Abstract
Study Design:
Systematic review.
Objective:
To compare outcomes of complete versus incomplete resection in primary intramedullary spinal cord ependymoma.
Methods:
A comprehensive search of the MEDLINE, CENTRAL, and Embase databases was conducted by 2 independent investigators. Random-effect meta-analysis and meta-regression with seven covariates were performed to evaluate the reason for the heterogeneity among studies. We also used individual patient data in the integrative analysis to compare complete and incomplete resection based on 4 outcomes: progression-free survival (PFS), overall survival (OS), postoperative neurological improvement (PNI), and follow-up neurological improvement (FNI).
Results:
A total of 23 studies were identified, including 407 cases. Significant heterogeneity among included studies was observed in risk estimates (I2 for PFS, FNI, and PNI were 49.5%, 78.3%, and 87.2%, respectively). The mean follow-up time across cases was 48.6 ± 2.35 months. Cox proportional multivariable analysis revealed that the complete resection can prolong PFS (model, hazard ratio = 0.18, CI 0.05-0.54, P = .004,) and improve the FNI (binary logistic regression, adjusted odds ratio = 16.5, CI 1.6-171, P = .019). However, PNI and OS were similar in patients with incomplete resected spinal cord ependymoma compared with complete resection (binary logistic regression respectively and Cox multivariable analysis, P > .5).
Conclusion:
The data presented in this study showed that OS was not significantly affected by the degree of surgery. However, complete resection of intramedullary ependymomas provides the optimal outcomes with longer PFS and better long-term neurological outcomes than incomplete resection.
Keywords: tumor, intramedullary tumor, ependymoma, overall survival
Introduction
Ependymoma accounts for 3% to 6% of all central nervous system tumors and is the most common primary spinal cord tumor in adults.1-3 It arises from the ependymal cells in the proximity of the cerebral ventricles, choroid plexus, and central canal of the spinal cord. The World Health Organization (WHO) has classified these tumors based on their histopathologic grades. Grade I tumors are myxopapillary ependymomas and subependymomas. Grade II tumors are referred to as ependymomas or classical ependymomas and grade III tumors are anaplastic ependymomas.4
Due to the relative rarity of the tumor, the prognostic factors for ependymomas are scarce, and the quality of current medical recommendations are very low.5 There is no consensus on the optimal ways to manage these tumors. There are 2 main surgical strategies, complete resection and incomplete resection (subtotal or partial resection of the tumor). Several reviews have evaluated the effects of different prognostic factors on outcomes such as overall survival. For instance, results of Lee et al6 support that the extent of resection is the strongest predictor of long-term survival. However, in a systematic review based on grade II ependymoma, Wang et al showed that the OS was not significantly different between complete and incomplete resection.
The aim of this systematic review is to compare outcomes of complete with incomplete resection and also evaluate other prognostic factors.
Methods
Search Strategy
A comprehensive electronic search of MEDLINE (1946 to present), CENTRAL, and Embase (1980 to present) was conducted in August 2018 (Appendix 1). Additionally, a list of references to related articles was reviewed. We did not have language limit for this literature search.
Selection Method
All published papers on intramedullary spinal cord tumors were tested using a search strategy. After deduplication, 4 reviewers (FS, MG, SH, MSN) independently screened the titles and abstracts of the studies with each record reviewed by 2 independent reviewers. After getting the full text of eligible papers, 3 reviewers independently reviewed them for inclusion and exclusion criteria (FS, MG, SH). Any discrepancy regarding the eligibility of studies was resolved by the fourth reviewer (VRM).
Inclusion and Exclusion Criteria
All studies reported the extent of surgical resection in patients with intramedullary ependymomas and described surgery-related outcomes were included in this study.
In order to avoid misleading data from some authors with less experience, we only included case series when they reported at least 10 cases and excluded all the case reports. In articles that described the outcomes of all types of tumors, only the data of patients with ependymomas was obtained for the analysis. If the patient underwent a second surgery in follow-up time, we only used the data before the second surgery. For the studies using the same database, we only included the one with higher number of cases and a more complete data. Exclusion criteria were the following: patients with a history of previous surgery or radiotherapy, patients with extramedullary tumors (filum terminale and cauda equina tumors were excluded), and studies without follow-up data, and those that did not determine the extent of resection.
Main Outcomes
Clinical and functional outcome measures were evaluated. Surgery outcomes included progression-free survival (PFS), overall survival (OS), postoperative neurological status improvement (PNI), and follow-up neurological improvement (FNI). Regardless of the functional classification method, all the PNI and FNI outcomes in the included studies with the report of preoperative functional status for each case were calculated.
Data Extraction
We utilized predeveloped forms. From each study, we collected general information (first author, year of study, country, journal), methods (study design, sample size), participants (age, gender, preoperative neurological score), extent of resection (complete resection, incomplete resection), outcomes of surgery, adjuvant treatment, tumor location, tumor length (number of involved spinal segments), time to progression, and follow-up time. Three review authors extracted data independently (FS, MG, SH). The fourth author reexamined the input data (MSN); there were minor errors that were corrected by focus discussion and reference to the original article.
To analyze the prognostic factors of intramedullary ependymomas, we grouped the extracted factors. The age of the patients was categorized into pediatric and adult groups (age <18 years and age ≥18 years). The extent of resection was divided into 2 subgroups: complete resection (CR) and incomplete resection (IR). When the authors reported a total resection of the tumor based on postoperative imaging or surgeon’s report (If there were discrepancies, we considered postoperative imaging results), we believe that the resection is CR. All the other resections such as subtotal resection, near-total resection, partial resection, and biopsy were classified as IR. The location of the tumors was divided into 2 groups: cervical and thoracolumbar spine. We used the highest level of the tumor for classification; for example, if the tumor was located in C3-T2, we classified it as a cervical tumor. The pathology of tumors was divided into 2 groups: advanced grade and low grade. We grouped WHO class III ependymomas as advanced grade and classes I and II as low grade.4 Tumor length was classified into 2 groups: less than 3 segments involvement and 3 or more segments. There were 3 methods for functional evaluation of patients in the studies: Frankel, JOA (Japanese Orthopedic Association), and McCormick. In this study, we used the McCormick7 method to study the data on preoperative functional evaluation of patients. Wherever available, we recorded the data and categorized it into high grade (grade III and more) and low grade for further analysis.
Assessment of Methodological Quality
The methodological quality of the included studies was evaluated using the JBI Critical Appraisal Checklist for descriptive/case series studies.8 We considered the studies with at least 5 of 10 criteria as sufficient methodological quality for inclusion.
Statistical Analysis
Meta-analysis
After investigating the literature, we did not find any published randomized clinical trials, and all the included studies were case series. We divided all included studies into 2 different series: a series of patients who underwent IR and a series of patients who underwent CR. We conducted a meta-analysis of the CR and IR series, respectively.
We performed a meta-analysis of every single outcome using Metaprop command with Freeman-Tukey double arcsine transformation to stabilize the variances.9 A random-effects meta-analysis was performed to evaluate the effect estimate for the series. Heterogeneity among studies was estimated using I2 statistics.10 Potential sources of heterogeneity were further investigated by arranging study groups based on potential relevant characteristics and meta-regression analysis. Seven covariates were chosen in our meta-regression: sex, age, tumor location, tumor length, adjuvant therapy, study location, and study year. These covariates were used together for meta-regression individually and together in a random-effects meta-regression model using Stata (StataCorp 2011. Stata Statistical Software: Release 14).
Integrative Analysis
We described the characteristics of patients with intramedullary ependymomas undergoing surgery. To compare CR and IR, we entered individual patient data from studies with a detailed report of individual patient outcomes into the integrative analysis.
PFS and OS were analyzed using Kaplan-Meier curves, and differences were assessed using log-rank or Breslow tests. This analysis was followed by Cox proportional hazards to adjust for confounding variables (age, gender, and histopathologic grade, the extent of resection, adjuvant therapy, tumor length, and tumor location). A hazard ratio (HR) with 95% confidence interval (CIs) was also estimated. The Pearson’s chi-square test was used to evaluate the improvement rates postoperative and follow-up neurological function. Then the unadjusted odds ratios were calculated. Then a binary logistic regression model used to measure the adjusted odds ratio for different factors (age, tumor length, preoperative score, tumor location, gender, adjuvant therapy, preoperative score, pathologic grade, and extent of resection). Analyses were performed using the SPSS software (version 25; IBM Corp). P < .05 was considered to be statistically significant.
Results
Results of the Search
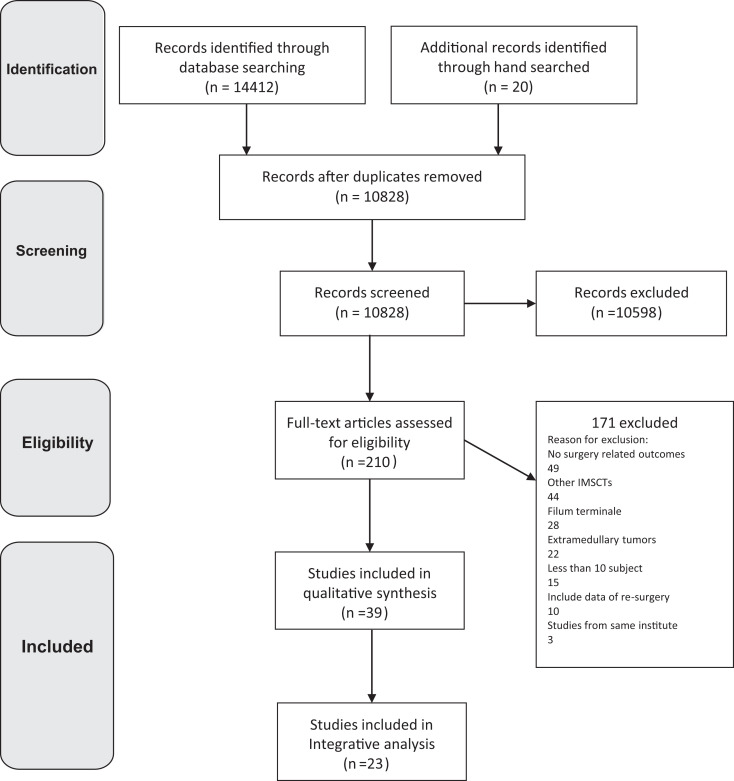
A total of 14432 studies were identified according to the search strategy, of which 3604 duplicates were deleted. In all, 10598 studies were excluded from the title and abstract screening phase. The full text of remaining articles was reviewed in detail and 39 papers met the inclusion criteria. Among the 171 excluded studies, 49 papers did not report surgery related outcomes, 44 addressed other intramedullary spinal cord tumors, 28 papers reported outcomes of filum terminale region, 22 described extramedullary lesions, 15 papers were case series with less than 10 subjects, and 10 studies included data of resurgeries. Also, 3 articles were excluded because their data has been published partly in other papers.11-13 Of the 39 included papers, 23 reported detailed patient information and were included in analysis. The flow diagram of the study is shown in Figure 1.
Figure 1.
The study flow diagram.
Description of Studies and Demographic Features
Thirty-nine studies met our inclusion criteria (sample sizes ranged from 10 to 221 patients; the total number of participants across studies was 1191).7,14-50,56 All the included studies were case series. The description of the studies is presented in Table 1.
Table 1.
Results of the Systematic Review on Outcomes of Surgery in Intramedullary Ependymomas.
| Author(s), year | n | EOR | Adjuvant therapy | Recurrence | Mortality | FNI | FU | High-grade pathology | Integrative | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aghakhani et al, 200841 | 10 | 9 CR 1 IR |
0 | 0 | 0 | 0 | 46 | 0 | a |
| 2 | Aghakhani et al, 201716 | 221 | 176 CR 45 IR | NR | 8 | NR | NR | 62 | 11 | |
| 3 | Andrade et al, 200930 | 29 | 23 CR 6 IR | 3 RT, 1 CH | 7 | 3 | NR | 38 | 0 | a |
| 4 | Asazuma et al, 199920 | 26 | 15 CR 11 IR | 10 RT, 1 CH | 8 | 6 | NR | 6 | NR | |
| 5 | Azzazi et al, 201143 | 17 | 9CR 8 IR (2B) |
NR | 0 | 0 | 7 | 6 | 0 | a |
| 6 | Brotch et al, 199824 | 93 | 86 CR 7 IR |
2 RT | 3 | 4 | NR | NR | 2 | |
| 7 | Chang et al, 200222 | 31 | 23 CR 8 IR |
4 RT | 6 | NR | NR | 33b | NR | |
| 8 | Epstein et al, 199329 | 23 | 23 CR | NR | NR | 0 | NR | 24 | NR | |
| 9 | Ge et al, 201632 | 28 | 21 CR 7 IR |
7 RT | 2 | 0 | 25 | 50 | NR | a |
| 10 | Han et al, 200834 | 13 | 12 CR 1 IR |
2 RT | 3 | 0 | 3 | 69 | 0 | a |
| 11 | Hanbali et al, 200231 | 15 | 13 CR 2 IR |
1 RT | 0 | 1 | 1 | 11 | 0 | a |
| 12 | Hejazi et al, 199817 | 36 | 33 CR 3 IR |
2 RT | NR | 0 | NR | 42 | 4 | |
| 13 | Hoshimaru et al, 199933 | 36 | 34 CR 2 IR |
NR | 1 | 0 | 14 | 55 | 1 | a |
| 14 | Hulshuf et al, 199323 | 34 | 17 CR 17 IR (4B) | 11 RT | 7 | NR | 14 | 60 | 3 | |
| 15 | Iwasaki et al, 200014 | 29 | 21 CR 8 IR |
3 RT | 1 | 0 | NR | 70 | 0 | |
| 16 | Joaquim et al, 200950 | 10 | 10 CR | NR | 0 | 0 | 4 | 37b | 0 | a |
| 17 | Kane et al, 199927 | 21 | 14 CR 7 IR |
5 RT | 3 | NR | NR | 102 | NR | |
| 18 | Kaner et al, 201037 | 11 | 11 CR | 0 | 1 | NR | 7 | 53 | NR | a |
| 19 | Karikari et al, 201138 | 17 | 13 CR 4 IR |
NR | 2 | NR | 11 | 42 | 0 | a |
| 20 | Kochbati et al, 200339 | 16 | 2 CR 14 IR (2B) | 16 RT | 2 | 0 | NR | 68 | 2 | a |
| 21 | Kucia et al, 201128 | 67 | 55 CR 12 IR (B) | NR | 3 | NR | NR | 32 | NR | |
| 22 | Kutluk et al, 201426 | 19 | 10 CR 9 IR (2B) |
13 RT 12 CH |
NR | NR | NR | 60 | 2 | |
| 23 | Lin et al, 200547 | 17 | 13 CR 4 IR |
3 RT | 1 | 1 | NR | 94 | 1 | a |
| 24 | Liu et al, 201348 | 19 | 16 CR 3 IR |
8 RT 6 CH |
3 | 5 | 0 | 60 | 19 | a |
| 25 | Lonjon et al, 199849 | 20 | 10 CR 10 IR (3B) | 6 RT | 3 | NR | 7 | 67b | 3 | a |
| 26 | McCormick et al, 19907 | 15 | 15 CR | 0 | 0 | 0 | 5 | 55 | 0 | a |
| 27 | Nakamura et al, 200821 | 33 | 30 CR 3 IR |
NR | NR | NR | 17 | 74 | NR | |
| 28 | Ohata et al, 199936 | 18 | 17 CR 1 IR |
1 RT | 0 | 0 | 1 | 86 | 2 | a |
| 29 | Peker et al, 200445 | 21 | 21 CR | 0 | 0 | 1 | 6 | 6 | 0 | a |
| 30 | Plotkin et al, 201144 | 10 | 5 CR 5 IR |
2 RT | 0 | NR | NR | 91 | 0 | a |
| 31 | Prokopienko et al, 201725 | 29 | 25 CR 4 IR |
7 RT | 2 | NR | NR | 108 | 3 | |
| 32 | Raco et al, 200519 | 68 | 55 CR 13 IR | NR | 6 | NR | NR | 85 | NR | |
| 33 | Safaee et al, 201442 | 12 | 8 CR 4 IR (1B) | 4 RT | 3 | 0 | NR | 60 | 0 | a |
| 34 | Stephen et al, 201235 | 11 | 10 CR 1 IR | 5 RT | 0 | 0 | NR | 51 | 0 | a |
| 35 | Svoboda et al, 201715 | 37 | 33 CR 4 IR |
1 RT | 5 | NR | NR | 114b | 1 | |
| 36 | Sweeny et al, 201618 | 17 | 16 CR 1 IR |
1 RT | 0 | NR | NR | 29 | 1 | |
| 37 | Tao et al, 201746 | 36 | 27 CR 9 IR |
2 RT | 1 | 0 | 30 | 42 | 0 | a |
| 38 | Wu et al, 201440 | 13 | 8 CR 4 IR |
0 | 0 | 0 | 11 | 68 | 0 | a |
| 39 | Yang et al, 201456 | 13 | 11 CR 2 IR |
2 RT | 1 | 0 | 9 | 72 | 0 | a |
Abbreviations: EOR, extent of the resection; RT, radiotherapy; FU, mean follow-up time in months; FNI, follow-up neurological improvement; CR, complete resection, IR, incomplete resection; NR, not reported.
a Included in the integrative analysis.
b Reported median to follow-up time.
Twenty-three studies, including 407 cases with reported detailed information (treatment strategy and follow-up data), were included in the analysis. Their methodological quality was assessed using the JBI Critical Appraisal Tool (Table 2). All of them have sufficient methodological quality for inclusion. The characteristics of the included patients are summarized in Table 3. The mean age was 35.2 ± 0.8 years with 43% of the patients being female.
Table 2.
Methodological Quality Assessment of Studies Included in the Meta-analysis Using the JBI Risk of Bias Assessment Tool.
| Authors, year | Clear inclusion criteria | Standard measurement | Valid methods | Consecutive Inclusion | Complete Inclusion | Clear reporting of Demographics | Clear reporting of clinical information | Clear reporting of outcomes | Clear reporting of clinic demographic | Appropriate statistical analysis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aghakhani et al, 200841 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 2 | Andrade et al, 200930 | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3 | Azzazi et al, 201143 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 4 | Ge et al, 201632 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Han et al, 200834 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| 6 | Hanbali et al, 200231 | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes |
| 7 | Hoshimaru et al, 199933 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8 | Joaquim et al, 200950 | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| 9 | Kaner et al, 201037 | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | Karikari et al, 201138 | Yes | yes | No | Yes | Yes | No | No | No | No | Yes |
| 11 | Kochbati et al, 200339 | Yes | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | Yes |
| 12 | Lin et al, 200547 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 13 | Liu et al, 201348 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 14 | Lonjon et al, 199849 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 15 | McCormick et al, 19907 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 16 | Ohata et al, 199936 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 17 | Peker et al, 200445 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 18 | Plotkin et al, 201144 | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| 19 | Safaee et al, 201442 | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| 20 | Stephen et al, 201235 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 21 | Tao et al, 201746 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 22 | Wu et al, 201440 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 23 | Yang et al, 201456 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes |
Table 3.
Demographic Properties of Patients Included in the Integrative Analysis.
| Demographic | Number of Patients |
|---|---|
| Total patients, n | 407 |
| Age, years, mean ± SEM | 35.2 ± 0.8 |
| ≤18 | 82 |
| >18 | 308 |
| Gender | |
| Female | 175 |
| Male | 188 |
| Tumor location | |
| Cervical | 248 |
| Thoracic | 95 |
| Lumbar | 47 |
| Pathology | |
| Low grade | 340 |
| High gradea | 28 |
| Complete resection | 318 |
| Incomplete resection | 89 |
| Biopsy | 11 |
| Adjuvant therapy | |
| RT | 62 |
| CHb | 7 |
| Preoperative neurologic dysfunctional situationc | |
| Low grade | 194 |
| High graded | 54 |
| Postoperative neurologic status | |
| Improved | 60 |
| Not improved | 226 |
| Follow-up neurologic status | |
| Improved | 122 |
| Not improved | 118 |
| Tumor length | |
| <3 | 80 |
| ≥3 | 243 |
| Follow-up, months, mean ± SD | 48.6 ± 2.35 |
| Recurrence | 30 |
| Death | 11 |
Abbreviations: RT, radiotherapy; CH, chemotherapy.
a Grade III tumors based on World Health Organization classification.
b All patients who underwent chemotherapy also underwent radiation.
c Number of patients reported preoperative functional status using McCormick classification
d GradeIII or more based on McCormick classification
Of the 407 patients, 318 patients underwent CR (47.2% female). Of these patients, 51 and 99 experienced PNI and FNI, respectively. Twelve patients experienced recurrence and seven mortalities were reported in the CR group.
The present study, 89 patients underwent IR (51.9% female). In this group, 18 of patients relapsed and 4 patients died during the follow-up. Among these patients, 9 and 23 experienced PNI and FNI, respectively. Eleven patients had biopsy alone (6 of them received adjuvant radiotherapy). No mortalities were reported but 2 patients experienced recurrence. There was no PNI or FNI report for the patients with biopsy.
Meta-analysis
Mortality
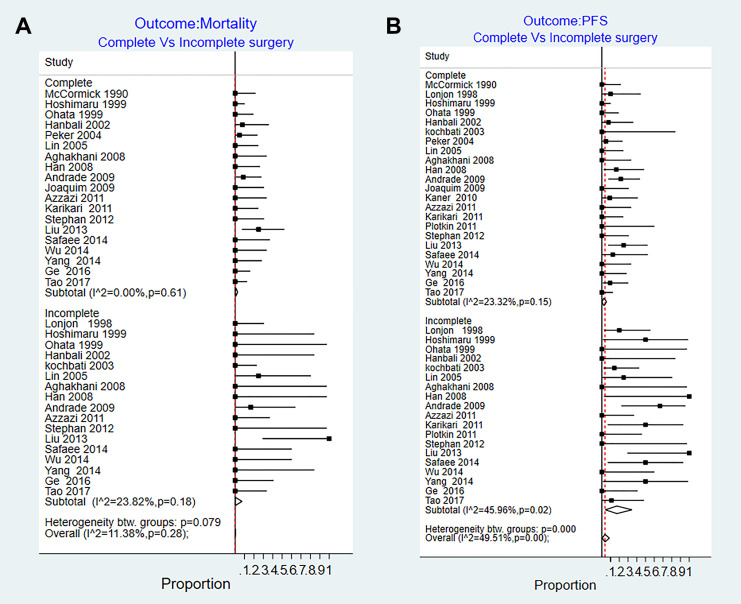
We identified 19 series that reported the mortality after CR. The risk of mortality ranged from 0% to 25%. Pooled risk estimate for CR group was 0.7% (95% CI = 0%-3%). There was no heterogeneity among the studies (I2 =0%, P = .6). We identified 17 series that reported post-IR mortality data. The mortality rate estimates for incomplete resection ranged from 0% to 25%. Pooled risk estimate for IR group was 0.3% (95% CI = 0%-3%) (I2 = 23.8%, P = .18) (Figure 2A).
Figure 2.
Results of the random effect meta-analysis on the studies that reported follow-up outcomes of complete and incomplete surgical resection in patients with intramedullary ependymomas. (A) Mortality rate and (B) tumor progression rate. PFS, progression-free survival.
None of the predefined factors were significant coefficient for explaining the heterogeneity: tumor length (0.000, P = .8), tumor location (0.000, P = .9), sex (0.001, P = .87), age (0.001, P = .5), adjuvant therapy (–0.002, P = .4), study year (–0.003, P = .8), and country (0.18, P = .3).
Progression-Free Survival
We identified 23 studies that reported the PFS after CR of ependymomas. The risk of tumor progression ranged from 0% to 25%. Pooled risk estimate for CR group was 2% (95% CI = 0%-6%, I2 = 23.3%, P = .15), with none of the sample characteristics were significantly associated with this heterogeneity on meta-regression analysis. In the 19 series that reported the risk of progression for patients underwent IR, the risk estimates ranged from 0% to 67%. Pooled risk estimate for IR was 17% (95% CI = 4%-35%). There was moderate heterogeneity among studies (I2 = 45.9%, P = .02) (Figure 2B).
None of the predefined factors had significant coefficient for explaining heterogeneity among the studies: tumor length (0.002, P = .4), tumor location (0.002, P = 0.2), sex (0.001, P = .7), age (0.000, P = .9), adjuvant therapy (0.000, P = .8), study year (0.001, P = .9), and country (0.000, P = .9).
Postoperative Neurological Improvement
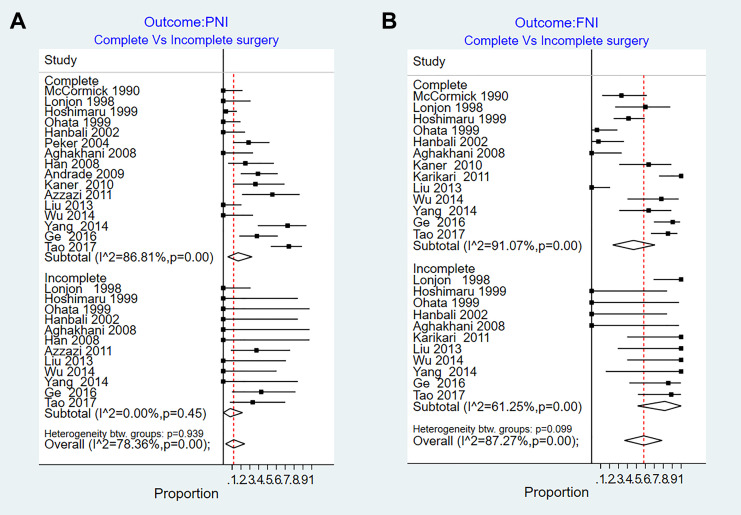
There are 16 series that evaluated neurological function after CR. The improvement rate estimates for CR ranged from 0% to 74%. Pooled improvement estimate was 17% (95% = CI 5%-32%, I2 = 86.8%, P < .01). There are 12 series that reported improvement rate for patients underwent IR. The risk estimates ranged from 0% to 43%. Pooled risk estimate was 8% (95% CI = 0%-22%, I2 = 0%, P = .45) (Figure 3A).
Figure 3.
Results of the random effect meta-analysis on the studies that reported follow-up outcomes of complete and incomplete surgical resection in patients with intramedullary ependymomas. (A) Postoperative neurological improvement (PNI) and (B) follow-up neurological improvement (FNI).
None of the predefined factors was significant coefficient for explaining the heterogeneity: tumor length (–0.003, P = .3), tumor location (0.000, P = .8), sex (–0.002, P = .6), age (0.002, P = .3), adjuvant therapy (–0.003, P = .4), study year (0.01, P = .3), and country (0. 02, P = 0.3)).
Follow-up Neurological Improvement
Thirteen series evaluated neurological function after CR. Pooled risk estimate for CR ranged from 0% to 100%. Pooled rate of improvement estimate was 47% (95% CI 24%-71%, I2 = 91.07%, P < .01). Eleven series reported improvement rate for patients with IR. The risk estimates ranged from 0% to 100%. Pooled risk estimate was 82% (95% CI 51%-100%, (I2 = 61.25%, P < .01) (Figure 3B).
None of the predefined factors significant coefficient for explaining the heterogeneity: tumor length (0.005, P = .2), tumor location (0.002, P = 0.6), sex (−0.004, P = .4), age (−.005, P = .08), adjuvant therapy (−0.008, P = .54), study year (0.003, P = .8), and country (0.005, P = .1).
Integrative Analysis
Progression-Free Survival
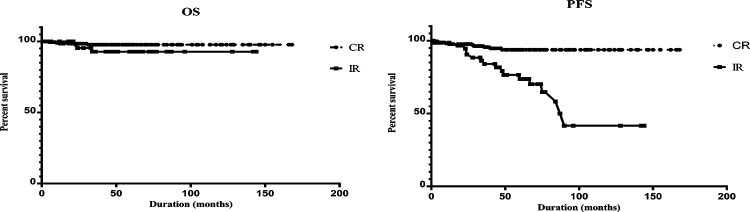
The mean time to progression was 210.0 ± 3.88 months and 155.1 ± 19.5 for the CR and IR group, respectively. Eleven of 248 cases who underwent CR had recurrence or died. On the other hand, 14 of 66 cases from IR group had tumor progression. Kaplan-Meier analysis showed a significant difference in the PFS between the CR and IR groups (log-rank test, P < .001) (Figure 4). Further univariate analysis by gender, age, tumor length, tumor location, and preoperative neurological function status and adjuvant therapy did not show a significant effect on PFS. However, there was also a significant difference in PFS according to histological grade (log-rank test, P = .005) (Table 4). We performed a multivariable Cox proportional hazards model for PFS using the following variables: age, gender, pathology grade, the extent of resection, adjuvant therapy, tumor length, and tumor location. The results showed that the CR was independently associated with improvement of PFS (P = .004, Table 4). In addition, pediatric patients had a reduced risk of PFS (HR = 5.36, 95% CI = 1.5-18.4, P = .008). Also, patients with low-grade pathology had a lower risk of progression than patients with advanced-grade pathology (HR = 0.18, 95% CI = 0.04-0.79, P = .02).
Figure 4.
Univariate analysis of progression-free survival and overall survival stratified by the extent of surgery. PFS, progression-free survival; OS, overall survival; CR, complete resection; IR, incomplete resection.
Table 4.
(A) Results of Kaplan-Meier Analysis and (B) Multivariable Cox Regression Analysis on Progression-Free Survival and Overall Survival in Patients With Intramedullary Ependymomas.
| A: Kaplan-Meier analysis | ||||||
|---|---|---|---|---|---|---|
| OS | PFS | |||||
| Mean, mo | SE | P | Mean, mo | SE | P | |
| Age | .57 | .08 | ||||
| Adult | 214.73 | 3.08 | 201.1 | 5.5 | ||
| Pediatric | 254.61 | 5.3 | 186.2 | 14.6 | ||
| Gender | .41 | .39 | ||||
| Female | 163.6 | 2.5 | 147.2 | 5.5 | ||
| Male | 249.3 | 4.6 | 206 | 8.2 | ||
| Tumor length | .49 | .8 | ||||
| <3 | 138.8 | 4.5 | 139.4 | 4.2 | ||
| ≥3 | 215.7 | 3.2 | 196.8 | 6.7 | ||
| Adjuvant therapy | .97 | .07 | ||||
| No | 214.4 | 3.4 | 202 | 5.7 | ||
| Yes | 248.5 | 7.8 | 175.6 | 16.9 | ||
| Pathology | <.001 | .005 | ||||
| High grade | 97.7 | 15 | 109.9 | 17.1 | ||
| Low grade | 257.4 | 1.7 | 200.1 | 6.3 | ||
| Surgery strategy | .31 | <.001 | ||||
| CR | 217.3 | 2.5 | 210.05 | 3.8 | ||
| IR | 243.5 | 3 | 155.1 | 19.5 | ||
| Preoperative neurological status | .79 | .12 | ||||
| High | 148.2 | 9.8 | 145.28 | 9.8 | ||
| Low | 163.3 | 2.6 | 157.3 | 4.5 | ||
| Tumor location | .46 | .17 | ||||
| Cervical | 214.2 | 3.5 | 203.8 | 6.8 | ||
| Thoracolumbar | 253.8 | 4.3 | 193.8 | 9.6 | ||
| B: Multivariable Cox regression analysis | ||||||
| OS | PFS | |||||
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Age | 0.465 | (0.045-4.8) | .5 | 5.36 | (1.5-18.4) | .008 |
| Pediatric vs adult | ||||||
| Gender | 0.618 | (0.08-4.46) | .6 | 2.58 | (0.8-7.6) | .08 |
| Female vs male | ||||||
| Tumor length | 3.833 | (0.37-39.13) | .2 | 0.9 | (0.2-3.8) | .99 |
| <3 vs ≥3 | ||||||
| Adjuvant therapy | 4.175 | (0.63-27.2) | .1 | 1.783 | (0.4-7.3) | .423 |
| No vs yes | ||||||
| Pathology | 0.013 | (0.002-0.088) | <.001 | 0.18 | (0.04- 0.79) | .023 |
| Low-grade vs high-grade | ||||||
| Surgery strategy | 0.741 | (0.078-7.06) | .79 431 267 | 0.18 | (0.05-0.54) | .004 |
| CR vs IR | ||||||
| Tumor location | 4.32 | (0.59-31.60) | .14 949 901 | 0.722 | (0.2-2.0) | .549 |
| Cervical vs thoracolumbar | ||||||
Abbreviations: OS, overall survival; PFS, progression-free survival; SE, standard error; HR, hazard ratio; CR, complete resection; IR, incomplete resection.
The mean time to progression for patients who underwent biopsy was 188 ± 50.59 months. Two patients in the biopsy group suffered from a recurrence during the follow-up. We performed a Kaplan-Meier analysis to compare PFS of the patients who underwent biopsy and other patients in the IR group. The analysis showed no significant difference (P = .04, log-rank test).
Overall Survival
Overall, a total number of 11 deaths were reported. The mean survival time was 217.3 months and 243.5 months for CR and IR group, respectively. The 5-year OS rate was 97.8% in the CR group and 95.5% in the IR group. Log-rank test did not show a significant difference in the OS between the CR and IR groups (log-rank test, P = .31) (Figure 4). Also, univariate analysis showed that OS was significantly different among patients with high- and low-grade pathology (Table 4). Age, gender, pathology grade, the extent of resection, adjuvant therapy, tumor length, and tumor location were included in the Cox regression model, of which only pathologic grade had a significant effect on OS (HR = 0.013, 95% CI = 0.002-0.088, P < .001) (Table 4).
Seven of 11 cases who underwent biopsy were followed up (mean follow-up = 85.7 months). Log-rank test did not show a significant difference in the OS between the biopsy group and other patients with IR (log-rank test, P = .4).
Neurologic Outcomes
Neurological function was evaluated by using Frankel classification system in 15 cases, JOA classification in 49 patients and McCormick classification in 277 patients. The postoperative neurological evaluation was reported in 286 patients. Compared with the preoperative evaluation, 60 patients showed neurological improvement after the surgery. There are 20.8% of patients who underwent CR experienced improvement. In the IR, the rate of PNI was 22% (Table 5). However, Pearson’s chi-square test revealed that this difference was not significant (P = .8). We performed a binary logistic regression model for PNI using the following variables: age, tumor length, preoperative neurological status, tumor location, gender, and extent of resection. The analysis showed that tumor length was the only independent prognostic factor of PNI (adjusted OR = 0.3, 95% CI = 0.1-0.7, P = .014).
Table 5.
Results of Univariate Chi-Square Analysis and Multivariable Binary Logistic Regression on Postoperative and Follow-up Neurological Status Improvement Compared With Preoperative Score.
| Risk factor | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| A: Postoperative neurological function improvement | ||||
| Age (pediatric vs adult) | 0.08 (0.01-0.6) | .002 | 5.7 (0.7-45.8) | .096 |
| Tumor length (<3 vs ≥3) | 1.5 (0.7-3) | .2 | 0.3 (0.1-0.7) | .014 |
| Preoperative score (low vs high) | 0.7 (0.3-1.8) | .5 | 2 (0.7-5.8) | .164 |
| Tumor location (upper vs lower) | 1.08 (0.6-1.7) | .8 | 0.5(0.1-2) | .353 |
| Sex (female vs male) | 1.06 (0.5-2) | .8 | 0.5(0.2-1.4) | .245 |
| Surgery type (CR vs IR) | 0.95(0.4-2) | .8 | 1.5 (0.3-6.2) | .574 |
| B: Long-term neurological function improvement | ||||
| Age (pediatric vs adult) | 1.1(0.5-2.2) | .7 | 014 (0.04-0.47) | .001 |
| Sex (female vs male) | 1.1 (0.6-1.9) | .6 | 0.5 (0.16-1.6) | .271 |
| Adjuvant therapy (no vs yes) | 1.2 (0.4-3.2) | .6 | 0.03 (0.001-1.4) | .076 |
| Tumor length (<3 vs ≥3) | 0.78 (0.4-1.4) | .4 | 1.08 (0.18-6.2) | .926 |
| Lesion site (upper vs lower) | 1.4 (0.7-2.7) | .2 | 1.8 (0.5-6.75) | .333 |
| Surgery type (CR vs IR) | 0.3 (0.15-0.85) | .01 | 16.5 (1.6-171) | .019 |
| Preoperative score (low vs high) | 0.2 (0.1-0.7) | .003 | 4.4 (1.16-17.3) | .03 |
| Pathology grade (low vs high) | 12 (1.5-99) | .002 | 0.15 (0.006-3.64) | .245 |
Abbreviations: CR, complete resection; IR, incomplete resection.
Neurological evaluation of FNI was reported in 225 patients. The mean follow-up was 48.6 ± 2.35 months (median = 39 months). Compared with the preoperative evaluation, 122 patients showed neurological improvement in long-term follow-up. Although 51% of patients treated with CR had improvement, the incidence of FNI in the IR group was 74% (Table 5). The univariate analysis showed that this difference was significant (Pearson’s chi-square test, P = .01). The multivariable binary logistic regression model for FNI used the following variables: age, tumor length, preoperative score, tumor location, gender, adjuvant therapy, preoperative score, pathologic grade, and extent of resection. Our analysis showed that CR resulted in better neurologic outcome compared with IR (adjusted OR = 16.5, 95% CI = 1.6-171, P = .019). In addition, multivariate analysis showed that preoperative neurological score and age were also independent risk factors for long-term neurologic outcomes (P = .001 for age and P = .03 for a preoperative score) (Table 5).
Discussion
Primary spinal cord ependymoma is very rare with limited data on treatment outcomes and prognostic factors. Ependymoma is more likely to have defined spinal cord planes of cleavage than other intramedullary spinal cord tumors and therefore the surgeons tend to perform CR to treat these tumors.20 To our knowledge, this is the most comprehensive study of intramedullary ependymomas comparing the outcomes of complete and incomplete resection.
This systematic review identified 23 studies with 407 individuals. The 4 main outcomes include OS, PFS, PNI, and FNI. The substantial heterogeneity between the studies included in the review was not unexpected since all the studies were case series. We performed the I2 statistics and the result shows that there was significant heterogeneity between studies for PFS, OS, PNI, and FNI. Then we used meta-regression method to evaluate seven factors for this heterogeneity. However, the analysis revealed that none of the sex, age, tumor location, tumor length, adjuvant therapy, study location, and study year could explain the heterogeneity. Since each study reported cases from a wide span of time, we could not evaluate the exact year of surgery in our meta-regression. Since magnetic resonance imaging and microsurgery methods have changed dramatically in the past decades, the heterogeneity of data could be explained by different year of surgery.
We compared CR and IR using individual patient data. Our results indicate that 78.13% of patients with ependymoma underwent CR. Univariate and multivariate analysis showed that CR was independently associated with improvement in PFS. These results are consistent with previous systematic reviews.51-53 Our analysis showed that postoperative adjuvant radiotherapy did not affect surgical outcomes. This result is consistent with a study conducted by Feldman et al, which reported no association between radiotherapy and overall tumor recurrence, regardless of the extent of resection.52 Wang et al54 also showed that adjuvant therapy could not improve outcomes after resection. However, Chen et al51 reported that adjuvant radiotherapy positively affected PFS. This discrepancy could be due to the population of this study which only included high-grade ependymomas.51 Surprisingly, Hamilton et al55 reported shorter PFS with radiotherapy. This could be due to the fact that the clinicians tend to start radiotherapy when tumors are more aggressive.
In our study, the 5-year OS incidence were 97.8% and 95.5% for IR and CR, respectively, showing that the surgery is associated with a definitive cure in most cases. Surprisingly, the Kaplan-Meier analysis showed no significant difference in the survival between the two groups. The multivariate Cox proportional hazard model showed that age, sex, tumor location, length of the tumor, and adjuvant radiotherapy does not affect this result. It appears that only grade of pathology affects OS (Table 4). This is inconsistent with the systematic review by Wang et al.54 The multivariate Cox model used to evaluate prognostic factors in spinal cord ependymomas showed that the extent of surgery did not increase OS (HR = 0.690, 95% CI = 0.119-3.977, P = .679). Wang et al54 conducted an integrative analysis on grade II ependymomas and found that the extent of surgery did not have a statistical effect on OS. However, most previous reviews claimed that CR is associated with better outcomes. For instance, Hamilton et al55 concluded that CR prolonged OS in ependymomas. The reason for this controversy might be the vast heterogeneity among the studies included in the analysis and failure to include important confounders in the analysis. For example, the integrative analysis performed by Oh et al53 evaluating 348 with spinal cord ependymomas showed that CR is associated with improvement of OS (HR = 0.07, P = .001). But this study only evaluated the use of adjuvant radiotherapy and pathologic grading as confounders.53
The results of this study showed that PNI is not affected by the extent of surgery. The multivariate analysis showed that the tumor length was the only independent prognostic factor of PNI. However, the results of the multivariate analysis indicated that CR resulted in better FNI than IR. This is by far the first integrative analysis evaluating neurologic outcomes regarding the extent of resection. However, since the meta-analysis shows that the heterogeneity between the included studies is significant, this will limit the application of these results in the clinical setting.
This study has several limitations. Due to the rarity of these tumors, all included papers are case series. In addition, different patient treatment plan in different institutions and surgeon experience may be disregarded and may affect the results of the comprehensive analysis. Finally, some studies with large samples were not included in our analysis because they did not report individual patients’ data, leading to a selection bias. A multicenter randomized trial is necessary to confirm the results of this study.
Conclusion
Our study shows that CR can prolong PFS and improve FNI. The overall survival was not significantly affected by the degree of surgery. Patients with spinal cord ependymoma undergone IR had a similar PNI and OS to patients undergone CR, which provides optimal outcomes with longer PFS and better FNI. Therefore, CR should be the primary goal of surgery for every spinal cord ependymoma. However, if the complete resection of the tumor is associated with significant risks of morbidity or even mortality, IR is still acceptable as it can achieve similar overall survival.
Supplemental Material
Supplemental Material, appendix1 for Complete Versus Incomplete Surgical Resection in Intramedullary Ependymomas: A Systematic Review and Meta-analysis by Farhad Salari, Mehdi Golpayegani, Mohsen Sadeghi-Naini, Sara Hanaei, Farhad Shokraneh, Ayat Ahmadi, Hamid Reza Khayat-kashani, Alexander R. Vacarro and Vafa Rahimi-Movaghar in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alexander R. Vacarro, MD, PhD, MBA  https://orcid.org/0000-0002-8073-0796
https://orcid.org/0000-0002-8073-0796
Vafa Rahimi-Movaghar, MD  https://orcid.org/0000-0001-7347-8767
https://orcid.org/0000-0001-7347-8767
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Chamberlain MC. Ependymomas. Curr Neurol Neurosci Rep. 2003;3:193–199. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010;10:240–247. [DOI] [PubMed] [Google Scholar]
- 3. Duong LM, McCarthy BJ, McLendon RE, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012;118:4220–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Figueiredo N, Brooks N, Resnick DK. Evidence-based review and guidelines for the management of myxopapillary and intramedullary ependymoma. J Neurosurg Sci. 2013;57:327–341. [PubMed] [Google Scholar]
- 6. Lee J, Parsa AT, Ames CP, McCormick PC. Clinical management of intramedullary spinal ependymomas in adults. Neurosurg Clin N Am. 2006;17:21–27. [DOI] [PubMed] [Google Scholar]
- 7. McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. [DOI] [PubMed] [Google Scholar]
- 8. Joanna Briggs Institute. The Joanna Briggs Institute Reviewers’ Manual 2014. The Joanna Briggs Institute; 2014. [Google Scholar]
- 9. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrante L, Mastronardi L, Celli P, Lunardi P, Acqui M, Fortuna A. Intramedullary spinal cord ependymomas—a study of 45 cases with long-term follow-up. Acta Neurochir (Wein). 1992;119:74–79. [DOI] [PubMed] [Google Scholar]
- 12. Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323–330. [DOI] [PubMed] [Google Scholar]
- 13. Innocenzi G, Raco A, Cantore G, Raimondi AJ. Intramedullary astrocytomas and ependymonas in the pediatric age group: a retrospective study. Childs Nerv Syst. 1996;12:776–780. [DOI] [PubMed] [Google Scholar]
- 14. Iwasaki Y, Hida K, Sawamura Y, Abe H. Spinal intramedullary ependymomas: surgical results and immunohistochemical analysis of tumour proliferation activity. Br J Neurosurg. 2000;14:331–336. [DOI] [PubMed] [Google Scholar]
- 15. Svoboda N, Bradac O, de Lacy P, Benes V. Intramedullary ependymoma: long-term outcome after surgery. Acta Neurochir (Wein). 2018;160:439–447. [DOI] [PubMed] [Google Scholar]
- 16. Aghakhani N, Messerer M, David P, Herbrecht A, Parker F. Épendymomes intramédullaires: étude rétrospective multicentrique française sur 221 cas. Neurochirurgie. 2017;63:391–397. [DOI] [PubMed] [Google Scholar]
- 17. Hejazi N, Hassler W. Microsurgical treatment of intramedullary spinal cord tumors. Neurol Med Chir (Tokyo). 1998;38:266–273. [DOI] [PubMed] [Google Scholar]
- 18. Sweeney KJ, Reynolds M, Farrell M, Bolger C. Gross total resection rates of grade II/III intramedullary ependymomas using the surgical strategy of en-bloc resection without intra-operative neurophysiological monitoring. Br J Neurosurg. 2017;31:364–368. [DOI] [PubMed] [Google Scholar]
- 19. Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972–981. [PubMed] [Google Scholar]
- 20. Asazuma T, Toyama Y, Suzuki N, Fujimura Y, Hirabayshi K. Ependymomas of the spinal cord and cauda equina: an analysis of 26 cases and a review of the literature. Spinal Cord. 1999;37:753–759. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura M, Ishii K, Watanabe K, et al. Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord. 2008;46:282. [DOI] [PubMed] [Google Scholar]
- 22. Chang UK, Choe WJ, Chung SK, Chung CK, Kim HJ. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133–139. [DOI] [PubMed] [Google Scholar]
- 23. Hulshof M, Menten J, Dito J, Dreissen J, Van den Bergh R, González DG. Treatment results in primary intraspinal gliomas. Radiother Oncol. 1993;29:294–300. [DOI] [PubMed] [Google Scholar]
- 24. Brotchi J, Fischer G. Spinal cord ependymomas. Neurosurg Focus. 1998;4:e2. [DOI] [PubMed] [Google Scholar]
- 25. Prokopienko M, Kunert P, Podgórska A, Marchel A. Surgical treatment of intramedullary ependymomas. Neurol Neurochir Pol. 2017;51:439–445. [DOI] [PubMed] [Google Scholar]
- 26. Kutluk T, Varan A, Kafalı C, et al. Pediatric intramedullary spinal cord tumors: a single center experience. Eur J Paediatr Neurol. 2015;19:41–47. [DOI] [PubMed] [Google Scholar]
- 27. Kane P, el-Mahdy W, Singh A, Powell MP, Crockard HA. Spinal intradural tumours: part II—intramedullary. Br J Neurosurg. 1999;13:558–563. [DOI] [PubMed] [Google Scholar]
- 28. Kucia EJ, Bambakidis NC, Chang SW, Spetzler RF. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Oper Neurosurg. 2011;68(1suppl operative):57–63. [DOI] [PubMed] [Google Scholar]
- 29. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79:204–209. [DOI] [PubMed] [Google Scholar]
- 30. de Andrade FG, de Aguiar PHP, Matushita H, et al. Intracranial and spinal ependymoma: series at Faculdade de Medicina, Universidade de São Paulo. Arq Neuropsiquiatr. 2009;67(3A):626–632. [DOI] [PubMed] [Google Scholar]
- 31. Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2002;51:1162–1174. [DOI] [PubMed] [Google Scholar]
- 32. Ge X, Wu Z, Zhang J, Zhang L. Surgical strategies and functional outcome of intramedullary cervicomedullary ependymoma. Turk Neurosurg. 2017;27:563–572. [DOI] [PubMed] [Google Scholar]
- 33. Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal, cord ependymomas: analysis of 36 cases. Neurosurgery. 1999;44:264–269. [DOI] [PubMed] [Google Scholar]
- 34. Han IH, Kuh SU, Chin DK, Kim KS, Jin BH, Cho YE. Surgical treatment of primary spinal tumors in the conus medullaris. J Korean Neurosurg Soc. 2008;44:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephen JH, Sievert AJ, Madsen PJ, et al. Spinal cord ependymomas and myxopapillary ependymomas in the first 2 decades of life: a clinicopathological and immunohistochemical characterization of 19 cases. J Neurosurg Pediatr. 2012;9:646–653. [DOI] [PubMed] [Google Scholar]
- 36. Ohata K, Takami T, Gotou T, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wein). 1999;141:341–347. [DOI] [PubMed] [Google Scholar]
- 37. Kaner T, Sasani M, Oktenoglu T, Solmaz B, Sarloglu AC, Ozer AF. Clinical analysis of 21 cases of spinal cord ependymoma: positive clinical results of gross total resection. J Korean Neurosurg Soc. 2010;47:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karikari IO, Nimjee SM, Hodges TR, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2015;76(suppl 1):S4–S13. [DOI] [PubMed] [Google Scholar]
- 39. Kochbati L, Nasr C, Frikha H, et al. Les épendymomes intramédullaires primitifs: étude rétrospective de 16 cas. Cancer Radiothér. 2003;7:17–21. [DOI] [PubMed] [Google Scholar]
- 40. Wu L, Yang T, Deng X, et al. Surgical outcomes in spinal cord subependymomas: an institutional experience. J Neurooncol. 2014;116:99–106. [DOI] [PubMed] [Google Scholar]
- 41. Aghakhani N, David P, Parker F, Lacroix C, Benoudiba F, Tadie M. Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery. 2008;62:1279–1285. [DOI] [PubMed] [Google Scholar]
- 42. Safaee M, Oh MC, Mummaneni PV, et al. Surgical outcomes in spinal cord ependymomas and the importance of extent of resection in children and young adults. J Neurosurg Pediatr. 2014;13:393–399. [DOI] [PubMed] [Google Scholar]
- 43. Azzazi A, Sakr S, Sedik M. Microsurgical treatment for intramedullary spinal cord ependymomas: radical surgical resection and outcome. Neurosurg Quart. 2011;21:97–102. [Google Scholar]
- 44. Plotkin SR, O’Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14:543–547. [DOI] [PubMed] [Google Scholar]
- 45. Peker S, Ozgen S, Ozek MM, Pamir MN. Surgical treatment of intramedullary spinal cord ependymomas: can outcome be predicted by tumor parameters? J Spinal Disord Tech. 2004;17:516–521. [DOI] [PubMed] [Google Scholar]
- 46. Tao X, Hou Z, Hao S, et al. The clinical features and surgical outcomes of spinal cord tanycytic ependymomas: a report of 40 cases. World Neurosurg. 2017;106:60–73. [DOI] [PubMed] [Google Scholar]
- 47. Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71:205–210. [DOI] [PubMed] [Google Scholar]
- 48. Liu X, Sun B, Xu Q, et al. Outcomes in treatment for primary spinal anaplastic ependymomas: a retrospective series of 20 patients. J Neurosurg Spine. 2013;19:3–11. [DOI] [PubMed] [Google Scholar]
- 49. Lonjon M, Goh KY, Epstein FJ. Intramedullary spinal cord ependymomas in children: treatment, results and follow-up. Pediatr Neurosurg. 1998;29:178–183. [DOI] [PubMed] [Google Scholar]
- 50. Joaquim AF, dos Santos MJ, Tedeschi H. Surgical management of intramedullary spinal ependymomas. Arq Neuropsiquiatr. 2009;67(2A):284–289. [DOI] [PubMed] [Google Scholar]
- 51. Chen P, Sui M, Ye J, Wan Z, Chen F, Luo C. An integrative analysis of treatment, outcomes and prognostic factors for primary spinal anaplastic ependymomas. J Clin Neurosci. 2015;22:976–980. [DOI] [PubMed] [Google Scholar]
- 52. Feldman WB, Clark AJ, Safaee M, Ames CP, Parsa AT. Tumor control after surgery for spinal myxopapillary ependymomas: distinct outcomes in adults versus children: a systematic review. J Neurosurg Spine. 2013;19:471–476. [DOI] [PubMed] [Google Scholar]
- 53. Oh MC, Ivan ME, Sun MZ, et al. Adjuvant radiotherapy delays recurrence following subtotal resection of spinal cord ependymomas. Neuro Oncol. 2013;15:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Cai R, Wang R, Wang C, Chen C. Outcome predictors in the management of intramedullary classic ependymoma: An integrative survival analysis. Medicine (Baltimore). 2018;97:e10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamilton KR, Lee SS, Urquhart JC, Jonker BP. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci. 2019;63:168–175. [DOI] [PubMed] [Google Scholar]
- 56. Yang T, Wu L, Yang C, Deng X, Xu Y. Clinical features and long-term outcomes of intraspinal ependymomas in pediatric patients. Childs Nerv Syst. 2014;30:2073–2081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, appendix1 for Complete Versus Incomplete Surgical Resection in Intramedullary Ependymomas: A Systematic Review and Meta-analysis by Farhad Salari, Mehdi Golpayegani, Mohsen Sadeghi-Naini, Sara Hanaei, Farhad Shokraneh, Ayat Ahmadi, Hamid Reza Khayat-kashani, Alexander R. Vacarro and Vafa Rahimi-Movaghar in Global Spine Journal