Abstract
Objective
We aimed to identify the factors that influence serum anti-Müllerian hormone (AMH) concentration measurements.
Methods
We collected serum samples between May and September 2018 and compared the effect on AMH concentration measured by ELISA of conditions including venepuncture, storage time, storage temperature, locations of the reaction microplate, and the use of the oral contraceptive pill and gonadotrophin-releasing hormone (GnRH).
Results
AMH concentration was not affected by food intake but was affected by haemolysis. It was also much higher in samples on the edge of the ELISA microtitre plate. AMH concentration increased after incubation at room temperature for 1 day, 4°C for 3 days, −20°C for 1 month and −40°C for 4 months, but no change occurred during storage at −80°C for 9 months. AMH concentration was high in patients following GnRH agonist treatment but was not affected by oral contraceptives.
Conclusions
No fasting is required prior to AMH measurement. Placement of serum samples on the edge of microtitre plates affects the results of the AMH ELISA. If serum samples cannot be assayed immediately, it is best to store them at −80°C. Basal AMH concentration cannot be used as a measure of ovarian reserve after GnRH agonist treatment.
Keywords: Anti-Müllerian hormone, clinical diagnosis, enzyme-linked immunosorbent assay, sample processing, microtitre plate, gonadotrophin-releasing hormone, oral contraceptive
Introduction
Anti-Müllerian hormone (AMH) is a member of the transforming growth factor-beta superfamily,1 which has roles in tissue differentiation and growth. After puberty, AMH is produced by primary ovarian follicles, and this production is maximal in small antral follicles and preantral structures, but subsequently wanes until ovulation.2 However, AMH is produced fairly consistently over the menstrual cycle.3 The in-depth study of AMH has led to the development of numerous clinical applications of the measurement of serum AMH concentration.4 To date, AMH measurements have been used for human fertility counselling,5 the prediction of menopause,6 the diagnosis of polycystic ovarian syndrome (PCOS),7,8 the prediction of the response to ovarian stimulation9,10 and as a prognostic marker for endometrial cancer.11 Thus, serum AMH concentration can be used to confirm clinical diagnoses and guide personalised therapy. Therefore, the accurate measurement of AMH concentration is important for clinicians. However, although most of the current methods for the measurement of AMH concentration are based on enzyme-linked immunosorbent assay (ELISA),12 differences between the methods are associated with differing results for a given sample.13,14 In addition, there are numerous smaller hospitals and reproductive medicine centres worldwide, at which AMH concentrations may not be able to be measured immediately.
In the present study, we aimed to identify the factors that influence the measured serum AMH concentration to recommend a standardised procedure for the clinical measurement of serum AMH concentration. To this end, we measured serum AMH under various conditions, including fasting, haemolysis, various storages temperatures, various storage times, differing position on the assay microplate, and in samples from patients who were using the contraceptive pill or had recently been administered a GnRH agonist (GnRH-a). We hope that this work will provide a theoretical basis for clinicians to assess their patients’ conditions using their circulating AMH concentrations.
Materials and methods
Patients
The sample size was determined as previously described.13,15 Female patients who participated in in vitro fertilisation (IVF) between May and September 2018 at the Reproductive Medicine Centre of the General Hospital of Ningxia University of Medicine were enrolled in the study. The age of the participants was 20 to 30 years and they had normal ovarian function; the infertility was the result of problems associated with their male partner. The study was approved by the Institutional Review Board of the General Hospital of Ningxia University of Medicine and written informed consent was obtained from each of the participating couples. The exclusion criteria were PCOS; rheumatoid arthritis; abnormal blood lipid profile, bilirubin concentration, haemoglobin concentration or haematological disease; and the use of a steroid hormone-derived drug within the preceding 3 months.
Sample processing
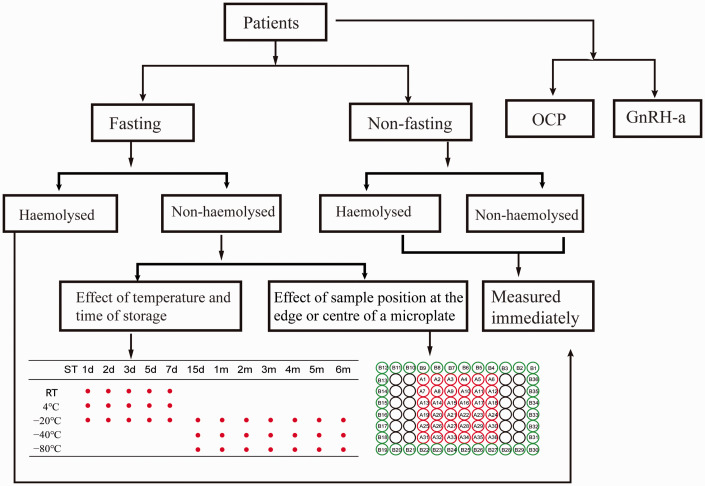
A fasting venous blood sample was collected from each participant. Half of each sample was placed into a condensation tube and left at room temperature for 30 minutes, after which it was centrifuged at 1260 × g for 10 minutes, and the separated serum was aliquoted for later assay. The other half of each sample was haemolysed by subjecting it to an overnight freeze–thaw cycle, after which it was centrifuged in the same way. A further blood sample was collected from each participant 2 hours after eating or drinking water, and this was processed in the same way. The experimental design is shown in Figure 1. Fasting non-haemolysed serum was stored at several different temperatures for several periods of time, and was analysed in two locations on the assay microplate to identify the factors influencing the measured serum AMH concentration. To determine the effect of position on the microplate, a centre position was compared with an edge position. Some of the participants were taking the oral contraceptive pill (OCP; drospirenone or ethinyloestradiol) over 28-day cycles, and AMH concentration was measured on the third day of the next menstrual cycle. For participants who had been administered a GnRH-a, AMH concentration was measured on the third day of menstruation and 14 days after the end of the GnRH-a course.
Figure 1.
Flow chart of the comparisons made in the present study.
OCP, oral contraceptive pill; GnRH-a, gonadotrophin releasing hormone agonist; RT, room temperature; d, day(s); m, month(s). “A” numbers reflect samples placed in the centre of the microplate and “B” numbers samples at the edge of the microplate.
AMH analysis
We used the AMH Gen II ELISA assay, reagent (KR-AMH-001) and Automatic ELISA workstation (DS2) (Guangzhou Kangrun Biological Technology Co., Ltd, Guangzhou, China). The AMH assay was performed in triplicate, in strict accordance with the procedure recommended by the manufacturer.
Statistical analysis
Data were analysed using SPSS 17.0 (SPSS, Chicago, IL, USA) or Prism 7.0 (GraphPad software, San Diego, CA, USA) and are expressed as means ± standard deviations (SDs). Each measurement was performed in triplicate. Analysis of variance, followed by the least significant difference test, was used to evaluate differences between multiple groups and the effect of position on the microplate was evaluated using a paired t-test. P < 0.05 was considered to represent statistical significance.
Results
Comparisons of measured AMH concentration between non-haemolysed and haemolysed samples, and between samples collected under fasting and non-fasting conditions
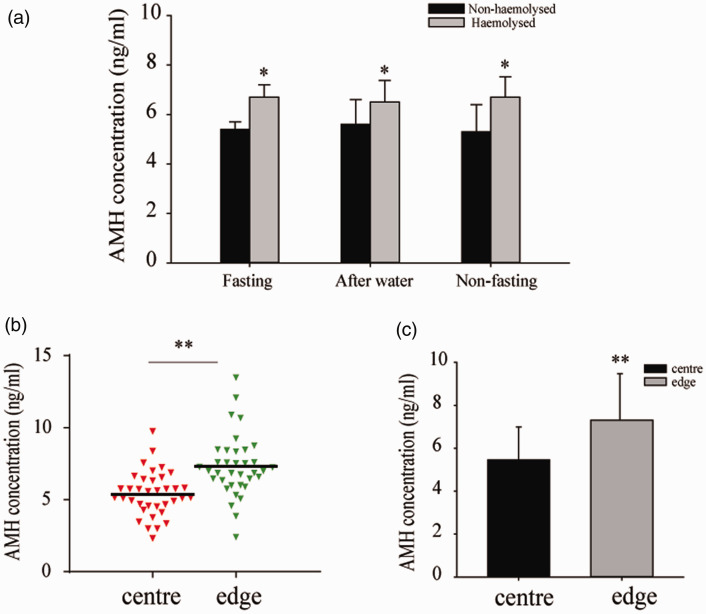
We recruited 155 patients. The measured AMH concentration did not differ between samples collected after fasting (5.753 ± 0.266 ng/mL), postprandially (5.623 ± 0.542 ng/mL) and after drinking water (5.749 ± 0.612 ng/mL). Irrespective of whether the samples were collected after fasting or after a meal, the AMH concentration in haemolysed samples was higher than those in non-haemolysed samples (P < 0.05). However, there was no significant difference between the concentrations before and after a meal when the samples were haemolysed (Figure 2a).
Figure 2.
Effects of fasting, haemolysis and position on the microtitre plate on the measured AMH concentration. (a) AMH concentration in non-haemolysed and haemolysed samples (n = 85 per condition) collected under fasting and non-fasting conditions. *P < 0.05 vs. fasting/non-haemolysed samples (Student’s t-test). (b, c) AMH concentrations in samples placed in the centre or at the edge of the ELISA microplate (n = 36 per location). **P < 0.01 (paired t-test). The data are presented as the mean ± SEM of at least three independent replicates.
AMH, anti-Müllerian hormone.
The position of the serum sample on the assay microplate affects the measured AMH concentration
The measured AMH concentration depended on the site of the serum sample on the assay microplate. The placement of the samples was as shown in Figure 1. The “A” samples were placed in the centre of the plate and the “B” samples were placed on the edges of the plate. The measured AMH concentration in the centre was 5.46 ± 1.56 ng/mL and that on the edge of the plate was 7.70 ± 2.17 ng/mL (P < 0.01) (Figure 2b, c).
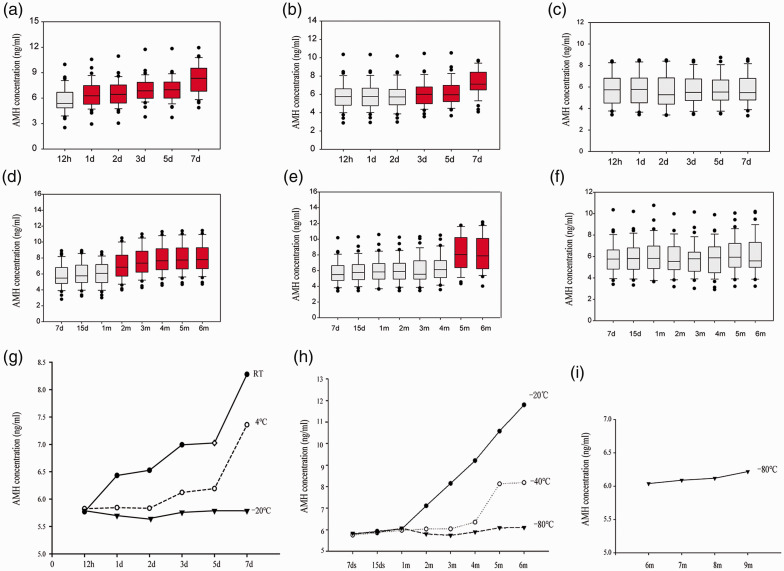
Storage time and temperature affect the accuracy of the measurement of AMH concentration
The AMH concentration increased progressively with storage time and temperature. When the serum samples were stored at room temperature (RT) or 4°C for 1 or 3 days, the measured AMH concentration was significantly higher than in fresh samples (P < 0.05). There was no significant increase in AMH concentration when the samples were stored at −20°C for 1 week, but it was significantly higher after 1 month and 4 months of storage at −20°C or −40°C (P < 0.05). However, there was no difference in the AMH concentration when the samples were stored at −80°C for 6 or 9 months (Figure 3).
Figure 3.
Effects of storage time and temperature on the measured AMH concentration. AMH concentration in samples stored at (a) RT, (b) 4°C, (c, d) −20°C, (e) −40°C and (f) −80°C. (g–i) The measured AMH concentration increased progressively with the duration of storage. N = 85 for each set of experiments. ANOVA was used to assess the differences between the groups. The red boxes represent P < 0.05 vs. samples tested immediately. AMH, anti-Müllerian hormone; RT, room temperature; d, day(s); m, month(s).
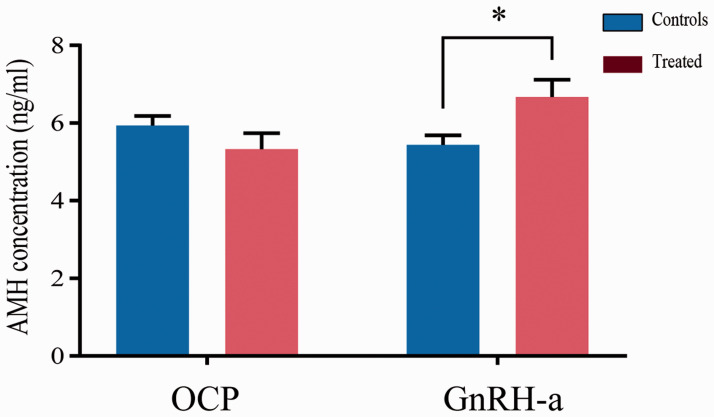
Effects of contraceptives and GnRH agonists on the measured AMH concentration
The measured AMH concentrations in samples from participants who were undergoing GnRH-a treatment (n = 35 participants) were higher than in those who were not (P < 0.05). However, OCP use (n = 35 participants) did not affect the measured AMH concentration (Figure 4).
Figure 4.
Effects of taking the OCP or recent GnRH-a administration on the measured AMH concentration. n = 35 participants took each of these types of medication *P < 0.05 (Student’s t-test). The results of at least three independent experiments per group are shown. AMH, anti-Müllerian hormone; OCP, oral contraceptive pill; GnRH-a, gonadotrophin releasing hormone agonist.
Discussion
AMH concentration shows little variation within and between menstrual cycles, and can therefore be assessed throughout at any stage of the cycle.16 The measurement of AMH concentration is becoming a highly useful tool in clinical medicine. It is not only a sensitive marker of ovarian reserve, but also facilitates the early diagnosis and the prediction of the recurrence of many tumours.17,18 In addition, it is a predictor of the final menstrual period.19 For IVF, AMH is a superior predictor of live birth than follicle-stimulating hormone.20 Therefore, the accuracy of the results of AMH assays is critical for appropriate decision-making by clinicians. Guidance for the measurement of AMH concentration suggests that serum AMH is unstable under certain storage and handling conditions, which implies that these factors might affect the measured concentrations. Furthermore, the use of the OCP is widespread, including for the treatment of PCOS.21 In women who participate in IVF, the administration of a GnRH-a is associated with a higher pregnancy rate.22 Finally, the measurement of AMH concentration is considered to be a reliable means of assessing ovarian reserve.23 Therefore, in the present study, we determined whether the OCP or GnRH-a affect the serum AMH concentration.
We first measured the AMH concentrations of serum samples collected under fasting and non-fasting conditions, and found no significant difference. This implies that eating and drinking water have no effect on the serum AMH concentration. This is important because it means that patients with hypoglycaemia, such as those with diabetes, who may suffer severe adverse effects if they fast,24 do not need to do so prior to the measurement of serum AMH concentration. Furthermore, this renders the measurement easier and more acceptable for patients in general.
Haemolysed samples are quite frequently obtained in the clinic, and haemolysis affects several clinical parameters.25 Although laboratory instruments are routinely equipped with systems that automatically detect and correct for the effects of haemolysis. It has been shown that the majority of errors in laboratory medicine occur in the extra-analytical phases of the testing process, and especially in the pre-analytical phase.26 Haemolysis can occur for non-biological reasons, during sample collection and handling, including from traumatic venepuncture, sample collection using inappropriate materials, inappropriate sample handling, inadequate storage conditions and re-centrifugation.27 To date, there have been no studies of the effect of haemolysis on serum AMH concentration.28 In the present study, we have shown that the measured AMH concentration significantly differs between haemolysed and non-haemolysed samples. However, in the haemolysed samples, there was no significant difference between samples collected under fasting and non-fasting conditions. The effect of haemolysis appears to be approximately linearly related to the final concentration of lysed erythrocytes in the sample. However, heterogeneous and unpredictable effects of haemolysis have been shown with respect to diverse parameters, which prevents the adoption of statistical means of correcting measured values according to the degree of haemolysis.25 In addition, the results of a previous study indicate that repeated fist clenching and unclenching during venepuncture may trigger acute changes in several routine clinical chemistry parameters,29 which may be the result of muscle contractions, haemolysis or both. Therefore, venepuncture should be performed without fist clenching.
The present findings indicate that blood sampling and the storage and transportation of the samples must be performed appropriately. This is the first study to determine the influence of the position of the serum sample on the ELISA microplate on the measured AMH concentration. An “edge effect” is present if the colour of peripherally located samples is deeper than that for those that are central, and may occur if there is a thermodynamic gradient across the plate. Here, we found that centrally located samples had lower measured AMH concentrations than peripherally located samples. Thus, the positioning of samples on a microtitre plate might affect the accuracy of the measurements made. Therefore, it is necessary for an adjustment to be designed to correct test results accordingly.
The duration and temperature of storage of blood samples are very important for the accuracy of many measurements, but some smaller reproductive medicine centres may not be able to immediately perform such assays. Kumar et al.30 found that the serum AMH concentration is stable for up to 7 days at 2 to 8°C, −20°C and −80°C. In the present study, the most striking change in serum AMH concentration was in samples that were incubated for over 24 hours at RT, and this implies that AMH measurements are not reliable under these circumstances. In addition, the measured AMH concentration was significantly higher after samples were stored for 3 days at 4°C. However, there was no significant change when samples were stored at −20°C for 1 week. In addition, the AMH concentration in serum samples incubated at RT for up to 7 days increased by 58% and that in samples incubated at −20°C for 5 days increased by 23% compared with fresh samples.15 The results of Fleming et al. were consistent with these findings,31 but the assessment in the present study was more thorough. Furthermore, the measured AMH concentration in samples stored at −40°C increased over a 4-month period, but it was stable for 9 months when the samples were stored at −80°C. Thus, an appropriate temperature should be selected, according to the period of time serum samples must be stored prior to assay, such that the AMH results are reliable. However, there are many assay providers and it may be that the most appropriate storage times and temperatures differ for each.
The present findings provide other useful information regarding for the measurement of AMH for clinical and research purposes. Treatment with goserelin, a gonadotrophin-suppressing drug, has been shown to reduce the concentration of AMH over several months,32 and the present data show that AMH concentration is increased by treatment with a GnRH-a. However, this effect on AMH varies among individuals. A previous study showed an approximate 13% increase in AMH concentration following 14 days of GnRH-a treatment, but the serum AMH decreased 7 days after the administration.33 These data indicate that the timing of the AMH measurement affects the type of difference in serum AMH concentration following GnRH-a treatment, but individual variation may also contribute to this.34 A limitation of the present study was that we only recruited relatively young patients, who have ovaries that would be more sensitive to GnRH-a. Thus, basal AMH concentration may not be a reliable marker of the ovarian response to long-term administration of such agonists.
The OCP is also widely used to treat reproductive endocrine diseases. In the present study, we found that AMH concentration was not significantly affected by the use of the OCP. Thus, basal AMH is a reliable marker of the ovarian response in patients who have been taking the OCP for 28 days. Kallio et al. found that the AMH concentration was 50% lower after 9 weeks of administration of the OCP, but that there was no significant difference after 5 weeks.35 The long-term use of contraceptives leads to changes in other hormones that would affect AMH concentration. The present study findings provide a theoretical basis for the accurate clinical use of serum AMH concentration measurements. Hormone treatment may cause changes in AMH, so the measured AMH concentration may not accurately reflect the ovarian reserve. Unfortunately, we did not measure the haemoglobin content of these samples to quantify the degree of haemolysis; therefore, we will determine the effect of the level of haemolysis on the measurement of serum AMH in a follow-up study to define a threshold value for haemoglobin.
In conclusion, we have shown that fasting is not necessary prior to the measurement of serum AMH concentration in the clinic. Furthermore, haemolysis and the placement of samples on the edge of the ELISA microtitre plate affects the accuracy of AMH measurements. If AMH concentrations cannot be measured immediately, samples should be stored at a suitable temperature. If serum samples are tested within 12 hours, they can be stored at RT, or they can be stored at 4°C for 2 days, at −20°C for 2 weeks, or at −40°C for 3 months, without a loss of accuracy. However, for long-term storage, a −80°C freezer is required. Finally, basal AMH concentration cannot be used as a reliable marker of ovarian reserve for patients who have recently undergone treatment with a GnRH-a.
Acknowledgements
We thank the Laboratory of Fertility Maintenance of Ningxia University of Medicine and the Clinical Pathogen Microbiology Laboratory of the General Hospital of Ningxia University of Medicine for providing the experimental platform.
Footnotes
Author contributions: Yun-xing Fu and Hui Wang participated in the study design, performed the experiments and drafted the manuscript. Rong Hu participated in the study design, directed the execution of the experiments and revised the article for intellectual content. Ting Hu and Fei-miao Wang participated in the sample collection. All the authors approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Leading Talent Project 2018 (grant no. KJT2016008), the National Natural Science Foundation of China (grant no. 81960277), the First-Class Discipline Construction Funded Project of Ning Xia Medical University and the School of Clinical Medicine (grant no. NXYLXK2017A05), the 2018 Advantageous Subjects Project of Ning Xia Medical University (grant no. XY201807) and a Ningxia Hui Autonomous Region Key Research and Development Plan project (grant no. 2019BFG02005).
ORCID iD: Yun-Xing Fu https://orcid.org/0000-0003-4372-1433
References
- 1.Kristensen SG, Andersen K, Clement CA, et al. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod 2014; 20: 293–308. [DOI] [PubMed] [Google Scholar]
- 2.Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014; 20: 370–385. [DOI] [PubMed] [Google Scholar]
- 3.Ledger WL. Clinical utility of measurement of anti-mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab 2010; 95: 5144–5154. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RA, Nelson SM, Wallace WH. Measuring anti-Mullerian hormone for the assessment of ovarian reserve: when and for whom is it indicated? Maturitas 2012; 71: 28–33. [DOI] [PubMed] [Google Scholar]
- 5.Seifer DB, Baker VL, Leader B. Age-specific serum anti-müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril 2011; 95: 747–750. [DOI] [PubMed] [Google Scholar]
- 6.Dolleman M, Faddy MJ, Van Disseldorp J, et al. The relationship between anti-Mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab 2013; 98: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 7.Saxena U, Ramani M, Singh P. Role of AMH as Diagnostic Tool for Polycystic Ovarian Syndrome. J Obstet Gynaecol India 2018; 68: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont A, Robin G, Catteau-Jonard S, et al. Role of anti-müllerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol 2015; 13: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broer SL, Van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013; 19: 26–36. [DOI] [PubMed] [Google Scholar]
- 10.Henry NL, Xia R, Schott AF, et al. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist 2014; 19: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowkielewicz M, Lipka A, Piotrowska A, et al. Anti-Müllerian Hormone Expression in Endometrial Cancer Tissue. Int J Mol Sci 2019; 20: 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh P, Smith K, Nelson SM. A single-centre evaluation of two new anti-Mullerian hormone assays and comparison with the current clinical standard assay. Hum Reprod 2014; 29: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 13.Han X, McShane M, Sahertian R, et al. Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Mullerian hormone measurement using the Beckman Coulter Gen II assay. Hum Reprod 2014; 29: 1042–1048. [DOI] [PubMed] [Google Scholar]
- 14.Pankhurst MW, Chong YH, McLennan IS. Enzyme-linked immunosorbent assay measurements of antimullerian hormone (AMH) in human blood are a composite of the uncleaved and bioactive cleaved forms of AMH. Fertil Steril 2014; 101: 846–850. [DOI] [PubMed] [Google Scholar]
- 15.Rustamov O, Smith A, Roberts SA, et al. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod 2012; 27: 3085–3091. [DOI] [PubMed] [Google Scholar]
- 16.Fanchin R, Taieb J, Lozano DH, et al. High reproducibility of serum anti-Mullerian hormone measurements suggests a multistaged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005; 20: 923–927. [DOI] [PubMed] [Google Scholar]
- 17.Karkanaki A, Vosnakis C, Panidis D. The clinical significance of anti-mullerian hormone evaluation in gynecological endocrinology. Hormones (Athens) 2011; 19: 95–103. [Google Scholar]
- 18.Dong Z, An J, Xie X, et al. Preoperative serum anti-Müllerian hormone level is a potential predictor of ovarian endometrioma severity and postoperative fertility. Eur J Obstet Gynecol Reprod Biol 2019; 240: 113–120. [DOI] [PubMed] [Google Scholar]
- 19.Steiner AZ. AMH as a predictor of the final menstrual period. J Clin Endocrinol Metab 2020; 105: e1908–e1909. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Zhang Y, Mensah V, et al. Discordant anti-müllerian hormone (AMH) and follicle stimulating hormone (FSH) among women undergoing in vitro fertilization (IVF): which one is the better predictor for live birth? J Ovarian Res 2018; 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witchel SF, Oberfield SE, Peña AS. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J Endocr Soc 2019; 3: 1545–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update 2017; 23: 560–579. [DOI] [PubMed] [Google Scholar]
- 23.Broer SL, Broekmans FJ, Laven JS, et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014; 20: 688–701. [DOI] [PubMed] [Google Scholar]
- 24.Hartling L, Dryden DM, Guthrie A, et al. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep) 2012: 1–327. [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G, Salvagno GL, Montagnana M, et al. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 2006; 44: 311–316. [DOI] [PubMed] [Google Scholar]
- 26.Simundic AM, Lippi G. Preanalytical phase–a continuous challenge for laboratory professionals. Biochem Med (Zagreb) 2012; 22: 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G, Plebani M, Di Somma S, et al. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci 2011; 48: 143–153. [DOI] [PubMed] [Google Scholar]
- 28.Lippi G, Luca Salvagno G, Blanckaert N, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med 2009; 47: 934–939. [DOI] [PubMed] [Google Scholar]
- 29.Lima-Oliveira G, Guidi GC, Salvagno GL, et al. Estimation of the imprecision on clinical chemistry testing due to fist clenching and maintenance during venipuncture. Clin Biochem 2016; 49: 1364–1367. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Kalra B, Patel A, et al. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods 2010; 362: 51–59. [DOI] [PubMed] [Google Scholar]
- 31.Fleming R, Nelson SM. Reproducibility of AMH. Hum Reprod 2012; 27: 3639–3641; author reply 3641-3632. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RA, Themmen AP, Al-Qahtani A, et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 2006; 21: 2583–2592. [DOI] [PubMed] [Google Scholar]
- 33.Su HI, Maas K, Sluss PM, et al. The impact of depot GnRH agonist on AMH levels in healthy reproductive-aged women. J Clin Endocr Metab 2013; 98: E1961–E19E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai J, Liu L, Zheng J, et al. Differential response of AMH to GnRH agonist among individuals: the effect on ovarian stimulation outcomes. J Assist Reprod Genet 2018; 35: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallio S, Puurunen J, Ruokonen A, et al. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril 2013; 99: 1305–1310. [DOI] [PubMed] [Google Scholar]