Abstract
Objectives
The COVID-19 pandemic has shocked the sports world because of the suspension of competitions and the spread of SARS-CoV-2 among athletes. After SARS-CoV-2 infection, cardio-pulmonary complications can occur and, before the resumption of sports competitions, a screening has been recommended. However, few data are available and discrepancies exist in the screening modalities. We conducted this prospective study to investigate the incidence of cardiovascular consequences following SARS-CoV-2 infection in young adult competitive athletes and the appropriate screening strategies for a safe return-to-play.
Methods
Ninety competitive athletes (24 ± 10 years) after asymptomatic or mildly symptomatic SARS-CoV-2 infection were screened by physical examination, blood testing, spirometry, 12‑lead resting ECG, 24-h ambulatory ECG monitoring, echocardiogram, and cardiopulmonary exercise testing (CPET).
Results
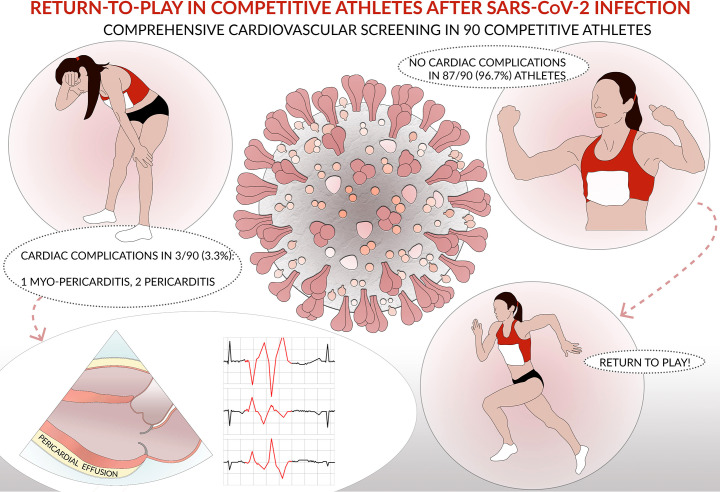
Sixty-four athletes (71.1%) were male, and most (76.7%) were mildly symptomatic. After SARS-CoV-2 infection, spirometry and resting ECG were normal in all athletes. Ambulatory ECG monitoring demonstrated <50/24 h supraventricular and ventricular premature beats in 53.3% and 52.2% of athletes, respectively, in the absence of malignant arrhythmias. CPET did not demonstrate cardiopulmonary limitations. Echocardiography showed pericardial effusion in 3 athletes (all females) with symptomatic SARS-CoV-2 infection (3.3%; 4.4% in the symptomatic group) with a definitive diagnosis of myopericarditis in 1 athlete (1.1%) and pericarditis in 2 athletes (2.2%).
Conclusions
Cardiac consequences of SARS-CoV-2 infection were found in 3.3% of competitive athletes. An appropriate screening primarily based on the detection of uncommon arrhythmias and cardiac symptoms should be recommended in competitive athletes after SARS-CoV-2 infection to detect a cardiac involvement and guarantee a safe return-to-play.
Keywords: COVID-19; SARS-CoV-2 infection; Myocarditis, pericarditis; Pre-participation screening; Return-to-play; Athletes
1. Introduction
SARS-CoV-2 is the causative virus responsible for the coronavirus disease of the 2019 (COVID-19) pandemic that rapidly spread worldwide with several implications on public health and society [1]. The COVID-19 pandemic has shocked the world of sport, initially for the suspension of competitions and training activities, then with the spread of SARS-CoV-2 among several athletes and entire sports teams [2]. In patients with SARS-CoV-2 infection, systemic inflammation and pulmonary complications can result in significant morbidity and mortality, and cardiovascular complications may occur, including myocarditis, pericardial inflammation, acute coronary syndromes, heart failure, arrhythmias, and venous thromboembolic events [1,3]. Although competitive athletes are usually young and healthy and may develop SARS-CoV-2 infection asymptomatically or with mild symptoms [2], concerns exist about the potential Covid-19 cardiac complications among athletes and the risk of myocardial and myopericardial involvement leading to sport-related arrhythmias and eventually sudden cardiac death (SCD). Accordingly, after SARS-CoV-2 infection and before the resumption of competitions, cardiovascular screening is recommended to exclude COVID-19-specific complications that may pose a risk to the athlete and decide whether, when and how to resume training and competitions [[4], [5], [6]]. However, uncertainties exist about the prevalence of cardiac complications after SARS-CoV-2 infection, and the optimal approach to screen competitive athletes after Covid-19 infection and different screening protocols have been recommended for a safe return to sports [2,5,6]. These recommendations are primarily based on expert opinion [5] and unfortunately, little data are currently available in competitive athletes. Therefore, we conducted this prospective study to investigate the incidence of cardiovascular complications following SARS-CoV-2 infection in young adult competitive athletes and to identify the appropriate screening tests for a safe return-to-play.
2. Methods
The study was conducted in professional and non-professional competitive athletes with previous asymptomatic or mildly symptomatic SARS-CoV-2 infection. Mild symptoms were defined as: nonspecific and self-limited fatigue, non-persistent fever, anosmia or ageusia, nausea, vomiting, diarrhoea, headache, cough, sore throat, and nasopharyngeal congestion. Athletes with severe infection requiring hospitalization or veteran athletes (i.e. >50 years of age [7]) were excluded from the study. The participants were evaluated according to the preparticipation screening protocol recommended before the 10th of December by the Italian Federation of Sports Medicine (FMSI) (https://www.fmsi.it/images/img/archivio/protocollo_FMSI_ripresa-att-sport_20200430-3.pdf) for resuming competitive training after the resolution of SARS-CoV-2 infection. According to the protocol, all competitive athletes were subjected to the following investigations:
-
−
Personal history and clinical profile: history of pulmonary or cardiovascular disease, comorbidities, familiar history for SCD or coronary artery disease, drug therapy, type and duration of symptoms related to SARS-CoV-2 infection or symptoms suggestive of cardiac involvement (i.e., palpitations, dyspnoea, syncope, typical or atypical chest pain).
-
−
Blood testing: blood count with formula, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), creatine phosphokinase (CPK), high-sensitivity troponin I (TnI), C reactive protein (CRP), lactic acid dehydrogenase (LDH), protein electrophoresis, D-dimer, ferritin, urine examination.
-
−
Twelve‑lead resting ECG: the interpretation of ECG was performed according to the current international criteria for the interpretation of ECG in athletes [8].
-
−
Twelve‑lead 24-h ambulatory ECG monitoring: the presence of rhythm abnormalities, conduction disturbances, supraventricular arrhythmias, ventricular arrhythmias, and other abnormal findings were investigated [8]; ventricular arrhythmias were classified as common or uncommon [[9], [10], [11], [12]], and further investigations were requested according to the current recommendations [11].
-
−
Echocardiography: biventricular size and function, wall motion abnormalities, the presence of valvular heart disease, and pericardial effusion were analyzed according to the standardized criteria applied to the general population and competitive athletes [13,14].
-
−
Cardiopulmonary exercise testing (CPET): Data on CPET methods are available on supplementary data methods.
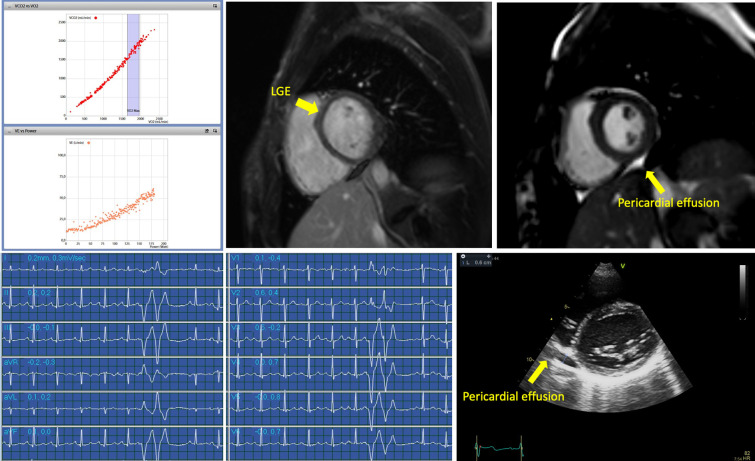
In case of positive abnormal findings, additional clinical investigations were performed according to the principles of good clinical practice and the current guidelines to rule out cardiovascular abnormalities, i.e. cardiac magnetic resonance (CMR) and chest computed tomography (CT). The diagnosis of myocarditis was based on the presence of 2 out of 3 diagnostic targets of the Lake Louise Criteria at CMR [15]: edema, hyperemia, and necrosis or scar—derived from signal intensity assessment in T2-weighted, early gadolinium enhancement and late gadolinium enhancement (LGE) CMR images. The diagnosis of pericarditis was based on the presence of at least 2 of the 4 following criteria: chest pain, pericardial rubs, new widespread ST-elevation or PR depression on ECG, pericardial effusion (new or worsening) [16].
2.1. Safety procedures
Data on safety procedures are available on supplementary data methods.
The Ethics Committee of the University of Siena approved the study.
3. Statistical analysis
Normal distribution of all continuous variables was examined using the Shapiro-Wilk test, and data are presented as mean ± SD. Categorical variables are expressed as percentages. According to data distribution, the unpaired t-test and the Mann-Whitney test were used to assess the between groups significance (asymptomatic vs symptomatic; males vs females). The chi-squared test was used for nominal data. A p value <0.05 was considered statistically significant. Statistics were performed using SPSS, version 21.0 (Statistical Package for the Social Sciences Inc., Chicago, Illinois, USA).
4. Results
Ninety competitive athletes with previous asymptomatic or mildly symptomatic SARS-CoV-2 infection were included in the study. The demographic characteristics of the study population are reported in Table 1. Sixty-four athletes (71.1%) were males, and most of the athletes practised mixed sport (i.e., soccer, volleyball…). Most athletes (76.7%) were mildly symptomatic, particularly for fever, anosmia and ageusia, with an average duration of symptoms of 9 ± 14 days. The main clinical findings are reported in Table 2. Spirometry and 12‑lead resting ECG after the infection were normal in all athletes. Twenty-four ambulatory ECG monitoring showed isolated supraventricular and ventricular premature beats (VPBs) in 53.3% and 52.2% of the cases, respectively, in the absence of malignant arrhythmias. The two athletes with a VPBs' count between 50 and 500 had common VPBs' with infundibular and fascicular origin, respectively, isolated, monomorphic and suppressed by exercise. Given that 12‑lead resting ECG and echocardiography were normal, no additional investigations were required. During CPET, neither VPBs nor St-T abnormalities were found, except for an athlete showing isolated VPBs and couplets at the peak and immediately after the effort. In this athlete, echocardiography demonstrated the presence of mild pericardial effusion. Normal biventricular dimension and function and no significative valvular regurgitation or stenosis were observed in the overall population, while mild pericardial effusion was found in 3 (3.3%) female athletes. CMR was performed in these 3 cases, and a final diagnosis of myopericarditis was reached in 1 athlete. The athlete with myopericarditis was a 22-year-old professional soccer player, mildly symptomatic for fever, cough, and asthenia for few days. The evaluation was performed 30 days after the negativization of the nasopharyngeal swab. Blood testing, spirometry, 12‑lead resting ECG were normal. Ambulatory ECG monitoring showed only 14 VPBs/24 h, although most of them were found during the training session. CPET parameters were in range except for the presence of VPBs and couplets during the effort, with a right bundle branch block morphology and a wide QRS, and echocardiography demonstrated mild pericardial effusion (Supplementary Fig. 1). CMR demonstrated the myocardial involvement with edema and mild LGE. A period of detraining of 3 months was prescribed; after this period, a complete clinical evaluation was repeated: echocardiography demonstrated the absence of pericardial effusion and ventricular arrhythmias were not detected during the exercise testing. According to this clinical data, the athlete was considered eligible for sports competitions. The other two athletes were young females with mild pericardial effusion, symptomatic for chest pain during SARS-CoV-2 infection; they were evaluated after 15 and 30 days respectively after infection's resolution, and they were asymptomatic at the time of evaluation, with normal ECG. In one athlete a mild increase in TnI was found. CMR performed in these two athletes confirmed the diagnosis of pericarditis, in absence of myocardial involvement. No arrhythmias were demonstrated. The athletes were disqualified from sports competitions for three months and considered eligible, after the resolution of pericardial effusion, the absence of cardiac symptoms and uncommon arrhythmias.
Table 1.
Demographic characteristics of the study population.
| Variables | N = 90 |
|---|---|
| Age, years | 24 ± 10 |
| Males, n (%) | 64 (71.1) |
| Asymptomatic n (%) | 21 (23.3) |
| Mild symptomatic n (%) | 69 (76.7) |
| Fever n (%) | 50 (72.5) |
| Cough n (%) | 20 (29.0) |
| Dyspnoea n (%) | 6 (8.7) |
| Asthenia n (%) | 31 (44.9) |
| Ageusia n (%) | 34 (49.3) |
| Anosmia n (%) | 35 (50.7) |
| Diarrhoea n (%) | 5 (7.3) |
| Headache n (%) | 25 (36.2) |
| Symptoms duration, days | 9 ± 14 |
| Cardiac complications n (%) | 3 (3.3) |
| BSA, m2 | 1.9 ± 0.3 |
| BMI, kg/m2 | 22.5 ± 2.9 |
| Type of sport | |
| Endurance, n (%) | 13 (14.5) |
| Mixed, n (%) | 76 (84.4) |
| Power, n (%) | 1 (1.1) |
| Skill, n (%) | 0 (0) |
BSA, body surface area. BMI, Body Mass Index.
Table 2.
Clinical findings in asymptomatic or mildly symptomatic Covid-19+ competitive athletes.
| Variables | N = 90 |
|---|---|
| Evaluation time from negativization | |
| <15 days, n (%) | 37 (41.1) |
| 16–30 days, n (%) | 12 (13.3) |
| >30 days, n (%) | 41 (45.6) |
| Abnormal blood tests, n (%) | 5 (5.5) |
| Abnormal spirometry at rest, n (%) | 0 (0) |
| Abnormal 12‑lead resting ECG n (%) | 0 (0) |
| Maximum HR, bpm | 177 ± 15 |
| % predicted maximum HR | 90 ± 5 |
| Resting SBP, mmHg | 117 ± 10 |
| Resting DBP, mmHg | 72 ± 8 |
| Maximum SBP, mmHg | 182 ± 22 |
| Maximum DBP, mmHg | 80 ± 9 |
| 24-h Holter ECG | |
| <50 SPVBs n (%) | 48 (53.3) |
| <50 PVBs n (%) | 47 (52.2) |
| 51–500 SPVBs n (%) | 2 (2.2) |
| 51–500 PVBs n (%) | 2 (2.2) |
| >500 SPVBs n (%) | 0 (0) |
| >500 PVBs n (%) | 0 (0) |
| PVBs during exercise testing, n (%) | 1 (1.1) |
| ST-T abnormalities during exercise, n (%) | 0 (0) |
| Echocardiographic abnormalities, n (%) | 3 (3.3) |
| Pericardial effusion, n (%) | 3 (3.3) |
| LV systolic dysfunction, n (%) | 0 (0) |
| Wall motion abnormalities n (%) | 0 (0) |
| LV pathological dilatation, n (%) | 0 (0) |
| RV systolic dysfunction, n (%) | 0 (0) |
| RV pathological dilatation, n (%) | 0 (0) |
| Pulmonary Hypertension, n (%) | 0 (0) |
HR, heart rate; SBP, systolic blood pressure; DPB, diastolic blood pressure; PVB, premature ventricular beats, SPVB, premature supraventricular beats, LV, left ventricle, RV, right ventricle.
CPET parameters are described in Table 3. No pulmonary or cardiac limitation to exercise was identified, peak exercise oxygen pulse was normal, and chronotropic competence was preserved. However, in the athlete with a definitive diagnosis of myopericarditis during CPET exercise-induced uncommon ventricular arrhythmias were observed.
Table 3.
Cardiopulmonary parameters in Covid-19+ competitive athletes.
| Variables | N = 90 |
|---|---|
| Peak Cycling Power Output, watt | 227 ± 62 |
| Peak METS | 11.2 ± 1.8 |
| Peak RER | 1.1 ± 0.1 |
| Oxygen desaturation n (%) | 0 (0) |
| Peak VO2, mL/min | 2781 ± 719 |
| Peak VO2/Kg, mL/min/Kg | 39.0 ± 6.6 |
| Peak VO2, %predicted | 95 ± 15 |
| VT1 VO2, mL/min | 1455 ± 397 |
| VT1 VO2/Kg, mL/min/Kg | 20.8 ± 5.5 |
| VT1 peak VO2, %measured | 53 ± 12 |
| VT1 HR, bpm | 125 ± 23 |
| VT2 VO2, mL/min | 2289 ± 639 |
| VT2 VO2/Kg, mL/min/Kg | 32.2 ± 5.8 |
| VT2 peak VO2, %measured | 84 ± 8 |
| VT2 HR, bpm | 160 ± 19 |
| VE/VCO2 slope | 26.7 ± 3.2 |
| Peak VE, l/min | 90.8 ± 22.8 |
| Peak VO2/HR, mL/min/bpm | 16.0 ± 4.7 |
| Peak VO2/HR, % | 105 ± 15 |
| Peak VO2/WR, mL/min/W | 10.4 ± 0.9 |
| BR % | 45.5 ± 15.8 |
METS: Metabolic Equivalent of Task; RER: Respiratory Exchange Ratio; VO2 oxygen uptake; VT1 first ventilatory threshold; VT2, second ventilatory threshold, VCO2, exhaled carbon dioxide; VE: ventilation; WR, Work Rate; HR heart rate.
The blood testing results are reported in Table 4 . In most athletes, the parameters were within the normal ranges after SARS-CoV-2 infection, except for an increase in TnI in one athlete (the athlete with pericarditis previously described), an increase in GGT in 1 athlete (1%), in CPK in 1 athlete (1%), and in D-dimer in 2 athletes (2%). In these two athletes, chest CT was performed, and pulmonary embolism was excluded; however, pulmonary bronchiolitis was observed in one athlete with a value of D-dimer = 3133. In this athlete, all other sources of thromboembolism were excluded. Given the absence of symptoms and cardiopulmonary limitations at CPET, new blood tests were required after 1 week, demonstrating a reduction (although not a complete normalization) of abnormal laboratory parameters. Accordingly, a gradual return-to-play was suggested, and further blood tests were required during the follow up to ensure a normalization of these parameters.
Table 4.
Blood test findings in Covid-19+ competitive athletes.
| Variables | N = 90 |
|---|---|
| Red blood cells, x106/ mm3 | 5.1 ± 0.4 |
| White blood cells, x103/mm3 | 6.3 ± 1.4 |
| Haemoglobin, g/L | 14.8 ± 1.1 |
| Hematocrit, % | 44.5 ± 3.5 |
| Mean corpuscular volume, fl | 88.1 ± 4.1 |
| Mean corpuscular haemoglobin, pg | 29.2 ± 1.6 |
| Platelets, x103/ mm3 | 232.5 ± 57.8 |
| Lymphocytes% | 36.7 ± 6.2 |
| Neutrophils% | 51.7 ± 7.2 |
| Monocytes% | 7.3 ± 1.9 |
| Eosinophils% | 3.4 ± 2.5 |
| Basophils% | 0.8 ± 0.5 |
| GOT, U/L | 24 ± 14 |
| GPT, U/L | 21 ± 16 |
| GGT, U/L | 20 ± 14 |
| Creatinine, mg/dl | 1.04 ± 0.2 |
| CPK, U/L | 232 ± 396 |
| LDH, U/L | 256 ± 106 |
| D-Dimer, ng/mL | 302 ± 495 |
| CRP, mg/L | 0.19 ± 0.46 |
| Ferritin, ng/mL | 111 ± 69 |
GOT, Glutamic Oxaloacetic Transaminase; GPT, Glutamic Pyruvic Transaminase; GGT, gamma-glutamyl transferase; CPK creatine phosphokinase; LDH, Lactate dehydrogenase; CRP, c-reactive protein.
Comparison between male and female athletes and between asymptomatic and mildly symptomatic athletes are available as supplementary data.
Fig. 1 summarizes the main findings of this study.
Fig. 1.
Central illustration summarizing the main findings of the study.
4.1. Safety
Data on safety results are available on supplementary data results.
5. Discussion
Although competitive athletes are usually young and healthy and may develop SARS-CoV-2 infection asymptomatically or with mild symptoms [2], concerns exist about the potential Covid-19 cardiac complications among athletes and the risk of cardiac involvement leading to sport-related arrhythmias and malignant events. This study investigated the prevalence of cardiac complications after SARS-CoV-2 infection in young adult competitive athletes screened before their return-to-play. An extensive clinical screening was conducted based on physical examination, 12‑lead resting ECG, blood test, 24-h Holter ECG monitoring, echocardiogram, and CPET. The main findings of this original prospective study are: i) cardiac complications occurred as myopericarditis and pericarditis in 3.3% of competitive athletes recovering from SARS-CoV-2 infection, particularly in women and in athletes with mildly symptomatic infection, confirming that screening is necessary in this population for a safe return-to-play; ii) the presence of cardiac symptoms and exercise-induced uncommon ventricular arrhythmias [9,10,17] identified by exercise testing allowed us to suspect a cardiac involvement, confirmed firstly by echocardiography, supporting the use of screening in competitive athletes after SARS-CoV-2 infection based on physical examination, 12‑lead resting ECG, and exercise testing, with additional tests indicated only in specific cases.
In patients with SARS-CoV-2 infection, systemic inflammation and pulmonary complications can result in significant morbidity and mortality, and cardiovascular complications may include myocarditis, pericardial inflammation, acute coronary syndromes, heart failure, arrhythmias, and venous thromboembolic events [1,3]. Myocarditis and pericarditis after COVID-19 could be related to several pathways. The SARS-CoV-2 virus binds to angiotensin-converting enzyme 2 (ACE2) in the upper airways and lungs after the protein spike on the virus is activated by transmembrane protease serine 2 [5,18]. The myocardium also contains a high concentration of ACE2 receptors, activation of which may exert direct toxic effects within the myocardium leading to myocarditis with lymphocyte-rich inflammatory histology, acute impairment of cardiac muscle function and potentially residual chronic scar with increased vulnerability to malignant ventricular arrhythmias [19]; the intense ‘cytokine storm’ that develops during severe COVID-19 illness may lead to cardiac dysfunction, similar to other forms of sepsis [19,20]. Cardiovascular involvement detected by CMR has been demonstrated in up to 78% of patients with recent COVID-19 illness, independent of preexisting conditions, severity and overall course of the acute illness, time from the original diagnosis or presence of cardiac symptoms [21]. The most prevalent abnormality was myocardial inflammation (defined as abnormal native T1 and T2 measures), detected in 60% of patients, followed by regional scar (32%) and pericardial enhancement (22%) [21]. COVID-19-related myocarditis has been described in adults and young and adolescent individuals [22,23]. Unfortunately, myopericarditis is a well-recognized cause of SCD during sport in athletes [24,25] and therefore competitive athletes recovering from COVID-19 may be theoretically at risk when they return to play. Few data are currently available regarding the cardiac involvement after SARS-CoV-2 infection in athletes. In this study, we found that 3.3% of competitive athletes had a cardiac involvement after SARS-CoV-2-infection (2 pericarditis, 1 myopericarditis). A recent research has demonstrated that more than 1 in 3 previously healthy college athletes recovering from COVID-19 infection showed imaging features of a resolving pericardial inflammation [3]: 48 athletes underwent both echocardiography and CMR, and abnormal findings were identified in 27 (56.3%) individuals with 19 athletes (39.5%) showing pericardial late enhancement with associated pericardial effusion. A recent CMR study [26], performed in 26 competitive college athletes with previously asymptomatic or mildly symptomatic COVID-19, demonstrated that four athletes (15%) had CMR findings consistent with myocarditis and 2 of them also had pericardial effusion: two of these four athletes with evidence of myocardial inflammation had mild symptoms (shortness of breath) while the other 2 were asymptomatic. Notably, eight athletes (30.8%) had LGE without concomitant T2 elevation. There were no diagnostic St-T segment changes, and ventricular volumes and function were within the normal range in all athletes by echocardiography; none had elevated serum levels of troponin I [26]. A lower prevalence of myocarditis (1.4%) detected by CMR was found in a population of 145 competitive student athletes recovering from COVID-19: one patient did not experience SARS-CoV 2 related symptoms but had abnormal serum troponin-I level and nonspecific ST–T-wave ECG abnormalities; the other patient had mild to moderate SARS-CoV-2 symptoms but had normal laboratory values [27]. The specificity of current CMR myocarditis criteria in the asymptomatic and otherwise healthy population is unknown, and the degree to which postinfectious CMR findings develop with common respiratory viral pathogens remains unknown [6,28]. In agreement with these findings, in this study, we demonstrated that young competitive athletes might develop myopericarditis and pericarditis after SARS-CoV-2 infection, even if the percentage of athletes found with cardiac complications were lower. In the athletes diagnosed with myopericarditis, blood test, spirometry, and 12‑lead ECG were normal. A myocardial involvement was suspected by the presence of exercise-induced VPBs with uncommon morphology at exercise testing and ambulatory ECG monitoring (including a training session) [9,10] and pericardial effusion at echocardiography. Conversely, the two athletes with pericarditis were symptomatic for chest pain. Therefore, cardiac complications were initially suspected in these athletes based on exercise-induced uncommon ventricular arrhythmias and cardiac symptoms.
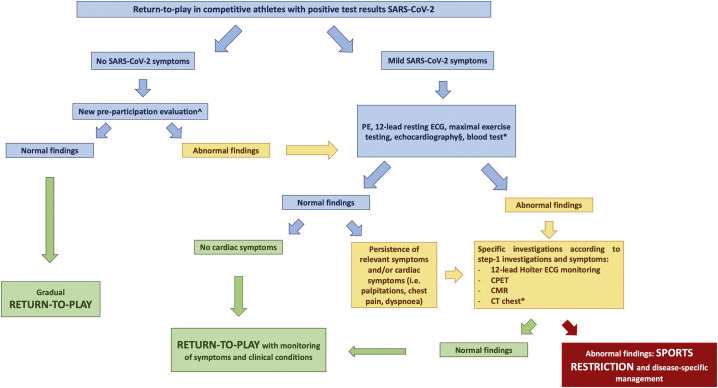
According to the present findings and in agreement with the recommendations currently proposed, SARS-CoV-2-positive competitive athletes should be adequately screened before the resumption of sport to exclude cardiopulmonary complications that may pose a risk to the athlete during the sports activity. However, discrepancies exist in terms of screening modalities among the different recommendations published. This heterogeneity is primarily due to the lack of data and differences among the countries in the pre-participation evaluation (PPE), leading to guidelines mainly based on experts' opinion. Indeed, some experts do not advocate cardiovascular risk stratification in athletes after SARS-CoV-2 infection with mild symptoms or in asymptomatic athletes that completely resolve during 10 days of self-isolation after a positive test result or symptom onset [6]. They also suggested that cardiovascular examinations (hs-cTn, 12‑lead resting ECG and echocardiography -and further tests if necessary-) should be considered on an individualized basis for athletes with protracted symptoms (≥10 days) and are recommended for athletes with prior moderate or severe COVID-19 [6,29]. Conversely, other authors have suggested for athletes with mild-to-moderate COVID-19 symptoms, who managed their condition at home and have recovered, a thorough clinical assessment with a medical history and physical examination, 12‑lead resting ECG and echocardiography, if they are free from symptoms at rest for 7 days and no sooner than day 10 from the onset of symptoms. Conversely, ECG, CMR, chest X-ray, lung function, hs-Ctn, D-dimer, CRP are recommended in athletes not recovered from COVID-19 ≥ 14 days from symptoms onset (particularly in case of fatigue, cough, chest pain, palpitation, or dyspnoea) and a more extensive evaluation in hospitalized athletes or in case of abnormal cardiac results [30]. Other experts reserve 12‑lead resting ECG, echocardiography and maximal exercise testing to athletes who are currently asymptomatic but had a debilitating illness lasting >7 days and/or experience symptoms compatible with myocarditis/pericarditis, and a complete evaluation (e.g. CMR and ambulatory ECG monitoring) to athletes who are experiencing persistent cardiac symptoms even after the acute infection has resolved or hospitalized or with reduced performance despite an appropriate re-training regimen [5]. Moreover, other authors proposed screen athletes with positive SARS-Cov-2 test results who remained asymptomatic with resting ECG and athletes recovering from symptomatic SARS-CoV-2 infection, without cardiac symptoms suggestive for myopericarditis, with resting ECG, exercise testing and echocardiography [2]. In line with this approach, our findings suggest that a PPE based on physical examination, resting ECG and exercise testing may identify athletes with cardiac consequences after asymptomatic or mildly symptomatic SARS-CoV-2 infection and that cardiac imaging and additional tests should be reserved for athletes with abnormal findings or with symptoms suggestive of cardiac involvement. The proposal of a clinical algorithm to screen athletes after COVID-19 is showed in Fig. 2 . Notably, in agreement with the study by Rajpal et al. [26], athletes with myopericarditis in our study did not show an abnormal ECG at rest; therefore, myopericardial involvement after COVID-19 could be missed by the use of resting ECG and troponin only, especially when the clinical evaluation is performed several days after infection resolution, as usually occurs in the clinical practice [26,31]. Conversely, exercise testing may elicit uncommon ventricular arrhythmias [9,10] in the case of myocarditis and may unmask myocardial involvement after SARS-CoV-2 infection, indicating a CMR. According to our findings, CPET, CMR and chest CT should be indicated only in selected cases, while their extensive application to all athletes after SARS-CoV-2 infection may have an unjustified impact on the costs of the return-to-play screening without a net clinical benefit.
Fig. 2.
Proposal of a clinical algorithm to screen competitive athletes recovering from SARS-CoV-2 infection before their return-to-play.
*The test is indicated on a case-by-case decision, based on clinical symptoms, individual risk profile and clinical course of Covid-19 infection.
^New pre-participation evaluation should be performed according to national modalities and recommendations.
§ in case of abnormal 12‑lead resting ECG, uncommon ventricular arrhythmias or cardiac symptoms.
In our study, cardiac complications were not found in athletes who had an asymptomatic SARS-CoV-2 infection. However, other authors demonstrated that myocardial and pericardial abnormalities could also be found in asymptomatic athletes recovering from COVID-19 infection when an extensive imaging screening, including CMR, was conducted [3,26]. Therefore, given that the screening should exclude potential life-threatening cardiac conditions and that exercise may result in accelerated virus replication, increased inflammation and cellular necrosis with a proarrhythmic myocardial substrate during the acute phase [32], further studies are needed to confirm whether asymptomatic athletes have a lower risk of cardiac complications as compared to symptomatic athletes. However, an extensive screening including CMR is neither feasible nor cost-effective; accordingly, we suggested a new PPE in young adult athletes recovering from SARS-CoV-2 infection, to be performed according to national guidelines for PPE in competitive athletes and, particularly in athletes after symptomatic SARS-CoV-2 infection, including an exercise testing. PPE is traditionally viewed as a tool to screen for unknown cardiovascular diseases that predispose the athlete to SCD, while in the COVID-19 era, it should be conducted not only to search for congenital or genetic cardiac disorders but also for possibly life-threatening cardiovascular sequelae due to COVID-19 [19].
6. Limitations
In this study, we found that all athletes with myopericardial involvement after COVID-19 were females. However, previous studies conducted in the general population observed that myopericarditis was significantly more common among men [33,34], in agreement also with the study by Rajpal et al. [26] conducted in young athletes. The present study was not powered to detect differences between men and women practising sport in terms of COVID-19-related cardiac complications; further studies are needed to investigate whether specific categories of athletes may be at higher risk of myopericarditis after SARS-CoV-2 infection.
All athletes enrolled in this study were Caucasians. Although data on the impact of ethnicity in COVID-19 patients remains limited, emerging data suggest that black, Asian, and minority ethnic individuals are at an increased risk of acquiring SARS-CoV-2 infection than white individuals with a worse clinical outcome from COVID-19 [[35], [36], [37]]. Whether these findings are influenced by ethnicity or socioeconomic status and whether they can also be applied to athletes is currently unknown and should prompt further investigation.
Finally, prognostic data on cardiac complications found after COVID-19 still lack in the general population and athletes. Further studies will describe the outcome of patients with COVID-19-related cardiac complications.
7. Conclusions
The COVID-19 pandemic has shocked the world of sport, and there is an ongoing concern about COVID-19-associated cardiac complications in athletes. In a population of young adult competitive athletes recovering from asymptomatic or mildly symptomatic SARS-CoV-2 infection, we found that 3.3% of athletes had myopericarditis or pericarditis, associated with exercise-induced ventricular arrhythmias or cardiac symptoms. Therefore, to exclude cardiac complications, screening should be conducted in SARS-CoV-2-positive athletes to allow a safe return-to-play and guarantee disease-specific management, including potential temporary sports restriction in case of abnormal findings until complete healing. A screening based on physical examination, ECG, exercise testing and cardiac imaging in case of abnormal findings may detect cardiovascular complications. In contrast, blood testing, spirometry and CPET are unnecessary in asymptomatic or mildly symptomatic athletes and should be performed only in selected cases.
Contributorship
LC, FD drafted the manuscript. FF, FT, NM, PS, SB, AP, and NC contributed to the data acquisition. LC, FD, NM, PS, and MB contributed to the interpretation of the data. FD analyzed the data, LC and FD contributed to the conception and the design of the study. SV, MF, MC, and MB critically revised the manuscript. All authors gave the final approval.
Funding
none.
Declaration of Competing Interest
none.
Acknowledgements
The authors wish to thank all the members of the medical staff and the nurses who actively participated in data collection. A special thanks to Massimo Capitani and to Franca Giuggia e Stefania Rosati for their valuable support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.05.042.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Clinical findings in a competitive athlete with myopericarditis after SARS-CoV-2 infection. Exercise-induced uncommon ventricular arrhythmias and mild pericardial effusion were demonstrated during the screening; accordingly, a cardiac magnetic resonance was performed, confirming pericardial effusion and demonstrating the presence of late gadolinium enhancement.
Supplementary Table 1. Comparison between male and female competitive athletes after Covid-19 infection.
Supplementary table 2. Comparison between asymptomatic vs. symptomatic competitive athletes after SARS-CoV-2 infection.
Supplementary data methods describing how CPET was conducted and the safety procedures.
References
- 1.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schellhorn P., Klingel K., Burgstahler C. Return to sports after COVID-19 infection. Eur. Heart J. 2020;41:4382–4384. doi: 10.1093/eurheartj/ehaa448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brito D., Meester S., Yanamala N., Patel H.B., Balcik B.J., Casaclang-Verzosa G., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc. Imaging. 2020;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gervasi S.F., Pengue L., Damato L., Monti R., Pradella S., Pirronti T., et al. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br. J. Sports Med. 2020;55:54–61. doi: 10.1136/bjsports-2020-102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia R.T., Marwaha S., Malhotra A., Iqbal Z., Hughes C., Borjesson M., et al. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European Association of Preventive Cardiology (EAPC) Eur. J. Prev. Cardiol. 2020;27(12):1242–1251. doi: 10.1177/2047487320930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.H., Levine B.D., Phelan D., Emery M.S., Martinez M.W., Chung E.H., et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2020;6:219–227. doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 7.Wilson M., O’Hanlon R., Prasad S., Deighan A., Macmillan P., Oxborough D., et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J. Appl. Physiol. (1985) 2011;110(6):1622–1626. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S., Drezner J.A., Baggish A., Papadakis M., Wilson M.G., Prutkin J.M., et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018;39(16):1466–1480. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D., Drezner J.A., D’Ascenzi F., Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br. J. Sports Med. 2019;54:1142–1148. doi: 10.1136/bjsports-2018-100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Ascenzi F., Zorzi A., Alvino F., Bonifazi M., Corrado D., Mondillo S. The prevalence and clinical significance of premature ventricular beats in the athlete. Scand. J. Med. Sci. Sports. 2017;27(2):140–151. doi: 10.1111/sms.12679. [DOI] [PubMed] [Google Scholar]
- 11.Heidbuchel H., Arbelo E., D’Ascenzi F., Borjesson M., Boveda S., Castelletti S., et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: ventricular arrhythmias, channelopathies, and implantable defibrillators. Europace. 2020;23:147–148. doi: 10.1093/europace/euaa106. [DOI] [PubMed] [Google Scholar]
- 12.D’Ascenzi F., Zorzi A., Alvino F., Bonifazi M., Mondillo S., Corrado D. Premature ventricular beats in young athletes: interpretation and diagnostic pathway. G Ital Cardiol (Rome) 2019;20(4):229–241. doi: 10.1714/3126.31076. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. (e14) [DOI] [PubMed] [Google Scholar]
- 14.Pelliccia A., Caselli S., Sharma S., Basso C., Bax J.J., Corrado D., et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018;39(21):1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T., et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J. Am. Coll. Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler Y., Charron P., Imazio M., Badano L., Baron-Esquivias G., Bogaert J., et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Florio A., Fusi C., Anselmi F., Cavigli L., Focardi M., Cameli M., et al. Clinical management of young competitive athletes with premature ventricular beats: a prospective cohort study. Int. J. Cardiol. 2021;330:59–64. doi: 10.1016/j.ijcard.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baggish A., Drezner J.A., Kim J., Martinez M., Prutkin J.M. Resurgence of sport in the wake of COVID-19: cardiac considerations in competitive athletes. Br. J. Sports Med. 2020;54(19):1130–1131. doi: 10.1136/bjsports-2020-102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 21.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trogen B., Gonzalez F.J., Shust G.F. COVID-19-associated myocarditis in an adolescent. Pediatr. Infect. Dis. J. 2020;39(8) doi: 10.1097/INF.0000000000002788. e204-e5. [DOI] [PubMed] [Google Scholar]
- 24.Peretto G., Sala S., Rizzo S., De Luca G., Campochiaro C., Sartorelli S., et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16(5):793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Corrado D., Basso C., Pavei A., Michieli P., Schiavon M., Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296(13):1593–1601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 26.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarski T.P., Simonetti O.P., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starekova J., Bluemke D.A., Bradham W.S., Eckhardt L.L., Grist T.M., Kusmirek J.E., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2020.7444. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J. Am. Coll. Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 29.Phelan D., Kim J.H., Elliott M.D., Wasfy M.M., Cremer P., Johri A.M., et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. JACC Cardiovasc. Imaging. 2020;13(12):2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson M.G., Hull J.H., Rogers J., Pollock N., Dodd M., Haines J., et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020;54(19):1157–1161. doi: 10.1136/bjsports-2020-102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark D.E., Parikh A., Dendy J.M., Diamond A.B., George-Durrett K., Fish F.A., et al. 2020. COVID-19 Myocardial Pathology Evaluated Through scrEening Cardiac Magnetic Resonance (COMPETE CMR) medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelan D., Kim J.H., Chung E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020;5:1085–1086. doi: 10.1001/jamacardio.2020.2136. [DOI] [PubMed] [Google Scholar]
- 33.Younis A., Mulla W., Matetzky S., Masalha E., Afel Y., Fardman A., et al. Sex-based differences in characteristics and in-hospital outcomes among patients with diagnosed acute myocarditis. Am. J. Cardiol. 2020;125(11):1694–1699. doi: 10.1016/j.amjcard.2020.02.040. [DOI] [PubMed] [Google Scholar]
- 34.Laufer-Perl M., Havakuk O., Shacham Y., Steinvil A., Letourneau-Shesaf S., Chorin E., et al. Sex-based differences in prevalence and clinical presentation among pericarditis and myopericarditis patients. Am. J. Emerg. Med. 2017;35(2):201–205. doi: 10.1016/j.ajem.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Vepa A., Bae J.P., Ahmed F., Pareek M., Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab. Syndr. 2020;14(5):1043–1051. doi: 10.1016/j.dsx.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClin. Med. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Comparison between male and female competitive athletes after Covid-19 infection.
Supplementary table 2. Comparison between asymptomatic vs. symptomatic competitive athletes after SARS-CoV-2 infection.
Supplementary data methods describing how CPET was conducted and the safety procedures.