tumour differentiation, degree | ||
| Well | 2 (8.69) | 3 (13.64) |
| Moderate | 18 (78.27) | 17 (77.27) |
| Poor | 3 (13.04) | 2 (9.09) |
| TNM stage, I–IV | ||
| I–II | 16 (69.6) | 16 (72.7) |
| III–IV | 7 (30.4) | 6 (27.3) |
| Lymph node metastasis | ||
| Positive | 23 (100) | 22 (100) |
| Negative | 0 | 0 |
Data presented as n (%) prevalence or mean ± SD.
CA 19-9, carbohydrate antigen 19-9; γ-GGT, γ- Glutamyl transferase; ALP, alkaline phosphatase; TNM, tumour node metastasis.
Immunohistochemical analysis of PRC1 expression
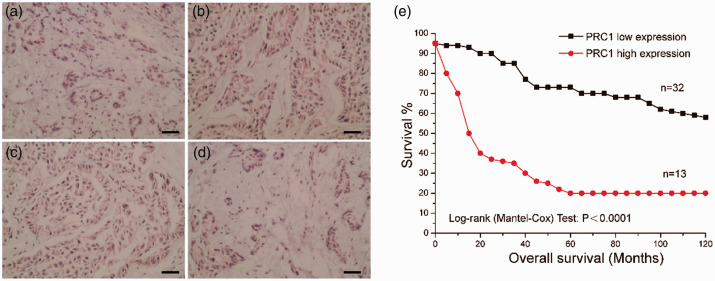
Expression of PRC1 in tumour and pericarcinomatous tissue from the 45 patients was examined by analysis of PRC1 proteins using immunohistochemistry (Figure 1). Low levels of PRC1 expression were observed in the cytoplasm of cells from normal control pericarcinomatous tissue (Figure 1a). Cancer tissues were found to be positive for PRC1 (Figure 1b–1d), and PRC1 expression was found to be significantly higher in cancer tissue than control tissue (P < 0.05; data not shown). This suggests that PRC1 may be closely related to the occurrence of CCA. Moreover, patients with CCA with high levels of PRC1 expression exhibited significantly shorter overall survival (P < 0.0001; Figure 1e) than patients with low PRC1 expression. The results suggest that highly expressed PRC1 and its related pathways may be involved in the pathogenesis of CCA.
Figure 1.
Expression of protein regulator of cytokinesis 1 (PRC1) in tumour and pericarcinomatous tissue from patients with cholangiocarcinoma (CCA), showing: (a) representative photomicrograph of low positive cytoplasmic PRC1 immunosignal in pericarcinomatous tissue (normal control); (b–d) representative photomicrographs of positive cytoplasmic PRC1 immunosignal in tumour tissues; and (e) plot showing overall survival in patients with CCA and high (relative level, 1.3–2.7) or low PRC1 expression (relative level, 0–1.3). Original magnification, × 200.
Western blot analysis of PRC1 expression
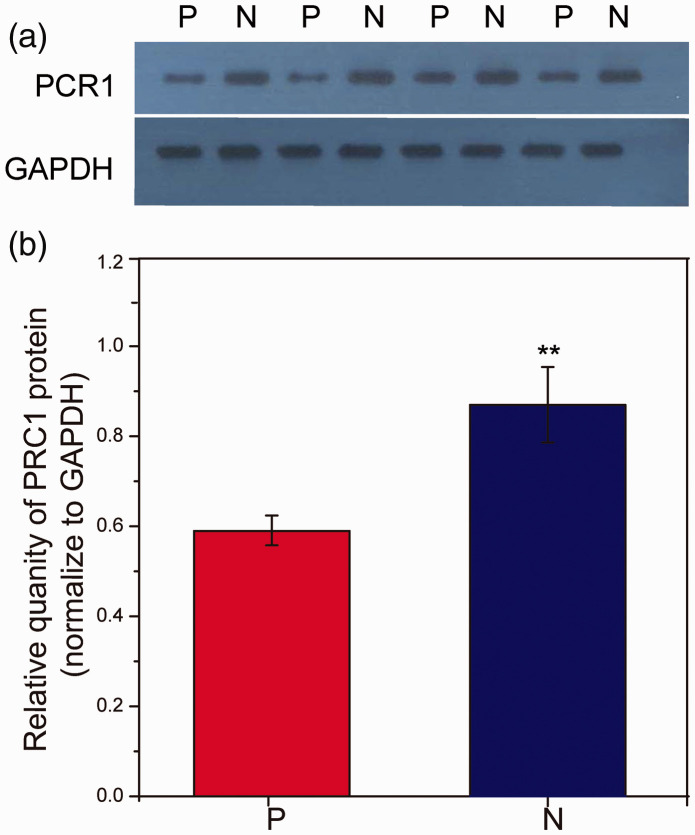
To investigate whether levels of PRC1 protein were altered in CCA, PRC1 levels in CCA tumour tissues and pericarcinomatous tissues from patients with CCA were analysed by western blot (Figure 2). Quantification of protein levels showed that PRC1 was expressed in both pericarcinomatous and tumour tissues, and levels of PRC1 protein were significantly higher in CCA tumour tissues (P < 0.01).
Figure 2.
Expression of protein regulator of cytokinesis 1 (PRC1) in tumour and pericarcinomatous tissue from 45 patients with cholangiocarcinoma, assayed in triplicate by western blot. PRC1 protein densitometric values were normalised to GAPDH internal control: (a) representative western blot panels showing PRC1 levels in tumour (N) and pericarcinomatous (P) tissues and comparable GAPDH reference levels among all samples; and (b) relative quantification of PRC1 levels in tumour (N) versus pericarcinomatous (P) tissues (normalised to GAPDH). **P < 0.01 versus pericarcinomatous tissues (Independent samples t-test).
Univariate analysis of the relationship between clinical characteristics and patient survival
Univariate analysis of the association between demographic/clinical variables and patient survival revealed that higher PRC1 levels were associated with lower survival duration in the population of patients with CCA (P < 0.05; Table 2). No other factors were found to be significantly associated with survival in the univariate analysis.
Table 2.
Univariate analysis of the relationship between demographic and clinical variables and patient survival.
| Variable | Overall survival |
|
|---|---|---|
| Hazard Ratio | Statistical significance | |
| Sex (male versus female) | 0.99 | NS |
| Age (>58 versus ≤58 years) | 1.01 | NS |
| CA 19-9 (>37 versus ≤37 ng/ml) | 1.03 | NS |
| γ-GGT (>50 versus ≤50 U/l) | 1.01 | NS |
| Tumour number (multiple versus single) | 1.49 | NS |
| Tumour size (>5 versus ≤5 cm) | 2.95 | NS |
| Tumour differentiation (well versus moderate versus poor) | 2.58 | NS |
| TNM stage (III–IV versus I–II) | 1.33 | NS |
| Lymph node metastasis (yes or no) | 1.53 | NS |
| PRC1 in tumour tissue (low or high) | 0.55 | P = 0.018 |
CA 19-9, carbohydrate antigen 19-9; γ-GGT, γ- Glutamyl transferase; TNM, tumour node metastasis; PRC1, protein regulator of cytokinesis 1.
NS, no statistically significant association (P > 0.05).
Multivariate analysis of the relationship between clinical characteristics and patient survival
Multivariate analysis confirmed that poor tumour differentiation, high TNM stage and high levels of PRC1 expression were associated with poor prognosis in the population of patients with CCA (P < 0.05; Table 3). No other factors were independently prognostic in the multivariable analysis.
Table 3.
Multivariate analysis of the association between clinical variables and patient survival.
| Variable | HR (95% CI) | Statistical significance |
|---|---|---|
| Tumour number (multiple versus single) | 2.03 (0.83, 2.57) | NS |
| Tumour size (>5 versus ≤5 cm) | 1.98 (1.13, 2.74) | NS |
| Tumour differentiation (well versus moderate versus poor) | 2.58 (1.93, 3.64) | P = 0.042 |
| TNM stage (III–IV versus I–II) | 3.21 (2.67, 4.26) | P = 0.029 |
| Lymph node metastasis (yes or no) | 2.24 (1.43, 3.27) | NS |
| PRC1 in tumour tissue (high or low) | 0.27 (1.58, 4.74) | P = 0.016 |
TNM, tumour node metastasis; PRC1, protein regulator of cytokinesis 1; HR, hazard ratio; CI, confidence interval.
NS, no statistically significant association (P > 0.05).
Logistic regression analysis of correlations between PRC1 expression and clinical characteristics
Logistic regression was used to analyse the correlation between PRC1 expression and clinical characteristics in patients with CCA (Table 4). Serum CA 19-9 concentration ≤37 ng/ml and γ-GGT was correlated with high levels of PRC1 expression (P < 0.0001). Additionally, regardless of whether the primary tumour site was a single tumour or multiple tumours, and whether the tumour was large or small, these characteristics were all related to PRC1 (P < 0.05). The lower the degree of tumour differentiation, the higher the expression of PRC1 (P < 0.05), i.e. out of patients with high levels of PRC1, most had moderate or poorly differentiated tumours, and in those with poorly differentiated tumours, 80% showed high PRC1 expression levels. High levels of PRC1 expression occurred significantly more frequently in patients with CCA with TNM stage III–IV, than in those with TNM stage I–II. These findings suggest that PRC1 correlates with clinical characteristics and may be involved in the occurrence and deterioration of patients with CCA.
Table 4.
Association between PRC1 levels and clinical characteristics in 45 patients with cholangiocarcinoma
| Expression of PRC1 in tumour
tissue |
|||
|---|---|---|---|
| Characteristic | Low | High | Statistical significance |
| Patients (n = 45) | 32 | 13 | P < 0.0001 |
| Sex | |||
| Male (n = 23) | 16 | 7 | P < 0.05 |
| Female (n = 22) | 16 | 6 | P < 0.05 |
| Age | |||
| >58 years | 18 | 5 | P < 0.001 |
| ≤58 years | 14 | 8 | P < 0.05 |
| CA 19-9 (ng/ml) | |||
| ≤37 | 28 | 10 | P < 0.05 |
| >37 | 4 | 3 | NS |
| γ-GGT (U/l) | |||
| ≤50 | 30 | 11 | P < 0.05 |
| >50 | 2 | 2 | NS |
| Tumour number | |||
| Single | 31 | 1 | P < 0.0001 |
| Multiple | 1 | 12 | P < 0.0001 |
| Tumour size (cm) | |||
| ≤5 | 29 | 2 | P < 0.001 |
| >5 | 3 | 11 | P < 0.05 |
| Tumour differentiation (degree) | |||
| Well differentiated | 2 | 3 | NS |
| Moderate differentiated | 29 | 6 | P < 0.001 |
| Poor differentiated | 1 | 4 | P < 0.05 |
| TNM stage (I–IV) | |||
| I–II (n = 32) | 26 | 6 | P < 0.001 |
| III–IV (n = 13) | 6 | 7 | NS |
| Lymph node metastasis | |||
| Positive | 32 | 13 | P < 0.005 |
| Negative | 0 | 0 | NS |
Data presented as n prevalence.
CA 19-9, carbohydrate antigen 19-9; γ-GGT, γ- glutamyl transferase; PRC1, protein regulator of cytokinesis 1.
NS, no statistically significant correlation (P > 0.05).
Discussion
Cholangiocarcinoma is a highly malignant primary liver tumour with strong genetic heterogeneity, however, current understanding of the pathogenesis of CCA is not comprehensive and key drivers involved in CCA carcinogenesis still need to be defined.17 Previously published bioinformatics analyses of the GSE76297 and GSE26566 datasets suggest that the PRC1 protein may be involved in CCA pathogenesis. Further analysis of survival information regarding PRC1 protein in CCA, using the TCGA database, showed a negative correlation between PRC1 expression and survival time.17,18 In the present study, PRC1 expression was analysed at the protein level to investigate its association with prognosis in patients with CCA. Using immunohistochemistry, western blot and correlation analysis, a very high correlation was observed between PRC1 levels and the prognosis of patients with CCA.
Immunohistochemical analysis of tumour tissues from patients with CCA showed significantly increased PRC1 levels in tumour tissue compared with pericarcinomatous tissue. In addition, western blot results further indicated that tumour tissues expressed higher levels of PRC1 in patients with CCA. PRC1 has been described as a tumour suppressor and oncogene, and research has shown abnormal expression of PRC1 in cancer. For instance, Obama et al.18 established a gene-expression profile for patients with CCA and found that PRC1 was upregulated in tumour tissues compared with noncancerous tissues. Tang et al.19 showed the important biological significance of PRC1 in tumour pathogenesis and prognosis of patients with non-small cell lung cancer.
Importantly, univariate analysis of the relationship between patient survival and PRC1, CA 19-9, tumour size, and tumour number, revealed that only high expression of PRC1 was significantly associated with patient survival. This suggests that PRC1 may be a biomarker of CCA pathogenicity and survival. To the best of the authors’ knowledge, the present data are the first to report a potential relationship between PRC1 and survival of patients with CCA, and these results may support the development of new guidance for CCA prognosis.
Another important finding of the present study was that tumour differentiation and TNM stage showed no significant association with patient survival in univariate analysis, while multivariate analysis revealed the opposite results. The correlation between PRC1 and patient survival remained statistically significant following multivariate analysis, which further supports a potential role of PRC1 in CCA progression. In recent years, symptoms, liver function tests and imaging results have been used as a means of diagnosing CCA in the clinic.20–22 With the development of high-throughput sequencing and other technologies, more and more molecular diagnostic technologies, such as genomics, proteomics, gene sequencing, have gradually made up for previous diagnostic techniques in chemotherapy.23 Candidate diagnostic biomarkers of CCA, such as PRC1, may inspire further research into CCA.
To date, no published studies have reported the significance of an association between clinical characteristics and PRC1 expression in patients with CCA. Unlike its association with serum hormone levels or tumour stage, the role of PRC1 in cancer has been previously reported. For example, PRC1 has been shown to be overexpressed in different cancers, such as bladder cancer, breast cancer, hepatocellular carcinoma, and pancreatic cancer. PRC1 was significantly inhibited by siRNA, and siRNA inhibition of PRC1 also reduced the proliferation of breast and bladder cancer cells. PRC1 overexpression has been shown to contribute to gastric cancer progression, and can be targeted by piperlongumine via a p53-independent mechanism.12,24–27 The present research indicated that the average level of PRC1 was elevated in cancer tissues of patients with CCA, and was significantly associated with multiple clinical characteristics. The results suggest that PRC1 levels in cancer tissues of patients with CCA may be an indicator of CCA progression and prognosis.
To the best of the authors’ knowledge, the present study is the first to propose a potential association between PRC1 and prognosis in patients with CCA. However, the results may be limited by the relatively small sample size and the single-centre research setting. In addition, no further research has been done on the relevant mechanism, and no animal model experiments have been conducted to verify the results. Further research is required to validate the results of the present study, and the authors plan to conduct relevant research in their next study.
Conclusions
In summary, PRC1 expression was determined in conjunction with prognosis in patients with CCA. The results provide a basis for the concept that overexpression of PRC1 in human CCA may be important in progression and poor prognosis. Moreover, elevated PRC1 expression may have clinical statistical significance in terms of clinical characteristics in patients with CCA, suggesting that PRC1 may serve as an oncogene and potential therapeutic biomarker for CCA.
Footnotes
Data accessibility statement: The data and materials are available on request from the corresponding author.
Author contributions: Qing Wang, Ying Chen conceived and designed experiments; Qing Wang, Ying Chen, Hua He, Weihui Lu performed experiments and data analysis; Hua He, Weihui Lu, Kanru Lin provided technical support, data collection and analysis; and Qing Wang, Ying Chen wrote the manuscript. All authors provided their final approval of the submitted and published version.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ying Chen https://orcid.org/0000-0001-8363-1024
References
- 1.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018; 15: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut 2001; 48: 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist 2016; 21: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013; 145: 1215–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritcher EGB, Voss JS, Brankley SM, et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology 2015; 149: 1813–1824.e1. [DOI] [PubMed] [Google Scholar]
- 6.Gonda TA, Viterbo D, Gausman V, et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin Gastroenterol Hepatol 2017; 15: 913–919.e1. [DOI] [PubMed] [Google Scholar]
- 7.Sun R, Chen T, Li M, et al. PTPN3 suppresses the proliferation and correlates with favorable prognosis of perihilar cholangiocarcinoma by inhibiting AKT phosphorylation. Biomed Pharmacother 2020; 121: 109583. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Jiang X, Li C, et al. LncRNA-MEG3 inhibits cell proliferation and invasion by modulating Bmi1/RNF2 in cholangiocarcinoma. J Cell Physiol 2019; 234: 22947–22959. [DOI] [PubMed] [Google Scholar]
- 10.Luo HW, Chen QB, Wan YPet al. Protein regulator of cytokinesis 1 overexpression predicts biochemical recurrence in men with prostate cancer. Biomed Pharmacother 2016; 78: 116–120. [DOI] [PubMed]
- 11.Yang XM, Cao XY, He Pet al. Overexpression of Rac GTPase activating protein 1 contributes to proliferation of cancer cells by reducing Hippo signaling to promote cytokinesis. Gastroenterology 2018; 155: 1233–1249.e22. [DOI] [PubMed]
- 12.Shimo A, Nishidate T, Ohta T, et al. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Sci 2007; 98: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Rajasekaran M, Xia H, et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut 2016; 65: 1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutz KO, Heilkenbrinker A, Lönne M, et al. Transcriptome analysis using next-generation sequencing. Curr Opin Biotechnol 2013; 24: 22–30. [DOI] [PubMed] [Google Scholar]
- 15.Chaisaingmongkol J, Budhu A, Dang H, et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 2017; 32: 57–70.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012; 142: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Long J, Xie F, et al. Transcriptomic analysis and identification of prognostic biomarkers in cholangiocarcinoma. Oncol Rep 2019; 42: 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obama K, Ura K, Li M, et al. Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma. Hepatology 2005; 41: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Xiao G, Behrens C, et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non-small cell lung cancer patients. Clin Cancer Res 2013; 19: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakeeb A, Lipsett PA, Lillemoe KD, et al. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg 1996; 171: 147–152. [DOI] [PubMed] [Google Scholar]
- 21.Qin XL, Wang ZR, Shi JS, et al. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol 2004; 10: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CY, Shiesh SC, Tsao HC, et al. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma–the influence of sampling time and hepatolithiasis. Hepatogastroenterology 2002; 49: 616–620. [PubMed] [Google Scholar]
- 23.Jusakul A, Kongpetch S, Teh BT. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr Opin Gastroenterol 2015; 31: 258–263. [DOI] [PubMed] [Google Scholar]
- 24.Kanehira M, Katagiri T, Shimo A, et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res 2007; 67: 3276–3285. [DOI] [PubMed] [Google Scholar]
- 25.Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res 2011; 17: 6040–6051. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Furukawa Y, Nakagawa H, et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene 2004; 23: 2385–2400. [DOI] [PubMed] [Google Scholar]
- 27.Zhan P, Zhang B, Xi GM, et al. PRC1 contributes to tumorigenesis of lung adenocarcinoma in association with the Wnt/β-catenin signaling pathway. Mol Cancer 2017; 16: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]