Abstract
Objective
Postoperative sore throat (POST) is an undesirable intubation-related complication after surgery. Several studies have investigated the efficacy of perioperative intravenous dexmedetomidine administration for the prevention of POST, but the results have been inconsistent. We aimed to summarize all existing evidence and draw a more precise conclusion to guide future clinical work.
Methods
PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure databases were comprehensively searched for all randomized controlled trials published before 1 February 2021 that investigated the efficacy of dexmedetomidine for the prevention of POST.
Results
Nine studies involving 400 patients were included in our meta-analysis. Compared with the control groups (i.e., saline and anesthetic drugs), perioperative intravenous use of dexmedetomidine significantly reduced the incidence of POST [risk ratio (RR): 0.56; 95% confidence interval (CI): 0.40–0.77; I2 = 0%) and coughing on the tube during extubation (RR: 0.58; 95% CI: 0.41–0.82; I2 = 0%). Additionally, patients in the dexmedetomidine group were more likely to develop bradycardia (RR: 2.46; 95% CI: 1.28–4.71; I2 = 0%) and hypotension (RR: 3.26; 95% CI: 1.14–9.33; I2 = 0%) during the administration of dexmedetomidine than those in the control group.
Conclusion
Perioperative intravenous administration of dexmedetomidine has a positive effect on the prevention of POST.
Keywords: Dexmedetomidine, postoperative sore throat, anesthesia, intubation-related complication, randomized control trial, meta-analysis
Introduction
Postoperative sore throat (POST) caused by transient irritation to the local mucosa of the oropharynx or trachea after intubation-related manipulations1–3 is one of the most undesirable intubation-related complications4 with an estimated incidence of 14.5% to 65%.1,5,6 POST can significantly reduce the patients’ satisfaction level7 and affect their recovery.8,9 Moreover, POST increases the cost of hospitalization for patients. In 2015, Mayhood et al. reported that patients with POST experienced a longer length of stay in postanesthesia care units and were discharged almost 1 hour later from these facilities than those without POST.10 Therefore, reducing the incidence and severity of POST is urgently needed to improve patients’ postoperative satisfaction and alleviate their burden of hospitalization costs.
Recently, several pharmacological and non-pharmacological approaches for the prevention of POST have gained widespread acceptance in clinical settings. These include the use of smaller endotracheal tubes,11 topical or intravenous application of corticosteroids or local anesthetics,12,13 ketamine gargle,14 topical application of magnesium15 and others. Dexmedetomidine, a selective α2-adrenaline receptor agonist, has a dose-dependent sedative effect on respiration with minimal depressive effects. It also attenuates the inflammatory response and inhibits pain signals.16–18
Several clinical experiments have been conducted to investigate the efficacy and safety of dexmedetomidine for the prevention of POST; however, there are inconsistencies among the results of these studies. For instance, several studies concluded that perioperative intravenous use of dexmedetomidine significantly reduced the incidence of POST, whereas other clinicians reported opposite findings.19
We aimed to summarize all existing evidence and perform a meta-analysis to assess the efficacy of perioperative intravenous dexmedetomidine administration for the prevention of POST and draw a more convincing conclusion, thereby providing guidelines for future clinical work.
Methods and materials
We conducted this systematic review and meta-analysis according to the rules of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).20 PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses that can be used as a basis for reporting systematic reviews of different types of research. This manuscript is a review article and does not involve a research protocol requiring approval by a relevant institutional review board or ethics committee. Informed consent was also not applicable.
Search strategies
The PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure (CNKI) databases were comprehensively searched for randomized controlled clinical trials (RCTs) published before February 2020 that investigated the efficacy of dexmedetomidine for the prevention of POST. In addition, the reference lists of all included studies were checked for any potential additional publications. Searches were performed again just before the final analysis to identify any further studies meeting the inclusion criteria. Unpublished studies were not assessed. We used the keywords of dexmedetomidine, alpha 2 adrenergic receptor agonists, endotracheal intubation, intratracheal intubation and intubation. The detailed search strategies for each database were presented in Supplementary Table 1.
Inclusion and exclusion criteria
For a published article to be included in our study, it had to meet the following criteria: (1) an RCT design, (2) investigated the efficacy of perioperative intravenous dexmedetomidine administration for the prevention of POST and (3) available full-text and data.
Studies were excluded if they were duplicate publications, reviews or meta-analyses, editorials, abstracts, comments, case reports, meetings or animal experiments.
Data extraction
Two reviewers (Xiaobin Wang and Dongmei Ai) independently screened the titles, abstracts and full texts, then selected relevant studies. The same two reviewers independently extracted the data from the studies according to a prespecified protocol with any disagreement settled by a third reviewer (Yuanhui Liu).
The following items were extracted: name of the first author; publication year; type of surgery; sample size (classified by the participants’ sex); participants’ age; anesthesia technique and type of intratracheal tube; method of dexmedetomidine use; and incidence of POST, coughing on the tube during the extubation process, postoperative hoarseness, bradycardia and hypotension.
The primary outcome of this meta-analysis was the incidence of POST, which was defined as sore throat after the extubation process with an unlimited pain level. The secondary outcomes included the incidence of coughing on the tube (the coughing response within the period from the first body movement to the time of extubation), postoperative hoarseness (alteration in vocal voice) and bradycardia (heart rate < 60 bpm) and hypotension (mean arterial pressure ≤60 mmHg or decreased by 30% compared with the baseline value) during the intervention.
Statistical synthesis and analysis
This meta-analysis was conducted using Review Manager (RevMan) Version 5.3 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Cochran’s Q test and the statistical I2 test were used to assess the statistical heterogeneity of the pooled results. If 0% ≤ I2 < 25%, the results showed no heterogeneity; if 25% ≤ I2 < 50%, the results showed a low level of heterogeneity; if 50%≤I2 < 75%, the results showed a medium level of heterogeneity; and if 75% ≤ I2 ≤ 100%, the results showed a high level of heterogeneity. Data were pooled from all eligible RCTs, and the Mantel–Haenszel method was used to calculate the risk ratio (RR) with 95% confidence intervals (CIs) for these dichotomous outcomes. A pooled estimate of RRs was computed using the DerSimonian and Laird random-effects model. This model provides an appropriate estimate of the average treatment effect when studies are statistically heterogeneous, and it typically yields relatively wide CIs resulting in a more conservative statistical claim.
The risk of bias assessment was performed using the Cochrane Collaboration tool (Cochrane, London, UK). We conducted subgroup analyses by classifying these included studies according to their different control drugs and intubation methods.
In addition, a sensitivity analysis was performed to assess the robustness of the results by excluding specific studies and applying different effect models. Finally, a funnel plot was used to assess potential publication bias. All included studies were represented by small circles. The X-axis refers to the RR value for each study included in this meta-analysis. The Y-axis refers to the standard error, which reflects the sample size. In other words, the larger the standard error, the smaller the sample size. The dotted line parallel to the Y-axis represents the synthesized RR values. Two reviewers (Dongmei Ai and Xiaobin Wang) independently synthesized the data with any disagreement settled by a third reviewer (Yuanhui Liu). A p-value of less than 0.05 was considered statistically significant.
Results
Literature search
The literature search identified 968 articles, including nine articles19–21,23–28 that met the inclusion criteria (Supplementary Figure 1). The characteristics of the nine studies involving 400 participants were summarized in Supplementary Table 2. All raw data extracted from the original articles were presented in the Supplemental Materials.
Bias assessment
As shown in the risk of bias graph (Supplementary Figure 2), one study2 was rated as high risk for performance bias because the researcher did not follow the rules of blinding to participants and personnel. Regarding publication bias, there was no significant asymmetry in the funnel plot (Supplementary Figure 3), suggesting the absence of significant publication bias.
Primary outcome
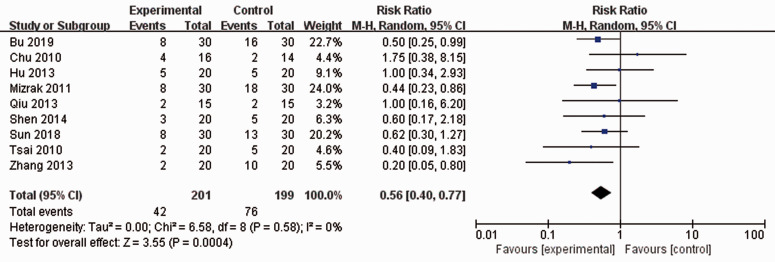
After synthesizing the data, the results showed that perioperative intravenous administration of dexmedetomidine significantly reduced the incidence of POST (RR: 0.56; 95% CI: 0.40–0.77; p = 0.0004; I2 = 0%) (Figure 1).
Figure 1.
Meta-analysis of nine randomized controlled trials on the perioperative administration of dexmedetomidine for the prevention of postoperative sore throat.
CI, confidence interval.
Subgroup analysis
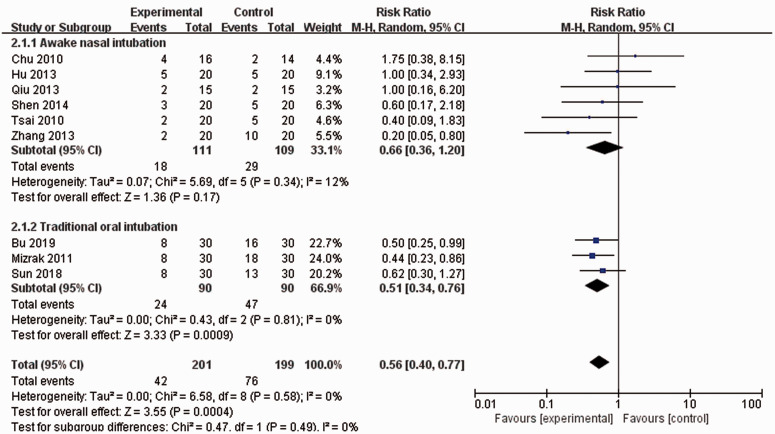
Awake nasal intubation vs. traditional oral intubation: Of the nine included studies, three studies21,23,24 reported intravenous administration of dexmedetomidine among patients undergoing traditional oral intubation, whereas the patients in six studies19,22,25–28 were infused with dexmedetomidine during awake nasal intubation. As shown in Figure 2, only patients in the traditional oral intubation group experienced a lower incidence of POST (RR: 0.51; 95% CI: 0.34–0.76; p = 0.0009; I2 = 0%) compared with those in the awake nasal intubation group (RR: 0.66; 95% CI: 0.36–1.20; I2 = 12%).
Figure 2.
Subgroup analysis by different intubation methods.
CI, confidence interval.
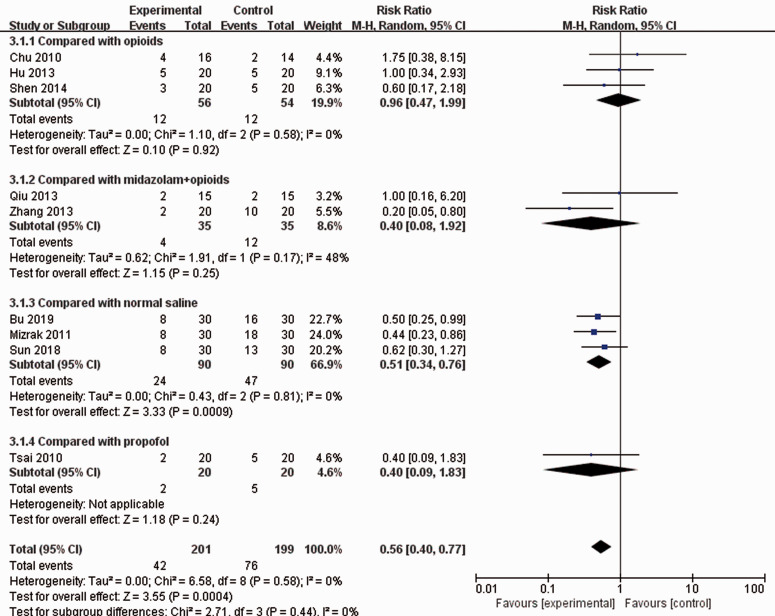
Different control groups: As shown in Figure 3, the infusion of dexmedetomidine did not show superiority over the administration of opioids (RR: 0.96; 95% CI: 0.47–1.99; I2 = 0%), midazolam plus opioids (RR: 0.40; 95% CI: 0.08–1.92; I2 = 48%) or propofol (RR: 0.40; 95% CI: 0.09–1.83). However, it had a positive effect on the prevention of POST compared with an equal volume of normal saline (RR: 0.51; 95% CI: 0.34–0.76; p = 0.0009; I2 = 0%).
Figure 3.
Subgroup analysis by different adjuvant drugs.
CI, confidence interval.
Secondary outcomes
We also synthesized data regarding coughing on the tube during the extubation process, postoperative hoarseness and hypotension and bradycardia during the infusion of dexmedetomidine. Hypotension: Patients in the dexmedetomidine group were more likely to experience hypotension during the administration of dexmedetomidine (RR: 3.26; 95% CI: 1.14–9.33; p = 0.03; I2 = 0%) (Supplementary Figure 4). Bradycardia: As shown in Supplementary Figure 5, patients in the dexmedetomidine group had a higher incidence of bradycardia than those in the control group (RR: 2.46; 95% CI: 1.28–4.71; p = 0.007; I2 = 0%). Postoperative hoarseness: The synthesized data showed that there was no significant difference between the dexmedetomidine group and the control group regarding the complication of postoperative hoarseness (RR: 0.79; 95% CI: 0.41–1.50; I2 = 0%) (Supplementary Figure 6). Coughing on the tube: Another advantage was that perioperative infusion of dexmedetomidine had a positive effect on the prevention of coughing on the tube during the extubation process compared with the control group (RR: 0.58; 95% CI: 0.41–0.82; p = 0.002; I2 = 0%) (Supplementary Figure 7).
Sensitivity analysis
We performed a sensitivity analysis by excluding the study of Bu et al,23 which was rated as high risk for performance bias (Supplementary Figure 8). We also changed the calculation model from a random-effects model to a fixed-effects model and re-performed the analysis (Supplementary Table 3). The results were similar after both sensitivity analyses, indicating the robustness of our meta-analysis.
Discussion
POST is an important risk factor affecting the recovery of patients after surgery.29 Several studies have reported various methods to reduce the incidence and severity of POST.11,13,15 Dexmedetomidine selectively activates α2-adrenaline receptors in the locus coeruleus and is a derivative of medetomidine, which inhibits the sympathetic nervous system and reduces the release of norepinephrine.30 It also attenuates the inflammatory response, significantly improves the sleep quality of critically ill patients, induces analgesia and reduces anesthetic requirements. Accordingly, it has been widely used as an adjunctive drug by anesthetists during clinical procedures and surgeries.17,18,31,32
Tracheal intubation may be one mechanism contributing to POST. Tracheal intubation, the most commonly used airway management method during general anesthesia, is safe for patients and convenient for anesthetists to manage patients’ airways. However, the manipulations in the oral cavity may cause transient irritation to the local mucosa of the oropharynx or trachea.1 During inhalational anesthesia with volatile anesthetic drugs, the intracuff pressure is reportedly increased by the diffusion of these volatile anesthetic drugs into the cuff, resulting in the formation of a local mucosa lesion.2,3 These injuries may cause several undesirable complications related to intubation, especially POST. POST is one of the most undesirable intubation-related complications4 that can result in a lower patient satisfaction level,7 longer length of hospital stay and longer recovery phase. Because of its anti-inflammatory effects, dexmedetomidine can be used to reduce the incidence and severity of POST.
Our meta-analysis based on a sample size of nine original studies suggested that only patients in the traditional oral intubation group experienced a lower incidence of POST, and no positive effect of dexmedetomidine on the prevention of POST was observed in the awake nasal intubation subgroup. The subgroup analysis based on different control drugs suggested the superiority of dexmedetomidine over normal saline for the prevention of POST; however, there was no significant difference between dexmedetomidine and other anesthetic drugs, such as fentanyl, sufentanil, midazolam plus fentanyl or propofol. Of note, the three studies that performed oral intubation all used normal saline as the control medication.19,23,24 In the awake nasal intubation subgroup, the control groups of all six20,21,25–28 studies were anesthetic drugs, including fentanyl, sufentanil, midazolam plus fentanyl and propofol, instead of normal saline because sedation was induced first during awake intubation. As a result, they could not compare dexmedetomidine with normal saline due to its lack of a sedative effect.
Hypotension and bradycardia are the most common adverse effects of dexmedetomidine and are caused by its inhibition of the sympathetic nervous system.33 It is not surprising that patients in the experimental group experienced a higher incidence of hypotension and bradycardia. However, these are transient adverse effects, and all patients in the included RCTs were effectively treated with atropine and vasoactive agents.34 Previous studies have demonstrated that the perioperative use of dexmedetomidine improved patient prognosis and shortened their length of hospital stay without long-term adverse events.35 Coughing on the tube may be life-threatening due to increased cerebral, intrathoracic, intraocular, intraabdominal and blood pressures, which may cause intracranial hemorrhage, postoperative wound hemorrhage, myocardial ischemia, tachycardia, bronchospasm and other life-threatening complications.36,37 Our results suggested that perioperative administration of dexmedetomidine significantly reduced the incidence of coughing on the tube during the extubation process, mostly because of its sedative effects.
The incidence of hoarseness after surgery was not reduced by the administration of dexmedetomidine. Sound production is a complicated process38 because it requires the use of several structures in a coordinated manner, such as the lungs, vocal tracts, vocal cords and vocal cavity.39 Vocal complications, such as hoarseness or vocal failures, may occur after inappropriate voice use and habits or vocal distortion.38 It is understandable that patients suffered hoarseness after surgery because of transient vocal distortion, which may be caused by compression of their vocal cords by the endotracheal tube during surgery. This may explain why the anti-inflammatory and sedative effects of dexmedetomidine did not reduce the incidence of postoperative hoarseness.
Regarding the dosage of medication, four studies19,21,26,27 reported a single bolus administration of 0.5 μg/kg or 1 μg/kg dexmedetomidine over 10 to 15 minutes before induction, whereas patients in the experimental group of the other five studies20,23–25,27 were administered a loading dose of 0.5 μg/kg to 1.5 μg/kg for 10 minutes followed by the continuous infusion of dexmedetomidine at a rate of 0.4 μg/kg/hour or 0.5 μg/kg/hour until the end of surgery or 30 minutes before the anticipated end of surgery.
Although there was no significant heterogeneity in our study, we still performed sensitivity analyses by excluding the high-risk study23 and changing the calculation model (random-effects model versus fixed-effects model). The results of the sensitivity analyses were similar to our previous results, indicating the stability and robustness of our meta-analysis.
Several limitations in our study should be acknowledged. First, the sample size of the nine RCTs in our study was relatively small. However, the practical and precise strategies used for comprehensive searches of four official databases, clear inclusion and exclusion criteria and strict consideration of study quality might have compensated for this limitation. Second, among the six studies comparing dexmedetomidine with other anesthetic drugs, only one study had a control group with propofol, two studies reported midazolam plus opioids as a control group, and three studies included control groups with opioids. All six studies were performed among patients undergoing awake intubation and included relatively small sample sizes. Therefore, further studies should be focused on comparing dexmedetomidine with different anesthetic drugs among patients undergoing awake intubation. Third, we stated that perioperative intravenous administration of dexmedetomidine reduced the incidence of coughing on the tube based on the patient populations of the studies in our meta-analysis who would likely be at low risk for these adverse events. The statements above regarding this being a relevant and important issue are applicable to high-risk patients. Therefore, further studies should be focused on patients that are at high risk, as mentioned in our discussion on the incidence of coughing on the tube. Finally, six of the nine articles analyzed were related to patients undergoing oral surgery under awake nasal intubation, and there was a limited number of studies reporting dexmedetomidine use during different types of surgeries under traditional oral intubation. Thus, future studies should address this research gap.
Conclusion
Perioperative intravenous administration of dexmedetomidine has a positive effect on the prevention of POST. However, this positive effect may only be evident among patients undergoing traditional oral intubation. This article contributes to the existing literature on treatment options for POST and may guide clinicians during their daily work.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yuanhui Liu https://orcid.org/0000-0003-2340-2566
Supplemental material: Supplemental material for this article is available online.
References
- 1.McHardy FE, Chung F. Postoperative sore throat: cause, prevention and treatment. Anaesthesia 1999; 54: 4444–4453. DOI: 10.1046/j.1365-2044.1999.00780.x [DOI] [PubMed] [Google Scholar]
- 2.Tu HN, Saidi N, Lietaut T, et al. Nitrous oxide increases endotracheal cuff pressure and the incidence of tracheal lesions in anesthetized patients. Anesth Analg 1999; 89: 187–190. DOI: 10.1097/00000539-199907000-00033 [DOI] [PubMed] [Google Scholar]
- 3.Bernhard WN, Yost LC, Turndorf H, et al. Physical characteristics of and rates of nitrous oxide diffusion into tracheal tube cuffs. Anesthesiology 1978; 48: 413–417. DOI: 10.1097/00000542-197806000-00007 [DOI] [PubMed] [Google Scholar]
- 4.McIntosh CA, Macario A. Managing quality in an anesthesia department. Curr Opin Anaesthesiol 2009; 22: 223–231. DOI: 10.1097/ACO.0b013e328324f810 [DOI] [PubMed] [Google Scholar]
- 5.Chandler M. Tracheal intubation and sore throat: A mechanical explanation. Anaesthesia 2002; 57: 155–161. DOI: 10.1046/j.1365-2044.2002.02329.x [DOI] [PubMed] [Google Scholar]
- 6.Loeser EA, Bennett GM, Orr DL, et al. Reduction of postoperative sore throat with new endotracheal tube cuffs. Anesthesiology 1980; 52: 257–259. DOI: 10.1097/00000542-198003000-00011 [DOI] [PubMed] [Google Scholar]
- 7.Lehmann M, Monte K, Barach P, et al. Postoperative patient complaints: a prospective interview study of 12,276 patients. J Clin Anesth 2010; 22: 13–21. DOI: 10.1016/j.jclinane.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Macario A, Weinger M, Carney S, et al. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 1999; 89: 652–658. DOI: 10.1097/00000539-199909000-00022 [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Abe R, Tanaka Y, et al. Tracheal intubation by trainees does not alter the incidence or duration of postoperative sore throat and hoarseness: a teaching hospital-based propensity score analysis. Br J Anaesth 2015; 115: 463–469. DOI: 10.1093/bja/aev234 [DOI] [PubMed] [Google Scholar]
- 10.Mayhood J, Cress K. Effectiveness of ketamine gargle in reducing postoperative sore throat in patients undergoing airway instrumentation: a systematic review. JBI Database System Rev Implement Rep 2015; 13: 244–278. DOI: 10.11124/jbisrir-2015-2045 [DOI] [PubMed] [Google Scholar]
- 11.Hu B, Bao R, Wang X, et al. The Size of Endotracheal Tube and Sore Throat after Surgery: A Systematic Review and Meta-Analysis. PLoS One 2013; 8: e74467. DOI: 10.1371/journal.pone.0074467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng F, Wang M, Yang H, et al. Efficacy of intracuff lidocaine in reducing coughing on tube: a systematic review and meta-analysis. J Int Med Res 2020; 48: 1–12. DOI: 10.1177/0300060520901872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuriyama A, Maeda H, Sun R, et al. Topical application of corticosteroids to tracheal tubes to prevent postoperative sore throat in adults undergoing tracheal intubation: a systematic review and meta-analysis. Anesthesia 2018; 73: 1546–1556. DOI: 10.1111/anae.14273 [DOI] [PubMed] [Google Scholar]
- 14.Thomas D, Bejoy R, Zabrin N, et al. Preoperative ketamine nebulization attenuates the incidence and severity of postoperative sore throat: A randomized controlled clinical trial. Saudi J Anaesth 2018; 12: 440–445. DOI: 10.4103/sja.SJA_47_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuriyama A, Maeda H, Sun R. Topical application of magnesium to prevent intubation-related sore throat in adult surgical patients: a systematic review and meta-analysis. Can J Anaesth 2019; 66: 1082–1094. DOI: 10.1007/s12630-019-01396-7 [DOI] [PubMed] [Google Scholar]
- 16.Giovannitti JA, Thoms SM, Crawford JJ. Alpha–2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog 2015; 62: 31–38. DOI: 10.2344/0003-3006-62.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014; 370: 444–454. DOI: 10.1056/NEJMra1208705 [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology 2014; 121: 801–807. DOI: 10.1097/ALN.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 19.Chu KS, Wang FY, Hsu HT, et al. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol 2010; 27: 36–40. DOI: 10.1097/EJA.0b013e32832e0d2b [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009; 151: 264–269. DOI: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 21.Mizrak A, Erbagci I, Arici T, et al. Dexmedetomidine use during strabismus surgery in agitated children. Med Princ Pract 2011; 20: 427–432. DOI: 10.1159/000324554 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Bai X, Zhou Q, et al. Comparison of the effects of dexmedetomidine and midazolam with fentanyl in patients with anticipated difficult intubation during awake blind nasal intubation. Hua Xi Kou Qiang Yi Xue Za Zhi 2013; 31: 253–256. [in Chinese]. DOI: 10.7518/hxkq.2013.03.009 [PubMed] [Google Scholar]
- 23.Bu X, Wan Z, Fang C, et al . Clinical observation of dexmedetomidine combined with visual double-chamber bronchial catheter in thoracoscopic surgery. Chin J Clin Healthc 2019; 22: 403–406. [in Chinese]. DOI: 10.3969/J.issn.1672-6790.2019.03.030 [Google Scholar]
- 24.Sun Z, He M, Li J. The application effect of dexmedetomidine combining with sevoflurane in the perioperative period of interventional treatment for patients with intracranial aneurysms. Journal of Qiqihar Medical University 2018; 39: 2257–2260. [in Chinese]. DOI: 10.3969/j.issn.1002-1256.2018.19.010 [Google Scholar]
- 25.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: a double-blinded randomized controlled trial. J Anesth 2013; 27: 211–217. DOI: 10.1007/s00540-012-1499-y [DOI] [PubMed] [Google Scholar]
- 26.Qiu L, Zhang L, Ji J. Sedation effect of dexmedetomidine on awake intubation in patients with difficult airway. Pharm Care Res 2013; 13: 208–212. [in Chinese]. DOI: 10.5428/pcar20130315 [Google Scholar]
- 27.Shen SL, Xie YH, Wang WY, et al. Comparison of dexmedetomidine and sufentanil for conscious sedation in patients undergoing awake fibreoptic nasotracheal intubation: a prospective, randomised and controlled clinical trial. Clin Respir J 2014; 8: 100–107. DOI: 10.1111/crj.12045 [DOI] [PubMed] [Google Scholar]
- 28.Tsai CJ, Chu KS, Chen TI, et al. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia 2010; 65: 254–259. DOI: 10.1111/crj.12045 [DOI] [PubMed] [Google Scholar]
- 29.Berning V, Laupheimer M, Nubling M, et al. Influence of quality of recovery on patient satisfaction with anaesthesia and surgery: a prospective observational cohort study. Anaesthesia 2017; 72: 1088–1096. DOI: 10.1111/anae.13906 [DOI] [PubMed] [Google Scholar]
- 30.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun 2005; 19: 493–499. DOI: 10.1016/j.bbi.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Pandharipande P, Ely EW, Maze M. Alpha-2 agonists: can they modify the outcomes in the postanesthesia care unit? Curr Drug Targets 2005; 6: 749–754. DOI: 10.2174/138945005774574515 [DOI] [PubMed] [Google Scholar]
- 32.Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology 1991; 74: 581–605. DOI: 10.1097/00000542-199103000-00029 [PubMed] [Google Scholar]
- 33.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology 2000; 93: 1345–1349. DOI: 10.1097/00000542-200011000-00030 [DOI] [PubMed] [Google Scholar]
- 34.Szumita PM, Baroletti SA, Anger KE, et al. Sedation and analgesia in the intensive care unit: evaluating the role of dexmedetomidine. Am J Health Syst Pharm 2007; 64: 37–44. DOI: 10.2146/ajhp050508 [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Liang Y, Dai Z, et al. Perioperative dexmedetomidine reduces delirium after cardiac surgery: a meta-analysis of randomized controlled trials. J Clin Anesth 2018; 50: 33–42. DOI: 10.1007/s12630-019-01440-6 [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez RM, Bjerke RJ, Drobycki T, et al . Prevention of endotracheal tube-induced coughing during emergence from general anesthesia. Anesth Analg 1994; 79: 792–795. DOI: 10.1213/00000539-199410000-00030 [DOI] [PubMed] [Google Scholar]
- 37.Stoelting RK. Blood pressure and heart rate changes during short-duration laryngoscopy for tracheal intubation: influence of viscous or intravenous lidocaine. Anesth Analg 1978; 57: 197–199. DOI: 10.1213/00000539-197803000-00009 [DOI] [PubMed] [Google Scholar]
- 38.Fellman D, Simberg S. Prevalence and risk factors for voice problems among soccer coaches. J Voice 2017; 31: 121.e9–121.e15. DOI: 10.1016/j.jvoice.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 39.Weikert M, Schlömicher-Thier J. Laryngeal movements in saxophone playing: video-endoscopic investigations with saxophone player. J Voice 1999; 13: 265–273. DOI: 10.1016/s0892-1997(99)80031-9 [DOI] [PubMed] [Google Scholar]