Significance
Metastasis is a leading cause of breast cancer-associated death. MDA-9/Syntenin expression is elevated and contributes at multiple nodal points in the metastatic process. Inhibition of MDA-9/Syntenin using a pharmacological inhibitor (PDZ1i), which blocks protein–protein interactions, suppresses metastasis in syngeneic mouse and human xenograft models. PDZ1i therapy robustly constrains breast cancer metastasis in syngeneic animals by inhibiting tumor cell-derived interleukin-1β secretion through deactivation of STAT3 and reducing infiltration of immune suppressor cells in the metastatic niche. Enhanced antitumor immunity correlates with expansion of interferon-γ–expressing T cells and conversion of the immunosuppressive microenvironment into an immunostimulatory tumor environment. Collectively, our findings highlight the essential function of MDA-9/Syntenin in immune tolerance, documenting a promising therapeutic strategy selectively targeting breast cancer metastasis.
Keywords: metastasis, breast cancer, MDA-9/Syntenin, IL-1β
Abstract
Melanoma differentiation associated gene-9 (MDA-9), Syntenin-1, or syndecan binding protein is a differentially regulated prometastatic gene with elevated expression in advanced stages of melanoma. MDA-9/Syntenin expression positively associates with advanced disease stage in multiple histologically distinct cancers and negatively correlates with patient survival and response to chemotherapy. MDA-9/Syntenin is a highly conserved PDZ-domain scaffold protein, robustly expressed in a spectrum of diverse cancer cell lines and clinical samples. PDZ domains interact with a number of proteins, many of which are critical regulators of signaling cascades in cancer. Knockdown of MDA-9/Syntenin decreases cancer cell metastasis, sensitizing these cells to radiation. Genetic silencing of MDA-9/Syntenin or treatment with a pharmacological inhibitor of the PDZ1 domain, PDZ1i, also activates the immune system to kill cancer cells. Additionally, suppression of MDA-9/Syntenin deregulates myeloid-derived suppressor cell differentiation via the STAT3/interleukin (IL)-1β pathway, which concomitantly promotes activation of cytotoxic T lymphocytes. Biologically, PDZ1i treatment decreases metastatic nodule formation in the lungs, resulting in significantly fewer invasive cancer cells. In summary, our observations indicate that MDA-9/Syntenin provides a direct therapeutic target for mitigating aggressive breast cancer and a small-molecule inhibitor, PDZ1i, provides a promising reagent for inhibiting advanced breast cancer pathogenesis.
Breast cancer remains the second leading cause of death among women in the United States (1). Prognosis for early-stage disease is favorable, whereas late-stage disease with tumor cell spread beyond the primary site (i.e., metastasis) frequently heralds poorer outcomes (1). Therapy of metastatic disease usually involves systemic chemotherapy combined with radiation, providing mostly palliative options to reduce metastatic outgrowth (2). Multiple unique and distinct biological steps and an interplay between transformed and nontransformed cells highlight complexities of the metastatic process, which habitually thwarts clinical intervention. In principle, targeting these processes independently or collectively could culminate in effective antimetastatic therapies.
Melanoma differentiation-associated gene-9 (mda-9), also known as Syntenin-1 or syndecan binding protein (SDCBP), was cloned in our laboratory using subtraction hybridization from terminal differentiating metastasis-derived human melanoma cells treated with interferon (IFN)-β and the protein kinase C activator, mezerein (3, 4) (designated as mda-9/Syntenin). Preferential elevated expression of mda-9/Syntenin is evident in histologically distinct tumors and contributes to several steps in the metastatic process (5). These include tumor cell invasion and migration (6, 7), induction of angiogenesis through secretion of proangiogenic factors (8–10), enhancement of epithelial–mesenchymal transition (EMT) (11, 12), regulation of the expression of integrins affecting cell-adhesion processes (13), exosome biogenesis and exosome-mediated signaling in cell–cell communication (14), and recently immune-modulation suppressing host-immune surveillance (15). Cancer cell-independent functions of MDA-9/Syntenin also contribute to metastatic progression by regulating immunosuppressive cell infiltration in the metastatic niche (16). Based on its relevance to the invasive and metastatic phenotype of cancers, MDA-9/Syntenin represents a prospective target for rational design of antimetastatic drugs.
Differential expression of MDA-9/Syntenin in cancer versus adjacent normal tissue is often a predictor of poor clinical outcomes (8). A relationship exists between MDA-9/Syntenin (SDCBP) and breast cancer in rat mammary tumors (genomic localization) (17) and in metastasis and clinical situations in human triple negative and other human breast cancers (11, 15, 18). MDA-9/Syntenin plays a pivotal role in EMT induction that includes initiation of Smad-dependent EMT through interaction with TGF-βR1, disrupting receptor internalization (11). Physical interaction between MDA-9/Syntenin and TGF-β activates small GTPases, Rho A, and CDC 42 (12). In addition, MDA-9/Syntenin enhances primary tumor growth and lung metastasis through immune evasion by up-regulating PD-L1 (program death ligand 1) through STAT3 activation, causing T cell apoptosis (15). In breast cancer, MDA-9/Syntenin affects tumor cell proliferation in estrogen receptor-negative breast cancer, causing cells to bypass the G1/S checkpoint promoting S-phase entry (19). MDA-9/Syntenin is also considered a potential antigen in breast cancer (20). These observations endorse MDA-9/Syntenin as a prospective target for the therapy of breast cancer metastasis.
Disturbing MDA-9/Syntenin protein:protein interactions is viewed as a viable strategy to disrupt key downstream signaling pathways regulating cancer cell invasion and metastasis (reviewed in ref. 5). Fragment-based drug discovery guided by NMR identified a first in-class interaction inhibitor of the PDZ1 domain of MDA-9/Syntenin, PDZ1i (21), displaying efficacy against glioblastoma multiforme, neuroblastoma, and prostate cancer (5, 13, 22, 23). PDZ1i suppresses cancer cell-autonomous and nonautonomous functions of MDA-9/Syntenin, culminating in strong antiinvasive and antimetastatic properties in vitro and in vivo, without inducing overt cytostatic or toxic effects in normal or most cancer cells. Informed by the crystal structure of MDA-9/Syntenin, peptide-based inhibitory molecules have been developed and validated in cell-based assays (24). Additionally, a genetic approach using adenovirus-mediated delivery of shmda-9 has shown efficacy in xenografted human melanoma (8) and prostate cancer (25) in nude mice. These investigations confirm MDA-9/Syntenin as a viable target for suppressing both primary and metastatic tumor growth and support further evaluation of PDZ1i on breast cancer metastasis.
Evidence from both experimental models and clinical studies show a relationship between abundance of tumor-infiltrating immune cells and metastasis (26). To create a permissive environment in a secondary site, disseminated tumor cells employ multiple strategies, including reducing host immune surveillance (27). In several mouse tumor models, myeloid-derived suppressor cells (MDSCs), a heterogeneous population of myeloid cells with immunosuppressive properties, are expanded in the blood, lymph nodes, and spleen (28). They help shape the microenvironment and metastatic niches by regulating both innate and adaptive immunity (29). In breast cancer mouse models, MDSCs accumulate in the lungs prior to metastatic spread (30) and promote immune suppression by producing reactive oxygen species and arginase (Arg-1) (31). Not surprisingly, various chemotherapeutic agents, such as gemcitabine (32), 5-flurouracil (33), and docetaxel (34) decrease MDSC accumulation in the tumors’ stroma, thereby enhancing antitumor immune responses (29). Similar soluble factors are operational in primary tumors and metastases, including granulocyte-macrophage colony-stimulating factor, interleukins (e.g., IL-6, IL-1β), and vascular endothelial growth factor (VEGF), causing MDSC infiltration. Tumor cells in the metastatic niche that produce various cytokines or growth factors also regulate this process.
We have now explored a potential role of MDA-9/Syntenin in breast cancer progression with specific emphasis on a relevant interleukin, IL-1β, representing an important inflammatory cytokine mediating cancer pathogenesis and tumor progression (35). Inflammation regulates fundamental pathways that are causative of the cancer phenotype, including proliferation, survival, and migration (36). IL-1β regulates tumor initiation/progression, angiogenesis, Th17 cell differentiation, and expansion of MDSCs (35). Additionally, IL-1β controls macrophage recruitment and invasion, and metastasis of cancer cells (35). Based on these seminal roles in orchestrating the neoplastic process, IL-1β represents a potential therapeutic target and its regulation deserves further analysis. We now confirm that MDA-9/Syntenin, which can be obstructed by the small-molecule inhibitor PDZ1i, regulates IL-1β, thereby directly controlling breast cancer pathogenesis.
Results
PDZ1i Inhibits Metastasis in a Syngeneic Breast Cancer Metastatic Mouse Model.
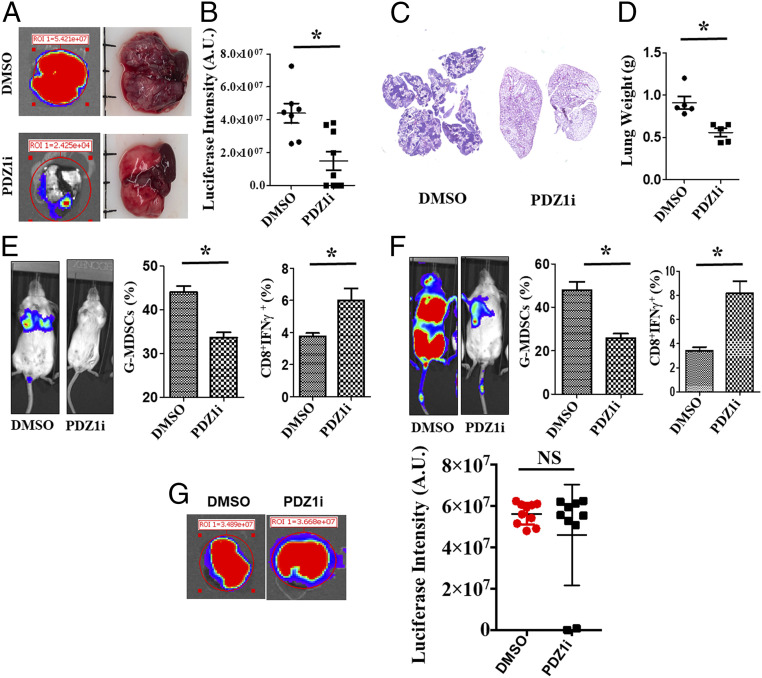
Given the well-established role of MDA-9/Syntenin in breast cancer metastasis progression and host-immune suppression (11, 12, 15), we initially determined the therapeutic impact of pharmacological inhibition of these pathways. 4T1-Luc cells were injected intravenously in BALB/c mice (Fig. 1) followed by intraperitoneal treatment with DMSO or PDZ1i on alternate days for 14 d. In addition to weekly imaging to monitor metastatic development in the lungs, ex vivo luciferase activity was also monitored in excised lungs (Fig. 1A) at day 14 (study endpoint). Region-of-interest (ROI) values for each lung between the DMSO- and PDZ1i-treated groups indicated an approximately twofold reduction in luciferase intensity in the lungs of PDZ1i-treated animals (Fig. 1B). Microscopic analysis of serial lung sections also confirmed a reduction in metastatic foci following PDZ1i vs. DMSO treatment (Fig. 1C). Moreover, the weight of lungs of PDZ1i-treated animals were less than DMSO-treated animals (Fig. 1D). Since immunosuppression is commonplace in metastasis (31), we determined if PDZ1i prevented immunosuppressive cell infiltration in the lungs focusing on accumulation of MDSCs in the lungs of tumor-bearing mice. DMSO-treated lungs contained significantly higher numbers (∼1.5-fold) of granulocytic MDSCs (G-MDSCs, CD11b+Ly6ClowLy6G+) than lungs of PDZ1i-treated animals at both day 7 (Fig. 1 E, Center) and day 14 (Fig. 1 F, Center). Reduction in MDSCs correlated with reduced metastasis in the lungs of PDZ1i-treated animals (Fig. 1 E and F, Left). Consistent with the reduction in MDSCs in PDZ1i-treated lungs, we observed an up-regulation (∼1.5- to 2-fold) in tumor-specific (4T1-Luc) T cells (CD8+IFN-γ+) at days 7 and 14 (Fig. 1 E and F, Right, respectively). Intriguingly, PDZ1i-mediated suppression of metastasis was not observed in NOD-SCID-γ (NSG) mice, emphasizing the importance of an intact immune system in pharmacological targeting of MDA-9/Syntenin in breast cancer (Fig. 1G). Collectively, reduction of MDSCs and systemic activation of T cells following treatment with PDZ1i provide support for an immunological role of MDA-9/Syntenin in promoting metastatic breast cancer growth in the lungs of syngeneic animals (Fig. 1).
Fig. 1.
PDZ1i inhibits breast cancer experimental lung metastasis in immunocompetent animals. (A) A cohort (n = 16) of animals were inoculated with 4T1-Luc cells through tail vein injections. After 12 h, mice received DMSO or PDZ1i at a dose of 30 mg/kg body weight intraperitoneally on alternate days during a 2-wk study period (three injections per week and six injections in total). Representative ex vivo bioluminescence (BLI) images (Left), as well as photographs of lungs are presented from DMSO- and PDZ1i-treated groups (Right). (B) Relative luciferase intensity was measured in individual animals at a single time point (day 15) and was plotted. (C) Lungs were processed for pathological evaluation. Representative H&E sections at lower magnification are presented. (D) The gross lung weight (at the end of the study) was determined and was plotted. (E and F) A cohort of animals were inoculated with 4T1-Luc cells and separated into control (DMSO) and therapeutic (PDZ1i, 30 mg/kg body weight) groups. A subset of animals (n = 5) were killed on day 7 (after three doses of PDZ1i) and lungs were analyzed for accumulation of MDSC populations using FACS. Spleens were collected and total splenocytes were stimulated with 4T1-Luc cell extracts. CD8+IFN-γ+ cells were analyzed through FACS. Similar experiments were also performed on other subsets of animals on day 15. In both E and F, the Left shows BLI images of representative animals from each group. Center and Right represent the average of G-MDSCs and CD8+IFN-γ+ in the corresponding groups. (G) A cohort of NSG mice were inoculated with 4T1-Luc cells by intravenous tail vein injections. Mice received DMSO or PDZ1i injections 12 h later at a dose of 30 mg/kg body weight and then received a similar dose of test agent intravenously every alternate day for 2 wk (three injections per week and six injections in total). Representative BLI images (Left) and relative luciferase intensity (Right) was measured and plotted graphically. NS: Not statistically significant. *P < 0.05 is considered as statistically significant.
Tumor Cell-Derived IL-1β Regulates MDSC Accumulation in PDZ1i-Treated Animals.
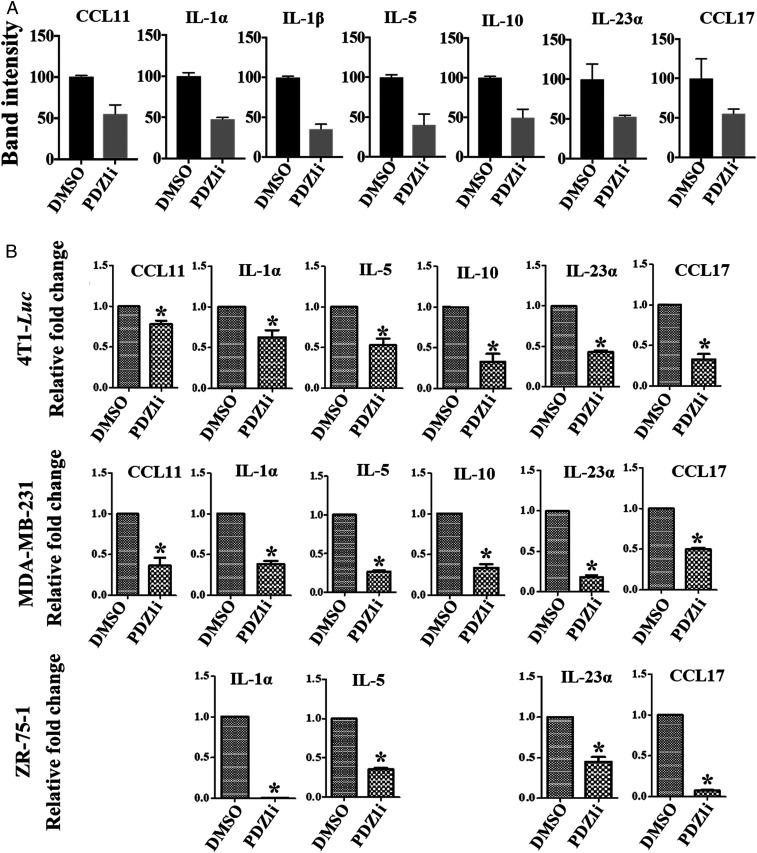
Tumor cell-derived cytokines/growth factors promote infiltration of immunosuppressive cells in the local niche (37). Accordingly, we analyzed a protein-based array consisting of 40 cytokines (SI Appendix, Fig. S1), predominantly involved in triggering inflammation in the tumor niche, using conditioned media from DMSO- or PDZ1i-treated 4T1-Luc cells (SI Appendix, Fig. S2). Multiple cytokines (CCL11, IL-1α, IL-1β, IL-5, IL-10, IL-23, CCL17) were down-regulated greater than twofold by PDZ1i, with IL-1β showing the greatest effect (Fig. 2A). Validation of these findings was obtained using semiquantitative RT-PCR for selected cytokines in PDZ1i-treated murine (Fig. 2 B, Top) and human breast cancer cell lines (Fig. 2 B, Middle and Bottom).
Fig. 2.
IL-1β, a proinflammatory cytokine, is robustly down-regulated by PDZ1i in tumor cells. (A) DMSO- or PDZ1i-treated (12 h) 4T1-Luc conditioned media were analyzed for expression of various growth factors/cytokines using protein-based cytokine arrays from R&D Biosystems. Data were captured and band intensity was measured using ImageJ software. Relative intensity changes (percentage of DMSO-treated group) are presented for representative cytokines that showed 50% or greater decreases in expression. (B) 4T1-Luc and two different human breast cancer cell lines, MDA-MB-231 and ZR-75-1, were treated for 12 h with PDZ1i and total RNA was prepared. qPCR was performed for selective cytokines (from A) and presented as relative fold-changes vs. the control (DMSO) group. *P < 0.05 is considered as statistically significant.
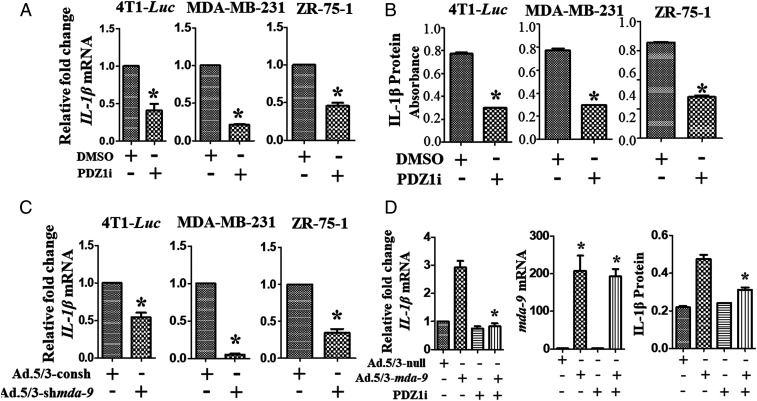
IL-1β is a mediator of MDSC infiltration and promotes tumor growth in the metastatic niche (38). We confirmed that PDZ1i down-regulates IL-1β mRNA and protein in three breast cancer cell lines using qPCR and ELISA (Fig. 3 A and B, respectively). To investigate the role of MDA-9/Syntenin in IL-1β regulation, mda-9/Syntenin expression was knocked down in breast cancer cell lines (SI Appendix, Fig. S3A), resulting in decreased IL-1β expression (Fig. 3C). To provide confirmation of specificity of regulation and inhibition of IL-1β expression by mda-9/Syntenin and PDZ1i, we used a T47D human breast carcinoma cell line, which does not express MDA-9/Syntenin (12). Expressing mda-9/Syntenin in T47D cells up-regulated IL-1β expression, which was prevented by PDZ1i at both mRNA and protein levels (Fig. 3D), supporting the specificity of MDA-9/Syntenin induction and PDZ1i disruption of MDA-9/Syntenin downstream signaling. Scrutinizing the Gene Expression Profiling Interactive Analysis (http://gepia.cancer-pku.cn) databases (GEPIA) indicated that MDA-9/Syntenin and IL-1β positively correlate in breast cancer patient samples (SI Appendix, Fig. S3B).
Fig. 3.
PDZ1i regulates IL-1β both transcriptionally and translationally in different breast cancer cell lines. (A and B) Indicated cells were treated with DMSO or PDZ1i for 24 h before processing for RNA or protein extraction. qPCR and ELISA were conducted to analyze mRNA or protein levels in cell lysates, respectively. (C) Indicated cell lines were genetically manipulated for MDA-9/Syntenin expression using an adenovirus (Ad.5/3) expressing shRNA for mda-9/Syntenin (Ad.5/3-shmda-9) and IL-1β mRNA levels were determined. Relative fold-changes in comparison with Ad.5/3 expressing control shRNA groups are presented. (D) T47D cells were induced to express mda-9/Syntenin using a viral-based expression approach and were analyzed for IL-1β mRNA and protein levels by qPCR (Left) and ELISA (Right), respectively. Expression of mda-9/Syntenin under different experimental conditions was confirmed by qPCR (Center). *P < 0.05 is considered as statistically significant.
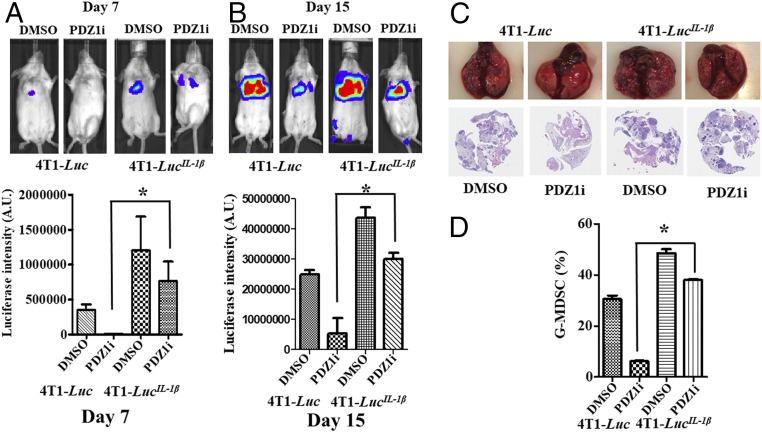
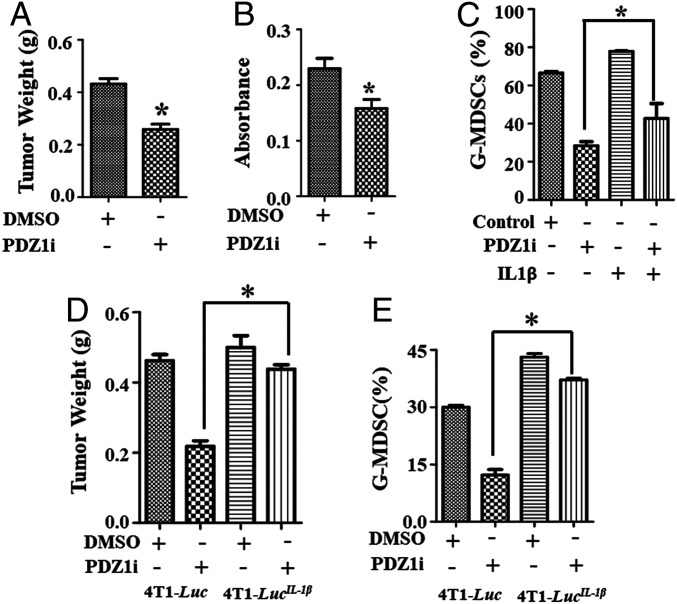
To define the functional relevance of IL-1β on cellular phenotypes in breast cancer cells, we engineered three stable 4T1-Luc clones to overexpress IL-1β, 4T1-LucIL−1β Cl.1, Cl.2, and Cl.3 (SI Appendix, Fig. S4). After confirming IL-1β expression by qPCR (SI Appendix, Fig. S4A) and ELISA (SI Appendix, Fig. S4B), Cl.3 was selected for further analysis. Intravenous injection of Cl.3 produced metastatic lesions in the lungs of mice, which were significantly inhibited by PDZ1i at days 7 and 15, as measured by luciferase intensity (Fig. 4 A and B) and pathological observations (Fig. 4C). At day 15, PDZ1i suppressed metastatic burden by ∼79% in animals receiving 4T1-Luc cells, however, the suppression of metastatic lesions was lower (∼30%) in animals injected with 4T1-LucIL−1β cells and treated with PDZ1i (Fig. 4B). This rescue was associated with increased accumulation of MDSCs in PDZ1i-treated animal lungs receiving 4T1-LucIL−1β cells in comparison with parental cells (Fig. 4D). Injection of 4T1-LucIL−1β cells enhanced G-MDSC infiltration in the lungs ∼1.6-fold and PDZ1i-induced suppression was less effective in comparison with 4T1-Luc–injected animals (Fig. 4D).
Fig. 4.
PDZ1i-mediated metastasis suppression is nullified in IL-1β overexpressing 4T1-Luc cells. (A and B) A cohort of animals (n = 20) were inoculated intravenously with either 4T1-Luc control (n = 10) or 4T1-LucIL-1β (n = 10) cells. After 12 h, half of the animals (n = 5) received either DMSO or PDZ1i (30 mg/kg body weight) intraperitoneally during the 2-wk study. Animals were subjected to live imaging on day 7 and day 15. Representative BLI imaging (Upper) and the average ROI (Lower) from each experimental group are presented. (C) Lungs were excised, photographed for gross pathology, and processed for histology (H&E-stained sections) and are presented (Upper and Lower, respectively). Magnification, 20×. (D) Three lungs from each group were processed and analyzed for the presence of G-MDSCs. The average values in different experimental conditions are plotted. *P < 0.05 is considered as statistically significant.
Next, we orthotopically implanted 4T1-Luc cells in the fourth mammary fat pad and treated the animals intraperitoneally with six doses of PDZ1i (or DMSO). PDZ1i inhibited primary tumor growth (∼40% in comparison with controls) (Fig. 5A) and reduced IL-1β expression in the tumor niche (Fig. 5B). Established tumors (∼1,000 mm3) received a single dose of PDZ1i (30 mg/kg) and were excised 6 h later, and MDSC infiltration vs. DMSO controls was determined. PDZ1i reduced accumulation of MDSCs by approximately twofold in tumor cell lysates (Fig. 5C) and suppression was partially reversed when recombinant IL-1β was given intratumorally. When orthotopically implanted 4T1-LucIL−1β cells were treated with PDZ1i for 14 d, minimal inhibition of tumor growth was evident (Fig. 5D) and MDSC infiltration was reduced (Fig. 5E). It is worth noting that IL-1β overexpression did not impact tumor growth (Fig. 5D, DMSO group from 4T1-Luc vs. 4T1-LucIL−1β), although it significantly enhanced G-MDSC accumulation (Fig. 5 D and E, DMSO group from 4T1-Luc vs. 4T1-LucIL−1β).
Fig. 5.
PDZ1i suppresses IL-1β expression and regulates primary tumor growth. (A) Following implantation of 4T1-Luc cells in the fourth mammary fat pad, mice were divided into two groups, vehicle control and PDZ1i, as described in Materials and Methods. Treatment started after 12 h. Tumors were excised on day 14 d and weighed. The average values from five mice are presented. (B) Tumor lysates were prepared and analyzed for IL-1β using ELISA. (C) Orthotopically implanted tumor-bearing mice received a single dose of DMSO or PDZ1i intraperitoneally 6 h after intratumoral injection of recombinant IL-1β (rIL-1β). Tumors were excised after 3 h of rIL-1β injection and analyzed for the accumulation of G-MDSCs. (D) Indicated cell lines were implanted in the fourth mammary fat pad and received DMSO or PDZ1i for 2 wk (injected intraperitoneally every alternate day, total of six injections). Tumor weight was measured and the average value of each group is plotted. (E) Tumors were analyzed for G-MDSCs by FACS and the average values are presented. *P < 0.05 is considered as statistically significant.
Role of STAT3 Inactivation in PDZ1i-Mediated IL-1β Suppression.
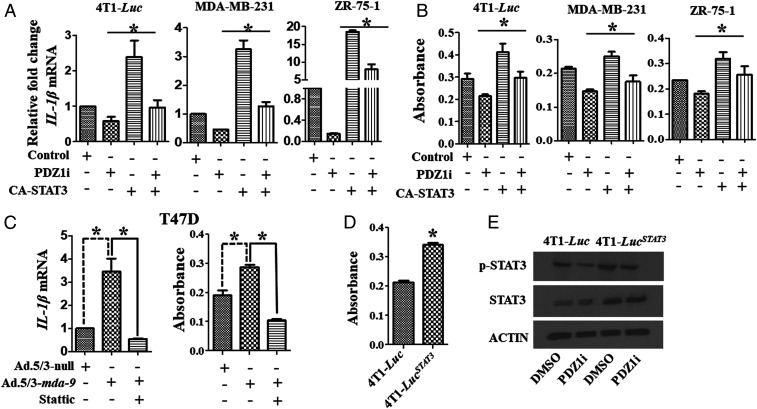
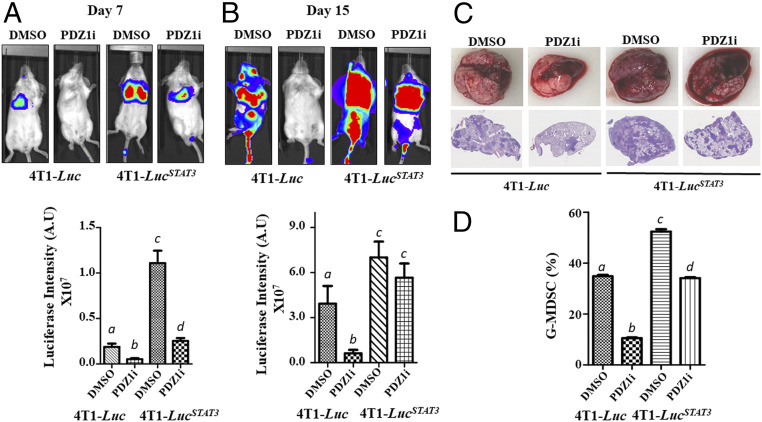
Since PDZ1i down-regulates IL-1β at an mRNA level, we predicted and now confirmed that this suppressive effect is transcriptional (Fig. 3 A and C). STAT3 activation stimulates production of various cytokines in breast cancer cells, including IL-1β (39), and PDZ1i treatment inactivates STAT3 in 4T1-Luc, MDA-MB-231, and ZR-75-1 cells (SI Appendix, Fig. S5), suggesting a presumed link between regulation of IL-1β and STAT3. To test this hypothesis, a constitutively active STAT3 (CA-STAT3) was transiently overexpressed in different breast cancer cell lines (both murine and human) followed by PDZ1i treatment. PDZ1i reduced IL-1β levels (both mRNA and protein) in control vector transfected murine and human breast cancer cell lines (Fig. 6 A and B). However, this suppression was partially reversed at both transcriptional and translational level when a constitutively active STAT3 was expressed. Additionally, mda-9/Syntenin–induced IL-1β expression was inhibited by Stattic, a nonpeptidic small-molecule STAT3 inhibitor, in MDA-9/Syntenin-expressing T47D cells, supporting a role of STAT3 in this regulation (Fig. 6C). Further support for this link derives from constitutively active STAT3 4T1-Luc cells (4T1-LucSTAT3), which secrete more IL-1β (Fig. 6D) and are less sensitive in terms of PDZ1i inhibition of IL-1β expression (Fig. 6 A, B, and E) than parental 4T1-Luc cells. To determine if the increased in vitro transformation-associated properties of 4T1-LucSTAT3 cells correlated with enhanced in vivo aggressiveness, we intravenously injected this cell line into mice (Fig. 7). This was indeed the case, as evidenced by enhanced luciferase intensity (Fig. 7 A and B) (at days 7 and 15) and pathological changes (Fig. 7C) (at day 15) in comparison with parental 4T1-Luc cells. The efficacy of PDZ1i was reduced in STAT3-overexpressing 4T1-LucSTAT3 cells, particularly at day 15 (Fig. 7). Moreover, PDZ1i failed to down-regulate MDSCs accumulation in the lungs of 4T1-LucSTAT3– vs. 4T1-Luc–injected mice (Fig. 7D). These data in composite support a role of mda-9/Syntenin in host immune suppression through the STAT3–IL-1β axis, which as demonstrated is genetically and pharmacologically targetable using mda-9sh and PDZ1i, respectively, providing therapeutic insights into how mda-9/Syntenin mediates cancer pathogenesis (Fig. 8).
Fig. 6.
PDZ1i-mediated deactivation of STAT3 down-regulates IL-1β transcription. (A and B) Indicated cells were transfected with a control vector or constitutively active STAT3 expression plasmid and 24 h posttransfection, cells were treated with DMSO or PDZ1i. Total cellular RNA and proteins were prepared and analyzed by qPCR and ELISA, respectively. Relative fold-changes of mRNA are presented. The absorbance value represents the amount of protein determined by ELISA. (C) mda-9/Syntenin expression was induced in T47D cells and cells were treated with Stattic, a STAT3 inhibitor. Total RNA and protein lysates were prepared and analyzed for IL-1β at mRNA and protein levels, respectively. (D) IL-1β expression in conditioned media from 4T1-Luc and 4T1-LucSTAT3 cells expressing an active STAT3 expression construct. (E) Indicated cells were treated with DMSO or PDZ1i for 24 h and Western blotting analysis was done with selected antibodies as shown in the figure. *P < 0.05 is considered as statistically significant.
Fig. 7.
Antimetastatic activity of PDZ1i is partially prevented in constitutively active STAT3-overexpressing 4T1-Luc cells (4T1-Luc STAT3). (A and B) A cohort of 20 animals were randomly divided into two groups and inoculated with control or STAT3-overexpressing cells. After 12 h, half of these mice received DMSO intraperitoneally and the remaining half received PDZ1i intraperitoneally at 30 mg/kg body weight (three times per week, total six injections). Mice were imaged once a week (on day 7 and day 15) before terminating the study. Representative photomicrographs from different experimental groups are presented (Upper). ROI values were calculated and the average value from five mice are presented for the two time points (Lower). Different letters indicate statistical significance among the corresponding groups. (C) Lungs were excised and representative photographs are presented (Upper). H&E sections covering the lungs in lower magnification are shown (Lower). Magnification, 20×. (D) Accumulation of MDSCs in the lung from different experimental groups were analyzed using FACS and the average values are plotted. Different letter in two variables are statistically significant (P < 0.05).
Fig. 8.
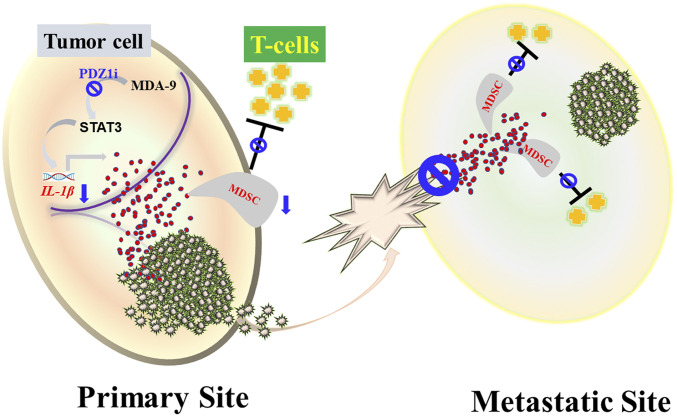
Schematic of the role of MDA-9/Syntenin in immune suppression and reversal of suppression by PDZ1i. In tumor cells, MDA-9/Syntenin mediates activation of STAT3 resulting in secretion of IL-1β that fosters mobilization of MDSCs in the tumor niche, establishing an immunosuppressive environment. PDZ1i, selectively inhibits MDA-9/Syntenin signaling, thereby obstructing the STAT3/IL-1β pathway preventing MDSCs accumulation and consequently altering the dynamics of immunosuppression, preventing metastatic breast cancer cell growth.
Discussion
mda-9/Syntenin is a promising molecular target in breast cancer progression and metastasis (11, 12, 15, 18). We focused on evaluating a first-in-class small-molecule inhibitor of MDA-9/Syntenin, PDZ1i (13, 21, 23), in breast cancer metastasis, emphasizing central elements controlling tumor immune tolerance. MDA-9/Syntenin provokes immune suppression in primary breast cancer growth through PD-L1 by promoting T cell apoptosis and knockdown of mda-9/Syntenin reduces metastatic competence (15). We demonstrate that MDA-9/Syntenin regulates tumor-specific expression of IL-1β and other proinflammatory cytokines mediated by STAT3, and enhances MDSC infiltration producing immune suppression. Blocking MDA-9/Syntenin with PDZ1i impedes breast cancer metastasis in syngeneic animals, reversing the immunosuppressive effects in the metastatic breast cancer niche providing therapeutic benefit against metastatic breast cancer.
The tumor microenvironment shapes tumor cell outgrowth and patient outcome and consists of immune-activating and -suppressing cells that are attracted by tumor-derived immune-regulatory molecules, chemoattractant molecules, and growth factors (40). Proinflammatory, proangiogenic and Th2-related cytokines, such as platelet-derived growth factor BB and IFN-γ induced protein-10 (IP-10) are major constituents of this microenvironment (41). High levels of tumor infiltrating lymphocytes in the tumor milieu also correlate with elevated levels of additional cytokines, including IL-7, IL-10, IL-1RA, IL-1β, Rantes, and VEGF (42). MDSCs, key suppressors of myeloid lineage, are well represented in the tumor microenvironment (43), promoting potent immunosuppressive effects by inducing antigen-specific T cell anergy, T cell tolerance (33). Accordingly, modulating the secretion of such factors and restoration of an immunostimulatory environment may provide an efficient direct approach to treat cancer. PDZ1i blocks IL-1β production resulting in reduced MDSC accumulation in metastatic sites (lungs), enhancing antitumor immunity. PDZ1i through inhibition of MDA-9/Syntenin signaling affects both cell-autonomous and noncell-autonomous functions in regulating cancer metastasis (13, 21, 23). Immune modulation by PDZ1i involves down-regulation of IL-1β at a transcriptional level in tumor cells (summarized in Fig. 8). Additionally, cytokine secretion by infiltrating tumor cells enhances further tumorigenic activity.
Inhibiting cytokine expression using antibodies has provided only transient improvement and limited long-term clinical responses in patients (44). In principle, obtaining tangible benefits may require blocking expression of multiple cytokines. PDZ1i blocks expression of numerous cytokines in addition to IL-1β in tumor cells, including CCL11, IL-10, and IL-23 that are germane to breast cancer metastasis. CCL11 levels correlate with infiltration of immunosuppressive Treg cells in breast cancer progression (45). IL-10, an immune-suppressive cytokine, directly promotes vascularization and immune escape during breast cancer progression (46). Additionally, breast cancer progression correlates with aberrant expression of IL-23 and its cognate receptor IL-23R (47). It is anticipated that PDZ1i-mediated reduction of these cytokines might influence the overall antimetastasis activities of this inhibitor.
The production of biologically active IL-1β requires both transcriptional and posttranslational regulation (48). Proinflammatory mediators, including IL-1β through engagement of its IL-1R canonical receptor, further augment transcription. LPS stimulates STAT3 tyrosine phosphorylation (at position 705) in murine macrophages (39). Using multiple approaches, including gain- and loss-of-function with expression plasmids and chemical inhibitors, we demonstrate that STAT3 regulates IL-1β expression. Inhibiting MDA-9/Syntenin activity suppresses IL-1β mRNA and protein expression. STAT3 activation by MDA-9/Syntenin is a general phenomenon in multiple cancers (9, 10, 23, 25, 42, 49), including breast cancer where MDA-9/Syntenin up-regulates PD-L1 by STAT3 activation (15). PDZ1i blocks STAT3 activation moderating prostate cancer progression (23). Moreover, PDZ1i reduces IL-1β in metastatic prostate cancer cells (23), likely involving an analogous mechanism of regulation as observed in breast cancer. These observations support broad overlapping molecular modes of action of PDZ1i in multiple cancer types overexpressing MDA-9/Syntenin.
STAT3 activation is crucial for both the intrinsic (cancer cell-centric) and extrinsic (cancer cell-mediated/secreted) pathways controlling cancer progression (50). STAT3 regulates tumor cell proliferation and survival, as well as secretion of multiple cytokines (or other growth factors, including proangiogenic factors) responsible for recruiting and activating immune suppression (50). A constitutively active STAT3-expressing 4T1 clone showed enhanced metastatic aggression and progression, IL-1β secretion, and infiltration of MDSCs in the metastatic niche and all of these phenotypic gains were impervious to PDZ1i-mediated suppression (Fig. 7). Although IL-1β is subject to multiple levels of control, including posttranslational and processing of mature IL-1β, we consider transcriptional control to be a primary regulator of expression, since PDZ1i down-regulates IL-1β at an mRNA level. A potential involvement of other transcription factors, such as NF-κB, an established activator of IL-1β (51), cannot be excluded based on a strong association between this transcription factor and MDA-9/Syntenin in defining cancer metastasis (52, 53). In total, our results suggest that PDZ1i down-regulates STAT3-mediated IL-1β expression, reducing the mobilization of MDSCs and enhancing antigen-specific systemic T cell activation, facilitating cytotoxic activity.
Chronically high IL-1β expression sustains activation of NF-κB, MAPK, AKT, and WNT signaling pathways providing a causal link in tumor development (54). IL-1β also promotes tumor growth through endothelial cell activation, tumor angiogenesis, and induction of immunosuppressive cells (55). MDSCs are expanded and migrate toward cancers (56), including 4T1 tumors. IL-1β may be produced by tumor cells and tumor microenvironment resident or infiltrating cells, including immune cells (57). In mice, global knockout of IL-1β suppresses breast cancer growth by decreasing CCR2+-infiltrating cells that have differentiated into immunosuppressive cells enhancing T cell-mediated immunity (57). Although, a potential role of IL-1β promoted by MDA-9/Syntenin expression in stromal cells remains to be shown, our previous study confirms a reduction in inflammatory cytokines in global knockout mda-9/syntenin mice (16). Since PDZ1i could affect both the tumor and microenvironment, it is likely that PDZ1i also down-regulates IL-1β in both compartments. It is also possible that an IL-1β feed-forward loop may exist between an established tumor and resident cells in the microenvironment and PDZ1i might also disrupt this communication.
In summary, MDA-9/Syntenin is an essential governor of cancer metastasis, controlling multiple pivotal and mandatory steps in this process that include invasion and intravasation, attachment in secondary organ sites, survival in the circulation, extravasation, modulation of the tumor microenvironment, and angiogenesis (reviewed in ref. 58). MDA-9/Syntenin also regulates chemoresistance, survival, and stemness in cancer stem cells (59, 60), further supporting potential as a therapeutic target for metastasis. Proof-of-concept supporting this possibility is provided by PDZ1i, a first-in-class small-molecule inhibitor targeting the PDZ1 domain of MDA-9/Syntenin, which possesses robust cancer invasion and metastasis inhibitory properties (5, 13, 21, 23). PDZ1i modifies the immune environment, selecting against growth and survival of metastatic breast cancer cells by down-regulating STAT3, inhibiting IL-1β expression, reducing mobilization of MDSCs in the metastatic niche, and enhancing antigen-specific systemic T cell activation. Our studies highlight tumor-derived IL-1β as a critical driver of breast cancer metastasis regulated by MDA-9/Syntenin (Fig. 8). They also confirm the antiinvasion and antimetastatic properties of PDZ1i as an efficacious inhibitor of breast cancer metastasis. PDZ1i represents a promising potential therapeutic agent for invasive and metastasic breast cancer, since it does not display toxicity in murine models and has acceptable ADME (absorption, distribution, metabolism, excretion) and excellent pharmacological properties (a half-life of ≥9 h when injected intraperitoneally). Detailed investigational new drug-enabling toxicological studies using an appropriate clinically relevant formulation are key next steps to progress PDZ1i from bench to bedside.
Materials and Methods
Reagents and Cell Culture.
PDZ1i was synthesized and characterized as described previously (21). Cell lines used in this study included T47D, ZR-75-1, and MDA-MB-231 (human breast cancer cell lines), which were obtained from the ATCC (American Type Culture Collection). The 4T1 (mouse mammary cancer cell line) cell line was described previously (61). All the cell lines were regularly monitored for mycoplasma contamination using a mycoplasma detection kit (Sigma). Constitutive STAT3- and IL-1β–overexpressing cells were developed using standard protocols (62).
Real-Time PCR.
RNA was isolated using a Qiagen kit and cDNAs were prepared using an ABI cDNA synthesis kit. Real-time PCR was performed using TaqMan probes and real time master mix obtained from Applied Biosystems. Data were analyzed using Graphpad prism software.
MDSCs Staining and T Cell Activation Assay.
Single-cell suspensions were prepared from lungs, as described previously (16). Equal numbers of cells were resuspended in FACS buffer (PBS containing 0.1% BSA and 0.04% EDTA-Na2). After surface staining with the appropriate antibody from BioLegend (e.g., for MDSCs, CD11b [Alexa fluor 594], Ly6C [Alexa fluor 488], and APC-Ly6G), flow cytometry analysis was performed using a BD FACS Canto flow cytometer. For analysis of T cell activation, tumors were digested with collagenase D (1 mg/mL) and DNase I (100 μg/mL), and cell suspensions were filtered through a 70-μm cell strainer, as previously described (63). The viable mononuclear cells were isolated using the Histopaque (Sigma-Aldrich) gradient and analyzed by FACS caliber (BD Biosciences). To determine the activation of CD8+ T cells, single-cell suspension prepared from 4T1-Luc were stimulated with PMA plus ionomycin in the presence of Golgi-stop for 6 h, followed by intracellular staining for IFN-γ–producing CD8+ cells.
Western Blotting.
Standard protocols were followed for Western blotting. The primary antibodies used were p-STAT3, STAT3, Actin (Cell Signaling Technology), MDA-9/Syntenin (Abnova). Secondary antibodies were purchased from Cell Signaling Technology. Fluorescently tagged antibodies used for Flow cytometry (CD3, CD8, IFN-γ, CD11b, Ly-6C, and Ly-6G) were obtained from BioLegend.
Animal Studies.
BALB/c female mice were used and obtained from Envigo. All animal studies were conducted using a protocol approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. 4T1-Luc cells (1 × 105 cells in 100 µL PBS) constitutively expressing a luciferase gene were injected in BALB/c mice through their tail veins. BALB/c mice were injected in the right second mammary gland with 4T1 luciferase-expressing cells (5 × 105 cells) in 100 µL serum-free medium. Animals were treated as described in the figure legends and, after 2 wk, mice were killed and the tumors were extracted for further study.
Statistical Analyses.
Statistical analyses were performed by ANOVA or t test using Graph pad prism software. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
The present study was supported in part by NIH/National Cancer Institute (NCI) Grants R01 CA244993 (to D.S. and P.B.F.) and CA099326 and CA229812 (to X.-Y.W.); Department of Defense Grant W81XWH1910489 (to J.W.L.); NCI Cancer Center Support Grant to the Virginia Commonwealth University (VCU) Massey Cancer Center (MCC) P30 CA016059 (to Robert Winn); the National Foundation for Cancer Research (W.K.C. and P.B.F.); and a Sponsored Research Agreement between VCU and InVaMet Therapeutics (to S.K.D.). P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research at the MCC. D.S. is the Harrison Foundation Distinguish Professor in Cancer Research at the MCC. X.-Y.W. holds the Harry and Judy Wason Chair in Cancer Research at the MCC. Services and products in support of the research project were generated by the VCU MCC Flow Cytometry Shared Resource and Cancer Mouse Models Core Laboratory, supported, in part, with funding from NIH/NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Competing interest statement: W.K.C. and P.B.F. are cofounders and have ownership interest in InVaMet Therapeutics, Inc. Virginia Commonwealth University and the Sanford Burnham Prebys Medical Discovery Institute have ownership interest in InVaMet Therapeutics, Inc.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2103180118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Liu F. C., et al., Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 8, 16939–16950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan L., Delay in breast cancer: Implications for stage at diagnosis and survival. Front. Public Health 2, 87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J. J., Jiang H., Fisher P. B., Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene 207, 105–110 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Grootjans J. J., et al., Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. U.S.A. 94, 13683–13688 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das S. K., et al., MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol. Res. 155, 104695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukerche H., et al., mda-9/Syntenin: A positive regulator of melanoma metastasis. Cancer Res. 65, 10901–10911 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Koo T. H., et al., Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene 21, 4080–4088 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Das S. K., et al., MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 73, 844–854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S. K., et al., The MDA-9/syntenin/IGF1R/STAT3 axis directs prostate cancer invasion. Cancer Res. 78, 2852–2863 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta S., et al., Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin. Cancer Res. 19, 4621–4633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwangbo C., et al., Syntenin regulates TGF-β1-induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-β type I receptor internalization. Oncogene 35, 389–401 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Menezes M. E., et al., MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 7, 80175–80189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhoopathi P., et al., Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene 38, 6781–6793 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imjeti N. S., et al., Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. U.S.A. 114, 12495–12500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., et al., Syntenin1/MDA-9 (SDCBP) induces immune evasion in triple-negative breast cancer by upregulating PD-L1. Breast Cancer Res. Treat. 171, 345–357 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Das S. K., et al., Knockout of MDA-9/Syntenin (SDCBP) expression in the microenvironment dampens tumor-supporting inflammation and inhibits melanoma metastasis. Oncotarget 7, 46848–46861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plasterer C., et al., Identification of a rat mammary tumor risk locus that is syntenic with the commonly amplified 8q12.1 and 8q22.1 regions in human breast cancer patients. G3 (Bethesda) 9, 1739–1743 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., et al., Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res. 15, R50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian X. L., et al., Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation. PLoS One 8, e60046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ternette N., et al., Immunopeptidomic profiling of HLA-A2-positive triple negative breast cancer identifies potential immunotherapy target antigens. Proteomics 18, e1700465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kegelman T. P., et al., Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc. Natl. Acad. Sci. U.S.A. 114, 370–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kegelman T. P., et al., Targeting tumor invasion: The roles of MDA-9/syntenin. Expert Opin. Ther. Targets 19, 97–112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S. K., et al., Suppression of prostate cancer pathogenesis using an MDA-9/Syntenin (SDCBP) PDZ1 small molecule inhibitor. Mol. Cancer Ther. 18, 1997–2007 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Liu J., et al., Syntenin-targeted peptide blocker inhibits progression of cancer cells. Eur. J. Med. Chem. 154, 354–366 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Talukdar S., et al., MDA-9/Syntenin (SDCBP) is a critical regulator of chemoresistance, survival and stemness in prostate cancer stem cells. Cancers (Basel) 12, 53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen L. M. E., Ramsay E. E., Logsdon C. D., Overwijk W. W., The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer 5, 79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X., et al., Circulating tumor cells in cancer patients: Developments and clinical applications for immunotherapy. Mol. Cancer 19, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younos I., et al., Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int. Immunopharmacol. 11, 816–826 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Ding Y., Guo N., Wang S., MDSCs: Key criminals of tumor pre-metastatic niche formation. Front. Immunol. 10, 172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H. H., et al., Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sceneay J., Parker B. S., Smyth M. J., Möller A., Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. OncoImmunology 2, e22355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki E., Kapoor V., Jassar A. S., Kaiser L. R., Albelda S. M., Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Vincent J., et al., 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Kodumudi K. N., et al., A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 16, 4583–4594 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A., Barajon I., Garlanda C., IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 281, 57–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens L. M., Werb Z., Inflammation and cancer. Nature 420, 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith H. A., Kang Y., The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. (Berl.) 91, 411–429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu S., et al., Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14, 408–419 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samavati L., et al., STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 46, 1867–1877 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Yuan Y., Jiang Y. C., Sun C. K., Chen Q. M., Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 35, 2499–2515 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Jabeen S., et al., Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. OncoImmunology 8, e1537691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinoza J. A., et al., Cytokine profiling of tumor interstitial fluid of the breast and its relationship with lymphocyte infiltration and clinicopathological characteristics. OncoImmunology 5, e1248015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becht E., Giraldo N. A., Dieu-Nosjean M. C., Sautès-Fridman C., Fridman W. H., Cancer immune contexture and immunotherapy. Curr. Opin. Immunol. 39, 7–13 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Weiner L. M., Surana R., Wang S., Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10, 317–327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R., Huang K., CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL-2 and TGF-β by CD4+ T cells via the STAT5 signaling pathway. Mol. Med. Rep. 21, 2522–2532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheikhpour E., et al., A survey on the role of interleukin-10 in breast cancer: A narrative. Rep. Biochem. Mol. Biol. 7, 30–37 (2018). [PMC free article] [PubMed] [Google Scholar]
- 47.Sheng S., Zhang J., Ai J., Hao X., Luan R., Aberrant expression of IL-23/IL-23R in patients with breast cancer and its clinical significance. Mol. Med. Rep. 17, 4639–4644 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Mayer-Barber K. D., Yan B., Clash of the cytokine titans: Counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell. Mol. Immunol. 14, 22–35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talukdar S., et al., Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells. Oncotarget 7, 54102–54119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H., Pardoll D., Jove R., STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cogswell J. P., et al., NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 153, 712–723 (1994). [PubMed] [Google Scholar]
- 52.Boukerche H., et al., Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene 29, 3054–3066 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boukerche H., Su Z. Z., Prévot C., Sarkar D., Fisher P. B., mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc. Natl. Acad. Sci. U.S.A. 105, 15914–15919 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinarello C. A., Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tulotta C., Ottewell P., The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer 25, R421–R434 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song X., et al., CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J. Immunol. 175, 8200–8208 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Bent R., Moll L., Grabbe S., Bros M., Interleukin-1 beta-A friend or foe in malignancies? Int. J. Mol. Sci. 19, 2155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das S. K., Sarkar D., Emdad L., Fisher P. B., MDA-9/Syntenin: An emerging global molecular target regulating cancer invasion and metastasis. Adv. Cancer Res. 144, 137–191 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Talukdar S., et al., MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc. Natl. Acad. Sci. U.S.A. 115, 5768–5773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talukdar S., et al., Regulation of protective autophagy in anoikis-resistant glioma stem cells by SDCBP/MDA-9/Syntenin. Autophagy 14, 1845–1846 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagahashi M., et al., Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 72, 726–735 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emdad L., et al., Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 21300–21305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huanfa Y.et al., Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T-cell response and antitumor immunity. Cancer Res. 71, 6611–6620 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.