Significance
The growth of coral reefs is threatened by the dual stressors of ocean warming and acidification. Despite a wealth of studies assessing the impacts of climate change on individual taxa, projections of their impacts on coral reef net carbonate production are limited. By projecting impacts across 233 different locations, we demonstrate that the majority of coral reefs will be unable to maintain positive net carbonate production globally by the year 2100 under representative concentration pathways RCP4.5 and 8.5, while even under RCP2.6, coral reefs will suffer reduced accretion rates. Our results provide quantitative projections of how different climate change stressors will influence whole ecosystem carbonate production across coral reefs in all major ocean basins.
Keywords: carbonate production, climate change, corals, calcification
Abstract
Ocean warming and acidification threaten the future growth of coral reefs. This is because the calcifying coral reef taxa that construct the calcium carbonate frameworks and cement the reef together are highly sensitive to ocean warming and acidification. However, the global-scale effects of ocean warming and acidification on rates of coral reef net carbonate production remain poorly constrained despite a wealth of studies assessing their effects on the calcification of individual organisms. Here, we present global estimates of projected future changes in coral reef net carbonate production under ocean warming and acidification. We apply a meta-analysis of responses of coral reef taxa calcification and bioerosion rates to predicted changes in coral cover driven by climate change to estimate the net carbonate production rates of 183 reefs worldwide by 2050 and 2100. We forecast mean global reef net carbonate production under representative concentration pathways (RCP) 2.6, 4.5, and 8.5 will decline by 76, 149, and 156%, respectively, by 2100. While 63% of reefs are projected to continue to accrete by 2100 under RCP2.6, 94% will be eroding by 2050 under RCP8.5, and no reefs will continue to accrete at rates matching projected sea level rise under RCP4.5 or 8.5 by 2100. Projected reduced coral cover due to bleaching events predominately drives these declines rather than the direct physiological impacts of ocean warming and acidification on calcification or bioerosion. Presently degraded reefs were also more sensitive in our analysis. These findings highlight the low likelihood that the world’s coral reefs will maintain their functional roles without near-term stabilization of atmospheric CO2 emissions.
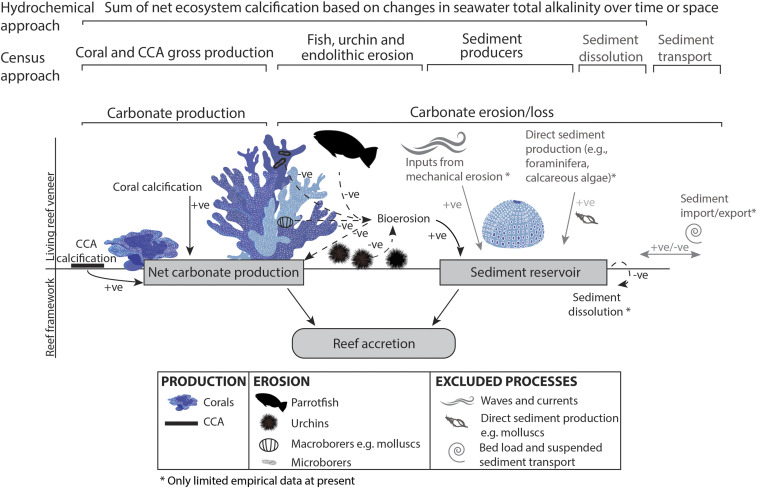
Coral reef ecosystems provide a habitat for a vast array of biodiversity (1, 2), yield billions of dollars of global revenue from fisheries and tourism (3, 4), and protect tropical shorelines from hazards such as storms (5). These functions are dependent on the maintenance of the framework structure of the reefs, the accumulation of which requires the net production of calcium carbonate by resident taxa. This net calcium carbonate production is a balance between gross production minus the loss due to physical, chemical, and biological erosion. However, the net calcium carbonate production and related potential vertical accretion of reefs is increasingly threatened by anthropogenic climate change (5). Vertical reef accretion is the product of a number of processes that include 1) biological net calcium carbonate production (gross production by calcifying taxa minus bioerosion), 2) net sediment production (gross production minus endolithic and pore water–driven dissolution), 3) sediment transport (import and export), 4) physical erosion, and 5) cementation rates (Fig. 1). We refer to this potential vertical accretion of reefs simply as “accretion” hereafter, and note that we focus on sediment dissolution and biological net carbonate production (hereafter referred to as “net carbonate production,” the measurement of which is referred to as “carbonate budgets”), perhaps the best quantified and largest contributors to accretion rates on reefs on short timescales.
Fig. 1.
Processes involved in net carbonate production and accretion on reefs as well as the associated methods typically employed to measure this. +ve = positive contribution to accretion with solid lines; −ve = negative contribution with dashed lines. Processes in gray are not included in most carbonate budgets or here. Here, we project the effects of ocean acidification and warming on CCA and coral calcification, chemical components of bioerosion, and sediment dissolution. Only chemical components of bioerosion are included in hydrochemical measurements, while direct sediment production by bioeroders is also included here.
Climate change will impact both the abundance and calcification rates of reef taxa responsible for producing calcium carbonate, such as corals and coralline algae (2, 6, 7), while simultaneously altering the bioerosion and recycling of this calcium carbonate by resident bioeroders, such as sponges and cyanobacteria (8, 9). Both net carbonate production and accretion are already declining regionally in response to fishing pressure, disease, and marine heatwaves (10–13). Such changes have profound implications for societally relevant ecosystem service provisioning (11), and rapid climate change impacts are projected to further exacerbate these negative trajectories. Specifically, ocean warming and associated marine heatwaves will reduce gross carbonate production rates on coral reefs, as coral cover is reduced by more frequent and severe mass bleaching events (14–16) and as elevated temperatures decrease the calcification rates of coral and coralline algae under more severe warming scenarios (6, 17). Ocean acidification is also projected to reduce the calcification rates of key taxa such as corals and coralline algae that form reef structures and associated sediments (6, 7, 18, 19) while further reducing accretion by increasing the dissolution of carbonate sediments (20) and enhancing rates of bioerosion (8, 9). Furthermore, the combined impacts of ocean warming and acidification are predicted to be amplified under higher CO2 emission scenarios (6, 19).
While the responses of reef-forming taxa to ocean warming and acidification have been the focus of considerable scientific effort in recent decades (2, 6, 7), quantitative predictions of the impacts of climate change on global coral reef net carbonate production and reef accretion are limited. Specifically, existing projections are largely theoretical, limited to specific locations, only include sea level rise and not ocean acidification or warming, or do not include some of the major processes controlling coral reef net carbonate production (5, 10, 20–23). For example, one prominent model provided important data on lagoon sediment dissolution rates (20), although the link between changes in these rates and forereef accretion is unclear. Other global-scale projections do not include the impacts of ocean warming or acidification (5). How the combined effects of changes in the mortality, calcification, and bioerosion rates of individual reef taxa will manifest spatially across different ocean basins due to ocean warming and acidification remains unresolved.
Predicting the trajectories of future net carbonate production is complicated by uncertainties around the magnitude of future declines in coral cover, which is likely to be one of the major drivers of future carbonate budgets of coral reefs; yet, estimating future coral cover is difficult. While coral cover is declining globally due to repeated mass coral bleaching (hereafter referred to as “bleaching”) and other local stressors, there is clear temporal and spatial variability of local anthropogenic impacts (16, 24–26). This makes estimating future coral cover a complex and heavily debated process, even on local scales (27–29). The impacts of marine heatwaves on coral mortality, recovery, and subsequent recruitment cannot be captured accurately in short-term laboratory experiments in the way that changes to calcification can, and currently available projections of coral cover into the future are encumbered with uncertainties that do not allow us to predict exact future cover for any specific region (16, 30, 31).
Here, we resolve these challenges of applying laboratory results to real coral reef locations by assessing changes in future carbonate budgets of reefs as a function of integrated robust estimates of the responses of major components of the carbonate budget to climate change as well as including estimates of changes in future coral cover. We collate or measure data from 233 locations on 183 distinct reefs globally (49% Atlantic, 39% in the Indian, and 11% in the Pacific Ocean) to quantify the impacts of ocean warming and acidification on coral reef net carbonate production and then use these data to estimate the impacts on net carbonate production and accretion by 2050 and 2100. We incorporate more than 800 empirically measured changes in net calcification rates of the main producers of calcium carbonate on coral reefs (corals and coralline algae), bioerosion rates, and sediment dissolution in response to ocean warming, acidification, and their interaction from 98 studies. We model the size of the effects of ocean acidification, ocean warming, and their interaction under contrasting Intergovernmental Panel on Climate Change emissions scenarios for representative concentration pathways (RCP) 2.6, 4.5, and 8.5 for the year 2050 and 2100. We then apply these estimated effects to reefs with previously measured rates of net carbonate production, where the cover of corals and coralline algae is well defined (SI Appendix, Table S1). Importantly, we account for the impact of reduced coral cover, which, in most locations, will be further diminished by more severe and frequent bleaching events (16, 25), including estimates of its impacts based on currently available information. We calculate region-specific projections of degree heating weeks (DHW), a commonly used metric that accounts for the severity and duration of marine heatwaves on corals (32) and combine them with reductions in coral cover that were measured after exposure to differing DHWs during the 2016 El Niño event (25). These models (Materials and Methods) are then used to explore the effects of ocean warming and acidification independently, and in interaction with each other, under each climatic scenario on rates of reef net carbonate production and accretion.
Results and Discussion
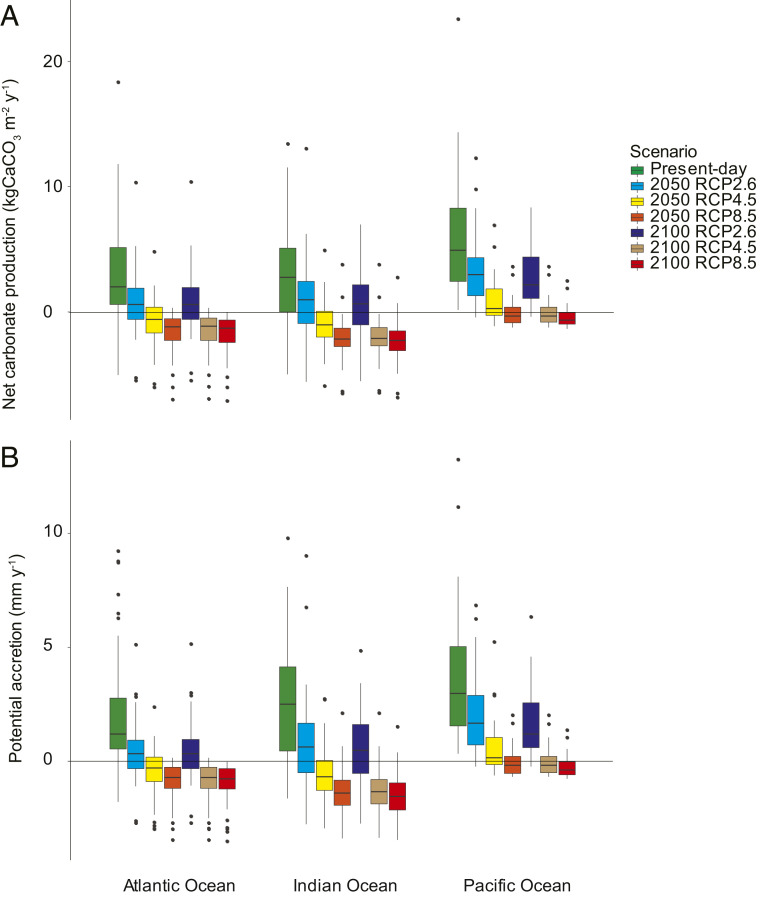
Net carbonate production in every coral reef at every site included in our analysis was reduced by each of the projected scenarios, with the extent of declines being dependent on scenario and location (Figs. 2–4 and SI Appendix, Figs. S1–S3). We project declines in net carbonate production so severe that reef accretion will cease globally by 2100 under RCP4.5 and 8.5 (Fig. 2) even under our optimistic estimates that do not factor for physical erosion. Even under RCP2.6, we project mean declines in global net carbonate production of 71% by 2050 and 77% by 2100. These declines are largely the result of reduced coral cover from bleaching events rather than from the direct impacts of ocean warming or acidification on calcification or bioerosion (SI Appendix, Figs. S1–S4). We further predict that median reef net carbonate production will switch from production to erosion under higher emissions scenarios. Specifically, we project 119% mean declines in global net carbonate production by 2050 and 148% by 2100 under RCP4.5, while we estimate declines in net carbonate production of 149% by 2050 and 155% by 2100 under RCP8.5.
Fig. 2.
(A) Net carbonate production rates (kg CaCO3 m−2 ⋅ y−1) and (B) potential vertical accretion rates (mm ⋅ y−1), presently and under the interactive effects of ocean acidification and ocean warming. These data account for reduced future coral cover due to mass bleaching events across three ocean basins for the mean of each of 183 reefs. Scenarios shown are three RCP scenarios (2.6, 4.5, and 8.5) by 2050 and 2100. Medians, 75% quartiles, 95% whiskers, and outliers are presented. See SI Appendix, Figs. S1 and S3 for the accretion and carbonate production rates projected under each stressor singularly. For accretion without sediment dissolution, see SI Appendix, Fig. S2.
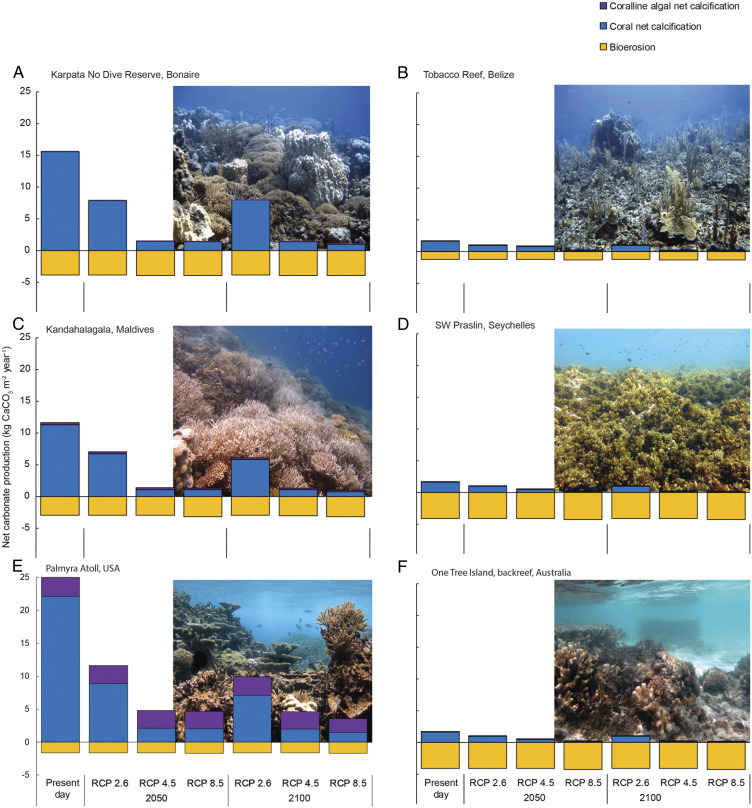
Fig. 4.
Examples of how the effects of ocean acidification, ocean warming, and mass coral bleaching are projected to impact net carbonate production through changes in bioerosion and net calcification of corals and of coralline algae. Displayed here are regions in the Atlantic (A and B), Indian (C and D), and Pacific Oceans (E and F) with high (A, C, and E) and low (B, D, and F) present-day net carbonate production. Scenarios are the same as in Fig. 1: present-day and RCP2.6, 4.5, and 8.5 in 2050 and 2100. Photo credits: A, B, and C were taken by Chris Perry; D was taken by Nicholas Graham; E was taken by Gareth Williams; and F was taken by Christopher Cornwall.
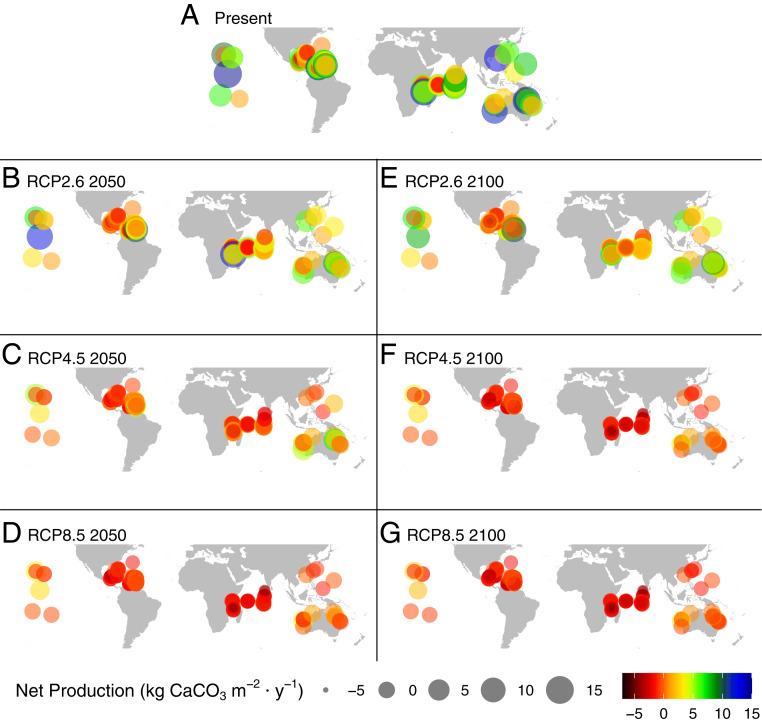
Fig. 3.
Location of study regions and their net carbonate production (kg CaCO3 m−2 ⋅ y−1) under the following scenarios: (A) present-day and projections of the interactive effects of ocean acidification, warming, and mass coral bleaching by 2050 at (B) RCP2.6, (C) RCP4.5, and (D) RCP8.5 and by 2100 under (E) RCP2.6, (F) RCP4.5, and (G) RCP8.5 occurring at each of 183 reefs. Present-day Palmyra reef is higher than 15 kg CaCO3 m−2 ⋅ y−1.
We project much lower decreases in net carbonate production when the impacts of coral bleaching are excluded. For example, we estimate net carbonate production will only be reduced 4% by 2050 and 3% by 2100 under RCP2.6. If CO2 emissions are kept to within RCP2.6, our meta-analysis of past experimental work forecasts relatively small declines (1 to 6%) in individual coral and coralline algal calcification (Table 1) and only small changes in bioerosion rates (4% declines to 6% increases). However, we estimate 15% declines in net carbonate production by 2100 under RCP4.5, while under RCP8.5, we estimate 58% declines in global net carbonate production by 2100. The scenarios without coral beaching would only manifest if coral thermal tolerances increased dramatically to the point where they are no longer impacted by coral bleaching events, which is a highly unlikely scenario. Nevertheless, these scenarios demonstrate the impacts that ocean warming and acidification will have on metabolic rates of remaining thermally tolerant corals and coralline algae. The declines in net carbonate production caused solely by the impacts of climate change on the processes of calcification and bioerosion could be construed as being relatively small (e.g., SI Appendix, Figs. S1 and S4). However, there is considerable natural variability in net carbonate production presently, and there are many heavily degraded reefs that already have low net carbonate production (33). To put these declines in net carbonate production rates into perspective, mean global declines in net carbonate production (e.g., SI Appendix, Fig. S4) predicted under RCP8.5 by ocean acidification alone outweighs the present-day net carbonate production rates for 31% of these reefs.
Table 1.
Mean percentage change on individual components of the carbonate budget by 2050 and 2100 relative to today, caused by ocean warming, acidification and their interactive effects. Values were calculated using multiple linear regressions of responses measured in the laboratory against region-specific increases in temperature, and decreases in pH. See Table S1 for a full study list. The 100% increase in sediment dissolution indicates full removal of all sediment within those scenarios
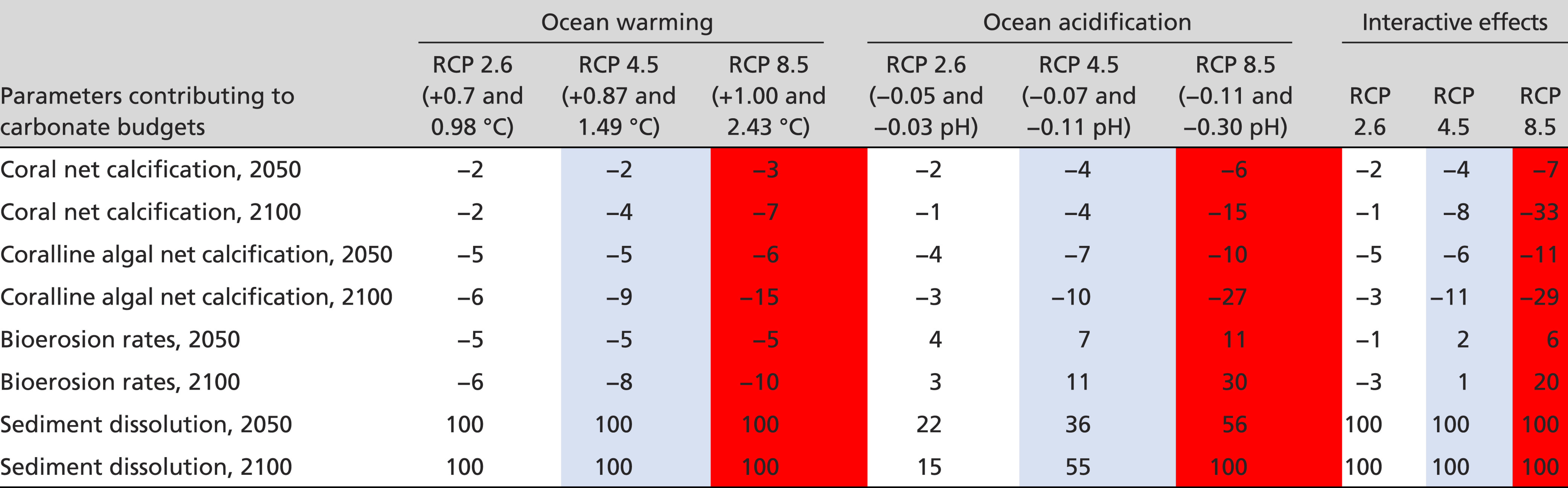 |
Values were calculated using multiple linear regressions of responses measured in the laboratory against region-specific increases in temperature and decreases in pH. See SI Appendix, Table S1 for a full study list. The 100% increase in sediment dissolution indicates full removal of all sediment within those scenarios. Different colors represent the different RCP scenarios (white 2.6, light blue 4.5, red 8.5).
We project that many presently degraded reefs will logically continue to have low levels of net carbonate production in the future (see examples in Fig. 4 B, D, and F) but that reefs with higher present-day production rates will follow trajectories that are largely related to three factors. These factors are 1) their present-day net carbonate production rates; 2) the biotic composition of the reef presently, where reefs with higher bioerosion fare worse and those with higher coralline algal carbonate production fare slightly better under scenarios with reduced coral cover; and 3) their geographic location, as projections of ocean warming and the associated timing and magnitude of loss of coral cover due to bleaching events varies by region (SI Appendix, Fig. S5–6). Reefs in the Pacific Ocean tend to fare better than others under most future scenarios, and those in the Atlantic fare the worst under RCP2.6 scenarios (Figs. 2–4). This is reflective of the generally higher present-day rates of net carbonate production in the Pacific Ocean and lower rates in the more heavily degraded coral reefs in the Atlantic Ocean (34, 35). Additionally, higher bioerosion rates in the Indian Ocean reefs (mean 2.90 kg CaCO3 m−2 ⋅ y−1 compared to 1.93 kg CaCO3 m−2 in the Atlantic Ocean and 1.52 kg CaCO3 m−2 in the Pacific Ocean) and higher contributions of coralline algal carbonate production in Pacific Ocean reefs (mean gross production = 1.08 kg CaCO3 m−2 ⋅ y−1 compared to 0.06 kg CaCO3 m−2 in the Atlantic and 0.31 kg CaCO3 m−2 in the Indian Oceans) alter the trajectories of these reefs.
The contribution of corals to gross carbonate production and its trajectory under climate change plays the largest role in dictating net carbonate production and accretion rates on reefs. Present-day rates of bioerosion are also important in determining net carbonate production under RCP8.5 once coral cover is severely reduced in our model (R2 = 0.85). However, our estimated changes in total bioerosion rates in situ are relatively small. This is because parrotfish and sea urchins—which erode mainly by biophysical means—contribute the greatest to total bioerosion at most of our sites (mean: 1.74 kg CaCO3 m−2 ⋅ y−1) as opposed to micro- or macrobioeroders living in/on the carbonate framework (mean 0.65 kg CaCO3 m−2 ⋅ y−1), which also can bioerode by chemical means. Coralline algal gross carbonate production plays a limited role on many reefs (mean gross production = 0.28 kg CaCO3 m−2 ⋅ y−1). However, our data illustrate that Pacific Ocean reefs with higher coralline algal carbonate production rates are more robust under scenarios where coral gross production is reduced by bleaching, but these reefs are comparatively more susceptible to the ocean acidification scenarios. This is due to the larger adverse effects of ocean acidification on the net calcification rates of coralline algae compared to corals (up to 12% in 2100 under RCP 8.5; Table 1). Coralline algae appear more robust to the impacts of marine heatwaves than corals (17), and thus we did not decrease their abundance here under ocean warming scenarios. All reefs with positive net carbonate production under RCP8.5 by 2100 had present-day coralline algal net carbonate production rates ≥1.8 kg CaCO3 m−2 ⋅ y−1. Together, this indicates that coralline algal calcification could initially act as a short-term substitute to provide carbonate in reefs heavily influenced by mass coral bleaching. However, coralline algal–dominated reefs will offer very different (or reduced) ecological services and structural complexity compared to coral-dominated reefs (11), and their capacity to produce carbonate will also be limited once ocean acidification intensifies. Conversely, their ability to support parrotfish and sea urchin bioeroders will also be reduced (36–38), which could therefore support slightly higher net carbonate production than equivalent coral-dominated reefs with equal gross carbonate production (33).
A major consequence of declining net carbonate production rates on reefs relates to their capacity to accrete at the same rate as rising sea levels. Median global coral reef accretion potential is estimated as 2.80 mm ⋅ y−1 in our present-day scenario (range: −1.77 to 13.20), but we project this will fall to −1.11 mm ⋅ y−1 (−3.51 to 1.51) under the interactive effects of coral bleaching, ocean warming, and acidification in RCP8.5. Global sea level rises of up to 15 mm ⋅ y−1 (range: 10 to 15) are projected by 2100 under RCP8.5 (39). While rates vary between regions, no reefs here maintain accretion rates that will match the projected global mean rates of sea level rise. However, rates of sea level rise are much lower under RCP2.6 (mean: 4 mm ⋅ y−1) (39), and, here, accretion rates only drop to 0.47 mm ⋅ y−1 (−2.75 to 6.31) (Fig. 2B). However, only four reefs still maintain rates of accretion that match mean increases in sea level rise by 2100 under RCP2.6. These aforementioned accretion rates assume sediment dissolution rates on reefs will equate to those measured on lagoon sediments (20). However, the link between the two processes is currently unknown. If sediment dissolution rates measured in lagoons are not accounted for here, mean accretion rates are likely to decline less in all future scenarios: −0.74 and 0.89 mm ⋅ y−1 under RCP8.5 and 2.6, respectively (SI Appendix, Fig. S2). While previous global-scale assessments have demonstrated the likelihood of lagoon sediments becoming sites of net sediment dissolution under future ocean acidification scenarios (20), we demonstrate here that this negative trajectory is likely to extend to whole reef–scale net carbonate production and that most reefs will likely suffer net erosion by 2100 under business-as-usual scenarios. However, it is possible that some shallower reefs may actually benefit from rising sea levels, with increasing accommodation space allowing for increased coral vertical growth (40). Indeed, this has been observed in the past (41) during much slower rates of sea level rise. It is unknown whether these possible gains in accretion will outweigh losses due to ocean warming and acidification, but determining when and where this could occur should be an urgent focus for future research.
We note that the capacity for reef-building taxa to gain tolerance to marine heatwaves, and ongoing ocean warming and acidification over the coming decades, is largely unknown (30, 31, 42–45). The trajectories of reef accretion projected here will be highly sensitive to changes in coral community thermal tolerance. The fast rate of environmental change relative to the time required for adaptation suggests it will be difficult for corals to maintain their current role, especially those with longer generation times. The only remaining corals after repeated mass bleaching events could be heat-sensitive species or phenotypes (30). However, it is unlikely that these heat-tolerant corals would maintain similar rates of gross carbonate production and cover as the current assemblages. If so, these possibilities represent the only real avenues for future reef persistence and slowing of rates of reef surface submergence for the majority of reefs under RCP4.5 and 8.5 scenarios as coral cover continues to decline under these emissions scenarios. In an analysis such as this, it is also not feasible to include other moderating effects, such as the effects of pH/temperature variability, light, nutrients, and water velocity in modifying responses at a site level (46–49). These could further modify trajectories of individual reefs. However, on a global scale, we would assume that the individual studies included here would encompass a range of environmental conditions, both the estimates of net carbonate production and the effects of climate change stressors on key ecological processes across sites.
Our results indicate that the net carbonate production and accretion of most the world’s coral reefs will be fundamentally reduced by ongoing climate change. Increasingly negative impacts are associated with higher levels of emissions and environmental change, and thus the two most contrasting futures for coral reef carbonate production are highlighted by this analysis: one in which the RCP2.6 stabilization scenario is achieved and the second in which emissions continue to rise under conditions similar to those predicted under RCP4.5 through to 8.5. Under RCP2.6, most coral reefs could maintain positive net carbonate production, with a small subset even having accretion rates that match sea level rise. If rapid action is taken to reduce CO2 emissions, there is a higher potential for coral reefs to maintain their many key functional roles in the future (50). In alternate scenarios where emissions are not curbed sufficiently, almost all coral reefs will suffer losses in net carbonate production so severe that it will halt their capacity to accrete vertically and no reefs will match sea level rise (given the caveats above). This will progressively limit their capacity to provide important services such as habitat for reef-associated taxa, protection of shorelines from wave action, and serving as centers of tourism and fisheries. Our projections here are likely optimistic given that we do not account for increasing storm frequency, which could further remove reef framework via physical erosion, nor do we include some other factors that reduce coral cover or calcification rates, such as disease, pollution, and frequent outbreaks of Crown of Thorns. Given the increased risk of globally declining coral cover and the mean global net decline in carbonate production predicted under current emissions trajectories, we must now markedly reduce CO2 emissions to have any possibility of sustaining positive carbonate production and reef accretion rates, thus maintaining the critical ecological and societal services that reefs provide.
Materials and Methods
Coral Reef Taxa Responses to Climate Change Scenarios.
We used data from published sources to calculate changes in calcification rates of different coral reef calcifying taxa to different climate change scenarios. We searched Web of Science with different combinations of “coral reef taxa” and “climate change stressor” terms. The different taxa terms were coralline algae, calcifying algae, crustose coralline algal (CCA), and coral. The different climate change stressor terms were warming, temperature, acidification, CO2, and climate change. The list of suitable publications was then cross-checked against the database on Pangaea, the list used in Kroeker et al. (7), and from Kornder et al. (6). This search was completed on February 24, 2017. We extracted direct values for calcification rates whenever they were listed when data were not deposited freely online. The remaining data were extracted from figures within publications using the software Datathief (https://www.datathief.org/). We only used data from tropical specimens. This resulted in 985 suitable calcification responses from 98 studies (SI Appendix, Table S1 and Fig. S7). This includes 58 studies examining the impacts of ocean acidification on coral calcification, 27 on ocean warming and corals, 16 on the interactive effects on coral, 13 on ocean acidification and coralline algal calcification, 6 on ocean warming and coralline algal calcification, and 5 on the interactive effects on coralline algal calcification. Some studies examined corals and coralline algae simultaneously. We do not include Halimeda spp. in our model because of the unclear nature of its eventual contribution to reef accretion and because of the larger uncertainty regarding the impacts of ocean acidification and warming on its calcification that was observed during our surveys of the literature.
Seawater pH from ocean acidification research and temperature data from ocean warming research were extracted or calculated along with net calcification measurements. Seawater carbonate chemistry was recalculated in some studies where inconsistencies were found between pH scales. Studies using the National Bureau of Standards scale were converted to the total scale (hereafter "pH") using the excel macro CO2sys (51) and method following ref. 52. Research examining the effects of ocean acidification were excluded if they did not present standardized measurements of seawater carbonate chemistry that could allow us to accurately determine pH on the total scale (53). In order to estimate the future responses of calcifying coral reef taxa to ocean acidification and warming, we employed a regression style approach similar to that used by Chan and Connolly (54) and by Cornwall and Eddy (55). Current experimental evidence supports a complex control of both dissolved inorganic carbon (DIC) and H+ concentrations in seawater on coral calcifying fluid chemistry rather than saturation state that is correlated with the ratio of DIC:H+ (56). We would also moderate results by the background total alkalinity at each site ideally, though this data are not available at the scale we would need to make accurate projections of its impacts on organism responses. However, pH is the primary driver of declining calcification under OA and therefore should well represent the responses to OA in the majority of regions (6, 56). For each study, the measured calcification rates for each species were linearly regressed against the temperature and pH values used in control and experimental treatments. We used linear regressions because the bulk of responses were linear; only two were not linear. We used the calculated linear regression slope and intercept of each study to estimate the calcification response at today’s pH in each region at pH projected under future scenarios at RCP2.6, 4.5, and 8.5 for the years 2050 and 2100. A weight corresponding to the relative SD of the reported responses was given to each study. We then calculated the mean weighted proportional changes in calcification of the different taxa between the present values and the six future scenarios (mean responses represented in Table 1). We acknowledge that the majority of research investigating the effects of ocean acidification and warming has been conducted in the laboratory, though its negative effects have also been observed in the field or in flumes exposed to the same seawater pH variability, light, and nutrients as the collection sites (23, 57).
Monthly data for the following variables were obtained from fully coupled models in the Coupled Model Intercomparison Project 5 (https://esgf-node.llnl.gov/projects/cmip5/) for all four RCP experiments [Moss et al. (58)]): sea surface temperature, surface pressure of CO2, and pH. If multiple model runs were available, they were averaged before creating a multi-model mean. All modeled data were remapped to a 1 × 1° resolution grid, and missing data were filled in the zonal direction using the National Center for Atmospheric Research Command Language’s (https://www.ncl.ucar.edu/) Poisson grid fill. The methods and models used to calculate DHWs can be found in Van Hooidonk et al. (59). The models used for the pH projections can be found in the supplementary materials of ref. 59.
We did not give a specific weight for each study and did not account for potential phylogenic relatedness because we did not want to overestimate the contribution of rare species, but rather considered that the most studied species are usually the most representative of reef diversity. Traditional log response ratio tests cannot easily be used to project quantitative changes in organism responses for a given difference in pH or pCO2 between controls and treatments. These do indicate whether the impacts are significantly different to zero or whether increasing pCO2 or temperature have larger effect sizes. This is the case for both pCO2 and temperature effects on coral and coralline algal calcification in our data set. The impacts of ocean acidification on coralline algae (ΔpCO2: P = 0.012) and corals (ΔpCO2: P < 0.001) and the effects of ocean warming on coralline algae (Δ°C from summer maximum: P < 0.001) and corals (Δ°C from summer maximum: P < 0.001) are all significant.
We also estimated the calcification rates at today’s temperature (i.e., 0 °C anomaly from that used in designated controls, unless stated otherwise in the study) and at all elevated temperatures from the designated controls. We examined regional changes in temperatures at each of our 183 reefs for each scenario (RCP2.6, 4.5, and 8.5) and year (2050 and 2100) and used weighted linear regressions (as above in the ocean acidification section) to determine regional changes in coral and coralline algal calcification rates as well as bioerosion and sediment dissolution rates as a function of temperature. Furthermore, we also estimated changes in calcification and bioerosion rates under the interactive scenarios of decreasing pH and temperature at all 183 reefs by employing experimental findings on their interactive effects via multiple weighted linear regressions as per above.
Bioerosion.
A similar approach was used to estimate coral reef bioerosion sensitivities to seawater pH and temperature using the linear regression approach mentioned above, where we examined the literature for experimental research estimating their effects. However, only 11 suitable studies were found (SI Appendix, Table S1). Experimental substrates were coral skeletons in all studies, except one that used coralline algae (8) and another that used rubble (9). We used the same linear regression approach as for the calcifying taxa described above to estimate bioerosion sensitivities, using only studies that independently tested pH and temperature to evaluate their separate effects. To evaluate the interaction of pH and temperature, we used the combined effects of pH and temperature at each reef due to the paucity of tests of their interactive effects available in the literature. Given the relatively few studies available, we neither distinguished between bioeroding agents (i.e., sponges or microbioeroders) nor substrates (i.e., corals, coralline algae, or rubble). We calculated the percent of change in bioerosion rate for each study under all RCP scenarios relative to present day and then took the median responses among studies to estimate the separate and combined sensitivities to pH and temperature.
Sediment Dissolution.
We used the same linear regression and associated methods as in the two sections above to determine coral reef carbonate sediment production sensitivities to seawater pH and temperature. We used both sediment dissolution rates obtained in the field or laboratory. This yielded 8 studies with 16 responses (SI Appendix, Table S1). For both RCP4.5 and 8.5 scenarios, we estimated declines in sediment production that were greater than 100% (i.e., transition from net production to net dissolution of CaCO3 material). The loss of CaCO3 sediment due to dissolution eclipsed the reported rates of sediment accumulation (sediment infilling) estimated by Perry et al. (5). Therefore, sediment infilling was simply removed from all future scenarios that estimated reef accretion rates. There are currently no estimates of sediment dissolution rates for forereef environments, only within lagoons (SI Appendix, Table S1). Therefore, we provide two sets of data to demonstrate the potential impacts if sediment dissolution rates on forereefs equal those in the lagoon: our Fig. 2 with sediment dissolution and SI Appendix, Fig. S2 that includes no sediment dissolution. See the main manuscript for a discussion of these differences. We also note here that we do not include changes in physical erosion due to storms or deposition events due to the absence of empirical data on event-driven physical loss rates.
Application of Coral Reef Taxa Responses.
We applied the calculated changes in calcification and bioerosion to existing coral reefs with different compositions of calcifying taxa as examples of how the effects of climate change would influence the carbonate budgets of different coral reefs. These reefs were those for which coral and coralline algal cover, and coral and coralline algal calcification rates in the Indian and Atlantic Oceans were available in Perry et al. (5), and some additional reefs in the Atlantic, Indian, and Pacific Oceans, for which we could obtain similar data (SI Appendix, Table S1 for the study list). We used all available data, but for the majority of the Pacific reefs, this data was measured using hydrochemical methods (i.e., using the total alkalinity anomaly technique) and not census-based methods that combine cover and calcification rates of individual taxa (60). Note that these two methods utilize slightly different data, where census-based methods also include bioerosion, but neither method includes the loss of reef framework through nonbiotic physical erosion (e.g., storms). Also, hydrochemical carbonate budgets could include some aspects of sediment and framework dissolution but not physical bioerosion. The hydrochemical methods especially need to be measured over several days and seasons in order to obtain accurate rates of carbonate production, and even the census-based approach can be strongly impacted by recent events at any one location. However, by employing both methods in our model for all three ocean basins, it gives a better mix of both reef flat and reef slope communities that would otherwise be difficult if we only used data obtained from one method. Irrespectively, both methods provided similar net carbonate production and accretion rates.
We applied the changes in net calcification of resident biota and bioerosion to the data sets for each reef. For sites not included in Perry et al. (5), we used a method similar to the census-based estimation of net carbonate production. To do this, the percentage cover of different calcifying taxa and that taxa’s individual calcification rates (either at the site or from the closest nearby location, see methods in ref. 5), were compared to the carbonate production obtained using total alkalinity anomaly techniques. In our example coral reefs, the mean net carbonate production of the corals and coralline algae were obtained and standardized to their surface area (CaCO3 kg ⋅ m−2 ⋅ y−1). The total calcification (termed here as TG for simplicity in our equations only) of the two taxa at each site were then determined by multiplying their mean net calcification rates (G) by their approximate surface area per meter (A). Then, ecosystem net carbonate production rates were divided by the sum of these mean calcification rates to determine the proportion of calcification attributed to each taxa. This is summarized by Eq. 1, including coral (C) and CCA calcification and reef rugosity as R:
| [1] |
For corals, we used the mean rugosity value of 1.8 from the extensive data set of ref. 5 when this value was not given for a particular reef. The proportional contribution of each taxa to gross carbonate production (PrG) is determined for each taxa, here using coral as an example:
| [2] |
To estimate the impacts on bioerosion rates, we assumed that the chemical portion of bioerosion was measured already in hydrochemical methods. These are denoted as micro- or macrobioeroders in Perry et al. (60) and encompass all bioeroders except parrotfishes and sea urchins. Most studies not included in ref. 5 did not directly measure bioerosion, though some did. The following methods apply for those studies that did not measure bioerosion directly. At our hydrochemical sites, we used the studies listed in SI Appendix, Table S1 to estimate chemical bioerosion (already included in hydrochemical studies) as 10% in Moorea, 40% at Lizard Island and other Australian reefs, and the mean of the two (25%) in other locations in the Pacific. In Indian and Atlantic Oceans sites, we used the median of the bioerosion measured in Perry et al. (5) for each ocean basin. We then deducted these micro- and macrobioerosion rates from the total bioerosion at hydrochemical sites and then deducted this adjusted bioerosion rate to the gross carbonate production rates in these locations. Thus, our estimates of bioerosion sensitivity to acidification based on laboratory studies are only applied to the chemical component of bioerosion in our future projections. This is represented in Eq. 3, where Bu is total bioerosion not incorporated in the hydrochemical sites, BT is the total bioerosion, BMi is bioerosion from microbioeroders, and BMa is bioerosion from macrobioeroders:
| [3] |
To determine sediment infilling, we used data from Perry et al. (5) or employed their methods to determine sediment infilling at the hydrochemical sites. For hydrochemical sites, we assumed that the ratio of parrotfish:sea urchin bioerosion in the Pacific was equal to the mean of the Atlantic and Indian Oceans ratios (0.917:0.083). For our sites in the Indian or Atlantic oceans, we took the mean ratios of these regions (0.934 and 0.065 and 0.880 and 0.119, respectively). Accretion rates were then determined as per the methods of Perry et al. (5, 60) that adds sediment infilling and biotic net carbonate production and then factors for the influence of community structure on framework stacking porosity following ref. 61.
We present all important output data distributions, and all future scenarios result in declines in these parameters across all reefs. Therefore, we do not use statistical tests to interpret differences in our modeled scenarios, as widely recommended for a number of reasons (62). In particular, the responses of each reef to the effects of the future scenarios we explore are not independent except for the initial composition of resident taxa. Additionally, our large samples sizes would result in infinitesimally small P values, large F values, etc., here if assessing statistical differences in declines in carbonate production or reef accretion rates from the present-day values.
It is elementary that global coral cover will continue to decline in the future (2). To assess the responses of future coral reefs to climate change, we applied our estimates of changes in net calcification to corals and coralline algae present on each reef. We carried out two different scenario types, one where either coral abundance remains the same as present-day covers and another where both ocean warming alone or its interactive effects with ocean acidification reduces coral abundance due to mass coral bleaching events of various magnitudes. During our literature review, we observed that corals were not always grown at temperatures exceeding their summer maximum at the site of collection in ocean warming research in “warming” treatments (SI Appendix, Fig. S7). Therefore, it was not always possible to estimate the effects of ocean warming on coral mortality under future summer temperatures, where the physiological effects of increasing temperatures will be the most severe for corals. Mortality estimates from laboratory research were also therefore not possible. The lack of data for responses at temperatures above summer maximums at the collection sites could partially be due to a disconnect between the timing of experiments, their ultimate goals, or the ability to grow corals above summer maximums for any length of time. To more accurately project the impacts of future ocean warming and marine heatwaves, a greater proportion of these should be carried out in summer by future researchers.
However, because changes in coral cover will greatly impact net carbonate production (13), we present best estimates of coral cover at each of our sites that use the null assumption that the effect of marine heatwaves on future coral communities would be equal to their impacts today. We use this null assumption because the effects of coral population acclimatization and microevolution in response to exposure to increasingly severe marine heatwaves are largely unknown. While there is some evidence that coral populations could increase their thermal tolerance after exposure to multiple coral bleaching events (44, 63–67), these changes in coral thermal tolerances are difficult to accurately quantify. Therefore, we project future occurrences of DHW at our sites using models that assume no adaptive capacity (59). DHW is a commonly used metric that accounts for heat stress caused by both the duration and intensity of marine heatwaves on coral reefs (32, 68). We then use data from the recent large-scale measurements of the impacts of DHW on long-term coral cover on coral communities on the Great Barrier Reef (25). This equates to −39, −60, −67, and −90% coral cover under 4, 6, 8, and 10 DHW, respectively. We used this data set because it provided estimates of coral cover in response to measured DHWs across many reefs that encompass a great variety in biotic and abiotic factors, unlike other available data sets that encompass only one or a few reefs. Here, we assume that if a coral reef community suffers two or more marine heatwaves of a certain DHW extent within one decade, these events will not allow them to recover to their original coral cover. Therefore, we reduce coral cover on each reef by a proportion that is equal to the measured in situ effects of differing DHW in 2 °C steps. For example, if reef X encounters a 4 DHW event in 2030 and 2034 under a particular RCP scenario, and its initial coral contribution to gross carbonate production is 8 kg CaCO3 m−2 ⋅ y−1, then its cover is reduced by 39% to contribute 4.88 kg CaCO3 m−2 ⋅ y−1. If the same reef encounters two 6 DHW events in the next decade, its coral contribution is then reduced to 60% of its original value. These DHW predictions for our sites are displayed in SI Appendix, Fig. S5. These data are from the Great Barrier Reef, and while we acknowledge future work could also find similar data from other regions closer to these study sites, we consider it covers an ideal range of habitats and taxa that many data sets do not.
Supplementary Material
Acknowledgments
Funding from the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies supported the initial workshop and supported C.E.C., M.S.P., K.D.A., M.T.M., M.G.-R., V.S., and R.J.L. S.C. was supported by an ARC Discovery Early Career Researcher Award. C.E.C. was supported by a Rutherford Discovery Fellowship from The Royal Society of New Zealand Te Apārangi. R.V.H. was supported by the National Oceanic and Atmospheric Administration Coral Reef Conservation Program. G.D.-P. was supported by grants from ARC (DP160103071) and the Great Barrier Reef Foundation. C.T.P. was supported by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015265118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information. Some previously published data were also used for this work (SI Appendix, Table S1).
References
- 1.Stuart-Smith R. D., Brown C. J., Ceccarelli D. M., Edgar G. J., Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560, 92–96 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Hughes T. P., et al., Coral reefs in the anthropocene. Nature 546, 82–90 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Deloitte Access Economics , Economic Contribution of the Great Barrier Reef (Deloitte Access Economics, 2013). [Google Scholar]
- 4.Costanza R., et al., The value of the world’s ecosystem services and natural capital. Nature 387, 253–260 (1997). [Google Scholar]
- 5.Perry C. T., et al., Loss of coral reef growth capacity to track future increases in sea level. Nature 558, 396–400 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Kornder N. A., Riegl B. M., Figueiredo J., Thresholds and drivers of coral calcification responses to climate change. Glob. Change Biol. 24, 5084–5095 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Kroeker K. J., et al., Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes-Nivia C., Diaz-Pulido G., Dove S., Relative roles of endolithic algae and carbonate chemistry variability in the skeletal dissolution of crustose coralline algae. Biogeosciences 11, 4615–4626 (2014). [Google Scholar]
- 9.Stubler A. D., Peterson B. J., Ocean acidification accelerates net calcium carbonate loss in a coral rubble community. Coral Reefs 35, 795–803 (2016). [Google Scholar]
- 10.Kennedy E. V., et al., Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 23, 912–918 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Perry C. T., Alvarez-Filip L., Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 33, 976–988 (2019). [Google Scholar]
- 12.Perry C. T., Morgan K. M., Bleaching drives collapse in reef carbonate budgets and reef growth potential on southern Maldives reefs. Sci. Rep. 7, 40581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry C. T., et al., Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4, 1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes T. P., et al., Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Hughes T. P., et al., Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hughes T. P., et al., Global warming impairs stock-recruitment dynamics of corals. Nature 568, 387–390 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Cornwall C. E., Diaz-Pulido G., Comeau S., Impacts of ocean warming on coralline algal calcification: Meta-analysis, knowledge gaps, and key recommendations for future research. Front. Mar. Sci. 6, 186 (2019). [Google Scholar]
- 18.Comeau S., Edmunds P. J., Spindel N. B., Carpenter R. C., The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398 (2013). [Google Scholar]
- 19.Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S., Hoegh-Guldberg O., Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U.S.A. 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyre B. D., et al., Coral reefs will transition to net dissolving before end of century. Science 359, 908–911 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Shaw E. C., Hamylton S. M., Phinn S. R., Incorporating benthic community changes into hydrochemical-based projections of coral reef calcium carbonate production under ocean acidification. Coral Reefs 35, 739–750 (2016). [Google Scholar]
- 22.Silverman J., Lazar B., Cao L., Caldeira K., Erez J. C. L., Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. 36, 1–5 (2009). [Google Scholar]
- 23.Dove S. G., Brown K. T., Van Den Heuvel A., Chai A., Hoegh-Guldberg O., Ocean warming and acidification uncouple calcification from calcifier biomass which accelerates coral reef decline. Commun. Earth Environ. 1, 55 (2020). [Google Scholar]
- 24.Bruno J. F., Selig E. R., Regional decline of coral cover in the indo-pacific: Timing, extent, and subregional comparisons. PLoS One 2, e711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes T. P., et al., Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Eakin C. M., et al., Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS One 5, e13969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClanahan T. R., Graham N. A. J., Darling E. S., Coral reefs in a crystal ball: Predicting the future from the vulnerability of corals and reef fishes to multiple stressors. Curr. Opin. Environ. Sustain. 7, 59–64 (2014). [Google Scholar]
- 28.Donner S. D., Skirving W. J., Little C. M., Oppenheimer M., Hoegh-Guldberg O., Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Frieler K., et al., Limiting global warming to 2 °C is unlikely to save most coral reefs. Nat. Clim. Chang. 3, 165–170 (2013). [Google Scholar]
- 30.Hughes T. P., et al., Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 9, 40–43 (2019). [Google Scholar]
- 31.Torda G., et al., Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 7, 627 (2017). [Google Scholar]
- 32.Maynard J. A., et al., ReefTemp: An interactive monitoring system for coral bleaching using high-resolution SST and improved stress predictors. Geophys. Res. Lett. 35, 1–5 (2008). [Google Scholar]
- 33.Molina-Hernández A., González-Barrios F. J., Perry C. T., Álvarez-Filip L., Two decades of carbonate budget change on shifted coral reef assemblages: Are these reefs being locked into low net budget states? Proc. Biol. Sci. 287, 20202305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roff G., Mumby P. J., Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Pandolfi J. M., et al., Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Taylor B. M., et al., Synchronous biological feedbacks in parrotfishes associated with pantropical coral bleaching. Glob. Change Biol. 26, 1285–1294 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Tzadik O. E., Appeldoorn R. S., Reef structure drives parrotfish species composition on shelf edge reefs in La Parguera, Puerto Rico. Cont. Shelf Res. 54, 14–23 (2013). [Google Scholar]
- 38.Lee S. C., Habitat complexity and consumer-mediated positive feedbacks on a Caribbean coral reef. Oikos 112, 442–447 (2006). [Google Scholar]
- 39.Pörtner H.-O., et al., IPCC (Technical Summary in IPCC, The Intergovernmental Panel on Climate Change, 2019).
- 40.Pratchett M. S., Anderson K. D., Hoogenboom M. O., Widman E., Baird A. H., Spatial, temporal and taxonomic variation in coral growth—Implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 53, 215–295 (2015). [Google Scholar]
- 41.Roff G., Reef accretion and coral growth rates are decoupled in Holocene reef frameworks. Mar. Geol. 419, 106065 (2020). [Google Scholar]
- 42.Bay R. A., Rose N. H., Logan C. A., Palumbi S. R., Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 3, e1701413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comeau S., et al., Resistance to ocean acidification in coral reef taxa is not gained by acclimatization. Nat. Clim. Chang. 9, 477–483 (2019). [Google Scholar]
- 44.Guest J. R., et al., Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One 7, e33353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratchett M. S., McWilliam M. J., Riegl B., Contrasting shifts in coral assemblages with increasing disturbances. Coral Reefs 39, 783–793 (2020). [Google Scholar]
- 46.Rivest E. B., Comeau S., Cornwall C. E., The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Change Rep. 3, 271–281 (2017). [Google Scholar]
- 47.Comeau S., et al., Flow-driven micro-scale pH variability affects the physiology of corals and coralline algae under ocean acidification. Sci. Rep. 9, 12829 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris L. A., Voolstra C. R., Quigley K. M., Bourne D. G., Bay L. K., Nutrient availability and metabolism affect the stability of coral-symbiodiniaceae symbioses. Trends Microbiol. 27, 678–689 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Schoepf V., Stat M., Falter J. L., McCulloch M. T., Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duarte C. M., et al., Rebuilding marine life. Nature 580, 39–51 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Lewis E., Wallace D. W. R., Program Developed for CO2 System Calculations, ORNL/CDIAC-105, Carbon Dioxide Inf (Anal. Cent., Oak Ridge Natl. Lab, Oak Ridge, TN, 1998). [Google Scholar]
- 52.Nisumaa A.-M., et al., EPOCA/EUR-OCEANS data compilation on the biological and biogeochemical responses to ocean acidification. Earth Syst. Sci. Data 2, 167–175 (2010). [Google Scholar]
- 53.Dickson A. G., Sabine C. L., Christian J. R., Guide to Best Practices for Ocean CO2 Measurements (North Pacific Marine Science Organization, 2007). [Google Scholar]
- 54.Chan N. C. S., Connolly S. R., Sensitivity of coral calcification to ocean acidification: A meta-analysis. Glob. Change Biol. 19, 282–290 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Cornwall C. E., Eddy T. D., Effects of near-future ocean acidification, fishing, and marine protection on a temperate coastal ecosystem. Conserv. Biol. 29, 207–215 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Comeau S., Cornwall C. E., DeCarlo T. M., Krieger E., McCulloch M. T., Similar controls on calcification under ocean acidification across unrelated coral reef taxa. Glob. Change Biol. 24, 4857–4868 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Mollica N. R., et al., Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. U.S.A. 115, 1754–1759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss R. H., et al., The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 (2010). [DOI] [PubMed] [Google Scholar]
- 59.van Hooidonk R., Maynard J. A., Manzello D., Planes S., Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Change Biol. 20, 103–112 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Perry C. T., Spencer T., Kench P. S., Carbonate budgets and reef production states: A geomorphic perspective on the ecological phase-shift concept. Coral Reefs 27, 853–866 (2008). [Google Scholar]
- 61.Kinsey D. W., Hopley D., The significance of coral reefs as global carbon sinks— response to Greenhouse. Palaeogeogr. Palaeoclimatol. Palaeoecol. 89, 363–377 (1991). [Google Scholar]
- 62.White J. W., Rassweiler A., Samhouri J. F., Stier A. C., White C., Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123, 385–388 (2014). [Google Scholar]
- 63.Vargas-Ángel B., Zapata F. A., Hernández H., Jiménez J. M., Coral and coral reef responses to the 1997 & 1998 El Niño event on the Pacific coast of Colombia. Bull. Mar. Sci. 69, 111–132 (2001). [Google Scholar]
- 64.Pratchett M. S., McCowan D., Maynard J. A., Heron S. F., Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS One 8, e70443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palumbi S. R., Barshis D. J., Traylor-Knowles N., Bay R. A., Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Gintert B. E., et al., Marked annual coral bleaching resilience of an inshore patch reef in the Florida keys: A nugget of hope, aberrance, or last man standing? Coral Reefs 37, 533–547 (2018). [Google Scholar]
- 67.DeCarlo T. M., et al., Acclimatization of massive reef-building corals to consecutive heatwaves. Proc. Biol. Sci. 286, 20190235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Hooidonk R., Maynard J. A., Planes S., Temporary refugia for coral reefs in a warming world. Nat. Clim. Chang. 3, 508 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. Some previously published data were also used for this work (SI Appendix, Table S1).