Significance
Identifying how past human populations altered ecosystems is critical for understanding current ecological diversity and for the management of both natural and cultural resources. This study presents evidence for an enduring ecological legacy of ancient people on the Colorado Plateau, where the complexity of archaeological sites correlates with the richness of culturally important plant species. This suggests the intentional or unintentional transport and cultivation of native plants on a scale that is often overlooked in the American Southwest, where exogenous domesticates (corn, beans, and squash) are emphasized. These results illustrate how even small-scale societies can affect ecosystems and highlight the importance of coupling archaeology, ecology, and tribal expertise for resource management.
Keywords: ethnobotany, archaeo-ecosystems, species richness, Solanum jamesii, Bears Ears
Abstract
Humans have both intentional and unintentional impacts on their environment, yet identifying the enduring ecological legacies of past small-scale societies remains difficult, and as such, evidence is sparse. The present study found evidence of an ecological legacy that persists today within an semiarid ecosystem of western North America. Specifically, the richness of ethnographically important plant species is strongly associated with archaeological complexity and ecological diversity at Puebloan sites in a region known as Bears Ears on the Colorado Plateau. A multivariate model including both environmental and archaeological predictors explains 88% of the variation in ethnographic species richness (ESR), with growing degree days and archaeological site complexity having the strongest effects. At least 31 plant species important to five tribal groups (Navajo, Hopi, Zuni, Ute Mountain Ute, and Apache), including the Four Corners potato (Solanum jamesii), goosefoot (Chenopodium sp.), wolfberry (Lycium pallidum), and sumac (Rhus trilobata), occurred at archaeological sites, despite being uncommon across the wider landscape. Our results reveal a clear ecological legacy of past human behavior: even when holding environmental variables constant, ESR increases significantly as a function of past investment in habitation and subsistence. Consequently, we suggest that propagules of some species were transported and cultivated, intentionally or not, establishing populations that persist to this day. Ensuring persistence will require tribal input for conserving and restoring archaeo-ecosystems containing “high-priority” plant species, especially those held sacred as lifeway medicines. This transdisciplinary approach has important implications for resource management planning, especially in areas such as Bears Ears that will experience greater visitation and associated impacts in the near future.
Local resource abundance is important for determining where in a given landscape humans decide to live. Nearby water, game, soil, and plants provide readily available wild resources for foraging and conditions that allow for cultivation (1–5). However, humans also modify their surrounding environments in order to increase the abundance and diversity of local plant (6–11) and animal (12–15) resources. Such “human niche construction” is a hallmark of ancient and modern societies (16, 17), having positive and negative impacts on global biodiversity while possibly creating enduring ecological legacies (18–21). This may be especially true for more sedentary and dense populations (22, 4) that are more likely to find investment worthwhile (23) and to produce unintentional impacts. Thus, variation in contemporary ecological diversity may in part reflect past land use dynamics and, therefore, be revealed through coupled archaeological and ecological research (24–33).
Coupled ecological and archaeological research has led to the discovery of altered patterns of succession resulting from 1) forest clearing and changes in canopy light regime (34, 35), 2) alterations of soil especially linked to food refuse (36, 37), 3) changes in fire regimes (38, 39), and, more rarely, 4) the importation of plant propagules from distant sites of collection (40, 41). Identifying such long-lost dynamics between humans and landscapes can inform conservation aimed at restoring site-specific artifacts, features, and the associated resource base past and present, here termed “archaeo-ecosystems” (42, 43). This would greatly facilitate cross-cultural management of public lands (44) in ways that promote Indigenous health, cultural reclamation, and sovereignty (7, 45). The linkages, however, between ecological legacies, archaeo-ecosystem restoration and cross-cultural management have yet to be systematically tested or practically applied.
Here, we offer a formal evaluation of this archaeo-ecosystem approach by using paired archaeological and ecological survey data focused on Puebloan occupation of a region known as Bears Ears on the Colorado Plateau in southeastern Utah (Fig. 1). Puebloan populations modified their environment by constructing terraces and check dams, developing blinds and wing traps, importing exogenous species, and setting fires (4, 22, 46), but investments were not uniform across the region. We test the hypothesis that locations with greater investment indicated by larger and more complex archaeological sites should today have higher richness of culturally significant plant species, here termed ethnographic species richness (ESR), as an enduring legacy of past investment. Our study expands previous work on ecological legacies by using field surveys to develop an explanatory model applied to 265 sites across one million acres of semiarid public lands. It documents the occurrence of uncommon and ethnographically significant plant species associated with those sites and infuses traditional ecological knowledge into proposed management actions for conserving these archaeo-ecosystems. Controlling for underlying environmental variation, our results indicate that past human habitation increases the diversity of plant species important for Indigenous subsistence.
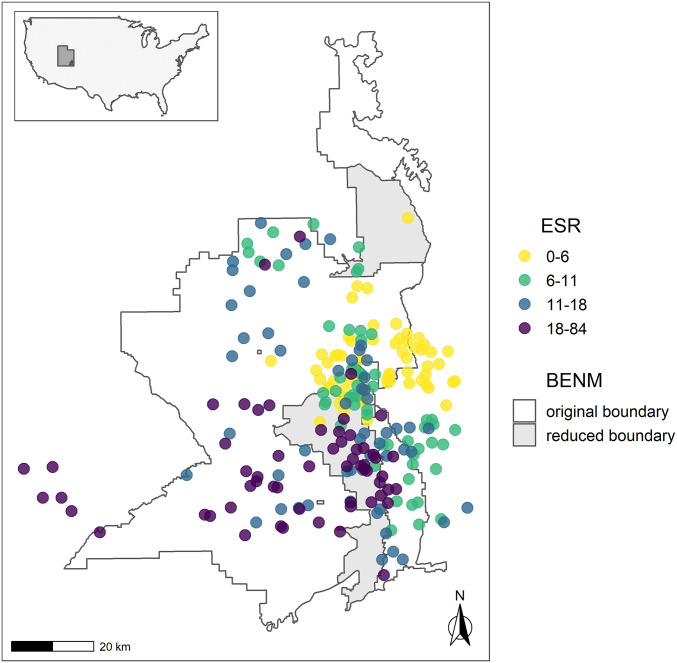
Fig. 1.
Location of Bears Ears National Monument in southeastern Utah. The predicted ESR at 265 known archaeological sites across the original and reduced monument boundaries and surrounding region are shown.
Results
Ethnographic Species Richness (ESR).
A total of 117 plant taxa with ethnographic significance occur among the 25 surveyed sites (SI Appendix, Table S1). ESR ranges from six to 27 per site with a mean of 16.25 (SD = 5.9) and a median of 18 (interquartile range = 7) (SI Appendix, Fig. S1). Sites with high ESR positively covary in space, indicating that sites with high ESR are likely to be close to one another, and vice versa (Fig. 1 and SI Appendix, Fig. S2). Eight species (Achnatherum hymenoides, Amelanchier utahensis, Artemisia tridentata, Elymus elymoides, Ephedra viridis, Gutierrezia sarothrae, Juniperus osteosperma, and Pinus edulis) are dominant components of regional vegetation types (47) and had frequencies of occurrence (f) between 0.60 and 1.00, indicating that they are generally abundant across the landscape and should not correlate with past human activity. However, at least 31 species of importance to at least four of the five tribal groups (SI Appendix, Table S1) occur with low to moderate frequency (0.10 to 0.60), most of which occur in the archaeological record of the Colorado Plateau (48, 49). These include relatively rare (Solanum jamesii f = 0.13, and Chenopodium sp. f = 0.10 to 0.20) and more common (Rhus trilobata, f = 0.40, Lycium pallidum f = 0.40, Shepherdia rotundifolia f = 0.40) plants that produce highly valued tubers, fruits, and seeds for a variety of uses (food, medicine, ceremony) (50).
Explanatory Model to Predict ESR.
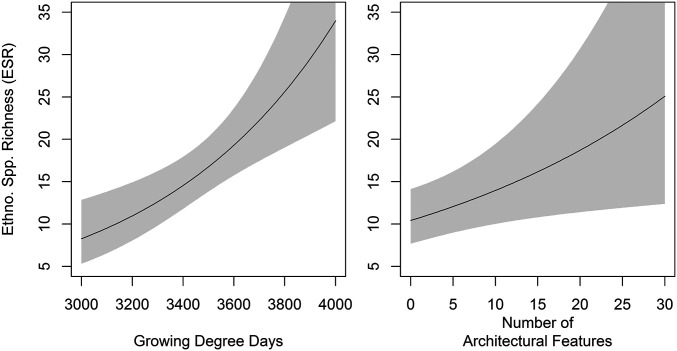
A multivariate model predicting ESR as a function of environmental and archaeological attributes explains 88% of the variation in observed ESR, with one significant environmental variable (growing degree days) and one significant archaeological variable (site complexity measured as the number of architectural features) (Fig. 2). ESR is highest where growing degree days are high (P = 0.0004), and the site has a large number of architectural features (P = 0.0182). Moisture index and site size negatively covary with ESR, but the relationship is not statistically significant (P > 0.05). As expected, given the positive autocorrelation in ESR, site coordinates are also a significant model term (P < 0.0001). Diagnostics indicate that the model adequately accounts for spatial covariance, as there is no meaningful spatial patterning in the residuals (SI Appendix, Fig. S3) and that coordinates are adequately smoothed (k = 1.15, P = 0.77). Model residuals are normally distributed and centered near zero (SI Appendix, Fig. S4).
Fig. 2.
Partial response plots for significant (P < 0.05) model terms showing how predicted ESR varies as a function of growing degree days and the number of archaeological site features when holding all other variables constant. Growing degree days (GDD) has the strongest effect, increasing ESR from ∼8 to above 30 across the observed range in GDD. The number of archaeological features has the next largest and only other significant effect, with the number of features increasing predicted ESR from ∼10 to ∼19 across the range of observed features. The results indicate that even when holding the environmental variables constant, the number of architectural features still has a significant effect on the expected number of culturally important plant species present today.
Predicted ESR across all known Puebloan sites in the study area indicate several peaks, including one centered around the major canyons of central Bears Ears (e.g., South Cottonwood, Mule, Dry, Arch, and Owl canyons, Cheese and Raisins). Some of these high ESR locations are within the current reduced boundaries of Bears Ears National Monument while others are not (Fig. 1).
Discussion
Our results reveal a clear ecological legacy of past human behavior: even when holding environmental variables constant, the richness of culturally important plants present today increases significantly as a function of site complexity and, therefore, past investment. As predicted, modern plant diversity is in part structured by the enduring legacy of past subsistence practices.
The environmental context of Puebloan sites determined the resource base, health, social structure, size, and persistence of human populations (51–53). Populations relied on the cultivation of domesticated plants, especially maize, beans, and squash (54). Wild plant resources often have higher return rates than domesticated crops (23, 55) and were essential for meeting nutritional, medicinal, and ceremonial needs. Their diversity and abundance varied and still vary widely across the landscapes of the Four Corners region and among its occupational sites. For example, coprolites from Antelope House in Canyon De Chelly contained macroremains of 22 ethnographic plant species, while those from Salmon Pueblo on the San Juan River contained only 12 species (table 2 in ref. 52). Occupants of both sites relied heavily on the three cultivated plants, but the former was occupied for nearly 750 y, while the latter was occupied for ∼200 y. Multiple factors contributed to these differences, but human exploitation of ecosystem variations and native plant diversity were essential components of successful habitation in this challenging, semiarid region, especially during periods of extreme climatic variation.
In the present study, high ESR was associated with the number of archaeological features, accompanied by substantial drainage systems (56) and the juxtaposition of several vegetation types (e.g., riparian forest, pinyon–juniper woodland, and semidesert scrub) that contained a broad range of ethnographic plant species. Many species would be present as wild populations occupying their own physiologically based environmental niches, and their resources would be easily gathered if widespread or locally abundant. These would include the high frequency pinyon, juniper, grass, and shrub species that characterize the dominant ecosystems of the region. However, low frequency species, especially those that are relatively rare or localized, but highly desirable, would require much greater effort to obtain. We suggest that individuals, possibly women seeking to reliably provision offspring (e.g., refs. 57 and 58), would find it advantageous to have these plants growing closer to their habitations. Once transported from distant wild populations, accidentally discarded or intentionally sown propagules of wild potato, goosefoot, wolfberry, or jimsonweed would likely find suitable habitat on canyon floodplains and maize terraces that are often associated with occupational sites in the Bears Ears region of the Colorado Plateau. In other words, the most complex archaeo-ecosystems in Bears Ears resulted from once xenic, but subsequently localized, ecological diversity supporting clusters of people that depended on, and therefore enhanced, the available plant resource base. Perennial species, often known to have lifespans measured in centuries and millennia (59, 60), would persist as relics or produce descendants even if habitat conditions were suboptimal.
Perhaps the best example of ESR enhancement from Bears Ears is that of the Four Corners potato. During surveys, it was found at seven archaeological sites, all of these beyond the climatic envelope modeled from 160 known occurrences (43) across the entire range of the species (largely in central Arizona and New Mexico). Within Bears Ears, the extant populations are small (nine to 300 stems), associated with alluvial terraces and drainage features (Fig. 3), and known to be genetically depauperate. At one site, plants grow out of pit houses along with an anomalous, small population of wolfberry (Lycium pallidum). Tubers of this potato species are produced in abundance and can persist for 14 y underground (61). They are nutritious and available during the winter and spring, therefore extending occupancy all year long (19, 48). Because of such ecological legacies, models may not predict the location or condition of living resources, especially those associated with well-developed archaeological sites.
Fig. 3.
Four Corners potato (Solanum jamesii) growing in sand at the base of slick rock waterfall, just above site 42SA244, a two-story cliff dwelling in Bears Ears. The species reproduces only by tubers that have very limited dispersal capability. The situation repeats itself among archaeological sites in southern Utah, Arizona, and New Mexico. Photos by Kari Gillen.
In addition to the significant relationship between the number of architectural features and high ESR, the model predicts that sites with high ESR tend to be located in the central region of Bears Ears as well as on Cedar and Mancos mesas (Fig. 1 and SI Appendix, Fig. S8). Our model identifies specific locations within Bears Ears that will require special management regimes, especially in light of increased visitation and the proposed development or expansion of resource extraction activities in the recently downsized national monument. Those special regimes should be cross-cultural, developed with tribal input (44, 62, 63), to emphasize the conservation and restoration of archaeo-ecosystems that contain “high priority” plant species, especially those held sacred as lifeway medicines. It is likely that such rare or uncommon species indicate ancient habitation or cultivation and provide insight into human subsistence behaviors. These plant populations should be documented in detail, monitored and targeted for special actions (e.g., visitation restrictions, improved footpaths, limited grazing, interpretive signage, and designated tour guides) that ensure their remarkable persistence. Formally embedding traditional ecological knowledge into land management decisions would improve federal stewardship and promote the longstanding linkages between Indigenous people and their ancestral lands.
Materials and Methods
Site Selection and Field Surveys.
Surveys of 25 documented and undocumented archaeological sites were conducted in the Bears Ears region of southeast Utah between September 2017 and September 2019. Nine sites were randomly selected from the State History Preservation Office database, 10 were vetted to include significant archaeological features (major dwellings, rock art), and six were opportunistically included during the course of field work. Access varied greatly across difficult terrain, but these represented a geographically dispersed set of sites encompassing the full range of environmental variation within the project area (Fig. 1). Survey teams were composed of both archaeologists and botanists with standardized datasheets so that artifact and feature distributions, dimensions, and abundances could be compared with ecosystem characteristics over the same georeferenced points and measured transects.
Species lists were compiled for each site and vouchered specimens mounted, labeled, and reposited in the Garrett Herbarium, Natural History Museum of Utah as well as on the Intermountain Regional Herbarium Network (https://intermountainbiota.org/portal/collections/index.php?catid=1). Scientific and common names, as well as taxonomic relations, conform to those compiled by the US Department of Agriculture Natural Resources Conservation Service PLANTS database (https://plants.sc.egov.usda.gov). Each specimen was annotated and provided a Navajo name and cataloged along with ethnographic information (42).
ESR.
Determination of ethnographic species and their traditional uses was made by consulting the Native American Ethnobotany Database (naeb.brit.org) and multiple secondary sources (47, 64–66). The focus was on five tribes (Navajo, Hopi, Ute Mountain Ute, Zuni, and Apache) that trace ancestry to or currently reside near the project region and use these plants for food, medicinal, ceremonial, and utilitarian purposes. Once a species was determined to have ethnographic significance, it was added to tallies for each study site to obtain an estimate of ESR.
Archaeological and Ecosystem Characteristics.
Archaeological site characteristics were determined by measuring the following: 1) site dimensions and 2) the number and type of habitation and other features and their metrics. These ranged from very small (e.g., 5 m × 5 m) to very large (336 m ×153 m) sites with zero to 21 features, including residential structures or room blocks, towers, granaries, slab-lined cists, rock art, earthen depressions, middens, agricultural terraces, check dams, etc. Likewise, these sites had very few (<10) to many (>500) artifacts (42, 43).
Ecosystem characterizations include dominant vegetation types, geomorphological features, and substrate characteristics. Sites ranged in elevation from ∼4,600 (Butler Wash, Comb Ridge) to 8,500 m (Elk Ridge), and, therefore, vegetation types included desert scrub, riparian, pinyon–juniper woodland, and ponderosa–aspen forest. Sites were located in canyons, near streams or dry drainages, atop mesas, along ridges, or beneath overhangs.
Landscape Variables.
Even though ecosystem survey areas (based upon sampling transect lengths) varied greatly between sites, there was no significant correlation between survey area and ESR (P > 0.05). This indicates that no matter how large the sampling area around an archaeological site was, the documented ESR was not affected (i.e., more species were not found in larger sampling areas as a methodological artifact). Secondly, the lack of a break in slope also suggests that sampling occurred in vegetation types with overlapping species compositions rather than wholly different plant communities.
Explanatory Model to Predict ESR.
We evaluate our predictions with a multivariate generalized additive model (67), examining how ESR varies as a function of both environmental (i.e., slope, moisture index, growing degree days) and archaeological predictors (i.e., site size, number of architectural features) and including controls for spatial autocorrelation (i.e., geographic location). As we expect environmental and archaeological predictors to vary linearly with ESR, they are included as parametric terms in the model. Coordinates are fit as a single smoothed Gaussian process, with the number of splines defined through generalized cross-validation. Model diagnostics include a check on whether or not coordinates are adequately smoothed and whether model residuals are normally distributed and contain spatial autocorrelation (67). We use the model output to predict ESR for all 265 well-documented Puebloan sites in the study area.
Supplementary Material
Acknowledgments
We thank the Department of the Interior Bureau of Land Management Monticello and Salt Lake City field offices, especially Cameron Cox, Don Hoffheins, Jared Lundell, Lydia DeHaven, Tyler Ashcroft, Nate Thomas, Allison Ginn, and Melanie Beckstand for funding our cooperative agreement (Cooperative Ecosystem Studies Unit L17AC00059) and for their advice throughout the project. B.F.C.’s efforts were in part supported by the NSF (Award DEB-1714972). L.A.L. and B.M.P.’s efforts were also supported in part by the NSF (Award BCS-1827414). We also thank our Indigenous collaborators (Karlos Baca, Christina Castro, and Elouise Wilson) for sharing their knowledge and expertise on plants in Bears Ears. Our field crews (Paul Allgaier, Alyssa Chapman, Anna Cohen, Kari Gillen, Rich Hiett, Allison Joyce, Drew Potter, Roxanne Lamson, Ellyse Simons, Heidi Simper, and Carolyn Spencer) helped us record, document, and measure archaeological and ecosystem characterizations. We thank Jaydee Dolinar and Kari Gillen for mounting all the plant specimens and to Elizabeth Johnson and Allison Izaksonas for repositing the collection in the Natural History Museum of Utah Garrett Herbarium and imaging them for online access. We thank Arie Leeflang for helping with the Utah State Historic Preservation Office site selection, Wes McCool for organizing and quality checking the archaeological data, and the Garrett Herbarium at the Natural History Museum of Utah for mounting specimens.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025047118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Adams K. D., et al., Lake-level fluctuations in the Lahontan basin, Nevada: Implications for the distribution of archaeological sites. Geoarchaeology 23, 608–643 (2008). [Google Scholar]
- 2.Benson L. V., Ramsey D. K., Stahle D. W., Petersen K. L., Some thoughts on the factors that controlled prehistoric maize production in the American Southwest with application to southwestern Colorado. J. Archaeol. Sci. 40, 2869–2880 (2013). [Google Scholar]
- 3.Kohler T. A., Parker S. C., Predictive models for archaeological resource location. Adv. Archaeol. Method Theory 9, 397–452 (1986). [Google Scholar]
- 4.Kohler T. A., et al., Modelling prehispanic Pueblo societies in their ecosystems. Ecol. Modell. 241, 30–41 (2012). [Google Scholar]
- 5.Vernon K. B., Yaworsky P. M., Spangler J., Brewer S., Codding B. F., Decomposing habitat suitability across the forager to farmer transition. Environ. Archaeol., 10.1080/14614103.2020.1746880 (2021). [DOI] [Google Scholar]
- 6.Anderson M. K., Tending the Wild: Native American Knowledge and the Management of California’s Natural Resources (University of California Press, Berkeley, CA, 2013), p. 558. [Google Scholar]
- 7.Fowler C. S., Applied ethnobiology and advocacy: A case study from the Timbisha Shoshone tribe of Death Valley, California. J. Ethnobiol. 39, 76–89 (2019). [Google Scholar]
- 8.Hankins D. L., The effects of indigenous prescribed fire on riparian vegetation in central California. Ecol. Process. 2, 24 (2013). [Google Scholar]
- 9.Lake F. K., Trails, fires, and tribulations: Tribal resource management and research issues in northern California. Occasion 5, 1–22 (2013). [Google Scholar]
- 10.Lake F. K., Christianson A. C., “Indigenous fire stewardship” in Encyclopedia of Wildfires and Wildland-Urban Interface (WUI) Fires, Manzello S. L., Ed. (Springer, 2019), pp. 1–9. [Google Scholar]
- 11.Trauernicht C., Brook B. W., Murphy B. P., Williamson G. J., Bowman D. M., Local and global pyrogeographic evidence that indigenous fire management creates pyrodiversity. Ecol. Evol. 5, 1908–1918 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird R. B., Tayor N., Codding B. F., Bird D. W., Niche construction and dreaming logic: Aboriginal patch mosaic burning and varanid lizards (Varanus gouldii) in Australia. Proc. Biol. Sci. 280, 20132297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codding B. F., Bird R. B., Kauhanen P. G., Bird D. W., Conservation or co-evolution? Intermediate levels of aboriginal burning and hunting have positive effects on kangaroo populations in Western Australia. Hum. Ecol. Interdiscip. J. 42, 659–669 (2014). [Google Scholar]
- 14.Rick T. C., et al., Millennial-scale sustainability of the Chesapeake Bay Native American oyster fishery. Proc. Natl. Acad. Sci. U.S.A. 113, 6568–6573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toniello G., Lepofsky D., Lertzman-Lepofsky G., Salomon A. K., Rowell K., 11,500 y of human-clam relationships provide long-term context for intertidal management in the Salish Sea, British Columbia. Proc. Natl. Acad. Sci. U.S.A. 116, 22106–22114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boivin N. L., et al., Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. U.S.A. 113, 6388–6396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens L., et al., Archaeological assessment reveals Earth’s early transformation through land use. Science 365, 897–902 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Fisher J. A., et al., Indigenous peoples’ habitation drives present-day forest biodiversity in British Columbia’s coastal temperate rainforest. People Nat. 1, 103–114 (2019). [Google Scholar]
- 19.Kinder D. H., Adams K. R., Wilson H. J., Solanum jamesii: Evidence for cultivation of wild potato tubers by ancestral Puebloan groups. J. Ethnobiol. 37, 218–240 (2017). [Google Scholar]
- 20.Power M. J., et al., Human fire legacies on ecological landscapes. Front. Earth Sci. 6, 151 (2018). [Google Scholar]
- 21.Sullivan A. P., Bird D. W., Perry G. H., Human behaviour as a long-term ecological driver of non-human evolution. Nat. Ecol. Evol. 1, 65 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Carrer F., An ethnoarchaeological inductive model for predicting archaeological site location: A case-study of pastoral settlement patterns in the Val di Fiemme and Val di Sole (Trentino, Italian Alps). J. Anthropol. Archaeol. 32, 54–62 (2013). [Google Scholar]
- 23.Mohlenhoff K. A., Codding B. F., When does it pay to invest in a patch? The evolution of intentional niche construction. Evol. Anthropol. 26, 218–227 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Briggs J. M., et al., Why ecology needs archaeologists and archaeology needs ecologists. Front. Ecol. Environ. 4, 180–188 (2006). [Google Scholar]
- 25.Dunn M., Coquile flora: An ethnobotanical reconstruction. Econ. Bot. 37, 349–359 (1983). [Google Scholar]
- 26.Fukasawa K., Akasaka T., Long-lasting effects of historical land use on the current distribution of mammals revealed by ecological and archaeological patterns. Sci. Rep. 9, 10697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heckenberger M. J., et al., Amazonia 1492: Pristine forest or cultural parkland? Science 301, 1710–1714 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Heckenberger M. J., Russell J. C., Toney J. R., Schmidt M. J., The legacy of cultural landscapes in the Brazilian Amazon: Implications for biodiversity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 197–208 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levis C., et al., Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355, 925–931 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Lullfitz A., et al., Contemporary distribution of Macrozamia dyeri (Zamiaceae) is correlated with patterns of Nyungar occupation in south-east coastal Western Australia. Austral Ecol. 45, 933–947 (2020). [Google Scholar]
- 31.McMichael C. N., Ecological legacies of past human activities in Amazonian forests. New Phytol. 229, 2492–2496 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mensing S. A., et al., Historical ecology reveals landscape transformation coincident with cultural development in central Italy since the Roman period. Sci. Rep. 8, 2138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oliveira E. A., et al., Legacy of Amazonian Dark Earth soils on forest structure and species composition. Glob. Ecol. Biogeogr. 29, 1458–1473 (2020). [Google Scholar]
- 34.Flinn K. M., Marks P. L., Agricultural legacies in forest environments: Tree communities, soil properties, and light availability. Ecol. Appl. 17, 452–463 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Hayashida F. M., Archaeology, ecological history, and conservation. Annu. Rev. Anthropol. 34, 43–65 (2005). [Google Scholar]
- 36.Cook-Patton S. C., Weller D., Rick T. C., Parker J. D., Ancient experiments: Forest biodiversity enhanced by native American middens. Landsc. Ecol. 29, 979–987 (2014). [Google Scholar]
- 37.Trant A. J., et al., Intertidal resource use over millennia enhances forest productivity. Nat. Commun. 7, 12491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delcourt P. A., Delcourt H. R., Ison C. R., Sharp W. E., Gremillion K. J., Prehistoric human use of fire, the eastern agricultural complex, and Appalachian oak-chestnut forests: Paleoecology of cliff palace pond, Kentucky. Am. Antiq. 63, 263–278 (1998). [Google Scholar]
- 39.Pausas J. G., Keeley J. E., A burning story: The role of fire in the history of life. Bioscience 59, 593–601 (2009). [Google Scholar]
- 40.Chepstow-Lusty A., Winfield M., Inca agroforestry: Lessons from the past. Ambio 29, 322–328 (2000). [Google Scholar]
- 41.Levis C., et al., How people domesticated amazonian forests. Front. Ecol. Evol. 5, 171 (2018). [Google Scholar]
- 42.Louderback L. A., Pavlik B. M., Codding B. F., “Archaeo-Ecosystems of the Four Corners: Ethnobotanical Surveys of Puebloan Sites in San Juan Co., Utah” (USDI Bureau of Land Management, Monticello, UT, 2018), p. 95. [Google Scholar]
- 43.Louderback L. A., Pavlik B. M., Codding B. F., Vernon B., Yaworsky P., “Archaeo-Ecosystems of the Four Corners: Ethnobotanical Surveys of Puebloan Sites in San Juan Co., Utah, Year 2” (USDI Bureau of Land Management, Monticello, UT, 2020), p. 63. [Google Scholar]
- 44.Zedler J. B., Stevens M. L., Western and traditional ecological knowledge in ecocultural restoration. San Franc. Estuary Watershed Sci. 16, 1–18 (2018). [Google Scholar]
- 45.Garnett S. T., et al., Healthy country, healthy people: Policy implications of links between indigenous human health and environmental condition in tropical Australia. Aust. J. Public Adm. 68, 53–66 (2009). [Google Scholar]
- 46.Herring E. M., Anderson R. S., San Miguel G. L., Fire, vegetation, and ancestral Puebloans: A sediment record from prater canyon in Mesa Verde National Park, Colorado, USA. Holocene 24, 853–863 (2014). [Google Scholar]
- 47.Heil K. D., O’Kane S. L., Reeves L. M., Clifford A., Flora of the Four Corners Region, Vascular Plants of the San Juan River Drainage: Arizona, Colorado, New Mexico, and Utah (Missouri Botanical Garden Press, 2013). [Google Scholar]
- 48.Louderback L. A., Pavlik B. M., Starch granule evidence for the earliest potato use in North America. Proc. Natl. Acad. Sci. U.S.A. 114, 7606–7610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geib P. R., Jolie E. A., “The rise of broad spectrum foraging on the Colorado Plateau during the early holocene” in The Archaic Southwest, Vierra B. J., Ed. (University of Utah Press, 2018), pp. 189–214. [Google Scholar]
- 50.Yarnell R. A., Implications of distinctive flora on Pueblo ruins. Am. Anthropol. 67, 662–674 (1965). [Google Scholar]
- 51.Adams K. R., “Archaeobotanical summary and conclusions” in Thirty-Five Years of Research at Salmon Ruins, New Mexico, Reed P. R., Ed. (Center for Desert Archaeology and Salmon Ruins Museum, 2006), pp. 867–873. [Google Scholar]
- 52.Reinhardt K. J., “Pathoecology of two ancestral Pueblo villages” in Case Studies in Environmental Archaeology, Reitz E. J., Scarry C. M., Scudder S. J., Eds. (Springer, 2008), pp. 191–209. [Google Scholar]
- 53.Sutton M. Q., Reinhard K. J., Cluster analysis of the coprolites from Antelope house: Implications for ancestral Pueblo diet and cuisine. J. Archaeol. Sci. 22, 741–750 (1995). [Google Scholar]
- 54.Bohrer V. L., Doebley J. F., “Cultivated plants from salmon ruin” in Thirty-Five Years of Research at Salmon Ruins, New Mexico, Reed P. R., Ed. (Center for Desert Archaeology and Salmon Ruins Museum, 2006), pp. 721–739. [Google Scholar]
- 55.Bowles S., Cultivation of cereals by the first farmers was not more productive than foraging. Proc. Natl. Acad. Sci. U.S.A. 108, 4760–4765 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall R. L., Dennis A. E., “Cultivated and gathered food plants” in Archaeological Investigations at Antelope House, Morris D. P., Ed. (National Park Service, 1986), pp. 110–141. [Google Scholar]
- 57.Hawkes K., O’Connell J. F., Jones N. G., Hunting income patterns among the Hadza: Big game, common goods, foraging goals and the evolution of the human diet. Philos. Trans. R. Soc. Lond. B Biol. Sci. 334, 243–250, (1991). [DOI] [PubMed] [Google Scholar]
- 58.Codding B. F., Bird R. B., Bird D. W., Provisioning offspring and others: Risk-energy trade-offs and gender differences in hunter-gatherer foraging strategies. Proc. Biol. Sci. 278, 2502–2509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowers J. E., Webb R. H., Rondeau R. J., Longevity, recruitment and mortality of desert plants in Grand Canyon, Arizona, USA. J. Veg. Sci. 6, 551–564 (1995). [Google Scholar]
- 60.Ferguson C. W., Annual Rings in Big Sagebrush – Artemisia Tridentata. Papers of the Laboratory of Tree-Ring Research No. 1 (University of Arizona Press, Tucson, 1964), p. 95. [Google Scholar]
- 61.Bamberg J., Observed limit of Solanum jamesii tuber dormancy at 14 years. Am. J. Potato Res. 91, 36 (2014). [Google Scholar]
- 62.Reyes-García V., et al., The contributions of Indigenous Peoples and local communities to ecological restoration. Restor. Ecol. 27, 3–8 (2019). [Google Scholar]
- 63.Long J. W., Lake F. K., Goode R. W., Burnette B. M., How traditional tribal perspectives influence ecosystem restoration. Ecopsychology 12, 71–82 (2020). [Google Scholar]
- 64.Dunmire W. W., Tierney G. D., Wild Plants of the Pueblo Province: Exploring Ancient and Enduring Uses (Museum of New Mexico Press, Santa Fe, 1995). [Google Scholar]
- 65.Moerman D. E., Native American Ethnobotany (Timber Press, Inc., 1998). [Google Scholar]
- 66.Moerman D. E., Native American Medicinal Plants: An Ethnobotanical Dictionary (Timber Press, Inc., 2009). [Google Scholar]
- 67.Wood S. N., Generalized Additive Models: An Introduction with R (CRC press, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.