Abstract
The default-mode network (DMN) in humans consists of a set of brain regions that, as measured with functional magnetic resonance imaging (fMRI), show both intrinsic correlations with each other and suppression during externally oriented tasks. Resting-state fMRI studies have previously identified similar patterns of intrinsic correlations in overlapping brain regions in rodents (A29C/posterior cingulate cortex, parietal cortex, and medial temporal lobe structures). However, due to challenges with performing rodent behavior in an MRI machine, it is still unclear whether activity in rodent DMN regions are suppressed during externally oriented visual tasks. Using distributed local field potential measurements in rats, we have discovered that activity in DMN brain regions noted above show task-related suppression during an externally oriented visual task at alpha and low beta-frequencies. Interestingly, this suppression (particularly in posterior cingulate cortex) was linked with improved performance on the task. Using electroencephalography recordings from a similar task in humans, we identified a similar suppression of activity in posterior cingulate cortex at alpha/low beta-frequencies. Thus, we have identified a common electrophysiological marker of DMN suppression in both rodents and humans. This observation paves the way for future studies using rodents to probe circuit-level functioning of DMN function.
Significance
Here we show that alpha/beta frequency oscillations in rats show key features of DMN activity, including intrinsic correlations between DMN brain regions, task-related suppression, and interference with attention/decision-making. We found similar task-related suppression at alpha/low beta-frequencies of DMN activity in humans.
Keywords: alpha, DMN, local field potentials, posterior cingulate, task-related interference
Introduction
The default-mode network (DMN) was initially recognized from the observation that, during externally oriented cognitive tasks, activity in these brain regions is lower during the task compared to a control “base-line” condition (Shulman et al. 1997; Raichle et al. 2001). Further work linked activity in default-mode brain regions with internally oriented cognitive processes including planning, reminiscing, mind-wandering, and imagining (Andrews-Hanna et al. 2010a; Raichle 2015). The DMN includes the medial temporal lobe subsystem and other limbic brain regions, posterior cingulate cortex, medial prefrontal cortex, and inferior parietal lobule (Andrews-Hanna et al. 2010b). Studies using functional magnetic resonance imaging (fMRI) show that regions within this brain-network are 1) intrinsically correlated with each other (Greicius et al. 2003, 2009) and 2) anticorrelated with frontoparietal and cingulo-opercular cognitive control brain regions (Greicius et al. 2003, 2009; Chai et al. 2012). Moreover, DMN activity is suppressed when subjects are properly engaged on sensorimotor or decision-making tasks that engages cognitive control circuits. Abnormal DMN activity and/or connectivity has been linked with depression (Sheline et al. 2009), schizophrenia (Kim et al. 2009), cognitive deficits after traumatic brain injury (Bonnelle et al. 2011), attention deficit disorder (Uddin et al. 2008), and other psychopathological disease states (Whitfield-Gabrieli and Ford 2012). These studies show that regulation of activity and connectivity within DMN brain regions is critically important for intact cognition and healthy emotion regulation. As such, it is clear that developing and validating a physiological model of DMN activity in rodents would allow for a deeper understanding of the processes that govern this network and may pave the way for developing DMN-targeted treatments.
fMRI studies have been conducted in rodents to characterize brain networks using methods similar to those used in humans, though measured primarily at rest (Upadhyay et al. 2011; Lu et al. 2012; Schwarz et al. 2013; Stafford et al. 2014; Gozzi and Schwarz 2016; Hsu et al. 2016; Schwarz and Gozzi 2017; Clemm von Hohenberg et al. 2018; Hinz et al. 2019). These studies have identified regions with both anatomic and functional homology to DMN in humans (including posterior cingulate cortex, lateral parietal cortex, and many parts of the medial temporal lobe). However, there have been few studies in rodents investigating whether activity in these homologous DMN brain regions are suppressed during an externally oriented visual task. Performing behavioral investigations with rodents in an magnetic resonance imaging machine is challenging for both technical and behavioral reasons, limiting our ability to measure task-related activity within DMN.
To investigate activity in DMN brain regions during an externally oriented task, we reanalyzed data (taken from a recently published manuscript (Fakhraei et al. 2021)), in which animals and humans perform a visual-based behavioral inhibition task. In this task, subjects had to either respond immediately (within 2 s) or wait for 2 s before responding based on different visual cues. Animals were implanted with electrodes to capture local field potentials in a set of 32 brain regions simultaneously (Fig. 1A,B). This included the putative DMN regions described above (posterior cingulate cortex, parietal cortex, hippocampus/medial temporal lobe), as well as frontal, sensorimotor cortex, striatum, and thalamus. In the prior paper we focused our investigation on identifying frontal and striatal activity associated with action or inhibition. Here we focused our investigation on identifying task-related suppression in previously identified homologs of the DMN.
Figure 1.
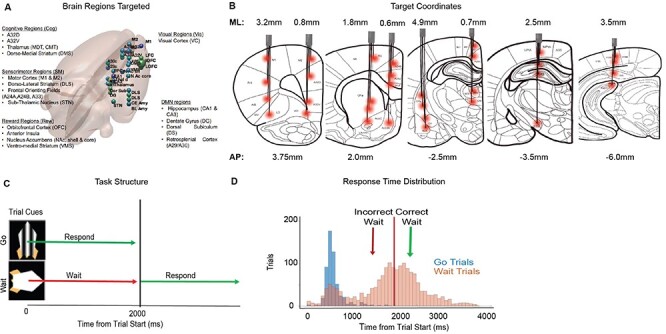
Target electrode locations and behavioral task. (A) 3D rendering of a rat brain with the relative location of all 32 electrodes marked. Target sites had some general involvement in the following domains of behavior: sensorimotor (motor cortex, dorsolateral striatum, and subthalamic nucleus), reward (orbitofrontal cortex, nucleus accumbens, and ventromedial striatum), cognitive (A32D/prelimbic cortex, A32V/infralimbic cortex, M2/premotor cortex, anterior insula, medio-dorsal thalamus, and dorsomedial striatum), visual (visual cortex), and default mode (hippocampus, dentate gyrus, dorsal subiculum, and amygdala). (B) Coronal rat brain sections (adapted from Paxinos & Watson, 7th Edition, 2013) with placement of the 8 cannulas marked. ML and AP (mm) coordinates are noted relative to bregma. Individual depths of all 4 wires in each cannula are visualized with a red dot at their target brain region (32 targets). (C) General structure of the go/wait task. Task was self-paced. Animals triggered visual stimuli by entering a noseport. Visual stimuli indicated the trial type to be either a “go” or “wait” trial. Animals would receive a reward if they responded within 2 s on a go trial or waited for 2 s prior to responding on a wait trial. (D) Response time distribution for different trial types (Go and Wait) from 1 animal.
Methods
In this paper, we reanalyzed data described in a recent publication (Fakhraei et al. 2021). In that publication we characterized prefrontal, motor, and striatal circuits linked with action preparation and inhibition. Here, in the same set of animals (n = 11) we were specifically focused on characterizing DMN suppression. We have included methods again for completeness.
Animal Methods
A total of 11 male Long–Evans rats obtained from Charles River Laboratories were used for these experiments. Rats were approximately 1-month-old weighing 150 g when received, and started training about 2 weeks after arrival. Rats were housed in pairs during training prior to electrode implantation, and individually housed postimplantation in a standard rat cage (10 × 10.75 × 19.5 in, Allentown, NJ, USA) with free access to food and on a standard light cycle (lights on at 6 am/off at 6 pm). Water scheduling (free access to water for 2 h/day) was initiated during training to maintain motivation for water reward in the task. Rats were weighed weekly to ensure that water scheduling did not lead to reduced food intake. Water was given ad libitum on days with no behavioral training. Repeated measurements were taken in animals across days, for a total sample size of 60 sessions.
Operant Chamber and Training
The custom-designed operant chamber used for this study consists of 5 nose-ports (NPs), each of which has an light-emitting diode (LED), infrared (IR) sensor and metal cannula that delivers water rewards. The chamber also contains two auditory tone generators, a house-light, a screen to display visual stimuli, and 5 peristaltic stepper motors/water pumps that deliver the water rewards into NPs. The chamber was controlled using Simulink (Mathworks) installed directly onto a Raspberry Pi system. The operant chamber is synchronized with electrophysiological signals using lab-streaming-layer protocol, designed to integrate multiple behavioral and physiological streams into a common timing (Ojeda et al. 2014, 2020). The design, operation, and software control of this chamber has been described in more detail previously (Buscher et al. 2020; Ojeda et al. 2020). Prior to training on the behavioral task, animals first went through a pretraining period (ca. 5–10 sessions), in which they learned that a noseport with an LED on signaled an available response port; that responding in such a port would trigger a water reward (after a delay of ca. 400 ms); and finally that there was a sequential nature to the task (animals start a “trial” by first entering the middle noseport 3, after which they could use either of the neighboring ports (2/4) to respond and collect an immediate reward). This “standard” pretraining paradigm is used for all types of behavioral paradigms in our lab, allowing us to develop self-triggered tasks (animals “trigger” the trial to start by entering the middle noseport). Animals advanced to the next stage of training when they were able to consistently perform at least 100 trials in 60 min.
Go-Wait (Action Delay) Task
Full details of this task have been described in a recent manuscript (Fakhraei et al. 2021). The rodent version of this task is self-paced. Each trial begins with a rat entering the middle NP, ensuring animals are in an identical position on every trial. After a short, fixed delay of 30 ms, a visual stimulus appears on the screen denoting the trial to be either a go trial (animal required to respond before 2 s to attain a reward) or wait trial (animal required to respond after 2 s to attain a reward) (Fig. 1C). On go trials the stimulus remains on the screen until the animal responds, thus minimally taxing attentional resources. On wait trials, the stimulus disappears after 2 s, or when animals respond (if earlier than 2 s). Thus on wait trials stimulus disappearance provides an additional visual cue for animals to respond, similar to the human design. If animals respond correctly, a 2-s water reward (total of 20 μl) is delivered after a delay of 400 ms. If animals respond incorrectly, the house-light flashes for a 5-s “time-out” period and no reward is given. Rewards are delivered using a stepper-motor, which have a loud sound when in operation, providing an auditory cue regarding reward delivery). Training on this task proceeded in two stages. In the first stage, animals were trained with a distribution of 75% go and 25% wait trials in order for them to learn to “respond”. Animals typically learned the “go” cue rapidly, within approximately 2 weeks. Once animals achieved greater than 75% accuracy on go trials, they proceeded to the next stage in which they are given a distribution of 25% go/75% wait trials. Animals were trained on this distribution of trials for an additional 12 weeks when performance on the task typically stabilized (with at least >80% accuracy on go trials, and ca. 50% performance on wait trials), after which they were implanted with local field potentials (LFP)-electrodes as described below. After implantation we waited 2 weeks to allow animal to recover from surgery prior to water scheduling and retrained animals on the task for an additional 1–2 weeks prior to recording. Recordings were typically conducted at least 2 times/week and were performed mostly with the 25% go/75% wait trial distribution (a few sessions were conducted with a 50/50 distribution). We measured reaction times on go and wait trials to assess behavior differences between the trial types (Fig. 1D, example from 1 animal). Reaction time was defined as the time from the start of the trial (visual display on) to the response in the noseport. All analyses described in this paper related to the animals are based on data from 60 recording sessions from 11 rats. All procedures were approved prior to the start of the study by the Animal Welfare and Ethics committee of the VA. Sessions used for physiological analysis had an average of 272 +/20.4 (standard error of the mean [SEM]) trials.
Surgery
Surgery was performed with the “sterile tip” method and all instruments were autoclaved prior to start. Surgeries were conducted under isoflurane anesthesia (SomnoSuite, Kent Scientific, CT, USA), with a body-temperature-controlled heating mat (VWR, PA, USA). Animals received a single dose of Atropine (0.05 mg/kg) to diminish respiratory secretions during surgery and a single dose of Dexamethasone (0.5 mg/kg) to decrease inflammation prior to surgery. A local anesthetic, Lidocaine (max 0.2 cc), was injected under the skin at the incision site while the animal was anesthetized but before surgery initiation. Implantation of electrodes was performed under stereotactic control. Using a Micro Drill (Stoelting, IL, USA) holes were drilled at eight predetermined stereotactic locations for electrodes, ground screw implanted over cerebellum and a minimum of 3 anchor screws attached for headstage stability. Electrodes were implanted as “bundles” of 4 50 μm tungsten wires (California Fine Wire, Grover Beach CA) that were precut to the appropriate length and secured within a 30-gauge, 8-mm long metal cannula (Mcmaster-Carr) prior to surgery. Thus, the depth (D/V position) of each electrode was fixed relative to the 4 other electrodes in the bundle. The electrode positions were secured with superglue (Loctite, Germany) and thus able to be positioned with minimal flexing during surgery. Electrodes were measured such that the cannula did not enter the brain.
Eight different bundles of electrodes were positioned across various A/P sites (Fig. 1A,B; Supplementary Fig. 1). All coordinates are described relative to Bregma (Paxinos & Watson, 7th Edition), and brain targets are likewise named as currently described in the latest edition of Paxinos and Watson. In this nomenclature, to help standardize rodent and nonrodent brain regions, subdivisions of anterior cingulate cortex are used to describe mid-line brain regions in rodents in lieu of or in addition to rodent-specific names (Vogt and Paxinos 2014; Laubach et al. 2018). Thus, prelimbic cortex we be described as A32D;infralimbic cortex as A32V. Specific parts of secondary motor cortex (also described as frontal orienting fields (Barthas and Kwan 2017)) will be listed as A24a, A24b, and A33). Electrode target locations were chosen to balance targeting as many potential brain regions of interest across the D/V axis while minimizing the total number of cannulas implanted. Electrode bundles were initially secured with superglue (Loctite, Germany) in the craniotomy and on the skull, followed by metabond (Parkell, NY, USA). The entire headstage apparatus was held to the skull with dental cement (Stoelting, IL, USA). Once all eight cannulas were placed and cemented to the skull, wires were threaded through the holes of a 36-channel electrode interface board (EIB) (Neuralynx, CO, USA) and bolted down using gold pins (Neuralynx, CO, USA). Placing the EIB board above the electrodes, dental cement was used to create a smooth headpiece encapsulating all the wires. This headpiece allowed attachment of the rat to a headstage (Intan, CA, USA) and a commutator (Plexon, TX, USA) inside the behavioral box. At the conclusion of surgery, rats received a single dose (1 mg/kg) of buprenorphine SR for pain management. Rats recovered from surgery on a heating pad to control body temperature and received SMZ-TMP in their drinking water (60 mg/kg per day for 8 days) to prevent infections.
Electrophysiology
Electrophysiology data were recorded using a 32-channel RHD headstage (Intantech Part C3324) coupled to a RHD USB interface board (Intantech, Part C3100) with an SPI interface cable. We used plug-in graphical user interface (GUI) (Open Ephys) software to acquire data. Data were recorded at 1000 Khz, with a band-pass filter set at 0.3 to 999 Hz during acquisition. Physiology data were integrated with operant chamber behavioral data using a lab-streaming-layer protocol (Ojeda et al. 2014), implemented with a customized plug-in written for plug-in GUI (https://github.com/aojeda/plugin-GUI), as described previously (Buscher et al. 2020; Ojeda et al. 2020).
LFP Analysis
Preprocessing Steps. To measure neural activity in brain regions linked to the task, we carried out standard preprocessing and time–frequency (TF) analyses using custom MATLAB scripts and functions from EEGLAB (Ramanathan et al. 2018). 1) Data epochs: We first extracted time-points for events of interest during the task (trial start and response). Time-series data were extracted for each electrode, from 2 s before to 5 s after each behavioral marker for each trial and organized into a 3D matrix (electrodes, times, trials). 2) Artifact removal: Noisy trials were removed. Trials with greater than ×4 the standard deviation in activity (measured across the time dimension) were treated as artifact and discarded. 3) Median reference: At each time-point, the “median” activity was calculated across all electrodes and subtracted from each electrode. 4) TF decomposition: A trial by trial TF decomposition (TF decomposition) was calculated using a complex wavelet function implemented within EEGLAB (newtimef function, using Morlet wavelets, with cycles parameter set to: [2, 0.7], frequency window of between 2 and 70 hz and otherwise default settings used) (Delorme and Makeig 2004). We calculated the analytic amplitude of the signal (using the abs function). 5) Baseline normalization: To measure evoked activity (i.e., change from baseline) we subtracted, for each electrode at each frequency, the mean activity within a baseline window between 1000 and 750 ms prior to the start of the trial. 6) Trial averaging: We next calculated the average activity across trials for specific trial types (go correct, wait correct or wait incorrect) at each time-point and frequency for each electrode, thus creating a 3D matrix (time, frequency, and electrode) for each behavioral session. 7) Comparison across animals: Prior to averaging across sessions/animals, we “z-scored” the data recorded from each behavioral session. This was accomplished by subtracting the mean and dividing by the standard deviation of activity in each electrode (at each frequency) over time. Z-scoring was helpful for normalizing activity measured from different animals prior to statistical analysis. These preprocessing steps resulted, for each session used in our data analysis, in a 3D TF–electrode matrix of dimensions 200 × 139 × 32, which was used for further statistical analyses as described in following section.
Statistical Analysis of TF Data
Statistical analyses were performed at the level of sessions, with a total of 60 sessions from 11 animals. We performed two main types of statistical analyses on the whole-brain TF-electrode (TFE) data. 1) Trial-type mean: Mean was calculated at each time and frequency point for each electrode across the 60 behavioral sessions. Statistical significance was estimated with a one-sample, two-sided t-test (t-test function in MATLAB), compared with the null hypothesis of Z = 0, with degree of freedom of 59 for these tests. Because we had already performed a “baseline” subtraction (as described above), this analysis was essentially capturing whether there was a significant increase or decrease in activity compared to baseline. False discovery rate (FDR)-correction was applied to the entire time-frequency-electrode matrix (32 electrodes, 200 time-points, and 137 frequencies from 1 to 70 Hz) to evaluate statistical significance at this level (FDR-corrected P-value threshold set to 0.05). To visualize significant time–frequency activations or deactivations, nonsignificant values were set to 0. FDR-correction was performed across all times–frequencies–electrodes for whole-brain correction.
In addition to the above analysis performed at all times/frequencies, we also calculated the mean analytic amplitude for each electrode across a specified time window and frequency band of interest. The mean/SEM of that data was calculated across 60 sessions, and we used a one-sample, two-sided t-test (again compared to the null hypothesis that Z = 0) to evaluate significance of the means. We applied a more stringent Bonferroni correction to the P-values from this data (corrected for 32 comparisons).
WPLI Analysis
Weighted-phase lag index (wPLI) was calculated as described in prior studies (Stam et al. 2007; Vinck et al. 2011; Bastos and Schoffelen 2016), using the Fieldtrip analytic toolbox. We first computed the cross-spectrum C(f) = X(f)Y*(f), of two real signals of x and y, with X and Y representing the Fourier-transform of x and y within a particular frequency band, and * indicates the complex conjugate. The wPLI was computed from this using the magnitude of the imaginary component by the following equation1
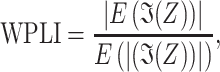 |
where Z indicates the complex nondiagonal part of C. wPLI was computed within each behavioral session separately for each trial type (go correct, wait correct, and wait incorrect). We performed two analyses of the wPLI data. First, we estimated the region to region wPLI during the first second of the task (the time period when maximal suppression occurred) for each trial type in each behavioral session. We then calculated the mean wPLI for each pairwise interaction across sessions (n = 60) for each trial type. Statistical analyses was conducted for each pairwise interaction, at each time-frequency point, using a one-sample, two-tailed t-test followed by FDR-correction across the entire 4D matrix (electrode–electrode–time–frequency, d.f. 59, with a total of 35 frequency points ca. 70 Hz and 51 time-points). The null hypothesis was a wPLI of 0.
Logistic Mixed-Effects Model
To understand how activity in DMN brain regions was linked with behavior, we extracted time-frequency data at a trial by trial level from all animals and assembled together into a matrix (trials, time, frequency, and session). A linear-mixed-effects (LME) model was performed with activity (at each time-frequency point for each electrode) as the dependent factor and response time (RT, time from start of trial to the response) as the independent factor. We included a set of nuisance variables to account for the animal in which trial data were taken from) in the model as well (Koerner and Zhang 2017). Data were FDR-corrected across the entire time–frequency–channel 3D matrix. A beta-value (slope) and an associated t-statistic/P-value was calculated at each time-frequency point (data were FDR-corrected across the entire 3D matrix of time, frequencies, and electrodes, with nonsignificant points set to 0). After FDR-correction, we calculated the “peak” significant t-stat within the time–frequency window of interest.
Histology
At completion of recording sessions wire tips were marked by passing 12 A current for 10s through each electrode (Nano-Z, Neuralynx). Rats were killed under deep anesthesia (100 mg/kg ketamine, 10 mg/kg xylazine IP) by transcardiac perfusion of physiological saline followed by 4% formalin. Brains were extracted and immersed in 4% formalin for 24 h and then stored in 30% sucrose 4% formalin until ready to be sectioned. Tissue was blocked in the flat skull position, and sectioned frozen in the coronal plane at 50
A current for 10s through each electrode (Nano-Z, Neuralynx). Rats were killed under deep anesthesia (100 mg/kg ketamine, 10 mg/kg xylazine IP) by transcardiac perfusion of physiological saline followed by 4% formalin. Brains were extracted and immersed in 4% formalin for 24 h and then stored in 30% sucrose 4% formalin until ready to be sectioned. Tissue was blocked in the flat skull position, and sectioned frozen in the coronal plane at 50 m. Brain slices of interest were Nissl stained using thionin to identify the course of the electrode tracks. Coarse electrode tracks (location of entire bundle of wires) were easily visualizable, though specific DV sites for each electrode within the bundle were less clearly visualizable. Because the DV sites were fixed prior to implantation; however, bundle location was used to make sure that electrodes were at least within the approximate AP/ML location (Supplementary Fig. 1).
m. Brain slices of interest were Nissl stained using thionin to identify the course of the electrode tracks. Coarse electrode tracks (location of entire bundle of wires) were easily visualizable, though specific DV sites for each electrode within the bundle were less clearly visualizable. Because the DV sites were fixed prior to implantation; however, bundle location was used to make sure that electrodes were at least within the approximate AP/ML location (Supplementary Fig. 1).
Human Methods
Task
Participants accessed a game-like cognitive task structured similarly to the task in animals. The basic task framework was modeled after the standard test of variables of attention (Greenberg and Waldman 1993). In this two-block task, visual stimuli of colored rockets appeared in either the upper or lower central visual field. The task sequence consisted of a central fixation “+” cue for 500 ms, followed by a rocket stimulus of either blue target color or other isoluminant nontarget color, presented for 100 ms. For blue rocket targets, participants were instructed to press the spacebar on the laptop keyboard as quickly as possible (“go” trials). For nontarget color rockets (isoluminant brown, mauve, pink, purple, and teal), the participant was instructed to withhold their response until the fixation “+” cue flashed briefly on the screen, at 2 s for a 100 ms duration (“wait” trials). Response feedback was provided for accuracy as a smiley or sad face emoticon presented 200 ms postresponse for 200 ms duration, followed by a 500 ms intertrial interval. Both task blocks lasted 5 min and consisted of 90 trials per block with 30/60 target/nontarget ratio in block 1 and 60/30 ratio in block 2. Stimuli were presented in a shuffled order. Four practice trials preceded the first task block, and participants received a percent block accuracy score at the end of each block with a series of happy face emoticons (up to 10).
Subjects
In total, 66 adult human subjects (mean age 24.5 ± 7.3 years, range 18–53 years, 41 females) participated in the cognitive task assessment. All human participants were healthy, with no current diagnosis for a psychiatric disorder and/or current/recent history of psychotropic medications. All participants provided written informed consent for the study protocol approved by the local institutional review board (IRB# 180140). All participants reported normal/corrected-to-normal vision and hearing and no participant reported color blindness. 95% of participants were right-handed.
Electroencephalography (EEG)
EEG data were collected simultaneous to the cognitive assessment using a 24-channel SMARTING device with a semidry and wireless electrode layout following standard 10/20 system. Data were acquired at 500 Hz sampling frequency at 24-bit resolution. Cognitive event markers were integrated using LSL and data files were stored in xdf format.
Behavioral Analysis
Data were analyzed for each type of stimulus, that is, go and wait. For each stimulus, signal detection sensitivity was computed as d’ = z(Hits)-z(False Alarms) (Heeger and Landy 2009). All d′ values were divided by max theoretical d′ of 4.65 to obtain scaled d′ in the 0–1 range. RTs are reported in seconds.
Neural Analysis
We applied a uniform processing pipeline to all EEG data consistent with that of the animal data processing (Balasubramani et al. 2020; Ojeda et al. 2020). This included: 1) data preprocessing, 2) computing event-related spectral perturbations (ERSP) for all channels, and 3) cortical source localization of the EEG data filtered within the relevant [8–20 Hz] frequency band.
1) Data preprocessing: Data preprocessing was conducted using the EEGLAB toolbox in MATLAB (Delorme and Makeig 2004). EEG data were resampled at 250 Hz, and filtered in the 1–45 Hz range to exclude ultraslow DC drifts at less than 1 Hz and high-frequency noise produced by muscle movements and external electrical sources at more than 45 Hz. EEG data were average referenced and epoched to the task stimuli as informed by the LSL time-stamps in the −1 s to +2 s stimulus time window. Epoched data were cleaned using the autorej function in EEGLAB to remove noisy trials (>5sd outliers rejected over max 8 iterations; 8.1 ± 5.1% of trials rejected per participant). EEG data were further cleaned by excluding signals estimated to be originating from nonbrain sources, such as electrooculographic, electromyographic or unknown sources, using the Sparse Bayesian learning (SBL) algorithm (https://github.com/aojeda/PEB) explained below (Ojeda et al. 2018, 2019).
2) ERSP calculations: We performed time-frequency decomposition of the epoched data using the continuous wavelet transform (cwt) function with the analytic Morlet (Gabor) wavelet in MATLAB’s signal processing toolbox. Baseline time-frequency (TF) data in the −750 ms to −550 ms time window prior to stimulus presentation were subtracted from the epoched trials (at each frequency) to observe the event-related synchronization and event-related desynchronization modulations (Pfurtscheller 1999). Here, we computed ERSP differences between correct-response go and wait stimuli, and statistically corrected the results across subjects using false discovery rate (FDR, P < 0.05).
3) Cortical source localization: Cortical source localization was performed to map the underlying neural source activations for the ERSPs using the block-Sparse Bayesian learning (SBL) algorithm (Ojeda et al. 2018, 2019) implemented in a recursive fashion. This is a two-step algorithm in which the first-step is equivalent to low-resolution electromagnetic tomography (Pascual-Marqui et al. 1994). LORETA estimates sources subject to smoothness constraints, that is, nearby sources tend to be coactivated, which may produce source estimates with a high number of false positives that are not biologically plausible. To guard against this, SBL applies sparsity constraints in the second step wherein blocks of irrelevant sources are pruned. Source space activity signals were estimated and the root mean square signals were partitioned into cortical regions of interest (ROIs) and artifact sources. ROIs were based on the standard 68 brain region Desikan–Killiany atlas (Desikan et al. 2006) using the Colin-27 head model (Pascual-Marqui et al. 1994). Activations from artifact sources contributing to EEG noise from nonbrain sources such as electrooculographic, electromyographic, or unknown sources, were removed to clean the EEG data. Cleaned subject-wise correct trial-averaged EEG data were then specifically filtered in the frequency band of interest [8–20 Hz], source-localized, and baseline subtracted to estimate their band-specific cortical ROI source signals. The source signal envelopes were computed in MATLAB (envelop function) by a spline interpolation over the local maxima separated by at least one time sample; we used this spectral amplitude signal for all neural analyses presented here. We focused on time periods for characterizing DMN suppression that were significant based on ERSP time–frequency maps and topography plots. The source-localized brain maps were statistically compared across subjects using t-test (P < 0.05).
Results
We have analyzed and reported the core findings regarding the task-related brain activity related to action and inhibition behaviors in a separate manuscript (Fakhraei et al. 2021). This manuscript is focused on a specific observation we made during the analysis of that data-set: the presence of task-related suppression of activity within rodent default-mode-network brain regions. In this paper, we describe these results in more detail. We were primarily interested in investigating whether DMN regions showed evidence of functional network relationships and evidence of task-related suppression during the behavioral inhibition task. On this task, we measured brain activity during two different trial types: on go trials, animals were required to respond within 2 s to collect a reward; on wait trials, animals were required to withhold their response for 2 s prior to responding. We initially analyzed activity only for correct trials. Animals showed a mean response time of 610 ± 20 ms on correct go trials, and 2510 ± 140 ms on correct wait trials, (P < 0.001, paired t-test between trial types, n = 60 sessions/11 animals). Example reaction times from one animal are presented in Figure 1D. By separately analyzing activation and suppression for each trial type, we were able to more clearly understand how DMN suppression occurs relative to behaviors on the task.
We began our analysis by focusing on time-frequency activity from posterior cingulate cortex electrode (A29c), guided by fMRI studies showing that, in rodents, A29c is a key hub of the DMN (Upadhyay et al. 2011; Lu et al. 2012; Stafford et al. 2014; Hsu et al. 2016). We performed a time-frequency analysis in this brain region with data normalized for each frequency (via subtraction) from a baseline period 700–1000 ms prior to the trial start. We next performed a one-sample, two-tailed t-test at each time-point and frequency across sessions (df: 59) to assess whether there was a significant activation or suppression in activity relative to baseline. We performed this analysis across all 200 time-points, 139 frequencies in all 32 electrodes from which we recorded. To account for the multiple-comparisons, data were FDR-corrected. We found striking differences in activity within posterior cingulate cortex (A29C) (Fig. 2A, P < 0.05, FDR-corrected. Nonsignificant time-points set to 0 on the image map for clarity). Posterior cingulate cortex showed a clear pattern of deactivation during the task (i.e., suppression from baseline) for both trial types, observable at multiple time-points and frequencies. The suppression spanned up to 20 Hz for both trial types at different frequencies. Figure 2B shows the mean/SEM power, on “go” trials (calculated between 0 and 2000s) and “wait” trials (calculated from 2000 to 4000 ms) across frequencies. For the purposes of comparison, we also calculated the mean time-frequency plot in dorsomedial prefrontal cortex (A32D), a prefrontal brain region involved in action and inhibition processes (Fakhraei et al. 2021). We found that this brain region shows some task-related suppression at theta-frequencies between 0-2000 ms on go trials and a similar desynchronization between 2000 and 4000 ms on wait trials, but at frequencies above theta (specifically, in alpha/beta-frequencies), we did not observe suppression temporally overlapping with suppression in posterior cingulate cortex (Fig. 2C). To better illustrate this, we plotted the mean power between 8 and 20 Hz for both A29C and A32D (Fig. 2D, df: 59). We found minimal suppression in A32D. In A29C, we observed an early suppression on both trial types (peak suppression of −0.66 +/− 0.1 SEM, (t(59) = −6.5, P = 2e-8), at 243 ms on go trials, and a peak suppression of −0.83, +/− 0.14 SEM at 272 ms, (t(59) = −5.8, P = 2e-07) on wait trials). On both trial types this was followed by a second suppression period (peak suppression of −1.1 +/− 0.14 SEM, (t(59) = −7.9, P = 8e-11 at 893 ms poststimulus on “go” trials and peak of −1.3 +/− 0.12 SEM, (t(59) = −10.3, P < 1e-11, at 2580 ms on “wait” trials). The fact that the early suppression occurs on both trial types argues that this suppression is linked with common, stimulus-related processing. The latter suppression seems to occur during the action/response period for both trial types, respectively. To better illustrate what suppression looks like, we plotted single trial examples for both go and wait trials (examples include both raw/unfiltered and filtered data for 5 traces for each trial types) (Fig. 2E).
Figure 2.
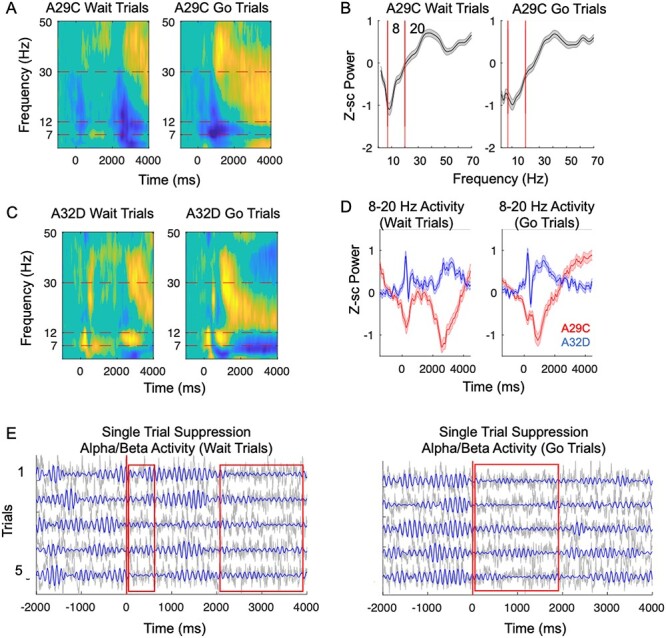
Alpha and beta suppression in DMN brain regions during task. (A,B). We performed a time–frequency decomposition in dorsomedial prefrontal cortex (A32D) and posterior cingulate cortex (A29C) (mean across 60 sessions, from 11 animals, data FDR-corrected across all times, frequencies, and electrodes. Nonsignificant values (FDR-adjusted P-value > 0.05) were set to 0 for the purpose of visualization). (A) On both go and wait trials, A29C (posterior cingulate cortex) shows distinct epochs of task-related suppression relative to baseline across theta, alpha, and beta-frequencies. (B) Shaded error bar shows mean posterior cingulate cortex (A29C) activity for “wait” trials (0–4000 ms) and “go” trials (0–2000 ms) at different frequencies, with 8–20 Hz frequency band highlighted by red bars. (C) A32D shows increased activity in alpha and beta-frequencies, but desynchronization at lower frequencies. (D) Mean TF activity within 8–20 Hz band plotted over time, showing for both wait and go trials, two distinct “peaks” of suppression. (E) Five examples of both go and wait trials from A29C. Gray line show the raw time-series and blue lines show the 8-20 Hz filtered activity from the same trials.
In these animals we also recorded simultaneous local field potentials from other brain regions as described previously (Fakhraei et al. 2021). This included field potentials in cognitive regions (dmPFC, vmPFC, and dorsomedial thalamus) (Hardung et al. 2017; Laubach et al. 2018), sensorimotor regions (motor/premotor brain regions, frontal orienting fields [A24a/A24b/A33] and dorsolateral striatum) (Vogt and Paxinos 2014; Kawai et al. 2015; Barthas and Kwan 2017; Ramanathan et al. 2018; Lemke et al. 2019; Veuthey et al. 2020), reward-related regions (nucleus accumbens core and shell, ventral striatum, and orbitofrontal cortex) (van Duuren et al. 2009; Haber and Knutson 2010; Bossert et al. 2012; Floresco 2015; Heilbronner et al. 2016; Izquierdo 2017), visual cortical regions and finally putative default-mode brain regions (A29C, A30C, posterior parietal cortex, and medial temporal lobe structures such as dorsal hippocampus (CA3, CA1) and dentate gyrus) (Lu et al. 2012; Hsu et al. 2016).
Using data from these additional electrodes, we next examined whether the suppression in oscillatory activity at alpha/low beta-frequencies during the task occurs only in posterior cingulate cortex (A29C/A30C) or whether similar suppression could be observed in other putative nodes of the DMN. We found clear evidence that task-related suppression within the alpha/low beta band (8–20 Hz) noted above occurred in many other putative DMN brain regions (Fig. 3A, P < 0.05, FDR-corrected across electrodes, times, and frequencies). To quantify this suppression we calculated the mean activity (in the frequency band noted) over the first 2000 ms poststimulus for go trials and over the first 4000 ms poststimulus for wait trials (Fig. 3B, bar plots). On go trials, A29C showed a mean evoked power of −0.54 ± 0.09, (t(59) = −5.8, P = 9.5e-6 FWE-adjusted for 32 electrodes) and on wait trials, A29C showed a mean evoked power of −0.48 ± 0.08 (t(59) = −5.8, P = 8.1e-6 Family wise error (FWE)-adjusted for 32 electrodes). Other DMN brain regions showed similar levels of deactivation during this time window (see Supplementary Table 1 for values from all electrodes). Regions associated with cognition generally showed increased activation during the task, including prefrontal cortex, orbitofrontal cortex and anterior insula. Anterior insula, classically part of a cognitive control/salience network that is highly anticorrelated with DMN regions in humans, showed the strongest pattern of activation at alpha/low beta-frequencies during this time window, with a mean activation of 0.59 ± 0.06, t(59) = 10, P = 8.8e-13, FWE-adjusted for 32 electrodes) on go trials and 0.39 ± 0.05, (t(59) = 7.6, P = 9e-9, FWE-adjusted).
Figure 3.
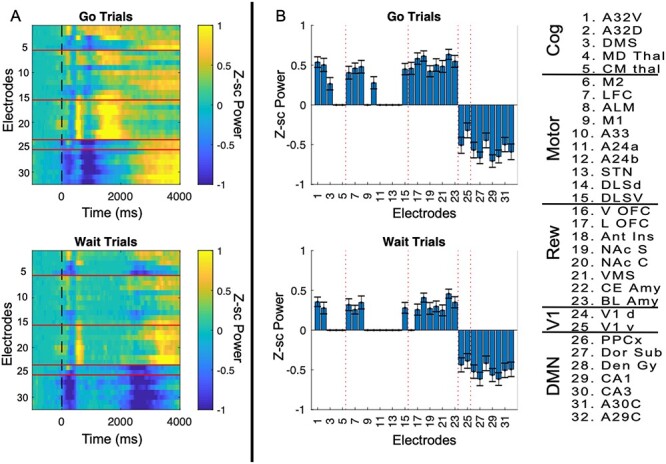
Alpha-power suppression across brain regions. (A) Average data from each electrode within alpha/low beta-frequencies (8–20 Hz) were plotted relative to trial onset for each electrode (n = 60 sessions) plotted separately for “go” and “wait” trials. We applied a one-sided t-test at each time point for each electrode (against the null hypothesis of 0) followed by an FDR-correction across 32 electrodes/200 time-points, with nonsignificant points set to 0. (B) Bar plots are displayed only for brain regions showing significant activation or suppression in in the 8–20 Hz band (Bonferroni adjusted for 32 brain regions). Mean/SEM and P-values for each electrode is displayed in Supplementary Table 1. Each electrode number (1–32) is labeled and grouped into cognitive, motor, reward, visual, or DMN functional categories. On both “go” and “wait” trials we found a significant task-related suppression of activity primarily in putative DMN brain regions.
To probe whether these putative DMN brain regions formed a functional network within the 8–20 Hz frequency band noted above, we estimated phase-lagged interactions using wPLI (Stam et al. 2007; Lau et al. 2012; Hardmeier et al. 2014; Bastos and Schoffelen 2016). In this method, the degree of phase-lag between two signals is first calculated and then further weighted by the imaginary coherence, thereby suppressing zero-phase lag interactions and minimizing small phase-lagged interactions (see methods for equation and description). This method suppresses activity that can arise due to volume-conduction artifacts and is generally considered a fairly conservative method of probing interactions (Vinck et al. 2011). We calculated the wPLI within the 8–20 Hz frequency band identified above for both go and wait trials (we used data during the first second in the task). We found that DMN brain regions show highly significant phase-lagged interactions with each other within the 8–20 Hz frequency band prior to the task (Fig. 4A, P < 0.05, FDR-corrected across 32 brain regions/200 time-points) on both trial types. This is further illustrated by the network graph (Fig. 4B, set at a 0.15 wPLI threshold). We also calculated the mean wPLI from A29 to all other brain regions (Fig. 4C, df: 59), to better illustrate the pattern of connectivity from A29C (posterior cingulate cortex) to other brain regions at the 8–20 Hz frequency band. wPLI values on go trials between A29C and other DMN brain regions ranged from 0.08 to 0.15 (d.f. = 59, all P < 1e-6, FWE-corrected for 32 brain regions) and on wait trials ranged from 0.0.07 to 0.2 (df = 59, all P < 1e-8, FWE-corrected for 32 brain regions). Full statistical results of connectivity values from A29C are in Supplementary Table 2. Finally, to statistically probe whether DMN regions showed greater “within-network” connectivity, we calculated the mean wPLI between DMN and regions from each grouping defined above (Fig. 4D, we calculated the mean wPLI from DMN regions to regions categorized as “cognitive,” “sensorimotor,” “reward,” or “visual,” and “DMN”). Connectivity within the DMN had a mean wPLI of 0.15 ± 0.01 on go trials and 0.15 ± 0.01 on wait trials in the 8–20 Hz frequency band. We next ran a repeated-measures analysis of variance (ANOVA, with regions as the main factor) to analyze whether there was significantly greater interconnectivity within the DMN compared to DMN to other brain (within the categorical groupings). The repeated-measures ANOVA was highly significant (F(4, 236) = 71, P = 1e-39 for go trials, and post hoc tests showed highly significant differences between connectivity within the DMN compared to DMN to all other networks (P < 5e-13 for all post hoc comparisons of DMN–DMN connectivity versus DMN to any other catalog of brain regions). Similar results were observed for wait trials F(4, 236) = 60, P = 3e-12 for all post hoc differences between intra DMN connectivity and connectivity between DMN and other brain networks). Thus, using an analysis of the wPLI between regions we established that DMN regions are strongly and specifically functionally interconnected at an 8–20 Hz frequency band.
Figure 4.
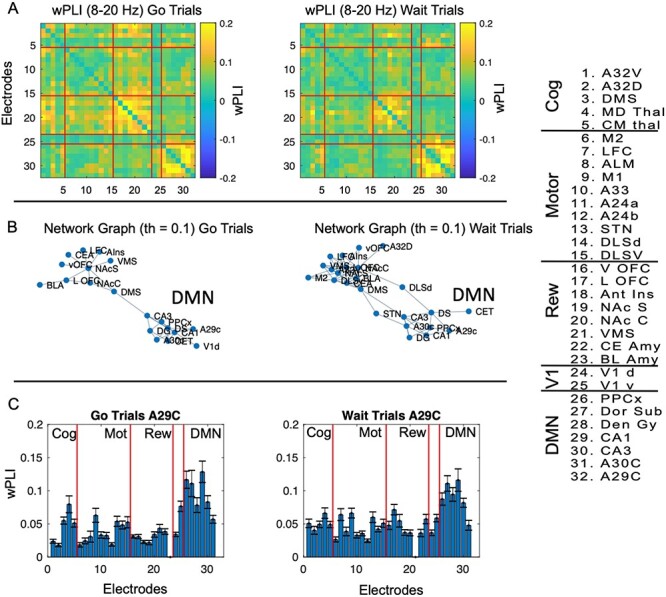
Alpha-frequency wPLI. (A) In each session we calculated the wPLI, averaged over the first second poststimulus separately for “go” and “wait” trials. We then calculated the average wPLI for each pair of electrodes across sessions (n = 60) within the 8–20 Hz frequency band over a time window of 0–1000 ms poststimulus, to generate a 32 × 32 matrix of “mean” wPLI values between regions. We applied a one-sided t-test to each pairwise value (against the null hypothesis of 0, with FDR-correction applied, FDR-adjusted P-value < 0.05). Nonsignificant pairwise relationships were set to 0 for easy visualization. We found strong evidence of inter-regional connectivity between default-mode brain regions for both trial types at alpha-frequencies. (B) We plotted the graph of connectivity matrices above (using wPLI threshold of 0.15) to illustrate the strong inter-regional relationships between DMN brain regions at alpha-frequencies for “go” and “wait” trials separately. (C) We calculated the mean wPLI for “go” and “wait trials” specifically between A29C and all other brain regions (d.f. = 59) to further illustrate the strong inter-regional wPLI from this seed to other DMN brain regions. Each electrode number (1–32) is labeled and grouped into cognitive, motor, reward, visual, or DMN functional categories. Full details of wPLI values are in Supplementary Table 2. (D) Connectivity (wPLI) values were calculated within DMN regions and from DMN to other brain regions (grouped according to panel above). wPLI was calculated in the time window noted above for the 8–20 Hz frequency band. We found that DMN regions were more strongly connected with each other compared to with regions from other networks within the 8–20 Hz band.
Prior work in humans has suggested that a lack of suppression of DMN activity is associated with interference on externally oriented cognitive tasks (Christoff et al. 2009; Bonnelle et al. 2011; Anticevic et al. 2012; Csifcsák and Mittner 2017). We hypothesized that if DMN brain regions interfered with performance, less suppression (i.e., greater activity) might be associated with worse performance. Specifically, we posited that greater DMN alpha/beta-activity would be associated with slower response times on go trials while, simultaneously, being associated with impaired waiting/faster response times on wait trials. For this analysis, we focused on the time period prior to 500 ms (when DMN suppression is greatest for both trial types). We used the LME model to analyze the relationship between power and reaction time, at the level of individual trials (Fig. 5A, 8451 wait trials, 5858 go trials). Animal was included as a factor in the LME model to account for animal-specific effects. FDR-correction was applied to the LME analysis across all electrodes, frequencies, and times. We observed that increased alpha and beta-activity in DMN brain regions (particularly posterior parietal cortex and posterior cingulate cortex, specifically A30c) was associated with worse performance across both go and wait trials (Fig. 5B,C, P < 0.05, FDR-adjusted across all electrodes, times, and frequencies). Strikingly, we found there was a significant positive relationship between alpha/low beta-activity and response time for go trials, indicating that less suppression (i.e., greater activity) was linked with slower response times and thus worse performance. By contrast, there was a significant negative relationship between activity and response time for wait trials within these frequency bands, suggesting that less suppression was associated with impaired waiting (faster reaction times). To estimate effects, we evaluated the T-stat values from the LME analysis. The max T-stat value in A29C on go trials (measured within the frequency band noted within the first 500 ms) was 8.5, P = 7e.-16; the min T-stat value for A29C on wait trials in the same time-frequency window was −7.7, P = 1e-13). The max T-stat value in posterior parietal cortex (PPC) on go trials (for alpha-frequencies in the first 500 ms) was 6, P-value = 3e-8; the min T-stat value for PPC on wait trials in the same time-frequency window was −7.6, P-value 4e-13. To show that not all brain regions follow this pattern, we also plotted the T-stat for the LME analysis for anterior insula (Fig. 5D). Anterior insula activity showed essentially the opposite relationship with behavior, with greater activity in the same time period associated with longer response times on wait trials and faster response times on go trials, consistent with a brain region in which activity was associated with improved performance on both trial types.
Figure 5.
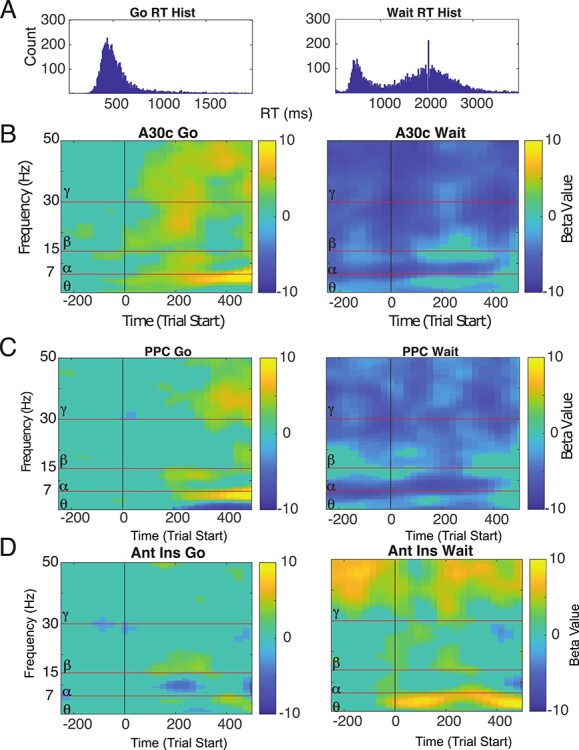
Relationship between alpha-power in DMN regions and behavior on the task. (A) The response time distribution (8451 wait trials, 5858 go trials) used for this analysis. In general, we assumed that faster response time on go trials and slower response time on wait trials were associated with better performance on this task. (B,C) We calculated the relationship between power and response time across all trials (from all animals) for all brain regions (FDR-corrected for all electrodes/frequencies/time-points). A30C and Posterior parietal cortex (PPCx) showed the strongest inverse relationship between alpha activity and behavior (nonsignificant time-points set to 0). Specifically, we found that increased power in A30C was associated with slower RT for go trials (positive relationship) and faster RT on wait trials (negative relationship), clear evidence that greater alpha-power in this brain region is linked with interference on the task. (D) Anterior insula shows the opposite relationship with behavior, with greater alpha-power associated with faster RTs on go trials (negative relationship) and greater theta/alpha-power showing slower RTs on wait trials (positive relationship). All imagesc plots were corrected using an FDR-correction (P < 0.05, across all time, frequency, and electrodes).
Prior work has suggested that alpha and low beta-frequency oscillations may be markers of DMN activity in humans (Jann et al. 2009; Knyazev et al. 2011; Capotosto et al. 2013, 2017; Mayhew et al. 2013; Mo et al. 2013). Thus, we surmised that the local field potentials observed in rodents may have a human correlate. To study this, we measured EEG activity in humans performing a similar cognitive task, with a specific focus on identifying and source-localizing desynchronization in the same frequency band as observed in rodents (8–20 Hz). Human subjects performed well on the task, with mean accuracy of 67.4 ± 2.58% on go trials and 77.41 ± 1.27% on wait trials (Fig. 6A), compared to rodent performances of 93.81 ± 1.18% and 40.70 ± 2.94%, respectively. Mean RT in humans was 0.45 ± 0.01 s and 2.25 ± 0.01 s for the two trial types compared to 0.61 ± 0.02 s and 1.69 ± 0.06 s in rodents.
Figure 6.
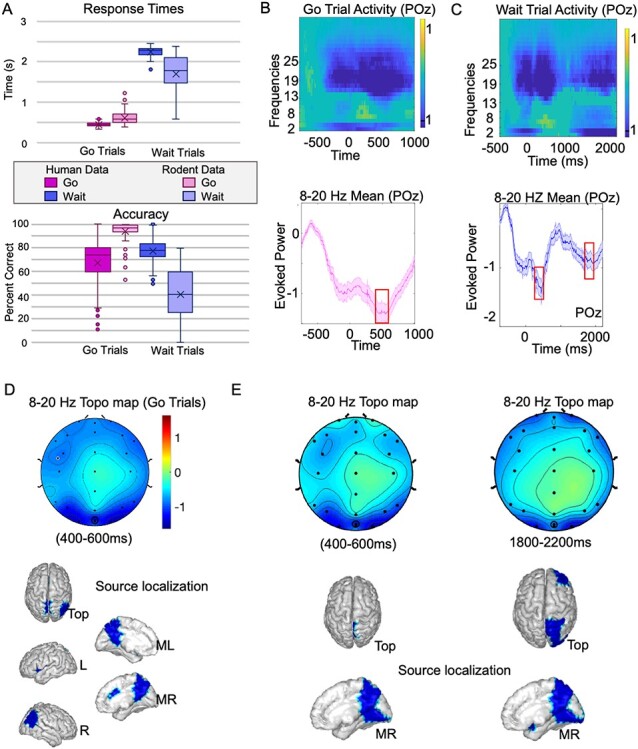
Evidence for DMN suppression of alpha-oscillations in humans. (A) Behavior for animals and humans on the task. (B) Evidence of alpha/low beta-desynchronization (i.e., suppression relative to baseline) is shown in electrode POz, the electrode nearest to posterior cingulate cortex in humans. Power within this [8–20 Hz] frequency band (baseline subtracted) shows suppression in activity during the trial, strongest between 400 and 600 ms (shaded error bar shows mean/SEM, n = 65 subjects) (C) Similar desynchronization can be observed on wait trials, though with two main peaks at 400 to 600 and 1800 to 2000 ms; note that in case of go-trials the correct-response would trigger a new trial by max 1500 ms, hence analyses > 1500 ms were not relevant to these stimuli. (D) Scalp topography (topo map) of go trials within the 8–20 Hz frequency band between 400 and 600 ms, and corresponding source localization demonstrates significant sources within this time-frequency band in DMN brain regions (particularly posterior cingulate cortex, precuneus, and right inferior parietal lobule). (E) Scalp topographies and corresponding source localizations for wait trials are shown for the dual peak suppression time windows (P < 0.05).
We first analyzed activity in the scalp electrode POz, the electrode in our montage closest to the posterior cingulate cortex. On go trials we observed a clear pattern of desynchronization in POz that overlapped with the 8–20 Hz pattern of desynchronization observed in rats (Fig. 6B, df: 64, P < 0.05, FDR-corrected). The mean power in the 8–20 Hz frequency band on go trials demonstrated a pattern of desynchronization with a peak value of −1.42 ± 0.18, (t(61) = −7.66, P < 0.0001), at 440 ms poststimulus. We calculated the scalp topography (topo map) averaged for activity in the 400–600 ms time window within the 8–20 Hz band (Fig. 6C). We then source-localized activity this activity following methods described previously (Ojeda et al. 2020; Balasubramani et al. 2021). Significant sources (P < 0.05) were localized to human DMN regions including posterior cingulate cortex, precuneus and right inferior parietal lobule. We followed a similar series of steps to examine desynchronization on wait trials within the 8–20 Hz frequency band (Fig. 6D). The POz electrode showed desynchronized activity for the duration of the wait period, with two clear peaks of desynchronization (similar to the two peaks observed in rodents). The first peak had mean power of −1.56 ± 0.22 (t(62) = −7.53, P < 0.0001) at 440 ms, and the second peak had mean power of −0.95 ± 0.13 (t(62) = −7.12, P < 0.0001) at 1940 ms. The topo maps and source-localized patterns of activity were estimated for both of these peaks (Fig. 6E), and again showed similar patterns of activity within DMN regions including precuneus and posterior cingulate cortex (P < 0.05). These data show that for both trial types, we observe similar patterns of desynchronization within the same frequency band (8–20 Hz) as observed in rodents that source-localize to DMN brain regions.
Discussion
In this manuscript we demonstrated that rodent posterior cingulate cortex (PCC) (A29C/A30C), along with parietal cortex, visual cortex, and temporal lobe structures show a suppression of oscillatory activity within alpha and low beta-frequencies during an externally oriented cognitive task. This set of brain regions comprises many of the same regions that were identified in prior fMRI studies in animals as being part of a rodent DMN (Lu et al. 2012; Hsu et al. 2016). Our data thus provide an electrophysiological correlate of DMN suppression in animals. We further show that these regions are interconnected at similar frequencies in which they show suppression, further proof of a functional network that is suppressed. We show, on a trial by trial basis, that less suppression of oscillatory activity in these brain regions is linked with worse performance on the task in rodents (slower reaction time on “go” trials and increased reaction time on “wait trials). Finally, we show task-related suppression of DMN in humans on a similar task at similar frequencies. These results support prior resting-state fMRI work suggesting that rats show homologous patterns of default-mode suppression similar to that described in humans. It is important to note that the brain regions comprising this putative DMN are quite different in rats and humans. Recent work has shown that core parts of the default-mode-network in primates have shown disproportionate expansion during recent primate evolution (Sneve et al. 2019) and show considerable variation even in humans (Reardon et al. 2018). It is thus striking that despite these differences we captured some common electrophysiological processes between species.
We found that DMN suppression was particularly strong at alpha and low-beta-frequencies in both animals and humans. A number of previous studies have suggested that posterior alpha/beta oscillations may be related to DMN function in humans. Simultaneous EEG-fMRI work have identified both alpha and beta power with DMN activity (Laufs et al. 2003; Moosmann et al. 2003; Hlinka et al. 2010; Mo et al. 2013) and during task states (Mayhew et al. 2013). Posterior alpha-oscillations have been directly linked with self-referential thinking (Moosmann et al. 2003; Knyazev et al. 2011, 2015). Moreover, both neurologic (Hsiao et al. 2013; Bonfiglio et al. 2014; Brueggen et al. 2017) and psychiatric (Clancy et al. 2020) diseases disrupt the relationship between alpha/low beta oscillations and DMN activity. Our findings are both consistent with and extend upon this prior work to observations in rodents. There has been recent, exciting work showing that lower-frequency oscillations in rodent and human posterior cingulate cortex are related to dissociated experiences that occur with dissociative anesthetics such as ketamine (Vesuna et al. 2020). It is unclear at present how this theta activity is linked with the alpha/low beta-activity we observe, an area that is ripe for further investigation.
We found several differences between the DMN suppression observed in humans and those found in animals. Although there is a general overlap in the timing of the desynchronization between animals and humans, it is not identical. These differences might arise from differences in behavior as well as slight differences in the task structure: the human task had a cue followed by the visual stimulus, while the rodent task was self-paced. Humans had a greater diversity of stimuli to respond to, and behavior itself was quite different: humans performed far better than rats on waiting less variability. Finally, while the human data were roughly balanced across gender, the rodent data were collected only in males. It is clear that further studies will need to demonstrate whether female rats also show similar alpha/beta-desynchronization in these putative DMN-like brain regions. However, it is striking that despite all of these differences, we still observed fairly similar patterns of desynchronization, localized to similar sources, across species for both go and wait trials.
There are also other limitations to our study analyzing rodent LFP. First, inaccurate electrode targeting could cause some degree of imprecision in our results. Generally, this imprecision could cause less significant patterns of activity; and a greater degree of “spatial spread” of the results. However, because we analyzed electrodes simultaneously in the same animals, it is hard to see how such imprecision would spuriously result in significant deactivation at particular brain sites; specificity of the deactivation to alpha/beta-frequencies; or significant network-level phase-lagged relationships—if anything, it would weaken most of our findings. The second major caveat is related to interpretation of local field potentials. In general, field potential around an electrode can only be understood when the structure is organized and laminar (Buzsáki and Draguhn 2004; Buzsáki et al. 2012) as occurs in hippocampus and cortex. Most of the major findings from this paper arise from cortical/hippocampal brain regions, but some electrodes reported in this study are in subcortical brain structures in which interpretation of neural oscillations are more complicated and less clear.
Having a simple and functional read-out of DMN activity (alpha/low beta-oscillations within posterior cingulate cortex) can provide a key anchor for many future mechanistic studies to better understand the physiologic basis of the DMN, genetic manipulations that can drive aberrant activity in these brain regions, and the effects of environmental or pharmacologic perturbations on DMN network activity. Pairing single unit recordings of DMN activity within A32D and A29C, along with thalamus, may provide key evidence for the relationships between alpha-oscillations and underlying brain activity within these core brain regions. Using optogenetics to specifically perturb brain circuits from one of these two brain regions, while monitoring activity in the other, can provide an opportunity to understand whether there are direct or indirect mechanisms that maintain anticorrelated activation patterns, and moreover can provide causal evidence for whether a lack of DMN suppression leads to cognitive difficulties. Calcium imaging studies can be used to better understand how activity in long-range projection neurons underlies the network-level phenomenon observed with LFP. Finally, even thpugh many studies have observed a link between DMN function and psychopathology, having a simple preparation to examine this will allow for easy investigation of how genetic, environmental, and behavioral manipulations affect this network, and whether certain medications or treatment approaches can preferentially target this brain system (Liddle et al. 2011; Tomasi et al. 2011). Thus, our findings open up opportunities upon which to develop a more detailed physiological/causal model of DMN function in a straightforward and tractable way that does not rely on fMRI.
Supplementary Material
Contributor Information
Leila Fakhraei, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Miranda Francoeur, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Pragathi P Balasubramani, Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Tianzhi Tang, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Sidharth Hulyalkar, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Nathalie Buscher, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Jyoti Mishra, Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Dhakshin S Ramanathan, Mental Health Service, VA San Diego Healthcare System., La Jolla, CA 92161, USA; Department of Psychiatry, UC San Diego, La Jolla, CA 92093, USA.
Notes
Conflict of Interest: None declared.
Funding
The Department of Veterans Affairs, Veterans Health Administration (Career Development Award: 7IK2BX003308 to D.S.R.); start-up funds from the UCSD Department of Psychiatry (to D.S.R. and J.M.); a Career Award for Medical Scientists Award from the Burroughs Welcome Fund (1 015 644 to D.S.R.); a National Alliance of Research for Schizophrenia and Depression (NARSAD) Young Investigators Award (to D.S.R.); a UCSD Institute for Neural Computation Award (to L.F.).
Author Contributions
L.F., M.F. P.B., T.T., S.H., N.B., J.M, and D.S.R. designed research. L.F., M.F., P.B., T.T., and S.H. conducted the research. L.F., M.F., and P.B. analyzed data. J.M. and D.R. wrote the paper.
References
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. 2010a. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 104.1:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010b. Functional-anatomic fractionation of the brain’s default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. 2012. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani PP, Ojeda A, Grennan G, Maric V, Le H, Alim F, Zafar-Khan M, Diaz-Delgado J, Silveira S, Ramanathan D, et al. 2021. Mapping cognitive brain functions at scale. Neuroimage. 231:117641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F, Kwan AC. 2017. Secondary motor cortex: where ‘Sensory’ meets ‘Motor’ in the rodent frontal cortex. Trends Neurosci. 40:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Schoffelen J-M. 2016. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Sys Neurosci. 9. doi: 10.3389/fnsys.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio L, Piarulli A, Olcese U, Andre P, Arrighi P, Frisoli A, Rossi B, Bergamasco M, Carboncini MC. 2014. Spectral parameters modulation and source localization of blink-related alpha and low-beta oscillations differentiate minimally conscious state from vegetative state/unresponsive wakefulness syndrome. PLoS One. 9:e93252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, Boissezon XD, Greenwood RJ, Sharp DJ. 2011. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 31:13442–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang H-L, Morales M, Shaham Y. 2012. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 32:4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggen K, Fiala C, Berger C, Ochmann S, Babiloni C, Teipel SJ. 2017. Early changes in alpha band power and DMN BOLD activity in Alzheimer’s disease: a simultaneous resting state EEG-fMRI study. Front Aging Neurosci. 9. doi: 10.3389/fnagi.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher N, Ojeda A, Francoeur M, Hulyalkar S, Claros C, Tang T, Terry A, Gupta A, Fakhraei L, Ramanathan DS. 2020. Open-source raspberry Pi-based operant box for translational behavioral testing in rodents. J Neurosci Methods. 342:108761. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science. 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. 2013. Resting-state modulation of alpha rhythms by interference with angular gyrus activity. J Cogn Neurosci. 26:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Baldassarre A, Sestieri C, Spadone S, Romani GL, Corbetta M. 2017. Task and Regions specific top-down modulation of alpha rhythms in parietal cortex. Cereb Cortex. 27:4815–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage. 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci. 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy KJ, Andrzejewski JA, Simon J, Ding M, Schmidt NB, Li W. 2020. Posttraumatic stress disorder is associated with alpha dysrhythmia across the visual cortex and the default mode network. ENeuro. 7:ENEURO.0053–ENEU20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemm von Hohenberg C, Weber-Fahr W, Lebhardt P, Ravi N, Braun U, Gass N, Becker R, Sack M, Cosa Linan A, Gerchen MF, et al. 2018. Lateral habenula perturbation reduces default-mode network connectivity in a rat model of depression. Transl Psychiatry. 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csifcsák G, Mittner M. 2017. Linking brain networks and behavioral variability to different types of mind-wandering. Proc Natl Acad Sci. 114:E6031–E6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134:9–21. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- van Duuren E, van der Plasse G, Lankelma J, Joosten RNJMA, Feenstra MGP, Pennartz CMA. 2009. Single-cell and population coding of expected reward probability in the orbitofrontal cortex of the rat. J Neurosci. 29:8965–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhraei L, Francoeur M, Balasubramani P, Tang T, Hulyalkar S, Buscher N, Claros C, Terry A, Gupta A, Xiong H, et al. 2021. Mapping large-scale networks associated with action, behavioral inhibition and impulsivity. ENeuro. 8:ENEURO.0406–ENEU20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. 2015. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 66:25–52. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz AJ. 2016. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 127:496–509. [DOI] [PubMed] [Google Scholar]
- Greenberg LM, Waldman ID. 1993. Developmental normative data on the test of variables of attention (T.O.V.A.). J Child Psychol Psychiatry. 34:1019–1030. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci. 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. 2009. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardmeier M, Hatz F, Bousleiman H, Schindler C, Stam CJ, Fuhr P. 2014. Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS One. 9:e108648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardung S, Epple R, Jäckel Z, Eriksson D, Uran C, Senn V, Gibor L, Yizhar O, Diester I. 2017. A functional gradient in the rodent prefrontal cortex supports behavioral inhibition. Curr Biol. 27:549–555. [DOI] [PubMed] [Google Scholar]
- Heeger D, Landy M. 2009. Signal detection theory. In: Goldstein B, editor. Encyclopedia of perception. SAGE Publications, pp. 887–892. https://nyuscholars.nyu.edu/en/publications/signal-detection-theory. [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. 2016. Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry. 80:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz R, Peeters LM, Shah D, Missault S, Belloy M, Vanreusel V, Malekzadeh M, Verhoye M, Van der Linden A, Keliris GA. 2019. Bottom-up sensory processing can induce negative BOLD responses and reduce functional connectivity in nodes of the default mode-like network in rats. Neuroimage. 197:167–176. [DOI] [PubMed] [Google Scholar]
- Hlinka J, Alexakis C, Diukova A, Liddle PF, Auer DP. 2010. Slow EEG pattern predicts reduced intrinsic functional connectivity in the default mode network: an inter-subject analysis. Neuroimage. 53:239–246. [DOI] [PubMed] [Google Scholar]
- Hsiao F-J, Wang Y-J, Yan S-H, Chen W-T, Lin Y-Y. 2013. Altered oscillation and synchronization of default-mode network activity in mild Alzheimer’s disease compared to mild cognitive impairment: an electrophysiological study. PLoS One. 8:e68792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L-M, Liang X, Gu H, Brynildsen JK, Stark JA, Ash JA, Lin C-P, Lu H, Rapp PR, Stein EA, et al. 2016. Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci. 113:E4541–E4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A. 2017. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 37:10529–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K, Dierks T, Boesch C, Kottlow M, Strik W, Koenig T. 2009. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage. 45:903–916. [DOI] [PubMed] [Google Scholar]
- Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, Kampff AR, Ölveczky BP. 2015. Motor cortex is required for learning but not for executing a motor skill. Neuron. 86:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, et al. 2009. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 30:3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV, Pylkova LV. 2011. The default mode network and EEG alpha oscillations: an independent component analysis. Brain Res. 1402:67–79. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Savostyanov AN, Bocharov AV, Dorosheva EA, Tamozhnikov SS, Saprigyn AE. 2015. Oscillatory correlates of autobiographical memory. Int J Psychophysiol. 95:322–332. [DOI] [PubMed] [Google Scholar]
- Koerner TK, Zhang Y. 2017. Application of linear mixed-effects models in human neuroscience research: a comparison with Pearson correlation in two auditory electrophysiology studies. Brain Sci. 7(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau TM, Gwin JT, McDowell KG, Ferris DP. 2012. Weighted phase lag index stability as an artifact resistant measure to detect cognitive EEG activity during locomotion. J NeuroEng Rehabil. 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR. 2018. What, if anything, is rodent prefrontal cortex? ENeuro. 5:ENEURO.0315–ENEU18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. 2003. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Nat Acad Sci USA. 100:11053–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke SM, Ramanathan DS, Guo L, Won SJ, Ganguly K. 2019. Emergent modular neural control drives coordinated motor actions. Nat Neurosci. 22:1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, Liddle PF. 2011. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 52:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. 2012. Rat brains also have a default mode network. Proc Natl Acad Sci. 109:3979–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew SD, Ostwald D, Porcaro C, Bagshaw AP. 2013. Spontaneous EEG alpha oscillation interacts with positive and negative BOLD responses in the visual–auditory cortices and default-mode network. Neuroimage. 76:362–372. [DOI] [PubMed] [Google Scholar]
- Mo J, Liu Y, Huang H, Ding M. 2013. Coupling between visual alpha oscillations and default mode activity. Neuroimage. 68:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. 2003. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 20:145–158. [DOI] [PubMed] [Google Scholar]
- Ojeda A, Bigdely-Shamlo N, Makeig S. 2014. MoBILAB: an open source toolbox for analysis and visualization of mobile brain/body imaging data. Front Hum Neurosci. 8. doi: 10.3389/fnhum.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda A, Kreutz-Delgado K, Mullen T. 2018. Fast and robust block-sparse Bayesian learning for EEG source imaging. Neuroimage. 174:449–462. [DOI] [PubMed] [Google Scholar]
- Ojeda A, Kreutz-Delgado K, Mishra J. 2021. Bridging M/EEG Source Imaging and Independent Component Analysis frameworks using biologically-inspired sparsity priors. Neural Computation. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda A, Buscher N, Balasubramani P, Maric V, Ramanathan D, Mishra J. 2020. SimBSI: an open-source Simulink library for developing closed-loop brain signal interfaces in animals and humans. Biomed Phys Eng Express. 6:035023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. 1994. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 18:49–65. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Da Silva FL. 2011. EEG-Event-Related Desynchronization (ERD) and Event-Related Synchronization. In Electroencephalography-Basic Principles, Clinical Applications and Related Fields. (pp. 935-948). Kluwer/Lippincott Williams & Wilkins. [Google Scholar]
- Raichle ME. 2015. The Brain’s default mode network. Annu Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci. 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan DS, Guo L, Gulati T, Davidson G, Hishinuma AK, Won S-J, Knight RT, Chang EF, Swanson RA, Ganguly K. 2018. Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nat Med. 24:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon PK, Seidlitz J, Vandekar S, Liu S, Patel R, Park MTM, Alexander-Bloch A, Clasen LS, Blumenthal JD, Lalonde FM, et al. 2018. Normative brain size variation and brain shape diversity in humans. Science. 360:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A. 2017. Mapping the connectional architecture of the rodent brain with fMRI. In: Tucci V, editor. Handbook of neurobehavioral genetics and phenotyping. John Wiley & Sons, Inc., pp. 527–551. [Google Scholar]
- Schwarz AJ, Gass N, Sartorius A, Risterucci C, Spedding M, Schenker E, Meyer-Lindenberg A, Weber-Fahr W. 2013. Anti-correlated cortical networks of intrinsic connectivity in the rat brain. Brain Connect. 3(5):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. 2009. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. 1997. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 9:648–663. [DOI] [PubMed] [Google Scholar]
- Sneve MH, Grydeland H, Rosa MGP, Paus T, Chaplin T, Walhovd K, Fjell AM. 2019. High-expanding regions in primate cortical brain evolution support supramodal cognitive flexibility. Cereb Cortex. 29:3891–3901. [DOI] [PubMed] [Google Scholar]
- Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, Lahvis GP, Lattal KM, Mitchell SH, David SV, et al. 2014. Large-scale topology and the default mode network in the mouse connectome. Proc Natl Acad Sci. 111:18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, Daffertshofer A. 2007. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 28:1178–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. 2011. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 54:3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, et al. 2008. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 169:249–254. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Chandran P, Miller L, Lee Y, Marek GJ, Sakoglu U, Chin C-L, Luo F, Fox GB, et al. 2011. Default-mode-like network activation in awake rodents. PLoS One. 6:e27839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna S, Kauvar IV, Richman E, Gore F, Oskotsky T, Sava-Segal C, Luo L, Malenka RC, Henderson JM, Nuyujukian P, et al. 2020. Deep posteromedial cortical rhythm in dissociation. Nature. 586:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veuthey TL, Derosier K, Kondapavulur S, Ganguly K. 2020. Single-trial cross-area neural population dynamics during long-term skill learning. Nat Commun. 11:4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA. 2011. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 55:1548–1565. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Paxinos G. 2014. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct. 219:185–192. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 8:49–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


