Significance
Employing an intergenerational model of diet restriction (DR) that reduces weight gain, we identify differences in carbohydrate metabolic enzyme gene content of DR versus full-fed (FF) pig gut microbiomes as animals experienced a phased-feeding program administered to farm-raised pigs during their growth cycle. Gnotobiotic mice harboring DR or FF pig microbiomes and fed a corn/soy-dominated pig diet disclosed that the DR microbiome has reduced capacity to produce butyrate, a key diet-derived energy source, and alters hepatic energy metabolism. Combining studies of farm animal microbiome development with functional assays of their microbiomes in gnotobiotic mice should help generate husbandry recommendations for promoting healthy growth of animals during this time of increasing food insecurity and mandates to eliminate subtherapeutic antibiotics for growth-promotion.
Keywords: gut microbiome, malnutrition, carbohydrate-active enzymes, metabolic regulation, feature selection/information theory
Abstract
The concept that gut microbiome-expressed functions regulate ponderal growth has important implications for infant and child health, as well as animal health. Using an intergenerational pig model of diet restriction (DR) that produces reduced weight gain, we developed a feature-selection algorithm to identify representative characteristics distinguishing DR fecal microbiomes from those of full-fed (FF) pigs as both groups consumed a common sequence of diets during their growth cycle. Gnotobiotic mice were then colonized with DR and FF microbiomes and subjected to controlled feeding with a pig diet. DR microbiomes have reduced representation of genes that degrade dominant components of late growth-phase diets, exhibit reduced production of butyrate, a key host-accessible energy source, and are causally linked to reduced hepatic fatty acid metabolism (β-oxidation) and the selection of alternative energy substrates. The approach described could aid in the development of guidelines for microbiome stewardship in diverse species, including farm animals, in order to support their healthy growth.
Undernutrition afflicts over 200 million children worldwide and accounts for 45% of mortality in children under 5 y (1). Children with acute malnutrition exhibit wasting (impaired ponderal growth), often accompanied by stunting (reduced linear growth), deficits in bone development, neurodevelopment, and immunity, as well as perturbed metabolism (2, 3). Epidemiologic studies indicate that acute malnutrition in children is not due to food insecurity alone and that perturbed gut microbial community development is a contributing factor; children with severe acute malnutrition (SAM) and moderate acute malnutrition (MAM; weight-for-length z-scores are, respectively, 2 to 3 and >3 SDs below World Health Organization mean values) have microbiota that appear “younger” (more immature) compared to those of chronologically aged-matched healthy children (4–6). Studies in gnotobiotic mice colonized with microbiota from healthy and undernourished children have provided evidence that immature microbiota can transmit features of undernutrition (5, 7). These tests of causality inspired development of microbiota-directed complementary foods (MDCFs) designed to repair the microbiota of undernourished children. A controlled feeding study, involving a small group of 12- to 18-mo-old Bangladeshi children with MAM, identified an MDCF formulation that repaired their microbiota; repair was associated with a marked change in their plasma proteome characterized by alterations in levels of key mediators of bone growth, metabolism, immune function, and neurodevelopment toward a healthy state (5). A larger, longer randomized controlled study showed that this MDCF produced a superior effect on ponderal growth compared to a ready-to-use supplementary food even though the caloric density of the MDCF was 20% lower (8).
These observations prompted us to examine the influence of the gut microbiome on weight gain in the domestic pig, Sus scrofa domesticus. We focused on this species for several reasons. First, pigs account for ∼35% of global meat intake, second only to poultry (9, 10). Production costs are heavily influenced by how efficiently feed is transformed into body mass, as well as the degree of growth uniformity across animals (11). Second, pigs have been used as a model for studying human nutrition and metabolism because of the many ways in which they are anatomically, physiologically, and metabolically similar to humans (12, 13). Third, most of the commercial pig industry raises animals in highly controlled farming systems engineered to promote efficient and consistent growth phenotypes. These systems typically include phased feeding programs that transition animals from early, more costly, readily digestible, nutrient-rich diets to later, less-expensive diets with less nutrient fortification where energy/nutrient extraction is more dependent on expressed metabolic activities encoded in the gut microbiome. A central premise of the current study is that in order to more fully realize the goal of predictable robust weight gain at affordable prices, additional knowledge is needed regarding codevelopment of the gut microbiome and host; this knowledge could allow diets to be formulated based on greater understanding of which components (features) of the community play key roles in transforming dietary components to products that the animals use to satisfy their growth requirements (14). The environmentally controlled settings for raising pigs provide great opportunities for performing longitudinal studies designed to delineate these interactions between diet, microbiome features, and host physiology. Finally, the need to focus on whether/how the gut microbiome contributes to growth is made more pressing by international mandates to eliminate use of subtherapeutic antibiotics for growth promotion of farm animals because of the spread of antibiotic-resistant organisms (15, 16).
In the present study, we developed an algorithm (entropy-based method for microbial ecology research, EMMER), based on the von Neumann entropy calculation from quantum information theory (17, 18), to identify representative characteristics of fecal microbiomes serially sampled from litters of pigs that were or were not subjected to maternal diet restriction (DR) in utero and then provided either ad libitum access to, or restricted amounts of, a sequence of diets commonly given to farm-reared pigs as they complete their growth cycle. A 45% lower weight was attained by DR compared to full-fed (FF) pigs by the third postnatal month and this difference was sustained for the remainder of the 5-mo-long study. DR microbiomes exhibited a significantly reduced representation of genes encoding enzymes involved in the degradation of polysaccharides from dominant components of diets administered after postnatal day 70. These differences in the DR microbiome were associated with diminished fecal levels of butyrate, a major source of host energy, and significant increases in plasma levels of triglycerides, glucogenic amino acids, and urea cycle precursors. Functional features of DR and FF fecal microbiomes, collected during the period of consumption of the corn/soy-rich “finisher” diet (the last given during the feeding program), were subsequently assayed in gnotobiotic mice under controlled feeding conditions where all animals were provided the same amount of the finisher phase pig diet. The results confirmed the reduced capacity of the DR microbiome to generate butyrate. Moreover, mice colonized with the DR microbiome also exhibited reduced fatty acid oxidation in the liver, a metabolic effect that could explain the redirection of amino acids from protein synthesis to replenish hepatic energy reserves in DR pigs. Marrying longitudinal studies of farm animal gut microbiome development and function, conducted in well-engineered farming systems, with gnotobiotic mouse models that incorporate the microbial communities and diets of the farm animals, provides an opportunity to develop an informed set of practices for microbiome husbandry that promotes healthy growth. The results could have substantial economic and societal impact during this time of increasing global food insecurity and when producing sufficient amounts of high-quality protein to feed a rapidly expanding human population is a major challenge (9).
Results
Diet Restriction in Gestating Sows and Their Offspring Results in Lower Birth Weight and Impaired Weight Gain.
Fig. 1A describes the experimental design. Four sows (mixture of Landrace and Yorkshire genetic backgrounds) were artificially inseminated using a boar with a Duroc genetic background. One group of two gilts received 2 kg/d of a standard breeder diet throughout the course of their gestation, while the two gilts in the other group received a restricted diet (1 kg/d for the first 35 d of pregnancy and subsequently 0.6 kg/d from gestational days 36 to 114). All piglets were vaginally delivered. The four lactating sows were given a “sow lactation diet” ad libitum; all of their offspring (n = 6 to 10 per litter) were given full access to sows’ milk during the period of lactation and to a first nursery diet (nursery 1) during the weaning period. When weaned (postnatal day 29), piglets were moved to individual pens in a common barn. Piglets from a given sow were separated from the offspring of the other sows. From this point forward, DR piglets received 40% less feed than the amount consumed by those born to FF sows, an amount we calculated would result in a ∼50% reduction in growth rate (19). Based on the typical growth phenotype of swine, where poorest performing animals grow at ∼80% of the rate of normal (20), we reasoned that a 50% rate reduction would represent moderately severe malnutrition. To achieve this level of consistent diet restriction, during the first 10 postnatal weeks, the amounts of nursery 1 and a second nursery diet (nursery 2) given to the DR group were adjusted weekly based on the amount of diet consumed by the FF cohort, while during postnatal weeks 11 to 22 the amounts of “grower” and “finisher” feed given were based on biweekly measurements of feed consumption by the FF group (see Dataset S1 for diet composition and nutritional analysis as well as the amount of each diet consumed by pigs in each treatment group, and Dataset S2 for their weights). Neither the maternal diet nor the diets fed to their offspring contained antibiotics.
Fig. 1.
Applying diet restriction to sows and their offspring. (A) Experimental design. (B–D) Weights at birth (B), postnatal day 28 (C), and from postnatal days 28 to 154 (D). Mean values ± SEM are shown. (E) Weight difference between FF and DR pigs. (F) Plasma IGF1 levels measured at the end of each dietary phase (postnatal day indicated in parenthesis). Open and closed circles distinguish the two litters within the DR and FF treatment groups (n = 17 and 13 animals, respectively). *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 (Mann–Whitney U test in B, C, and F; linear mixed-effects model [treatment and time set as fixed effects and animals as the random effect] in D).
Piglets born to the two DR sows had significantly lower birth and weaning weights compared to FF sow-derived piglets (P < 0.001 and P < 0.01, respectively; Mann–Whitney U test) (Fig. 1 B and C and Dataset S2). This result is consistent with the fetal growth impairments of DR reported previously (21). There were statistically significant differences in weight gain between DR and FF animals throughout the 18-wk period when the nursery, grower, and finisher diets were consumed (P < 0.001; linear mixed-effects model; treatment and time set as fixed effects and animals as random effect) (Fig. 1D). By postnatal day 77, the targeted 45 to 50% difference in weight between the two groups had been achieved and was maintained for the remainder of the study (i.e., through postnatal day 154) (Fig. 1 D and E and Dataset S2). Pigs were raised under conditions designed to minimize exposure to enteropathogens; none of the animals in any of the treatment groups exhibited evidence of diarrheal disease. The impaired weight gain of DR animals was accompanied by statistically significant lower plasma IGF1 levels at the end of each of the five dietary phases compared to FF animals (Fig. 1F).
Distinguishing Microbiome Features of FF and DR Pigs.
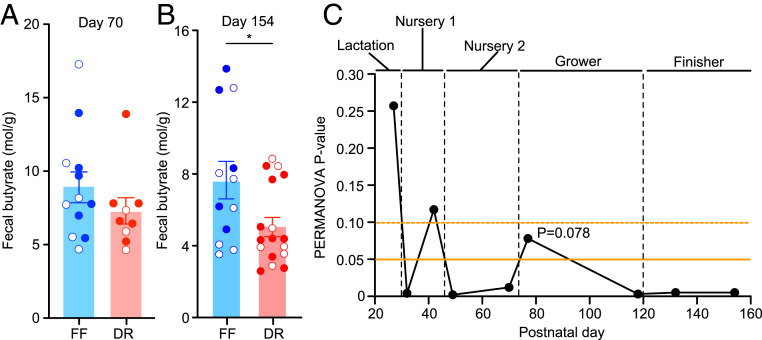
Corn and soy, the two principal ingredients in finisher diets, are rich in polysaccharides. The ability of the microbiome to ferment these polysaccharides is a determinant of feed conversion efficiency (22); butyrate generated from fermentation is a major contributor to the energy intake of pigs (23, 24). Gas chromatography-mass spectrometric (GC-MS) quantification of short-chain fatty acids (acetate, propionate, butyrate) in fecal samples disclosed no differences in their levels between the two groups at the end of nursery phase 2 treatment (postnatal day 70), but statistically significantly lower butyrate levels in DR pigs at the end of the finisher diet phase (postnatal day 154; P < 0.05; Mann–Whitney U test) (Fig. 2 A and B). This finding raised two questions: What features distinguish the configurations of the gut microbiomes of DR and FF pigs, specifically those components involved in the catabolism of polysaccharides? What are the metabolic adaptations of the host to diet restriction and to what extent are they causally related to the gut microbiome?
Fig. 2.
Effects of diet and diet restriction on fecal butyrate and fecal CAZyme gene abundances. (A and B) Fecal levels of butyrate measured at postnatal days 70 (A) and 154 (B). Open and closed circles indicate litter membership for each treatment group. Mean values ± SEM, are shown. *P < 0.05 (Mann–Whitney U test). (C) P values of PERMANOVA tests of DR versus FF microbiome configurations projected on PCA plots based on the abundances of information-rich CAZymes. Fecal samples collected at the indicated time points from both litters in each treatment group were included in the analysis.
Microbial community datasets typically contain disproportionately high numbers of features (species/strains, genes, and so forth) relative to the number of biospecimens being characterized. Moreover, these features can be redundant, noisy, or not relevant to the biological question being considered. Consequently, feature selection algorithms are often employed to identify highly informative subsets of characteristics that can be used for analyses or to generate testable hypotheses for follow-up experiments. Algorithms, such as Random Forests and indicator species analysis, have been used to select microbial community features that differentiate treatment groups or time points; they are generally not suitable when the microbiome configurations of different groups or across time points are highly similar (SI Appendix, Fig. S1). We developed a flexible feature selection algorithm (EMMER) that can accommodate a range of microbiome-related datasets, including those that consist of a single treatment group, or multiple groups with highly similar characteristics, or multiple groups with highly dissimilar characteristics. EMMER quantifies “information content” using von Neumann entropy (vNE), a generalization of Shannon entropy (17, 18). For each dataset, the algorithm first identifies a set of “information-rich features” whose removal has a significant impact on overall vNE (SI Appendix, Fig. S2 A–D). EMMER iterates the information-rich feature identification step for each treatment group/time point being considered (SI Appendix, Fig. S2E). The relationship between treatment groups/time points can be visualized, based on these information-rich features using principal component analysis (PCA) since EMMER and PCA have a shared mathematical basis (SI Appendix, Fig. S2 E–G). PERMANOVA is then applied to test whether microbiomes from different groups/time points are significantly different in PCA space (SI Appendix, Fig. S2H). If significant differences are found, EMMER can then identify a subset of information-rich features that differentiate treatment groups or time points (SI Appendix, Fig. S3). This iterative strategy enables EMMER to select distinguishing features within and between groups of interest (SI Appendix, Supplementary Results).
Fig. 2C illustrates how EMMER was applied to genes that encode carbohydrate active enzymes (CAZymes) in the gut microbiomes of DR and FF pigs. CAZymes include enzymes involved in the recognition and metabolism of various complex carbohydrates, including oligosaccharides, polysaccharides, and glycoconjugates (25). DNA prepared from fecal samples, collected at the beginning and end of each of the dietary phases from animals in each of the two treatment groups, was subjected to shotgun sequencing (n = 181 fecal DNA samples) (Dataset S2). We identified 987 genes among the 15,885,872 genes in the combined DR and FF dataset that had CAZyme assignments and that were present in ≥33% of samples obtained at a given time point in a given treatment group. These CAZymes were subjected to feature selection using EMMER. A total of 55 information-rich CAZymes were selected from the DR dataset and 65 in the FF dataset; 22 were present in both datasets, yielding 98 unique CAZyme features (see their annotations in Dataset S2). An analysis of CAZyme-based microbiome configurations revealed a temporal pattern of separation between the FF and DR groups; this separation was statistically significant on postnatal day 49 (7 d after initiation of the nursery 2 diet) and, with the exception of a single measured timepoint (day 77), remained statistically significant (P < 0.05; PERMANOVA) during subsequent exposure to the grower and finisher diets (Fig. 2C and Dataset S2). A similar temporal pattern was observed when information-rich bacterial taxa (amplicon sequence variants identified from PCR of 16S rRNA genes) were used to characterize microbiota configuration (SI Appendix, Fig. S2H and Dataset S2).
The nursery 2, grower and finisher diets are all rich in corn and soy, with the representation of corn increasing with each diet change (Dataset S1). Dry corn kernels contain ∼70% starch, 2% cellulose, and 3% oligosaccharides by weight (26–28) while soybean meal contains 19% polysaccharide, 3% sucrose, 2% stachyose, and 2% raffinose (29). The representation of an information-rich CAZyme gene identified by EMMER that encodes a predicted endo-β-1,4-glucanase belonging to a glycoside hydrolase (GH) family involved in cellulose degradation (GH8), exhibits a distinct temporal pattern in its abundance, rising from being undetectable during consumption of the grower phase diet (postnatal days 77 to 118), to prominence during the finisher phase (postnatal days 132 to 154) in both DR and FF microbiomes (Fig. 3A). Two other information-rich CAZyme genes that are involved in starch metabolism, one encoding GlgB, a GH13_9 family member with specificity for 1,4 α-glucan branching structures, and another specifying a GH13_12 pullulanase, demonstrate temporal and statistically significant group-specific differences in their abundances. Their representation becomes significantly lower in DR compared to FF microbiomes as animals transition from the grower diet to the finisher diet and then fall to undetectable levels in both groups of pigs by the end of the finisher phase (Fig. 3 B and C). An information-rich GH13_31 α-glucosidase gene involved in starch degradation, which does not exhibit significant differences in its abundance during the grower phase between DR and FF microbiomes, abruptly falls to levels below the limits of detection after the grower-finisher phase transition (Fig. 3D). Sucrose bound or encapsulated within the corn and soybean components of the finisher diet escapes digestion by host small intestinal sucrose-isomaltase but is liberated by microbial processing of the diet in the distal gut. Genes encoding two CAZymes, one a sucrose-6-phosphate hydrolase (GH32) and the other a sucrose hydrolase (GH32), manifest statistically significant lower abundances in DR compared to FF microbiomes during the finisher phase (Fig. 3 E and F).
Fig. 3.
The representation of information-rich CAZymes in the fecal microbiomes of DR versus FF animals as a function of diet and time. CAZymes associated with degradation of cellulose (A) and starch (B–D), and sucrose metabolism (E and F). Gene numbers in the microbiome datasets are noted as are the corresponding CAZyme annotations. Mean values ± SEM are plotted. *P < 0.05; **P < 0.01; ***P < 0.005 (unpaired t test). CPM: read counts per million.
Distinguishing Metabolic Features of FF and DR Pigs.
To characterize metabolic adaptations during diet restriction, we collected nonfasting plasma samples at the end of each dietary phase from all DR and FF pigs. On postnatal day 154, there were no statistically significant differences in plasma insulin levels between the two groups (70.3 ± 30.6 pmol/L [FF] versus 58.8 ± 19.3 pmol/L [DR]; mean ± SD; P = 0.22, Mann–Whitney U test), nor were there statistically significant differences in plasma glucose levels (Dataset S2). However, in the nonfasted state, DR animals had significantly higher plasma triglyceride levels (P < 0.05; Mann–Whitney U test) (Fig. 4A); this difference was not accompanied by differences in levels of ketones, nonesterified fatty acids, 3-hydroxybutyrate, or lactate compared to their FF counterparts (P > 0.05; Mann–Whitney U test) (Dataset S2). [Note that pigs are a hypo-ketonemic species with diminished capacity to generate ketones from fatty acids (30).]
Fig. 4.
Metabolic phenotyping of DR and FF pigs. (A–F) Plasma metabolites. Open and closed circles distinguish the two litters within the FF and DR treatment groups. Mean values ±SEM are plotted. *P < 0.05; ***P < 0.005; ****P < 0.001 (Mann–Whitney U test).
Undernourished children exhibit reductions in fatty acid oxidation when fasting (31). Reduced fatty acid metabolism prompts use of amino acids for gluconeogenesis and to generate energy via their catabolism to TCA cycle intermediates; replenishment of these amino acids in the nonfasted state comes at the expense of protein synthesis. Interestingly, the glucogenic amino acids alanine and glycine, which accounted for >40% of free amino acids in plasma at postnatal day 154, were significantly elevated in nonfasting DR compared to FF animals (Fig. 4 B and C and Dataset S2). This increase in glucogenic amino acids was accompanied by significant increases in the urea cycle metabolites citrulline, ornithine, and arginine (Fig. 4 D–F and Dataset S2). Significantly higher levels of alanine, citrulline, ornithine, and arginine in DR pigs were first evident at the end of nursery 2 diet phase (postnatal day 70) (Fig. 4 B–F and Dataset S2), coinciding with the time when we observed a marked difference in weight gain (Fig. 1 D and E) and significant differences in microbiome CAZyme configuration (Fig. 2C).
These findings suggest that nonfasting DR pigs have a higher rate of catabolism of amino acids in their livers to generate glucose and cellular energy, with increased urea cycle activity to convert the resulting byproduct, ammonia, to urea (32). This may be especially important in pigs given their attenuated ability to produce ketones as an alternative energy source for the brain. Moreover, redirecting amino acids from synthesis of muscle protein to replenishing energy reserves could contribute to their impaired growth and weight gain. Based on this reasoning, we turned to gnotobiotic mice colonized with representative DR or FF pig microbial communities to address two questions. Is the gut microbiome of DR pigs less efficient at fermenting polysaccharides prominently represented in the corn-rich finisher diet? Does the gut microbiome play a causal role in suppressing fatty acid utilization and effecting a shift to alternative sources of energy in DR pigs?
Metabolic Phenotyping of Gnotobiotic Mice Colonized with the Microbiomes of Postnatal Day 154 DR or FF Pigs.
Feeding practices (FF versus DR) and microbial community composition are two factors that need to be considered when interpreting the results of our pig experiment. For example, previous studies showed that reductions in food intake can result in higher digestibility of crude fibers (33). To disentangle the effects of diet restriction and microbiome configuration, we conducted functional assays of DR and FF microbiomes, sampled from pigs on postnatal day 154, using gnotobiotic mice fed the pig finisher diet. [Postnatal day 154 fecal samples were used rather than those from earlier time points when discordance in the abundances of information rich CAZyme first became evident because differences in butyrate levels were not evident until the later time point (Fig. 2 A and B and Dataset S2).]
In the first experiment, the diet was provided ad libitum while in the second recipients of both types of donor microbiome were given the same amount of diet each day. In the first experiment, just weaned 4.5-wk-old germ-free male C57BL/6J mice were placed on pig finisher diet that was administered ad libitum for the duration of the experiment. Two days later, animals were colonized with intact uncultured fecal communities obtained from a representative 154-d-old DR or FF pig (n = 6 mice/pig donor microbiome; all recipients of a given pig microbiome were singly housed in cages placed in the same gnotobiotic isolator) (Fig. 5A). Microbial community donors (pig 18 from the DR group and pig 11 from the FF group in Dataset S3) were selected based on two criteria: 1) the information-rich CAZyme gene content of their microbiomes, and 2) two metabolic parameters—fecal butyrate and plasma triglyceride levels (Dataset S2).
Fig. 5.
Functional characterization of the fecal microbiomes of DR and FF pigs in gnotobiotic mice. (A) Design of the ad libitum feeding experiment. Germ-free C57BL/6J mice (n = 6 per arm) were colonized at 4.5 wk of age with a representative fecal microbiome from a postnatal day 154 DR or FF pig. Mice were fed the pig finisher diet ad libitum for 21 d and were then killed after a 4-h fast. (B and C) Butyrate levels in cecal contents (B) and fecal samples (C) quantified by GC-MS. (D) Cecal glucose levels measured by LC-QQQ-MS. (E) Design of the controlled feeding experiment. Germ-free C57BL/6J mice (n = 6 per arm) were colonized at 4.5 wk of age with the same representative DR and FF microbiomes used in the ad libitum study. Both groups were subjected to a controlled feeding regimen where all animals in both groups were given the same amount of pig finisher diet each day for 17 d. Mice were then killed following a 4-h fast. (F and G) Levels of cecal (F) and fecal (G) butyrate. (H–J) Levels of cecal glucose (H), taurocholic acid (I), and amino acids (J). (K) Serum triglycerides. (L and M) Liver acylCoA and acylcarnitine concentrations. (N) Lactate concentration in gastrocnemius muscle. *P < 0.05; **P < 0.01; ***P < 0.005 (Mann–Whitney U test).
On experimental day 21, mice were killed after a 4-h fast and their cecal contents and feces were collected. Of the 18 information-rich CAZyme genes we had identified in postnatal day 154 pigs (Dataset S2), 16 were present in the cecal and fecal microbiomes of the two groups of recipient mice (the same 16 in both groups) (Dataset S4). Epididymal fat weights were not significantly different between mice harboring the FF compared to DR microbiome (0.7 ± 0.1 mg/g [mean ± SD] body weight versus 0.6 ± 0.1 mg/g [DR], respectively; P = 0.2, Mann–Whitney U test). However, GC-MS analysis of cecal contents and fecal samples revealed that butyrate levels were significantly higher in those with the FF donor microbiome (P < 0.005, Mann–Whitney U test) (Fig. 5 B and C and Dataset S4). Follow-up targeted liquid chromatography-triple quadrupole mass spectrometry (LC-QQQ-MS) of cecal contents also disclosed significantly lower levels of glucose in ad libitum fed mice containing the transplanted FF pig microbiome (P < 0.05, Mann-Whitney U test) (Fig. 5D and Dataset S4).
To isolate the impact of microbiome composition on host physiology, we performed a follow-up, controlled feeding experiment where each mouse in each treatment group was given the same amount of the finisher diet per day (Fig. 5E). Two days after introduction of the same DR and FF microbiomes used for the ad libitum feeding experiment, 4.5-wk-old germ-free C57BL/6J male recipients (n = 6 per group) were singly housed. Each mouse initially received 3 g of the finisher diet per day for 7 d, followed by 3.3 g/d for 10 additional days. To investigate the effect of microbiome composition on host fatty acid utilization and energy metabolism, we killed mice in both groups on experiment day 21 after a 4-h fast and collected their cecal contents, liver, gastrocnemius muscle, and serum. Body weights, measured every 2 d, were not significantly different between the two groups at any time point during the 17-d controlled feeding period (weight gain, 4.8 ± 5.6% [mean ± SD; FF] and 7.8 ± 2.7% [DR]; normalized to the first time point where weights were measured after initiation of controlled feeding [experimental day 7]; P > 0.05; linear mixed-effects model [donor microbiome and time set as fixed effects and animals as random effects]) (Dataset S5). As with the ad libitum feeding experiment, we found the same 16 postnatal day 154 information-rich CAZyme genes represented in the cecal communities of recipient mice (Dataset S5). [Based on assignments of taxonomy to the 16S rDNA dataset (34), 77% and 90% of species represented in the DR and FF communities were transferred to recipient mice, respectively].
Gnotobiotic mice colonized with the DR and FF microbiomes and fed a controlled amount of pig finisher diet recapitulated findings from the first pig-to-mouse microbial community transplant experiment, namely: 1) animals containing the DR pig microbiome exhibited significant reductions in cecal and fecal butyrate (P < 0.05 and P < 0.01, respectively; Mann–Whitney U test) (Fig. 5 F and G); and 2) cecal glucose levels were higher in DR mice, although the increase did not achieve statistical significance (P = 0.065; Mann–Whitney U test) (Fig. 5H and Dataset S5).
To extend these findings, we examined the functional metabolic potential of the transplanted fecal microbiomes using the microbial community SEED (mcSEED) database (35, 36). In silico mcSEED-based metabolic reconstructions combine homology- and genome context-based evidence with known sets of enzymatic reactions and nutrient transporters in order to group genes into “microbial community subsystems” that capture and project variations in particular metabolic pathways/modules across thousands of microbial genomes (35, 36). We assigned microbiome-encoded proteins to a collection of 104 mcSEED subsystems that encompass the metabolism of 19 amino acids, 10 vitamins, 40 sugars, and 6 fermentation products projected over 2,600 annotated reference bacterial genomes using procedures described in ref. 5. The results revealed the subsystems/pathways annotated as “sucrose, fructose, and fructooligosaccharides” and “glycolysis” had a significantly lower representation in the transplanted DR compared to FF microbiomes (P < 0.05; Mann–Whitney U test) (Dataset S5). The lower abundances of genes corresponding to the glycolysis pathway is consistent with the higher glucose levels that we had documented in the cecums of mice harboring the DR community. The “butyrate fermentation” pathway was also less prominent in the microbiomes of mice colonized with the pig DR community compared those harboring the pig FF community. Although the difference did not reach statistical significance (P = 0.08; Mann–Whitney U test) (Dataset S5), this finding is consistent with the significantly lower butyrate levels in DR versus FF cecal samples.
The representation of the mcSEED “bile acid transformation” subsystem was significantly lower in the transplanted DR microbiome (P < 0.05; Mann–Whitney U test) (Dataset S5), suggesting a reduced capacity to deconjugate bile acids. Consistent with this observation, LC-QQQ-MS analysis of cecal contents revealed that the major conjugated bile acid present in the mouse gut, taurocholic acid, was present at statistically significantly higher concentrations in mice colonized with the DR microbiome (P < 0.05, Mann–Whitney U test) (Fig. 5I and Dataset S5). Notably, plasma levels of a major conjugated bile produced by pigs, glycohyodeoxycholic acid, were also significantly higher in DR pigs than in their FF counterparts (P < 0.05, Mann–Whitney U test) (Dataset S2). In addition, the representation of pathways for branched-chain amino acid and tryptophan metabolism were significantly reduced in the DR compared to FF microbiomes (P < 0.05, Mann–Whitney U test) (Dataset S5B), as were the levels of these amino acids (P < 0.05, Mann–Whitney U test) (Fig. 5J).
As in DR pigs, mouse recipients of the DR pig microbiome exhibited significantly higher levels of serum triglycerides compared to mice harboring the FF pig microbiome (P < 0.05, Mann–Whitney U test) (Fig. 5K and Dataset S5). Targeted MS also disclosed that the DR donor microbiome recipients had significantly lower hepatic levels of C14:1-, C16:1-, and C18:1-acylCoAs, plus medium and long-chain acylcarnitines (P values varying from <0.05 to <0.005, Mann–Whitney U test) (Fig. 5 L and M and Dataset S5) as well as significantly higher levels of lactate in their gastrocnemius muscle (P < 0.05, Mann–Whitney U test) (Fig. 5N and Dataset S5). However, in this controlled feeding study, there were no statistically significant increases in alanine, glycine, and urea cycle intermediates in sera collected from fasted mice containing the DR compared to FF microbiomes (P > 0.05, Mann–Whitney U test) (Dataset S5). Nevertheless, our findings support the hypothesis that the microbiome is causally related to selection of alternative energy substrates. In both the pig and mouse models, the DR microbiome was linked to reduced hepatic fatty acid metabolism (β-oxidation). In pigs, the reduction in fatty acid catabolism was linked to an apparent increase in amino acid utilization, whereas in mice, the alternate fuel appears to be glucose, as suggested by the significant increase in gastrocnemius muscle lactate levels. The lack of increase in the glucogenic amino acids alanine and glycine in the DR mice may reflect their use, when these animals were in a fasted state, for hepatic gluconeogenesis in order to provide a pool of substrate for peripheral tissues, such as skeletal muscle. Predominant use of alanine and pyruvate for gluconeogenesis in both groups of mice would also obviate increases in urea cycle intermediates.
Discussion
In this report, we examine the adaptations, metabolic features, and host effects of the gut microbiomes of pigs subjected to DR in utero and as they grew to maturity. As noted in the Introduction, engineered commercial farming systems for rearing pigs include phased feeding programs that smoothly transition animals from early, readily digestible diets with high nutritional value to later diets with less fortification and less digestible components. Microbial biotransformation of these components yields metabolic products that the animals utilize as sources of energy to satisfy their growth requirements (14). The goal of these phased-diet programs is to support sustained rapid weight gain with minimum total feed costs. A central premise of our study is that achieving predictable robust weight gain at an affordable price requires additional knowledge of the codevelopment of the microbiome and host, with diets formulated based on an understanding of which components (features) of the gut community play a key role in nutrient processing. In this sense, our focus on the diet restriction model in pigs is linked to efforts to understand which features in the developing microbiomes of pigs (and malnourished children) are causally linked to the regulation of various facets of growth, including weight gain. Knowledge of these features sets the stage for deliberate manipulation of their expressed properties: for example, through “rational” design of diets that contain nutrients that can be utilized by key growth-promoting microbial community components.
We developed a feature selection algorithm, EMMER, based on the vNE calculation from quantum information theory, to identify distinguishing characteristics of the microbiomes from serially sampled pigs that were or were not subjected to diet restriction in utero, and then either fed ad libitum, or provided with 40% less feed per day than their FF counterparts. The 45% reduction in weight resulting from the DR protocol was accompanied by reductions in the representation of microbial genes encoding a number of CAZymes, including those associated with degradation of carbohydrates that are the principal components of the finisher diets used prior to market.
Our previous “xenomicrobiota” transplant studies (37) showed that it is possible to successfully capture components of microbial communities harvested from a variety of foreign ecosystems in the guts of recipient germ-free mice. Therefore, we transplanted pig microbiomes into germ-free mice to establish whether the microbiome plays a causal role in mediating DR-associated metabolic phenotypes. Donor microbiomes were selected according to an EMMER-based determination that they exemplified CAZyme features that distinguish DR from FF pigs at a time when 1) their weight and metabolic phenotypes are highly discordant, and 2) their diet is dominated by ingredients requiring microbial biotransformation for its energy content to be recovered by the host. An additional element of our experimental strategy was to reenact interactions between the farm animal’s diet and their gut microbiome by feeding gnotobiotic mice the same diet. In this case, mouse recipients of DR and FF pig microbiomes were subjected to a controlled feeding protocol designed to eliminate differences in the amount of finisher-phase pig diet they consumed, thereby allowing us to avoid a potential confounder in assigning functional differences to their implanted microbial communities.
Butyrate represents a major source of energy for the colonic epithelium (38–40). Studies in pigs have documented how dietary supplementation with sodium butyrate after weaning results in improved growth performance between the first and second postnatal months (41, 42). Metabolic reconstructions of serially sampled DR and FF pig microbiomes, GC-MS analysis of fecal short-chain fatty acids, and functional characterization of these microbiomes in gnotobiotic mice disclosed that the DR microbiome is less capable of metabolizing the corn-dominated finisher diet to butyrate.
Hepatic steatosis has been reported in 4-mo-old pigs that had experienced undernutrition in the preceding 7 wk (43). Consistent with this report, we found that DR pigs had significantly higher nonfasting plasma triglyceride levels without accompanying differences in levels of insulin, glucose, ketones, nonesterified fatty acids, 3-hydroxybutyrate, or lactate. Our controlled feeding experiment in gnotobiotic mice revealed that a DR microbiome transmitted a metabolic phenotype indicative of reduced hepatic β-oxidation (lower levels of liver acylcarnitines and acylCoAs and significantly elevated serum triglyceride levels) without accompanying changes in serum levels of the products of fatty acid catabolism. These findings, combined with our observation that plasma levels of glucogenic amino acids and urea cycle precursors become significantly elevated in DR pigs at a time when significant differences in their microbiome CAZyme configurations appear, support the notion that reductions in butyrate generation and liver fatty acid metabolism act together to compromise the weight gain of DR pigs.
Our ability to recapitulate metabolic differences between DR and FF pigs in gnotobiotic mice after transfer of DR and FF pig microbiomes provides evidence that the microbiome contributes to host metabolic phenotypes. Our findings are also compatible with the view that pig microbiomes adapt to (reconfigure in response to) and then reinforce diet-induced metabolic phenotypes; when transferred into germ-free-mice, and given a diet similar to which they had previously adapted, they then promote the phenotypes that gave rise to these community configurations.
Identifying new ways to ensure the availability of sources of high-quality dietary protein is a pressing problem at this time of increasing global food insecurity. The approach we describe in this report should be applicable to other species where improvements in husbandry that optimize growth are being sought. The approach also provides opportunities to delineate basic mechanisms that connect expressed gut microbiome functions to various facets of host biology. Envisioned outcomes include identification of point-of-care microbiome and microbiome-linked host biomarkers that could be used to: 1) guide decision-making about the environments where animals are raised prior to and after weaning and the selection of ingredients used for feed (e.g., during periods of marked fluctuations in cost); 2) assess the extent to which host genetics impacts growth via effects on the gut microbiome; 3) define the prevalence of and assess the risk for dysbioses, including those produced by enteropathogens; and 4) develop new prebiotic, probiotic, or synbiotic formulations that promote healthy growth phenotypes at a time when use of subtherapeutic antibiotics for growth promotion is now being prohibited (44). Finally, while we did not define the effects of DR on the maternal microbiome in the present study, introducing the microbiome of pregnant DR and FF sows into pregnant germ-free mice and allowing the community to be transmitted to their pups provides an opportunity to develop maternal diets that promote the nutritional health of sows and healthy growth of their offspring.
Materials and Methods
Experiments Involving Pigs.
Husbandry.
Protocols for breeding, feeding, housing, sampling, and killing animals belonging to the FF and DR groups were approved by the North Carolina State University Institutional Animal Care and Use Committee. All studies were performed under the supervision of a veterinarian. The diet treatments followed procedures described in ref. 21. Briefly, four 6-to-8-mo-old gilts (mixture of Landrace and Yorkshire genetic background) were divided into two groups. Two weeks before artificial insemination, MATRIX (Altrenogest; Intervet) was given to all animals (16 mg in their daily feed) to synchronize estrus. Gilts received semen from a single sire (Duroc genetic background). Gilts in the FF group also received 2.5 kg/d of a gestation diet, supplemented with a micronutrient mixture and docosahexaenoic acid (DHA), from the time of estrus synchronization to the time of insemination, after which the amount of diet was reduced to 2 kg/d during farrowing (Dataset S1). DR gilts received the gestation diet without micronutrient/DHA supplementation according to the following schedule: 2.5 kg/d during estrus synchronization, 1 kg/d during the first 35 d after insemination, and 0.6 kg/d from gestational days 36 to 114.
All piglets were vaginally delivered and maintained with their sows. During lactation, sows received a sow lactation diet ad libitum. Piglets in all four litters were given unrestricted access to their sow’s breastmilk. At postnatal day 29, piglets were weaned and each member of a litter was maintained in individual pens to avoid competition for food. The individual pens containing littermates were clustered together and separated from pens containing members of other litters by a minimum of two empty pens. Piglets in the FF group were fed ad libitum for the remainder of the experiment. Pigs in the DR group received 40% less feed per day than the amount consumed by their FF counterparts (Dataset S1). The amount of food consumed by each pig was assessed by measuring feed disappearance each day. Piglets were weighed every week for the first three diet phases and every 2 wk during the last two diet periods (Dataset S2); the resulting dataset was analyzed by applying linear mixed-effects models built using R (v3.5) (45) and the lmerTest package (v3.1.2) (46).
Biospecimen collection.
Fecal samples were collected from pigs by swabbing the rectum with a cotton-tipped swab. Following sample collection, the tip of each swab was clipped and transferred to a sterile microfuge tube. Bulk fecal samples were collected into sterile cryocups on postnatal days 70 (fresh samples from a subset of animals) and 154 (samples from all animals). When a fresh sample could not be directly obtained from a 154-d-old pig, recently evacuated feces were collected from the floor of their pen. All rectal swabs and bulk fecal samples were immediately frozen on dry ice and stored at −80 °C. Nonfasted blood samples were collected from the jugular vein into EDTA-containing tubes (BD) at the end of each dietary phase. Plasma (∼1 mL per sample) was obtained after centrifugation (1,300 × g for 20 min at 4 °C), then aliquoted into sterile microfuge tubes and stored at −80 °C.
Quantifying IGF1 and insulin levels.
IGF1 and insulin levels were measured in nonfasting pig plasma samples using the human IGF-I/IGF-1 Quantikine ELISA Kit (catalog no. DG100B, R&D Systems) and the Human/Canine/Porcine Insulin Quantikine ELISA Kit (catalog no. DINS00, R&D Systems), following the manufacturer’s protocol.
Measurement of plasma and cecal metabolites.
Plasma levels of triglycerides and cholesterol were measured according to ref. 47 while nonesterified fatty acids, acylcarnitines, acylCoAs, amino acids, and glucose plus fecal short-chain fatty acids were quantified using protocols described in ref. 5. Plasma levels of bile acids were quantified using protocols described in ref. 48 with minor modifications. Briefly, ice-cold methanol was mixed with 20 μL of plasma (1:1 [vol/vol]) and shaken for 2 min in a bead beater (Biospec Products) at maximum setting without adding beads. After centrifugation (20,800 × g for 10 min at 4 °C), the supernatant was transferred to an Eppendorf tube and dried in SpeedVac at room temperature. Dried samples were resuspended in 5% ethanol (in water). After centrifugation (20,800 × g for 10 min at 4 °C), bile acids were measured in the supernatants with a 1290 Infinity II UHPLC system coupled to an Agilent 6470 Triple Quadrupole LC/MS system equipped with a Jet Stream electrospray ionization source. The flow rate was set at 0.3 mL/min for the 150 mm × 2.1 mm Waters BEH C18 1.7-μm particle column. The capillary column was maintained at 325 °C with a sheath gas flow of 40 (arbitrary units), an aux gas flow of 5 (arbitrary units) and a sweep gas flow of 3 (arbitrary units) for both positive and negative injections. The spray voltage was 4.5 kV for the positive-ion injection and 4 kV for the negative-ion injection. Mobile phases for positive-ion mode were 0.1% formic acid in water and 0.1% formic acid in acetonitrile, while for negative-ion mode they were 5 mM ammonium bicarbonate in water and 5 mM ammonium bicarbonate in 95:5 acetonitrile:water.
Metabolomic data generated from pig biospecimens (177 metabolites measured in 30 animals) were first subjected to a Grubbs’ test (49) to identify any measurements of given analyte that could be formally classified as an “outlier”; these measurements (39 of 5,310; 0.7%) were subsequently removed prior to statistical tests of differences between treatment groups (outlier values are indicated with an asterisk in Dataset S2).
Fecal bacterial V4-16S rDNA amplicon sequencing.
Each fecal sample on a frozen swab tip (Dataset S2) was resuspended in 650 μL buffer A (200 mM NaCl, 200 mM Trizma base, 20 mM EDTA) by shaking in a Mini-Beadbeater-96 (BioSpec Products) for 20 s. A 500 μL aliquot of the resuspended sample was then transferred to a new tube containing 0.1-mm diameter zirconia/silica beads, 500 μL of a phenol:chloroform:isoamyl alcohol mixture (25:24:1), and 210 μL 20% SDS. DNA was extracted by bead-beating, purified (Qiaquick columns; Qiagen), eluted into 100 μL Qiagen buffer EB (Qiagen), quantified (Quant-iT dsDNA broad range kit; Invitrogen) and the mixture was adjusted to 1 ng/μL. Variable region 4 of the bacterial 16S rRNA gene was amplified by PCR using the following conditions: denaturation (94 °C for 2 min) followed by 26 cycles of 94 °C for 15 s, 50 °C for 30 s, and 68 °C for 30 s, followed by incubation at 68 °C for 2 min (4). Amplicons were quantified, pooled, and sequenced (Illumina MiSeq instrument; paired-end 250-nt reads). Amplicon sequence variants were generated from demultiplexed paired-end reads with DADA2 (50) and taxonomy was assigned based on the DADA2-formatted training dataset [GreenGenes Database Consortium, v13.8 (34)] in R (v3.5) (45).
Shotgun sequencing of fecal community DNA.
Fecal DNA was prepared and subjected to shotgun sequencing using methods described in ref. 5, with minor modifications. Libraries were generated from DNAs isolated from fecal samples collected at the first and the last sampling point of each dietary phase (n = 181) (Dataset S2B) using the Nextera XT kit (Illumina) with the reaction volume reduced to 2.5 μL (51). Libraries were then pooled and sequenced using an Illumina NovaSeq instrument (4.14 × 107 ± 1.01 × 107 paired-end 150-nt reads per sample (mean ± SD). Demultiplexed reads were quality filtered (Sickle; v1.33) (52) and Nextera adapter sequences were trimmed (cutadapt; v1.11) (53). Reads assigned to the host genome (Ensemble Sus scrofa genome; Sscrofa11.1) and the genomes of two dominant ingredients of the pig diets, corn (Ensemble Zea mays genome; B73_RefGen_v4) and soybean (Ensemble Glycine max genome; Glycine max v2.1), were removed using Bowtie2 (54) yielding 3.53 × 107 ± 1.02 × 107 (mean ± SD) reads per sample (Dataset S2). Filtered reads were subsequently assembled into contigs (Megahit; v1.1.4) (55). The resulting contigs were annotated with Prokka (v1.12) (56). Open reading frames identified by Prokka were clustered at 80% identity with MMSeq2 (–min-seq-id: 0.8) (57). Genes were given CAZyme annotations using the CAZy database (25). Additional functional information for each CAZyme-annotated gene was obtained by querying the National Center for Biotechnology Information nonredundant protein sequence database with DIAMOND (v0.9.19) (58) as well as by using a manually curated database developed by one of the coauthors (B.H.). Genes were assigned to metabolic pathways by querying the mcSEED database using DIAMOND (blastp; e-value < 0.001; percent identity >80%) (59). The number of reads assigned to each gene was normalized by gene length in order to express gene abundance as read counts per million (CPM). To calculate the CPM value for each gene, we first divided the reads assigned to that gene by its length in kilobases, then divided the sum of all reads per kilobase (RPK) values for all genes in a sample by one million and, finally, divided the RPK value for a gene by the per million scaling factor.
EMMER.
See SI Appendix, Supplementary Results for details of the EMMER algorithm. The Pandas (v0.42.2) (60) and NumPy (v1.16.4) (61) packages were employed for data processing and calculations of vNE. After selecting information-rich features, results were visualized with matplotlib (v3.1.0) (62). The Procrustes test (SciPy package; v1.3.0) (63) was applied to quantify the degree of similarity between PCA plots. Linear regression (sklearn package; v0.21.3) (64) was used to describe the relationship between the number of information-rich features and the dissimilarity score between PCA plots; the results were utilized to optimize the threshold selected for information-rich feature calling. PERMANOVA (scikit-bio package; v0.5.5) was used to test the statistical significance of differences in the projections of FF and DR pig microbial community samples on PCA plots.
Experiments Involving Gnotobiotic Mice.
Experiments involving mice were performed using protocols approved by the Washington University Animal Studies Committee. Germ-free C57BL/6J animals were housed in plastic flexible film gnotobiotic isolators (Class Biologically Clean) at 23 °C under a strict 12-h light cycle (lights on at 0600 hours).
Pulverized fecal samples that had been obtained on postnatal day 154 from the selected DR pig (animal no. 18) and FF pig (animal no. 11) were weighed; a 200-mg aliquot was placed in 50-mL conical plastic tubes which were brought into an anaerobic chamber (Coy Laboratory Products) containing an atmosphere of 75% N2, 20% CO2, and 5% H2. Once in the chamber, each sample was resuspended in sterile PBS/0.05% L-cysteine-HCl (1:10 [wt/vol]). After adding 2-mm-diameter glass beads (VWR, catalog no. 26396-506), the tube was gently vortexed for 20 s. The resulting mixture was passed through a 100-μm-pore diameter nylon cell strainer (BD Falcon); the filtrate was subsequently mixed with an equal volume of PBS/0.05% l-cysteine-HCl/30% glycerol, and aliquoted into 1.8-mL glass vials (E-Z vials, Wheaton). Tubes were crimped with covers containing a PTFE/gray butyl liner (Wheaton) and stored at −80 °C. Just prior to colonization, the clarified fecal sample was thawed within the gnotobiotic isolator and a 200-μL aliquot was administered to each recipient mouse via an oral gavage needle (catalog no. 7901; Cadence Science).
Animals were initially weaned onto an autoclaved sterile Teklad Global 18% Protein Rodent Diet (2018S; Envigo) and fed ad libitum. A finisher diet comparable to that given to pigs (Dataset S1) was sterilized by γ-irradiation (30 to 50 kGy); administration of this diet was initiated 2 d prior to gavage of pig microbiomes. Two groups of germ-free animals were colonized at 4.5 wk of age with fecal microbiomes from the selected DR and FF pigs (n = 6 mice per group; all animals singly-housed). After 21 d of ad libitum consumption of the finisher diet, animals in the first experiment were killed after a 4-h fast and cecal contents plus fecal samples were collected.
In the second experiment, 2 d after gavage of the representative DR and FF donor microbiomes, animals were singly housed (n = 6 mice per group) and given 3 g of the finisher diet per day for 7 d followed by 3.3 g of the diet per day for 10 d. The health status of all mice was monitored by daily observation and by measuring their weights every 2 d. On day 17 of the controlled feeding protocol, mice were subjected to a 4-h fast, blood was obtained by retro-orbital phlebotomy and animals were killed. Samples of liver, gastrocnemius muscle, cecal contents, and feces were obtained, snap frozen in liquid nitrogen, and stored at −80 °C. Serum was recovered from clotted blood samples after centrifugation (4,000 × g, 10 min, 4 °C) and aliquots were stored at −80 °C.
Serum levels of triglycerides were measured as described in ref. 47. Short-chain fatty acids, monosaccharides, disaccharides, and amino acids were quantified in fecal samples and acylcarnitine and acylCoA levels were measured in liver using targeted MS (5). Glycolytic and TCA cycle metabolites were quantified in gastrocnemius muscle following protocols described in ref. 7. Unadjusted P values from Mann–Whitney U tests are reported.
Methods used for isolating and sequencing cecal and fecal DNA were identical to those applied to pig fecal samples with the exception that shotgun reads assigned to the Mus musculus genome (Ensemble; GRCm38-mm10) were removed. HMMER (v3.1) (65) was used to determine how many of the 18, postnatal day 154, information-rich CAZymes could be detected in recipients of the representative DR and FF microbiomes. Each CAZyme gene, along with highly similar homologs identified by MMSeq2 in the pig shotgun sequencing dataset were aligned using Clustal Omega (v1.2.4) (66). Each resulting alignment then served as the input for constructing an HMM profile specific for one of the 18 information-rich CAZyme genes. Mouse shotgun sequencing data were subsequently searched using HMMER and each of these profiles was used to establish how many of the postnatal day 154 information-rich CAZyme genes could be identified in the cecal and fecal microbiomes of DR and FF recipient animals. A CAZyme gene was deemed “present” if a homolog with a bit score greater than 50 was found.
Supplementary Material
Acknowledgments
We thank Maria Karlsson, Marty Meier, Sabrina Wagoner, Justin Serugo, Su Deng, Jessica Hoisington-López, Kaitlyn Walker, Kaylyn Goranson, and Andrew Smith for superb technical assistance and Arjun Raman and Zachary Beller for helpful discussions during development of EMMER (entropy-based method for microbial ecology research). This work was supported by NIH Grant DK30292 (to J.I.G.) and the Bill & Melinda Gates Foundation (X.L.). H-W C. is the recipient of a “Government Scholarship to Study Abroad Predoctoral Fellowship, Taiwan.” J.I.G. is the recipient of a Thought Leader award from Agilent Technologies.
Footnotes
Competing interest statement: J.I.G. is a cofounder and N.P.M. is an employee of Matatu, Inc., a company characterizing the role of microbiota development and diet-by-microbiome interactions in animal health. This study received no funding from Matatu, Inc. No experimental or computational methods or datasets arising from this project were provided to Matatu, Inc., nor was any intellectual property belonging to Matatu, Inc. used in these studies. H-W.C., M.C.H., D.O., J.C., V.L., B.H., O.I., M.J.M., C.B.N., and M.J.B. are not affiliated with and do not receive financial support from Matatu. J.O. has conducted experimental animal trials for Matatu under research service agreements with his University (NCSU).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024446118/-/DCSupplemental.
Data Availability
Bacterial V4-16S rDNA amplicon sequencing and shotgun sequencing datasets in their raw format prior to postprocessing and data analyses have been deposited at the European Nucleotide Archive, https://www.ebi.ac.uk/ena/browser (accession no. PRJEB38446). The Python3 implementation of the EMMER algorithm can be accessed in GitHub (https://github.com/HWChang/emmer/). All other study data are included in the article and supporting information.
References
- 1.World Health Organization , Global nutrition report. https://www.who.int/nutrition/globalnutritionreport/en/. Accessed 23 November 2020.
- 2.Bartz S., et al., Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 99, 2128–2137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giallourou N., et al., Metabolic maturation in the first 2 years of life in resource-constrained settings and its association with postnatal growths. Sci. Adv. 6, eaay5969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S., et al., Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehrig J. L., et al., Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 365, eaau4732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman A. S., et al., A sparse covarying unit that describes healthy and impaired human gut microbiota development. Science 365, eaau4735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanton L. V., et al., Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351, aad3311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R. Y., et al., A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 384, 1517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G., et al., Production and supply of high-quality food protein for human consumption: Sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 1321, 1–19 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Ritchie H., Roser M., Meat and dairy production. https://ourworldindata.org/meat-production. Accessed 23 November 2020.
- 11.Patience J. F., Rossoni-Serão M. C., Gutiérrez N. A., A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 6, 33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller E. R., Ullrey D. E., The pig as a model for human nutrition. Annu. Rev. Nutr. 7, 361–382 (1987). [DOI] [PubMed] [Google Scholar]
- 13.Victora C. G., et al., What global maternal and child nutrition can learn from animal science. Lancet Glob. Health 5, e749–e751 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Han I. K., et al., Application of phase feeding in swine production. J. Appl. Anim. Res. 17, 27–56 (2000). [Google Scholar]
- 15.Lusk L. J., Norwood F. B., Pruitt R., Consumer demand for a ban on antibiotic drug use in pork production. Am. J. Arg. Econ. 88, 1015–1033 (2006). [Google Scholar]
- 16.Marshall B. M., Levy S. B., Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 24, 718–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Neumann J., Mathematische Grundlagen der Quantenmechanik (Springer, Berlin, 1932). [Google Scholar]
- 18.Bergou J. A., Hillery M., Introduction to the Theory of Quantum Information Processing (Springer, New York, NY, 2013). [Google Scholar]
- 19.National Research Council , Nutrient Requirement of Swine (National Academies Press, 2012). [Google Scholar]
- 20.Knauer M. T., Hostetler C. D., US swine industry productivity analysis, 2005 to 2010. J. Swine Health Prod. 21, 248–252 (2013). [Google Scholar]
- 21.Lima H. K., et al., Supplementation of maternal diets with docosahexaenoic acid and methylating vitamins impacts growth and development of fetuses from malnourished gilts. Curr. Dev. Nutr. 2, nzx006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regassa A., Nyachoti C. M., Application of resistant starch in swine and poultry diets with particular reference to gut health and function. Anim. Nutr. 4, 305–310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varel V. H., Activity of fiber-degrading microorganisms in the pig large intestine. J. Anim. Sci. 65, 488–496 (1987). [DOI] [PubMed] [Google Scholar]
- 24.Yen J. T., Nienaber J. A., Hill D. A., Pond W. G., Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J. Anim. Sci. 69, 2001–2012 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Cantarel B. L., et al., The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 37, D233–D238 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earle F. R., Curtis J. J., Hubbard J. E., Composition of the component parts of the corn kernel. Cereal Chem. 23, 504 (1946). [Google Scholar]
- 27.Watson S. A., Ramstad P. E., Corn: Chemistry and Technology (American Association of Cereal Chemists, St. Paul, MN, 1987). [Google Scholar]
- 28.Food and Agriculture Organization of the United Nations , Maize in Human Nutrition (Food and Agricultural Organization of the United Nations, Rome, 1992). [Google Scholar]
- 29.National Animal Nutrition Program , National Ingredient Database. https://animalnutrition.org/feed-composition-database-signup?destination=feed-composition-database. Accessed 23 November 2020.
- 30.Adams S. H., Odle J., Plasma β-hydroxybutyrate after octanoate challenge: Attenuated ketogenic capacity in neonatal swine. Am. J. Physiol. 265, R761–R765 (1993). [DOI] [PubMed] [Google Scholar]
- 31.Hoffman D. J., Sawaya A. L., Verreschi I., Tucker K. L., Roberts S. B., Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from São Paulo, Brazil. Am. J. Clin. Nutr. 72, 702–707 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Mallet L. E., Exton J. H., Park C. R., Control of gluconeogenesis from amino acids in the perfused rat liver. J. Biol. Chem. 244, 5713–5723 (1969). [PubMed] [Google Scholar]
- 33.Cunningham H. M., Friend D. W., Nicholson J. W. G., The effect of age, body weight, feed intake and adaptability of pigs on the digestibility and nutritive value of cellulose. Can. J. Anim. Sci. 42, 167–175 (1962). [Google Scholar]
- 34.Callahan B. J., The RDP and GreenGenes taxonomic training sets formatted for DADA2. Zenodo. 10.5281/zenodo.158955. Accessed 5 February 2019. [DOI]
- 35.Aziz R. K., et al., The RAST server: Rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overbeek R., et al., The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 42, D206–D214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seedorf H., et al., Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159, 253–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roediger W. E. E., Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21, 793–798 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roediger W. E. W., Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83, 424–429 (1982). [PubMed] [Google Scholar]
- 40.Sakata T., Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 58, 95–103 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Piva A., et al., Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 1, 35–41 (2002). [Google Scholar]
- 42.Lu J. J., Zou X. T., Wang Y. M., Effects of sodium butyrate on the growth performance, intestinal microflora and morphology of weanling pigs. J. Anim. Feed Sci. 17, 568–578 (2008). [Google Scholar]
- 43.Lykke M., et al., Malnutrition induces gut atrophy and increases hepatic fat infiltration: Studies in a pig model of childhood malnutrition. Am. J. Transl. Res. 5, 543–554 (2013). [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Khalaifah H. S., Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 97, 3807–3815 (2018). [DOI] [PubMed] [Google Scholar]
- 45.R core team, R: A Language and Environment for Statistical Computing. Version 3.5. https://www.R-project.org/. Accessed 2 May 2018.
- 46.Kuznetsova A., Brockhoff P. B., Christensen R. H. B., lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017). [Google Scholar]
- 47.Newgard C. B., et al., A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dey N., et al., Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell 163, 95–107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grubbs F. E., Sample criteria for testing outlying observations. Ann. Math. Stat. 21, 27–58 (1950). [Google Scholar]
- 50.Callahan B. J., et al., DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baym M., et al., Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS One 10, e0128036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi N. A., Fass J. N., Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files. Version 1.33. https://github.com/najoshi/sickle. Accessed 13 August 2018.
- 53.Martin M., Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 17, 10–12 (2011). [Google Scholar]
- 54.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D., Liu C.-M., Luo R., Sadakane K., Lam T.-W., MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Seemann T., Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Steinegger M., Söding J., MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Buchfink B., Xie C., Huson D. H., Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Rodionov D. A., et al., Micronutrient requirements and sharing capabilities of the human gut microbiome. Front. Microbiol. 10, 1316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinney W., Data structures for statistical computing in Python (Proceedings of the 9th Python in Science Conference, 2010), pp. 51–56. [Google Scholar]
- 61.Oliphant T. E., A Guide to NumPy (Massachusetts Institute of Technology, Cambridge, MA, 2006). [Google Scholar]
- 62.Hunter J. D., Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). [Google Scholar]
- 63.Virtanen P.et al.; SciPy 1.0 Contributors , SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedregosa F., et al., Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011). [Google Scholar]
- 65.Eddy S. R., Accelerated profile HMM searches. PLOS Comput. Biol. 7, e1002195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sievers F., et al., Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bacterial V4-16S rDNA amplicon sequencing and shotgun sequencing datasets in their raw format prior to postprocessing and data analyses have been deposited at the European Nucleotide Archive, https://www.ebi.ac.uk/ena/browser (accession no. PRJEB38446). The Python3 implementation of the EMMER algorithm can be accessed in GitHub (https://github.com/HWChang/emmer/). All other study data are included in the article and supporting information.