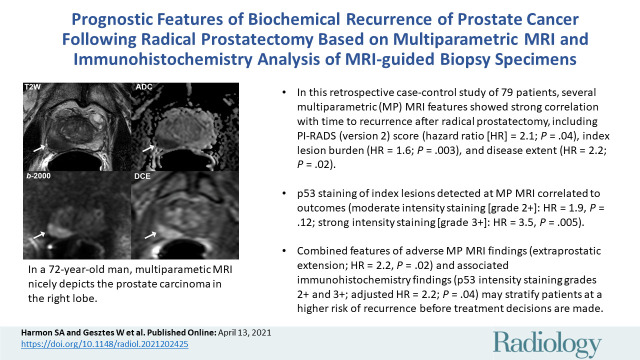
Abstract
Background
Although prostate MRI is routinely used for the detection and staging of localized prostate cancer, imaging-based assessment and targeted molecular sampling for risk stratification are an active area of research.
Purpose
To evaluate features of preoperative MRI and MRI-guided biopsy immunohistochemistry (IHC) findings associated with biochemical recurrence (BCR) of prostate cancer after surgery.
Materials and Methods
In this retrospective case-control study, patients underwent multiparametric MRI before MRI-guided biopsy followed by radical prostatectomy between 2008 and 2016. Lesions were retrospectively scored with the Prostate Imaging Reporting and Data System (PI-RADS) (version 2) by radiologists who were blinded to the clinical-pathologic results. The IHC staining, including stains for the ETS-related gene, phosphatase and tensin homolog, androgen receptor, prostate specific antigen, and p53, was performed with targeted biopsy specimens of the index lesion (highest suspicion at MRI and pathologic grade) and scored by pathologists who were blinded to clinical-pathologic outcomes. Cox proportional hazards regression analysis was used to evaluate associations with recurrence-free survival (RFS).
Results
The median RFS was 31.7 months (range, 1–101 months) for 39 patients (median age, 62 years; age range, 47–76 years) without BCR and 14.6 months (range, 1–61 months) for 40 patients (median age, 59 years; age range, 47–73 years) with BCR. MRI features that showed a significant relationship with the RFS interval included an index lesion with a PI-RADS score of 5 (hazard ratio [HR], 2.10; 95% CI: 1.05, 4.21; P = .04); index lesion burden, defined as ratio of index lesion volume to prostate volume (HR, 1.55; 95% CI: 1.2, 2.1; P = .003); and suspicion of extraprostatic extension (EPE) (HR, 2.18; 95% CI: 1.1, 4.2; P = .02). Presurgical multivariable analysis indicated that suspicion of EPE at MRI (adjusted HR, 2.19; 95% CI: 1.1, 4.3; P = .02) and p53 stain intensity (adjusted HR, 2.22; 95% CI: 1.0, 4.7; P = .04) were significantly associated with RFS.
Conclusion
MRI features, including Prostate Imaging Reporting and Data System score, index lesion burden, extraprostatic extension, and preoperative guided biopsy p53 immunohistochemistry stain intensity are associated with biochemical relapse of prostate cancer after surgery.
© RSNA, 2021
Online supplemental material is available for this article.
See also the editorial by Costa in this issue.
Summary
Multiparametric MRI provides prognostic information, and pairing information from MRI with molecular characterization from biopsy samples may assist prostate cancer risk stratification.
Key Results
■ In this retrospective case-control study of 79 patients, several multiparametric MRI features showed strong correlation with time to recurrence after radical prostatectomy, including Prostate Imaging Reporting and Data System (version 2) score (hazard ratio [HR], 2.10; P = .04), index lesion burden (HR, 1.55; P = .003), and disease extent (HR, 2.18; P = .02).
■ The p53 immunohistochemistry (IHC) staining of index lesions detected at multiparametric MRI correlated to outcomes (moderate intensity staining [2+ intensity]: HR, 1.92, P = .12; strong intensity staining [3+ intensity]: HR, 3.46, P = .005).
■ Combined features of adverse multiparametric MRI findings (extraprostatic extension; adjusted HR, 2.19, P = .02) and associated IHC findings (p53 intensity staining 2+ and 3+; adjusted HR, 2.22; P = .04) may stratify patients at a higher risk of recurrence before treatment decisions are made.
Introduction
Prostate cancer exhibits a broad spectrum of biologic aggressiveness, and determining appropriate treatment requires accurate diagnosis and risk assessment (1). Risk stratification according to clinical (eg, prostate-specific antigen [PSA] level) and histopathologic (eg, Gleason score) characteristics can help distinguish indolent versus aggressive disease likely to result in a biochemical recurrence (BCR) after definitive local treatment (1). However, addressing the diversity of aggressiveness across intermediate risk groups remains challenging (2), leading to unnecessary interventions in some patients with low- to intermediate-risk disease and undertreatment of other men who may be harboring lethal variants of clinically identical disease.
An important component for more accurately characterizing prostate cancers is precise sampling of the most aggressive features within a lesion. Multiparametric MRI has become an established imaging technique for the identification of intraprostatic disease for diagnosis and staging of prostate cancer (3). Its clinical implementation has resulted in improved detection and more accurate biopsy sampling of clinically significant prostate cancer, and it also decreases the detection of indolent low-risk prostate cancer (4–6). However, the potential for enhanced disease characterization using both multiparametric MRI features and tissue features from biopsy material must be better understood.
Tissue-based biomarker studies have resulted in several potential biologic correlates of adverse disease and poor prognosis in prostate cancer. Some of these can be efficiently evaluated with clinical-grade immunohistochemistry (IHC) assays. Documented prevalence of TP53 and androgen receptor defects in the genomic landscape of metastatic disease provide a strong rationale for investigating these transcription factors in localized disease (7). Additionally, the tumor suppressor gene phosphatase and tensin homolog (PTEN) within the phosphoinositide 3-kinase pathway and the oncogene ETS-related gene (ERG) are associated with aggressive prostate cancer and poor outcomes (8–10). The objective of this study was to evaluate features of preoperative MRI and MRI-guided biopsy IHC findings of prostate cancer associated with BCR after surgery.
Materials and Methods
Patient Overview
This retrospective case-control study was compliant with the Health Insurance Portability and Accountability Act and approved by appropriate institutional review boards at each institution (approval numbers: 16-CN-115 for center A [imaging and surgery] and HU-CPDR-90–3894 for center B [immunohistochemistry]). Informed consent was waived.
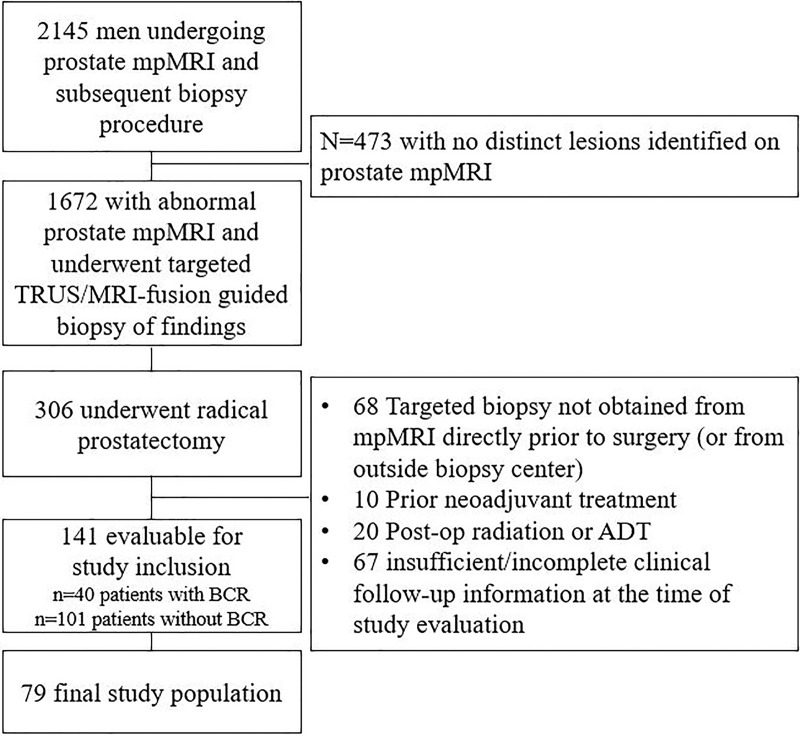
Eligibility for retrospective evaluation and study inclusion required patients to have undergone MRI of the prostate at center A followed by image-guided biopsy of the index lesions based on MRI findings and subsequent radical prostatectomy between 2008 and 2016 with follow-up data available. Patients with evidence of disease recurrence were identified, and control patients with no recurrence were matched from a pool of 141 eligible patients selected according to Gleason grade for radical prostatectomy, preoperative PSA level, and age. Patient selection was prioritized according to the availability of tissue specimens and patients with at least 2 years of follow-up, when available, and propensity scoring was completed to evaluate selection. Additional exclusion criteria included any neoadjuvant therapy before radical prostatectomy or insufficient follow-up information (Fig 1). This study population was reported in a previous publication, which aimed to evaluate the use of MRI-targeted, systematic, or combined prostate biopsy in an attempt to define the most effective method for prostate cancer diagnosis (6).
Figure 1:
Flowchart shows determination of final study population. ADT = androgen deprivation therapy, BCR = biochemical recurrence, mpMRI = multiparametric MRI, TRUS = transrectal US.
MRI Scans
Patients were scanned with a 3.0-T scanner (Achieva, Philips) using a combination of a 16-channel surface-array coil (Sense cardiac surface coil, Philips Medical Systems) and an endorectal coil (BPX-30, Bayer) (n = 74 patients) or a 32-channel cardiac coil (Sense, Invivo) (n = 5 patients). T2-weighted diffusion-weighted sequences, with a high b value and apparent diffusion coefficient (ADC) mapping, and dynamic contrast-enhanced sequences were performed. The complete list of multiparametric MRI acquisition protocols is shown in Table E1 (online).
MRI Evaluations
All imaging findings were evaluated by a genitourinary radiologist (B.T., with more than 12 years of cumulative experience who evaluates more than 3000 MRI scans per year) at the time of acquisition for biopsy targeting. For patients undergoing imaging before the introduction of Prostate Imaging and Reporting Data System (PI-RADS) (version 2), an in-house scoring system was used (11). A retrospective evaluation was performed by the same genitourinary radiologist who followed PI-RADS (version 2) guidelines with a minimum washout period of 24 months since the initial clinical interpretations. Additionally, regions of interest for each detected lesion were manually contoured with the T2-weighted images by using commercial software (version 6.6.10; MIM), which enabled spatial referencing to images from all other sequences to ensure lesion features were encompassed in entirety within the region of interest.
Total lesion burden was defined as the ratio of all lesion volumes to total prostate volume. Index lesions were defined as lesions visible at MRI that also had the highest suspicion score and highest Gleason grade at targeted biopsy. Index lesion burden was defined as the ratio of index lesion volume to total prostate volume. Retrospective grading of imaging-based suspicion for extraprostatic involvement at multiparametric MRI was also reported according to the study by Mehralivand et al (12). Quantitative ADC metrics, including median, 10th percentile, and total diffusivity index, defined as the ratio of lesion volume to median ADC (13), were extracted for index lesions using regions of interest resampled to the spatial resolution of the ADC images.
Targeted Biopsy and Specimen Processing
Patients with suspicious lesions underwent MRI and transrectal US fusion–guided biopsy. Biopsy targets were selected based on multiparametric MRI findings, and biopsies were performed using the UroNav platform (Invivo). All procedures were performed by either one urologist (P.A.P.) or one interventional radiologist (B.J.W.), both of whom have performed more than 1500 MRI and transrectal US fusion–guided biopsies for more than 10 years. All specimens were coded by the original target location before tissue processing. One experienced genitourinary pathologist from center A (M.J.M., with more than 25 years of experience in the interpretation of prostate histopathologic specimens) analyzed the resultant biopsy cores. The index lesion was defined as the lesion visible at MRI that also had the highest suspicion score and highest Gleason grade at targeted biopsy.
All patients underwent subsequent robotic-assisted radical prostatectomy at center A. Clinical assessments of the prostate specimens were recorded for every patient, including findings of extraprostatic extension (EPE), presence of seminal vesicle involvement, presence of perineural invasion, presence of lymphovascular invasion, and final primary and secondary Gleason scores.
Histopathologic and IHC Evaluation
Biopsy specimens corresponding to presurgical imaging-based index lesions were accessioned, serially sectioned at 5-μm intervals, and de-identified of any clinical information for IHC analysis at center B. Hematoxylin-eosin staining was performed along with the characterization of IHC protein expression for five prostate cancer–related oncoproteins—androgen receptor, PSA, ERG, PTEN, and p53. All slides were reviewed by an expert genitourinary pathologist at center B (I.A.S., with more than 25 years of experience) who was blinded to all patient clinical data and outcomes. Staining procedures are reported in Table E2 (online). IHC findings were reported as follows.
ERG.—Absence of ERG staining in all cancerous cells with ERG-positive staining in endothelial cells as positive controls was classified as an ERG-negative finding. Positive staining of cancerous cells in the core specimen were considered positive for ERG protein expression. The rare phenomenon of variable ERG protein expression in a mosaic pattern within a single neoplastic gland or cluster of glands was classified as ERG hybrid.
PSA.—Staining was classified as either positive or negative in cases of uniform staining appearance of cancerous cells within the core specimen. Tumor specimens with positive PSA staining except for aggregates of absent or weakly staining cells were classified as variable.
Androgen receptor.—Staining was classified as either positive (ie, cancerous cells with detectable androgen receptor protein) or negative (ie, cancerous cells without androgen receptor protein).
PTEN.—PTEN status was interpreted as positive (ie, normal expression with strong or weak intensity staining), negative (ie, absent or nondetectable PTEN tumor suppressor gene), or variable (ie, PTEN positive with various degrees of focally deleted cell populations).
p53.—Staining was evaluated according to nuclear accumulation in cancer cells, assessed as either negative or positive. Lesions with evidence of positive p53 staining were further categorized according to the percentage of cancerous cells expressing any nuclear protein accumulation, estimated incrementally, and the highest nuclear accumulation stain intensity was rated, as follows: (a) rare, less than 1% of cells; (b) 1+ intensity, weak intensity staining with less than 5% of cells; (c) 2+ intensity, moderate intensity staining with less than 10% of cells; and (d) 3+ intensity, strong intensity staining with more than 10% of cells.
Outcome Measures
All patients were followed up after surgery with serial serum PSA measurements, per standard clinical guidelines (16). Recurrence was defined as serum PSA levels greater than or equal to 0.2 ng/mL at two consecutive measurements or clinical assessment consistent with recurrence leading to altered patient treatment, including positive imaging findings consistent with recurrent disease and/or subsequent salvage radiation therapy or systemic treatments. Recurrence-free survival (RFS) was defined as the number of days from radical prostatectomy to the day the patient experienced a recurrence event. Patients not experiencing disease recurrence were censored to the last appropriate examination date.
Statistical Analysis
Propensity scoring was evaluated to assess quality and potential bias of the final cohort selection compared with the selection pool on the basis of predefined variables (ie, age, preoperative PSA level, and final Gleason grade group). The area under the curve from the propensity scores model before and after patient selection calculated from a linear regression model for binary association with BCR groups was 0.82 (95% CI: 0.74, 0.89) and 0.65 (95% CI: 0.52, 0.77), respectively.
The association of continuous clinical (ie, PSA level, PSA density, and age) and radiologic (ie, total lesion burden, index lesion burden, and ADC) metrics with binary recurrence outcome was evaluated with the nonparametric Wilcoxon test. Association of remaining categoric data with recurrence was reported with the Fisher exact test. Cox proportional hazard regression analyses were conducted to evaluate associations between clinical and imaging variables with RFS. In a multivariable analysis, forward and backward selection methods were used to identify a parsimonious model. Univariable predictors with P < .2 were considered for inclusion in the initial nonparsimonious model. Backward selection was performed using Bayesian information criteria. A Kaplan-Meier analysis was completed for a combination of selected variables in the parsimonious model, and the log-rank test was used for comparison between groups. All reported P values were two-sided, and P < .05 indicated a statistically significant difference. Statistical analyses were conducted with R software (version 3.4.1, the R Foundation for Statistical Computing).
Results
Patient Characteristics
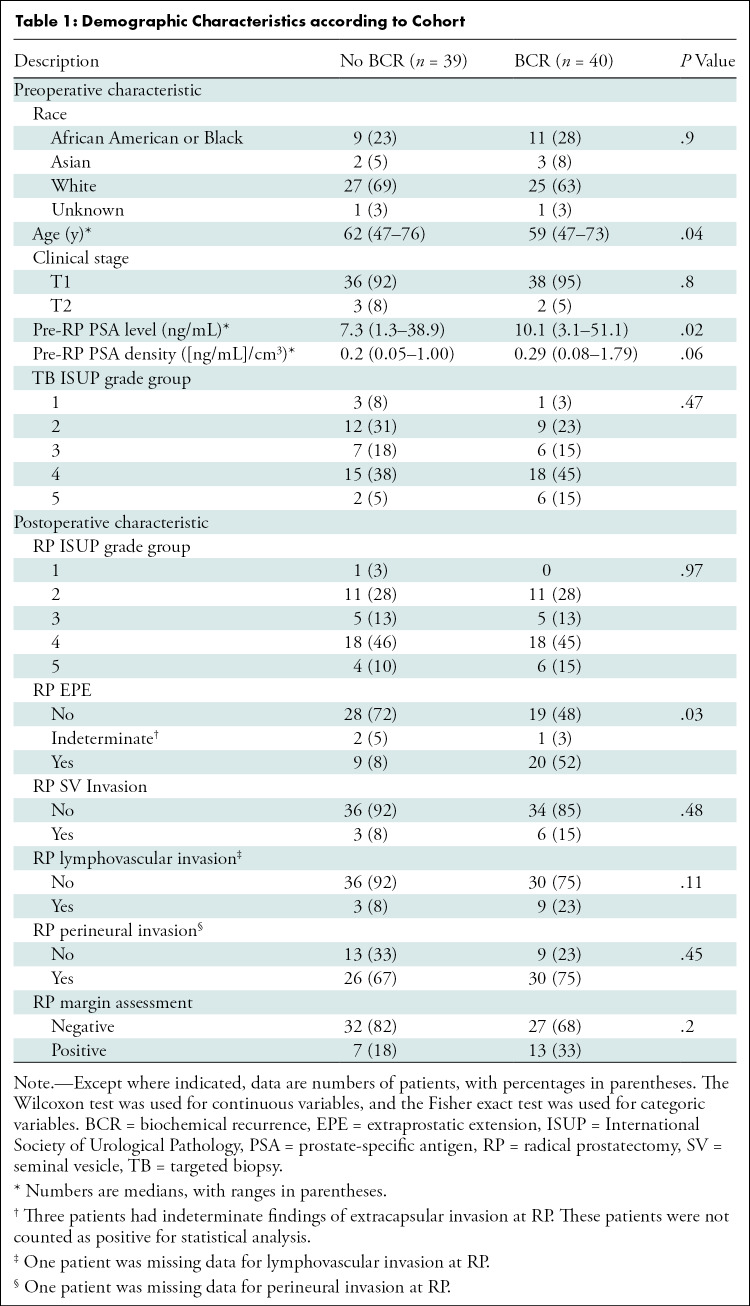
Thirty-nine patients (median age, 62 years; range, 47–76 years) without BCR, or non-BCR, and 40 patients (median age, 59 years; range, 47–73 years) with BCR were included. The median RFS was 14.6 months (range, 1–61 months) for patients with BCR and 31.7 months (range, 1–101 months) for patients without BCR. A summary of patient demographic information, surgical outcomes, radiologic finding, and IHC findings are presented in Tables 1 and 2, respectively. After case-control selection, no significant difference between the BCR and non-BCR groups was observed for radical prostatectomy International Society of Urological Pathology grade groups (P = .47); however, age (P = .04), and PSA level (P = .02) remained different between the groups (Table 1).
Table 1:
Demographic Characteristics according to Cohort
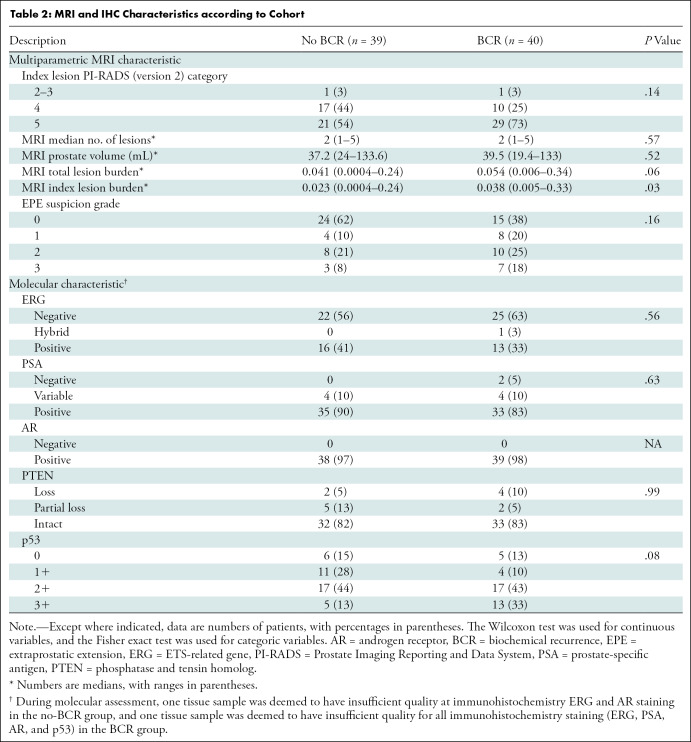
Table 2:
MRI and IHC Characteristics according to Cohort
MRI Characteristics
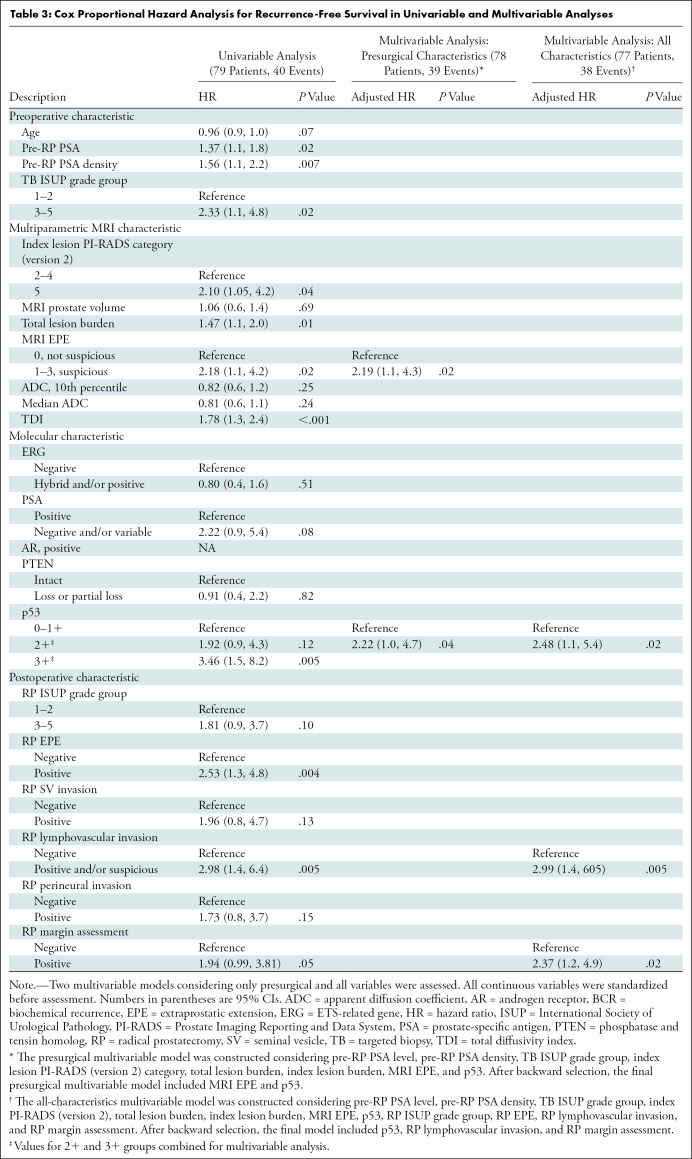
In total, 145 lesions were prospectively detected at multiparametric MRI (median number of lesions, two; range, one to five lesions per patient). Summary characteristics are provided in Table 2. A representative high-risk multiparametric MRI lesion is shown in Figure 2. In the univariable analysis, MRI characteristics demonstrating a significant association with shorter RFS included an index lesion with a PI-RADS score of 5, total lesion burden, index lesion burden, any grade 1–3 MRI-based EPE suspicion, and total diffusivity index (Table 3). The most significant of these features in the univariable analysis was index lesion burden (HR, 1.55; 95% CI: 1.2, 2.1; P < .003), whereas MRI-based EPE suspicion showed the highest risk relationship with RFS (HR, 2.18; 95% CI: 1.1, 4.2; P = .02).
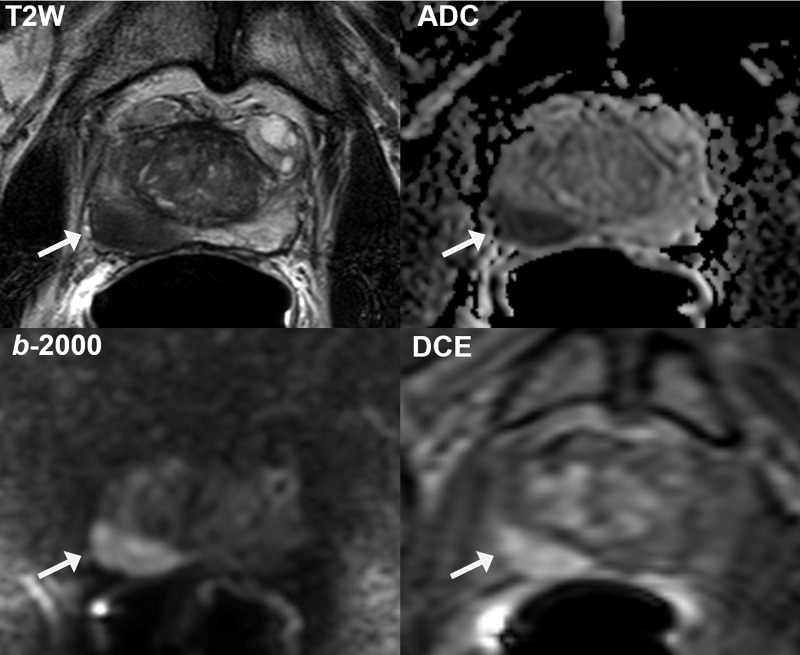
Figure 2:
Representative multiparametric MRI scans obtained in 72-year-old African American man with serum prostate-specific antigen concentration of 7.32 ng/mL show large Prostate Imaging Reporting and Data System (version 2) category 5 lesion (arrow) in right midbase peripheral zone (volume, 2.3 mL; index lesion burden, 0.07). The lesion is well visualized on T2-weighted (T2W) image, apparent diffusion coefficient (ADC) map, high b-value (2000 sec/mm2) diffusion-weighted image (b-2000), and dynamic contrast-enhanced (DCE) image, with grade 3 features suspicious for extraprostatic extension. Targeted biopsy revealed Gleason grade 4+3 tumor (International Society of Urological Pathology grade group 3). Final histopathologic findings revealed this to be the only location of malignancy, with final Gleason grade 4+3 tumor (International Society of Urological Pathology grade group 3) encompassing 20% of right prostatic lobe and no evidence of extraprostatic extension or seminal vesicle invasion at pathologic examination.
Table 3:
Cox Proportional Hazard Analysis for Recurrence-Free Survival in Univariable and Multivariable Analyses
IHC Characteristics
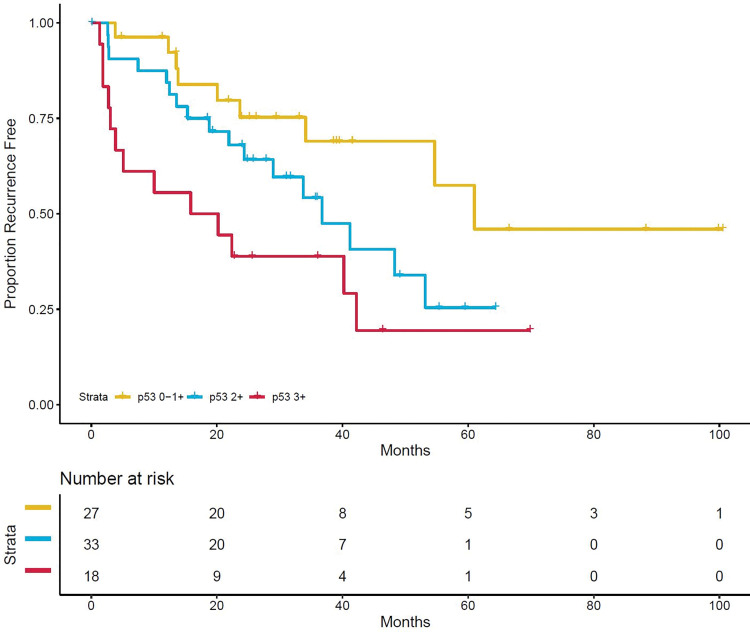
Within index lesions, all specimens demonstrated positive androgen receptor nuclear staining (Table 2). Any ERG positivity was observed in 30 of the 79 patients (38%), whereas partial or complete PTEN loss was observed in 13 (16%). Representative IHC images are shown in Figure 3, demonstrating Gleason grade 4+3 (International Society of Urological Pathology grade group 3) with PTEN loss and 3+ nuclear staining of p53. Association of p53 positivity with RFS is shown in Figure 4. In the univariable analysis, only p53-positive cases with 2+ and 3+ stain intensity and partial and/or complete PSA loss (lack of stain positivity) were significant or were trending significance for poorer RFS (Table 3). The frequency of p53 2+ and 3+ positivity was 72% (39 of 54) for biopsy cores with International Society of Urological Pathology grade group 3–5 tumors compared with 52% (13 of 25) for biopsy cores with International Society of Urological Pathology grade group 1–2 tumors (P = .13).
Figure 3:
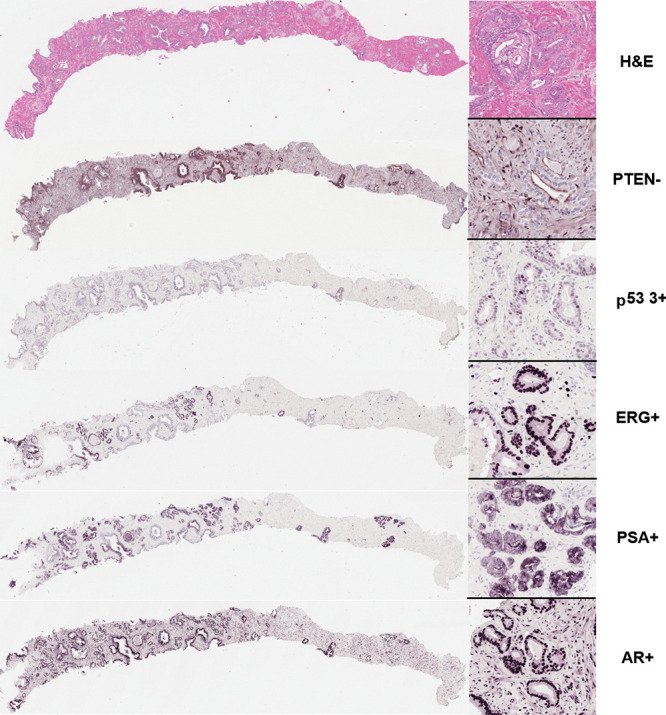
Representative immunohistochemical stains from MRI and transrectal US–targeted biopsy core of index lesion. Hematoxylin-eosin (H&E) stain shows Gleason grade 3+4 (International Society of Urological Pathology grade group 2) prostate adenocarcinoma involving approximately one-third of core length. Immunohistochemistry revealed focal loss of phosphatase and tensin homolog (PTEN) expression in part of tumor and 3+ nuclear staining intensity of p53. ETS-related gene (ERG), prostate-specific antigen (PSA), and androgen receptor (AR) were all positive in tumor regions. Images scanned at × 40 magnification.
Figure 4:
Kaplan-Meier curve of recurrence-free survival by p53 stain intensity. One patient is not shown because of missing data. Censored patients are denoted by hash marks.
Multivariable Analysis
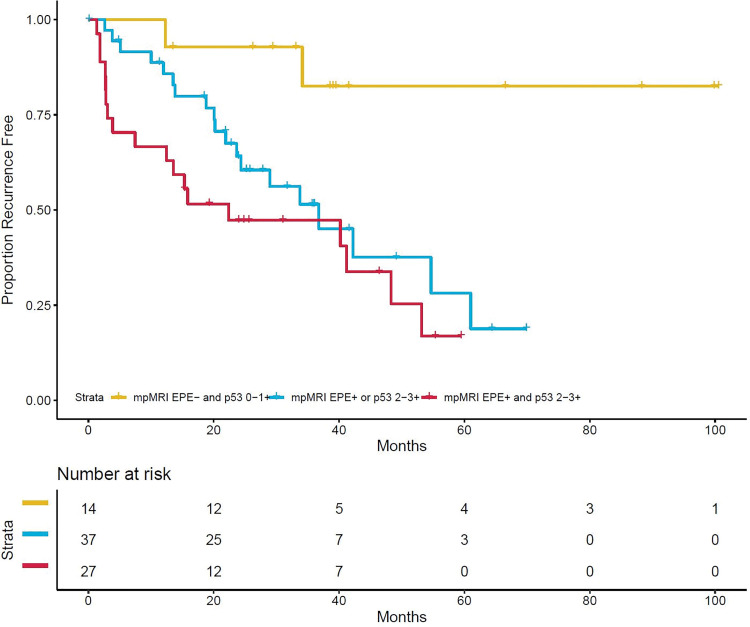
Two parsimonious multivariable models were evaluated, one considering only presurgical characteristics (eg, clinical, multiparametric MRI, and IHC) and another considering addition of surgical outcomes (Table 3). Because of heterogeneity in scan acquisition, quantitative ADC metrics were not included in multivariable modeling. In the presurgical model, MRI-based EPE suspicion and p53 positivity (2+ and 3+) were the only variables remaining significant with an adjusted HR of 2.19 (95% CI: 1.1, 4.3; P = .02) and an adjusted HR of 2.22 (95% CI: 1.0, 4.7; P = .04), respectively. Combined, patients without MRI-based EPE suspicion and low and/or no p53 expression had significantly prolonged RFS compared with patients with one or both characteristics (log-rank test P < .001, Fig 5). After addition of surgical outcomes, p53 positivity (intensity 2+ and 3+) remained significantly associated with worse RFS, along with positive surgical margins and lymphovascular invasion at radical prostatectomy (Table 3).
Figure 5:
Kaplan-Meier curve of recurrence-free survival by combined multiparametric MRI-based extraprostatic extension suspicion (grades 1–3) and p53 positivity (intensity 2+ and 3+). One patient is not shown because of missing data. Censored patients are denoted by hash marks. EPE = extraprostatic extension, mpMRI = multiparametric MRI.
Discussion
Presurgical assessment of recurrence risk could inform treatment planning and follow-up for patients with localized prostate cancer. The recently increased availability of prostate multiparametric MRI for lesion depiction and biopsy targeting, along with the availability of immunohistochemistry (IHC) markers relevant to prostate cancer, may provide valuable prognostic information when combined. Our study showed that an MRI index lesion with a Prostate Imaging Reporting and Data System score of 5, MRI total lesion burden, MRI index lesion burden, any MRI-based extraprostatic extension (EPE) suspicion (grade 1–3), and p53 expression at IHC from targeted biopsy of the MRI index lesion demonstrated significant association with biochemical recurrence after radical prostatectomy. In a multivariable analysis, multiparametric MRI-based EPE suspicion (adjusted hazard ratio [HR], 2.19; 95% CI: 1.1, 4.3; P = .02) and high p53 expression at IHC (adjusted HR, 2.22; 95% CI: 1.0, 4.7; P = .04) remained significantly associated with risk of biochemical recurrence.
The primary clinical use of prostate MRI is for the identification of intraprostatic lesions that are suspicious for cancer to provide diagnostic targeting for biopsy and treatment planning or patient staging. However, evidence shows that MRI features also have an association with BCR. Most notably, PI-RADS scoring and lesion volume have shown independent prognostic ability in several large cohorts (17–19). We reported similar results in relation to these previous studies, finding that patients with an index lesion PI-RADS score of 5 have significantly shorter RFS, consistent with findings by Faiena et al (17), in a cohort of 326 patients. PI-RADS guidelines associated category 5 lesions with distinct intraprostatic lesion sizes larger than 1.5 cm or having definite EPE or other invasive behavior (20). Herein, we have further validated the significance of suspicious features of EPE at imaging using the structured local staging system published by Mehralivand et al (12), which demonstrated strongly independent association with poorer outcomes. The importance of prognostic substratification of lesion burden and extraprostatic findings warrants future studies.
The use of MRI for guiding biopsies to the most suspicious regions of the prostate gland may be important for increasing the sampling efficiency sensitivity for key molecular markers. We observed that p53 positivity (intensity 2+ and 3+) at targeted biopsy of a multiparametric MRI index lesion was associated with worse RFS, even when adjusting for surgical outcomes. The prognostic value of detecting focally elevated expression of p53 foci was anticipated on the basis of previous studies (21–23). Prostate cancer cells harboring focal TP53 mutations in primary tumors can be detected in lethal metastatic tumors (24). As a future perspective, targeted biopsy of index lesions with EPE suspicion and further evaluation of these targeted biopsy cores with p53 can potentially further inform the clinicians for prediction of RFS.
The rate of PTEN loss was unexpectedly low in this population, observed in 16% of patients. There are several possible explanations for this, including the inability to detect these tumors at MRI and/or the PTEN IHC may have had a limited quantitative range to detect single allele loss or inactivation. Thus, only complete loss of PTEN was reliably detected in this patient cohort.
Results from a previous study have suggested that lesion ADC characteristics at MRI are significantly associated with BCR (25). The only metric to demonstrate an association with RFS was the total diffusivity index. Although not widely used in prostate cancer, this metric may reflect the burden of restricted diffusion within the lesion as compared with a single metric, such as minimum ADC. Quantitative imaging characterization was limited in this study because of heterogeneous scan acquisition protocols, limiting comparison to previously published studies on the association of MRI and prostate cancer gene expression (26,27,28). Further studies capturing the full extent of disease are warranted to understand the relationships between genomic drivers, imaging-based features, and patient outcomes.
The main limitations of this retrospective study included limited patient selection, a single expert radiologist for interpretation and region of interest delineation, heterogeneous scan acquisition protocols, and a lack of standardized interval follow-up. In this case-control population, patient selection was retrospectively completed according to clinical characteristics of pathologic grading, preoperative PSA level, and age across both cohorts. Margin assessment and standardized interval follow-up were not included in patient selection. This was designed to allow for the comparison of molecular and imaging features independent of final histologic grade. Bias in the selected population and a lack of external validation require further analysis in a prospective, independent group. Furthermore, only biopsy tissue samples from index multiparametric MRI lesions were assessed. Future studies should validate the biopsy-based molecular findings with molecular findings at final surgical-pathologic examination. Additionally, PI-RADS was not uniformly used at the initial image assessment because it was not available at the time of image interpretation and biopsy guidance phases for all patients included in this study. Finally, this study was conducted at two expert centers with combined expertise in both imaging and molecular assessment of prostate cancer. Both imaging-based techniques—acquisition and reading—have some variability in nonexpert settings.
In conclusion, our findings in this retrospective study indicated that several features for local aggressive prostate cancer at multiparametric MRI and targeted biopsy are associated with biochemical relapse after surgery, most notably MRI-based suspicion of extraprostatic extension and p53 stain intensity at immunohistochemistry of MRI index lesion biopsy.
S.A.H. and W.G. contributed equally to this work.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USUHS), The Henry M. Jackson Foundation for the Advancement of Military Medicine, the Department of Defense (DoD) or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. Supported in part by the Uniformed Services University of the Health Sciences and Center for Prostate Disease Research fund (HU0001-10-2-0002).
Disclosures of Conflicts of Interest: S.A.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has patent planned, pending, or issued. Other relationships: disclosed no relevant relationships. W.G. disclosed no relevant relationships. D.Y. disclosed no relevant relationships. S.M. disclosed no relevant relationships. Y.M. disclosed no relevant relationships. T.S. disclosed no relevant relationships. J.S. disclosed no relevant relationships. J.C. disclosed no relevant relationships. I.L.R. disclosed no relevant relationships. S.S. disclosed no relevant relationships. M.J.M. disclosed no relevant relationships. B.J.W. Activities related to the present article: institution holds Cooperative Research and Development Agreement with Philips. Activities not related to the present article: institution has grants/grants pending; institution has patents planned, pending, or issued with Philips; receives royalties from Philips; institution receives reimbursement from Philips for travel, accommodations, and meeting expenses. Other relationships: institution has patents pending with Philips; institution has patents issued and licensed; receives royalties; institution receives payment from Philips as a licensee of intellectual property. P.A.P. disclosed no relevant relationships. P.L.C. disclosed no relevant relationships. A.D. disclosed no relevant relationships. I.A.S. disclosed no relevant relationships. B.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives royalties from the U.S. government; has cooperative research and development agreement with Philips and NVIDIA. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ADC
- apparent diffusion coefficient
- BCR
- biochemical recurrence
- EPE
- extraprostatic extension
- ERG
- ETS-related gene
- HR
- hazard ratio
- IHC
- immunohistochemistry
- PSA
- prostate-specific antigen
- PTEN
- phosphatase and tensin homolog
- PI-RADS
- Prostate Imaging Reporting and Data System
- RFS
- recurrence-free survival
References
- 1.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17(5):479–505. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol 2016;69(3):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkbey B, Mani H, Aras O, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 2012;188(4):1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;378(19):1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padhani AR, Barentsz J, Villeirs G, et al. PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology 2019;292(2):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahdoot M, Wilbur AR, Reese SE, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020;382(10):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley DA, Dang HX, Zhao SG, et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018;174(3):758–769.e9[Published correction appears in Cell 2018;175(3):889.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 2011;17(20):6563–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troyer DA, Jamaspishvili T, Wei W, et al. A multicenter study shows PTEN deletion is strongly associated with seminal vesicle involvement and extracapsular extension in localized prostate cancer. Prostate 2015;75(11):1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovics G, Liu A, Shaheduzzaman S, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 2005;24(23):3847–3852. [DOI] [PubMed] [Google Scholar]
- 11.Gaur S, Harmon S, Mehralivand S, et al. Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018;48(5):1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehralivand S, Shih JH, Harmon S, et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 2019;290(3):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imaging Biol 2011;13(5):1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furusato B, Tan SH, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis 2010;13(3):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidenberg HB, Sesterhenn IA, Gaddipati JP, et al. Alteration of the tumor suppressor gene p53 in a high fraction of hormone refractory prostate cancer. J Urol 1995;154(2 Pt 1):414–421. [DOI] [PubMed] [Google Scholar]
- 16.Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw 2018;16(5S):620–623. [DOI] [PubMed] [Google Scholar]
- 17.Faiena I, Salmasi A, Mendhiratta N, et al. PI-RADS Version 2 Category on 3 Tesla Multiparametric Prostate Magnetic Resonance Imaging Predicts Oncologic Outcomes in Gleason 3 + 4 Prostate Cancer on Biopsy. J Urol 2019;201(1):91–97. [DOI] [PubMed] [Google Scholar]
- 18.Tan N, Shen L, Khoshnoodi P, et al. Pathological and 3 Tesla Volumetric Magnetic Resonance Imaging Predictors of Biochemical Recurrence after Robotic Assisted Radical Prostatectomy: Correlation with Whole Mount Histopathology. J Urol 2018;199(5):1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenkrantz AB, Ream JM, Nolan P, Rusinek H, Deng FM, Taneja SS. Prostate Cancer: Utility of Whole-Lesion Apparent Diffusion Coefficient Metrics for Prediction of Biochemical Recurrence After Radical Prostatectomy. AJR Am J Roentgenol 2015;205(6):1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griewe GL, Dean RC, Zhang W, et al. p53 Immunostaining guided laser capture microdissection (p53-LCM) defines the presence of p53 gene mutations in focal regions of primary prostate cancer positive for p53 protein. Prostate Cancer Prostatic Dis 2003;6(4):281–285. [DOI] [PubMed] [Google Scholar]
- 22.Stackhouse GB, Sesterhenn IA, Bauer JJ, et al. p53 and bcl-2 immunohistochemistry in pretreatment prostate needle biopsies to predict recurrence of prostate cancer after radical prostatectomy. J Urol 1999;162(6):2040–2045. [DOI] [PubMed] [Google Scholar]
- 23.Bauer JJ, Sesterhenn IA, Mostofi KF, McLeod DG, Srivastava S, Moul JW. p53 nuclear protein expression is an independent prognostic marker in clinically localized prostate cancer patients undergoing radical prostatectomy. Clin Cancer Res 1995;1(11):1295–1300. [PubMed] [Google Scholar]
- 24.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013;123(11):4918–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon MY, Park J, Cho JY, et al. Predicting biochemical recurrence in patients with high-risk prostate cancer using the apparent diffusion coefficient of magnetic resonance imaging. Investig Clin Urol 2017;58(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoyanova R, Pollack A, Takhar M, et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget 2016;7(33):53362–53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamshidi N, Margolis DJ, Raman S, Huang J, Reiter RE, Kuo MD. Multiregional Radiogenomic Assessment of Prostate Microenvironments with Multiparametric MR Imaging and DNA Whole-Exome Sequencing of Prostate Glands with Adenocarcinoma. Radiology 2017;284(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee D, Fontugne J, Gumpeni N, et al. Molecular alterations in prostate cancer and association with MRI features. Prostate Cancer Prostatic Dis 2017;20(4):430–435. [DOI] [PubMed] [Google Scholar]