Abstract
In recent times, the Coronavirus disease (caused by COVID-19) is evidently observed to be the extremely contagious one with high fatality rate worldwide. In March 2020, the disease was declared a “global pandemic” by the World Health Organization (WHO). So far, there is no known/effective vaccine or medicine. In this paper, we propose and analyze an SEIR compartment model. We also compare and analyze the case study of India and Brazil. The model system is discussed by using MATLAB (2018a) software and the numerical results are verified graphically.
Keywords: COVID-19, Epidemic, SEIR model, Stability analysis, Case study: India and Brazil
1. Introduction
Coronavirus disease 2019 (COVID-19), that has generated a pandemic, is primarily a respiratory illness, caused by a novel coronavirus that spreads from person to person, poses a serious public health risk with its high contagion rate. In most cases, the infected people perceive mild respiratory symptoms that usually disappear on their own, but some people develop severe illness, like pneumonia [1]. The virus is transmitted through contact with an infected person or via respiratory droplets when an infected person coughs or sneezes.
Since December 2019, an outbreak of a new coronavirus named SARS-COV-2 has been reported in many countries and has infected thousands of people all over the world with a high mortality rate. The World Health Organization has declared COVID-19 to be a pandemic. First it was identified in Wuhan city, Hubei Province of China on December 31, 2019 [2]. The symptoms of COVID-19 appearing within 2–14 days after infection include fever, cough, a running nose and difficulty in breathing. There is a higher risk of infection if one has been in a contaminated area or if one has been in close contact with a person infected with the new coronavirus. There is also a higher risk if one suffers from co-morbidities. In recent times, most of the countries are in the clutches of COVID-19 and a large number of people are infected. COVID-19, has seriously affected the lives of people in multiple ways and incurred huge losses to the economy.
Coronavirus is common among animals. However, animal to human transmission is reportedly rare. It causes damage to the respiratory tract ranging from the common cold to severe conditions like SARS [3]. The outbreak of Coronavirus (officially known as Covid-19), which started in China, has so far killed 567,657 people and infected 12,844,410 people across the globe (as on July 12, 2020) [4]. In India, 849,553 confirmed cases and 22,674 deaths have been reported so far according to official figures released by the Union Ministry of Health and Family Welfare (MoHFW) [5].
Mathematical models can be viable tools in analyzing the spread and control of infectious disease; for instance, the epidemic model developed by Kermack and Mckendrick in 1927 [6]. In epidemiology there are different models to predict and explain the dynamics of an epidemic. The data of the Covid-19 outbreak can also be studied through various mathematical models such as SIR, SEIR (Susceptible, Exposed, Infected and Recovery), SIQR (Susceptible, Infectious, Quarantined and Recovered) and so on [[7], [8], [9], [10], [11], [12]]. Tang, Wang, Li and Bragazzi [13] presented a compartmental deterministic model that would integrate the clinical development of the disease, the epidemiological status of the patient and the measures for intervention. In this situation, several studies have been conducted using real time data of the affected countries and distinct characteristics of the outbreak [14,15] have been examined. Kamrujjaman, Ghosh and Islam [16] developed the Susceptible Exposed Infectious Recovered model (SEIR) to clarify the dynamics of COVID-19 case. The SEIR model divides the population into four parts, namely the susceptible S(t), the exposed E(t), the infected I(t) and the recovered R(t) at time t. Several researchers have worked on mathematical modeling of the novel coronavirus. It exposes that those SIS, SIR and SEIR models can reflect the dynamics of various epidemics well. Concurrently, different models have been used to model the COVID-19 [[17], [18], [19], [20], [21], [22], [23], [24]]. Its one variant, SEIR [25] is considered to be the most appropriate modeling technique for COVID-19. It may be observed that the specifications of this virus require more complex models in the days to come.
Some of the frequently asked questions that need to be answered are as follows. How many people exactly recover from COVID-19 over a certain period of time? How many people are infected from the disease? How many people died from it? Recently, several mathematical models suitable for the study of the dynamics and evolution of this pandemic have been published as an attempt to answer the above questions.
In the present work we introduce and analyze the most basic transmission model for a directly transmitted infectious disease caused by bacteria, viruses or fungi. Direct transmission occurs through individual-to-individual contact, through a sneeze or cough or through skin-skin contact.
The purpose of the present work is:
-
1.
Study of the dynamical behavior of the model and its stability.
-
2.
Confirmation of the results by numerical simulation to control the spread of COVID-19.
-
3.
Comparison and analysis of the scenarios of two countries, namely India and Brazil.
-
4.
Validation and discussion of the model in COVID-19 cases of a state named West Bengal in India.
Brazil is the fifth largest country in the world with India at the seventh position. It has a much lower population density than India. However, Brazil shows higher number of confirmed cases and a higher mortality rate than India. The above observations motivate us to conduct a comparative study of COVID -19 cases between India and Brazil.
The article is organized as follows:
In Section 2 we describe the SEIR Model and study the equilibrium points. Section 3 is devoted to the discussion of stability analysis and stability criterion of the model. The case studies of India and Brazil are presented in Section 4. In Section 5 we perform numerical simulation using MATLAB. We discuss the comparison between India and Brazil in Section 6. In Section 7, we exhibit the validation of the model. Finally, Section 8 includes conclusion of the paper.
2. Model formulation
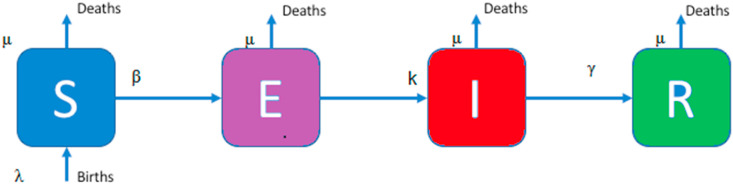
Consider a Susceptible –Exposed –Infected – Recovered (SEIR) model for the present epidemic. The SEIR disease transmission model is based on several strong assumptions [26]. The population (N) is divided into four classes: the susceptible individuals (S), the exposed individuals (E), the infected individuals (I) and the recovered individuals (R) at any time t 0 (see Fig. 1 ). Therefore, we have
| (i) |
with initial condition S(0) = S0 > 0, E(0) = E0 ,I(0) = I0 > 0 and R(0) = R0 .
Fig. 1.
The Diagram of the SEIR model.
2.1. Positivity and boundedness of solutions
Theorem
All the variables are non-negative for all t0.
The closed regionis positive invariant for the system (i).
Proof. From the equation (i), we get
We have,
Now
We have,
Also.
We have,
Now
We have, .
Again .
Therefore,
(ii) If< 0 then .
Therefore, the equation (ii) is bounded by .
Then we get S, E ,I and as positive function.
2.2. Basic reproduction number, disease-free equilibrium state and epidemic equilibrium state
The basic reproduction number , is the number ofsecondary infections that one infected person would produce in a fully susceptible population through the entire duration of the infectious period. provides a threshold condition for the stability of the disease-free equilibrium point (for most models).
The disease-free equilibrium point is locally asymptotically stable when < 1 i.e., the disease dies out. The disease-free equilibrium point is unstable when > 1 i.e., the disease establishes itself in the population or an epidemic occurs. Since, the considered model has disease-free equilibrium at (), the basic reproduction number can be found analytically.
The basic reproduction number () for the COVID-19 model can be obtained from the leading eigen value of the matrix FV−1 [27] where,
F = and V = .
Therefore, the reproduction number
| (iii) |
3. Stability analysis
The equilibrium points are obtained by equating the right hand side of the equations in system (i) to zero.
0.
The two equilibrium points are given by = ( ) which is the disease-free equilibrium point and which is the unique epidemic point of the system (i), where.
= ,
= ,
= ,
= .
With given by equation (iii). In case of an epidemic, will exist only when >1.
3.1. Theorem
The disease-free equilibrium of the system is locally stable if < 1 and unstable if > 1.
Proof: From equation (i) we consider
The Jacobian matrix is,
At the equilibrium point = ( ), the Jacobian matrix becomes,
Therefore, its characteristic equation is
The characteristic roots are , , and ( k) ().
The first three roots are negative and the last one is will be negative if <1 and positive if >1.
Hence the equilibrium point is locally asymptotically stable if <1 and unstable if >1.
3.2. Theorem
If >1, the epidemic equilibrium is locally asymptotically stable.
Proof: We consider the equations
The Jacobian matrix.
J = .
At the equilibrium point, the Jacobian matrix becomes,
J() = .
Therefore, its characteristic equation is = 0.
Or, () ( = 0, where
By Routh-Hurwitz Criterion, the system (i) is locally asymptotically stable if.
Thus, is a locally asymptotically stable equilibrium point.
3.3. Theorem
The disease-free equilibrium of the system (i) is globally asymptotically stable if < 1.
Proof: Considering the following linear Lyapunov function
With Lyapunov derivative (where a dot represents differentiation with respect to time)
Substituting the expression for from (i), we have
| (iv) |
Little perturbation from equation (iv) with the reproduction number (iii) gives:
| (v) |
Substituting the expression of obtained from equation (v) we have:
Since,, it follows that
Hence if , then . Hence, by LaSalle''s extension to Lyapunov's principle [28,29], the disease free equilibrium points is globally asymptotically stable.
3.4. Theorem
If >1, the epidemic equilibrium is globally asymptotically stable.
Proof: Consider the model (i) and >1, so that the epidemic equilibrium of model exists.
We consider the following non-linear Lyapunov function of Goh-Volterra type:
With Lyapunov derivative (where a dot represents differentiation with respect to time)
| (vi) |
Substituting the value from (i) into (vi), we have
| (vii) |
At steady state from equation (i) we have:
| (viii) |
Substituting equation (viii) into (vii) gives:
| (ix) |
Further simplification gives:
| (x) |
Collecting all infected class without single star (*) from (x) and equating to zero:
| (xi) |
A little perturbation of steady state from (i) and (xi) resulted into:
| (xii) |
Substituting the expression from (xii) into (x) gives:
| (xiii) |
Finally, since the arithmetic mean exceeds the geometric mean, we have
Thus, for >1.
Hence, is a Lyapunov function, by LaSalle's Invariance Principle [29], the epidemic equilibrium is globally asymptotically stable.
4. Case study
In this section we observe the scenario of COVID-19 pandemic in India and Brazil.
4.1. India
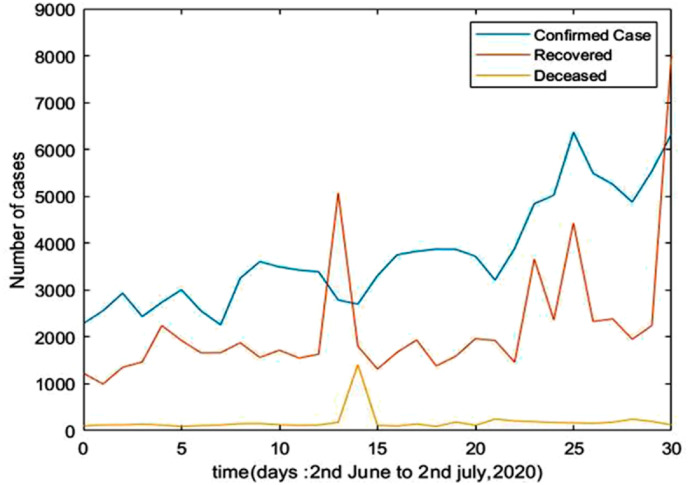
We have considered the number of confirmed cases, death cases and recovered cases of India as given in Ref. [4]. The first case of COVID-19 was reported on January 30, 2020. During the month of February, the number of cases reported was only 3 and remained the same during the whole month. In March it was noted that the number of cases started increasing. However, an explosion in the number happened ultimately in May 2020. To describe the spread of COVID-19 using SEIR model, few considerations and assumptions were made due to limited availability of data [4,13,30,31]. We have considered the number of infected, recovered and deceased cases from 2nd June to July 2, 2020 [32] which is depicted in Fig. 2 . The estimated values of the parameters given in Table 1 are as follows: effective contact rate 0.476, birth rate of susceptible 0.0182 and mortality rate 0.0073. We obtain the recovery rate 0.286.
Fig. 2.
Confirmed cases, Recovered cases and Deceased cases with respect to time (Days).
Table 1.
Model parameters and their descriptions.
| Notation | Interpretations |
|---|---|
| Effective contact rate | |
| Birth rate of susceptible | |
| Mortality rate | |
| Progression rate exposed to infected | |
| Recovery rate |
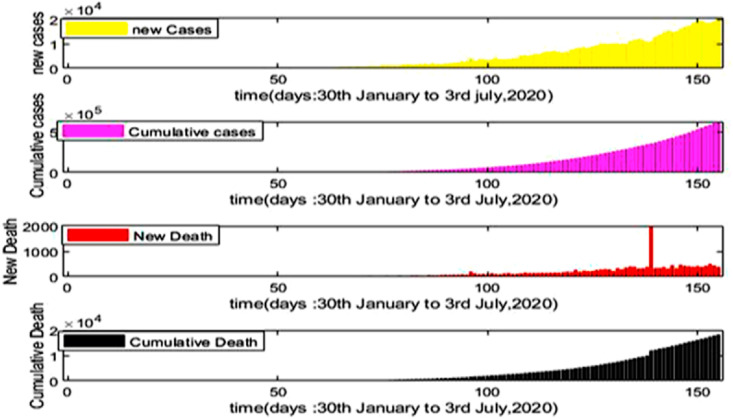
In Fig. 3 , the bar diagram shows the number of new cases as well as cumulative new cases and new death along with cumulative death from 30th January to 3rd July 2020 in India due to COVID-19 [32]. Therefore, we obtain an estimation of the value of from the equation (iii) as 3.67.
Fig. 3.
New cases, Cumulative cases and New death, Cumulative death with respect to time (Days).
In this study, we have only predicted the number of confirmed cases. We have used only time series data for confirmed cases and death cases. An increase in active cases is an alarming situation because as discussed earlier, India is one of the largest populations in the whole world.
4.2. Brazil
The first case in Brazil was a 61-year-old man who had returned from Lombardy (Italy) and tested positive for the virus. The study of evolution of COVID 19 and its prediction in Brazil is based on the data provided by Ref. [5] during the period of 26th February to July 3, 2020. The estimated values of the parameters given in Table 1 are as follows: effective contact rate 0.4417, birth rate of susceptible 0.01867 and mortality rate 0.00626. We obtain the recovery rate 0.07143. Therefore, we obtain an estimation of the value of from the equation (iii) as 2.8421.
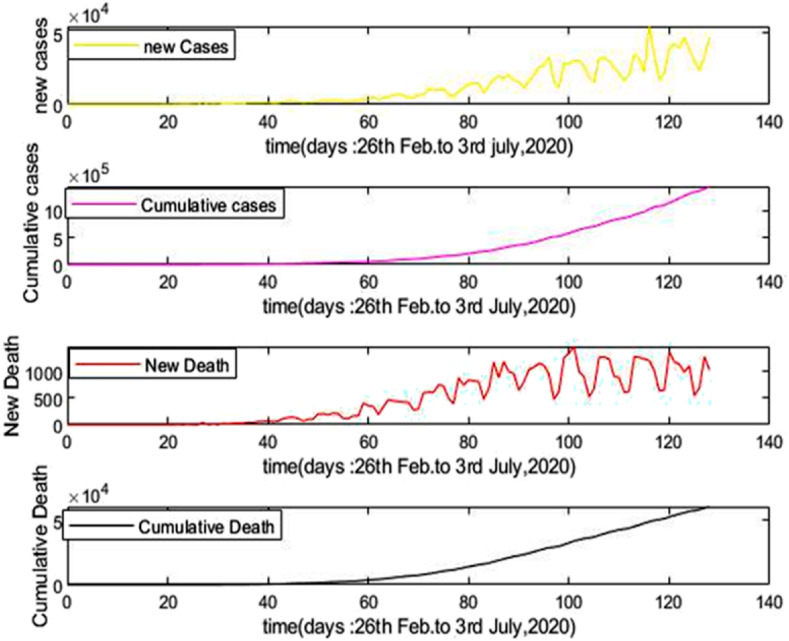
Fig. 4 shows the number of new cases as well as cumulative cases and new death as well as cumulative death from 26th February to July 3, 2020 of Brazil due to COVID-19.
Fig. 4.
New cases, Cumulative cases and New death, Cumulative death with respect to time (Days).
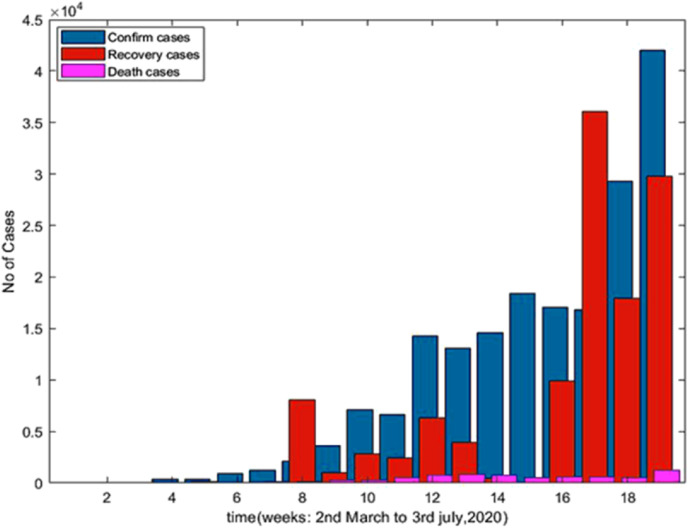
Fig. 5 is a diagrammatic representation of confirmed cases, death cases and recovered cases during the period of 2nd March to July 3, 2020 in Brazil. It is observed that the confirmed cases are gradually increasing with respect to death cases from 2nd March to July 3, 2020.
Fig. 5.
Confirmed cases, Recovered cases and Death cases with respect to time (Days).
5. Numerical simulation
In this section, we implement meticulous numerical validation of the results obtained analytically. We have used mathematical software MATLAB (2018a) to numerically approximate the solution of our model system (i) [14,16].
The estimated values of the parameters in the case of COVID-19 in India and Brazil are as follows:
6. Comparison between India and Brazil
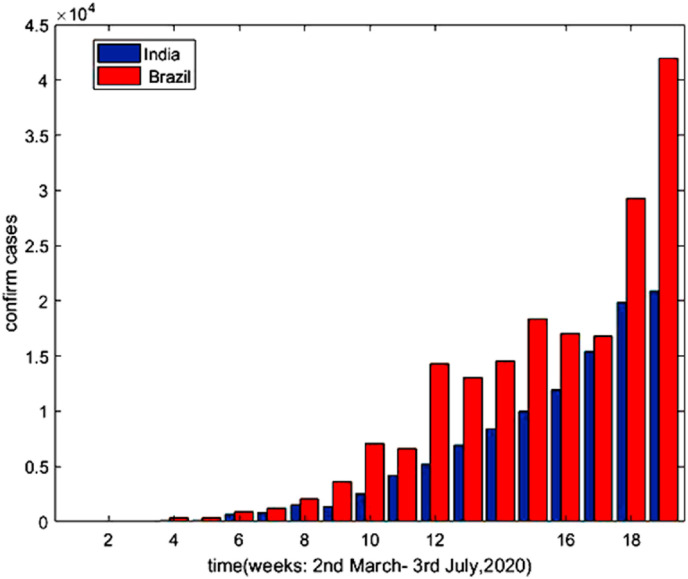
Fig. 9 is the graphical representation of comparison of confirmed cases during 2nd march to 3rd July 2020 between India and Brazil, according to the data provided in Ref. [5] and Ref. [32]. Although the number of infected cases gradually increases during this period, Brazil shows a much higher rate of increase than India.
Fig. 9.
Confirm Cases with respect to time (Days).
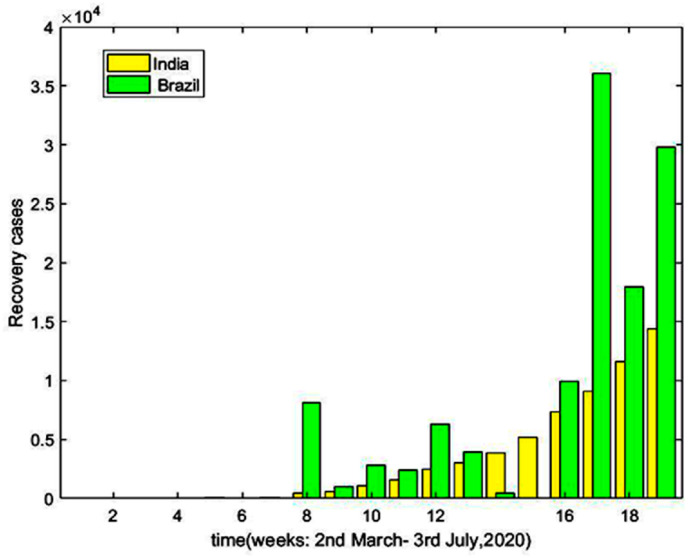
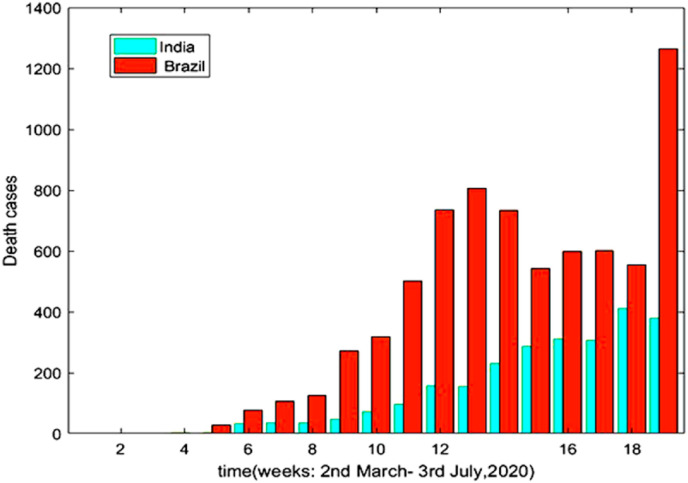
Fig. 10 and Fig. 11 are the graphical representation of comparison of recovered cases and death cases respectively during 2nd march to 3rd July 2020 between India and Brazil, according to the data provided in Ref. [5] and Ref. [32]. There is a gradual increase in the recovery rate as well as death rate for both the countries during this period. The representation also indicates a greater recovery rate and death rate for Brazil.
Fig. 10.
Recovery Cases with respect to time (Days).
Fig. 11.
Death Cases with respect to time (Days).
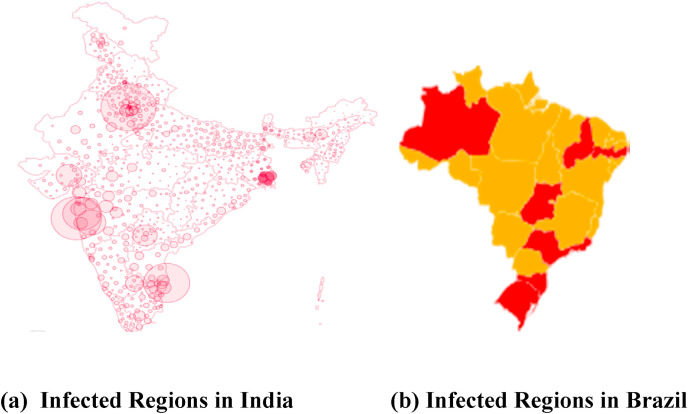
Fig. 12 shows that the geographical distribution of infected cases on 3rd July 2020 of India and Brazil. It should be noted that the average testing rate (per million) in both the countries are way behind the testing rate in some of the developed countries of the world. It is expected that with an increase in the testing rate there will be an increase in the infected regions in both the countries.
Fig. 12.
Infected region.
7. Model validation and discussion
In this section, the infection rate is taken into consideration for validation of the solution obtained from Equation (i) with real time data. For as given in Table 2 and if the population does not follow lockdown (i.e., E I), we have from the equation (i),
Table 2.
Parameter values of proposed model of system (i).
Then, .
Therefore,
| (xiv) |
being the initial number of infected cases.
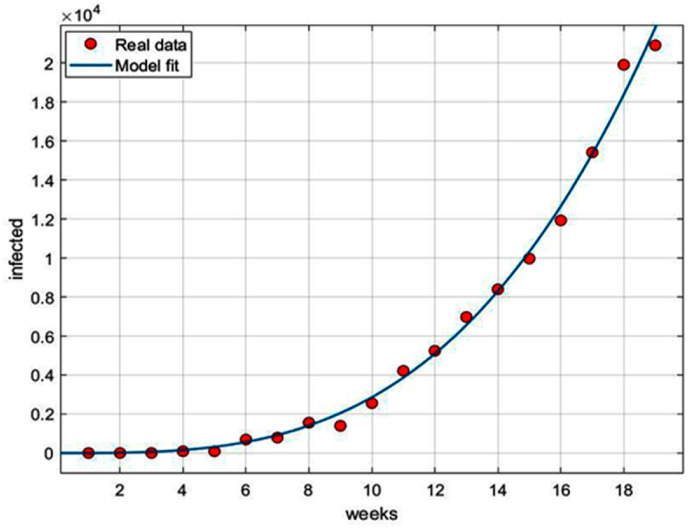
Fig. 13 shows the goodness of fit of Eq. (xiv) with the real time data of total infected cases in India as reported from 2nd March to July 3, 2020. Fitted parameters are with 95% confidence interval as (0.1952, 0.239) and with 95% confidence interval as (228.8, 508.2).
Fig. 13.
Infected cases.
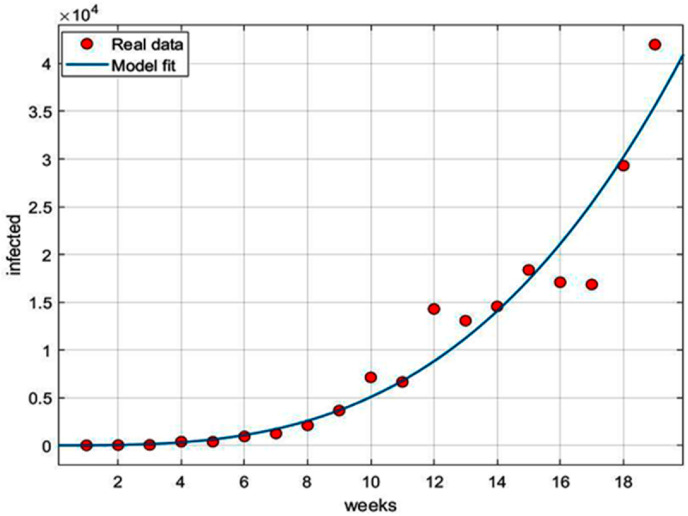
Fig. 14 shows the goodness of fit of Eq. (xiv) with the real time data of total infected cases in Brazil as reported from 2nd March to July 3, 2020. Fitted parameters are, with 95% confidence interval as (0.173, 0.2603) and with 95% confidence interval as (151.3, 1085).
Fig. 14.
Infected cases.
8. Conclusion
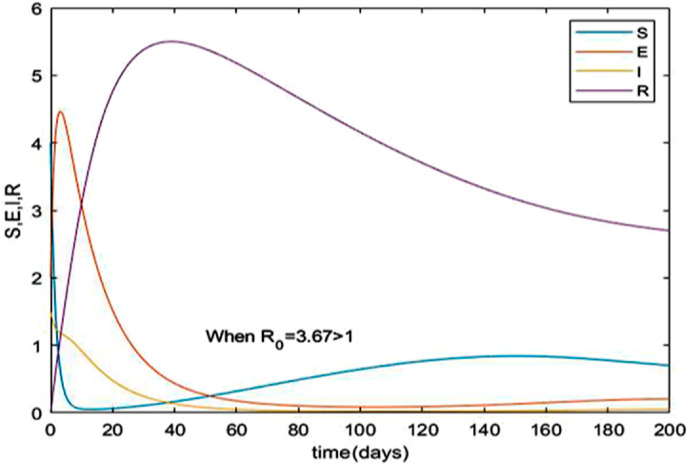
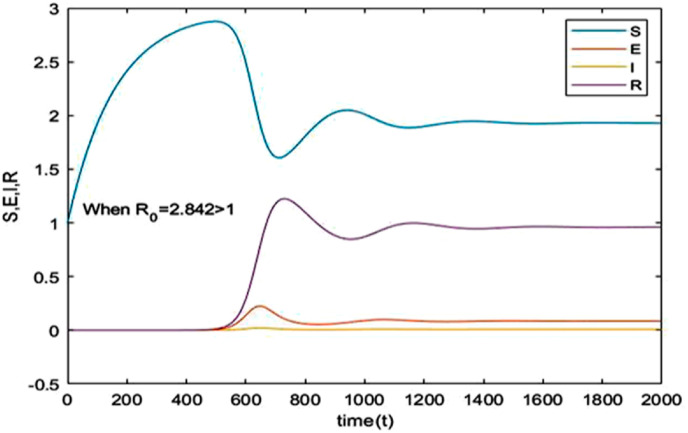
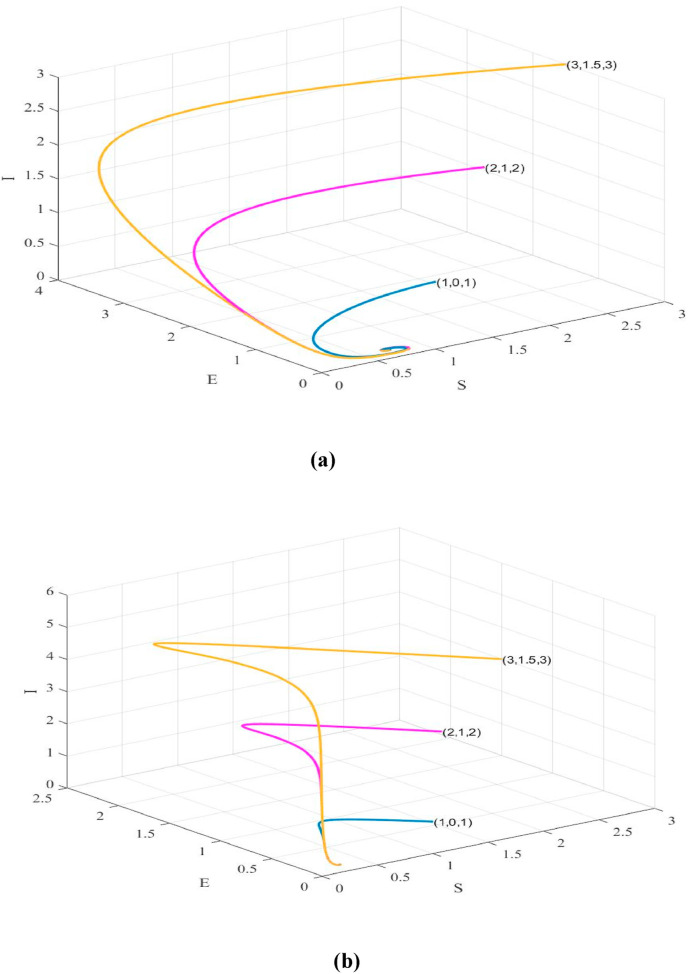
The Susceptible-Exposed-Infection-Recovered (SEIR) model is used in this paper to study the dynamical behavior and stability of the model characterizing the spread of COVID-19. Graphical representations of comparison of the spread of the disease and its mortality rates are extensively discussed for India and Brazil. The essential parameters namely effective contact rate , birth rate of susceptible mortality rate and recovery rate are estimated for both countries using the current data [5,32] and are shown in Table 2. The reproduction number () given by equation (iii) is 3.67 for India and 2.8421 for Brazil which gives a clear indication that India will be encountering an increased number of infected cases in near future. Fig. 6 and Fig. 7 indicates a time series plot of the SIER model for given in case of India and Brazil respectively. It may be observed that the COVID-19 scenario will prevail for a much longer period of time than expected. Fig. 8 shows that the infected cases exhibit a rapid ascent and reaches its peak for different increasing values of contact rate .
Fig. 6.
Time series plot of equation (i) with parameter values for India given in Table 2.
Fig. 7.
Time series plot of equation (i) with parameter values for Brazil given in Table 2.
Fig. 8.
Shows that global stability of in S-E-I plane using different initial values ((1,0,1),(2,1,2) and (3,1.5,3)) and the values of parameters for (a) India (b) Brazil.
We have compared the analytical results of infected cases obtained in equation (iv) with the numerical data in Figs. 13 and 14. The graphs are in good agreement with the numerical simulations performed with the help of MATLAB.
From our study of the present situation it is recommended that the rate of disease transmission needs to be controlled, otherwise a huge proportion of population will be affected within an extremely short period of time. Among the popular preventive measures are enforcement of lockdown, curfews and specification of containment zones. Mainly, the government's strategy must be to restrict the contamination by reducing unnecessary mobilization and restricting unwanted social interactions.
We wish to extend our study towards a modified SEIR compartmental model accounting for infection from undiagnosed individuals and for different levels of population isolation in order to evaluate effects of contact reduction in the epidemic temporal dynamics.
CRediT authorship contribution statement
Subrata Paul: Conceptualization, Methodology. Animesh Mahata: Data curation, Software, Investigation. Uttam Ghosh: Software, Validation. Banamali Roy: Supervision, Writing – review & editing.
Acknowledgement
The authors are grateful to the reviewers for their valuable comments and suggestions.
References
- 1.Richman D.D., Whitley R.J., Hayden F.G. John Wiley & Sons; 2016. Clinical Virology. [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for disease control and prevention Novel coronavirus. 2019. https://www.cdc.gov/coronavirus/2019-ncov 2020.
- 4.World. Health Organization Coronavirus world health organization. 2020. https://www.who.int/health-topics/coronavirus Available: 19.
- 5.https://www.worldometers.info/coronavirus/.
- 6.Kermack N.O., Mackendrick A.G. Contribution to mathematical theory of epidemics. P. Roy. Soc. Lond. A.Mat. US. 1927:700–721. [Google Scholar]
- 7.Ji C., Jiang D., Shi N. Multigroup SIR epidemic model with stochastic perturbation. Phys. Stat. Mech. Appl. 2011;390(10):1747–1762. [Google Scholar]
- 8.Liu M., Bai C., Wang K. Asymptotic stability of a two group stochastic SEIR model with infinite delays. Commun. Nonlinear Sci. Numer. Simulat. 2014;19(10):3444–3453. [Google Scholar]
- 9.Sun R., Shi J. Global stability of multi-group epidemic model with group mixing and nonlinear incidence rates. Appl. Math. Comput. 2011;218(2):280–286. [Google Scholar]
- 10.Yang Q., Mao X. Extinction and recurrence of multi-group SEIR epidemic models with stochastic perturbations. Nonlinear Anal. R. World Appl. 2013;14(3):1434–1456. [Google Scholar]
- 11.Yu J., Jiang D., Shi N. Global stability of two-group SIR model with random perturbation. J. Math. Anal. Appl. 2009;360(1):235–244. [Google Scholar]
- 12.Yuan C., Jiang D., O'Regan D., Agarwal R.P. Stochastically asymptotically stability of the multi-group SEIR and SIR models with random perturbation. Commun. Nonlinear Sci. Numer. Simulat. 2012;17(6):2501–2516. [Google Scholar]
- 13.Tang B., Wang X., Li Q., Bragazzi N.L., Tang S., Xiao Y., Wu J. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J. Clin. Med. 2020;9(2):462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari A. 2020. Modeling and Analysis of COVID-19 Epidemic in India. medRxiv preprint. [Google Scholar]
- 15.Fredj H.B., Cherif F. ELSEVIER; 2020. Novel Corona Virus Disease Infection in Tunisia: Mathematical Model and the Impact of the Quarantine Strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamrujjaman Md, Ghosh U., Islam Md. S. Pandemic and the dynamics of SEIR model. Case COVID- 2020;19 [Google Scholar]
- 17.Nadim Sk. S., Ghosh I., Chattopadhyay J. 2020. Short-term Predictions and Prevention Strategies for Covid-2019: A Model Based Study. arXiv preprint arXiv:2003.08150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucharski A., Russell T., Diamond C., Liu Y., Edmunds J., Funk S., Eggo R. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473–3099(20)30144–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quilty, Billy J., Clifford S., et al. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-ncov) Euro Surveill. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Wang Z., Dong Y., Chang R., et al. Phase-adjusted estimationof the number of coronavirus disease 2019 cases in wuhan, China. Cell. Discov. 2020;6(10) doi: 10.1038/s41421–020–0148–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferretti L., Wymant C., Kendall M., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S., Musa S., Lin Q., Ran J., et al. Estimating the unreported number of novel coronavirus (2019-nCoV) cases in China in the first half of january 2020: a data-driven modelling analysis of the early outbreak. J. Clin. Med. 2020;9:388. doi: 10.3390/jcm9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S., Lin Q., Ran J., et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Joseph T., Leung Kathy, Leung Gabriel M. Now casting and forecasting the potential domestic and international spread of the 2019-ncov outbreak originating in wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson R.M., May R.M. Oxford University Press; Oxford: 1991. Infectious Diseases of Humans. [Google Scholar]
- 26.Murray J. third ed. Springer-Verlag; Heidelberg: 2002. Mathematical Biology I. [Google Scholar]
- 27.Diekmann O., Heesterbeek J.A.P., Roberts M.G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface. 2009;7(47):873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perko L. Springer; 2000. Differential Equations and Dynamical Systems. [Google Scholar]
- 29.Li M.Y., Smith H.L., Wang L. Global dynamics of an SEIR epidemic model with vertical transmission. SIAM J. Appl. Math. 2001;62:58. [Google Scholar]
- 30.Victor A.O. 2020. Mathematical Predictions for COVID-19 as a Global Pandemic. medRxiv preprint. [Google Scholar]
- 31.Martcheva M. Springer; New York: 2015. An Introduction to Mathematical Epidemiology. [Google Scholar]
- 32.India COVID-19 Tracker 2020. https://www.covid19india.org/2020 Retrieved. 07-13.