Abstract
Background:
Testosterone (T) deficiency (TD) in men and women and estrogen (E) deficiency (ED) in women increasingly affects the overall health and quality of life of patients. T implants have seen increased utilization over the past decade. We evaluated continuation rates and adverse events that occurred during T therapy by reviewing practitioner reported data on compressed human-identical T implants for the treatment of TD in both men and women collected over 7 years.
Methods:
This was a retrospective review of data collected prospectively from men and women from 2012 and 2019. Men who had the clinical syndrome of TD received subcutaneous T implant therapy. Women who presented with symptoms of TD and/or ED underwent T implant and/or estradiol implant therapy. The clinics spanned multiple specialties including obstetrics and gynecology, internal medicine, family practice, and urology. Data were entered into a secure, custom tracking App, using Azure App Services and MS SQL Database integrated with a proprietary dosing site and industry-leading Pharmacy Dispensing software (BioTracker®).
Results:
Over the 7 years, 1,204,012 subcutaneous implant procedures were performed for 376,254 patients; 85% of the procedures were performed in women. Of the women, 54% of were premenopausal, and 46% were postmenopausal. The overall continuation rate after two insertions was 93%. The overall complication rate was <1%. Most common secondary response reported was pellet extrusion, which was more common in men (<3%) than women (<1%).
Conclusions:
This study is the largest reported retrospective study to evaluate the continuation and complication rates of T pellet implants. The safety of subcutaneous hormone pellet implants in men and women appears to be better than other routes of administration of bioidentical hormone replacement therapy. Further investigations on short- and long-term benefits of this modality are ongoing and could expand the overall utilization of this method.
Keywords: estradiol pellet implantation, pellet, sex hormone deficiency, testosterone pellet implantation
Introduction
Considerations for testosterone therapy in men
The clinical syndrome of hypogonadism in men has become a global medical problem affecting multiple health conditions including, but not limited to, diabetes, metabolic syndrome, decreased bone mineral density, and cardiovascular mortality and negatively impacts general health and quality of life.1–3 Although the prevalence of testosterone deficiency (TD) has been reported to include up to 38% of males, the definition of TD has varied as to what biochemical thresholds, if any, correlate with the clinical syndrome.1 Numerous studies have shown the protective effect of testosterone (T) on the heart. One study showed that achieving optimal serum levels effectively treats the short-term symptoms of TD and prevents the long-term diseases that have been reported to result from TD.4 Specifically, this study showed that, among 80,000 male veterans, T therapy led to reductions in the hazard ratios (HRs) for all-cause mortality [HR 0.40 (confidence interval, CI 0.39–0.43), myocardial infarction [HR 0.70 (CI 0.59–0.83)], and stroke [HR 0.57 (CI 0.40–0.82)]. In another study, compared with treated TD in men, untreated TD in men was associated with an 88% higher death rate.5 Since the 1990s, the use of T therapy in men has been increasing progressively year over year; in fact, there was a 300% increase in androgen replacement therapy in men from 2001 to 2011.6 This increase has been driven by clinical experience and research demonstrating that otherwise healthy men could experience symptoms and reduced quality of life (QOL) related to TD and often respond to T optimization.1
Considerations for T therapy in women
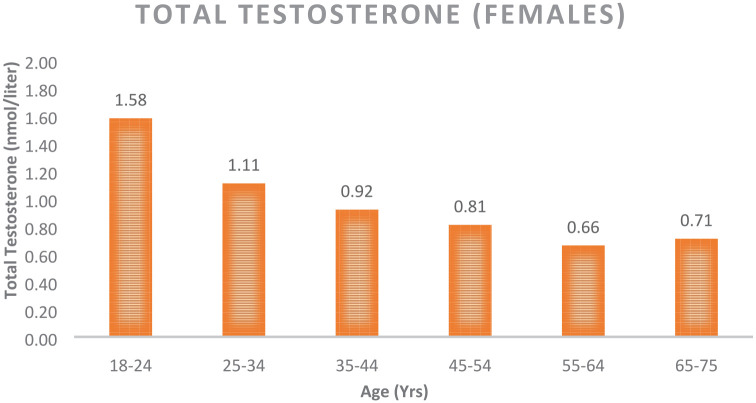
Although T is known as the “male” hormone, in 2002 Dimitrakakis et al. stated T is the most abundant biologically active gonadal hormone throughout the female lifespan.7 T is increasingly recognized as a vital hormone in women. In 2018, an International Consensus Group met regarding the use of T in women and agreed unanimously that T was the most important hormone for women regardless of the decade of life. Data were presented at the 2nd Androgen Society Meeting (April 2019, New Orleans, LA, USA) and has been published in Testosterone Insufficiency and Treatment in Women: International Expert Consensus.8 This paper stands in stark contrast to the Global Consensus Position Statement on the Use of Testosterone Therapy for Women by Davis et al., stating that “The only evidence-based indication for the use of testosterone in women is for the treatment of postmenopausal women who have been diagnosed as having HSDD after formal biopsychosocial assessment.”9 This paper unfortunately did not consider the literature supporting T use for brain, heart, breast, and bone protection in women.8 There is a medical need to perform additional validation studies confirming the importance of T use for general health in women. T promotes downstream physiological processes via functional androgen receptors (ARs), which are located in almost all tissues, including the breast, heart, blood vessels, gastrointestinal tract, brain, bladder, uterus, vagina, ovaries, skin, bone, bone marrow, muscle, joints, and adipose tissue.10 Use of the validated menopause rating scale (MRS) questionnaire has yielded objective evidence that T treatment significantly reduces symptoms in pre- and postmenopausal women.11 The clinical syndrome of TD in women has been associated with similar medical conditions to their male counterparts (see above). By the age of 40, women have reduced their production of T and many are T deficient with associated symptoms (see Figure 1).12
Figure 1.
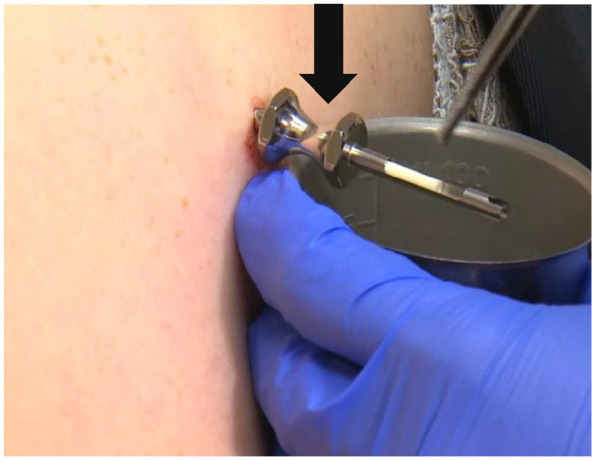
Pellet insertion. The stainless steel trocar (as indicated by the arrow) with the T pellet loaded is ready for deployment. The full procedure can be found at www.biotemedical.com. The insertion site was usually the upper gluteal area or the lower abdomen. Men were instructed to avoid strenuous exercise that involved the gluteal muscles, baths and swimming for 7 days. Women were given the same restrictions for a total of 3 days. The longer time for gluteal rest in males was secondary to the larger number and volume of pellets men receive to achieve optimal levels. Women with a uterus were prescribed micronized progesterone capsules to be taken nightly to reduce the incidence of uterine bleeding from the E pellets. E, estrogen; T, testosterone.
Treatment delivery modalities for T therapy in men and women
Human-identical T is not effectively absorbed via oral administration, and synthetic T (e.g., methyl T) is characterized by poor absorption and poor bioavailability.13 Various strategies for T delivery have been used, including parenteral injections, patches, creams, and pellets. Parenteral injections of synthetic T lead to very high spikes in T levels and large unpredictable variations in blood levels, leading to volatility and an increase in adverse effects,14 and the use of synthetic T is associated with an increased risk of hepatotoxicity.15 Furthermore, the intramuscular injection of T cypionate and T enanthate, which are esters in an oil base, promotes tissue granulomas, allergic reactions, and fluctuations in serum T levels.16 In an attempt to avoid the variability of transdermal absorption seen with creams, a T patch was developed and studied. In 2004, the United States (US) Food and Drug Administration (FDA) declined to approve a T patch, on the basis of a 30% rate of side effects in the submitted prospective study.17
Neither of these treatment modalities would be ideal for long-term hormone optimization, and their acceptability limits their usefulness. With better bio-availability and predictable absorption reported with T sub-cutaneous pellet implants, in order to fill the gap in knowledge in the world literature, this study was performed to test the long-term safety and patient retention rates of this route of administration of T. Studies have shown that oral estrogen (E) therapies likewise have a poor long-term continuation rates and higher risk than non-oral routes of administration. About 35% of users of oral E replacement therapy (ERT) patients stopped their therapy after the first prescription, and 76–81% of the women had stopped by the end of the third year.18 This study also looked at the long-term safety and patient retention rates of this route of administration of estradiol.
Sub-cutaneous hormone implant therapy has been shown promise in terms of treatment efficacy and adverse events. T implant therapy was first utilized 1930s,19 and subcutaneous hormone pellet implants were first applied in clinical practice in 1949 when Greenblatt introduced estradiol implants as a hormone replacement therapy (HRT) for hysterectomy patients.20 This form of therapy has not received much attention in the medical community or in scientific investigations in the US. This occurred presumably because of the widespread use of equine E (Premarin). However, hormone pellets continued to be utilized in Europe and Australia. In the 1990s, subcutaneous pellet therapy was re-established in the US, most likely due to a pharmacokinetic publication that clearly delineated how the hormones in the pellets were absorbed and how they offered predictable absorption and bioavailability.21 Hormone pellet implants develop a capillary network about them, and their absorption is based on cardiac output. Stated simply, the greater your cardiac output through exercise or stress, the more of your needed hormones will be absorbed. This is in stark contrast to injectables and their oil base, which allows for only time release of the hormones. The rates of adverse events associated with various T delivery systems are significantly higher (creams and gels >10%, injections >10%, and patches >30%) than those associated with implant therapy, limiting the applicability of these other delivery systems.
Context for current study
One of the major advantages of pellet therapy over other forms of treatment is its convenience, with few annual visits required to achieve normalization of hormone levels. Despite the long history of the use of T and estradiol implants as HRTs, aside from two reports by one research group describing 13 years of use of fused crystalline T pellets,22,23 there is no prospective outcome data on compressed pellets containing either T or estradiol for hormone optimization in men and women. Thus, we evaluated continuation rates and adverse events that occurred during therapy with T and E sub-cutaneous implant therapy. To this end, we retrospectively assessed adverse events in our proprietary tracking program to evaluate if our methodology offered a safe modality to deliver T and E to both males and females as compared with historic side-effect profile of other deliver modalities.
Materials and methods
BioTE medical
BioTE® Medical is a comprehensive training center in Las Colinas, TX, USA, that has trained and certified more than 5000 practitioners in hormone optimization and subcutaneous hormone pellet therapy, including physicians and nurse practitioners, in 50 states of the US and in Puerto Rico. Additional training was provided to the practitioners and their staff in order to enable data to be collected in a consistent way.
Ethics statement
The study was submitted to the Integreview Institutional Review Board (IRB), which classified it as exempt from IRB approval (Integreview Protocol #001) because of the retrospective evaluation of deidentified data. Informed consent for the study was waived by the IRB committee; however, informed consent for the procedure was obtained by the practitioner at the prior to the procedure.
Patient prescreening
Males and females older than 25 years of age were included in the study. There were no exclusions for prior hormone use, body mass index (BMI), smoking history, or other co-morbid medical conditions. Patients who could benefit from subcutaneous hormone optimization with pellets were identified by means of validated questionnaires similar to the MRS and androgen deficiency in the aging male (ADAM).11,24 Although the MRS is not indicated for premenopausal women, the symptoms described in the questionnaire overlap with those that women experience during the premenopausal period, making this questionnaire appropriate for our purposes. The questionnaires were administered by trained medical assistants or nurses. TD was confirmed with laboratory panels that evaluated follicle-stimulating hormone (FSH), estradiol, total T, vitamin D, and a thyroid panel [thyroid stimulating hormone (TSH), free T4, free T3, thyroid peroxidase (TPO) antibodies] in females and total and free T, hematocrit (Hct), prostate-specific antigen (PSA), estradiol, vitamin D, and a thyroid panel (as above) for men. The laboratory tests were performed by three laboratories (LabCorp, Quest, and CPL), which used similar methodologies [liquid chromatography–mass spectrometry (LCMS) or chemical luminescence] with similar reference ranges (15–70 ng/dl) and males (300–1000 ng/dl). Although free T was measured by the same methodology, the reference ranges varied by laboratory. Interpretation was performed by the patient medical care provider.
Male dosing
A proprietary dosing algorithm was used to guide the individualized dose each patient received. This algorithm was based on T levels, age, weight, demographics, and comorbidities.
Female dosing
A proprietary dosing algorithm was used to guide the individualized dose each patient received. This algorithm was based on T levels, age, weight, demographics, and comorbidities.
Pellet implantation procedure
All procedures were performed by certified BioTE® practitioners. T and E implants were manufactured by two 503b outsource pharmacies (Anazao Health and Apothecary Health). They were made in sterile rooms using United States Pharmacopeia (USP) T and estradiol powder and steric acid as a binding agent. The powder was compressed using an automated pellet press and the resulting pellet sterilized with electronic beam. A BioTE® patented stainless-steel trocar, obturator, and blunt plunger were used to administer the pellets (Figure 2).
Figure 2.
Changes in androgen levels in adult females.
Patient follow up
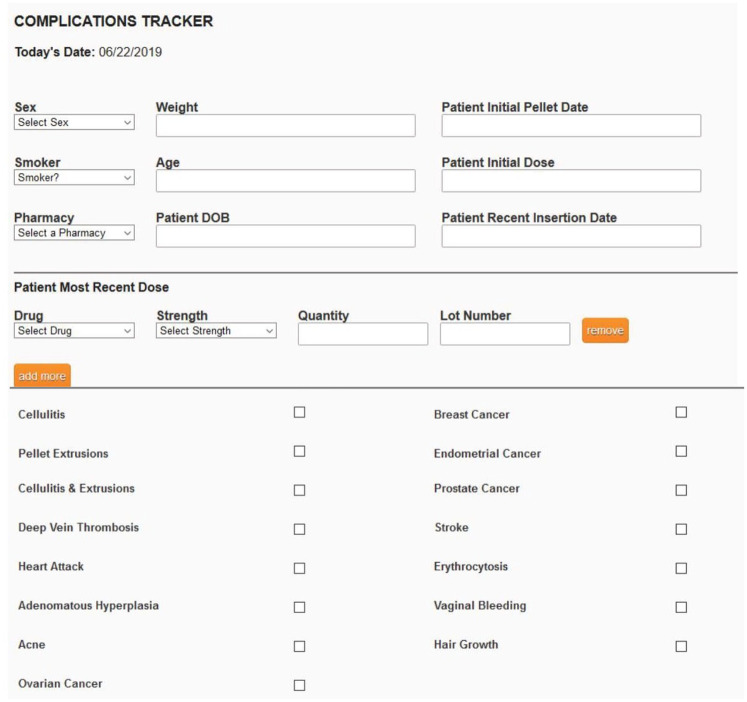
After the first pellet implantation, patients were followed up at 4–6 weeks to assess symptom resolution and re-evaluate serum hormone levels. The BioTE method has established target postinsertion T ranges were 150–250 ng/dl for women and 900–1100 ng/dl for men. The next round of pellet therapy was scheduled by the patients based on the return of symptoms. On average, women returned for the next round of pellet therapy at 3–4 months after the procedure, and men returned at 4–5 months. Annually after the first pellet insertion, the laboratory values of the serum hormones included in the panel performed prior to the first insertion were evaluated. Patients were given postprocedural instruction sheets with guidance to call their practitioner in case of pellet extrusion or signs of cellulitis, bleeding, or other complications. Practitioners were asked to report the complications and secondary responses of their patients including demographic data, dosing, where the pellets were manufactured, and lot numbers to BioTE Medical electronically using BioTracker® (Figure 3) every 2 weeks during the study period. The proprietary program, BioTracker®, was used to track this information for later evaluation (Figure 3). Nonreporting practitioners were identified and asked to update their data and patients were included in the study.
Figure 3.
BioTracker® (BioTE® Medical proprietary compliance software).
Data extraction
From 2012 through May of 2019, records were analyzed retrospectively for each patient and each pellet implantation procedure. The patient records included demographic data; MRS and ADAM questionnaire responses; laboratory evaluations of FSH, estradiol, total T, vitamin D, and a thyroid panel for females and total and free T, Hct, PSA, estradiol, vitamin D, and a thyroid panel for males; any medical co-morbidities, and details of the implantation procedure including the dosage and lot numbers of the pellets. There were no exclusions related to BMI or smoking history. Thus, while some patients were smokers, it was not one of the inclusion/exclusion criteria. In addition, follow-up visit information, including hormone levels, side effects, and complications, was included in the medical records.
The continuation rate is defined as the incidence of a patient undergoing an implant procedure to have a subsequent implant procedure within 12-months of the initial procedure. BioTracker® preserved the database for the retrospective analysis.
Results
Patients
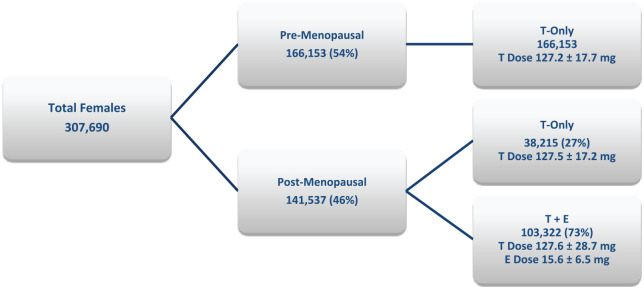
The BioTE® online proprietary password-protected dosing site (dosingsite.com) was used to assist in the implementation of the proper dose of the pellets and the individualization of therapy. The dosing was based on a combination of demographic characteristics, symptoms, and laboratory findings. Over the 7 years in which data were collected, 1,204,012 subcutaneous implant procedures were performed in 376,254 patients; 85% of the procedures (1,018,516) were performed in females, and 15% (185,496) were performed in males. Of the females, 54% were premenopausal, and 46% were postmenopausal (Figure 4). The age range of the women was 25–92 years and that of men was 35–85 years. TD has many causes, the most common etiology is the age-related decline in both men and women. Many of the women were on birth control pills (BCPs) or HRT (oral, creams, and patches) at the onset of the study.
Figure 4.
Dosing comparison among female patients (mean ± 1 SD).
E, estrogen; SD, standard deviation; T, testosterone.
Continuation rate
More than 70% of providers complied, encompassing more than 85% of the total procedures reported during the study period. To evaluate continuation rates, we used data submitted through BioTracker® to calculate the continuation rate of pellet use among the individuals over the 7 years of the study. Our data showed that 43% patients discontinued pellet therapy after the initial pellet insertion. The specific reasons for high levels of discontinuation after the initial pellet insertion were not captured as part of the data capture. The overall continuation rate after two pellet insertions was 93.3%; 89.2% in men, 94.3% in women, 94.9% in pre-menopausal women and 93.6% in post-menopausal women (Table 1). Completing two pellet insertions exponentially increased the continuation rate.
Table 1.
Number of implants and procedures per patient (patients that continued pellet therapy after initial pellet insertion).
| Mean (SD) | |
|---|---|
| Age, years | |
| Males | 55.6 ± 8.3 years |
| Females | 58.5 ± 8.6 years |
| Implants per procedure | |
| Males | 9.2 ± 2.1 |
| Females | 2.1 ± 0.6 |
| Procedures per patient | |
| Males | 11.3 ± 3.4 |
| Females | 14.5 ± 4.7 |
| Continuation rate (%) | 93.3 |
| Males (%) | 89.2 |
| Females (%) | 94.3 |
| Pre-menopausal (%) | 94.9 |
| Post-menopausal (%) | 93.6 |
SD, standard deviation.
Complications
Overall complications
From the 1,204,012 procedures, adverse effects (one or more complications) were observed in only 1% of cases (Tables 2–4). The three most common reported complications were male extrusions (4786 events, <3%), female extrusions (3621 events, <1%), male cellulitis/infection (765 events, <1%), female cellulitis/infection (285 events, <1%), and bleeding (<1%). Among the patients’ demographic characteristics, only increased activity in the first week after pellet implantation and a higher number of inserted pellets significantly predicted complications, and these factors increased the likelihood of cellulitis and extrusions. In addition, poor adherence to the prescribed regimen of micronized progesterone was a predictor of increased vaginal bleeding in postmenopausal female patients.
Table 2.
Complication data: insertions (2012–2019).
| Complication type | Gender | Complications (n) | Procedures with complications (%) |
|---|---|---|---|
| Pellet extrusions | Female | 3621 | 0.356a |
| Male | 4786 | 2.580a | |
| Cellulitis | Female | 285 | 0.028a |
| Male | 765 | 0.412a | |
| Total complications | 9457 | 0.785 | |
| Total procedures | 1,204,012 | ||
Calculated based on female/male procedures as applicable
Table 3.
Complication data: male patients (2012–2019).
| Complication type | Complications (n) | Patients with complications (%) |
|---|---|---|
| Myocardial infarction (non-fatal) | 46 | 0.067 |
| Prostate cancer | 37 | 0.054 |
| Deep vein thrombosis | 9 | 0.013 |
| Stroke (non-fatal) | 8 | 0.012 |
| Total complications | 100 | |
| Total male patients | 68,564 |
Table 4.
Complication data: female patients (2012–2019).
| Complication type | Complications (n) | Patients with complications (%) |
|---|---|---|
| Endometrial cancer | 26 | 0.008 |
| Myocardial infarction (non-fatal) | 11 | 0.004 |
| Deep vein thrombosis | 40 | 0.013 |
| Stroke (non-fatal) | 34 | 0.011 |
| Breast cancer | 248 | 0.081 |
| Total complications | 359 | |
| Total patients | 307,690 |
Extrusions
The most common complication was extrusion. Among men, extrusions occurred either very early (4–7 days after insertion) or much later (>60 days after insertion); 66% of the extrusions had only one pellet extruded, and the rest had two or more pellets extruded. The average time from the procedure to the first extrusion was 65 days, and the range was 4–150 days. Patients with late extrusions had no notable differences in age, activity, weight, percentage body fat, or occupation compared with those with early extrusions. Extrusions in men declined by half after the introduction of the semipermeable membrane as part of the postoperative dressing requirement. We were experiencing 4% extrusions when the dressing was simply 4 × 4 gauze and tape.
Among females, extrusions were exceedingly rare, occurring in less than 0.1% of individuals. Most of these individuals experienced extrusions after 42 days.
Cellulitis
The next most frequent complication was cellulitis. This complication occurred in less than 1% of the individuals undergoing pellet insertions. Males with a BMI < 25 were more likely to develop cellulitis compared with those with a higher BMI. The results were statistically significant at the 95% confidence level (single sample t-test was used to compare the differences between the two male groups).
In women, cellulitis occurred in less than 0.1% of individuals who underwent pellet insertions.
Bleeding
The BioTracker® was not used to track bleeding during the procedure, but individuals returning to the office at a later time for bleeding were reported using the BioTracker® program. Operative site bleeding was exceedingly rare, with a rate of 0.1% of total pellet insertion procedures.
Manual pressure for a few minutes controlled any oozing that may have occurred at the site. Overall insertion procedures had less than 5 ml of blood loss. Blood loss was increased somewhat in individuals taking nonsteroidal analgesics, aspirin, anti-coagulants, or omega-3 fish oil supplements, in those with a higher BMI, and in those who received a greater number of T 200 mg pellets. Vaginal bleeding occurred in approximately 1–2% of the postmenopausal patients who received estradiol. The vaginal bleeding was attributed to non-adherence in taking the oral micronized progesterone prescribed in the majority of patients reported on Bio-Tracker. They were therefore not included in the complication tables above. We did track and report the cases of endometrial carcinoma.
Secondary reactions
Other secondary reactions that were anticipated but not reported were histamine reactions to the preservative in the local anesthetics, and the patients responded well to antihistamines. A hyperemic area at the insertion site occurred rarely. There were no allergic reactions to the pellets, and no keloids were reported. There was some hyperpigmentation at the incision site in many of the individuals, but this was a secondary reaction that was not followed or reported.
Subdermal fibrosis was identified by palpation and resolved spontaneously, usually by the next pellet insertion. Alternating sides of the gluteal area and/or abdomen for injections allowed for complete resolution and avoided delaying the next insertion.
Discussion
In order to evaluate continuation rates and adverse events that occurred during hormone implant therapy in men and women, we have described a retrospective analysis of a very large number of T and estradiol pellet procedures performed in men and women and report very low complication rates. Over the 7-year period of the study, 1,204,012 subcutaneous implant procedures were performed in 376,254 people, with an overall complication rate of less than 1%. Our observational study provides confirmation of the safety and, indirectly, the efficacy of pellet therapy.
The literature discussing TD in women is sparse compared with that published for TD in men. Many more studies have reported on the treatment of menopausal symptoms using estrogen. In the Cardozo study of subcutaneous pellet implants,25 there was a marked response to treatment. Hot flashes improved in all patients, with a reduction in depression among 99% of cases and improvements in libido in 92% of cases. In both pre- and postmenopausal patients receiving T implants, the median 4-week postinsertion serum level was 299 ng/dl, which was 4–6 times the upper limit of normal for endogenous production. After 1 year, there were no adverse drug reactions.26 In our study, the fact that 54% of the women were premenopausal patients demonstrates the extent of TD in premenopausal women and the demand for treatment. This observation is consistent with that of Davison et al. showing that total T levels drop by 50% in women by the age of 40 years.12 Moreover, the lack of fluctuating serum levels and increase in symptom-free days contributed to making pellet therapy the preferred modality over others.27 Our study confirms that pellet therapy has a high efficiency for HRT and that utilizing the BioTE method of dosing and administration is safest due to the low complication rate.
There are several minor limitations of this study. First, the self-reporting of the certified providers. This allowed for selection bias and possible underreporting. The large sample size and number of procedures mitigates this limitation. The self-reporting of events by the patients also could allow for selection bias and under reporting; however, the common secondary reactions to pellet therapy usually require an intervention and office visit, also mitigating this limitation.
Because our study focused on complications from pellet therapy, some secondary reactions that occur with T use in women, such as body hair/facial hair, acne, and hair thinning, were not analyzed, representing another limitation of this study.
With the high continuation rate seen in our study, it is reasonable to hypothesize that the incidence of serious secondary reactions may be low. In addition, the results of studies conducted over the past 80 years regarding this therapy and its use on five continents have been uniformly positive with regard to the benefits versus the risks of T therapy.20,26
In summary, this study is the largest retrospective study to evaluate the continuation and complication rates of both T and estradiol pellet implants. This study of 7 years of extensive utilization of subcutaneous hormone pellet therapy demonstrates that TD and ED can be safely treated with T and estradiol pellets. The very high continuation rate supports the ease of the procedure, its tolerability, and its safety. The results also suggest that, through advanced training and certification with BioTE, experienced practitioners have been able to limit adverse events and improve the acceptability of subcutaneous hormone implant therapy for hormone optimization. These findings, and the complication rate below 1%, support pellet therapy as a modality that lends itself to long-term human-identical T and E optimization therapy. Up to this point, regimens for disease management have consisted of medications with multiple side effects, including reducing QOL; however, the high continuation rate of pellet therapy in our study provides an opportunity for disease prevention in these patients.
Importantly, the safety and efficacy data presented here should be used to motivate more studies on which chronic diseases could be prevented and treated by resolving TD in men and women.
Footnotes
Author contribution(s): GD: Conceptualization; Formal analysis; Writing-original draft; Writing-review & editing.
Conflict of interest statement: GD is the Founder and Chief Medical Officer of BioTE Medical.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials: Confidential access to primary data and proprietary methods will be considered upon receipt of specific requests.
ORCID iD: Gary S. Donovitz  https://orcid.org/0000-0002-8727-2388
https://orcid.org/0000-0002-8727-2388
References
- 1. Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: international expert consensus resolutions. Mayo Clin Proc 2016; 91: 881–896. [DOI] [PubMed] [Google Scholar]
- 2. Pantalone KM, Faiman C. Male hypogonadism: more than just a low testosterone. Cleve Clin J Med 2012; 79: 717–725. [DOI] [PubMed] [Google Scholar]
- 3. Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004; 27: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 4. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J 2015; 36: 2706–2715. [DOI] [PubMed] [Google Scholar]
- 5. Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab 2012; 97: 2050–2058. [DOI] [PubMed] [Google Scholar]
- 6. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011 [published correction appears in JAMA Intern Med. 2013 Aug 12;173(15):1477]. JAMA Intern Med 2013; 173: 1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimitrakakis C, Zhou J, Bondy CA. Androgens and mammary growth and neoplasia. Fertil Steril 2002; 77: 26–33. [DOI] [PubMed] [Google Scholar]
- 8. Donovitz G, Schwartz E, Miller C, et al. Testosterone insufficiency and treatment in women: international expert consensus. Ponce, PR: MSP Medicina Y Salud Publica, https://medicinaysaludpublica.com/testosterone-insufficiency-and-treatment-in-women-international-expert-consensus/ (2019, accessed 4 September 2019). [Google Scholar]
- 9. Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab 2019; 104: 4660–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson JD. Role of dihydrotestosterone in androgen action. Prostate Suppl 1996; 6: 88–92. [PubMed] [Google Scholar]
- 11. Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas 2011; 68: 355–361. [DOI] [PubMed] [Google Scholar]
- 12. Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005; 90: 3847–3853. [DOI] [PubMed] [Google Scholar]
- 13. Greenspan FS, Gardner DG, Shoback DM. Greenspan’s basic & clinical endocrinology. New York, NY: Lange Medical Books/McGraw-Hill, 2004. [Google Scholar]
- 14. Layton JB, Meier CR, Sharpless JL, et al. Comparative safety of testosterone dosage forms [published correction appears in JAMA Intern Med. 2015 Jul; 175: 1248]. JAMA Intern Med 2015; 175: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson FL, Lerner KG, Siegel M, et al. Association of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet 1972; 2: 1273–1276. [DOI] [PubMed] [Google Scholar]
- 16. Aakvaag A. Testosterone action, deficiency, substitution edited by Nieschlag E., Behre H. M. Editorial assistant S. Nieschlag. Springer Verlag, New York, Berlin, Heidelberg, 1990. Int J Androl 1992; 15: 282. [Google Scholar]
- 17. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000; 343: 682–688. [DOI] [PubMed] [Google Scholar]
- 18. Ettinger B, Li D-K, Klein R. Continuation rates with postmenopausal hormone therapy. Menopause 1996; 3: 185–189. [Google Scholar]
- 19. Hamilton JB. Treatment of sexual underdevelopment with synthetic male hormone substance. Endocrinol 1937; 21: 649–654. [Google Scholar]
- 20. Greenblatt RB, Suran RR. Indications for hormonal pellets in the therapy of endocrine and gynecic disorders. Am J Obstet Gynecol 1949; 57: 294–301. [DOI] [PubMed] [Google Scholar]
- 21. Jockenhövel F, Vogel E, Reinhardt W, et al. Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res 1997; 2: 293–298. [PubMed] [Google Scholar]
- 22. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 1990; 71: 216–222. [DOI] [PubMed] [Google Scholar]
- 23. Handelsman DJ. Clinical pharmacology of testosterone pellet implants. In: Nieschlag E, Behre HM. (eds) Testosterone. Berlin, Heidelberg: Springer, 1998. [Google Scholar]
- 24. Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative adam questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res 2010; 22: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cardozo L, Gibb DMF, Tuck SM, et al. The effects of subcutaneous hormone implants during the climacteric. Maturitas 1984; 5: 177–184. [DOI] [PubMed] [Google Scholar]
- 26. Glaser R, York AE, Dimitrakakis C. Incidence of invasive breast cancer in women treated with testosterone implants: a prospective 10-year cohort study. BMC Cancer 2019; 19: 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glaser RL, Dimitrakakis C. Reduced breast cancer incidence in women treated with subcutaneous testosterone, or testosterone with anastrozole: a prospective, observational study. Maturitas 2013; 76: 342–349. [DOI] [PubMed] [Google Scholar]