Abstract
The phylogeny and taxonomy of powdery mildews, belonging to the genus Erysiphe, on Lonicera species throughout the world are examined and discussed. Phylogenetic analyses revealed that sequences retrieved from Erysiphe lonicerae, a widespread powdery mildew species distributed in the Northern Hemisphere on a wide range of Lonicera spp., constitutes a complex of two separate species, viz., E. lonicerae (s. str.) and Erysiphe ehrenbergii comb. nov. Erysiphe lonicerae occurs on Lonicera spp. belonging to Lonicera subgen. Lonicera (= subgen. Caprifolium and subgen. Periclymenum), as well as L. japonica. Erysiphe ehrenbergii comb. nov. occurs on Lonicera spp. of Lonicera subgen. Chamaecerasus. Phylogenetic and morphological analyses have also revealed that Microsphaera caprifoliacearum (≡ Erysiphe caprifoliacearum) should be reduced to synonymy with E. lonicerae (s. str.). Additionally, Erysiphe lonicerina sp. nov. on Lonicera japonica in Japan is described and the new name Erysiphe flexibilis, based on Microsphaera lonicerae var. flexuosa, is introduced. The phylogeny of Erysiphe ehrenbergii and E. lonicerae as well as other Erysiphe species on honeysuckle is discussed, and a survey of all species, including a key to the species concerned, is provided.
Citation: Bradshaw M, Braun U, Götz M, Takamatsu S (2020). Taxonomy and phylogeny of the Erysiphe lonicerae complex (Helotiales, Erysiphaceae) on Lonicera spp. Fungal Systematics and Evolution 7: 49–65. doi: 10.3114/fuse.2021.07.03
Keywords: Ascomycota, epitypification, Erysiphe ehrenbergii, E. flexibilis, E. lonicerina, Lonicera, new taxa, powdery mildew, systematics
INTRODUCTION
Lonicera, the largest genus in the family Caprifoliaceae, comprises around 180 species of deciduous or evergreen shrubs as well as woody climbers that are largely distributed in temperate to subtropical regions of the Northern Hemisphere (Mabberley 2008). Various species of this genus are cultivated shrubs that are popular in gardens and parks. For example, Lonicera japonica and L. tatarica are commonly found throughout Europe (Roloff & Bärtels 2008). Lonicera is usually divided into two subgenera, one for species with 3-flowered cymes and perfoliate leaves that usually comprise twining shrubs (subgen. Lonicera), and one for species with 2-flowered cymes and free leaves (subgen. Chamaecerasus). The division of Lonicera into two subgenera has recently been confirmed by molecular methods (Theis et al. 2008, Nakaji et al. 2015). The formal naming of the two subgenera has been controversially discussed and depends on the lectotypification of this genus. The first valid lectotypification of Lonicera was accomplished by Hitchcock & Green (1929) who designated Lonicera caprifolium as lectotype. However, more recently, Hara (1983) proposed L. xylosteum as lectotype. Although the original lectotypification is valid, some authors, such as Nakaji et al. (2015), did not accept L. caprifolium as lectoptype and preferred to follow Hara (1983). As long as there is no accepted proposal to change the lectotypification of this genus, the first lectotypification should be followed (Art. 9.19).
Powdery mildew, caused by Erysiphe spp., is a common, widespread, detrimental fungal disease of plants within the Lonicera genus (Braun & Cook 2012). Salmon (1900: 142) noted that the European powdery mildew on Lonicera spp. was a variety of Microsphaera alni (sensu latissimo), which, at the time, encompassed all Microsphaera species that are morphologically similar to M. penicillata (= M. alni). Microsphaera ehrenbergii, introduced for a powdery mildew on Lonicera tatarica, was reduced to synonymy with M. alni var. lonicerae. The current taxonomy of Erysiphe lonicerae was first established by Jaczewski (1927). Following Jackzewski (1927), Blumer (1933, 1967) recognized the European Lonicera powdery mildew as a species of its own (including M. ehrenbergii on L. tatarica as synonym). Braun (1982b) treated the later taxon as a morphologically distinguished variety of M. lonicerae and introduced the combination M. lonicerae var. ehrenbergii. Braun & Takamatsu (2000) later introduced the new nomenclature Erysiphe lonicerae var. ehrenbergii. Braun & Cook (2012) recognised six Erysiphe species, all belonging in sect. Microsphaera, viz., Erysiphe caprifoliacearum var. caprifoliacearum and var. flexuosa, E. erlangshanensis, E. lonicerae var. lonicerae and var. ehrenbergii, E. lonicerae-ramosissimae, E. magnusii, and E. miurae. However, this treatment was just based on morphology.
The application of molecular methods for powdery mildews is commonly used to clarify unresolved taxonomic problems. In the current study, we conducted phylogenetic analyses of the E. lonicerae complex to determine whether the L. tatarica powdery mildew should be treated as a variety of E. lonicerae or if it should be separated into a species of its own. We included epitypifications of Erysiphe lonicerae and Microsphaera ehrenbergii with ex-epitype reference sequences, as well as a survey of Erysiphe species on Lonicera spp. and a key to the various species concerned. The phylogenetic analysis conducted is the basis for a revised taxonomic treatment of E. lonicerae s. lat.
MATERIALS AND METHODS
Collections examined
Morphological examinations and phylogenetic analyses were based on herbarium specimens deposited in BPI, GLM, HAL, TNS, TSU-MUMH, and WTU. The collections used for the present examinations are cited under the particular species in the taxonomic chapter.
DNA extraction, amplification (PCR), and phylogeny
Sequences were obtained in the USA as described by Bradshaw & Tobin (2020). DNA extractions were accomplished by the Chelex method (Walsh et al. 1991, Hirata & Takamatsu 1996). PCR was accomplished using the primer pairs PM10 (5’-GGCCGGAAAGTTGTCCAAAC-3’) / PM28R (5’-ACGTTCACTTTCATTCCGCG-3’) (Bradshaw & Tobin 2020). If PCR was unsuccessful a nested approach was accomplished using the Primers AITS (5’-CGATTGAATGGCTAAGTGAGG-3’) (Bradshaw & Tobin 2020) / TW14 (5’-GCTATCCTGAGGGAAACTTC-3’) (Mori et al. 2000) followed by PM10 / PM28R or PM10 / PM11 (for the ITS) and PM28F / PM28R (for the 28S) (Bradshaw & Tobin 2020). DNA was purified by isopropanol precipitation. Purified amplicons were sent to Eurofins (Luxembourg) to be directly sequenced in both the forward and reverse direction using the primer pairs above. In Germany, sequences were obtained as described by Bradshaw et al. (2017). Whole-cell DNA was extracted from infected leaves with the DNeasy plant mini kit (Qiagen, Germany), following the manufacturers protocol. For the first PCR reaction the primers PM1 (5’-TCGGACTGGCCYAGGGAGA-3’) (Cunnington et al. 2003) / TW14 were used followed by ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) (White et al. 1990) / PM2 (5’-TCACTCGCCGTTACTGAGGT-3’) (Cunnington et al. 2003) and PM5 (5’-TTGCTTTGGCGGGCCGGG-3’) / NLP2 (5’-GGTCCCAACAGCTATGCTCT-3’) (Mori et al. 2000) Amplicons were purified (MSB Spin PCRapace Kit; Stratec Biomedical AG, Germany) and sequenced in both directions (LGC Genomics GmbH, Germany) using the primers mentioned before. Consensus sequences were generated and edited (CLC Main Workbench 20.0, Qiagen Digital Insights, Germany). The sequences obtained in the present study were deposited in GenBank under the accession numbers MN211362–MN211363, MN277391–MN277395, MW045561–45570, and MW0455571–MW0455573 (Table 1).
Table 1.
List of fungi, hosts, vouchers, and NCBI accession numbers of the sequences used in this study. ‘na’ indicates information not available. ‘*’ indicates ITS and 28S rDNA sequences were deposited separately. ID in parenthesis shows 28S rDNA sequence.
| Fungal name | Host | Voucher | NCBI ID (ITS+28S rDNA) | References |
|---|---|---|---|---|
| Erysiphe abeliicola | Abelia spathulata | MUMH4472 | LC010069 | Takamatsu et al. (2015) |
| Abelia ×grandiflora | KUS-F25628 | JQ710724 | Cho et al. (2012b) | |
| E. aquilegiae | Cimicifuga simplex | TPU-495 | AB000944 (AB022405)* | Takamatsu et al. (1998) |
| Clematis apiifolia | MUMH277 | LC009938 | Takamatsu et al. (2015) | |
| Clematis stans | MUMH293 | LC009944 | Takamatsu et al. (2015) | |
| Clematis terniflora | MUMH98 | AB015929 (LC009920)* | Takamatsu et al. (1999) | |
| E. blasti | Lindera umbellata | MUMH2 | AB015918 (LC009905)* | Takamatsu et al. (1999) |
| E. caricae | Carica papaya | KW58355 | LC009901 | Takamatsu et al. (2015) |
| E. cornicola | Cornus controversa | MUMH90 | AB000941 (AB022389)* | Takamatsu et al. (1998) |
| Cornus controversa | YNMH12992 | AB015924 | Takamatsu et al. (1999) | |
| E. corylacearum | Corylus sieboldiana | MUMH199 | LC009928 | Takamatsu et al. (2015) |
| Corylus sp. | Dai13042 | JX880086 | Liu et al. (2013) | |
| E. corylopsidis | Corylopsis pauciflora | MUMH4174 | AB478988 (AB478984)* | Shiroya & Takamatsu (2009) |
| Corylopsis spicata | MUMH4104 | AB478990 (AB478986)* | Shiroya & Takamatsu (2009) | |
| E. deutziae | Deutzia crenata | KUS-F24694 | GU196146 | Park et al. (2012) |
| Deutzia crenata | K(M)140025 | DQ861917 | Denton & Henricot (2007) | |
| E. diervillae | Weigela hortensis | TPU-1669 | AB015931 (LC010087)* | Takamatsu et al. (1999) |
| Weigela hortensis | MUMH28 | AB015932 | Takamatsu et al. (1999) | |
| E. ehrenbergii | Lonicera alpigena | MUMH1434 | LC009989 | Takamatsu et al. (2015) |
| Lonicera caerulea | MUMH1440 | LC009993 | Takamatsu et al. (2015) | |
| Lonicera xylosteum | MUMH1438 | LC009992 | Takamatsu et al. (2015) | |
| Lonicera ×bella | MUMH1435 | LC009990 | Takamatsu et al. (2015) | |
| Lonicera tatarica | G566307 | MN211362 | This study | |
| Lonicera tatarica | G566299 | MN211363 | This study | |
| Lonicera tatarica | GLM-F57507 | MN277392 | This study | |
| E. erlangshanensis | Lonicera maackii | MUMH2586 | LC010053 | Takamatsu et al. (2015) |
| E. flexibilis | Lonicera involucrata | WTU-F072459 | MW045572 | This study |
| Lonicera involucrata | WTU-F071966 | MW045573 | This study | |
| E. helwingiae | Helwingia japonica | MUMH110 | AB015916 | Takamatsu et al. (1999) |
| E. hommae | Elsholtzia ciliata | MUMH167 | LC009926 | Takamatsu et al. (2015) |
| E. huayinensis | Isodon longitubus | MUMH30 | AB015914 | Takamatsu et al. (1999) |
| Isodon umbrosus | MUMH4644 | LC010072 | Takamatsu et al. (2015) | |
| E. ligustri | Ligustrum obtusifolium | MUMH264 | AB295456 | Seko et al. (2011) |
| Ligustrum obtusifolium | MUMH2244 | LC010012 (AB571057)* | Seko et al. (2011) | |
| E. lonicerae | Lonicera caprifolium | G566366 | MN277393 | This study |
| Lonicera caprifolium | GLM-F81562 | MN277394 | This study | |
| Lonicera caprifolium | GLM-F64253 | MN277395 | This study | |
| Lonicera ciliosa | UBC-F2283 | MW045561 | This study | |
| Lonicera ciliosa | WTU-F072457 | MW045562 | This study | |
| Lonicera ciliosa | WTU-F071967 | MW045563 | This study | |
| Lonicera ciliosa | WTU-F071969 | MW045564 | This study | |
| Lonicera ciliosa | WTU-F071968 | MW045565 | This study | |
| Lonicera ×heckrotti | BPI557673 | MW045566 | This study | |
| Lonicera hispidula | WTU-F072462 | MW045567 | This study | |
| Lonicera japonica | MUMH2481 | LC010020 | Takamatsu et al. (2015) | |
| Lonicera japonica | MUMH835 | LC009963 | Takamatsu et al. (2015) | |
| Lonicera japonica | JGSJYH002 | MF623858 | na | |
| Lonicera japonica | OE2015PMCS202 | KY660868 | na | |
| Lonicera japonica | KUS-F27344 | KT748729 | Lee et al. (2016) | |
| Lonicera japonica | Jinyinhua | KY203965 | na | |
| Lonicera japonica | WTU-F071970 | MW045568 | This study | |
| Lonicera japonica | MUMH4501 | MW045569 | This study | |
| Lonicera periclymenum | MUMH1436 | LC009991 | Takamatsu et al. (2015) | |
| Lonicera periclymenum | OE2015PM30CS | KY660891 | na | |
| Lonicera periclymenum | OE2015PM36CS | KY660928 | na | |
| Lonicera periclymenum | OE2014PM24CS | KY653206 | na | |
| Lonicera prolifera | BPI557665 | MW045570 | This study | |
| Lonicera sp. | OE2015PM51CS | KY660907 | na | |
| Lonicera sp. | OE2015PM141CS | KY653175 | na | |
| Lonicera sp. | GLM-F78413 | MT277391 | This study | |
| E. lonicerina | Lonicera japonica | MUMH601 | LC009953 | Takamatsu et al. (2015) |
| Lonicera japonica | MUMH4464 | MW045571 | This study | |
| E. linderae | Lindera praecox | MUMH4379 | LC010067 | Takamatsu et al. (2015) |
| E. macleayae | Macleaya cordata | TPU-1873 | AB016048 (LC010092)* | Takamatsu et al. (1999) |
| Macleaya microcarpa | KUS-F24459 | JQ681217 | Park et al. (2012) | |
| E. magnoliae | Magnolia obovata | MUMH5227 | JX235965 | Takamatsu et al. (2013) |
| Magnolia obovata | KUS-F26388 | JX235964 | Takamatsu et al. (2013) | |
| E. miranda | Viburnum sargentii | MUMH2561 | LC010033 | Takamatsu et al. (2015) |
| E. miurae | Lonicera morrowii | MUMH1216 | LC009980 | Takamatsu et al. (2015) |
| Lonicera vidalii | MUMH1209 | LC009979 | Takamatsu et al. (2015) | |
| E. ornata | Betula pubescens | MUMH2560/DB53529 | LC010032 | Takamatsu et al. (2015) |
| Betula pubescens | MUMH2565/DB53525 | LC010036 | Takamatsu et al. (2015) | |
| E. paeoniae | Paeonia coriacea | MUMH1449 | AB257436 | Takamatsu et al. (2006) |
| Paeonia lactiflora | MUMH146 | AB257438 | Takamatsu et al. (2006) | |
| E. penicillata | Alnus incana | MUMH1432 | LC009987 | Takamatsu et al. (2015) |
| E. pulchra | Cornus florida | na | AY870864 | Shi et al. (2009) |
| Cornus kousa | TPU-1731 | AB015935 (LC010089)* | Takamatsu et al. (1999) | |
| E. sedi | Kalanchoe blossfeldiana | KUS-F24911 | JX173288 | Cho et al. (2012a) |
| Sedum sp. | MUMH2576 | LC010046 | Takamatsu et al. (2015) | |
| E. staphyleae | Staphylea bumalda | MUMH16 | AB015922 (LC009908)* | Takamatsu et al. (1999) |
| E. symphoricarpi | Symphoricarpos albus | MUMH1428 | AB078970 | Kiss et al. (2002) |
| Symphoricarpos albus | MUMH974 | AB078969 (LC009970)* | Kiss et al. (2002) | |
| E. syringae-japonicae | Syringa vulgaris | MUMH1916 | LC010006 (AB571060)* | Seko et al. (2011) |
| Syringa vulgaris | MUMH1736 | AB295458 | Seko et al. (2011) | |
| E. vanbruntiana | Sambucus sieboldiana | MUMH17 | AB015925 (LC009909)* | Takamatsu et al. (1999) |
| E. viburni | Viburnum opulus | VPRI22168 | AF298541 | Cunnington et al. (2003) |
| Viburnum sieboldii | MUMH1 | LC009904 | Takamatsu et al. (2015) | |
| E. viburni-plicati | Viburnum plicatum | MUMH794 | AB863612 | Meeboon & Takamatsu (2015) |
| Erysiphe sp. | Baptisia australis | MUMH897 | LC009967 (LC009966)* | Takamatsu et al. (2015) |
| Sophora flavescens | MUMH89 | LC009919 | Takamatsu et al. (2015) | |
| Pseudoidium neolycopersici | Solanum lycopersicon | MUMH66 | AB032483 (LC009912)* | Kiss et al. (2005) |
| Solanum lycopersicon | DNA231 | AB032484 | Kiss et al. (2005) |
These sequences were aligned with the reference sequences of Erysiphe species shown in Table 1 by MUSCLE implemented in MEGA 7.0 (Kumar et al. 2016). Phylogenetic trees were constructed by maximum parsimony (MP) and maximum likelihood (ML) methods in PAUP* v. 4.0 (Swofford 2003) and raxmlGUI v. 1.3 (Silvestro & Michalak 2012), respectively, according to the procedures of Meeboon et al. (2020). All sites were treated as unordered and unweighted, with gaps treated as missing data. Strength of the respective brances was evaluated with 1 000 bootstrap (BS) values (Felsenstein 1985).
Morphology
All fungal structures were examined by light microscopy, using an Olympus BX50 or Zeiss Axio imager A1 microscope. Distilled water and lactic acid were used as mounting media, and aniline blue (cotton blue) was used to stain colourless structures. If possible, measurements of 30 conidia and other structures were made at a magnification of × 1 000, and the 95 % confidence intervals were determined (extreme values in parentheses).
RESULTS
Phylogeny of Erysiphe lonicerae s. lat.
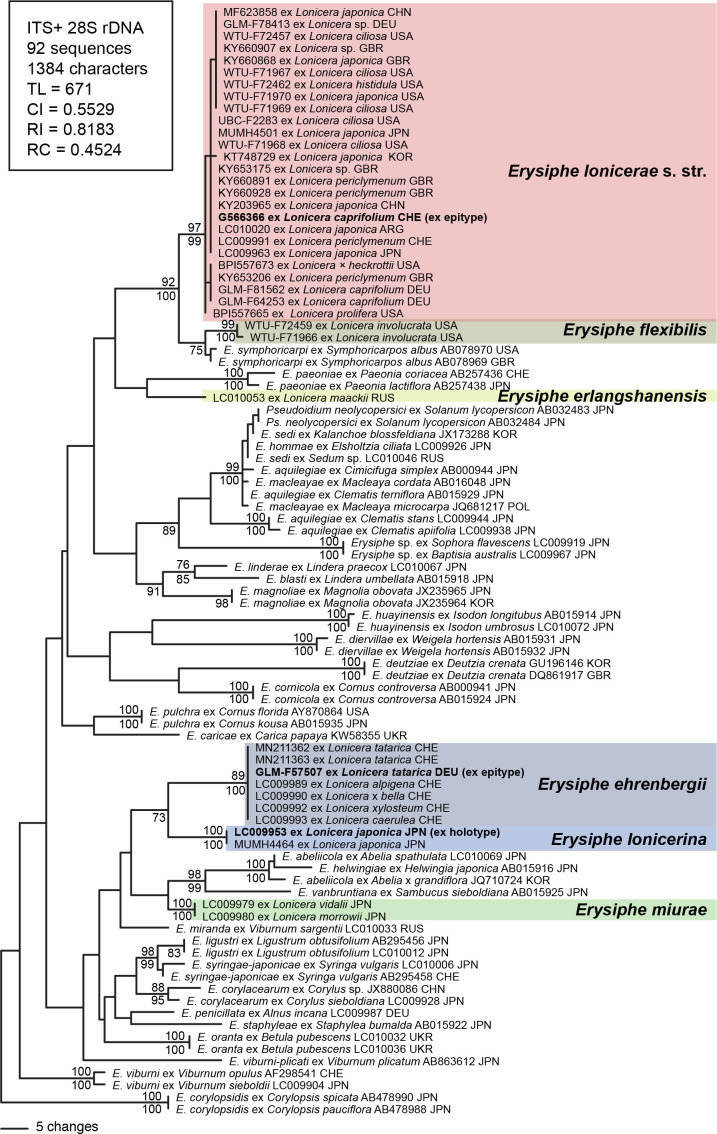
Fourty nuc-rDNA sequences including ITS regions, 5.8S rDNA and 5’-end of 28 rDNA from Erysiphe spp. on Lonicera species were aligned with the sequences of closely related Erysiphe species retrieved from DNA databases. The alignment matrix consisted of 92 sequences and 1 384 characters, of which 284 (20.5 %) characters were variable and 216 (15.6 %) were informative for parsimony analysis (TreeBASE ID: S27001). Two sequences of E. coryloposidis were used as outgroup in accordance with Takamatsu et al. (2015). About 7 × 105 equally most parsimonious trees with 671 steps were constructed by the MP analysis. Tree topologies were almost consistent among the trees, except for branching orders of the terminal branches and branch length. One of the trees is shown in Fig. 1. A phylogenetic tree generated from ML analysis was almost identical to the MP tree, and thus only BS values were shown on the MP tree. The 40 sequences from Erysiphe on Lonicera spp. were devided into six distinct clades strongly supported by high BS values. These six clades were not monophyletic and roughly separated in two groups: one group consisting of E. lonicerae s. str., E. flexibilis, and E. erlangshanensis, and another group of E. ehrenbergii, E. lonicerina, and E. miurae.
Fig. 1.
Phylogenetic tree of Erysiphe species on Lonicera spp. based on combined DNA sequences of internal transcribed spacer (ITS) region and the 5’-end of the 28S rDNA (including domains D1 and D2). Horizontal branch lengths are proportional to the number of substitutions that were inferred to have occurred along a particular branch of the tree. BS (≥ 70 %) values by the maximum parsimony (MP) and maximum likelihood (ML) methods are shown above and below the branches, respectively.
Taxonomy
Taxonomic revision of Erysiphe lonicerae s. lat.
On account of the present phylogenetic revision, Erysiphe lonicerae s. lat. has to be divided into two separate species. The two species are phylogenetically and morphologically different and will be referred to as E. lonicerae s. str. and Erysiphe ehrenbergii. E. ehrenbergii is based on Microsphaera ehrenbergii, but it is now not applied as it was in previous applications, in which it was confined only to powdery mildew on Lonicera tatarica, either as a species of its own – as it was originally introduced – or as a variety of Erysiphe/Microsphaera lonicerae, as the phylogenetic analyses revealed a co-evolution between the powdery mildews concerned along with the subgenera of Lonicera.
Erysiphe ehrenbergii (Lév.) U. Braun, M. Bradshaw & S. Takam., comb. nov. MycoBank MB837569.
Basionym: Microsphaera ehrenbergii Lév., Ann. Sci. Nat., Bot., Sér. 3, 15: 381. 1851.
Synonyms: Microsphaera lonicerae var. ehrenbergii (Lév.) U. Braun, Mycotaxon 15: 127. 1982.
Erysiphe lonicerae var. ehrenbergii (Lév.) U. Braun & S. Takam., Schlechtendalia 4: 10. 2000.
Illustrations: Magnus (1898: pl. II, figs 13–15), Blumer (1933: 298, fig. 108 A–C), Braun (1982a: 128, fig. 12b; 1984: 234, fig. 29, pl. 5; 1987: 329, pl. 104; 1995: 278, pl. 55, fig. B), Sałata (1985: 168, fig. 67), Chen et al. (1987: 203, fig. 104), Simonyan (1994: 161, fig. 35), Liu (2010: 106, fig. 49), Braun & Cook (2012: 478, fig. 551).
Exsiccatae [on Lonicera coeruleum (L.co.), L. tatarica (L.t.), L. xylosteum (L.x.)]: Allescher & Schnabl, Fungi Bav. 530 (L.t.). Erikss., Fungi Paras. Scand. 144 (L.t.). Fuckel, Fungi Rhen. Exs. 696 (L.t.). Kochm., Mycoth. Polon. 132 (L.t.). Krieger, Fungi Saxon. Exs. 1224 (L.t.). Krypt. Exs. 2619 (L.t.). Lepik, Fungi Estonici Exs. 256–258 (L.t.). Linh., Fungi Hung. Exs. 357 (L.x.). Lundell & Nannf., Fungi Exs. Suec. 1483 (L.t.). Neger, Forstschädl. Pilze 155 (L.co.). Rabenh., Fungi Eur. Exs. 556, 2651 (L.t.). Rabenh., Klotzschii Herb. Viv. Mycol. 473 (L.t.). Rehm, Ascomyc. 847 (L.x.). Syd., Mycoth. Germ. 2521 (L.t.). Thüm., Mycoth. Univ. 450 (L.t.). Weese, Eumyc. Sel. Exs. 630 (L.t.).
Description: Mycelium on leaves, amphigenous, effuse or in thin white patches, persistent or almost so on the upper leaf surface, less developed and evanescent below; hyphae branched, septate, hyaline, thin-walled, smooth, 2–7 μm wide; hyphal appressoria slightly to distinctly lobed, occasionaly almost nipple-shaped, 3–7 μm diam, solitary or occasionally in opposite pairs. Conidiophores arising from superficial hyphae, erect, terminal on mother cells, basal septum at the junction with the mother cell or slightly elevated (up to 8 μm), 45–90 μm long; foot-cells straight to moderately curved or sinuous (conidiophores with straight and curved-sinuous foot-cells mixed in all collections), 15–40 × 5–9 μm, width uniform throughout, followed by 1–3(–4) cells mostly shorter than the foot-cell, sometimes about as long as the foot-cell or only slightly longer (above all the secondary cell), 8–30 μm long, forming solitary conidia. Conidia cylindrical, subcylindrical to ellipsoid-ovoid, 23–39 × 10–17 μm, length/width ratio 1.9–3.2 (on average 2.4, N = 30), ends more or less truncated, apex rounded in primary conidia, conidial germination not observed. Chasmothecia amphigenous, on the upper leaf surface gregarious to scattered, below scattered, subglobose, 60–120 μm diam; peridial cells polygonal, rounded to irregularly shaped, 8–30 μm diam. Appendages 5–22, number of appendages correlated with the size of the chasmothecia, more or less equatorial, radiating, stiff, straight to curved, 1–2.5 times as long as the chasmothecial diam (60–255 μm), 6–10 μm wide below, aseptate or with a single basal septum, sometimes with a second or even third elevated septum, up to 70 μm from the base, colourless or brown below the septa, i.e., when two or three septa are formed pigmented part up to 70 μm long, wall thickened, 1.5–3 μm wide below, thinner towards the tip, smooth to verruculose, apex 3–5(–6) times regularly to somewhat irregularly dichotomously branched, loose to dense, primary branches short or oblong, 15–55 μm in length, tips of the ultimate branchlets straight or mixed with a few curved ones for a long time or up to the end of the season, a higher percentage recurved when fully mature, but straight and curved ones always mixed. Asci 2–6, broad ellipsoid, obovoid to saccate, sessile to short-stalked, 35–60 × 20–40 μm, hyaline, wall 1–3 μm thick, terminal oculus indistinct, 5–15 μm diam, 3–5-spored. Ascospores broad ellipsoid-ovoid, 14–26 × 9–14 μm, at first pale yellowish or olivaceous, later colourless.
Type: Léveillé (1851: pl. 8, fig. 22 – lectotype, designated by Braun 1987: 329). Epitype: Germany, Sachsen-Anhalt, Salzlandkreis, Calbe (Saale), Wartenberg, on Lonicera tatarica, 27 Sep. 2002, H. Jage (GLM-F57507; designated here, MycoBank MBT394140); ex-epitype sequence – MN277392.
Additional materials examined: Armenia, Gyumri (previously Leninakan), park, on Lonicera tatarica, 7 Sep. 1969, S. Simonyan (HAL 155 F). Germany, Sachsen, Landkreis Zwickau, Limbach-Oberfrohna, on Lonicera coerulea, 15 Jul. 2006, F. Dämmrich (GLM-F89964); Hessen, Vogelsbergkreis, east north east of Schlitz, on Lonicera tatarica, 22 Jul. 2000, H. Jage (GLM-F48342); Sachsen, Leipzig-Neustadt, on Lonicera tatarica, 22 Sep. 1994, H. Jage (GLM-F48823); Sachsen, Landkreis Zwickau, Reinsdorf, on Lonicera tatarica, 25 Sep. 1994, H. Jage (GLM-F48831); Sachsen, Görlitz-Rauschwalde, on Lonicera tatarica, 18 Aug. 2004, H. Boyle (GLM-F53683); Sachsen, Zwickau, on Lonicera tatarica, 20 Sep. 2006, H. Boyle & S. Hoeflich (GLM-F78413); Sachsen, Freiberg, on Lonicera tatarica, 1 Aug. 2007, F. Klenke (GLM-F104594); Sachsen-Anhalt, Halle (Saale), centre, green belt, on Lonicera tatarica, 18 Sep. 1977, U. Braun (HAL 152 F); Sachsen-Anhalt, Köthen, centre, college garden, on Lonicera tatarica, Sep. 1983, U. Braun (HAL 666 F); Sachsen-Anhalt, Gräfenhainichen, Hohenlubast, on Lonicera tatarica, 3 Oct. 1987, H. Jage (GLM-F50556); Sachsen-Anhalt, Landkreis Wittenberg, Kemberg, on Lonicera tatarica, 4 Oct. 1999, H. Jage (GLM-F50068); Sachsen-Anhalt, Landkreis Wittenberg, south of Kemberg, Pinus sylvestris forest, on Lonicera tatarica, 17 Oct. 1999, H. Jage (GLM-F477578); Sachsen-Anhalt, Wittenberg, on Lonicera tatarica, 15 Oct. 1999, H. Jage (GLM-F47531); Sachsen-Anhalt, Landkreis Wittenberg, Oranienbaum-Wörlitz, OT Wörlitz, park, on Lonicera tatarica, H. Jage (GLM-F47553); Sachsen-Anhalt, Magdeburg, centre, on Lonicera tatarica, 15 Nov. 2000, H. Jage et al. (GLM-F47206); Sachsen-Anhalt, Landkreis Wittenberg, Kemberg, on Lonicera tatarica, 9 Sep. 2000, H. Jage (GLM-F49074); Sachsen-Anhalt, Wittenberg, on Lonicera tatarica, 27 Sep. 2000, H. Jage (GLM-F49161); Sachsen-Anhalt, Landkreis Bitterfeld, Muldestausee, Pouch, Schloßpark, on Lonicera tatarica, 9 Oct. 2000, H. Jage (GLM-F49382); Thüringen, Landkreis Gotha, Friedrichroda, on Lonicera tatarica, 25 Sep. 2004, H. Jage (GLM-F65111); Hessen, Schwalm-Eder-Kreis, Bad Zwesten, OT Oberurff-Schiffelborn, on Lonicera xylosteum, 14 Jul. 2007, C. Klenke (GLM-F104900); Sachsen, Vogtlandkreis, Pöhl, OT Jocketa, on Lonicera xylosteum, 4 Sep. 1999, H. Jage & F. Klenke (GLM-F103509); Sachsen, Landkreis Görlitz, Beiersdorf, on Lonicera xylosteum, 2 Sep. 2004, H. Boyle (GLM-F53837); Sachsen, Landkreis Sächsische Schweiz-Osterzgebirge, Bad Schandau, Waltersdorf-Sellnitz, on Lonicera xylosteum, 9 Sep. 2009, F. Klenke (GLM-F102908). Finland, Nylandia, Helsinki, Engel Park, on Lonicera tatarica, 12 Jul. 1976, P. Alanko (HAL 150 F). Russia, Moscou, Lomonosov University campus, on Lonicera tatarica, 20 Jul. 1977, U. Braun (HAL 151 F). Switzerland, Genève, Jardin botanique, on Lonicera alpigena, 4 Sep. 1995, A. Boley (HAL 602 F); Genève, Jardin botanique, on Lonicera ×bella, 27 Oct. 1997, A. Bolay (G 566324); Bern, Jardin botanique, on Lonicera coerulea, 25 Oct. 1995, A. Bolay (G 566363); Genève, Jardin botanique, on Lonicera coerulea, 26 June 2001, A. Bolay (G 566364); ibid., 9 Jul. 2002, A. Bolay (G 566365); ibid., 18 Oct. 2006, A. Bolay (G 566326); Genève, Jardin botanique, on Lonicera pyrenaica, 20 Jun. 1995, A. Boley (G 566358); ibid., 28 Aug. 1995, A. Boley (G 566346, HAL 600 F); ibid., 4 Oct. 2000, A. Bolay (G 566345); ibid., 5 Oct. 2010, A. Bolay (G 566347); Genève, Jardin botanique, on Lonicera tatarica, 29 Sep. 1997, A. Bolay (G 566348); NE, Neuchâtel, on Lonicera tatarica, 11 Oct. 2002, A. Bolay (G 566349); Genève, Jardin botanique, on Lonicera xylosteum, 8 Sep. 1999, A. Bolay (G 566350, G 566351); ibid., on Lonicera xylosteum, 19 Aug. 2002, A. Bolay (G 566352).
Host range and distribution: on species of Lonicera subgen. Chamaecerasus; Lonicera alpigena (Europe: Austria, France, Germany, Switzerland, former Yugoslavia), L. ×bella (Europe: Estonia, Romania, Russia, Switzerland), L. coerulea (Europe: Germany, Switzerland), L. microphylla (Asia: China), L. fragrantissima (Europe: Russia, Slovakia, Ukraine), L. nigra (Europe: France, Germany, Romania, Russia, Slovakia, Spain, Switzerland, Ukraine, former Yugoslavia), L. pyrenaica (Europe: Romania, Switzerland), L. tatarica (Asia: China, Japan, Kazakhstan, Kyrgyzstan, Russia, Far East; Caucasus: Armenia; Europe: Austria, Belarus, Czech Republic, Estonia, Finland, Germany, Hungary; Europe: Latvia, Lithuania, Netherlands, Norway, Poland, Romania, Serbia, Slovakia, Sweden, Switzerland, UK, Ukraine; North America: Canada, USA: Kentucky, Maine, Minnesota, North Dakota, Iowa, New York, Pennsylvania, Wisconsin), L. xylosteum (Caucasus: Armenia; Europe: Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Lithuania, the Netherlands, Norway, Poland, Romania, Russia, Slovakia, Sweden, Switzerland, former Yugoslavia) [Amano 1986; Braun (1987, 1995) Heluta (1989), Paulech (1995), Grigaliūnaitė (1997), Girilovich et al. (2005), Braun & Cook (2012); https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm].
Notes: The asexual morph of E. ehrenbergii develops in early summer to early autumn (in Central Europe in June to September), either together with the sexual morph or it ceases with the chasmothecial formation (in Europe it is initiated in early summer, usually July, but lasting into November). In collections from October to November, infections are usually inconspicuous, mostly hypophyllous, without any trace of mycelium or the asexual morph. In Erysiphe ehrenbergii, the tips of the ultimate branchlets, of the terminal branched part of the chasmothecial appendages, are straight, with a few somewhat recurved tips (in most collections, especially in mature chasmothecia collected from September to November). A higher percentage of recurved tips have been observed, but only in a few specimens, collected in autumn. This characteristic is in contrast to Erysiphe lonicerae s. str., which is characterised by having frequently recurved tips in fully mature collections collected later in the season. Additionally, the asexual morph differs slightly in that the conidia in E. lonicerae tend to be longer (25–55 × 11–24 μm vs. 23–39 × 10–17 μm in E. ehrenbergii).
Erysiphe flexibilis M. Bradshaw, U. Braun & S. Takam., stat. et nom. nov. MycoBank MB837570. Fig. 2.
Fig. 2.
Erysiphe flexilis (HAL 3348 F). A. Hyphae. B. Hyphal appressoria. C. Conidiophores. D. Conidia. E. Conidia in a short “false chain”. Scale bar = 10 μm. U. Braun del.
Basionym: Microsphaera lonicerae var. flexuosa U. Braun, Mycotaxon 15: 129. 1982 [non Erysiphe flexuosa (Peck) U. Braun & S. Takam., 2000].
Synonyms: Microsphaera caprifoliacearum var. flexuosa (U. Braun) U. Braun, Nova Hedwigia 39: 229. 1984.
Erysiphe caprifoliacearum var. flexuosa (U. Braun) U. Braun & S. Takam., Schlechtendalia 4: 6. 2000.
Etymology: Flexibilis – referring to the flexuous chasmothecial appendages.
Illustrations: Braun (1982a: 128, fig. 12c; 1984: 232, pl. 4, fig. 23b; 1987: 432, pl. 197), Braun & Cook (2012: 444, fig. 518).
Exsiccatae: Solh., Mycolf. Saximon. Exs. 1321.
Description: Mycelium amphigenous, white, effuse or in thin patches; hyphae straight to somewhat sinuous, branched at right angles, thin-walled, hyaline, smooth, septate, hyphal cells 35–70 μm long and 3–6 μm wide; hyphal appressoria solitary or occasionally in opposite pairs, nipple-shaped to slightly lobed, 3–6 μm diam. Conidiophores arising from the upper surface of hyphal mother cells, in the middle of the mother cell or somewhat towards one septum, erect, straight or occasionally somewhat flexuous, 60–100 μm long, width of the conidiophores mostly somewhat increasing from base to top, foot-cells cylindrical or subcylindrical, straight or somewhat curved to slightly sinuous at the very base, basal septum at the junction with the mother cells or slightly elevated (to 5 μm), foot-cells 30–58 μm long and 5–7 μm wide, followed by 1–2 shorter cells, 12–40 μm long and 6–9 μm wide, conidia formed singly, occasionally adhering in short “false chains” (but not catenescent). Conidia ellipsoid-ovoid to subcylindrical, ends truncated to rounded, 24–43 × 11–19 μm, length/width ratio 1.7–3.2 (on average 2.6, N = 30). Chasmothecia amphigenous, scattered to gregarious, 65–120 μm diam; peridium cells irregularly polygonal, 10–25 μm diam. Appendages more or less equatorial, 4–15, 1.5–3.5(–4) times as long as the chasmothecial diam, 6–10 μm wide below, flexuous, septate or with a single basal septum, hyaline, wall thickened below, thinner towards the tip, smooth to rough, apex 3–6 times tightly and relatively regularly branched, branches of all orders usually short, not elongated, tips of the ultimate branchlets straight. Asci 3–10, broad obovoid-saccate, 40–60 × 25–40 μm, subsessile or short-stalked, 4–5-spored. Ascospores ellipsoid-ovoid(-subglobose), 15–24 × 8–14 μm, colourless.
Type: USA, Wyoming, Centennial, on Lonicera involucrata, 22 Aug. 1911, E. Bartholomew [Barthol., Fungi Columb. 3720] (K, s.n. – holotype). Isotypes: Barthol., Fungi Columb. 3720 (e.g. BPI 556098, 556100, 556101; FLAS-F-01605; MSC0250454; NEB59550, 59595; PUL 21000; WIS-F24361; WSP 3918). Reference sequence (retrieved from WTU-F-072459, on L. involucrata, USA, Washington): MW045572.
Host range and distribution: on host species of Lonicera subgen. Chamaecerasus; Lonicera canadensis (North America: Canada; USA: New York, Ohio, Wisconsin), L. involucrata (North America: Canada; USA, Washington, Wyoming).
Notes: Braun (1982a) described Microsphaera lonicerae var. flexuosa with Lonicera involucrata as type host [the citation of a collection on L. ciliosa as type of this variety in Braun & Cook (2012) is incorrect]. Sequences obtained from powdery mildew on Lonicera involucrata form a separate clade in a sister position to the Erysiphe symphoricarpi clade, supporting the status that this powdery mildew is a distinct species. The name Microsphaera lonicerae var. flexuosa is available for the taxon involved, which is morphologically very close to E. symphoricarpi (except for more compact and more regularly branched terminal parts of the chasmothecial appendages, in contrast to apically more loosely and irregularly brached appendages in E. symphoricarpi, and nipple-shaped or only slightly lobed hyphal appressoria and wider conidiophores, 5–7 μm wide below and 7–9 μm wide above, in contrast to distinctly lobed hyphal appressoria and narrower conidiophores, 4–6 μm wide below and 5–7 μm wide above, in E. symphoricarpi). The allocation of var. flexuosa to E. caprifoliacearum, now a synonym of E. lonicerae s. str., was undoubtedly wrong.
Braun (1984) and Braun & Cook (2012) listed Lonicera canadensis, L. ciliosa, and L. involucrata as host species for this North American variety. Lonicera involucrata as well as L. canadensis are species of Lonicera subgen. Chamaecerasus. The powdery mildew on L. canadensis coincides morphologically with collections on L. involucrata, but the identification as E. flexibilis is still in need of phylogenetic confirmation. Powdery mildew on L. ciliosa (Lonicera subgen. Lonicera) has to be excluded from Erysiphe flexibilis and pertains to E. lonicerae s. str. (see Fig. 1). A German collection of Erysiphe on L. involucrata has been examined (Sachsen, Görlitz-Rauschwalde, on Lonicera involucrata, 28 Sep. 2008, S. Hoeflich, GLM-F91168, only asexual morph), however, it is unclear if it constitutes an introduction of E. flexibilis or if the host was infected by E. ehrenbergii.
Erysiphe lonicerae DC., Fl. franç. 6: 107. 1815 (s. str.)
Synonyms: Alphitomorpha divaricata var. lonicerae (DC.) Schltdl., Verh. Ges. Naturf. Freunde Berlin 1: 49. 1819.
Erysibe divaricata var. lonicerae (DC.) Link, Sp. pl. 4, 6(1): 113. 1824.
Erysiphe penicillata var. lonicerae (DC.) Fr., Syst. mycol. 3: 244. 1829.
Microsphaera lonicerae (DC.) G. Winter, in Rabenhorst’s Krypt.-Fl., Pilze, 1(2): 36. 1884.
Microsphaera penicillata var. lonicerae (DC.) W.B. Cooke, Mycologia 44: 572. 1952.
Microsphaera caprifoliacearum U. Braun, Mycotaxon 14: 369. 1982, syn nov. [type: Canada, London, on Lonicera sp., Sep. 1896, J. Dearness [Ellis & Everh., Fungi Columb. 1032] (PH 41544 – holotype); isotypes: Ellis & Everh., Fungi Columb. 1032 (e.g., BPI 557667, C0285718F, CBRU1231, CLEM-F2589, FLAS-F01592, ISC-F-0087668, MSC0219819, NEB 59557, PUL 18885)]. Reference sequence (retrieved from BPI557673, USA, on Lonicera ×heckrottii): MW045566.
Erysiphe caprifoliacearum (U. Braun) U. Braun & S. Takam., Schlechtendalia 4: 6. 2000.
Illustrations: Léveillé (1851: pl. 9, fig. 26), Salmon (1900: pl. 1, figs 19–20), Jaczewski (1927: 323, fig. 83), Blumer (1933: 299, fig. 109; 1967: 257, fig. 89), Golovin (1956: 337, fig. 12), Sandu-Ville (1967: 268, fig. 47), Braun (1982a: 128, fig. 12a; 1982b: 371, fig. 1; 1982c: 322, fig. 7; 1984: 232, pl. 4, Fig. 23a; 1987: 328, pl. 103, 432, pl. 196; 1995: 278, pl. 55, fig. A), Gorlenko (1983: 45, fig. 12), Eliade (1990: 445, pl. 12, fig. 52), Fakirova (1991: 82, pl. 28, fig. 2), Nomura (1992: 266, fig. 174; 1997: 143, fig. 176), Simonyan (1994: 160, figs 33–34), Paulech (1995: 218, fig. 105), Grigaliūnaitė (1997: 125, fig. 75), Kim et al. (2007: 6, figs 1–2), Braun & Cook (2012: 442, fig. 518 A, 477, fig. 550).
Exsiccatae [on Lonicera caprifolium (L. ca.), L. periclymenum (L. p.)]: Kari, Fungi Exs. Fenn. 264 (L. ca). Kochm. & Sałata, Mycoth. Polon. 654 (L. p.). Krypt. Exs. 124 (L. ca.). Kunze, Fungi Sel. Exs. 319 (L. p.). Lundell & Nannf., Fungi Exs. Suec. 1482 (L. ca.). Thüm., Mycoth. Univ. 1056 (L. ca.).
Description: Mycelium on leaves, amphigenous, effuse or in thin white patches, subcircular to irregular, sometimes covering the entire leaf surface, persistent or evanescent (persistent on the upper leaf surface, evanescent below); hyphae branched, branching at right angle, septate, hyaline, thin-walled, smooth, (1.5–)2–6(–7) μm wide; hyphal appressoria solitary, usually with a single appressorium per cells, occasionally with up to four per cell, nipple-shaped to lobed, 2–8 μm diam. Conidiophores arising from superficial hyphae, erect, terminal on mother cells, in the middle of the mother cell or towards one septum, 50–120 μm long (without conidia); foot-cells (12–)18–55(–75) × 4–10 μm, straight, cylindrical or subcylindrical to moderately curved at the base or sinuous, in most cases majority of foot-cells curved-sinuous, followed by 1–3 cells, 8–40 μm long, shorter than the foot cell or about as long, second cell sometimes somewhat longer, basal septum at the junction with the supporting hypha or only slightly elevated (up to 5 μm), conidia formed singly. Conidia narrowly cylindrical, subcylindrical or somewhat ellipsoid-cylindrical, 25–45(–55) × 11–24 μm, length/width ratio 1.7–4.5 (on average 2.8, N = 30), apex rounded to almost truncated, base truncated or almost so, germ tubes perihilar, short to moderately long, terminally unlobed to lobed (in vivo). Chasmothecia amphigenous, scattered to gregarious, subglobose, 70–120 μm diam, peridial cells polygonal, rounded to irregularly shaped, 8–28 μm diam. Appendages (3–)5–16(–20), more or less equatorial, radiating, stiff, straight to curved or longer ones somewhat flexuous, 1–3 times as long as the chasmothecial diam (90–290 μm), stalk simple or occasionally forked in the lower half, 7–12 μm wide below, 6–10 μm wide above, aseptate or with a single basal septum, colourless or pigmented at the base, wall thickened, to 4 μm wide below, thinner towards the tip, smooth to verruculose, apex 3–6 times regularly to irregularly dichotomously branched (branched part 25–85 μm diam), primary and secondary branches short or oblong, 10–30 μm, tips of the ultimate branchlets at first straight, at most mixed with a few recurved ones, but the majority distinctly recurved when fully mature and in specimens collected late in the season. Asci 3–12, broad ellipsoid, obovoid to saccate, subsessile to short-stalked, 40–70 × 25–50 μm, hyaline, wall 1–2 μm thick, terminal oculus indistinct, 8–15 μm diam, (2–)3–5-spored. Ascospores broad ellipsoid-ovoid, rarely subglobose, 15–24 × 8–15 μm, colourless or sometimes yellowish.
Type: France, on Lonicera caprifolium, 1813, J.F. de Chaillet (as “Erysiphe lonicerae, No. 503”), in herb. de Candolle (G 00122106 – holotype). Epitype: Switzerland, Genève, Jardin botanique, on Lonicera caprifolium, 16 Jul. 1997, A. Bolay (G 566366; designated here, MycoBank MBT394141); ex-epitype sequence – MN277393.
Additional materials examined: Germany, Bayern, Lindau, on Lonicera caprifolium, 29 Jun. 2008, H. Boyle & S. Hoeflich (GLM-F81552); Sachsen, Landkreis Görlitz, Herrnhut, OT Oberrennersdorf, on Lonicera ×heckrotii, 6 Sep. 2003, collector unknown (GLM-F51351); Sachsen, Görlitz, centre, on Lonicera periclymenum, 14 Sep. 2005, H. Boyle (GLM-F70452); Sachsen, Landkreis Görlitz, Johnsdorf, Kurpark, on Lonicera periclymenum, 12 Aug. 2007, H. Boyle & S. Hoeflich (GLM-F78479); Sachsen, Landkreis Bauzen, Bischofswerda, on Lonicera periclymenum, 12 Aug. 2007, H. Boyle & S. Hoeflich (GLM-F81067); Sachsen-Anhalt, Salzlandkreis, Calbe (Saale), on Lonicera caprifolium, 6 Sep. 1998, H. Jage (GLM-F49931); Sachsen-Anhalt, Saalekreis, Mücheln, OT Branderoda, on Lonicera caprifolium, 2 Aug. 1999, H. Jage (GLM-F46805); Sachsen-Anhalt, Burgenlandkreis, Freyburg, on Lonicera caprifolium, 27 Jun. 1999, U. Richter (GLM-F49112); Sachsen-Anhalt, Zeitz, Moritzburg, medical plant garden, on Lonicera japonica, 21 Oct. 2004, H. Jage (GLM-F66310); Sachsen-Anhalt, Landkreis Börde, Wolmirstedt, west of OT Mose, Pinus sylvestris forest, on Lonicera periclymenum, 25 Jul. 2001, H. Jage (GLM-F56796, GLM-F58634); Sachsen-Anhalt, Altmarkkreis Salzwedel, Arendsee, east of OT Schrampe, on Lonicera periclymenum, 26 Sep. 2001, H. Jage (GLM-F56815); Sachsen-Anhalt, Burgenlandkreis, Weißenfels, on Lonicera periclymenum, 8 Sep. 2002, H. Jage (GLM-F57487); Sachsen-Anhalt, Landkreis Harz, Quedlinburg, on Lonicera periclymenum, 16 Sep. 2003, H. Boyle (GLM-F51395); Sachsen-Anhalt, Altmarkkreis Salzwedel, Beetzendorf-Diesdorf, southwest of Beetzendorf, on Lonicera periclymenum, 14 Aug. 2004, H. Jage (GLM-F62570); Thüringen, Landkreis Nordhausen, about 1.5 km east of Bleicherode, “Eckstein”, on Lonicera caprifolium, 19 Aug. 2004, W. Schulz (GLM-F64253). New Zealand, Auckland, Waikumete cemetery, on Lonicera japonica, 22 Jul. 2001, C.F. Hill (HAL 1658 F). Switzerland, GE, Lullier, Centre horticole, on L. japonica, 28 Oct. 1996, A. Bolay (G 566367, 566368); ibid., 4 Nov. 1996, A. Bolay (G 566369).
Host range and distribution: on host species of Lonicera subgen. Lonicera and Lonicera japonica; Lonicera caprifolium (Europe: Austria, Belgium, Estonia, Finland, France, Germany, Greece, Hungary, Italy, the Netherlands, Norway, Poland, Serbia, Sweden, Switzerland, Turkey, Russia, UK), L. dioica (North America: Canada; USA: Connecticut, Illinois, Iowa, Michigan, Minnesota, New York, Pennsylvania, Vermont, Wisconsin), L. etrusca (Europe: France, Russia, Spain, Switzerland, UK), L. flava (North America: USA: Illinois, Indiana, Iowa, Ohio), L. glaucescens (North America: Canada; USA: Nebraska, Wisconsin, Wyoming, Virginia), L. ×heckrottii (Europe: Germany; North America: Oklahoma), L. hirsuta (North America: Canada; USA: New York, Michigan, Minnesota, Wisconsin), L. japonica (Asia: China, Japan; Europe: Germany, Switzerland, UK; North America: USA: Florida; South America, Argentina), L. parviflora (North America: USA: Connecticut, Vermont, Wisconsin), L. periclymenum (Europe: France, Germany, Lithuania, the Netherlands, Norway, Poland, Romania, Sweden, Switzerland, UK; North America: Canada), L. prolifera [= L. sullivantii] (North America: USA: Colombia, Iowa, Michigan, Wisconsin), L. sempervirens (Asia: Korea; Europe: Estonia; North America: USA: Florida, Michigan, Minnesota, Pennsylvania, Wisconsin) [Amano (1986), Braun (1987, 1995), Grigaliūnaitė (1997), Rusanov & Bulgakov (2008), https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm].
Notes: The development of mycelium and the asexual morph of Erysiphe lonicerae s. str. is comparable with E. ehrenbergii: it commences in early summer (in Europe in June), with a chasmothecial formation from June to November. The conidial formation also ceases with the start of the formation of the sexual morph.
Sequences retrieved from North American Erysiphe samples on Lonicera ciliosa, belonging to Lonicera subgen. Lonicera, are identical with European sequences obtained from L. caprifolium (type host of E. lonicerae) and L. periclymenum and fall into the Erysiphe lonicerae s. str. clade. Powdery mildew on Lonicera ciliosa has previously been referred to as Erysiphe caprifoliacearum var. flexuosa (≡ Microsphaera lonicerae var. flexuosa), described from North America with a collection on Lonicera involucrata (Lonicera subgen. Chamaecerasus) as type material (Braun 1982a). However, M. lonicerae var. flexuosa constitutes a separate species. Sequences retrieved from Erysiphe on L. involucrata in North America form a sister clade to E. symphoricarpi distant from the E. lonicerae s. str. clade. Chasmothecia on L. ciliosa in North America are barely distinguishible from European collections of E. lonicerae. The only difference being that the chasmothecial appendages are somewhat longer and more flexuous in the North American collections compared to the European collections in which the appendages are somewhat shorter and more stiff. The more flexuous nature of the appendages in “var. flexuosa” on L. ciliosa is correlated with the appendage length and might be a modification. Long, flexuous appendages have also been observed in some European specimens. The structure of the branched apices of the appendages in “var. flexuosa” on L. ciliosa and in European collections of E. lonicerae s. str. is not differerent, i.e., in all collections the tips of the ultimate branchlets are at first (and for a longer time) straight, but tend to become recurved in fully mature specimens later in the season.
Erysiphe caprifoliacearum was described by Braun (1982b), based on differences between North American collections on Lonicera spp., with more regularly branched chasmothecial appendages with recurved tips, and European collections of E. lonicerae s. lat., characterised by having usually straight ultimate tips. However, recent comprehensive examinations of a wide range of European specimens collected from late spring to late autumn have shown that E. lonicerae s. str. on hosts of Lonicera subgen. Lonicera and Lonicera japonica is characterised by having branched chasmothecial appendages with ultimate tips that become recurved when fully mature later in the season. Since other traits of the chasmothecia are similar between E. lonicerae s. str. and E. caprifoliacearum, it is justified to reduce the latter species to synonymy with E. lonicerae s. str. The reduction of E. lonicerae s. str. and E. caprifoliacearum to synonomy is also supported by the present phylogenetic analyses and the host range of E. caprifoliacearum. Braun & Cook (2012) listed the following host species for E. caprifoliacearum: Lonicera dioica (= L. glauca auct.), L. flava, L. glaucescens, L. ×heckrottii, L. hirsuta, L. oblongifolia, L. parviflora, and L. prolifera (= L. sullivantii). The citation of “L. semenovii [= L. glauca]” in Braun & Cook (2012) was based on a confusion of the name L. glauca. North American records of powdery mildew on Lonicera glauca refer to L. glauca Hill, [which is a synonym of L. dioica (Rehder 1903)] and not L. glauca Hook. f. & Thomson, described from the Himalayas (which is a synonym of L. semenovii). All North American host species of E. caprifoliacearum, except for L. oblongifolia, pertain to Lonicera subgen. Lonicera, which is in agreement with the host range of E. lonicerae s. str. and supports the synonymy of the two species. The record of E. caprifoliacearum on L. oblongifolia (Lonicera subgen. Chamaecerasus) remains unclear and requires re-examinations of the host identity and morphology of the powdery mildew involved.
Lee et al. (2016) published a record of Erysiphe lonicerae on Lonicera japonica from Korea with a sequence that clusters in the E. lonicerae s. str. clade described with relatively large chasmothecia, 100–140 μm diam. The record of Erysiphe lonicerae on Lonicera sempervirens from Korea (Kim et al. 2007) seems to belong to E. lonicerae s. str. but should be confirmed by sequence data. Powdery mildew on Lonicera japonica in Florida (Alfieri et al. 1984) most likely belongs to Erysiphe lonicerae s. str., but due to the occurrence of a second powdery mildew, E. lonicerina, on this host, morphological and phylogenetical confirmations are necessary.
Erysiphe lonicerina S. Takam., sp. nov. MycoBank MB837571. Fig. 3.
Fig. 3.

Erysiphe lonicerina (TNS-F-87428 – holotype). A. Unlobed hyphal appressorium. B. Conidia. C. Conidium with Pseudoidium-type germ tube. D. Conidiophores with straight foot-cell. E. Conidiophore with slightly curved-sinuous foot-cell. Scale bars = 10 μm.
Etymology: Epithet composed of Lonicera, the name of the host genus, and the Latin suffix -ina (= belonging to).
Diagnosis: Phylogenetically distinct from Erysiphe ehrenbergii and E. lonicerae s. str.; the asexual morph differs from the latter two species in having conidiophores with almost exclusively straight foot-cells and longer conidia, 40–65 μm.
Description: Mycelium amphigenous, white, persistent; hyphae almost straight to somewhat sinuous, 4–7 μm wide, branching at right angles; hyphal appressoria well-developed, simply lobed, rarely nipple shaped, in opposite pairs or single. Conidiophores arising from hyphal cells, terminal on mother cells, erect, 70–128 × 9–15 μm, foot-cells usually straight, sometimes slightly curved or curved-sinuous at the very base, 34–68 × 7–12 μm, followed by 2–4 shorter cells, forming conidia singly. Conidia almost cylindrical to ellipsoid-ovoid, 40–65 × 15–20 μm, length/width ratio 2.2–4 (on average 3.0, N = 30), germ tubes perihilar, Pseudoidium-type. Sexual morph unknown.
Type: Japan, Chiba Prefecture, Chiba-shi, Chiba University, Nishi-Chiba Campus, on Lonicera japonica, 27 Nov. 1998, S. Takamatsu (TNS-F-87428 – holotype); ex-holotype sequence – LC009953 (ITS).
Additional material examined: Japan, Nara Prefecture, Sakurai-shi, Mt. Ryumon, on Lonicera japonica, 28 Oct. 2006, S. Takamatsu (TSU-MUMH 4464).
Host range and distribution: On Lonicera japonica, Asia (Japan).
Notes: Sequences belonging to Erysiphe lonicerina form a sister clade to the E. ehrenbergii clade. Erysiphe lonicerina differs from E. ehrenbergii and E. lonicerae s. str. in that it has almost exclusively straight conidiophore foot-cells and longer conidia (40–65 × 15–20 μm vs. 25–45(–55) × 11–24 μm in E. lonicerae and 23–39 × 10–17 μm in E. ehrenbergii).
Notes on additional Erysiphe spp. on Lonicera spp.
Erysiphe erlangshanensis (Y.N. Yu) U. Braun & S. Takam., Schlechtendalia 4: 8. 2000.
Basionym: Microsphaera erlangshanensis Y.N. Yu, in Yu & Lai, Acta Mycol. Sin. 2(2): 91. 1983.
Illustrations: Vasyagina et al. (1961: 300, fig. 97), Tanda et al. (1977: 29, pl. VIII, 30, pl. IX), Yu & Lai (1983: 92, fig. 2), Braun (1987: 424, pl. 189), Chen et al. (1987: 191, fig. 97), Shin (1988: 108, fig. 24; 2000: 102, fig. 30), Nomura (1992: 236, fig. 154; 1997: 127, fig. 155), Braun & Cook (2012: 459, fig. 550).
Descriptions: Tanda et al. (1977: 21), Braun (1987: 424), Chen et al. (1987: 190), Otani (1988: 218), Shin (1988: 106; 2000: 103), Nomura (1992: 235; 1997: 126), Braun & Cook (2012: 459).
Type: China, Prov. Sichuan, Erlangshan, on Lonicera hispida, 15 Sep. 1958, Liu & Song (HMAS 37844 – holotype).
Notes: Braun & Cook (2012) circumscribed the host range and distribution of E. erlangshanensis as follows: Lonicera chrysantha, L. gracilipes, L. hispida, L. karelinii, L. maackii, L. nummulariifolia, L. rubrechtiana, L. subsessilis, L. tatarica, and L. vidalii in Asia (Central Asia, China, Iran, Japan, Korea, Nepal, Russian Far East). All host species pertain to Lonicera subgen. Chamaecerasus. Khodaparast et al. (2000) recorded E. erlangshanensis on L. nummulariifolia from Iran, and Heluta (2004) confirmed L. maackii and L. rubrechtiana as hosts in the Far East of Russia. This species is characterised and distinguished from all other Erysiphe species on Lonicera by its very short chasmothecial appendages. The asexual morph of this species, characterised by having straight conidiophore foot-cells, was described and illustrated in detail in Shin (2000). A sequence retrieved from E. erlangshanensis on L. maackii from the Russian Far East clusters separately from E. lonicerae and all other sequenced Erysiphe spp. on Lonicera, supporting its status as a species of its own. However, because this species is widespread in Asia on a wide range of host species, more sequence data from different regions and hosts are necessary for proving the genetic uniformity of this species.
Erysiphe lonicerae-ramosissimae (Tanda) U. Braun & S. Takam., Schlechtendalia 8: 33. 2002.
Basionym: Microsphaera lonicerae-ramosissimae Tanda, Mycoscience 41: 158. 2000.
Illustrations: Tanda (2000: 158, fig. 3), Braun & Cook (2012: 479, fig. 582).
Description: Braun & Cook (2012: 478).
Type: Japan, Kanagawa Pref., Yokohama, Aoba-ku, Kodomonokuni children’s park, on Lonicera ramosissima, 2 Nov. 1980, S. Tanda (TUAMH 1462 – holotype).
Notes: This species is morphologically barely distinguishable from E. ehrenbergii. Lonicera ramosissmima is a species of Lonicera subgen. Chamaecerasus, supporting the assumption that E. lonicerae-ramosissimae might be a synonym of E. ehrenbergii. The final clarification of the synonymy requires sequence data retrieved from powdery mildew on L. ramosissima.
Erysiphe magnusii (S. Blumer) U. Braun & S. Takam., Schlechtendalia 4: 10. 2000.
Basionym: Microsphaera magnusii S. Blumer, Beitr. Krypt.-Fl. Schweiz 7(1): 299. 1933.
Illustrations: Blumer (1933: 300, fig. 110, 301, fig. 111), Golovin (1956: 339, fig. 13), Vasyagina et al. (1961: 301, fig. 98), Braun (1987: 355, pl. 127; 1995: 283, pl. 65), Eliade (1990: 445, pl. 12, fig. 53), Fakirova (1991: 82, pl. 28, fig. 1), Simonyan (1994: 162, fig. 36), Paulech (1995: 212, fig. 100), Braun & Cook (2012: 481, fig. 586).
Descriptions: Golovin (1956: 338, 359; 1960: 148), Vasyagina et al. (1961: 299), Blumer (1967: 260), Junell (1967: 59), Eliade (1973: 203; 1990: 442), Bunkina (1979: 91), Sałata (1985: 169), Braun (1987: 356; 1995: 172), Bunkina (1991: 109), Fakirova (1991: 89), Simonyan (1994: 161), Paulech (1995: 211), Bolay (2005: 63), Braun & Cook (2012: 481).
Type: Switzerland, Bern, Bremgartenwald, on Lonicera nigra, Aug. 1894, F. v. Tavel [ex herb. Volkart] (ZT – lecotype, designated by Braun 1987).
Notes: The host range and distribution of E. magnusii can be summarised as follows (Braun 1995, Braun & Cook 2012): Lonicera alpigena, L. coerulea, L. chamissoi, L. nigra, L. orientalis [= L. caucasica], L. pallasii [≡ L. coerulea subsp. pallasii], L. stenantha, L. xylosteum, Lonicera sp., Asia (Central Asia, Russia, Far East), Caucasus (Armenia), Europe (Austria, Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Italy, the Netherlands, Norway, Poland, Romania, Russia, Slovakia, Spain, Sweden, Switzerland, Ukraine, former Yugoslavia). The record of this species from Ukraine on Lonicera nigra was published by Heluta et al. (2018). Erysiphe magnusii has not been phylogenetically confirmed, but is readily distinguishable from all other species of Erysiphe on Lonicera spp. by its very long flexuous chasmothecial appendages. The tips of the ultimate branchlets of the terminal branched part of chasmothecial appendages are distinctly recurved when mature, but in a few collections (e.g., Switzerland, VD, Le Sentier, Tête du Lac, on Lonicera nigra, 4 Aug. 2005, A. Bolay, G 5666325) the tips remained straight for a longer time and only became recurved relatively late in the season. Past collections might have been confused with and misidentified as E. lonicerae.
Erysiphe miurae (U. Braun) U. Braun & S. Takam., Schlechtendalia 4: 11. 2000.
Basionym: Microsphaera miurae U. Braun, Mycotaxon 16: 420. 1983.
Illustrations: Braun (1983: 423, fig. 5; 1987: 437, pl. 201), Nomura (1992: 212, fig. 136; 1997: 114, fig. 137), Liu (2010: 108, fig. 50), Braun & Cook (2012: 484, fig. 592 A).
Descriptions: Braun (1987: 436), Otani (1988: 224), Nomura (1992: 211; 1997: 114), Braun & Cook (2012: 483–484).
Type: Japan, Pref. Uzen, Omagari, on Lonicera morrowii, 3 Nov. 1908, Miura (TNS-F-214110 – holotype).
Notes: Two sequences rertrieved from Japanese Erysiphe collections on Lonicera morrowii (type host of Microsphaera miurae) and L. vidalii cluster together in a clade distant from all other clades (Fig. 1), supporting Erysiphe miurae as a species of its own. The morphology of the powdery mildew on L. morrowii sequenced corresponds well with E. miurae, in that it includes chasmothecia with appendages about 1–1.5 times as long as the chasmothecial diam.
All host species of Erysiphe miurae belong to Lonicera subgen. Chamaecerasus. Braun & Cook (2012) recorded E. miurae from Asia (China, Japan) on Lonicera chamissoi, L. ciliata, L. japonica, L. morrowii, and L. orientalis. Braun (2002) recorded E. miurae on Lonicera glehnii from the Russian Far East, and Liu (2010) published a detailed description and illustration of this species on Lonicera tatarinovii from China (Inner Mongolia). The identity of Lonicera “ciliata” is unclear (confused doubtful name). Braun & Cook (2012: 484, fig. 592 B) assigned an examined Chinese specimen on L. japonica to E. miurae (China, Prov. Liaoning, Sep. 1974, Zhang, HMAS 37845). However, this record is unclear and doubtful. The E. miurae on L. japonica is a fully developed mature sample that most likely pertains to Erysiphe lonicerae s. str., which is known to occur on L. japonica in China. Fully mature specimens of E. lonicerae s. str. on L. japonica with recurved tips of the ultimate branchlets of the branched apex of the chasmothecial appendages are easily confusable with the sexual morph of E. miurae.
Besides the host plants of E. miurae included in Braun & Cook (2012), Kobayashi (2007) listed Lonicera korolkovii, L. maackii, L. orientalis, L. sempervirens (subgen. Chamaecerasus), L. strophiophora, L. tatarica, and L. vidalii as host plants in Japan. Lonicera vidalii is a confirmed host species of E. miurae (see Fig. 1). Lonicera maackii, L. tatarica, and L. vidalii have been recorded from Korea as hosts of E. erlangshanensis (Shin 2000). It is possible that the host ranges of E. erlangshanensis and E. miurae may overlap. Future research should confirm the host ranges by further examinations that include sequences. Until future host range analyses are confirmed, the confusion of the two Asian Erysiphe species by Kobayashi (2007) cannot be excluded. Additionally, a more in depth phylogenetic analysis from Asian collections on Lonicera spp., identified as E. erlangshanensis and E. miurae, are urgently needed to confirm the identity of collections on particular host species in Asia.
Unclear, not yet assignable records of Erysiphe lonicerae s. lat. [≡ Microsphaera lonicerae] and Microsphaera penicillata [= M. alni] s. lat.
There are numerous records of Erysiphe lonicerae s. lat. on a wide range of additional host species that are still unconfirmed. These records are often referring to collections made in botanical gardens and parks, i.e., the Lonicera spp. concered grew outside their natural ranges as ornamental shrubs or cultivated for scientific purposes.
All of the listed host records referred to as Erysiphe lonicerae (s. lat.) relate to species belonging to Lonicera subgen. Chamaecerasus (Bunkina 1979, 1991; Amano 1986; Karis 1987; Braun 1987, 1995): Lonicera altmannii [= L. humilis] (Russia), L. ×amoena (Estonia, Russia), L. caucasica (Russia), L. chamissoi (Japan, Russia), L. chrysantha (Russia), L. edulis (Estonia, Russia), L. glehnii (Russia), L. gracilipes (Japan), L. hispida (China), L. iberica (Armenia, Russia), L. karelinii [= L. heterophylla] (Russia), L. korolkowii (Finland, Russia, UK), L. maackii (Japan, Russia), L. maximowiczii [= L. sachalinensis] (Russia), L. microphylla (Russia), L. myrtillus [= L. angustifolia var. myrtillus] (Russia), L. numullariifolia [= L. arborea] (Russia), L. orientalis (Japan, Romania, Russia), L. praeflorens (Russia), L. prostrata (China, Russia), L. ruprechtiana (Estonia, Russia), L. stenantha (China), L. syringantha [≡ L. rupicola var. syringantha] (Romania), L. utahensis (USA: Wyoming, Solheim, Mycofl. Saximont Exs. 609).
Some of these plant species are known to be host species of Erysiphe erlangshanensis (L. gracilipes, L. hispida, L. maackii, L. ruprechtiana) as well as E. miurae (L. chamissoi, L. glehnii, L. maackii).
The following records published from North America under Microsphaera alni and M. penicilla on Lonicera spp. pertaining to Lonicera subgen. Chamaecerasus are also unclear and in need of re-examination: L. hispidula (USA: Floria, Alfieri et al. 1984), L. oblongifolia (Canada; USA: Michigan, Ohio, Wisconsin; Anonymous 1960, Amano 1986).
Key to species of Erysiphe on Lonicera
1. Foot-cells of the conidiophores almost exclusively straight; conidia very long, 40–65 × 15–20 μm; on Lonicera japonica, Japan .................................................................................................................................................................... Erysiphe lonicerina
1* Foot-cells of the conidiophores either straight to curved-sinuous and conidia shorter, on average < 40 μm, or only conidia shorter .................................................................................................................................................................................................. 2
2. Chasmothecial appendages long and flexuous, 1.5–10 times as long as the chasmothecial diam .................................................... 3
2* Chasmothecial appendages shorter, usually 0.8–2.5 times as long as the chasmothecial diam, and stiff, straight to curved ........... 4
3. Chasmothecial appendages 1.5–3.5(–4) times as long as the chasmothecial diam, apex tightly and rather regularly brached, tips straight and remaining so; on Lonicera involucrata (and probably L. canadensis), North America .................. Ersiphe flexibilis
3* Chasmothecial appendages very long, 2–10 times the chasmothecial diam, apex 2–5 times branched, tips of the ultimate branchlets recurved when mature; on Lonicera spp., Asia, Europe ................................................................................ Erysiphe magnusii
4(2*) Tips of the ultimate branchlets of the branched apex of the chasmothecial appendages always straight, only with a few somewhat recurved tips, also in fully mature samples, or straight for a longer time, even when asci and ascospores are already developed, but numerous tips becoming recurved when fully mature, i.e., in older samples and above all late in the season (Erysiphe lonicerae s. lat. complex) ............................................................................................................................................ 5
4* All or almost all tips of the ultimate branchlets recurved from the very beginning of maturity when asci and ascospores commence to develop; Asian and North American species .......................................................................................................................... 7
5. Chasmothecia 70–85 μm diam; appendages with a septum near the middle; asci usually 3; on Lonicera ramosissima, Japan .................................................................................................................................................. Erysiphe lonicerae-ramosissimae
5* Appendages aseptate or with 1–3 septa in the lower half; asci 2–8; on other host species .............................................................. 6
6. Conidia 25–45(–55) × 11–24 μm, length/width ratio 1.7–4.5 (on average 2.8, N = 30); ultimate tips of the branched part of the chasmothecial appendages straight for a longer time, only with a few recurved tips, but numerous tips becoming recurved in fully mature samples, above all late in the season, appendages aseptate or with a single basal septum; on host species of Lonicera subgen. Lonicera and L. japonica ................................................................................................................................................................ Erysiphe lonicerae s. str.
6* Conidia 23–39 × 10–17 μm, length/width ratio 1.9–3.2 (on average 2.4, N = 30); ultimate tips of the branched part of the chasmothecial appendages straight, only with a few recurved ones throughout the season, appendages not infrequently with 1–2 or even 3 elevated septa and then pigmented below the septa; on host species of Lonicera subgen. Chamaecerasus ................................................................................................................................................................... Erysiphe ehrenbergii
7(4*) Appendages 1–2 times as long as the chasmothecial diam .............................................................................. Erysiphe miurae
7* Appendages shorter, 0.5–1.25 times the chasmothecial diam, usually somewhat shorter than the diam ............................................................................................................................................................ Erysiphe erlangshanensis
DISCUSSION
The clarification of the phylogeny and taxonomy of Erysiphe lonicerae, including its variety ehrengergii on Lonicera tatarica, was the initial objective of the present examinations with the main goal to ascertain whether the powdery mildew on L. tatarica (L. lonicerae var. ehrenbergii) represented a species of its own or a synonym of L. lonicerae. Sequences (rDNA: ITS and 28S, the standard markers for powdery mildews) were retrieved from a wide range of collections originally identified as E. lonicerae and used for the construction of a phylogenetic tree, supplemented by corresponding sequences available in GenBank. The results of the current study revealed that the new sequences retrieved from powdery mildew on L. tatarica, and all other available sequences obtained from E. lonicerae s. lat. on various other Lonicera spp., formed six distinct, distant clades, representing six clearly separated species.
One clade, including sequences from Erysiphe lonicerae on Lonicera caprifolium, the type host of this species, has to be referred to as E. lonicerae s. str. It encompases sequences retrieved from E. lonicerae on L. caprifolium and L. periclymenum from Europe as well as on L. ciliosa, L. ×heckrotii and L. prolifera from North America. All host species pertain to Lonicera subgen. Lonicera (= subgen. Caprifolium), suggesting that E. lonicerae s. str. co-evolved with host species of this subgenus of Lonicera. However, sequences of powdery mildew on L. japonica also cluster within the E. lonicerae s. str. clade. Lonicera japonica currently tends to be assigned to subgen. Chamaecerasus sect. Nintooa, which is, however, polyphyletic (Nakaji et al. 2015). Previously, sect. Niontooa and L. japonica have been assumed to be a link between the two subgenera of Lonicera because its members have hollow branches and a climbing habit (traits that are also to be found in subgenus Lonicerae) yet the leaves subtending the inflorescence are not fused (Rehder 1903, Theis et al. 2008).
Lonicera japonica is a popular cultivated shrub native to Asia (Japan, Korea and East China), and is currently established in several parts of Europe, on the Azores and Madeira, South Africa, Australia, New Zealand, North and South America and Hawaii (Roloff & Bärtels 2008, Wu et al. 2011). Erysiphe lonicerae s. str. on L. japonica is also known from China and Japan, i.e., from the natural range of this host species. Lonicera caprifolium and L. periclymenum are European species, and L. ciliosa, L. ×heckrotii and L. prolifera are native to North America. Thus, E. lonicerae is a powdery mildew species that is widly distributed in the Northern Hemisphere. Phylogenetic analyses revealed that E. caprifoliacearum, of which L. ×heckrotii and L. prolifera are common hosts, should be reduced to synonomy with E. lonicerae (s. str.). The inclusion of Lonicera ciliosa in the host range of Erysiphe lonicerae has been confirmed by results of the present phylogenetic and morphological analyses and is supported by the placement of this host in Lonicera subgen. Lonicera.
Powdery mildew on L. ciliosa was originally assigned to Microsphaera lonicerae var. flexuosa by Braun (1982a). Braun (1984) went on to treat this powdery mildew as variety of M. caprifoliacearum. Most recently powdery mildew on L. ciliosa has been assigned to Erysiphe caprifoliacearum var. flexuosa (Braun & Takamatsu 2000, Braun & Cook 2012). It should be noted that L. ciliosa was not the type host of E. caprifoliacearum var. flexuosa. Phylogenetic analyses of North American Erysiphe collections on Lonicera involucrata, the type host of var. flexuosa, have shown that another species, that is phylogenetically allied to and morphologically close to Erysiphe symphoricarpi, is involved. The new name Erysiphe flexibilis, based on Microsphaera lonicerae var. flexuosa, is introduced for this North American powdery mildew on L. involucrata.
The second, larger clade, is composed of sequences retrieved from powdery mildew that was previously referred to as Erysiphe lonicerae (s. lat.) on various Lonicera spp., including the type host of E. lonicerae var. ehrenbergii, L. tatarica. This clade is distant from the E. lonicerae clade. The sequences in this clade have been retrieved from powdery mildew on various Lonicera spp. belonging to Lonicera subgen. Chamaecerasus (the largest subgenus of this genus). The involvement of the two clades of E. lonicerae s. lat. as well as the two subgenera of Lonicera suggests a high degree of co-evolution between these powdery mildews and their host species. Because the Lonicera tatarica powdery mildew is included in this clade and represents a species of its own, the name Microsphaera ehrenbergii is available for the species involved, and thus the new combination Erysiphe ehrenbergii is introduced. There also exists a few morphological characteristics that differentiate E. ehrenbergii and E. lonicerae. The conidia in E. lonicerae tend to be longer, 25–55 × 11–24 μm, with a length/width ratio of 1.7–4.5 (on average 2.8), and the ultimate tips of the branched part of chasmothecial appendages become frequently recurved when fully mature (vs., conidia 23–39 × 10–17 μm, length/width ratio 1.9–3.2, on average 2.4 in E. ehrenbergii, ultimate tips of the branched part of chasmothecial appendages remaining straight, also in mature and late collections, only with a few recurved ones).
There is an additional Erysiphe species on Lonicera japonica in Japan, yet it is only known in its asexual morph, i.e., chasmothecia have not yet been found. Sequences belonging to this species on L. japonica form a sister clade to the E. ehrenbergii clade. This species, described as E. lonicerina, differs from E. ehrenbergii and E. lonicerae s. str. in having almost exclusively straight conidiophore foot-cells and longer conidia, 40–65 × 15–20 μm, vs. 25–45(–55) × 11–24 μm in E. lonicerae and 23–39 × 10–17 μm in E. ehrenbergii.
There are several other species of Erysiphe sect. Microsphaera on Lonicera, viz., E. magnusii in Europe, and the Asian species E. erlangshanensis and E. miurae. These three species are morphologically easily distinguishable from E. loniceraea s. lat. (see Braun & Cook 2012). A few sequences retrieved from E. erlangshanensis and E. miurae suggest that these taxa represents separate species. Future research should obtain sequences from samples of E. magnusii and additional ones from E. erlangshanensis and E. miurae to confirm these taxa as separate species in phylogenetic analyses (see Fig. 1).
ACKNOWLEDGEMENTS
We are obliged to the curators of the herbaria BPI, G, GLM, MUMH and WTU for the possibility to examine specimens in their keeping.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
REFERENCES
- Alfieri SA, Jr, Langdon KR, Wehlburg C, et al. (1984). Index of Plant Diseases in Florida (Revised). Florida Department of Agriculture and Consumer Services Division of Plant Industry, Bulletin 11: 1–389. [Google Scholar]
- Amano (Hirata) K. (1986). Host Range and Geographical Distribution of the Powdery Mildew Fungi. Japan Scientific Societies Press, Tokyo. [Google Scholar]
- Anonymous (1960). Index of Plant Diseases in the United States. [U.S.D.A. Agriculture Handbook 165.]. Washington. [Google Scholar]
- Blumer S. (1933). Die Erysiphaceen Mitteleuropas unter besonderer Berücksichtigung der Schweiz. Beiträge zur Kryptogamenflora der Schweiz 7: 1–483. [Google Scholar]
- Blumer S. (1967). Echte Mehltaupilze (Erysiphaceae). G. Fischer Verlag, Jena. [Google Scholar]
- Bolay A. (2005). Les Oïdiums de Suisse (Erysiphacées). Cryptogamica Helvetica 20: 1–173. [Google Scholar]
- Bradshaw M, Tobin PC. (2020). Sequencing herbarium specimens of a common detrimental plant disease (powdery mildew). Phytopathology 110: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Bradshaw M, Braun U, Götz M, et al. (2017). Powdery mildew of Chrysanthemum × morifolium: phylogeny and taxonomy in the context of Golovinomyces species on Asteraceae hosts. Mycologia 109: 508–519. [DOI] [PubMed] [Google Scholar]
- Braun U. (1982a). Descriptions of new species and combinations in Microsphaera and Erysiphe. Mycotaxon 14: 369–374. [Google Scholar]
- Braun U. (1982b). Descriptions of new species and combinations in Microsphaera and Erysiphe (II). Mycotaxon 15: 121–137. [Google Scholar]
- Braun U. (1982c). Morphological studies in the genus Oidium (III). Zentralblatt für Mikrobiologie 137: 314–324. [Google Scholar]
- Braun U. (1983). Descriptions of new species and combinations in Microsphaera and Erysiphe (III). Mycotaxon 16: 417–424. [Google Scholar]
- Braun U. (1984). A short survey of the genus Microsphaera in North America. Nova Hedwigia 39: 211–243. [Google Scholar]
- Braun U. (1987). A monograph of the Erysiphales (powdery mildews). Beihefte zur Nova Hedwigia 89: 1–700. [Google Scholar]
- Braun U. (1995). The Powdery Mildews (Erysiphales) of Europe. G. Fischer Verlag, Jena. [Google Scholar]
- Braun U. (2002). Ersiphe miurae and E. syringae-japonicae – new records from Russia. Mikologiya i Fitopatologiya 36(2): 15–16. [Google Scholar]
- Braun U, Cook RTA. (2012). Taxonomic Manual of the Erysiphales (Powdery Mildews). CBS Biodiversity Series 11: 1–707. [Google Scholar]
- Braun U, Takamatsu S. (2000). Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences – some taxonomic consequences. Schlechtendalia 4: 1–33. [Google Scholar]
- Bunkina IA. (1979). Muchnisto-rosyanye griby (sem. Erysiphaceae) Dal’nego Vostoka. Dal’nevostochny Gosudarstvenny Universitet, Vladivostok. [Google Scholar]
- Bunkina IA. (1991). Erysiphales. In: Nizshie rasteniya, griby i mokhoobraznye Sovetskogo Dal’nego Vostoka. Griby, Tom 2, Askomicety, Erizifal’nye, Klavitsipital’nye, Gelotsial’nye (Azbukina ZM, ed.). Nauka, Leningrad: 11–142. [Google Scholar]
- Chen GQ, Han SJ, Lai YQ, et al. (1987). Flora Fungorum Sinicorum. Vol. 1, Erysiphales. Science Press, Beijing. [Google Scholar]
- Cho SE, Park MJ, Kim JY, et al. (2012a). First report of powdery mildew caused by Erysiphe sedi on Kalanchoe blossfeldiana in Korea. Plant Disease 96: 1701. [DOI] [PubMed] [Google Scholar]
- Cho SE, Park JH, Lee SK, et al. (2012b). Occurrence of powdery mildew caused by Erysiphe abeliicola on glossy abelia in Korea. Research in Plant Disease 18: 133–138. [Google Scholar]
- Cunnington JH, Takamatsu S, Lawrie AC, et al. (2003). Molecular identification of anamorphic powdery mildews (Erysiphales). Australasian Plant Pathology 32: 421–428. [Google Scholar]
- Denton G, Henricot B. (2007). First report of powdery mildew on Deutzia spp. in the UK. Plant Pathology 56: 353–353. [Google Scholar]
- Eliade E. (1973). Studiul monografic al speciilor de Microsphaera Lév. (Erysiphaceae) din Flora Romaniei. Lucrările Grădinii Botanice din Bucureşti 1975–1976: 187–218. [Google Scholar]
- Eliade E. (1990). Monografia Erysiphaceelor din România. Lucrările Grădinii Botanice din Bucureşti 1989–1990: 105–574. [Google Scholar]
- Fakirova IF. (1991). G’bute v B’lgariya, 1 tom, razred Erysiphales (Fungi Bulgaricae, 1 tomus, ordo Erysiphales). Izdatel’stvo na B’lgarskata Akademiya na Naukite. [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenetics: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Girilovich IS, Gulis VI, Khramtsov AK, et al. (2005). Micromycetes of state national park of Republik Belarus II. Powdery mildew fungi. Mikologiya i Fitopatologiya 39: 24–30. [Google Scholar]
- Golovin PN. (1956). Materialy k monografii muchnisto-rosyanykh gribov (semeystvo Erysiphaceae) v SSSR (rody Arthrocladiella, Podosphaera, Microsphaera). Trudy Botanichekogo Instituta Imeni V. L. Komarova Akademii Nauk SSSR, Ser. 2, 10: 309–366. [Google Scholar]
- Golovin PN. (1960). Muchnisto-rosyanye griby parazitiruyushchie na kul’turnykh i poleznykh rasteniyakh. Izdatel’stvo Akademii Nauk SSSR, Moskva, Leningrad. [Google Scholar]
- Gorlenko MV. (1983). Muchnisto-rosyanye griby Moskovskoj Oblasti – Semejstvo Erysiphaceae. Moskva. [Google Scholar]
- Grigaliūnaitė B. (1997). Mycota Lithuaniae. Vol. 3, Erysiphales 1. Mokslo ir Enciklopedijų Leidybos Institutas, Vilnius. [Google Scholar]
- Hara H. (1983). A revision of Caprifoliaceae of Japan with reference to allied plants in other districts and the Adoxaceae. Academia Scientific Book Inc., Tokyo. [Google Scholar]
- Heluta VP. (1989). Flora Gribov Ukrainy. Muchnistorosyanye griby (Flora Fungorum RSS Ucrainicae, Ascomycetes, Erysiphales). Naukova Dumka, Kiev. [Google Scholar]
- Heluta VP. (2004). First records of powdery mildew fungi (Erysiphales) from the Lazovskiy reserve (Russian Far East). Mikologiya i Fitopatologiya 38: 15–19. [Google Scholar]
- Heluta VP, Hayova VP, Tykhonenko YuYa. (2018). Hryby Natsionalnoho pryrodnoho parku «Cheremoskyi». Pryroda Zakhidnoho Polissya ta Prylehlykh Terytoriy 15: 117–129. [Google Scholar]
- Hirata T, Takamatsu S. (1996). Nucleotide sequence diversity of rDNA internal transcribed spacers extracted from conidia and cleistothecia of several powdery mildew fungi. Mycoscience 37: 283–288. [Google Scholar]
- Hitchcock AS, Green ML. (1929). Standard species of Linnaean genera of Phanerogamae (1753–1754). In: International Botanical Congress. Cambridge (England), 1930. Nomenclature. Proposals by British Botanists. His Majesty’s Stationery Office, London: 111–195. [Google Scholar]
- Jaczewski AA. (1927). Karmanny opredelitel’ gribov. Vyp. 2. Muchnisto-rosyanye griby. Mikologicheskaya Laboratoriya Imeni Professora A.A. Jaczewskogo, Gosudarstvennogo Instituta Opytnoy Agronomii, Leningrad. [Google Scholar]
- Junell L. (1967). Erysiphaceae of Sweden. Symbolae Botanicae Upsaliensis 14: 1–117. [Google Scholar]
- Karis H. (1987). Eesti Jahukastelised (Erysiphaceae). The Powdery Mildews (Erysiphaceae) of Estonia. Valgus, Tallinn. [Google Scholar]
- Khodaparast SA, Hedjaroude GA, Ershad D, et al. (2000). A study on the identification of Erysiphaceae in Gilan Province, Iran (I). Rostaniha 1: 53–63. [Google Scholar]
- Kim KH, Lee SK, Shin HD. (2007). First report of powdery mildew caused by Erysiphe lonicerae var. lonicerae on Lonicera sempervirens in Korea. New Disease Reports 15: 6. [Google Scholar]
- Kiss L, Bolay A, Takamatsu S, et al. (2002). Spread of the North American snowberry powdery mildew fungus, Erysiphe symphoricarpi (syn. Microsphaera symphoricarpi), to Europe. Mycological Research 106: 1086–1092. [Google Scholar]
- Kiss L, Takamatsu S, Cunnington JH. (2005). Molecular identification of Oidium neolycopersici as the causal agent of the recent tomato powdery mildew epidemics in North America. Plant Disease 89: 491–496. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. (2007). Index of fungi inhabiting woody plants in Japan. Host, Distribution and Literature. Zenkoku-Noson-Kyoiku Kyokai Publishing Co., Ltd. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee CK, Cho SE, et al. (2016). First report of powdery mildew caused by Erysiphe lonicerae var. lonicerae on Lonicera japonica in Korea. Plant Disease 100: 856. [DOI] [PubMed] [Google Scholar]
- Léveillé JH. (1851). Organisation et disposition méthodique des espèces qui composent le genre Erysiphé. Annales des Sciences Naturales, Botanique, Ser. 3, 15: 109–179. [Google Scholar]
- Liu TZ. (2010). The Erysiphaceae of Inner Mongolia. Inner Mongolia Science and Technology Press, Chifeng. [Google Scholar]
- Liu Y, Liu CJ, Liu SY, et al. (2013). A preliminary study on powdery mildew of Corylus heterophylla in Tieling, Liaoning province. Journal of Fungal Research 11: 24–26. [Google Scholar]
- Mabberley DJ. (2008). Mabberley’s Plant-book: A portable dictionary of plants. 3rd ed. Cambridge University Press, Avon. [Google Scholar]
- Magnus P. (1898). Der Mehltau auf Syringa vulgaris in Nordamerika. Berichte der Deutschen Botanischen Gesellschaft 16: 63–70. [Google Scholar]
- Meeboon J, Takamatsu S. (2015). Erysiphe viburni-plicati and Podosphaera photiniae, two new species of Erysiphales (Ascomycota) from Japan. Mycoscience 56: 14–23. [Google Scholar]
- Meeboon J, Takamatsu S, Braun U. (2020). Morphophylogenetic analyses revealed that Podosphaera tridactyla constitutes a species complex. Mycologia 112: 244–266. [DOI] [PubMed] [Google Scholar]
- Mori Y, Sato Y, Takamatsu S. (2000). Evolutionary analysis of the powdery mildew fungi nucleotide sequences of the nuclear ribosomal DNA. Mycologia 92: 74–93. [Google Scholar]
- Nakaji M, Tanaka N, Sugawara T. (2015). A molecular phylogenetic study of Lonicera L. (Caprifoliaceae) in Japan based on chloroplast DNA sequences. Acta Phytotaxonomica Geobotanica 6: 137–151. [Google Scholar]
- Nomura Y. (1992). Erysiphaceae of Japan. Yotsukaido C., published by the author. [Google Scholar]
- Nomura Y. (1997). Taxonomical Study of Erysiphaceae of Japan. Yokendo, Tokyo. [Google Scholar]
- Otani Y. (1988). Seiya Ito’s Mycological Flora of Japan. Vol. 3, Ascomycotina, No. 2, Onygenales, Eurotiales, Ascosphaeriales, Microascales, Ophiostomales, Elaphomycetales, Erysiphales. Yokendo, Tokyo. [Google Scholar]
- Park MJ, Choi YJ, Hong SB, et al. (2010). Powdery mildew of crenate deutzia caused by Erysiphe deutziae in Korea. The Plant Pathology Journal 26: 294. [Google Scholar]
- Park MJ, Cho SE, Piatek M, et al. (2012). First report of powdery mildew caused by Erysiphe macleayae on Macleaya microcarpa in Poland. Plant Disease 96: 1376. [DOI] [PubMed] [Google Scholar]
- Paulech C. (1995). Flóra Slovenska, X/1, Mycota (Huby), Ascomycetes (Vreckaté), Erysiphales (Múčnatkovaré). VEDA, Bratislava. [Google Scholar]
- Rehder A. (1903). Synopsis of the genus Lonicera. Annual Report of the Missouri Botanical Garden 14: 27–232. [Google Scholar]
- Roloff RA, Bärtels A. (2008). Flora der Gehölze. Bestimmung, Eigenschaften und Verwendung. Mit einem Winterschlüssel von Bernd Schulz. 3., korrigierte Auflage. Eugen Ulmer, Stuttgart (Hohenheim). [Google Scholar]
- Rusanov VA, Bulgakov TS. (2008). Powdery mildew fungi of Rostov region. Mikologiya I Fitopatologiya 42: 314–322. [Google Scholar]
- Sałata S. (1985). Flora Polska, Grzyby (Mycota), Tom XV, Workowce (Ascomycetes), Mącznikowe (Erysiphales). Państwowe Wydawnictwo Naukowe, Warszawa-Kraków. [Google Scholar]
- Salmon E. (1900). A monograph of the Erysiphaceae. Memoirs of the Torrey Botanical Club 9: 1–292. [Google Scholar]
- Seko Y, Heluta V, Grigaliunaite B, et al. (2011). Morphological and molecular characterization of two ITS groups of Erysiphe (Erysiphales) occurring on Syringa and Ligustrum (Oleaceae). Mycoscience 52: 174–182. [Google Scholar]
- Shi A, Kantartzi SK, Mmbaga MT, et al. (2009). Differentiation of two pathogens of powdery mildew disease in flowering dogwood (Cornus florida) by PCR-mediated method based on ITS Sequences. Journal of Phytopathology 157: 274–279. [Google Scholar]
- Shin HD. (1988). Erysiphaceae of Korea. Thesis, Department of Agricultural Biology, Graduate School of Seoul National University. [Google Scholar]
- Shin HD. (2000). Erysiphaceae of Korea. Plant Pathogens of Korea 2: 1–320. [Google Scholar]
- Shiroya Y, Takamatsu S. (2009). Erysiphe corylopsidis sp. nov., a new powdery mildew fungus found on Corylopsis spicata and C. pauciflora. Mycoscience 50: 409–414. [Google Scholar]
- Silvestro D, Michalak I. (2012). raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. [Google Scholar]
- Simonyan SA. (1994). Mikoflora Armenii. VII. Muchnistorosyanye griby Armenii (por. Erysiphales). Izdatel’stvo AN Armenii, Yerevan. [Google Scholar]
- Swofford DL. (2003). PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.0b10. Sunderland, Massachusetts: Sinauer Associates. [Google Scholar]
- Takamatsu S, Hirata T, Sato Y. (1998). Phylogenetic analysis and predicted secondary structures of the rDNA internal transcribed spacers of the powdery mildew fungi (Erysiphaceae). Mycoscience 39: 441–453. [Google Scholar]
- Takamatsu S, Bolay A, Limkaisang S, et al. (2006). Identity of a powdery mildew fungus occurring on Paeonia and its relationship with Erysiphe hypophylla on oak. Mycoscience 47: 367–373. [Google Scholar]
- Takamatsu S, Cho SE, Meeboon J, et al. (2013). Revisiting Erysiphe magnoliae with morphological and molecular data. Sydowia 65: 13–20. [Google Scholar]
- Takamatsu S, Ito H, Shiroya Y, et al. (2015). First comprehensive phylogenetic analysis of the genus Erysiphe (Erysiphales, Erysiphaceae) I. The Microsphaera lineage. Mycologia 107: 475–489. [DOI] [PubMed] [Google Scholar]
- Tanda S. (2000). Two new species of Erysiphaceae from Japan. Mycoscience 41: 155–160. [Google Scholar]
- Tanda S, Nomura A, Matsunami Y. (1977). Powdery mildews of the new hosts in Japan (III). Journal of Agriculture Science [Tokyo Nogyo Daigaku] 22: 15–30. [Google Scholar]
- Theis N, Donoghue JM, Li J. (2008). Phylogenetics of the Caprifolieae and Lonicera (Dipsacales) based on nuclear and chloroplast DNA sequences. Systematic Botany 33: 776–783. [Google Scholar]
- Vasyagina MP, Kuznetsova MN, Pisareva NF, et al. (1961). Muchnisto-rosyanye griby. Flora Sporovykh Rastenii Kazakhstana, Tom III. Alma-Ata. [Google Scholar]
- Walsh P., Metzger D., Higuchi R. (1991). Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10: 506–513. [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al., eds. PCR protocols: a guide to methods and applications. San Diego, California: Academic Press: 315–332. [Google Scholar]
- Wu Z-Y, Raven PH, Hong D, Eds. (2011). Flora of China. Volume 19: Cucurbitaceae through Valerianaceae, with Annonaceae and Berberidaceae. Science Press/Missouri Botanical Garden Press, Beijing/St. Louis. [Google Scholar]
- Yu YN, Lai YQ. (1983). Taxonomic studies on the genus Microsphaera of China. V. New and known species of Microsphaera on family Caprifoliaceae. Acta Mycologica Sinica 2: 89–95. [Google Scholar]