Abstract
The COVID-19 pandemic triggered an unparalleled pursuit of vaccines to induce specific adaptive immunity, based on virus-neutralizing antibodies and T cell responses. Although several vaccines have been developed just a year after SARS-CoV-2 emerged in late 2019, global deployment will take months or even years. Meanwhile, the virus continues to take a severe toll on human life and exact substantial economic costs. Innate immunity is fundamental to mammalian host defense capacity to combat infections. Innate immune responses, triggered by a family of pattern recognition receptors, induce interferons and other cytokines and activate both myeloid and lymphoid immune cells to provide protection against a wide range of pathogens. Epidemiological and biological evidence suggests that the live-attenuated vaccines (LAV) targeting tuberculosis, measles, and polio induce protective innate immunity by a newly described form of immunological memory termed “trained immunity.” An LAV designed to induce adaptive immunity targeting a particular pathogen may also induce innate immunity that mitigates other infectious diseases, including COVID-19, as well as future pandemic threats. Deployment of existing LAVs early in pandemics could complement the development of specific vaccines, bridging the protection gap until specific vaccines arrive. The broad protection induced by LAVs would not be compromised by potential antigenic drift (immune escape) that can render viruses resistant to specific vaccines. LAVs might offer an essential tool to “bend the pandemic curve,” averting the exhaustion of public health resources and preventing needless deaths and may also have therapeutic benefits if used for postexposure prophylaxis of disease.
Keywords: trained immunity, nonspecific effects of live vaccines, interferon, SARS-CoV-2
Like all living organisms, human beings are exposed to a variety of pathogens. How do we almost always stop the infections by different microbes, even those that we have never encountered before? When we are infected, why do the vast majority of pathogens disappear after only a few days or a week, causing very mild or no disease? Obviously, this cannot be due to the virus simply running out of cells to infect. It also cannot be a result of a very specific adaptive immune response consisting of B lymphocytes and the antibodies they produce, and of T lymphocyte-mediated cellular immunity. Antibodies develop through a complex multistep process and reach their needed levels of activity only after many days or weeks (1). Both specific antibodies and T cells are crucial for the host defense against prolonged infections, but they need time to be activated, and they are far less, if at all, involved during the first hours and days of an infection.
Our innate immune response represents the first line of defense against invading pathogens. Clinically, the fever that is often the first symptom of a viral infection is also the first manifestation of our antiviral defense system in action. Moreover, for 95% of all species—including plants and all invertebrate animals—innate immune responses are the only protection available (2, 3). Only vertebrates developed lymphocytes to mediate adaptive immune responses. These cells form the backbone of our highly pathogen-specific adaptive immunity that protects us when our first line of defense fails to keep the invaders at the gate. Unlike the days and weeks needed to activate adaptive immunity, its innate counterpart engages within minutes and hours to protect our bodies from a wide spectrum of pathogens (4). It is clear that innate immunity in the absence of antibodies and T cellular immune response is sufficient to prevent illnesses caused by many systemic pathogens from advancing, while the adaptive immune responses are crucial when the first line of defense is overcome (Fig. 1). The outcome of any infection depends on the race between the pathogen and the host defense systems. It stands to reason that increasing the efficacy of innate immunity and enhancing defense pathways abrogated by SARS-CoV-2 could substantially mitigate infection from the start or potentially even prevent them.
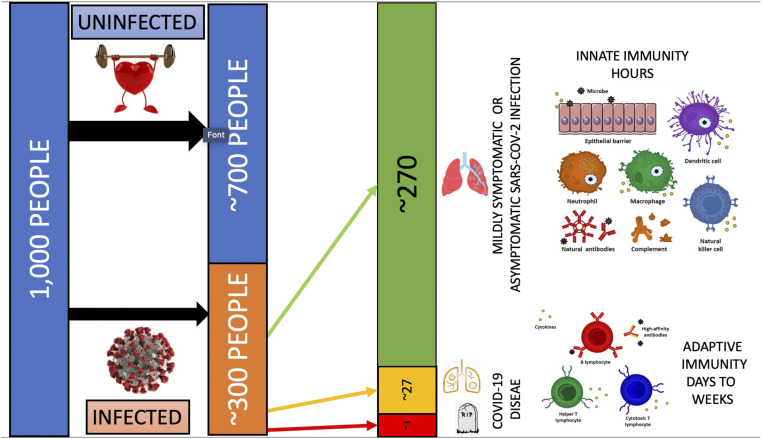
Fig. 1.
This figure illustrates how a healthy innate immune system protects most people within the population against infections. With an already robust or trained innate immune system, the overwhelming majority of people infected with a new pathogen, such as SARS-CoV-2, are able to eradicate the infection early during the asymptomatic or mildly symptomatic phase of infection. Without prior exposure or vaccination (e.g., with a messenger RNA vaccine), adaptive immunity takes days to weeks to kick in and is often suppressed in severe cases, contributing to a self-perpetuating and injurious hyperinflammatory response. The innate immune system often loses potency with age, certain comorbidities, immunosuppression, and with genetic susceptibility.
Table 1 presents a comparison of innate and adaptive immune systems. The power of innate immunity lies in its broad, nonspecific activation, and the ability to inhibit multiple pathogens. It is based on sensors called pattern-recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) and activate multiple pathways that render a host resistant to infection (5). In the case of RNA viruses like SARS-CoV-2, the recognition trigger lies within the nucleotide sequence of the RNA of the virus. The response is immediate and begins with stimulating key host cellular receptors, such as Toll-like receptor (TLR)3 and TLR7, leading to activation of natural killer (NK) cells, monocytes, and expression of interferon (IFN) genes. In turn, IFN serves as a mediator by binding IFN receptors on the host cell surface and launching expression of IFN-stimulated genes (ISG) (6). Proteins coded by these genes induce the so-called antiviral state by making cells unable to support virus replication.
Table 1.
Comparing the characteristics of the two forms of immune response
| Innate | Adaptive |
| Mediated by both myeloid and lymphoid (NK, T) cells | Involves lymphoid cells (B and T lymphocytes) |
| Based on direct phagocytosis and killing of microbes, release of cytokines and chemokines, NK-mediated killing of virus-infected cells | Mechanistically involve antibodies and specific T cells |
| Works almost immediately | Takes 1 to 2 wk to develop following exposure to a pathogen or vaccine |
| Present in all multicellular organisms | System is present in vertebrates but not in invertebrates or plants |
| Broadly specific, can be effective against groups of microorganisms | Specific to one microorganism or even strain |
Stimulation of PRRs by PAMPs leads to the induction of a family of cytokines called IFNs. There are three classes of IFN molecules, including type 1 (α, β, ε, κ, ω), type 2 (γ), and type 3 (λ). In most cases, stimulation of PRRs by PAMPs initially leads to the induction of type 1 IFN, which in turn leads to the production of several ISGs. ISGs have pleiotropic effects and can lead to induction of the cellular direct antiviral state, macrophage activation, stimulation of NK cells and cytotoxic T lymphocytes, and the proliferation of T-helper cells (7). Type 1 IFNs also have antiinflammatory effects due to down-regulation of several proinflammatory cytokines, including of tumor necrosis factor-α and interleukins 1 and 8. Exogenous recombinant IFNs have been approved to treat several viruses, including human papilloma virus, hepatitis C virus, and hepatitis B virus.
In contrast, the defining characteristic of the adaptive immune response is its singular pathogen-specificity and immunologic memory that allows the body to produce antibodies and activate T cells faster if reinfected by that particular pathogen. The induction of adaptive immunity that may last a lifetime (e.g., measles, polio) is the underlying principle behind prophylactic vaccines. However, the very high specificity of adaptive immunity is the “Achilles heel” of vaccines, because though some degree of cross-reactivity may exist (8), they protect predominantly against one pathogen, and often against only one specific strain. For this reason, vaccines relying on protective antibodies and T cells may lose effectiveness if mutations emerge in viral protective epitopes. If this happens, new pathogen-specific vaccines will have to be developed from scratch (as is the case with influenza vaccines). In contrast, viruses cannot easily develop resistance to innate immunity that will continue ensuring broad protection against pathogens, even if they undergo antigenic drift.
The durability and memory of the adaptive immunity system are often cited as its superiority over innate immune responses. However, recent results summarized in this report will show that these distinguishing characteristics between adaptive and innate stimuli may not be as widely apart as first thought. Indeed, some vaccines (e.g., influenza and HIV) induce adaptive immune response that lasts only months (9, 10), and long-term innate immunity activation may also last that long. Innate immunity is also now known to adapt to previous insults, indeed to develop a de facto innate immune memory, that facilitates stronger responses to subsequent pathogen attacks of heterogeneous nature (11). This innate immune memory, also called “trained innate immunity,” is mediated by epigenetic, transcriptional, and functional reprogramming of innate immune cells and their bone marrow progenitors. Trained innate immunity will be described below in more detail. In this article, we propose that during a pandemic (or epidemic), medical science could rapidly harness the power of innate immunity to induce partial protection against new (such as SARS-CoV-2) or reemerging pathogen threats and suggest that this might be achieved through the repurposing of some established live-attenuated vaccines (LAVs), which are powerful inducers of innate immunity.
Innate Immunity Is Key in Controlling the SARS-CoV-2 Pandemic
Several observations make clear the central importance of innate immunity in controlling SARS-CoV-2. First, human coronaviruses, including SARS, Middle Eastern respiratory syndrome (MERS), and SARS-CoV-2 have evolved special mechanisms to suppress immune responses. The viruses devote part of their genetic information to code for proteins that specifically target our innate immune mechanisms, namely the IFN response pathways, by inhibiting TLR3/7 signaling (12–15). Second, the expression of human genes favoring innate immune responses correlates with better clinical response. Indeed, in one study these were the only immune response genes correlated with a favorable prognosis (16). Third, specific mutations that reduce IFN production or function correlate with severe morbidity and mortality (17). In line with other studies that show a correlation between innate immune expression and better prognosis are the findings that correlate severe morbidity and mortality with mutations that lead to faulty type I IFN production or production of neutralizing autoantibodies targeting type I IFNs (18, 19). Consistent with this, therapeutic use of type I IFN (IFN-x2b) in the viral phase of COVID-19 reduced the duration of detectable virus in the respiratory tract and improved the prognosis (20, 21).
Finally, control of coronaviruses by bats is almost exclusively associated with an appropriate balancing of innate immune responses between resistance and tolerance. Many bat species can safely harbor coronaviruses that cause diseases in other mammals, including humans (22). Bats show an unusually high number of activated NK cells, as well as a constitutive expression of IFN. These studies also show a lesser inflammatory response to these viruses (23). Collectively, these findings present very strong arguments that innate immunity is critical to the control of coronavirus diseases. At the onset of the pandemic, this provoked some of us to consider strategies based on stimulation of innate immunity as a complement to disease-specific vaccines by bridging the period until their development and to overcome some of their limitations (11, 24, 25).
Specific Vaccines: A Crucial Tool Against COVID-19, yet More Help Is Needed
Vaccines are among our most successful public health interventions (26), allowing us to overcome many deadly contagions, and avoid “plague” measures of shutdowns and social distancing. We are laser-focused on the pursuit of a vaccine as the antidote to the clinical carnage, social devastation, and economic crisis caused by SARS-CoV-2. The rapid development of several highly effective vaccines using advanced new mRNA technology is an extraordinary achievement in the battle against COVID-19. It is hoped that widespread vaccination will significantly decrease the spread of SARS-CoV-2. However, mass deployment cannot happen in time to save tens of thousands human lives, tens of millions more jobs, and avert the severe hunger facing several million children. Many hurdles remain.
First, specific COVID-19 vaccines appear to be highly protective, in some cases reaching 95% clinical efficacy. However, efficacy refers only to prevention of illness, not to prevention of infection or onward spread, the real key to reaching herd immunity. It will take several months to undertake postmarketing evaluation to ascertain whether vaccines stop virus transmission in younger adults, the age group responsible for most spread. As preventing infection requires a higher level of antibodies than preventing serious disease, the effectiveness for preventing infection is likely to be lower than clinical efficacy. The safety of specific vaccines in pregnant women—a high-risk group representing a significant proportion of healthcare and aged care workforce—has not yet been established.
If vaccines are effective in preventing transmission, achieving herd immunity will require 60 to 70% of the population to be vaccinated (an estimate that is being revised upwards) (27).
Second, while the storage requirement at very cold temperatures and need for an ultracold chain for mRNA vaccine present difficulties (28), a bigger challenge is vaccine nationalism: the hoarding of vaccines by rich countries while the spread continues in poorer countries, just a plane ride away. An even bigger challenge is producing enough vaccines for the world’s population, hindered by intellectual property protections that are impeding the scale-up of the first successful vaccines and limiting access to billions of people.
Third, even once these obstacles are overcome, turning vaccines into vaccinations is contingent upon trust. Even the best biomedical solutions require social traction for uptake. The politicization of the pandemic, erosion of trust in authorities and vaccines, together with concerns about the safety of newly developed vaccines are likely to limit uptake and prevent creation of population immunity (29). The use of time-tested LAVs could help to overcome this problem.
Unlike lifelong immunity to polio, measles, and smallpox, immunity to coronaviruses appears to be evanescent, lasting only months to years (30–32). Reinfection cases underscoring limited natural immunity are increasingly reported. Furthermore, the extent to which the virus can mutate has not been clearly elucidated. Mutations in new variant strains, such as 501.V2, may affect vaccine efficacy, which combined with increased contagiousness would raise herd immunity thresholds, making it more difficult to reach. Other mutations may have the potential to stymie durable antibody-dependent immunity (33).
Another major obstacle to eliminating an infectious disease presents when the pathogen has substantial nonhuman reservoirs. Some other major infectious pathogens, like polio and measles, do not have notable animal reservoirs, and despite very effective vaccines we have not been able to eradicate them. In contrast, SARS-CoV-2 is able to cross between humans and multiple animal species, including pangolins, bats, turtles, snakes, mink, cats, and gorillas (34).
Vaccines based on induction of adaptive immunity response are very important. In the absence of effective treatment or reliable cure, the capacity of vaccines to prevent serious disease and death is especially vital for vulnerable groups, such as the elderly. However, such new vaccines generating specific antibodies and T cells cannot be immediately available during this or any future pandemic. These caveats to a future dependent solely on vaccines based on adaptive immunity demand an expansion of our current protective armament.
LAVs as Potent Inducers of Innate Immunity: Epidemiological Evidence for Beneficial Nonspecific Effects of Vaccines
The prevailing paradigm in vaccinology focuses almost exclusively on adaptive immunity based on pathogen-specific antibodies and T cells. However, vaccine impact is also achieved through a series of beneficial nonspecific effects (NSE). Accumulating evidence suggests that LAVs provide protection not only against the target infectious agent, but also against a broad range of other pathogens (35).
The first indication came from observations made almost 100 y ago in Paris with Bacillus Calmette–Guérin (bacillus Calmette–Guérin) vaccine against tuberculosis (36). Calmette, one of the inventors of bacillus Calmette–Guérin, noted a fourfold greater decline in child mortality not caused by tuberculosis in bacillus Calmette–Guérin-vaccinated children compared with unvaccinated peers.
In the 1950s to 1960s, early clinical trials of oral polio vaccine (OPV) revealed nonspecific protective effects of immunization. Multicenter prospective clinical trials of OPV during seasonal outbreak of influenza showed that vaccination with OPV reduced the incidence of influenza two- to fourfold (33, 34).
A systematic examination of the beneficial NSEs of LAVs started with the discovery that a measles vaccination campaign in 1979 in Guinea-Bissau, West Africa, reduced all-cause mortality by almost 70%, much more than could be explained by the prevention of measles infection (37). The finding, when confirmed in other low-income settings, led to the formulation of the hypothesis that measles vaccine has NSE, strengthening the immune system and providing increased protection against a broad range of infections (38). Since then, decades of clinical-epidemiological observations support the beneficial protective effect of LAVs and in particular bacillus Calmette–Guérin, OPV, smallpox vaccine, and measles vaccines (35, 39). For example, randomized trials showed that bacillus Calmette–Guérin (40), live measles vaccine (41), and OPV (42) reduce all-cause mortality much more than anticipated from their effects on the target disease alone. Historical cohort studies found the same beneficial NSE of smallpox vaccine (38). The reduced mortality is mainly due to a reduction in mortality due to respiratory infections (35). Vaccinations against tuberculosis and smallpox have been associated with better long-term survival (38). Intriguingly, the beneficial NSE of LAV may become more pronounced with subsequent doses (43). For example, OPV campaigns in West Africa have been associated with a 25% reduction in all-cause mortality, with each additional dose reducing mortality by a further 14% (44).
Table 2 compares characteristics of protective effects of LAVs based on stimulating innate immunity and pathogen-specific vaccines inducing adaptive immune responses. Difficulty in characterizing off-target effects of LAVs, controversy over needing to change immunization schedules, concern over limited manufacture of nonprofitable vaccines, as well as potential contribution to vaccine hesitancy have delayed much needed large-scale randomized controlled trials and research into NSE of vaccines. Emerging evidence and recent publications of robust data support the association between bacillus Calmette–Guérin, neonatal mortality, and malaria risk (45–47), and underscore the imperative for research to examine and exploit this effect.
Table 2.
Advantages and disadvantages of conventional adaptive immune response vaccines versus innate immune-stimulating vaccines through use of “old” LAVs for the SARS-CoV-2 pandemic
| Characteristic | Innate broadly specific LAVs | Adaptive (specific) vaccines |
| Economics | Cheap, but likely little market value | Expensive, but great market value |
| Availability | Not a problem for many of these vaccines | Unavailable during the emergence of a new pathogen |
| Speed of action | Immediate | Weeks |
| Safety record | Known and mainly very safe | Unknown for new vaccines (needs 1 to 2 y or longer) |
| Efficacy | Clearly for their target pathogen and numerous results for broader effects | Efficacious for SARS-CoV-2 in standard setting, still unclear in high-risk groups and for what duration |
| Durability | Weeks to months to years | Extremely variable a few months to a lifetime |
| Memory | Thought to be none; but recent results for bacillus Calmette–Guérin reveal innate immune memory (trained innate immunity) | Strong for B cells (antibodies) and T cell responses |
In 2014, a World Health Organization-commissioned review at the recommendation of the Strategic Advisory Group of Experts on vaccines concluded that LAVs reduced child mortality by more than expected by specific prevention of the target diseases (37). The same patterns were observed in high-income settings: For example, in the United States, having a live vaccine as the most recent vaccine was associated with a halving of the risk of hospitalization for nontargeted infections (48). The review advised more research regarding the beneficial heterologous effects of LAVs, and in particular if a different sequence of immunizations could exploit beneficial effects (48). These studies have yet to be conducted.
Animal studies provide additional evidence of the off-target protective effects of LAVs. For example, immunization of mice with cold-adapted live-attenuated influenza H3N2 vaccine protected them from disease upon challenge with respiratory syncytial virus (RSV), accompanied by induction of cytokines and infiltration of respiratory tract with leukocytes (49). Immunization with inactivated influenza vaccine as a control did not induce a protective effect against RSV. The effect of attenuated influenza vaccine was reduced in TLR3/TLR7− mice, supporting other evidence that these pathways are involved in the activation of innate immunity. An experimental vaccine against whooping cough made from live-attenuated Bordetella pertussis also prevented inflammation caused by unrelated respiratory infections, such as influenza and RSV in mice (50), and reduced noninfectious inflammation, including contact dermatitis (51).
Trained Innate Immunity: Immunological Mechanisms
Two important immunological mechanisms are believed to contribute to the beneficial NSE of live vaccines: heterologous cellular immunity and trained innate immunity. In the 1960s and 1970s, the seminal studies of Mackaness et al. (52, 53) demonstrated protection induced by bacillus Calmette–Guérin vaccination against infections with Listeria monocytogenes or Salmonella typhimurium. Similar heterologous protection against infections dependent on T cell activation has been later reported in additional studies (for review, see refs. 54, 55). Interestingly, some of these studies have shown that interaction of T cells with macrophages is crucial for this protection to be effective, raising the possibility of long-term adaptation and increased antimicrobial activity in innate immune cells (56).
Accumulating recent evidence suggests that some types of vaccines, especially LAVs that closely mimic natural infection, induce long-term enhancement of antimicrobial function of innate immune cells that contributes to protection from subsequent reinfection. The term “trained immunity” refers to this functional reprogramming of innate immune cells, such as myeloid and NK-cells (57).
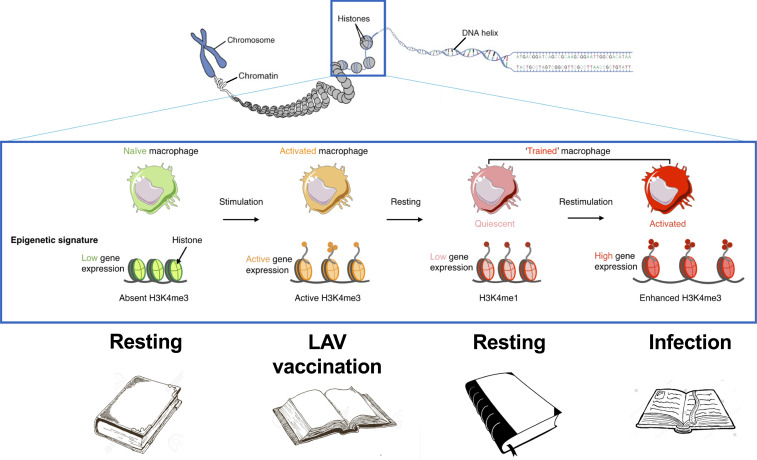
The induction of trained immunity by LAVs is mediated by a complex, finely tuned, interplay between immunological signals, cell metabolism, and epigenetic reprogramming (58). Cellular metabolism is rewired to increase glycolysis (59), glutaminolysis (60), and cholesterol synthesis (61) to provide the energy and building blocks for cells, and for modulation of epigenetic processes. At the same time, chromatin architecture is changed at the loci encoding genes important for host defense, with more open chromatin characterized by the histone marks H3K4me1, H3K4me3, and H3K27Ac (62). The methylation and acetylation of histones after vaccination with a LAV “mark” the gene necessary for host defense: upon infection with a pathogen, these genes will be transcribed quicker and stronger, improving the immune response and the survival of the host. This process can be compared with putting a bookmark in a book: if needed, the book can be opened more easily at the right place (Fig. 2).
Fig. 2.
The epigenetic mechanism induced by LAVs in innate immune cells and their precursors: methylation and acetylation of histones after vaccination “mark” the gene necessary for host defense, leading to long-term changes in chromatin architecture leading to stronger expression upon subsequent stimuli.
Induction of trained immunity has been reported to mediate at least in part the nonspecific biological effects of bacillus Calmette–Guérin vaccination through a NOD2-dependent mechanism (63). Importantly, it has also been demonstrated that long-term functional reprogramming of innate immune responses is mediated by transcriptional and epigenetic processes in the myeloid cell progenitors in the bone marrow, explaining the long-term effect of bacillus Calmette–Guérin on circulating myeloid cells (64, 65). The booster effect of bacillus Calmette–Guérin on cell function extends beyond myeloid cells to other innate immune cell populations, such as NK cells (66). Altogether, the accumulating evidence during the last decade supports the concept that trained innate immunity is responsible for a large part of the heterologous protective effects of LAVs. Since most of this evidence has been provided by studies on bacillus Calmette–Guérin vaccination, studies to decipher induction of trained immunity by measles-mumps-rubella (MMR), OPV, and other LAVs are urgently needed. In the case of MMR, it will be important to assess the NSE of each attenuated strain separately, as it may become important to have multiple LAVs available for serial administration.
Of note, live-attenuated Salmonella Typhi strain Ty21a has recently been shown to induce long-term training of innate immunity (67).
Epidemiological Evidence for the Effects of LAVs against COVID-19
The COVID-19 pandemic spurred research into the protective effects of LAVs against COVID-19. A mission to China at the end of January prompted the Director General of the World Health Organization to consider whether bacillus Calmette–Guérin could protect healthcare workers on the frontlines of the outbreak. In mid-February, the world’s first conference on NSE of vaccines led many researchers present at the conference to initiate clinical trials of bacillus Calmette–Guérin vaccine against COVID-19 (25, 68). There are currently ∼20 bacillus Calmette–Guérin trials that will provide estimates on the effect of bacillus Calmette–Guérin against infections, including COVID-19, in health care workers and elderly. Similar studies are ongoing with respect to OPV (69) and MMR (70). Table 3 provides a listing of currently available LAVs along with their salient characteristics.
Table 3.
Existing LAVs and their characteristics
| Criteria | Measles/MMR | OPV | Bacillus Calmette–Guérin |
| Route | Subcutaneous | Oral | Intradermal |
| Combination vaccines | MMR vaccine and MMR combined with Varicella vaccine | bOPV (OPV1 and OPV3) | Alone |
| Contraindications | Immunosuppression, pregnancy, HIV with CD4 T cell counts <15% | Immunosuppression, HIV, pregnancy | Immunosuppression, active tuberculosis, pregnancy |
| Adverse events | Serum sickness like arthralgias. Febrile seizures. Rare but severe allergic (anaphylactic) reactions. | Vaccine associated paralytic polio (VAPP) (1/million) only in unvaccinated children | Disseminated disease in immunosuppressed (CGD, IFN gamma defects) |
| Rare complication | SSPE (0.7/million) | VAPP (1/million) only in unvaccinated children | Bacillus Calmette–Guérin osteitis |
| Stimulation of innate immunity | Yes | Yes | Yes |
A recent observational study of healthcare workers in Los Angeles showed that the seroprevalence of anti–SARS-CoV-2 IgG, as well as incidence of self-reported COVID-19 symptoms, were significantly decreased among healthcare workers with a history of bacillus Calmette–Guérin vaccination compared with those without bacillus Calmette–Guérin vaccination. No effect was associated with a history of inactivated vaccines, such as meningococcal, pneumococcal, or influenza vaccination (71).
Several observational studies have examined the association between recent LAV vaccination and COVID-19 risk. A Dutch study showed fewer COVID symptoms in bacillus Calmette–Guérin-vaccinated cohorts compared with unvaccinated cohorts (72). A study from the United Arab Emirates showed bacillus Calmette–Guérin revaccination was associated with protection against COVID-19 (73). A recent Mexican study found less severe illness in individuals recently vaccinated with MMR (74).
Economic Analysis of LAVs and COVID-19
Effective response to a global pandemic is not possible without thorough economic evaluation. Its purpose is to guide resource allocation to where value for money is the highest, typically to interventions that maximize outcomes per million dollars spent. Economic evaluation of the pathogen-specific effects of vaccines looks at costs in terms of the vaccine itself, its delivery systems and, if the vaccine is in short supply, in terms of amount of vaccine available.* The evaluations look at outcomes that depend on context: cases averted, deaths averted, time off from work averted, and medical and hospitalization costs averted. Mortality reduction is sometimes explicitly valued in dollar terms but more usually not (for discussion, see ref. 75). Lee et al. (76) and Li et al. (77) provide extensive reviews of the effectiveness research underpinning economic analysis of 10 important pathogen-specific vaccines. As far as we are aware, only Byberg et al. (78) have undertaken a cost-effectiveness analysis that explicitly incorporates vaccines’ NSE. Their analysis concluded that the NSE, in the context of measles herd immunity, proved to be more important in determining effectiveness and cost-effectiveness than the intended measles-specific effect.
The economic attractiveness of LAVs to stimulate innate immunity and protection against COVID-19 will depend on (currently unknown) effectiveness and duration. Elaborate modeling is sometimes utilized for economic evaluations, but the magnitude of parameter and model uncertainty suggests that, at this point, elaborate efforts would add little insight while adding cost in terms of time and lack of transparency. Initial efforts should aim to generate first order or approximate cost-effectiveness (CEA) and benefit-cost (BCA) assessments. CEA’s typically assess cost per death or infection averted, whereas BCAs go beyond CEA to assign monetary value to reductions in morbidity and mortality. Economic evaluations of vaccine effectiveness often center on calculations of the number of deaths averted per 1,000 individuals immunized. Lee et al. (76) concluded, for example, that at 16.5 deaths averted per 1,000 immunizations (first dose), measles had the highest performance by this metric. Costs, and sometimes, dollar-valued benefits are added to complete the analysis. Delivery complexity, such as cold-chain requirements or ease of administration (as with OPV), will often play an important role in addition to costs narrowly defined (79). Some of the specific vaccines now licensed for use against COVID-19 require exceptional refrigeration with attendant cost and noncost barriers to implementation. LAV use against COVID-19 will, if proven effective, have significant advantages on the cost side of the economic evaluation.
Distinctive features that are typical for nonpathogen-specific use of existing LAVs (but not for pathogen-specific vaccines) will in all likelihood play an important part in economic evaluations. These features include (depending on the LAVs):
-
•
Effectiveness against multiple pathogens;
-
•
More rapid elicitation of a protective immunity;
-
•
Well-understood safety profiles;
-
•
Population familiarity with and acceptance of many of the LAVs;
-
•
Preexisting manufacturing capacity and licensing; and,
-
•
Possibility that (current or future) vaccine stockpiles could be diverted to pandemic responses.
Assessment of value for money depends critically on the specifics of proposed use. Table 4 summarizes possible uses of LAVs against COVID-19 into four broad categories: 1) bridge (while waiting for COVID-19 specific vaccine, or should the new vaccine fail or not be deployed); 2) to boost response to COVID-19 specific vaccine; 3) as a quick response ring vaccination of a specific population to contain an outbreak; and 4) potentially therapeutically or as postexposure prophylaxis. Initial (and still ongoing) economic evaluation of the first and third uses are pointing to favorable BCA ratios. This appears to result from a (plausibly) high number of deaths averted per 1,000 immunizations and from the exceptionally low cost of most of the existing LAVs. It is worth adding an observation concerning ring use. Responding to sudden disease flare-ups require effective containment of the outbreak before it has a chance to spread. The immediate effectiveness of LAVs provides an important advantage over slower acting (10 to 20 d) pathogen-specific vaccines. This suggests a potential role for LAV vaccination even after pathogen-specific vaccines are available. Before they are available it would be the only feasible approach in addition to ring “vaccination” with convalescent plasma or monoclonal antibodies.
Table 4.
Potential uses of live attenuated vaccines LAVs against COVID-19
| Use | Population addressed |
| “Bridge” use (until SARS-CoV-2–specific vaccine becomes available) | General populations |
| • Responders (medical, fire, police) | |
| • At-risk populations (hourly workers, gig workers, undocumented residents) | |
| OPV incremental to SARS-CoV-2 specific vaccine | As an adjuvant concomitant to administration of a COVID-19 vaccine |
| • To boost responses in elderly or individuals with comorbidity | |
| Ring use (ring use will generally require single administration of vaccines in well-defined populations as a quick response to appearance of infection) | Quenching postpandemic flare-ups |
| • Quenching prepandemic sparks | |
| • Institutionalized elderly and caretakers | |
| • Prisoners and guards | |
| • Other institutionalized groups | |
| Therapeutic use (hypothetical) | • Infected individuals immunized early after detection of infection |
It is worth expanding on the potential use of LAVs in environments, like today’s with COVID-19, where effective disease-specific vaccines are becoming available. In this context, use of LAVs could best be thought of not as substitutes for disease-specific vaccines, but rather as complements to them. Two different but important pathways exist for a LAV to add protection to a vaccination program that would otherwise offer only a single vaccine. First, in a rapidly unfolding pandemic, early interventions have important value in reducing secondary infections, as well as infection in the person vaccinated. While the adaptive immune system can take 3 to 6 wk to respond to a vaccine, this report has stressed that the innate immune system response is almost immediate. During a pandemic, 4 wk is a long time even if the vaccine was immediately available. Simultaneous administration of a COVID-19 vaccine and a LAV would add protection during the critical period prior to effectiveness of the covid vaccine. This is quantitatively important: during midpandemic the incidence of COVID-19 infection can easily lie between 1 and 5 infections per 1,000 persons per month. Even a LAV with only 50% efficacy could then prevent several more primary infections per 1,000 vaccinees than would a vaccination schedule offering only a COVID-19 vaccine. With an Reff of 2, the total number of infections prevented could be twice the number of primary infections prevented. And even after 4 to 5 wk, the LAV could plausibly add effectiveness to the COVID-19 vaccine alone.
COVID-19 vaccine manufacturing constraints and delivery logistics (two doses, cold-chain requirements) could plausibly leave half or more of the world’s population unvaccinated by the end of 2021. A second complementary use for a LAV would then be to provide protection during what will often be long delays prior to administration of the COVID-19 series. Again, that use would not be an alternative to a COVID-19 vaccine but a way of providing interim protection with a two-vaccine schedule of “LAV soon, COVID-19 vaccine when available.” OPV’s familiarity in many parts of the world, low cost, and easy delivery logistics may make it particularly suited to the role of first vaccine in a two-vaccine schedule.
Three final but more general points on economics are worth making. First, it is widely understood that the macroeconomic and social consequences of COVID-19 have been severe. Cutler and Summers (80) have documented this for the United States and Lau and Xiong (81) have done so for China as a whole and by province. Economic consequences in China, while less severe than in the United States, are surprisingly large given the rapidity with which the Chinese government brought the epidemic under control. Second, economic evaluations increasingly include examination of the impact on equity. Valuable as the COVID-19–specific vaccines are, early indications suggest that their introduction will exacerbate inequity within and across countries. The potential low cost and rapid availability and widespread acceptance of LAVs suggest their potential for attenuating these inequities. Finally, the massive public resources that have been dedicated to developing new vaccines with protectable intellectual property that can generate large returns for the developing companies stands in stark contrast to the paltry public resources that have been dedicated to assessing the efficacy of existing generic LAVs. This is likely due to both the economic benefits that accrue to the developers of new technologies but also the scientific prestige that accrues to the scientists. Public policy should recognize and counteract these influences for the public good.
Conclusions and Recommendations
In the war against infectious disease, COVID-19–specific vaccines are our preferred weapons of mass salvation. But they are not a panacea. The quest for specific SARS-CoV-2 vaccines started as soon as the sequence of the novel coronavirus was shared on January 11, 2020. The development of several efficacious vaccines 10 mo after the sequence was published and subsequently shared by the Chinese Center for Disease Control and Prevention is an incredible scientific success.
Vaccines give hope that the pandemic will be halted within a year or two, but we have also learned hard lessons about the limitations inherent in the development of completely new vaccines. One of the most important limitations relates to the time needed not only for the development, but for mass deployment. Challenges include scaled-up manufacture, logistics of rollout, vaccine nationalism, vaccine hesitancy, and governmental and intergovernmental regulations to ensure equitable distribution within and across countries.
Even in the case of a microorganism such as SARS-CoV-2, for which it appears that the development of a vaccine is not particularly difficult, it is still a minimum of 1.5 to 2 y until a safe and effective vaccine can be produced, tested, distributed, and delivered to the global population. In this period of time, countless lives have been lost, and economic havoc has been unleashed in the world economy. This could be even more tragic in the case of a pandemic caused by a microorganism for which the development of a vaccine is more difficult, transmission is more rapid, or the herd immunity more difficult to achieve. This can always happen in any future epidemic.
There are other unknown aspects about novel specific vaccines. In the case of COVID-19, most specific vaccines are focused on the spike protein of SARS-CoV-2. There are unanswered questions about the durability of antibodies to the spike protein, based on its glycoprotein structure and high mannose side chains reminiscent of the HIV glycoprotein 120 envelope protein (9, 82, 83) and the flu virus Hemagglutinin (10), to which antibodies last less than 6 mo. A greater suspicion arises from studies of the seasonal coronaviruses in which the spike antibodies have been shown to lack durability (84) and by clinical evidence that these antibodies decline within a few months (85). Furthermore, the efficacy of specific vaccines in preventing infection and onward spread remains to be demonstrated. Safety in pregnant women—a high-risk group representing significant proportion of healthcare and aged care workforce—has not been established. Finally, if SARS-CoV-2 undergoes antigenic drift similar to influenza viruses, vaccines targeting spike protein may lose their effectiveness. Indeed, just weeks after the rollout of the first COVID-19 vaccines, some variants of SARS-CoV-2 were found to be partially resistant to immunity induced by the original strain, prompting attempts to develop modified vaccines. In contrast, the antigenically altered viruses will remain vulnerable to a robust innate immune response, boosted by LAVs.
The above considerations prompted many to consider an evaluation of the use of already available LAVs to protect not just for SARS-CoV-2 but also for future pandemics, which will inevitably occur. The potential benefits of LAVs in the battle against pandemics include: 1) use as a bridge vaccination in the beginning of a pandemic, until specific vaccines are developed; 2) to boost responses in the at-risk groups, such as older adults and those with certain comorbidities, for whom classic vaccination is often inefficient; and 3) to be used in combination with specific vaccines, increasing their effectiveness and durability of the immune response. Another application of LAV-induced stimulation of innate immunity would be emergency postexposure prophylaxis. Vaccination of asymptomatic contacts of a COVID-19 case or a PCR+ individual could prevent or attenuate the disease. With their nonspecific or heterologous effects in boosting innate immunity, LAVs could have an important role in health promotion. Furthermore, from a logistical perspective, they can be immediately deployed if enough stocks were available. Many existing vaccines that could be used for this purpose are inexpensive and easy to administer (for example, the cost of one dose of OPV is $0.15, and it can be easily delivered orally). Failing to consider such a highly cost-effective intervention in the urgent battle against pandemics would be a large negligence on the part of the scientific and medical community.
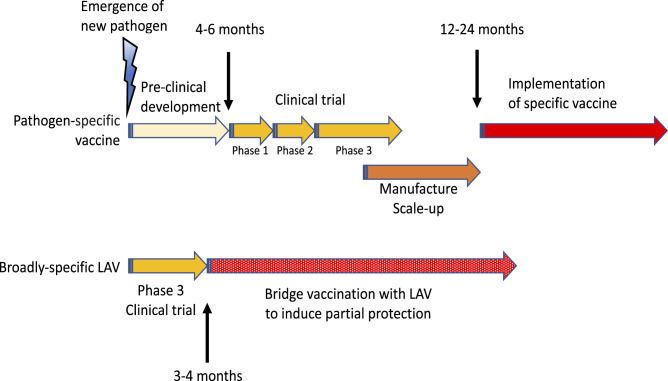
The time from the emergence of a pathogen to mass deployment of an effective LAV stimulating innate immunity could be reduced to months instead of the 1.5 to 2.0 y for a specific vaccine (Fig. 3). Such emergency vaccination with LAVs at the start of new pandemics could only be possible if several scientific and logistical issues are resolved upfront. They include ensuring that immunocompromised individuals be protected from potential side effects of live vaccines, as well as resolving supply issues. Repurposing existing vaccines should not come at the cost of reducing availability of vaccines for their primary indication. If a strategic stockpile of such vaccines were created ahead of time, the response could be faster and more effective. Vaccines preactivating innate immune responses could potentially provide useful bridge vaccination until the development of specific vaccines.
Fig. 3.
Specific vaccines against a new pathogen require extensive preclinical and clinical development and scaling up their production. Because of the urgency, some of the steps can be combined (e.g., phases 1 and 2, or 2 and 3 of clinical trials). On the other hand the existing LAVs only need phase 3 trial of their efficacy. They can either be stockpiled in advance or their manufacture could be ramped up in parallel with testing. Therefore, LAVs can be deployed sooner and used for bridge vaccination to provide (partial) protection and limit the spread of the pandemics until the development of specific vaccines.
Comparative characteristics of existing LAVs and the specific SARS-CoV-2 vaccines presented in this paper argue that they both have important roles to play in our response to the pandemic. One point is clear: There is immense readiness and massive financial support for the novel specific vaccines, but very little for the (nonspecific) LAVs, despite their potential to prevent needless suffering and help mitigate social and economic carnage in any future pandemic. Thus, support for this strategy to exploit the nonspecific effect of LAVs to protect high-risk populations, such as healthcare workers and the elderly, as well as low-income populations worldwide, thereby reducing social and economic inequities, rests on governments, philanthropy, and nonprofit foundations.
It is critically important from both scientific and public health perspectives that we complete rigorous trials evaluating the effectiveness of LAVs in preventing COVID-19 or mitigating its severity. The findings from these trials will inform if and how we could incorporate LAVs into our toolkit against future pandemics. It will never be possible for us to eradicate all pathogens, nor should we attempt to do so. Instead, perhaps we can emulate bats, whose robust innate immune system allows them to flourish while playing host to a plethora of coronaviruses.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
*By short supply, we simply mean that there are limits on the amount that can actually be purchased in the short run, making vaccine availability a separate constraint, in addition to money. This is the case today for COVID-19–specific vaccines, with important implications for equity.
Data Availability
All study data are included in the article.
References
- 1.Callow K. A., Parry H. F., Sergeant M., Tyrrell D. A., The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 105, 435–446 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netea M. G., Schlitzer A., Placek K., Joosten L. A. B., Schultze J. L., Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25, 13–26 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Netea M. G., Quintin J., van der Meer J. W., Trained immunity: A memory for innate host defense. Cell Host Microbe 9, 355–361 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Huber-Lang M., Lambris J. D., Ward P. A., Innate immune responses to trauma. Nat. Immunol. 19, 327–341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyama S., Ishii K. J., Coban C., Akira S., Innate immune response to viral infection. Cytokine 43, 336–341 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Birra D., et al., COVID 19: A clue from innate immunity. Immunol. Res. 68, 161–168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoggins J. W., Interferon-stimulated genes: What do they all do? Annu. Rev. Virol. 6, 567–584 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Welsh R. M., Che J. W., Brehm M. A., Selin L. K., Heterologous immunity between viruses. Immunol. Rev. 235, 244–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis G. K., DeVico A. L., Gallo R. C., Antibody persistence and T-cell balance: Two key factors confronting HIV vaccine development. Proc. Natl. Acad. Sci. U.S.A. 111, 15614–15621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis C. W., et al., Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science 370, 237–241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea M. G., et al., Trained immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 181, 969–977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei X., et al., Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 11, 3810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen C. K., et al., SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 9, 1418–1428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J. Y., et al., The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 286, 198074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young B. E., et al., Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: An observational cohort study. Lancet 396, 603–611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arunachalam P. S., et al., Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Made C. I., et al., Presence of genetic variants among young men with severe COVID-19. JAMA 324, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meffre E., Iwasaki A., Interferon deficiency can lead to severe COVID. Nature 587, 374–376 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Bastard P.et al., HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort , Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q., et al., Interferon-α2b treatment for COVID-19. Front. Immunol. 11, 1061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davoudi-Monfared E., et al., A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 64, e01061-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K., Bats and coronaviruses. Viruses 11, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespi B., Evolutionary medical insights into the SARS-CoV-2 pandemic. Evol. Med. Public Health 2020, 314–322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chumakov K., Benn C. S., Aaby P., Kottilil S., Gallo R., Can existing live vaccines prevent COVID-19? Science 368, 1187–1188 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Curtis N., Sparrow A., Ghebreyesus T. A., Netea M. G., Considering BCG vaccination to reduce the impact of COVID-19. Lancet 395, 1545–1546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty M., Buchy P., Standaert B., Giaquinto C., Prado-Cohrs D., Vaccine impact: Benefits for human health. Vaccine 34, 6707–6714 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Omer S. B., Yildirim I., Forman H. P., Herd immunity and implications for SARS-CoV-2 control. JAMA 324, 2095–2096 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Kartoglu U. H., Moore K. L., Lloyd J. S., Logistical challenges for potential SARS-CoV-2 vaccine and a call to research institutions, developers and manufacturers. Vaccine 38, 5393–5395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puri N., Coomes E. A., Haghbayan H., Gunaratne K., Social media and vaccine hesitancy: New updates for the era of COVID-19 and globalized infectious diseases. Hum. Vaccin. Immunother. 16, 2586–2593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellam P., Barclay W., The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J. Gen. Virol. 101, 791–797 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long Q. X., et al., Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Tillett R. L., et al., Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 21, 52–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q., et al., The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 182, 1284–1294.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiwari R., et al., COVID-19: Animals, veterinary and zoonotic links. Vet. Q. 40, 169–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benn C. S., Fisker A. B., Rieckmann A., Sørup S., Aaby P., Vaccinology: Time to change the paradigm? Lancet Infect. Dis. 20, e274–e283 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Calmette A., Preventive vaccination against tuberculosis with BCG. Proc. R. Soc. Med. 24, 1481–1490 (1931). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins J. P., et al., Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 355, i5170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieckmann A., et al., Vaccinations against smallpox and tuberculosis are associated with better long-term survival: A Danish case-cohort study 1971-2010. Int. J. Epidemiol. 46, 695–705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aaby P., Benn C. S., Developing the concept of beneficial non-specific effect of live vaccines with epidemiological studies. Clin. Microbiol. Infect. 25, 1459–1467 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Biering-Sørensen S., et al., Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: A randomized controlled trial. Clin. Infect. Dis. 65, 1183–1190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aaby P., et al., Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: Randomised controlled trial. BMJ 341, c6495 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund N., et al., The effect of oral polio vaccine at birth on infant mortality: A randomized trial. Clin. Infect. Dis. 61, 1504–1511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benn C. S., Fisker A. B., Whittle H. C., Aaby P., Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: A review. EBioMedicine 10, 312–317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen A., et al., National immunisation campaigns with oral polio vaccine may reduce all-cause mortality: An analysis of 13 years of demographic surveillance data from an urban African area. Clin. Infect. Dis., 10.1093/cid/ciaa1351 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Berendsen M. L., et al., BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa. BMJ Glob. Health 4, e001862 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen K. J., et al., Seasonal variation in the non-specific effects of BCG vaccination on neonatal mortality: Three randomised controlled trials in Guinea-Bissau. BMJ Glob. Health 5, e001873 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassat Q., Moncunill G., Dobaño C., Making sense of emerging evidence on the non-specific effects of the BCG vaccine on malaria risk and neonatal mortality. BMJ Glob. Health 5, e002301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardenheier B. H., McNeil M. M., Wodi A. P., McNicholl J. M., DeStefano F., Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005-2014. Clin. Infect. Dis. 65, 729–737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y. J., et al., Non-specific effect of vaccines: Immediate protection against respiratory syncytial virus infection by a live attenuated influenza vaccine. Front. Microbiol. 9, 83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cauchi S., Locht C., Non-specific effects of live attenuated pertussis vaccine against heterologous infectious and inflammatory diseases. Front. Immunol. 9, 2872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavanagh H., et al., Attenuated Bordetella pertussis vaccine strain BPZE1 modulates allergen-induced immunity and prevents allergic pulmonary pathology in a murine model. Clin. Exp. Allergy 40, 933–941 (2010). [DOI] [PubMed] [Google Scholar]
- 52.MacKaness G. B., The immunological basis of acquired cellular resistance. J. Exp. Med. 120, 105–120 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanden R. V., Lefford M. J., Mackaness G. B., The host response to Calmette-Guérin bacillus infection in mice. J. Exp. Med. 129, 1079–1107 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selin L. K., et al., Heterologous immunity: Immunopathology, autoimmunity and protection during viral infections. Autoimmunity 44, 328–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welsh R. M., Selin L. K., No one is naive: The significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2, 417–426 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Mackaness G. B., The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J. Exp. Med. 129, 973–992 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Netea M. G., et al., Trained immunity: A program of innate immune memory in health and disease. Science 352, aaf1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netea M. G., et al., Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng S. C., et al., mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arts R. J., et al., Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bekkering S., et al., Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146.e9 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Saeed S., et al., Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleinnijenhuis J., et al., Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 109, 17537–17542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaufmann E., et al., BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Cirovic B., et al., BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinnijenhuis J., et al., BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennington S. H., et al., Nonspecific effects of oral vaccination with live-attenuated Salmonella Typhi strain Ty21a. Sci. Adv. 5, eaau6849 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Vrieze J., Can a century-old TB vaccine steel the immune system against the new coronavirus? Science, (2020). https://www.sciencemag.org/news/2020/03/can-century-old-tb-vaccine-steel-immune-system-against-new-coronavirus. Accessed 28 April 2021.
- 69.Clinical Trials.gov, “OPV as potential protection against COVID-19” (U.S. National Library of Medicine, 2020). https://clinicaltrials.gov/ct2/show/NCT04445428. Accessed 28 April 2021.
- 70.Dryden J. Global trial to test whether MMR vaccine protects front-line health-care workers against COVID-19. (2020). https://medicine.wustl.edu/news/global-trial-to-test-whether-mmr-vaccine-protects-front-line-health-care-workers-against-covid-19/. Accessed 28 April 2021.
- 71.Noval Rivas M., et al., BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of healthcare workers. J. Clin. Invest. 131, e145157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moorlag S. J. C. F. M., et al., BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 33, 108387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amirlak I., et al., Effectiveness of booster BCG vaccination in preventing Covid-19 infection. medRxiv [Preprint] (2020). https://www.medrxiv.org/content/10.1101/2020.08.10.20172288v1 (Accessed 28 April 2021). [DOI] [PMC free article] [PubMed]
- 74.Larenas-Linnemann D. E., Rodríguez-Monroy F., Thirty-six COVID-19 cases preventively vaccinated with mumps-measles-rubella vaccine: All mild course. Allergy 76, 910–914 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Robinson L. A., Hammitt J. K., Jamison D. T., Walker D. G., Conducting benefit-cost analysis in low- and middle-income countries: Introduction to the special issue. J. Benefit Cost Anal. 10 (suppl. 1), 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee L. A., et al., The estimated mortality impact of vaccinations forecast to be administered during 2011-2020 in 73 countries supported by the GAVI Alliance. Vaccine 31 (suppl. 2), B61–B72 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Li X., et al., Estimating the health impact of vaccination against 10 pathogens in 98 low and middle income countries from 2000 to 2030. MedRxiv [Preprint] (2019). https://www.medrxiv.org/content/10.1101/19004358v1 (Accessed 28 April 2021). [DOI] [PMC free article] [PubMed]
- 78.Byberg S., et al., Cost-effectiveness of providing measles vaccination to all children in Guinea-Bissau. Glob. Health Action 10, 1329968 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lambert P.-H., Bloom B. R., The Vaccine Book (Academic Press, 2003). [Google Scholar]
- 80.Cutler D. M., Summers L. H., The COVID-19 pandemic and the $16 trillion virus. JAMA 324, 1495–1496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lau L. J., Xiong Y., The COVID-19 Epidemic in China (World Scientific, 2020). [Google Scholar]
- 82.Watanabe Y., Bowden T. A., Wilson I. A., Crispin M., Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta, Gen. Subj. 1863, 1480–1497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fouts T. R., et al., Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc. Natl. Acad. Sci. U.S.A. 112, E992–E999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edridge A. W. D., et al., Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 26, 1691–1693 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Ibarrondo F. J., et al., Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 383, 1085–1087 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.