Abstract
Introduction
Vessel wall magnetic resonance imaging can improve the evaluation of intracranial atherosclerotic disease. However, pathological validation is needed to improve vessel wall magnetic resonance imaging techniques. Human pathology samples are not practical for such analysis, so an animal model is therefore needed.
Materials and methods
Watanabe heritable hyperlipidemic rabbits and apolipoprotein E knockout rabbits were evaluated against New Zealand white wild-type rabbits. Evaluation of intracranial arteries was performed with vessel wall magnetic resonance imaging and pathological analysis, rating the presence and severity of disease in each segment. Two-tailed t-tests were performed to compare disease occurrence and severity prevalence among rabbit subtypes. Sensitivity and specificity were calculated to assess the diagnostic accuracy of vessel wall magnetic resonance imaging.
Results
Seventeen rabbits (five Watanabe heritable hyperlipidemic, four apolipoprotein E knockout and eight New Zealand white) were analysed for a total of 51 artery segments. Eleven segments (five Watanabe heritable hyperlipidemic and six apolipoprotein E knockout) demonstrated intracranial atherosclerotic disease on pathology. Disease model animals had lesions more frequently than New Zealand white animals (P<0.001). The sensitivity and specificity of vessel wall magnetic resonance imaging for the detection of intracranial atherosclerotic disease were 68.8% and 95.2%, respectively. When excluding mild cases to assess vessel wall magnetic resonance imaging accuracy for detecting moderate to severe intracranial atherosclerotic disease lesions, sensitivity improved to 100% with unchanged specificity.
Conclusion
Intracranial atherosclerotic disease can be reliably produced and detected using 3T vessel wall magnetic resonance imaging-compatible Watanabe heritable hyperlipidemic and ApoE rabbit models. Further analysis is needed to characterize better the development and progression of the disease to correlate tissue-validated animal findings with those in human vessel wall magnetic resonance imaging studies.
Keywords: Animal model, intracranial atherosclerosis, MRI, vessel wall imaging
Introduction
Ischemic stroke is a leading cause of death and long-term disability. Intracranial atherosclerotic disease (ICAD) causes 15% of ischemic strokes in the United States and up to 50% in some populations, representing the most common etiology of ischemic stroke worldwide.1–4 ICAD pathophysiology and natural history are incompletely understood. As a result, there is significant variability in diagnostic and treatment approaches. Identifying the optimal treatment for the large number of individuals with ICAD therefore remains a difficult problem.
Vessel wall magnetic resonance imaging (vwMRI) techniques offer new information for the management of ICAD.5–9 In general, vwMRI improves the characterization of vascular disorders by allowing direct visualization of vessel wall pathology as opposed to inferring minimal information from standard luminal imaging techniques. While promising as a diagnostic and prognostic tool for ICAD evaluation, vwMRI currently lacks support from histopathological confirmation, the gold standard in the assessment of human disease. This paucity of tissue data is true for nearly all ICAD investigation and is a particular problem when considering vwMRI investigations of ICAD. Such pathology studies are essentially impossible to perform in humans for a number of reasons, including the prohibitive morbidity of biopsy and typical timing of diagnosis late in the course of the disease.
In settings such as this, in which a disease is difficult to characterize adequately in humans, animal models must be used to elucidate critical natural history and pathophysiological data. The barrier to progress is the lack of a vwMRI-compatible animal model for preclinical studies and, in particular, one using 3T magnet strength and sequences that can be easily transitioned to human studies and clinical practice. One potential candidate is the Watanabe heritable hyperlipidemic (WHHL) rabbit model, which is thus far the most extensively utilized atherosclerotic animal known to develop ICAD.10–13 Despite being the most commonly studied, the mechanism by which it develops ICAD does not replicate the way in which ICAD develops in the vast majority of humans.11–15 Furthermore, the supply of these animals has recently proved unreliable, so the need for a more viable model is acute (M. Shiomi, WHHL rabbits at Kobe and elsewhere, personal communication, 2017). Diet-based models exist for atherosclerosis, such as producing disease in wild-type New Zealand white (NZW) rabbits fed high cholesterol diets. However, systemic effects typically lead to animal demise before ICAD occurs.16 Additional rabbit models of extracranial atherosclerosis exist, most notably using apolipoprotein E (ApoE) knockout rabbits, yet investigation for the development of intracranial atherosclerosis is lacking to date.13,17,18 This study seeks to demonstrate the ability to perform vwMRI effectively in rabbits and assess the suitability of the ApoE knockout rabbit as a potential alternative to the WHHL model ICAD analysis, using histopathological correlation that is not available in human studies.
Methods
All animal investigation was performed according to a protocol approved by the institutional animal care and use committee at our academic medical center. All rabbits underwent serial vwMRI studies on a 3T Prisma scanner (Siemens Healthineers, Erlangen, Germany) using an ankle coil over the head to obtain pre and post-contrast delay alternating with nutation for tailored excitation (DANTE) T1 variable flip angle turbo spin echo (SPACE), T2 SPACE and time-of-flight (TOF) magnetic resonance angiography (MRA).16 Table 1 summarizes the acquisition parameters. For gadolinium contrast, the Multihance (gadobenate dimeglumine; Bracco, Milan, Italy) dose was calculated by animal mass (0.2 ml/kg, 0.1 mmol/kg).
Table 1.
vwMRI scan parameters.
| Sequence | 3D TOF | T1w SPACE pre | 3D MPRAGE | DWI | T2w SPACE | T1w SPACE post |
|---|---|---|---|---|---|---|
| Orientation | Axial | Obl-Axial | Obl-Axial | Obl-Axial | Obl-Axial | Obl-Axial |
| Resolution (mm) | 0.3 | 0.2 | 0.2 | 0.7 | 0.2 | 0.2 |
| Slice thick (mm) | 0.3 | 0.2 | 0.2 | 2.0 | 0.2 | 0.2 |
| No. of slices | 36 per slab 3 slabs | 240 | 160 | 64 | 240 | 240 |
| TR/TE (ms) | 18/4.5 | 550/26 | 8.9/3.9 | 5000/74 | 550/26 | 550/26 |
| Preparation | Sup sat | DANTE prep = 150 ms | IR prepTI = 350 ms | b = 0/1000 s/mm2Fat saturation | DANTE prep = 150 ms | DANTE prep = 150 ms |
| Scan time (m:s) | 4:41 | 8:34 | 1:24 | 2:50 | 8:15 | 8:34 |
vwMRI: vessel wall magnetic resonance imaging; 3D: three-dimensional; TOF: time-of-flight; SPACE: variable flip angle turbo spin echo; MPRAGE: magnetization prepared rapid acquisition gradient echo; DWI: diffusion-weighted imaging; DANTE: delay alternating with nutation for tailored excitation.
The initial investigation was performed on mature WHHL rabbits. During the course of the study, additional WHHL rabbits became unavailable due to logistical limitations at the breeding facility (M. Shiomi, WHHL rabbits at Kobe and elsewhere, personal communication, 2017). Mature ApoE knockout rabbits fed a custom 2% cholesterol diet (Envigo Teklad Diets, Madison, WI, USA) underwent the same imaging protocol. In addition, wild-type NZW rabbits were evaluated, according to the same protocol, to serve as normal controls.
vwMRI images obtained closest to euthanasia were analyzed by a board-certified radiologist with certificate of added qualification in neuroradiology. Basilar and internal carotid artery segments were each rated as having no, mild, moderate, or advanced ICAD on vwMRI. Grading was based on subjective assessment of plaque volume, signal abnormality, stenosis and enhancement.
When determined appropriate to evaluate a certain stage of disease or dictated by failure to thrive, euthanasia was performed with perfusion fixation following a standardized protocol. General endotracheal anesthesia was initiated and maintained using isoflurane. The hair over the neck was shaved and a midline incision over the trachea was made with a scalpel. Using blunt dissection, the right carotid artery was isolated. Isolating the artery under gentle tension with vessel loops, the artery was entered with a 22 gauge intravenous catheter. The catheter was gently advanced over the needle, which was used for forming an arteriotomy. The needle was removed and a 0.018 inch wire was advanced into the catheter. The catheter was removed over the wire and a 5 French micropuncture catheter was advanced over the wire. The catheter was secured by suture over the vessel segment with the indwelling catheter. The inner catheter and wire were removed, and perfusion was performed by delivering a steady flow of 2% paraformaldehyde and 5% glutaraldehyde solution with a perfusion pump. With perfusate flowing, a jugular vein was transected to exsanguinate the animal with the aim of achieving fixation with few blood cells in the vessels. The animal was decapitated, and the brain, with intact intracranial arteries, was harvested and placed in a container with formalin.
Pathological preparation and analysis was performed by a specialized veterinary pathologist. After 2 weeks in formalin, harvested brains were sliced to prepare slides oriented to optimize the cross-sectional orientation of the proximal intracranial arteries. Slides were prepared after hematoxylin and eosin (H&E) staining. Light microscopic analysis was performed to assess the presence of ICAD.16 As with vwMRI evaluation, basilar and internal carotid artery segments were each rated by the pathologist according to specialty standards as having no, mild, moderate, or advanced ICAD based on the presence and severity of histological findings of atherosclerosis: arterial wall thickening, smooth muscle hypertrophy, inflammatory cell infiltration, neointimal formation, lipid deposition and lumen remodeling.
Statistical analysis was performed on a by-segment basis. To evaluate in the best way samples of these sizes in which normal distribution cannot be assumed, two-tailed Wilcoxon–Mann–Whitney ranked sum tests were performed to compare disease burden among rabbit subtypes. Analysis was performed when considering disease burden in a binary fashion and also on the ordinal severity scale. Sensitivity and specificity were calculated to assess the diagnostic accuracy of vwMRI. Statistical analysis was performed using R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Seventeen rabbits and 51 segments underwent evaluation, including five WHHL (24.0–34.4 months at euthanasia, mean 28.7 ± 4.3), four ApoE knockout (37.8–46.8 months, mean 42.8 ± 4.2) and eight NZW rabbits (7.1–27.6 months, mean 12.5 ± 7.7). vwMRI and histopathology images representative of normal vessels and severe disease are provided in Figures 1 and 2, respectively. Table 2 lists vwMRI and pathology results for each animal. Five (33.3%) vessel segments had ICAD identified on pathology (two mild, two moderate, one severe) in WHHL animals, while six (50.0%) ApoE knockout vessel segments had lesions (five mild, one moderate). No lesions were identified in NZW control animals. The sensitivity and specificity of vwMRI for the detection of ICAD were 68.9% and 95.2%, respectively, driven by two false positives and five false negatives. When excluding cases found to have mild disease on pathology to assess vwMRI accuracy for detecting moderate to severe ICAD lesions, sensitivity improved to 100% with unchanged specificity.
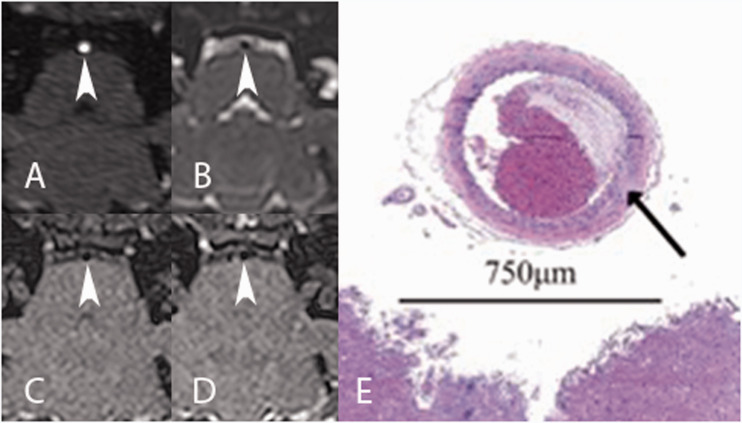
Figure 1.
Arrowheads demonstrate a normal basilar artery in a wild-type New Zealand white (NZW) rabbit on (a) three-dimensional time-of-flight (TOF) magnetic resonance angiography (MRA); (b) T2 variable flip angle turbo spin echo (SPACE); (c) non-contrast T1 delay alternating with nutation for tailored excitation (DANTE); (d) post-contrast T1 DANTE images. (e) Photomicrograph of hematoxylin and eosin (H&E) stained slice at 50× magnification at the corresponding level demonstrates normal basilar artery wall (arrow) with the brainstem margin at the bottom of the image. Blood products and clot fill the lumen after suboptimal perfusion to clear the vessel.
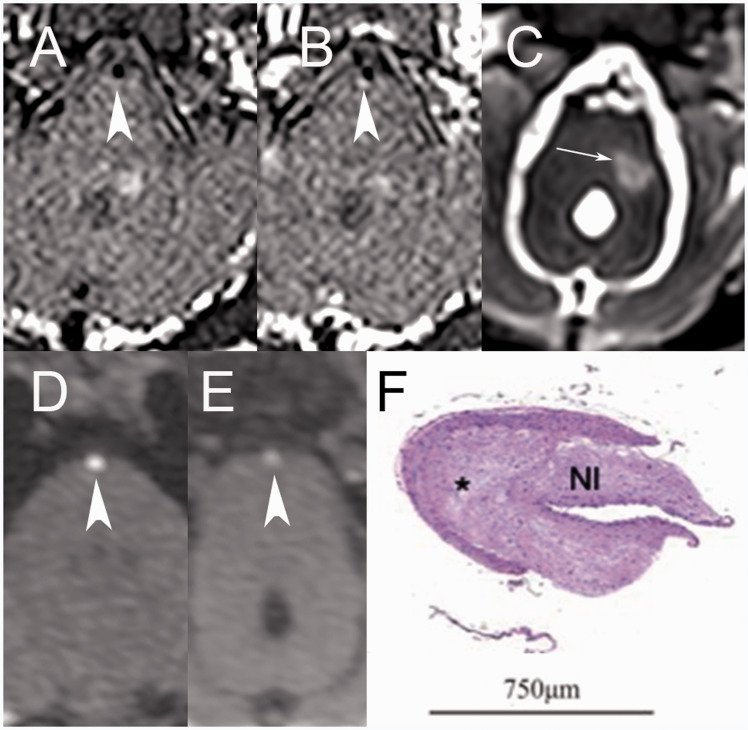
Figure 2.
(a) Pre and (b) post-contrast T1 delay alternating with nutation for tailored excitation (DANTE) sequences demonstrate enhancing plaque (arrowheads) at the distal basilar artery of a Watanabe heritable hyperlipidemic (WHHL) rabbit, which was rated as severe. (c) T2 variable flip angle turbo spin echo (SPACE) images of an adjacent slice through the brainstem demonstrates an associated left middle cerebellar peduncle infarct (white arrow). (d) Normal and (e) abnormal segments of the basilar artery are noted by arrowheads on three-dimensional time-of-flight (TOF) magnetic resonance angiography (MRA), with the abnormal segment corresponding to that demonstrated on T1 DANTE images. (f) Photomicrograph of the equivalent segment of the distal basilar artery stained with hematoxylin and eosin (H&E) and magnified 50× demonstrates asymmetrical hypertrophic neointima (NI) formation. Composed of smooth muscle cells, extracellular matrix with lipid deposition (*) and smooth muscle hypertrophy, findings are consistent with atherosclerotic plaque formation that was rated advanced.
Table 2.
Animal vessel segment findings.
|
ICAD Severity on vwMRI |
ICAD severity on pathology |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Strain | Age (months) | Sex | Mass (kg) | Right ICA | Left ICA | Basilar | Right ICA | Left ICA | Basilar |
| 1 | WHHL | 28.0 | M | 2.66 | None | None | None | None | None | Mild |
| 2 | WHHL | 31.7 | F | 3.41 | None | None | Moderate | None | None | Moderate |
| 3 | WHHL | 24.0 | M | 2.68 | Mild | Mild | Advanced | None | None | Advanced |
| 4 | WHHL | 34.4 | F | 3.31 | None | None | None | None | None | None |
| 5 | WHHL | 25.6 | M | 3.8 | None | None | Moderate | Mild | None | Moderate |
| 6 | ApoE | 47.0 | M | 4.4 | None | None | None | None | None | None |
| 7 | ApoE | 38.3 | M | 4.79 | Moderate | None | None | Moderate | None | None |
| 8 | ApoE | 36.3 | M | 4.8 | None | None | None | None | Mild | Mild |
| 9 | ApoE | 46.1 | M | 4.33 | Mild | Mild | None | Mild | Mild | Mild |
| 10 | NZW | 8.1 | F | 3.49 | None | None | None | None | None | None |
| 11 | NZW | 10.6 | F | 3.37 | None | None | None | None | None | None |
| 12 | NZW | 7.1 | F | 4.28 | None | None | None | None | None | None |
| 13 | NZW | 21.5 | F | 2.9 | None | None | None | None | None | None |
| 14 | NZW | 9.9 | F | 3.56 | None | None | None | None | None | None |
| 15 | NZW | 7.7 | F | 3.59 | None | None | None | None | None | None |
| 16 | NZW | 7.7 | F | 2.4 | None | None | None | None | None | None |
| 17 | NZW | 27.6 | F | 4.33 | None | None | None | None | None | None |
vwMRI: vessel wall magnetic resonance imaging; ICAD: intracranial atherosclerotic disease; ICA: internal carotid artery; ApoE: apolipoprotein E; NZW: New Zealand white; WHHL: Watanabe heritable hyperlipidemic.
Two-tailed Wilcoxon–Mann–Whitney ranked sum tests confirmed that disease model animals had lesions more frequently than NZW animals (U = 216, z = 2.56, P = 0.010). There was no statistical difference in the presence of any ICAD between WHHL and ApoE knockout animals (U = 75, z = –0.708, P = 0.478). The observed presence of moderate or advanced disease noted in WHHL rabbits compared to ApoE knockout rabbits was not statistically significant (U = 41.5, z = 0.278, P = 0.780).
Discussion
ICAD causes more ischemic strokes worldwide than any other etiology.1–3 In general, it is thought that atherosclerosis develops due to systemic processes that cause lesions to develop near points of stress, such as bifurcations or turns in vessels. However, this scheme has been best validated in the systemic circulation, with little investigation to date examining the pathophysiology of ICAD.19,20 Intracranial and extracranial arteries are derived from different germ cell layers and have different molecular and anatomical features, so it cannot be assumed that atherosclerosis in these two vascular beds involves identical pathophysiological processes.21–23 Few investigations to date have explored the differences between ICAD and extracranial atherosclerosis, but ICAD management strategies still largely mirror those for extracranial disease, ignoring these potential differences.
To develop improved treatment algorithms, a better understanding of ICAD pathophysiology and natural history is needed. However, such data cannot be effectively acquired from human investigations alone. Most ICAD is diagnosed at very late stages, and confounding from other diseases of late age and the treatment of these diseases often occurs. In addition, as mentioned above, histopathological confirmation cannot be performed without unacceptable morbidity. To study ICAD effectively in a longitudinal way that allows characterization of the full course of the disease, we must rely on an effective animal model.
Many insightful studies of atherosclerosis have been performed in rabbits, investigating peripheral and coronary disease.13 Their size makes both open and endovascular surgical procedures possible, allowing for superior translatability to humans than rodent models. They are large enough for high resolution non-invasive imaging, and their lack of a rete mirabile allows for endovascular access to the cerebral circulation unlike other large animals such as swine.13 Despite the utility of rabbit models, much less research has been performed on intracranial vessels.
WHHL rabbits have previously been demonstrated to develop ICAD; these animals lack low density lipoprotein (LDL) receptors, which causes them to predictably develop atherosclerosis.10,11,13,24,25 WHHL research led to the pathophysiological cause of familial hypercholesterolemia, but this finding suggests they may be a suboptimal model for ICAD.11 Familial hypercholesterolemia patients typically do not develop intracranial disease despite their profound systemic atherosclerotic burden, and nearly all ICAD patients do not have familial hypercholesterolemia, so this model could offer spurious vessel biology data with poor correlation to the preponderance of humans with ICAD.26–28 As a practical consideration, WHHL rabbits have poor health in general, and ICAD has only been described in them after the induction of hypertension, which can further complicate their care and limit longevity.14,15 Coupled with logistical difficulties that make a steady supply of WHHL animals impossible, the need for a better ICAD model becomes apparent (M. Shiomi, WHHL rabbits at Kobe and elsewhere, personal communication, 2017).
Other avenues exist for inducing atherosclerosis in rabbits. Some investigators have induced disease in NZW rabbits by feeding them atherogenic diets, but such an approach has not proved effective for ICAD.13,16 ApoE knockout has been used for disease investigation in a variety of species, and a rabbit strain that can be selectively bred was created in recent years.17,18,29,30 This current investigation sought to evaluate such ApoE knockout rabbits against WHHL rabbits when evaluated with both vwMRI and histopathology. Both WHHL and ApoE knockout animals were found to harbor ICAD lesions. No ICAD was identified in NZW control animals. A non-significant trend was noted for more advanced disease to be seen in WHHL rabbits compared to ApoE knockout specimens. This research showed that vwMRI studies were able reliably to detect ICAD lesions, particularly those that proved to be moderate or severe on pathology.
While both WHHL and ApoE knockout animals demonstrated ICAD, as desired for an animal model, the limitations of the current analysis bear noting and should be addressed in future investigations. First, none of the WHHL animals underwent induction of hypertension as has been described previously.15 This may limit comparison to previous studies of ICAD in WHHL rabbits. In comparing WHHL and ApoE knockout animals, ApoE knockout animals were fed an atherogenic diet. Both WHHL and ApoE knockout animals were older than their NZW counterparts. While NZW animals are not expected to develop ICAD, closer age matching could be performed in future studies. In addition, earlier stages of ICAD in younger animals could also be investigated to provide a more complete view of the disease course.
While both WHHL and ApoE knockout rabbits were found to harbor ICAD lesions, ApoE knockout rabbits are likely to represent the better option given supply limitations with WHHL animals and the potential for poor fidelity to human ICAD. In addition to the need for more investigation generally, future efforts should focus on means for accelerating the progression of ICAD in ApoE knockout rabbits to allow for more efficient investigation.
Conclusion
ICAD can be reliably produced and detected using 3T vwMRI-compatible rabbit models. Mature WHHL and ApoE knockout rabbits demonstrated ICAD. Further analysis is needed to characterize better the development and progression of disease in these animal models. vwMRI sequences can be used to analyze the disease in these model animals with high sensitivity and specificity, particularly with moderate to severe disease. Given comparable findings between the two models, ApoE knockout rabbits can be considered a suitable alternative to WHHL rabbits given supply constraints and concerns about their translatability to human ICAD.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_1971400920980153 for Rabbit models of intracranial atherosclerotic disease for pathological validation of vessel wall MRI by J Scott McNally, Adam de Havenon, Seong-Eun Kim, Chuanzhuo Wang, Shuping Wang, Matthew S Zabriskie, Dennis L Parker, Hediyeh Baradaran and Matthew D Alexander in The Neuroradiology Journal
Acknowledgements
This paper was presented at the International Society for Magnetic Resonance in Medicine (ISMRM) Annual Meeting August 2020, Paris (Virtual).
Footnotes
Author contributions: MDA and MSZ performed data collection and analysis. JSM and MDA wrote the manuscript. All remaining authors participated in data analysis and edited the manuscript.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by NIH S10OD018482, NIH R01HL127582, NIH/NINDS K23NS105924, American Heart Association transformational grant 19TPA34910194, American Heart Association scientist development grant 17SDG33460420 and the Joe Niekro research grant from the Joe Niekro Foundation and Society of NeuroInterventional Surgery Foundation.
ORCID iDs: Matthew D Alexander https://orcid.org/0000-0002-7534-5842
Adam de Havenon https://orcid.org/0000-0001-8178-8597
References
- 1.Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158–159. [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke 1986; 17: 648–655. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014; 45: 663–669. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Baradaran H, Al-Dasuqi K, et al. Gadolinium enhancement in intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. J Am Heart Assoc 2016; 5: e003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossa-Basha M, Shibata DK, Hallam DK, et al. Added value of vessel wall magnetic resonance imaging for differentiation of non-occlusive intracranial vasculopathies. Stroke; a Journal of Cerebral Circulation 2017; 48: 3026–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossa-Basha M, Alexander M, Gaddikeri S, et al. Vessel wall imaging for intracranial vascular disease evaluation. J Neurointerv Surg 2016; 8: 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander MD, Yuan C, Rutman A, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry 2016; 87: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander MD, de Havenon A, Kim SE, et al. Assessment of quantitative methods for enhancement measurement on vessel wall magnetic resonance imaging evaluation of intracranial atherosclerosis. Neuroradiology 2019; 61: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Havenon A, Muhina HJ, Parker DL, et al. Effect of time elapsed since gadolinium administration on atherosclerotic plaque enhancement in clinical vessel wall MR IMAGING STUDies. AJNR Am J Neuroradiol 2019; 40: 1709–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliev G, Burnstock G. Watanabe rabbits with heritable hypercholesterolaemia: a model of atherosclerosis. Histol Histopathol 1998; 13: 797–817. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JL, Kita T, Brown MS. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med 1983; 309: 288–296. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Zhang J, Li H, et al. Hyperlipidemia-associated gene variations and expression patterns revealed by whole-genome and transcriptome sequencing of rabbit models. Sci Rep 2016; 6: 26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanni AE. The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim 2004; 38: 246–256. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Shiomi M. Cerebral atherosclerosis occurs spontaneously in homozygous WHHL rabbits. Atherosclerosis 2001; 156: 57–66. [DOI] [PubMed] [Google Scholar]
- 15.Kong J, Tamaki N, Asada M. Early lesions of cerebral atherosclerosis from induced hypertension in Watanabe heritable hyperlipidemic rabbits. Kobe J Med Sci 2000; 46: 87–101. [PubMed] [Google Scholar]
- 16.Zabriskie M, Wang S, Kim S, et al. New Zealand white rabbits fed high cholesterol diets develop morbid systemic diseases before intracranial atherosclerosis is detected. J Vet Sci Med Diagnosis 2019; 8. [Google Scholar]
- 17.Ji D, Zhao G, Songstad A, et al. Efficient creation of an APOE knockout rabbit. Transgenic Res 2015; 24: 227–235. [DOI] [PubMed] [Google Scholar]
- 18.Zabriskie MS, Wang C, Wang S, et al. Apolipoprotein E knockout rabbit model of intracranial atherosclerotic disease. Anim Model Exp Med 2020; 3: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke; a Journal of Cerebral Circulation 2006; 37: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009; 54: 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasjaunias P, Berenstein A, ter Brugge KG. Clinical Vascular Anatomy and Variations. 2001. 10.1007/978-3-662-10172-8 (accessed 29 November 2020). [DOI]
- 22.Postiglione A, Nappi A, Brunetti A, et al. Relative protection from cerebral atherosclerosis of young patients with homozygous familial hypercholesterolemia. Atherosclerosis 1991; 90: 23–30. [DOI] [PubMed] [Google Scholar]
- 23.Alexander MD, De Havenon AH, Mossa-Basha M, et al. How far can we take vessel wall MRI? The tissue is still the issue. AJNR Am J Neuroradiol 2020; In press. [DOI] [PMC free article] [PubMed]
- 24.Rosenfeld ME, Tsukada T, Gown AM, et al. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 1987; 7: 9–23. [DOI] [PubMed] [Google Scholar]
- 25.Shiomi M, Ito T, Shiraishi M, et al. Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis 1992; 96: 43–52. [DOI] [PubMed] [Google Scholar]
- 26.Ding X, Li C, Yu K, et al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: a systematic review and meta-analysis. Int J Neurosci 2014; 124: 834–840. [DOI] [PubMed] [Google Scholar]
- 27.Lindenholz A, van der Kolk A, van der Schaaf I, et al. Vascular risk factors and intracranial atherosclerosis at 7T vessel wall MRI in Caucasian ischemic stroke and TIA patients. Paris, France: International Society for Magnetic Resonance in Medicine, 2018. [Google Scholar]
- 28.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014; 383: 984–998. [DOI] [PubMed] [Google Scholar]
- 29.Brousseau ME, Hoeg JM. Transgenic rabbits as models for atherosclerosis research. J Lipid Res 1999; 40: 365–375. [PubMed] [Google Scholar]
- 30.Taylor JM. and Fan J. Transgenic rabbit models for the study of atherosclerosis. Front Biosci 1997; 2: d298–d308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-neu-10.1177_1971400920980153 for Rabbit models of intracranial atherosclerotic disease for pathological validation of vessel wall MRI by J Scott McNally, Adam de Havenon, Seong-Eun Kim, Chuanzhuo Wang, Shuping Wang, Matthew S Zabriskie, Dennis L Parker, Hediyeh Baradaran and Matthew D Alexander in The Neuroradiology Journal