Abstract
Background and purpose
Magnetic resonance imaging (MRI) of the brain in scrub typhus meningoencephalitis is non-specific, and in the majority of the cases, conventional MRI fails to detect any abnormality. However, autopsy reports depict central nervous system involvement in almost all patients. There is therefore a need for research on the quantitative assessment of brain parenchyma that can detect microstructural abnormalities. The study aimed to assess the microstructural integrity changes of scrub typhus meningoencephalitis by using different diffusion tensor imaging (DTI) parameters.
Methods
This was a retrospective analysis of scrub typhus meningoencephalitis. Seven patients and seven age- and sex-matched healthy controls were included. Different DTI parameters such as apparent diffusion coefficient (ADC), fractional anisotropy (FA), relative anisotropy (RA), trace, volume ratio (VR) and geodesic anisotropy (GA) were obtained from six different regions of subcortical white matter at the level of the centrum semiovale. Intergroup significant difference was determined by one-way analysis of variance followed by Tukey’s post hoc test. Receiver operating characteristic curves were constructed to determine the accuracy of the DTI matrices.
Results
There was a significant decrease in FA, RA and GA as well as an increase in ADC and VR in the subcortical white matter in patients with scrub typhus meningoencephalitis compared to controls (p < 0.001). The maximum sensitivity of the DTI parameters was 85.7%, and the maximum specificity was 81%.
Conclusion
There was an alteration of subcortical white-matter integrity in scrub typhus meningoencephalitis that represents the axonal degeneration, myelin breakdown and neuronal degeneration. DTI may be a useful tool to detect white-matter abnormalities in scrub typhus meningoencephalitis in clinical practice, particularly in patients with negative conventional MRI.
Keywords: Scrub typhus, meningoencephalitis, diffusion tensor imaging, anisotropy
Introduction
The scrub typhus is a zoonotic disease caused by Orientia tsutsugamushi, classified taxonomically as bacteria. Approximately one million new cases are detected annually, and about one billion people may be at risk for this disease.1 Still, it remains underdiagnosed because of its non-specific clinical presentation.2 Meningoencephalitis is a common complication of scrub typhus.3 Magnetic resonance imaging (MRI) findings of scrub typhus meningoencephalitis have been reported sparingly, limited to case reports only.4–6 Moreover, even in patients who are neurologically symptomatic, the majority of conventional MRI does not demonstrate any abnormality, and the MRI findings are also non-specific.4,7,8 On the other hand, central nervous system (CNS) involvement is depicted at autopsy in almost all patients.9 There is therefore a need for research on the quantitative assessment of brain parenchyma that can detect microstructural abnormalities that may be missed by conventional MRI.
Diffusion tensor imaging (DTI) is an ideal method for the quantitative assessment of microstructural neurovascular events that rely on the diffusion of water molecules. The quantitative assessment of DTI and apparent diffusion coefficient (ADC) has been used to demonstrate white-matter abnormality in CNS vasculitis, alcohol dependence and so on.10–12 The study aimed to assess the microstructural integrity changes of scrub typhus meningoencephalitis by using different DTI parameters.
Methods
The data of scrub typhus patients from April 2013 to January 2020 were reviewed retrospectively. A positive Weil–Felix agglutination test or immuno-chromatographic card test for the detection of immunoglobulin M (IgM) antibodies to O. tsutsugamushi with clinical features of meningoencephalitis was included in the study. Meningoencephalitis is defined by the presence of a headache and/or nuchal rigidity, with either altered sensorium or focal neurological deficits. Data that were inadequate to confirm the diagnosis or data where an MRI of the brain with DTI was not available were excluded from the study. An equal number of healthy subjects were also included as controls. Controls were defined as those having a normal-appearing MRI of the brain where an MRI was performed for other reasons. As an institute protocol, MRI were conducted using a 1.5T MAGNETOM® Avanto MR Scanner (Siemens, Germany).
Data analysis
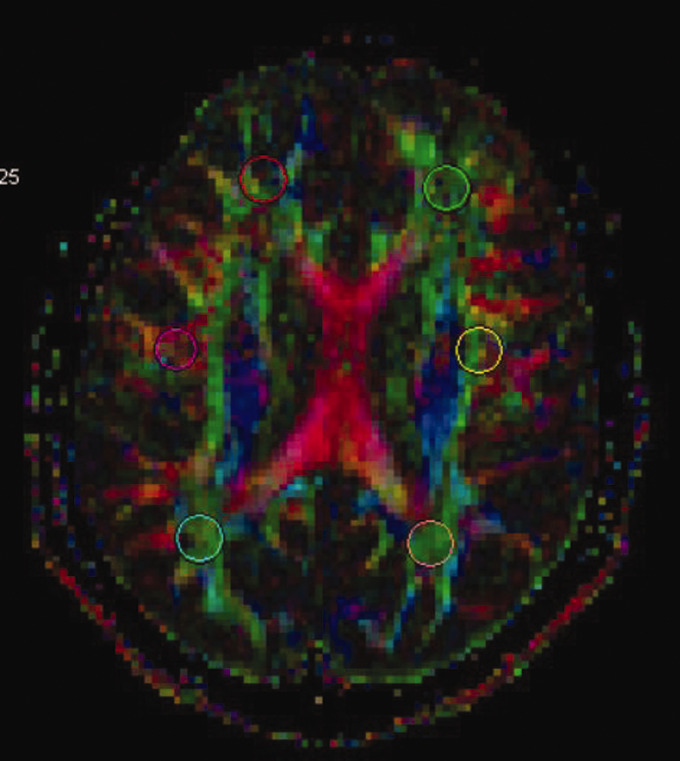
T2WI/FLAIR, GRE and DWI were inspected visually for any parenchymal lesion. DTI images were analysed with the Siemens Neuro 3D Task Card (Siemens, Germany). The regions of interest with 30 volumes were drowned over the diffusion tensor images in six areas of bilateral subcortical white matter in the anterior, central and posterior regions of the centrum semiovale (Figure 1). Values were collected for fractional anisotropy (FA), relative anisotropy (RA), apparent diffusion coefficient (ADC), trace, volume ratio (VR) and geodesic anisotropy (GA).
Figure 1.
The six regions of interest made in the anterior, central and posterior subcortical white matter in the centrum semiovale.
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows v23 (IBM Corp., Armonk, NY). Analysis of variance (ANOVA) was performed to determine the overall significant difference of FA, RA, ADC, trace, VR and GA in scrub typhus patients compared to healthy controls. A p-value of <0.05 was considered significant. Tukey’s post hoc tests were performed to determine honest significant differences. The significance level was set at p < 0.05.
Finally, receiver operating characteristic (ROC) curve was constructed to determine the accuracy of the DTI parameter. The thresholds for the DTI parameters of interest were identified using the Youden Index. Sensitivity and specificity at designated threshold levels with their 95% confidence interval (CI) were calculated. A p-value of <0.05 was considered significant.
Results
Demographics
Twenty patients fulfilled the criteria of having been diagnosed with scrub typhus meningoencephalitis. However, only seven patients had an MRI scan of the brain with a DTI study. The mean age of the seven patients was 29.42 years, and included five males and two females. An equal number of age- and sex-matched healthy participants with a normal-appearing MRI scan were included as the control group. The mean age of the healthy control group was 33 years, and also included five male patients and two female patients. The indications for the MRI scan of the brain of the healthy controls included the following: occipital neuralgia (one patient), headache (two patients), hemi-facial spasm (one patient), seizure disorder (one patient) and pituitary microadenoma (one patient).
Conventional MRI findings
The T2WI/FLAIR imaging findings are summarised in Table 1. The MRI was negative in four patients, and three patients had non-specific findings.
Table 1.
The Laboratory test and conventional MRI findings of the scrub typhus meningoencephalitis patients.
| Sl No | Test positive | Conventional MRI findings |
|---|---|---|
| 1 | IgM at immuno-chromatographic card test | Normal |
| 2 | IgM immuno-chromatographic card test | Gyral swelling, lacunar infarcts, vacuities |
| 3 | IgG immuno-chromatographic card test | Normal |
| 4 | IgM immuno-chromatographic card test | Normal |
| 5 | IgM immuno-chromatographic card test | Bilateral basal ganglia and white-matter infarcts, right frontal subcortical haemorrhage |
| 6 | IgM immuno-chromatographic card test | Bilateral white-matter infarcts, meningitis, vacuities |
| 7 | Weil–Felix agglutination test | Normal |
MRI: magnetic resonance imaging; IgM: immunoglobulin M.
DTI findings
Intergroup significant difference
A one-way ANOVA test was conducted that revealed a significant difference in the FA (p < 0.001), RA (p < 0.001), ADC (p < 0.001), VR (p < 0.001) and GA (p < 0.0001) values between the healthy controls and scrub typhus meningoencephalitis patients (Table 2).
Table 2.
A one-way ANOVA test for intergroup significant difference.
| Sum of squares | df | Mean square | F | p | ||
|---|---|---|---|---|---|---|
| FA | Between groups | 0.593 | 2 | 0.296 | 35.475 | 0.000 |
| Within groups | 0.677 | 81 | 0.008 | |||
| Total | 1.270 | 83 | ||||
| RA | Between groups | 0.533 | 2 | 0.267 | 32.386 | 0.000 |
| Within groups | 0.667 | 81 | 0.008 | |||
| Total | 1.201 | 83 | ||||
| ADC | Between groups | 0.298 | 2 | 0.149 | 22.039 | 0.000 |
| Within groups | 0.548 | 81 | 0.007 | |||
| Total | 0.846 | 83 | ||||
| TRACE | Between groups | 0.000 | 2 | 0.000 | 0.035 | 0.965 |
| Within groups | 0.063 | 81 | 0.001 | |||
| Total | 0.063 | 83 | ||||
| VR | Between groups | 0.323 | 2 | 0.161 | 26.557 | 0.000 |
| Within groups | 0.492 | 81 | 0.006 | |||
| Total | 0.815 | 83 | ||||
| GA | Between groups | 1.850 | 2 | 0.925 | 29.527 | 0.000 |
| Within groups | 2.537 | 81 | 0.031 | |||
| Total | 4.387 | 83 | ||||
ANOVA: analysis of variance; FA: fractional anisotropy; RA: relative anisotropy: ADC: apparent diffusion coefficient; VR: volume ratio; GA: geodesic anisotropy.
Honest significant difference between healthy control versus positive MRI and healthy control versus negative MRI
On further application of Tukey’s post hoc test, there was a significant increase in ADC values (p < 0.001) and VR values (p < 0.001) of MRI-positive and MRI-negative patients compared to healthy controls. On the other hand, the FA (p < 0.001), RA (p < 0.001) and GA (p < 0.0001) values of MRI-positive and MRI-negative patients were significantly decreased compared to healthy controls (Table 3).
Table 3.
Tukey’s post hoc tests for honest significant difference between healthy control versus positive MRI and healthy control versus negative MRI.
| Dependent variable | Mean difference (I–J)a | Standard error | p | 95% CI |
|||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| FA | Control | +MRI | 0.20224603 | 0.02575125 | 0.000 | 0.1407637 | 0.2637284 |
| –MRI | 0.12788214 | 0.02338974 | 0.000 | 0.0720380 | 0.1837262 | ||
| RA | Control | +MRI | 0.19294183 | 0.02556686 | 0.000 | 0.1318997 | 0.2539839 |
| –MRI | 0.11918571 | 0.02322225 | 0.000 | 0.0637415 | 0.1746299 | ||
| ADC | Control | +MRI | –0.15233889 | 0.02316308 | 0.000 | –0.2076418 | –0.0970359 |
| –MRI | –0.06372917 | 0.02103891 | 0.009 | –0.1139606 | –0.0134978 | ||
| VR | Control | +MRI | –0.15265952 | 0.02195707 | 0.000 | –0.2050831 | –0.1002360 |
| –MRI | –0.08693036 | 0.01994349 | 0.000 | –0.1345464 | –0.0393143 | ||
| GA | Control | +MRI | 0.36232778 | 0.04985725 | 0.000 | 0.2432912 | 0.4813643 |
| –MRI | 0.21547500 | 0.04528509 | 0.000 | 0.1073547 | 0.3235953 | ||
aThe mean difference is significant at the 0.05 level.
CI: confidence interval.
ROC curve analysis for diagnostic accuracy
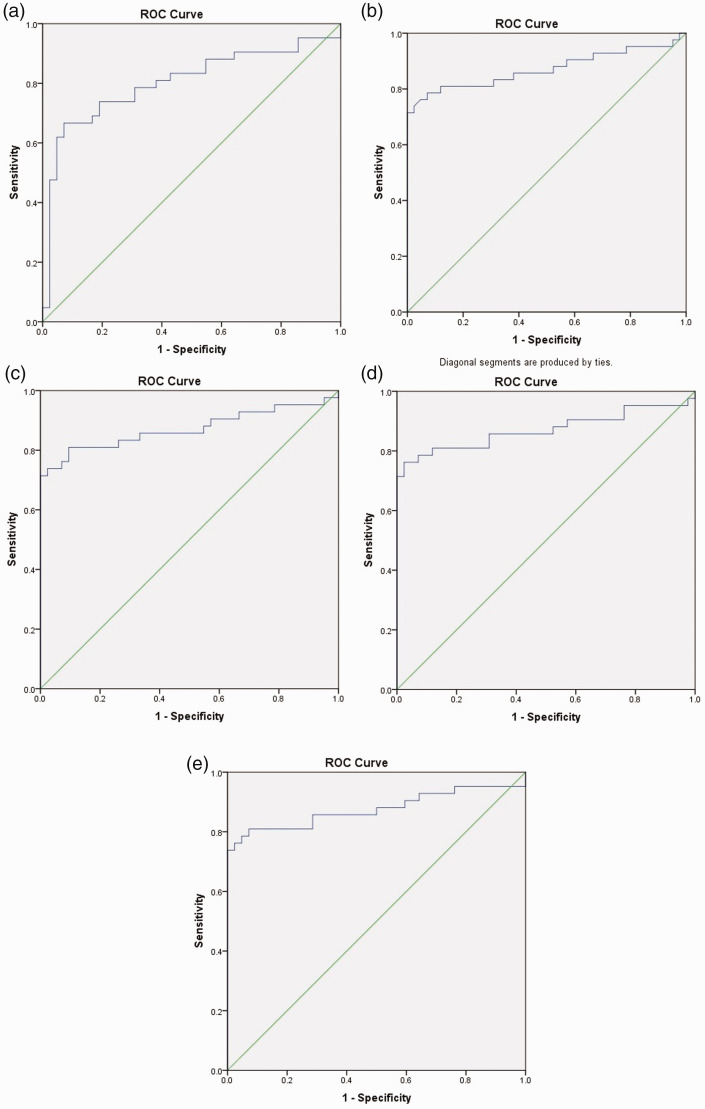
A ROC curve analysis for each of the DTI parameters (ADC, FA, RA, VR and GA) to determine the accuracy for assessment of the white-matter abnormality in scrub typhus meningoencephalitis is given in Figure 2 and Table 4. The best ROC area under the curve (AUC) was obtained for GA with a value of 0.876 (p < 0.001). Applying the cut-off value of FA, RA, ADC, VR and GA obtained from the ROC analysis, DTI abnormality in six of the seven patients was detected, indicating white-matter abnormality (Figure 3). These DTI values could not be identified as one of the four patients whose conventional MRI was negative.
Figure 2.
(a) Receiver operator characteristic (ROC) curve analysis of the apparent diffusion coefficient of scrub typhus meningoencephalitis to the healthy control. (b) ROC curve analysis of the fractional anisotropy of scrub typhus meningoencephalitis to the healthy control. (c) ROC curve analysis of the relative anisotropy of scrub typhus meningoencephalitis to the healthy control. (d) ROC curve analysis of the volume ratio of scrub typhus meningoencephalitis to the healthy control. (e) ROC curve analysis of the geodesic ratio of scrub typhus meningoencephalitis to the healthy control.
Table 4.
Receiver operating characteristic curve analysis with area under the ROC curve to determine the accuracy, sensitivity, and specificity of diffusion tensor imaging parameters for assessment of subcortical white-matter in scrub typhus meningoencephalitis.
| AUC | 95% CI | p-Value | Optimal cut-offa | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| ADC | 0.803 | 0.702–0.903 | 0.000 | 0.756 | 76.2% | 69% |
| FA | 0.871 | 0.786–0.956 | 0.000 | 0.400 | 85.7% | 50% |
| RA | 0.871 | 0.786–0.956 | 0.000 | 0.350 | 85.7% | 57.1% |
| VR | 0.870 | 0.784–0.957 | 0.000 | 0.800 | 88.1% | 47.6% |
| GA | 0.876 | 0.791–0.961 | 0.000 | 0.561 | 81% | 81% |
aCut-off values for continuous variables were obtained using the Youden Index.
AUC: area under the curve.
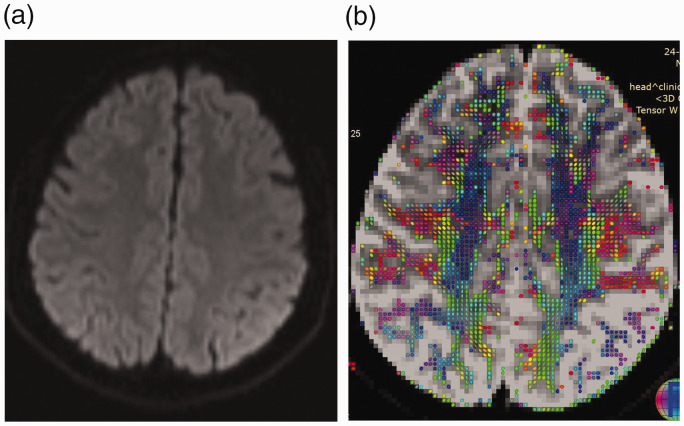
Figure 3.
(a) Diffusion-weighted image of a patient with scrub meningoencephalitis reveals no abnormality. (b) Diffusion tensor imaging shows significant reduction of white-matter tracts in the left parietal area compared to the right side.
Discussion
Scrub typhus is a public health problem in the Asia Pacific region.13 Meningoencephalitis, a potentially fatal complication of scrub typhus, is known to occur. The exact nature of the interaction of scrub typhus or its antigen to human brain parenchyma and pathogenesis is still incompletely understood. Probable hypotheses include: (a) the O. tsutsugamushi is an obligatory intracellular organism, spread through the cerebrospinal fluid via monocytes, and enters via the luminal cell membrane to the endothelium where it replicates and is released into the perivascular space14; (b) it induces microvessel angiitis that predominantly involves the grey matter;14,15 and (c) white-matter involvement is rare and may be due to microinfarcts, a breakdown of the blood–brain barrier or cerebral oedema secondary to vasculitis.5,14
In the present study, we quantitatively assessed the microstructural integrity of the subcortical white matter by using different DTI matrices. The results demonstrate the diffuse alteration of the subcortical white matter integrity, which occurs even in the normal-appearing brain of patients with scrub typhus meningoencephalitis.
ADC describes the magnitudes of water molecule diffusion and reflects the diffusion status in post brain infarction periods.16 Trace provides the total diffusivity of a media.17 FA is the magnitude of anisotropic diffusion. It represents the alterations in the axonal diameter, fibre density or myelin structure.18 RA is a normalised standard deviation that represents the ratio of the anisotropic fragment of the diffusion to the isotropic fragment.19 FA and RA vary between 0 (isotropic diffusion) and 1 (2 for RA; infinite anisotropy). VR is the ratio of the ellipsoid volume to the volume of a sphere of radius λ. VR ranges from 1 (isotropic diffusion) to 0. GA (Riemann distance) measures the distance of a diffusion tensor to the nearest isotropic tensor. GA increases more monotonically than FA. GA may have an advantage over FA for diffusion data with high anisotropy, but it is more susceptible to noise. FA is based on a simple comparison of the tensor eigenvalues. GA, however, measures the deviation of the tensor from the ‘nearest’ isotropic tensor in the associated log-Euclidean metric.
In our study, the overall diffusivity of the scrub typhus patients was equal to the healthy control group. There was no significant difference in trace in both groups. However, the FA, RA and GA were significantly reduced, and GA and ADC values were significantly increased. From this DTI analysis, we can hypothesise that a neurovascular event is a component of scrub typhus meningoencephalitis. We conjecture that the vasculitic process initiated by scrub typhus meningoencephalitis may cause tissue hypoxia, the breakdown of the blood–brain barrier and subsequently vasogenic oedema, as evident by increased ADC. Tissue hypoxia is also responsible for the axonal degeneration and myelin breakdown of the white-matter fibre at the subcortical region, along with the disruption of the neuronal cells.
DTI analysis may be a useful diagnostic tool in clinico-radiological practice to establish the diagnosis of scrub typhus meningoencephalitis, particularly for patients with normal-appearing brain parenchyma, by determining microstructural-level neurovascular events. Moreover, DTI is highly sensitive (88.1%) and specific (81%) for the same.
This study has several limitations. The MRI for scrub typhus meningoencephalitis was performed sparsely, resulting in a small number of cases that could be used in the study. However, we were able to show statistically significant differences in ADC and anisotropic DTI parameters with a valuable conclusion. The DTI study was performed with a 1.5T MRI using a diffusion gradient in 20 orientations. This results in a lower signal-to-noise ratio compared to 3T MRI.20 Similarly, using 30 or more directions would have provided a more accurate diffusion tensor value. One of the cases (case no. 5) had a positive IgM immuno-chromatographic card test for scrub typhus as well as acid-fast bacilli in cerebrospinal fluid. This may result in a false estimation of diffusion tensor from tubercular meningitis.
Conclusion
In conclusion, there is an alteration of the different anisotropic matrices in scrub typhus meningoencephalitis. This represents the vasogenic oedema following microinfarct secondary to the vasculitis induced by scrub typhus meningoencephalitis. Subsequently, there is axonal degeneration and myelin breakdown of the white-matter fibre at the subcortical region, along with disruption of the neuronal cells. Further study of scrub typhus meningoencephalitis is needed in order to predict patient diagnosis and clinical outcome.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Pranjal Phukan https://orcid.org/0000-0001-9210-0217
References
- 1.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 2003; 16: 429–436. [DOI] [PubMed] [Google Scholar]
- 2.Jamil M, Lyngrah KG, Lyngdoh M, et al. Clinical manifestations and complications of scrub typhus: a hospital based study from north eastern India. J Assoc Physicians India 2014; 62: 19–23. [PubMed] [Google Scholar]
- 3.Jamil MD, Hussain M, Lyngdoh M, et al. Scrub typhus meningoencephalitis, a diagnostic challenge for clinicians: a hospital based study from North-East India. J Neurosci Rural Pract 2015; 6: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin CH, Hong HS, Yi BH, et al. CT and MR imagings of semicircular canal aplasia. J Korean Soc Radiol 2009; 61: 9–15. [Google Scholar]
- 5.Yum KS, Na SJ, Lee KO, et al. Scrub typhus meningo-encephalitis with focal neurologic signs and associated brain MRI abnormal findings: literature review. Clin Neurol Neurosurg 2011; 113: 250–253. [DOI] [PubMed] [Google Scholar]
- 6.Dharmasaroja P. A case of subcortical heterotopia presenting with focal motor seizures and sensory loss. Neurol India 2016; 64: 787. [DOI] [PubMed] [Google Scholar]
- 7.Neyaz Z, Bhattacharya V, Muzaffar N, et al. Brain MRI findings in a patient with scrub typhus infection. Neurol India 2016; 64: 788. [DOI] [PubMed] [Google Scholar]
- 8.Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry 2015; 86: 761–766. [DOI] [PubMed] [Google Scholar]
- 9.Jeong YJ, Kim S, Wook YD, et al. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics 2007; 27: 161–172. [DOI] [PubMed] [Google Scholar]
- 10.White ML, Hadley WL, Zhang Y, et al. Analysis of central nervous system vasculitis with diffusion-weighted imaging and apparent diffusion coefficient mapping of the normal-appearing brain. Am J Neuroradiol 2007; 28: 933–937. [PMC free article] [PubMed] [Google Scholar]
- 11.Chua TC, Wen W, Slavin MJ, et al. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol 2008; 21: 83–92. [DOI] [PubMed] [Google Scholar]
- 12.Reijmer YD, Brundel M, De Bresser J, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care 2013; 36: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017; 11: e0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai H, Sohn S, Seong Y, et al. Central nervous system involvement in patients with scrub typhus. Clin Infect Dis 1997; 24: 436–440. [DOI] [PubMed] [Google Scholar]
- 15.Allen AC, Spitz S. A comparative study of the pathology of scrub typhus (tsutsugamushi disease) and other rickettsial diseases. Am J Pathol 1945; 21: 603. [PMC free article] [PubMed] [Google Scholar]
- 16.Shen JM, Xia XW, Kang WG, et al. The use of MRI apparent diffusion coefficient (ADC) in monitoring the development of brain infarction. BMC Med Imaging 2011; 11: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tae WS, Ham BJ, Pyun SB, et al. Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol 2018; 14: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori S. New image contrasts from diffusion tensor imaging: theory, meaning, and usefulness of DTI-based image contrast. Introduction to diffusion tensor imaging. Amsterdam: Elsevier Science, 2007, pp.69–84. [Google Scholar]
- 19.Dong Q, Welsh RC, Chenevert TL, et al. Clinical applications of diffusion tensor imaging. J Magn Reson Imaging 2004; 19: 6–18. [DOI] [PubMed] [Google Scholar]
- 20.Polders DL, Leemans A, Hendrikse J, et al. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J Magn Reson Imaging 2011; 33: 1456–1463. [DOI] [PubMed] [Google Scholar]