Abstract
Background
SARS-CoV-2 is associated with a severe inflammatory response contributing to respiratory and systemic manifestations, morbidity, and mortality in patients with coronavirus disease 2019 (COVID-19).
Methods
Tocilizumab (TCZ) efficacy on mortality and length of hospital stay was retrospectively evaluated in patients who received TCZ and compared with that in controls with a similar severity of COVID-19. The primary endpoint was survival probability on day 28. The secondary endpoints included survival at day 14 and length of hospital stay.
Results
Of the 148 patients included in the study, 62 received TCZ and standard of care, whereas 86 served as a control group and received only standard of care. The two groups were similar, although TCZ-treated patients were more likely to exhibit hypertension (46.7% vs. 29.8%), chronic kidney disease (14.5% vs. 1.1%), and high Charlson score (1.18 vs. 1.00; p = 0.006) and less likely to receive corticosteroid treatment (48.5% vs. 93.0%). TCZ was associated with lower mortality on both day 28 (16.1% vs. 37.2%, p = 0.004) and day 14 (9.7% vs. 24.4%, p = 0.022). The hospital stay was longer in the TCZ-treated than in the control group (15.6 ± 7.59 vs.17.7 ± 7.8 days, p = 0.103). Ten patients (16.0%) in the TCZ-treated group developed infections.
Conclusion
TCZ was associated with a lower likelihood of death despite resulting in higher infection rates and a non-significant longer hospital stay.
Keywords: Tocilizumab, COVID-19, Hospital stay, Survival, SARS-CoV2, Interleukin-6
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), first reported in Wuhan, China, has been declared a pandemic, and as of 03 January 2021, more than 85 million cases and 1.8 million deaths have been reported [1,2]. Most patients with COVID-19 experience mild illness; however, the elderly and those with co-morbidities, such as diabetes and hypertension, are at an increased risk of contracting severe COVID-19 [3]. Critical illnesses associated with respiratory failure, shock, or multiple organ failure occur in only 5% of infected patients but contribute considerably to patient deaths. Mortality rates as high as 50% have been reported in critical patients, particularly in the elderly [4].
Following the entry of SARS-CoV-2 in pneumocytes and other cells with angiotensin-converting enzyme-II receptors, the virus activates both the innate and adaptive immune system and results in a robust pro-inflammatory reaction. As a result, cytokines and chemokines, including interleukin (IL)-1β, IL-6, granulocyte-macrophage colony-stimulating factor, and tumour necrosis factor-α, are released [5]. The release of these cytokines generates a severe inflammatory response, leading to increased vascular permeability and the exudation of fluids and cells in the alveoli, resulting in adult respiratory distress syndrome, respiratory failure, and other worse outcomes. Post-mortem studies have revealed diffuse damage to the alveoli, the formation of a hyaline membrane, and intra-alveolar oedema [6].
Among the released pro-inflammatory cytokines, IL-6 plays a pivotal role in the development of severe complications in COVID-19. A recent meta-analysis shows that patients with severe or critical SARS-CoV-2 infections tend to have higher IL-6 levels and decreased survival. The mean IL-6 concentration is 2.9-fold higher in patients with severe COVID-19 than in those with the non-complicated disease (95% CI: 1.17–7.19) [7]. For this reason, tocilizumab (TCZ) is increasingly being used in the treatment of COVID-19, albeit with varying outcomes [8]. TCZ is a recombinant humanised anti-interleukin-6 receptor (IL-6R) monoclonal antibody that blocks both soluble and membrane-bound IL-6 receptors. It has been approved by the Food and Drug Administration for the treatment of rheumatoid arthritis and to dampen the exuberant cytokine storm following chimeric antigen receptor (CAR)-T cell therapy [9]. Several observational and randomised clinical trials (RCTs) show conflicting results on the effectiveness of TCZ in moderate, severe, and critical COVID-19. Multiple observational studies and meta-analyses show increased survival in patients treated with TCZ and supportive therapy [[10], [11], [12], [13], [14], [15], [16]]. In contrast, a recent publication of four randomised placebo-controlled trials shows no difference in mortality compared with standard of care or a placebo. The pooled risk ratio (RR) for mortality of the four trials is 1.09 (95% CI: 0.80–1.49). The adverse-event profile was similar in the two groups. Despite the lack of a mortality benefit, the meta-analysis showed a decreased risk of mechanical ventilation; the pooled RR for this analysis is 0.71 (95% CI: 0.52–0.96) [16].
Studies from different geographic zones have yielded different treatment outcomes and mortality rates [10]. The aim of the current study was to describe the clinical, laboratory, and radiological characteristics of severe/critical COVID-19 and the outcome after TCZ in Saudi Arabia.
Methods
This was a retrospective observational case-control study conducted in two centres in Riyadh, Saudi Arabia: Prince Sultan Military Medical City (PSMMC) and Imam Abdulrahman Al Faisal Hospital (IAFH). Both hospitals are accredited tertiary care centres in the country’s capital, Riyadh. Patients from PSMMC received TCZ and were considered the intervention group, whereas patients from IAFH who did not receive TCZ acted as the control group. Both groups were matched being admitted at the same admission period and with similar disease severity. Patients who were at least 18 years old and were positive for COVID-19 were included in the study. According to hospital protocol, patients who were pregnant, with active infection, with predisposition to bowel perforation or with active gastrointestinal bleeding were excluded. Patients who died within 48 h from admission were also excluded from the study. The diagnosis of SARS-CoV-2 infection was confirmed based on respiratory samples (nasopharyngeal or oropharyngeal swabs) using the Abbott m2000 system SARS-CoV-2 assay, a real-time reverse transcription-polymerase chain reaction (RT-PCR) targeting the SARS-CoV-2 genomic regions encoding the RdRp and N genes. The primary outcome was mortality at 28 days. The secondary outcome was mortality at day 14 and length of hospital stay.
Patients with severe/critical COVID-19 pneumonia who were admitted to the PSMMC and IAFH from 20 April to 10 June 2020 were analysed and the clinical outcomes were compared between the cohort who received TCZ and the group who were managed without TCZ. Severe COVID-19 was defined if at least one of these was detected: respiratory rate ≥30 per min, SpO2 ≤93% in room air, or PaO2/FiO2 ≤300 mmHg. Patients were considered critical if respiratory failure, hypotension, or any organ failure was present. The demographics, co-morbidities, date of hospitalisation, oxygen status, and clinical and laboratory data of the patients were retrieved from the electronic medical and intensive care unit (ICU) records. Radiological images were reported by a radiologist and confirmed by two pulmonary physicians (SH, EB). All positive blood and respiratory cultures were assessed and recorded if they were considered as infections or colonisations. Only infections that followed treatment with TCZ were considered in the treatment arm.
Furthermore, clinical and laboratory features of cytokine release syndrome (CRS) were sought in all patients. The predictive criteria for COVID-19 cytokine storm included the following: signs and symptoms of COVID-19 in the presence of positive SARS-CoV-2 RT-PCR test, ground-glass opacity determined using computed tomography or chest X-rays, and high levels of ferritin and serum C-reactive protein (CRP). Additionally, variables including lymphopenia, elevated D-dimer, and lactate dehydrogenase were documented. All patients in the TCZ group received one to two doses of TCZ (Actemra®, Roche Holdings AG, Basel), 400–800 mg every 12 h. Like the control group, patients in the TCZ arm received standard of care and supportive and therapeutic modalities, based on the discretion of the treating physician and the COVID-19 guidelines of the local hospital. Further therapeutic modalities included hydroxychloroquine; favipiravir, the antiretrovirals lopinavir/ritonavir(kaetra); interferon; prophylactic or therapeutic anticoagulation, convalescent plasma, systemic corticosteroids, and antimicrobials, when indicated.
Close monitoring of the treatment response and safety through screening for viral hepatitis and latent tuberculosis was performed in patients who received TCZ. The study was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients in the TCZ group while admission consent was used in the control group. Ethical approval was obtained from the Institutional Review Board of PSMMC, Riyadh, Saudi Arabia.
Data were analysed using Statistical Package for Social Studies (SPSS 22; IBM Corp., New York, NY, USA). Continuous variables are expressed as mean ± standard deviation and categorical variables as percentages. The t-test was used for continuous variables, whereas the Chi-square and Fisher’s exact tests were used for categorical variables. Survival curves were estimated using the Kaplan–Meier method followed by a log-rank test to compare the TCZ-treated group and the control group. Cox regression was used to calculate the hazard ratios. A p-value <0.05 was considered statistically significant.
Results
Table 1 shows the demographic variables, co-morbidities, and symptoms of patients treated with TCZ (n = 62) and without TCZ (n = 86). In general, the two groups were well-matched; they were similar with regards to sex, co-morbidities, and treatment regimens. The mean age ± SD of the TCZ group was 57.4 ± 14.3 years, with 82% being males. Patients treated with TCZ were more likely to be hypertensive (p = 0.027) and have chronic kidney disease (p = 0.002). As a result, the Charlson score, an indicator of comorbid illnesses, was higher in the TCZ group (1.18 vs. 1.00, p = 0.006). However, patients in the control group had more symptoms and presented earlier. A significant difference was noted between the TCZ-treated and control groups in the duration of symptoms (p = 0.005).
Table 1.
Baseline clinical variables of the study population.
| Demographic variables | Control (n, 86) | Tocilizumab (n, 62) | p Value |
|---|---|---|---|
| Male/female (n) | 72/14 (n) | 51/11 (n) | 0.815 |
| Age (years) | 52.8 ± 12.9 | 57.4 ± 14.3 | 0.043 |
| BMI (mean ± SD) | 29.7 ± 6.5 | 29.7 ± 5.7 | 0.970 |
| Comorbidities | |||
| Diabetes mellitus (yes) | 50 (58.1%) | 30 (48.4%) | 0.240 |
| Hypertension (yes) | 26 (30.2%) | 29 (46.8%) | 0.040 |
| Cardiac problem (yes) | 11 (12.8%) | 6 (9.7%) | 0.558 |
| Bronchial asthma (yes) | 11 (12.8%) | 7 (11.3%) | 0.783 |
| Chronic kidney disease (yes) | 1 (1.2%) | 9 (14.5%) | 0.002 |
| CLD (yes) | 0 | 3 (4.8%) | 0.070 |
| CTD (yes) | 0 | 1 (1.6%) | 0.416 |
| Stroke (yes) | 1 (1.2%) | 3 (4.8%) | 0.195 |
| DLP (yes) | 22 (25.6%) | 14 (22.6%) | 0.472 |
| Immunosuppressant (yes) | 1 (1.2%) | 4 (6.5%) | 0.029 |
| Charlson score | 1.00 ± 0.34 | 1.18 ± 0.42 | 0.002 |
| Symptoms | |||
| Fever (yes) | 64 (74.4%) | 55 (88.7%) | 0.031 |
| Cough (yes) | 56 (65.5%) | 50 (80.6%) | 0.039 |
| Sputum (yes) | 41 (47.7%) | 6 (9.7%) | 0.0001 |
| Sore throat (yes) | 3 (3.5%) | 13 (21.0%) | 0.001 |
| Chest pain (yes) | 35 (40.7%) | 4 (6.5%) | 0.0001 |
| Myalgia (yes) | 58 (67.4%) | 10 (16.1%) | 0.0001 |
| Arthralgia (yes) | 11 (12.8%) | 5 (8.1%) | 0.361 |
| Fatigue (yes) | 4 (4.7%) | 28 (45.2%) | 0.0001 |
| SOB (yes) | 70 (81.4%) | 47 (75.8%) | 0.410 |
| Headache (yes) | 3 (3.5%) | 9 (14.5%) | 0.015 |
| Confusion (yes) | 2 (2.3%) | 0 | 0.336 |
| NV | 2 (2.3%) | 13 (21.0%) | 0.0001 |
| Diarrhea/abdominal pain (yes) | 14 (16.3%) | 8 (12.9%) | 0.569 |
| Systolic blood pressure | 122.3 ± 21 | 129.9 ± 16 | 0.052 |
| Duration of symptoms | 4.99 ± 2.37 | 6.39 ± 3.11 | 0.005 |
| Days from admission till TCZ | 2.71 ± 1,42 |
Tables 2 and 3 show the clinical variables and medication history of the patients treated with and without TCZ. Significant results were observed in WBC count (p = 0.020), lymphocyte count (p = 0.040), creatinine (p = 0.0001), and O2 on room air (p = 0.001). Notably, 93% (80/86) of the control patients received steroids, whereas only 48.5% (30/62) received steroids in the TCZ group.
Table 2.
Baseline clinical /laboratory variables of the study population.
| Clinical variables | |||
|---|---|---|---|
| Variables | Control (n, 87) | Tocilizumab (n, 62) | p Value |
| WBC | 10.9 ± 5.09 | 6.84 ± 3.98 | 0.020 |
| Lymphocytes | 1.05 ± 0.730 | 1.05 ± 0.484 | 0.040 |
| Platelets | 258 ± 115 | 208 ± 99.4 | 0.050 |
| ALT | 65.3 ± 63.6 | 49.4 ± 37.6 | 0.210 |
| INR | 1.21 ± 0.448 | 1.05 ± 0.119 | 0.068 |
| Creatinine | 97.5 ± 57.72 | 139 ± 187.3 | 0.0001 |
| D-dimer | 4.55 ± 12.2 | 2.31 ± 4.43 | 0.096 |
| Ferritin | 817 ± 545 | 1470 ± 1757 | 0.205 |
| LDH | 445 ± 190 | 525 ± 375 | 0.064 |
| Procalcitonin | . ± . | 0.68 ± 1.79 | |
| CRP (positive/negative) | 79/7 | 56/1 | 0.0001 |
| O2 in RA (93/>93) | 60/0 | 38/22 | 0.001 |
| IL-6 | 1135 ± 235 | ||
| Medication | |||
|---|---|---|---|
| Antibiotic (yes/no) | 86/0 | 62/0 | NA |
| Antiviral (yes/no) | 78/8 | 14/48 | 0.0001 |
| Antifungal (yes/no) | 1/85 | 8/54 | 0.004 |
| HCQ | 40/46 | 8/54 | 0.0001 |
| Triple therapy | 10/76 | 8/54 | 0.815 |
| Kaletra | 48/38 | 2/60 | 0.0001 |
Table 3.
Symptoms and clinical variables of the study population.
| Variables | Groups | Baseline | p Value | Day 3 | p Value | Day 7 | p Value | Day 10 | p Value | Day 14 | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Control | <38 | 0.099 | <38 | – | <38 | – | <38 | – | <38 | – |
| Treated | 37.84 | 36.9 | 37.06 | 36.7 | 36.9 | ||||||
| HR | Control | 93.3 | 0.621 | 96 | 0.122 | 95.1 | 0.782 | 95.1 | 0.778 | – | |
| Treated | 92.4 | 83.6 | 86.0 | 85.1 | 85.3 | ||||||
| RR | Control | 26.8 | 0.056 | 26.6 | 0.113 | 26.8 | 0.021 | 26.8 | 0.085 | – | |
| Treated | 23.5 | 22.5 | 22.4 | 21.7 | 20.6 | ||||||
| O2 in RA | Control <93/>93 | 60/0 | 0.001 | 0/85 | 0.0001 | 0/82 | 0.009 | 0/86 | 0.004 | 0/86 | 0.001 |
| Treated <93/>93 | 38/22 | 11/51 | 5/57 | 5/50 | 6/36 |
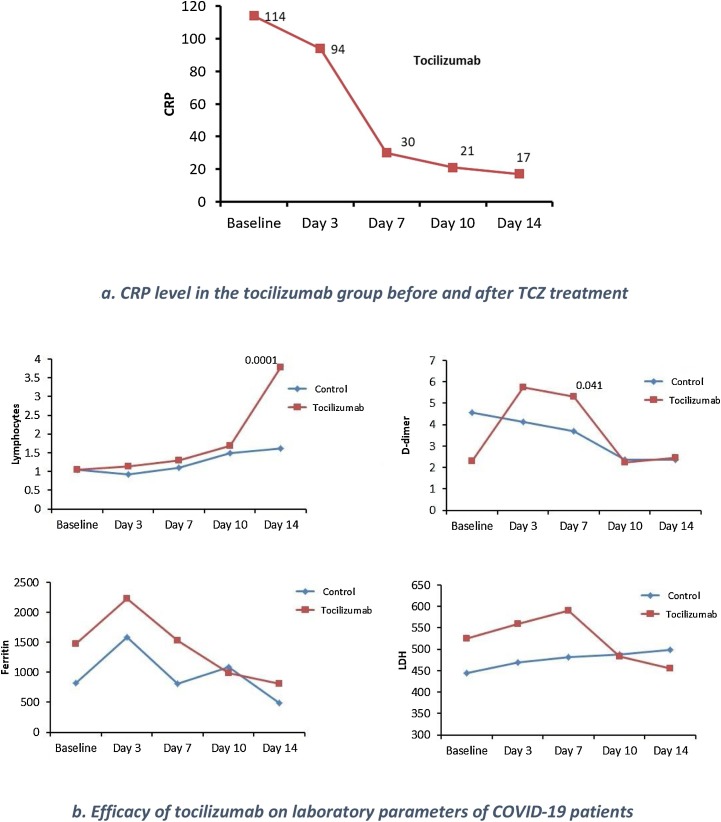
There were clear differences between the control and TCZ groups in laboratory parameters, such as lymphocytes, ferritin, LDH, and D-dimer, throughout the study period from baseline to day 14 (Fig. 1a). The mean IL-6 level in the TCZ cohort was 1135 ± 235 pg/mL, whereas it was not determined in the control group. A follow-up of IL-6 levels was not performed, although CRP, a surrogate for IL-6, was documented before and after TCZ treatment. There was a considerable decline in CRP level following treatment with TCZ (Fig. 1b). Serum ferritin and D-dimer levels initially increased after TCZ treatment, then declined. By day 14, a total of 67 and 19 patients were on ventilators in the control and TCZ group, respectively (p = 0.001).
Fig. 1.
Efficacy of tocilizumab on certain laboratory parameters of COVID-19 patients.
(a) Lymphocytes(X109), D-dimer, ferritin and lactic acid dehydrogenase (LDH) levels through D14. The baseline is the day of initiation of tocilizumab (TCZ). The TCZ group is shown in red, the control in blue. Observations are shown for baseline, D3, D7, D10, and D14.
(b) C-reactive protein (CRP) in mg/L in the tocilizumab group at baseline, D3, D7, D10, and D14.
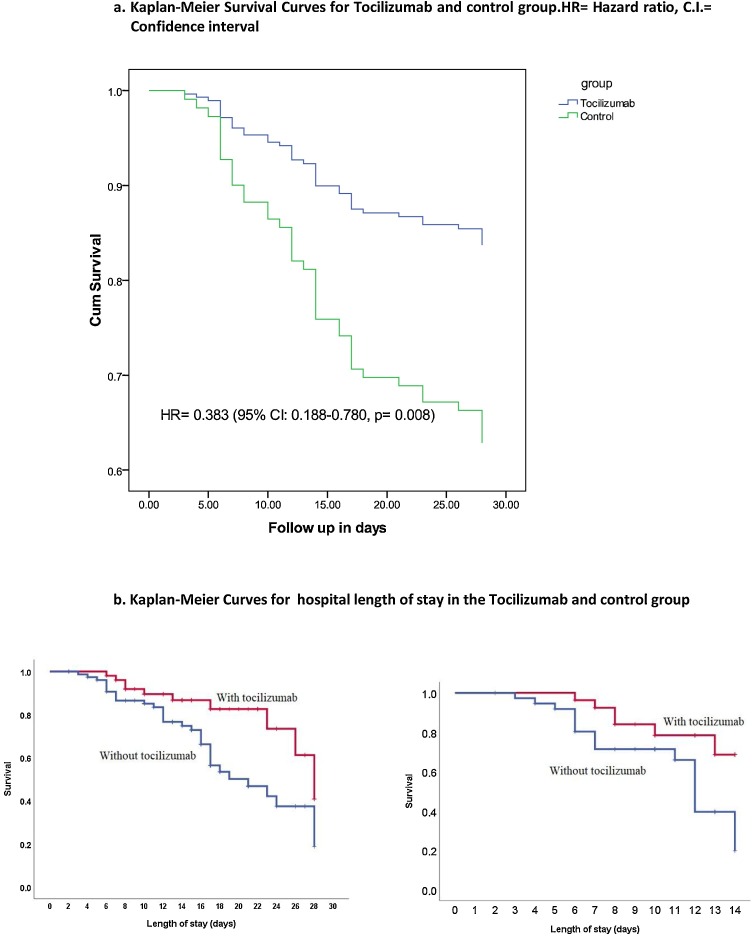
The primary endpoint was death by day 28 and the secondary endpoints included death by day 14 and length of hospital stay (TCZ = 51 and control = 78) (Fig. 2 ). The log-rank test showed a statistical difference in mortality between patients with COVID-19 who received TCZ therapy, HR = 0.354 (95%CI: 0.174−0.722) p = 0.04). There was a significant difference in the 28-day (TCZ = 62 and control = 86) fatality rate among the TCZ-treated patients and control group (16.1% vs. 37.2%, p = 0.004). The fatality rate at day 14 was lower in the TCZ-treated patients than in the control patients (9.7% vs. 24.4%, p = 0.022). Multivariate analysis showed TCZ as the only independent predictor for a favourable outcome (OR 0.201; 95% CI 0.063−0.634; p < 0.006). In contrast, the length of hospital stay was longer, though not significant, in the TCZ-treated group than that in the control group (17.7 ± 7.8 vs. 15.6 ± 7.59 days, p = 0.103: Table 4 ).
Fig. 2.
Kaplan–Meier Survival Curves for Tocilizumab and control group.
(a) 28 days (b) 14 days.
Table 4.
Outcome of patients treated with and without tocilizumab.
| Mortality | Status | Control (n = 86) |
Tocilizumab (n = 62) |
p Value | ||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Day 14 mortality (149) | Live | 65 | 75.6 | 56 | 90.3 | 0.022 |
| Died | 21 | 24.4 | 6 | 9.7 | ||
| Day 28 mortality | Live | 5 | 62.8 | 52 | 83.9 | 0.005 |
| Died | 32 | 36.8 | 10 | 16 | ||
| LOS (mean) | 15.6 ± 7.59 | 17.7 ± 7.8 | 0.103 | |||
Ten (16.0%) patients in the TCZ group developed infection: five contracted hospital/ventilator-associated pneumonia, whereas the other five developed blood-stream infections. Candidemia occurred in four patients (one case each for an unidentified Candida species, C. albicans, C. tropicalis, and C. dubliniensis), with two of them having concomitant coagulase-negative bacteraemia. One patient had cytomegalovirus viraemia. On the other hand, 6 patients (7.0%) from the control group developed HAP, with Klebsiella pneumoniae (2 patients) and Acinetobacter baumanii in the other 4 individuals.
Discussion
In this retrospective study, the efficacy of TCZ was evaluated in a cohort of patients with severe/critical COVID-19. Overall, the fatality rate was significantly decreased in the TCZ group by day 28 as well as day 14 of the study. Unexpectedly, the length of hospital stay was longer in the TCZ group than that in the control patients.
Several factors might have contributed to the beneficial outcomes observed in this study. First, most patients in the TCZ-treated cohort presented symptoms towards the second week of their illness (6.39 days). Their average IL-6 level was extremely high at 1135 ± 235 pg/mL. Such timing could have matched with the occurrence of a cytokine storm resulting in an optimal effect of treatment [[17], [18], [19]]. Following a viraemic phase, a minority of patients progress to a severe hyperinflammatory phase, which is characterised by high levels of cytokines, including IL-6, similar to that observed in these patients, with a significant elevation in CRP, ferritin, and D-dimer levels [20,21]. Lymphopenia, the most characteristic feature of COVID-19 CRS recovered in the TCZ group of this study [22]. Additionally, the inflammatory marker CRP, a surrogate for IL-6 and a marker of severity, decreased in these TCZ-treated patients, indicating a response to the IL-6 blocker [23]. CRP levels dropped significantly between day 0 (114 mg/L) and day 10 (21 mg/L) of treatment (Fig. 1b). However, in many studies, a drop in inflammatory markers does not always translate to an improvement in patient outcomes. It is not yet clear whether there is a pathway involving IL-6 which, when interrupted, results in survival benefit, or if it is simply a marker of severe disease (like severe sepsis).
Second, almost 50.0% of the TCZ-treated patients received steroids at different stages of their illness. This could have confounded these findings. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial shows the incidence of death to be lower in dexamethasone-treated patients than in the group that received usual care among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI: 0.51–0.81), and non-ventilated patients on oxygen supplements (23.3% vs. 26.2%; rate ratio, 0.8; 95% CI: 0.72–0.94) [21]. Nevertheless, although a larger number of patients in this study control group (93% vs. 48%) received steroids, a higher mortality rate was observed among them. Worth mentioning, the combination of steroids and TCZ might have had an additive effect in decreasing the mortality in this TCZ-treated cohort. This combination was previously shown to result in improved survival, quicker respiratory improvement, and a decreased likelihood of invasive ventilation [24,25].
Another possible explanation for the improvement in this cohort could be geographically related. Remarkably, there seems to be a variation in the outcomes of trials from different countries. Based on seven retrospective studies, a recent systematic review concluded that there is no convincing evidence to support the use of TCZ in patients with COVID-19 [12]. However, two trials from France included in that review show that the mortality rate is lower in the TCZ-treated group than the control group (RR = 0.44, 95% CI: 0.22–0.89) [26]. The difference in outcome is believed to be related to the population characteristics and the overall quality of care, including the availability of ICU beds, during the surge of the pandemic.
Overall, a large amount of data currently available on the effect of TCZ on the mortality of patients with severe COVID-19 present conflicting views. A large observational study, the Stop-Covid trial, with 3924 patients reveals a risk difference of 9.6% (95% CI: 3.1%–16.0%) in the TCZ-treated group [27]. Similarly, a systemic review of 23studies including 6279 patients showed an overall decrease in mortality [28]. A ssubgroup analysis including studies with only severe cases revealed lower mortality (RD: −0.12; CI: −0.18 to −0.06; p < 0.01) and need for mechanical ventilation (RD: −0.11; CI: −0.19 to −0.02; p = 0.01) in TOC group compared to SOC group.
The best evidence available was recently published in four RCTs, which suggested no mortality benefit when TCZ is used in the treatment of COVID-19 [29]. However, the results of these studies may be affected by the timing of randomization in the course of the disease [30]. It is worth noting that these trials were small with a total number of 771 patients. The pooled RR for mortality in the four trials is 1.09 (95% CI: 0.80–1.49). Despite a lack of survival benefit, these trials show a decreased risk of mechanical ventilation (pooled RR = 0.71, 95% CI: 0.52–0.96). The profile of side effects, particularly infection risk, was similar in the two groups [[31], [32], [33]]. In the current study a reduction in the risk of mechanical ventilation in the TCZ-treated group was observed. By day 14, a total of 67 and 19 patients were under mechanical ventilation in the control and TCZ group, respectively (p = 0.001). This may be of benefit in countries where there is a shortage of ventilators or those with busy ICUs. Clearly, larger randomised trials that also perform a cost-benefit analysis are needed to justify the benefits of this intervention.
In this study, patients who received TCZ were more likely to develop an infection than untreated patients, driven primarily by a large increase in ventilator-associated pneumonia and bloodstream infection. A total of 10 patients (16.0%) developed infections including candidemia, yet only one patient with complicated infection succumbed. This underscores that infection was not a major contributory factor to mortality in the TCZ-treated cohort. On the other hand, the control group developed infection in 7.0% of the cases. Evidence for increased risk following one or two doses of tocilizumab is still lacking. Recent RCTs do not disclose a higher risk of infections or adverse events with this medication. Similarly, TCZ therapy is not associated with increased infections in CRS patients following CAR-T cell therapy [34]. The higher infection rate in the patients included in this study may be related to critical illness and ICU intervention rather than TCZ use.
The trend to prolonged length of hospital stay (TCZ 17.7 vs. Control 15.6 days, p = 0.103) in these patients was difficult to explain. Previous studies and meta-analyses show that TCZ use decreases both the number of days of ventilator use and length of the hospital stay [35]. The higher rate of secondary infections and the need for extended parenteral therapy may have played a role in this group. Furthermore, early deaths in the control group could have shortened their hospital stay.
This study has several limitations. First, it was a small retrospective study that lacked blinding and randomisation. RCTs are needed to confirm these findings. Second, the adverse events in the TCZ-treated group and complicating infections in the control group were not addressed properly. The strength of this study, however, includes the addition of a control arm and its multi-centre nature. In addition, IL-6 quantitation was performed in almost all patients in the TCZ-treated group. Although IL-6 quantitation was not documented following TCZ therapy, CRP, a surrogate of IL-6, was obtained following treatment.
In conclusion, this study showed that the mortality rate was significantly lower in patients treated with TCZ than in those who were not treated; however, there was a non-significant trend towards a prolonged hospital stay in the TCZ group. Although the secondary infection rate was high, it was likely related to ICU intervention rather than TCZ treatment. It is difficult to draw a firm conclusion in view of the retrospective nature and the small sample size. Nonetheless, this is one of the few trials on the effect of tocilizumab in our area. There seems to be a variation in the outcome of trials from different geographical areas. Obviously, more data is needed to confirm the efficacy of TCZ-treated patients with COVID-19, particularly from RCTs.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Data sharing statement
No data sharing as this manuscript and the data were not published elsewhere.
Acknowledgements
The authors express their appreciation to all patients who participated in this study with all content and cooperation.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. Retrieved 23 September 2020.
- 2.World Health Organization (WHO) 2020. Novel coronavirus — China.https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ [Google Scholar]
- 3.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020:e2141. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alattar R., Ibrahim T.B.H., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;8:2042–2049. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parr J.B. Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Intern Med. 2021;181(1):12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 10.Sirimaturos M., Gotur D.B., Patel S.J., Dreucean D., Jakowenko N., Cooper M.H. Clinical outcomes following tocilizumab administration in mechanically ventilated coronavirus disease 2019 patients. Crit Care Explor. 2020;2(10):e0232. doi: 10.1097/CCE.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzghari S.K., Acuna V.S. Supportive treatment with tocilizumab for COVID-19. A systematic review. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2020;27:244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klopfenstein T., Zayet S., Lohse A., Balblanc J.C., Badie J., Royer P.Y. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50(5):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2020;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020;126(10):1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluccio F., Ergonenc T., Garcia Martos A., Allam A.E. Treatment algorithm for COVID-19: a multidisciplinary point of view. Clin Rheumatol. 2020;39(7):2077–2084. doi: 10.1007/s10067-020-05179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diorio C., Shaw P.A., Pequignot E., Orlenko A., Chen F., Aplenc R. Diagnostic biomarkers to differentiate sepsis from cytokine release syndrome in critically ill children. Blood Adv. 2020;4(20):5174–5183. doi: 10.1182/bloodadvances.2020002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikulska M., Nicolini L.A., Signori A., Di Biagio A., Sepulcri C., Russo C. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klopfenstein T., Zayet S., Lohse A., Selles P., Zahra H., Kadiane-Oussou N.J. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int J Infect Dis. 2020;99:491–495. doi: 10.1016/j.ijid.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz M., Haghbin H., Abu Sitta E., Nawras Y., Fatima R., Sharma S. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2020;93:1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 29.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundararaj Stanleyraj J., Sethuraman N., Gupta R., Thiruvoth S., Gupta M., Ryo A. Treating COVID-19. are we missing out the window of opportunity? J Antimicrob Chemother. 2021;76(2):283–285. doi: 10.1093/jac/dkaa442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parr J.B. Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Intern Med. 2020;181:12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 32.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-09-18.htm.
- 34.Rosas I., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1056/NEJMoa2028700. 2020.2008.2027.20183442, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eimer J., Vesterbacka J., Svensson A.K., Stojanovic B., Wagrell C., Sönnerborg A. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J Intern Med. 2020;289:434–436. doi: 10.1111/joim.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]