Abstract
Study Design.:
Retrospective cohort study.
Objectives:
To clinically evaluate saphenous nerve somatosensory-evoked potentials (SSEPs) as a reliable and predictable way to detect upper lumbar plexus injury intraoperatively during lateral lumbar trans-psoas interbody fusion (LLIF).
Methods:
Saphenous nerve SSEPs were obtained by stimulation of inferior medial thigh with needle electrodes and recording from transcranial potentials. The primary outcome was measured by testing reproducibility of SSEPs at baseline, changes during the procedure, and relevance to standard modalities. Significant SSEP changes were compared with actual postoperative nerve complications. The sensitivity and specificity of saphenous SSEPs to detect postoperative lumbar plexus nerve injury was calculated.
Results:
A total of 62 patients were included in the study. Reliable saphenous SSEPs were recorded on the LLIF approach side in 52/62 patients. Persistent saphenous SSEP reduction of amplitude of >50% in 6 cases was observed during expansion of the tubular retractor or during the procedure. Two of 6 patients postoperatively had femoral nerve sensory deficits, and 5 of 6 patients had mild femoral nerve motor weakness, all of which resolved at an average of 12 weeks postoperatively (range 2-24 weeks). One patient had saphenous SSEP changes but demonstrated intraoperative recovery and had no postoperative clinical deficits. Saphenous SSEPs demonstrated 52% to 100% sensitivity and 90% to 100% specificity for detecting postoperative femoral nerve complications.
Conclusion:
Saphenous SSEPs can be used to detect electrophysiological changes to prevent femoral nerve injury during LLIF. Intraoperative SSEP recovery after amplitude reduction or loss may be a prognostic factor for final clinical outcome.
Keywords: lateral interbody fusion, neuromonitoring, femoral nerve injury, saphenous nerve, somatosensory-evoked potentials
Introduction
Lateral lumbar trans-psoas interbody fusion (LLIF) is a minimally invasive retroperitoneal technique that has resulted in improved patient-reported outcomes and good to excellent satisfaction ratings while possibly reducing the risks related to traditional anterior or posterior approach surgeries.1,2 Compared with anterior surgery, LLIF may allow for smaller incisions, decreased risk of vascular or visceral injuries, decreased operative time, decreased blood loss, shorter hospital stays, and quicker recovery.1,3-5 Compared with posteriorly based interbody fusions, LLIF does not require entry into the spinal canal or foramina, and nerve root retraction is avoided; furthermore, a larger cage can be placed with the LLIF technique, which may provide a more favorable biomechanical and biological environment for eventual arthrodesis.6,7
Despite these advantages, the trans-psoas approach can result in a high incidence of nerve injury because of direct injury or prolonged retraction of the lumbar plexus, and some studies have reported motor weakness in up to 33.6% of patients and sensory complications in up to 75% of patients postoperatively.3,4,8-10 High anatomical variability of the lumbar plexus and potentially increased tension on the plexus resulting from intraoperative patient positioning make it difficult to establish a reproducible safe zone for the lateral trans-psoas approach. At L4-L5, a safe entry zone is even more challenging to establish because the lumbar plexus occupies up to 50% of the dorsal-ventral disc space.11
In an attempt to minimize iatrogenic nerve injury, intraoperative neuromonitoring (IONM), traditionally electromyography (EMG), is utilized.1 However, unimodal IONM with EMG may be insufficient because EMG has low specificity for detecting iatrogenic nerve injury, particularly secondary to compression, stretch, or focal ischemia that can occur with retractor deployment.8,12,13 Several studies have noted postoperative nerve deficits after LLIF in the setting of normal EMG readings,10,14,15 and subsequent studies have questioned the utility and accuracy of EMG during LLIF.16,17
Somatosensory-evoked potentials (SSEP) may be used in addition to EMG during LLIF as a multimodal approach to IONM to minimize iatrogenic nerve injury. SSEPs are sensitive to ischemic changes, but standard SSEP monitoring traditionally only tracks the lower lumbosacral plexus (L4-S2) via the posterior tibial nerve or the peroneal nerve. The upper lumbar plexus (L2-L4), at risk during LLIF, gives rise to the femoral nerve, which divides into anterior and posterior divisions in the thigh and continues as the saphenous nerve. In the distal thigh, the saphenous nerve becomes superficial between tendons of the sartorius and gracillis muscles where it can be monitored.16 The purpose of this study was to clinically evaluate saphenous nerve SSEPs as a reliable and predictable way to detect upper lumbar plexus injury intraoperatively during LLIF.
Methods
Institutional review board approval was obtained for this study prior to data collection. The medical records of 62 consecutive patients who underwent LLIF between 2013 and 2015 at a single institution were retrospectively reviewed. All patients undergoing LLIF during the study time period at a single institution were included. Patients in whom lower-extremity neuromonitoring was not possible were excluded. Patient demographics and surgical details were reviewed and collected using our institution’s electronic medical record.
Intraoperative saphenous nerve SSEPs were obtained by stimulation of the inferior medial thigh with needle electrodes and somatosensory cortex transcranial recordings (Figure 1). Saphenous nerve SSEPs were added to standard IONM modalities, including posterior tibial SSEP, EMG, and motor-evoked potential. EMG was conducted in a standard fashion by placing subdermal needle electrodes in the lower-extremity musculature associated with the lumbar surgical levels being operated on. SSEPs were obtained prior to incision, and baseline data was established. All IONM was performed by a certified neurophysiologist who was remotely supervised by a neurologist. Neuromuscular blockade was used for intubation only. All IONM was performed under complete absence of neuromuscular blockade and with the use of intravenous, as opposed to inhalational, anesthesia. A threshold criterion of a 10% increase in SSEP latency and/or a 50% decrease in SSEP amplitude was used to alert the attending surgeon for intervention.
Figure 1.
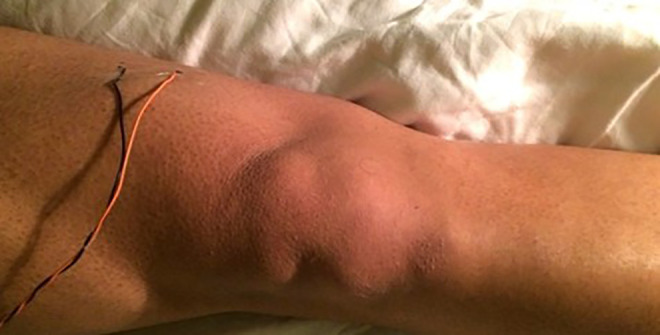
Example of saphenous nerve electrode placement.
Surgical technique was performed in the standard lateral lumbar trans-psoas approach fashion as described by Ozgur et al.1 The same retractor was used in each case, which consisted of an expandable blade retractor that was placed over the largest dilator. The retractor was secured to the operating table and expanded for all cases. No cases involved placing pins into the vertebral bodies to allow for docking.
The primary outcomes included the reproducibility of saphenous SSEPs at baseline and changes in saphenous SSEP latency and amplitude during the LLIF procedure (Figure 2). The study group was defined as those patients with saphenous SSEP peak latency increases greater than 10% and/or amplitude decreases greater than 50%. The remainder of the patients were considered the control group. Patient symptoms and clinical neurological deficits were retrospectively reviewed and correlated with IONM changes. Motor deficits were confined to weakness with hip flexion or adduction, or knee extension (strength ≤ 4 out of 5 on the Medical Research Council (MRC) scale). Sensory deficits included anterolateral thigh or groin numbness and/or dysesthesia. Sensitivity, specificity, and associated 95% CIs were calculated. Parametric univariate analysis was used to compare operative times between patients with and without SSEP signal changes.
Figure 2.
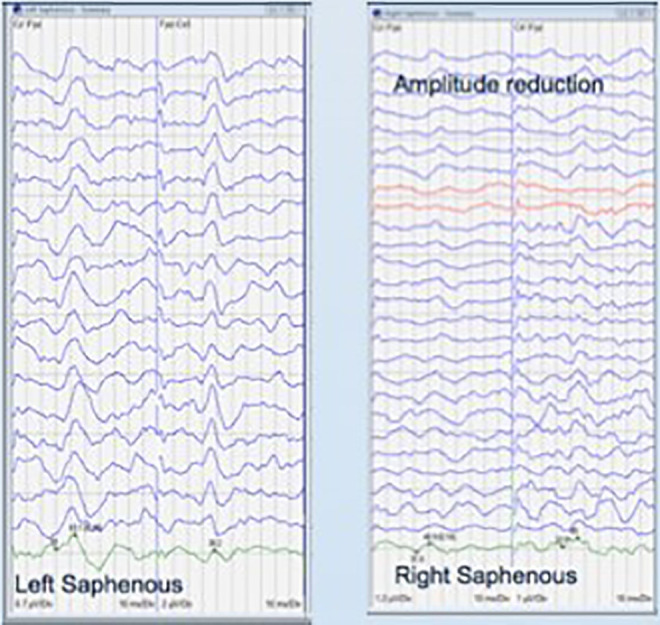
Examples of normal and reduced amplitude saphenous somatosensory-evoked potential tracings.
Results
The case series consisted of 62 patients with an average age of 62.9 years; 77% were female. Average follow-up was 6 months. A total of 69 levels were operated on, of which 11 were L1-2, 20 were L2-3, 22 were L3-4, and 16 were L4-5. Reliable SSEPs were recorded on the approach side in 52 of 62 patients.
Reduction of saphenous SSEP amplitude >50% and/or increased latency >10% occurred in 7 of 52 cases (study group) during expansion of the tubular retractors. The remaining 45 patients had normal saphenous SSEPs throughout the LLIF procedure, although 4 of these 45 patients had changes in other IONM modalities, including EMG changes. None of these 4 patients had significant clinical deficits postoperatively. Operative time was 245 minutes for patients with SSEP changes and 319 minutes for patients without SSEP changes (P = .08).
Among the 7 patients with SSEP changes, 1 patient had complete IONM recovery intraoperatively and had no postoperative clinical deficits. The remaining 6 patients had partial but incomplete IONM recovery intraoperatively. Two of the 6 patients with persistent intraoperative changes had postoperative femoral nerve sensory deficits; 5 of 6 patients had mild hip flexion weakness. All deficits were resolved at an average of 12 weeks postoperatively (range 2-24 weeks). True clinical positives and negatives with regard to saphenous SSEP detecting postoperative femoral nerve complications are listed in Table 1. The sensitivity and specificity analysis are presented in Table 2.
Table 1.
Saphenous SSEP Detection of Postoperative Femoral Nerve Complications.
| Clinical Positive | Clinical Negative | Total | |
|---|---|---|---|
| Saphenous SSEP positive | 6 | 0 | 6 |
| Saphenous SSEP negative | 0 | 46 | 46 |
| Total | 6 | 46 | 52 |
Abbreviation: SSEP, somatosensory-evoked potential.
Table 2.
Estimates of Population Prevalence, Sensitivity, and Specificity of Saphenous SSEP Detection of Postoperative Femoral Nerve Complications.
| Estimated Value | 95% CI Lower Limit | 95% CI Upper Limit | |
|---|---|---|---|
| Disease prevalence | 0.115 | 0.048 | 0.241 |
| Sensitivity | 1 | 0.517 | 1 |
| Specificity | 1 | 0.904 | 1 |
Discussion
LLIF was developed as a minimally invasive alternative for interbody fusion that can potentially avoid the approach-related complications associated with anterior and posterior direct approaches.1 Recent studies have demonstrated improved clinical and radiographic outcomes in patients undergoing LLIF for degenerative pathology2,18-21; however, this minimally invasive technique has resulted in a new set of approach-related nerve complications during interbody fusion as a result of traversing of the psoas muscle, which contains the lumbar plexus.3,4,8-10
The high incidence of nerve injury after LLIF resulted in several studies attempting to use IONM to minimize the risk of lumbar plexus injury. Tohmeh et al22 used free-run and triggered EMG intraoperatively in a series of 102 patients undergoing LLIF, and 17.5% of patients experienced postoperative sensory deficits. Similarly, Le et al23 demonstrated a 19.1% rate of postoperative ipsilateral thigh numbness while using EMG IOMN, although the incidence over time did decrease. Several other studies have also demonstrated relatively high rates of postoperative nerve complications in the setting of normal IONM EMG readings,10,14,15 and researchers have concluded that stand-alone EMG is largely inadequate for monitoring trans-psoas approaches to the lumbar spine.16,24
The use of SSEPs to detect intraoperative nerve damage in the lumbar spine remains an area of interest. Duncan et al17 were the first to describe lumbar nerve root injury likely secondary to ischemia/distraction, with associated SSEP changes in the setting of normal EMG; however, this was during transforaminal lumbar interbody fusion. The results of this study demonstrate that saphenous SSEPs can be recorded during LLIF to detect electrophysiological changes and potentially prevent femoral nerve/upper lumbar plexus injury during LLIF. Among the 46 without persistent SSEP changes, all 46 (100%) had normal postoperative exams without evidence of nerve injury. Six patients demonstrated persistent intraoperative SSEP changes and met threshold values, and all 6 (100%) demonstrated postoperative motor or sensory weakness, of which all resolved within 12 months. Silverstein et al16 performed a similar study in 2014 in a smaller cohort (46 patients), assessing the efficacy of using saphenous nerve SSEPs during LLIF. In their study, 4 patients demonstrated persistent intraoperative SSEP changes, and 3 (75%) patients demonstrated postoperative nerve deficits.16 Similar to the current study, intraoperative SSEP changes demonstrated a high sensitivity and specificity for postoperative nerve deficits. Finally, the current study was unable to establish reliable SSEP signals in 16% of patients, which is also similar to the study by Silverstein et al,16 in which 11% of patients had unreliable signals. The ability to establish reliable saphenous SSEP signals can be affected by body habitus, limb length, depth of the saphenous nerve, medical comorbidities, anesthetic agents, and intraoperative hemodynamics. To the authors’ knowledge, Silverstein et al are the only other set of authors to assess the use of saphenous nerve SSEPs during LLIF.
Although the findings of our study demonstrate possible benefit of saphenous SSEP monitoring during LLIF, there are several limitations. First, saphenous SSEPs were not reliably established in 10 (16%) patients, similar to the study by Silverstein et al.16 Second, this was a retrospective study subject to selection bias, although a consecutive series of patients was utilized in an attempt to minimize this bias. Third, the descriptions of nerve complications, including motor and sensory deficits, were not precisely defined. Fourth, we did not have the ability to assess retractor times; operative times were similar between the 2 groups, but this comparison may be subject to type II error. Fifth, we did not conduct a cost-benefit analysis because we did not have data available to us to assess how a potential saphenous SSEP signal change affected the surgery in terms of prolonging operative time or changing the anesthesia plan; the lack of a control group prevents analysis of other potential cost-benefits (return to operating room, return to work) related to postoperative neuropraxia that may be decreased with saphenous SSEP monitoring. Finally, this was a relatively small case-control series of 62 patients from a single institution, which thereby limits the generalizability of the results; however, it is the largest study to date assessing the efficacy of this IONM technique.
In conclusion, postoperative nerve complications after LLIF continue to occur, even with the use of EMG IONM. Several studies have examined the use of multimodal IONM techniques to better detect and prevent nerve complications during LLIF.16,24-26 The use of saphenous SSEPs as part of a multimodal approach to IONM during LLIF may be an effective technique to prevent lumbar plexus nerve injury. In the present study, saphenous SSEPs demonstrated 52% to 100% sensitivity and 90% to 100% specificity for detecting postoperative femoral nerve complications and may be a prognostic factor for final clinical outcome. A prospectively designed study with a larger cohort is needed to more accurately and precisely investigate the utility of this IONM technique.
Footnotes
- Nickul Jain (NSJ): Consultant for Spineart, Research support from Globus, Committee member, NASS
- Daniel Yanni (DY): Surgeon education consultant for Zimmer Biomet, Spinal Elements. Ceronovus education consultant
- Sam Bederman (SB) : SpineArt - consulting, royalties; Alphatec - consulting, royalties; Mighty Oak Medical – consulting.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ram Alluri, MD  https://orcid.org/0000-0001-5919-707X
https://orcid.org/0000-0001-5919-707X
References
- 1. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. [DOI] [PubMed] [Google Scholar]
- 2. Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976). 2010;35(26, suppl):S302–S311. [DOI] [PubMed] [Google Scholar]
- 3. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. Spine (Phila Pa 1976). 2010;35(26, suppl):S322–S330. [DOI] [PubMed] [Google Scholar]
- 4. Arnold PM, Anderson KK, McGuire RA, Jr. The lateral transpsoas approach to the lumbar and thoracic spine: a review. Surg Neurol Int. 2012;3(suppl 3):S198–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbagallo G, Albanese V, Raich A, Dettori J, Sherry N, Balsano M. Lumbar lateral interbody fusion (LLIF): comparative effectiveness and safety versus PLIF/TLIF and predictive factors affecting LLIF outcome. Evid Based Spine Care J. 2014;5:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laws CJ, Coughlin DG, Lotz JC, Serhan HA, Hu SS. Direct lateral approach to lumbar fusion is a biomechanically equivalent alternative to the anterior approach: an in vitro study. Spine (Phila Pa 1976). 2012;37:819–825. [DOI] [PubMed] [Google Scholar]
- 7. Ploumis A, Wu C, Fischer G, et al. Biomechanical comparison of anterior lumbar interbody fusion and transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2008;21:120–125. [DOI] [PubMed] [Google Scholar]
- 8. Ahmadian A, Deukmedjian AR, Abel N, Dakwar E, Uribe JS. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: diagnostic standardization. J Neurosurg Spine. 2013;18:289–297. [DOI] [PubMed] [Google Scholar]
- 9. Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J. 2012;21:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sofianos DA, Briseño MR, Abrams J, Patel AA. Complications of the lateral transpsoas approach for lumbar interbody arthrodesis: a case series and literature review. Clin Orthop Relat Res. 2012;470:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regev GJ, Chen L, Dhawan M, Lee YP, Garfin SR, Kim CW. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34:1330–1335. [DOI] [PubMed] [Google Scholar]
- 12. Houten JK, Alexandre LC, Nasser R, Wollowick AL. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine. 2011;15:280–284. [DOI] [PubMed] [Google Scholar]
- 13. Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139–144. [DOI] [PubMed] [Google Scholar]
- 14. Cahill KS, Martinez JL, Wang MY, Vanni S, Levi AD. Motor nerve injuries following the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;17:227–231. [DOI] [PubMed] [Google Scholar]
- 15. Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011;15:11–18. [DOI] [PubMed] [Google Scholar]
- 16. Silverstein J, Mermelstein L, DeWal H, Basra S. Saphenous nerve somatosensory evoked potentials: a novel technique to monitor the femoral nerve during transpsoas lumbar lateral interbody fusion. Spine (Phila Pa 1976). 2014;39:1254–1260. [DOI] [PubMed] [Google Scholar]
- 17. Duncan JW, Bailey RA, Baena R. Intraoperative decrease in amplitude of somatosensory-evoked potentials of the lower extremities with interbody fusion cage placement during lumbar fusion surgery. Spine (Phila Pa 1976). 2012;37:E1290–E1295. [DOI] [PubMed] [Google Scholar]
- 18. Louie PK, Haws BE, Khan JM, et al. Comparison of stand-alone lateral lumbar interbody fusion versus open laminectomy and posterolateral instrumented fusion in the treatment of adjacent segment disease following previous lumbar fusion surgery. Spine (Phila Pa 1976). 2019;44:E1461–E1469. [DOI] [PubMed] [Google Scholar]
- 19. Ahmadian A, Bach K, Bolinger B, et al. Stand-alone minimally invasive lateral lumbar interbody fusion: multicenter clinical outcomes. J Clin Neurosci. 2015;22:740–746. [DOI] [PubMed] [Google Scholar]
- 20. Castellvi AE, Nienke TW, Marulanda GA, Murtagh RD, Santoni BG. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res. 2014;472:1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16:329–333. [DOI] [PubMed] [Google Scholar]
- 22. Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14:31–37. [DOI] [PubMed] [Google Scholar]
- 23. Le TV, Burkett CJ, Deukmedjian AR, Uribe JS. Postoperative lumbar plexus injury after lumbar retroperitoneal transpsoas minimally invasive lateral interbody fusion. Spine (Phila Pa 1976). 2013;38:E13–E20. [DOI] [PubMed] [Google Scholar]
- 24. Chaudhary K, Speights K McGuire K, White AP. Trans-cranial motor evoked potential detection of femoral nerve injury in trans-psoas lateral lumbar interbody fusion. J Clin Monit Comput. 2015;29:549–554. [DOI] [PubMed] [Google Scholar]
- 25. Silverstein J, Block J. The utility of transcranial motor evoked potentials (MEPs) for intraoperative monitoring of femoral nerve function for retroperitoneal transpsoas access to the spine. Neurodiagn J. 2014;54:356. [Google Scholar]
- 26. Block J, Silverstein JW, Ball HT, et al. Motor evoked potentials for femoral nerve protection in transpsoas lateral access surgery of the spine. Neurodiagn J. 2015;55:36–45. [DOI] [PubMed] [Google Scholar]


