To the editor:
As coronavirus disease 2019 (COVID-19) vaccinations are administered globally on a massive scale, rare adverse events are being reported. We report a case of anti–neutrophil cytoplasmic antibody (ANCA) glomerulonephritis 2 weeks after receiving the COVID-19 (Moderna) vaccine.
A 52-year-old white man presented with headache and weakness 2 weeks after receiving his second dose of the Moderna (mRNA-1273) vaccine on April 15, 2021. Headache started the day after his second vaccination and was associated with weakness. Vitals were stable, and physical examination was unremarkable. His medical history included hypertension, and he was treated with amlodipine. He had no allergies and denied illicit drug use.
The initial laboratory results showed a creatinine of 8.41 mg/dl (baseline 1.11 mg/dl, 8 months prior), blood urea nitrogen of 82 mg/dl, sodium of 129 mEq/l, potassium of 5.0 mEq/l, bicarbonate of 21 mEq/l, and hemoglobin of 14.6 g/dl. Toxicology screen was negative. Urinalysis had 1+ proteinuria and microscopic hematuria with dysmorphic red blood cells. Renal ultrasound revealed no hydronephrosis. I.v. hydration was initiated. Additional serologic workup showed positive cytoplasmic ANCA titers and antibodies to proteinase-3 (PR3). Myeloperoxidase-O antibody was negative. Anti–glomerular basement membrane antibody was negative, and C3/C4 levels were normal. Despite hydration, creatinine worsened to 10.42 mg/dl, and after the return of the positive cytoplasmic ANCA, pulse dose steroids were begun.
A kidney biopsy (Figure 1 ) revealed cellular crescents and fibrinoid necrosis in 38 of 46 glomeruli, with some tubular injury. Immunofluorescence showed segmental fibrin staining the glomerular capillary loops, confirming fibrinoid necrosis. No immune complex–mediated deposits were seen on electron microscopy. Interstitial fibrosis and tubular atrophy were mild.
Figure 1.
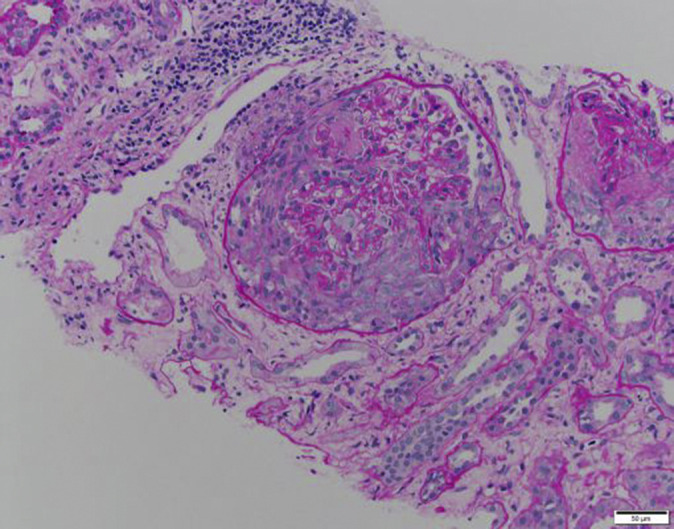
Periodic acid–Schiff stain showing a glomerulus with a cellular crescent arising in the Bowman’s space and destroying the mesangiocapillary architecture. Segmental capillary loop necrosis is also noted. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
On the basis of serologic and biopsy findings, a diagnosis of pauci-immune necrotizing and crescentic glomerulonephritis was made. Rituximab was initiated at 375 mg/m2, but the patient developed severe dyspnea and declined further doses. One dose of cyclophosphamide 7.5 mg/kg (per CYCLOPS trial dosing) was given; however, hemodialysis was initiated for hyperkalemia and worsening renal function. Currently, he continues to require dialysis, while on prednisone, and with a plan to repeat cyclophosphamide 2 weeks after the first dose.
With millions of doses of vaccines being administered worldwide for COVID-19, rare reports of adverse events are emerging, such as cases of minimal change disease.1 , 2 To our knowledge, this is the first reported case of ANCA glomerulonephritis after receiving the COVID-19 vaccine. ANCA vasculitis has previously been reported after influenza vaccination.3 In our case, the temporal association suggests a neutrophilic immune response to mRNA as a potential trigger. It is possible that the enhanced immune response after a second dose could be responsible for triggering PR3 antibodies. ANCA glomerulonephritis has been known to occur with certain medications such as hydralazine,4 infections, and with malignant tumors.5 With medication-induced ANCA vasculitis, usually myeloperoxidase-O titers are positive. However, in hematological malignancies, PR3 titers can be positive. PR3 is a bactericidal protein expressed by neutrophilic granules. Derangements in its expression and function have been linked to hematological malignancies and pauci-immune vasculitis. Greater analysis of the immune response induced by mRNA vaccines could provide better insight into the mechanism of various autoimmune reactions, including ANCA vasculitis.
References
- 1.D'Agati V.D., Kudose S., Bomback A.S. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100:461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toru W. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017;13:188–196. doi: 10.2174/1573397113666170517155443. [DOI] [PubMed] [Google Scholar]
- 4.Santoriello D., Bomback A.S., Kudose S. Anti-neutrophil cytoplasmic antibody associated glomerulonephritis complicating treatment with hydralazine. Kidney Int. 2021;100:440–446. doi: 10.1016/j.kint.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Folci M., Ramponi G., Shiffer D. ANCA-associated vasculitides and hematologic malignancies: lessons from the past and future perspectives. J Immunol Res. 2019;2019:1732175. doi: 10.1155/2019/1732175. [DOI] [PMC free article] [PubMed] [Google Scholar]


