Abstract
During the current COVID-19 pandemic, several emerging cases of laryngotracheal stenosis following prolonged intubation and tracheostomy are being reported. The patients’ pre-existing comorbidities, the disease itself and the pronation maneuvers increase the risk for endolaryngeal and tracheal damage. In this commentary, we report 4 such patients with acquired severe laryngotracheal stenosis. We describe their airway lesions, the surgical treatment they received, and the outcomes.
Keywords: COVID-19, laryngotracheal stenosis
Introduction
Following the first wave of COVID-19 pandemic, the Laryngotracheal Stenosis Committee of the European Laryngological Society (ELS) alerted the medical and scientific communities of the possibility of a surge in the number of airway injuries1 secondary to prolonged intubation and tracheostomy—and suggested that specialists in this field should see these patients at the earliest.
The various airway lesions due to prolonged intubation (PI) are: vocal cord(s) edema/granuloma, vocal cord(s) immobility—either due to recurrent laryngeal nerve palsy or ankylosis of one- or both cricoarytenoid joints, posterior glottic stenosis (PGS), vocal cords synechiae, subglottic retention cysts/ulcers/and stenosis. The endotracheal tube occupies the posterior glottis and the subglottis and following a PI may denude the airway mucosa and progressively develop PGS. Only a wait-and-see policy in suspected early laryngotracheal injuries (LTIs) might lead to scaring of these lesions and subsequently laryngotracheal stenosis with serious consequences. In several cases of acute LTIs, an early endoscopic management might avoid an open airway intervention and also significantly reduce long-term patient morbidity.2,3 The various airway lesions due to tracheostomy are: tracheal stenosis and malacia, and an A-shape deformity that typically manifests several weeks after decannulation. After the cannula is removed, persisting stomal perichondritis weakens the tracheal cartilages, and manifests an A shape that dynamically obstructs the airway.4,5 Lesions common to the PI and tracheostomy are: cuff and tip of the tube/cannula lesions and tracheoesophageal fistula.
Apart from avoiding an airway complication in the ICUs, emergency doctors, general practitioners, physicians, and other medical professionals should be aware of such injuries and their eventual sequelae. These patients should have an early referral to centers with adequate expertise in managing laryngotracheal stenosis. This report describes emerging cases of laryngotracheal complications secondary to prolonged intubation and tracheostomy in COVID-19 patients.
Methods
Institutional review board acceptance for the study (ref. 2020-01500 CER-VD) and appropriate patients’ consents were obtained.
Case 1
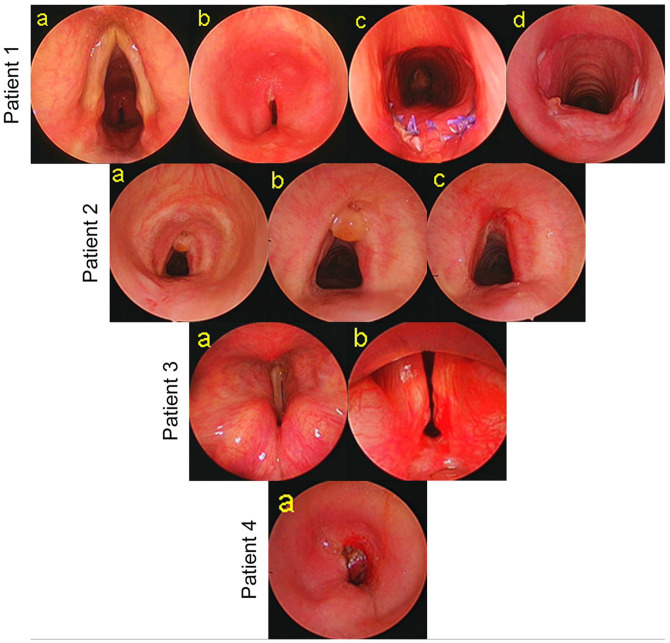
A 53-y-old man was hospitalized in the ICU of the referring hospital in March 2020 for COVID-19 severe bilateral pneumonitis and ARDS (Figure 1. #1). He was intubated (endotracheal tube ETT # 8.0, external diameter ED 11 mm) for 5 weeks, following which he received a percutaneous dilatation tracheostomy (PDT) and a Shiley cannula 8.0 (ED 10.9 mm) was inserted. The cannula was removed and tracheostomy closed medically after 8 days. He had past medical history significant for hypertension. His BMI was 30.5 kg/m2. He was discharged home after 7 weeks of hospitalization. Approximately, 3 months following decannulation, he started developing dyspnea on exertion that severely affected his daily routine. Endoscopy at that hospital showed a severe A-shape deformity at the tracheostomy site with severe tracheal obstruction. He received diode laser excision and stent insertion in the trachea. The stent was removed after 1 month and within weeks; he had recurrence of his respiratory symptoms. He was then referred to our clinic. Endoscopy at our clinic confirmed severe grade upper tracheal stenosis, malacia at the site of the tracheostomy with 90% obstruction of the tracheal lumen. The distal airway was normal. The patient underwent a single stage tracheal resection (excision of 4 tracheal rings) and cricotracheal anastomosis. The postoperative endoscopy was optimal. Symptomatically he improved dramatically and left the hospital on the 22nd postoperative day. Six months after the surgery, the patient has no respiratory complaints, feeds and drinks well without aspiration, and has a good voice. The last endoscopy showed a non-collapsible optimal airway.
Figure 1.
Laryngotracheal stenosis. Patient # 1: (a) endoscopic view of the larynx and trachea, (b) severe grade tracheal stenosis, (c) 10 days after single stage tracheal resection and anastomosis (TRA), and (d) 2-months after TRA. Patient # 2: (a, b) endoscopic view of A-shape deformity and granuloma, and (c) view after endoscopic treatment. Patient # 3: (a) endoscopic view of bilateral immobile vocal cords in median position and (b) severe posterior glottic stenosis. Patient # 4: (a) severe grade tracheal stenosis.
Case 2
In April 2020, a 68-y-man at a regional hospital received a PDT after 27 days of intubation for severe COVID-19 pneumonia and ARDS (Figure 1. #2). He had a Shiley cannula size 8.0. His comorbidities included chronic renal disease, hypertension, and obesity. After discharge from the hospital, the patient experienced progressive breathlessness over 8 weeks before being referred to our clinic. Endoscopy showed a mild grade tracheal stenosis at the tracheostomy site, mildly collapsing A-shape deformity and a granuloma obstructing 20% of the tracheal lumen. The granuloma was excised with cold steel instruments that improved his symptoms. He has remained asymptomatic since then.
Case 3
A 67-y-man was intubated (ETT 8.0) in March 2020 for COVID-19 related ARDS at our institution (Figure 1. #3). His comorbidities included ischemic heart disease that needed coronary stents, severe obesity, moderate to severe degree obstructive sleep apnea, diabetes mellitus and hypertension. Surgical tracheostomy (Shiley 8.0) was done after 26 days of intubation. Following his discharge from the hospital, he developed progressive dyspnea, occasional aspiration to liquids, and had no voice complaints. Flexible nasolarygoscopy showed bilateral vocal cords immobility and severely narrowed glottic space. Rigid laryngotracheoscopy showed severe posterior glottic stenosis with bilateral cricoarytenoid ankyloses. Distal airway was normal.
Laryngeal exposure at suspension micro-laryngoscopy was impossible in the patient. Therefore, we performed a left posterior cordotomy using a Lumenis Ultrapulse® DUO laser fiber (Lumenis Inc, Israel) delivered through a rigid bronchoscope. The laser fiber was stuck to the telescope using Steristrips 3M™. Additionally, the distal vents of the bronchoscope were occluded by Steristrips to maintain adequate ventilation and avoid air leaks. Intermittently, the bronchoscope was passed in the distal trachea and optimal ventilation was confirmed by observing the chest movements and on auscultation. At the last endoscopy, the patient had an optimal airway, no aspiration, but severe dysphonia.
Case 4
At a regional hospital, a 58-year-man with chronic renal disease received a percutaneous tracheostomy for severe COVID-19 disease conducive to ARDS, 17 days after intubation. An attempt to surgically close the tracheostomy by the local doctors led to development of an immediate onset cervico-thoracic subcutaneous emphysema and pneumomediastinum. Follow up endoscopy at our institution showed severe tracheal stenosis with 70% luminal obstruction and a large tracheostomy defect (Figure 1. #4). A tracheostomy cannula (Shiley 8.0) was reinserted. At the time of writing this report—the patient is fine, breathes through the cannula, has no feeding complaints and is scheduled for a single stage tracheal resection and anastomosis in the next few weeks.
Discussion
Endotracheal intubation and tracheostomy are well known causes of laryngotracheal stenosis (LTS). The degree and depth of injury mainly depends on duration of intubation, size of the tracheal tube, depth of sedation, patient’s general conditions (cardiovascular diseases, diabetes, and obesity playing an ominous role), and superimposing local infections.3,5 As far as the long-term outcomes and complications of tracheostomy are concerned, currently we are lacking prospective randomized trials comparing open versus percutaneous approaches.1,2,4 Percutaneous dilatation tracheostomy may cause fractures of tracheal cartilages and the increased trauma caused by the dilation force can lead to increased stenosis formation, in comparison to open techniques.6
Few weeks are needed before declaring a definite stabilization of the patient’s respiratory condition following an extubation or decannulation. Laryngotracheal stenosis following scarring of acute injuries do not manifest immediately after an ICU discharge. Instead, they manifest 3 to 4 weeks later when the patient is at home, and when normal respiratory conditions re-establish.7
Several mechanisms8 specifically related to COVID-19 treatment that increase the susceptibility of the larynx and the trachea to intubation and tracheostomy complications are: (1) pronation maneuvers, (2) prothrombotic and antifibrinolytic state of the patients affecting the laryngo-tracheal and oesophageal microcirculation—thus predisposing the mucosa to more ischemia and necrosis, (3) high viral replication in the tracheal epithelium could weaken the mucosa, (4) chronic high dose corticosteroid use thins down the tracheal mucosa, (5) lower PaO2/FiO2 ratio causes increased hypoxia of the laryngo-tracheal mucosa, (6) emotional and physical exhaustion of the care givers can add to the iatrogenic trauma, (7) existing comorbidities. The posterior glottis and subglottis endure most of the trauma and the injuries could evolve into uni- or bilateral cricoarytenoid fixation as was seen in one of our patients (#4). Use of larger tracheostomy cannula causes more iatrogenic tracheal damage. Additional suboptimal nursing and local infection causes tracheal stenosis and A-shape deformity. As recommended by the LTS committee of the European Laryngology Society1 it is prudent to see these patients early in clinics having expertise in managing LTS. It is important that the medical and nursing staff know the pathophysiology of the LTS development, especially because these patients spend several days in rehabilitation centers after long ICU stays.
Glottic, subglottic and tracheal stenosis can be treated endoscopically or by an open surgery and several publications in this context can be found in the literature.1 For posterior glottic stenosis (PGS), a rib cartilage graft can be inserted endoscopically or following a larygofissure and posterior cricoid cartilage split to expand the inter-arytenoid space. This intervention works well in children. However, in adults, the calcification of laryngeal and rib cartilages makes the surgery difficult and the results are uncertain. Posterior cordotomy or arytenoid reductions under suspension micro-laryngoscopy (SML) are other options for PGS. However, these procedures can be challenging in patients with COVID-19 and having comorbidities, as was the case in patient #3. He had extreme obesity and exposure of the larynx under SML was impossible. We therefore used the CO2 laser fiber and a rigid bronchoscope with modifications that allowed adequate ventilation of the patient and simultaneous treatment of his PGS.
Flexible bronchoscopy is necessary to diagnose A-shape deformity.3,4 With the patient breathing spontaneously, the larynx is sprayed with local anesthesia and the tracheostomy cannula removed. The stoma site is then blocked in the neck using a moist sterile gauze piece and simultaneous observation of the endotracheal airway obstruction is performed. Obstruction due to an A-shape deformity is a dynamic airway collapse and responds best to a segmental tracheal resection and anastomosis.
In our limited experience while treating LTS in these patients, we observed that the treatment is challenging because of their preexisting comorbidities and the sequelae secondary to long and complicated ICU stays. Surgeons must be well trained to choose the appropriate treatment option that best suits their patient. Management strategies during the current and future months of the pandemic must aim at reducing laryngotracheal injuries.
Conclusion
In the coming months of the COVID-19 pandemic, we might see a spurt of laryngotracheal stenosis cases following prolonged endotracheal intubation and tracheostomy. Physicians must have the knowledge of these lesions and consider an early referral to specialized centers. Treatment should be tailor-made as per the characteristics of larygotracheal stenosis and the patient comorbidities.
Footnotes
Funding:The author received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: K Sandu: concept, collection of data, manuscript writing and editing.
Patient Consent Confirmation Statement: All patients consent was secured to publish the findings of this case study.
Summary
In the coming months, laryngotracheal stenosis will be seen as a complication following prolonged intubation and tracheostomy in COVID-19 patients. To mitigate infection, use of larger than age-appropriate endotracheal tubes and tracheostomy cannulas, pronation maneuvers increase endo-laryngotracheal trauma, and could be responsible for the stenotic sequelae. These patients have severe comorbidities and hence treatment needs to be tailor made.
Role of the author: concept, data acquisition, manuscript writing, edition.
References
- 1. Piazza C, Filauro M, Dikkers FG, et al. Long‑term intubation and high rate of tracheostomy in COVID‑19 patients might determine an unprecedented increase of airway stenoses: a call to action from the European Laryngological Society. Eur Arch Otorhinolaryngol. 2021;278:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huston MN, Matthew R. Acute laryngeal injury after intubation—does wait and see mean wait and scar? JAMA Otolaryngol Head Neck Surg. 2021;147:237-238. [DOI] [PubMed] [Google Scholar]
- 3. Lowery AS, Malenke JA, Bolduan AJ, Shinn J, Wooten CT, Gelbard A. Early intervention for the treatment of acute laryngeal injury after intubation. JAMA Otolaryngol Head Neck Surg. 2021;147:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monnier P, ed. Pediatric Airway Surgery. Management of Laryngotracheal Stenosis in Infants and Children. Springer; 2011. [Google Scholar]
- 5. Li M, Yiu Y, Merrill T, Yildiz V, deSilva B, Matrka L. Risk factors for posttracheostomy tracheal stenosis. Otolaryngol Head Neck Surg. 2018;159:698-704. [DOI] [PubMed] [Google Scholar]
- 6. Pancani S, Virga A, Spina R, Peris A, Corvi A. Experimental measurement of forces during percutaneous dilatational tracheostomy. Proc Inst Mech Eng Part H J Eng Med. 2018;232:423-433. [DOI] [PubMed] [Google Scholar]
- 7. De Kleijn BJ, Wedman J, Zijlstra JG, Dikkers FG, van der Laan BFAM. Short- and long-term complications of surgical and percutaneous dilatation tracheotomies: a large single-center retrospective cohort study. Eur Arch Otorhinolaryngol. 2019;276:1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiacchini G, Tricò D, Ribechini A, et al. Evaluation of the incidence and potential mechanisms of tracheal complications in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021;147:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]