Cerebral small vessel diseases (SVDs) are a group of related pathologies that collectively account for over 25% of ischemic strokes and more than 40% of all dementias (1, 2). Although genetic forms have been identified, sporadic SVDs are the most common and become prevalent with increasing age. The causes of sporadic SVDs remain poorly understood, and no treatment options are currently available. SVDs can occur in any organ in the body. However, the brain’s microvasculature is uniquely susceptible to dysfunction. In tissues such as skeletal muscle, metabolic demand is met in part by an organ-wide dilation of the vasculature that lowers the resistance to flow so that increased demand is satisfied by a surge of blood flow throughout the tissue. In contrast, the skull imposes an essentially fixed volume to prevent global increases in the amount of blood in the brain. Thus, nature has evolved mechanisms unique to the cerebral circulation to rapidly redirect blood flow to brain regions with higher metabolic activity at the cost of diminished flow elsewhere (3, 4). This process is termed functional hyperemia. It involves communication between active brain regions and the cerebral vasculature by loosely defined processes known as “neurovascular coupling” (5–7).
Neurovascular coupling is disrupted in cerebral SVDs (1, 2), and the diminished state of functional hyperemia contributes to vascular cognitive impairment and dementia. In PNAS, Dabertrand et al. (8) demonstrate the molecular basis for the loss of functional hyperemia for a particular SVD and, impressively, show how the dysfunction may be reversed. In particular, impaired functional hyperemia is rescued by exogenously supplying the minor phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2), to a mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (9). This is the predominant genetic SVD and a model for more common sporadic forms of SVD. The findings by Dabertrand et al. (8) may set the stage for the development of treatments for impaired neurovascular coupling and dementias associated with cerebral SVDs.
The vasculature of the brain is organized as a hierarchy (10). The pial arteries form a highly interconnected network that spans the surface of the cortex. Dynamic changes in the diameter of different branches allow the pia to shuttle blood to areas of acute metabolic need (11). This network sources penetrating arterioles that dive into the parenchyma and, in turn, supply a vast, interconnected network of capillaries that provide energetic substrates to all brain cells. Despite the great numbers of paths for blood cells to take as they journey from a penetrating arteriole to their eventual exit through a penetrating venule and then vein, the capillaries provide the greatest resistance to flow in the brain (12).
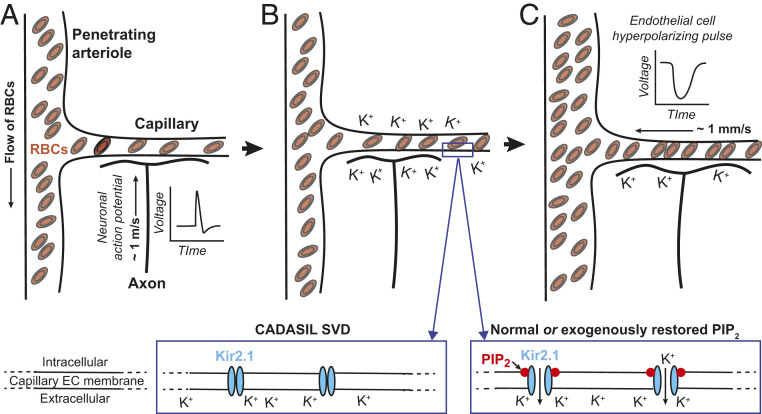
An emerging model of functional hyperemia in the brain focuses on the capillaries. While the density of the capillary network varies between different regions of the brain, the typical distance from a location in the neocortical parenchyma to the nearest capillary is quite small, about 13 µm (13). Drawing on this implicit, intimate relation between neurons and capillaries, Longden et al. (14) hypothesized that the brain uses the capillary network as a sensory web to detect elevated neuronal activity and subsequently signal upstream penetrating arterioles and pial arteries to dilate. The mechanism involves K+ ions and the inwardly rectifying K+ channel Kir2.1 (Fig. 1). Potassium ions are released during every neuronal action potential, and in principle, the local [K+] can approach 10 mM in the vicinity of capillaries (14, 15). This concentration is sufficient to activate Kir2.1, whose threshold for opening is raised by the increase in extracellular [K+]. This leads to the onset of a regenerative, hyperpolarizing pulse that propagates to adjacent endothelial cells via gap junctions, thereby stimulating further Kir2.1 channel activity to spread the signal. Upon reaching upstream arterioles, this hyperpolarizing signal is conveyed through gap junctions to overlying smooth muscle cells, causing them to relax. The relaxation of arteriole smooth muscle and subsequent dilation of the penetrating and pial vessels leads to an increase in the pressure head on the capillary network and an increase in blood flow through the region with heightened activity. Finally, activation of the transient receptor potential V4 (TRPV4) cation channel and possibly other mechanisms lead to the recovery to the membrane potential in endothelial cells.
Fig. 1.
The initial signaling events in functional hyperemia. (A and B) Neuronal action potentials induce hyperpolarizing pulses in capillary endothelial cells via buildup of extracellular [K+] to open Kir2.1 channels. Opening of these channels further requires binding of PIP2. (C) Hyperpolarizing pulses in capillary endothelial cells (ECs), caused by K+ flux through the opened Kir2.1 channels, are electrically conducted to upstream penetrating arterioles and induce dilation of the penetrating arterioles by relaxation of the associated smooth muscle. This leads to an elevated pressure head on the capillary network and an increase in red blood cell (RBC) flux. (Inset) Loss of PIP2 in the CADASIL small vessel disease (SVD) model prevents the opening of Kir2.1 channels and blocks functional hyperemia, while normal or restored levels of PIP2 support opening of Kir2.1 and a normal hemodynamic response.
Exploration of the K+/Kir2.1-based model makes use of an innovative ex vivo cerebral microvascular preparation that comprises a cannulated, pressurized penetrating arteriole segment with an intact capillary tree (14). Direct stimulation of the tree with a bolus of K+ leads to a rapid dilation of upstream arterioles in the parenchyma. This response is blocked by infusion with low concentrations of the known Kir2.1 channel blocker BaCl2 and is absent in endothelial cell-specific Kir2.1-knockout mice. Stimulation of the vibrissae, a classic sensory pathway in neurovascular physiology, induces functional hyperemic responses in the vibrissa primary somatosensory (vS1) cortex. Functional hyperemia is blunted by infusion with BaCl2 and impaired in endothelial cell-specific Kir2.1-knockout mice. The obligatory role of PIP2 in the Kir2.1-based mechanism for the control of functional hyperemia was observed in follow-up studies (Fig. 1, Inset). In particular, Harraz et al. (16) showed that prolonged stimulation of Gq G-protein–coupled receptors, which signal through phospholipase C (PLC) in capillary endothelial cells, will cripple the K+/Kir2.1-based mechanism for the control of functional hyperemia. Interestingly, the underlying mechanism is the consumption of PIP2 by the Gq signaling pathway.
Dabertrand et al. (8) demonstrate that the K+/Kir2.1-based neurovascular coupling mechanism is absent in CADASIL SVD mice due to twofold diminished activity of capillary endothelial cell Kir2.1 channels. This deficit leads to a significantly blunted vibrissa stimulation-induced functional hyperemic response in the vS1 cortex. Dabertrand et al. (8) go on to rescue the suppressed Kir2.1 currents in three ways. First, they show that the addition of PIP2 to the intracellular recording solution used with isolated capillary endothelial cells from CADASIL SVD mice restores the Kir2.1 currents. Second, applying a synthetic dipalmitoyl form of PIP2, diC16-PIP2, to the external solution that perfuses their preparation rescues the capillary-to-arteriole dilation in response to stimulation of a capillary tree with K+. Last, infusing mice with PIP2 restores vibrissa stimulation-induced functional hyperemia in vivo in CADASIL SVD mice.
Investigations into the cause of reduced PIP2 levels initially focused on processes that degrade PIP2. In particular, conventional wisdom pointed to PLC-mediated hydrolysis of PIP2 downstream of GqPCR signaling. However, inhibition of PLC did not affect blunted Kir2.1 current activity, which ruled out the degradation pathway. Subsequent investigation addressed PIP2 synthesis by lipid kinases, which are low ATP-affinity enzymes that require high ATP levels (17). Notably, ATP levels were depressed in brain capillaries from CADASIL SVD mice. This suggests that a decrease in ATP synthesis in brain capillary endothelial cells is the ultimate cause of diminished PIP2 levels, which drives the cascade that impairs Kir2.1 channel activity and leads to the failure of neurovascular coupling in CADASIL SVD mice.
Collectively, the data of Dabertrand et al. (8) demonstrate that the molecular basis of faulty neurovascular coupling associated with CADASIL SVD is an exclusively capillary Kir2.1 channelopathy that is caused by PIP2 depletion. These findings provide proof-of-concept for the therapeutic potential of PIP2 replacement. More generally, they spell out the complete molecular sequence for a cerebral SVD. However, questions remain. Is the PIP2 depletion-dependent impairment in microvascular function unique to the CADASIL SVD model, or does it contribute to other cerebral SVDs? A recent report demonstrating that PIP2 treatment restores impaired functional hyperemia in the 5xFAD mouse model of Alzheimer’s disease (18) provides intriguing evidence for a generalized PIP2-centric model of cerebral microvascular dysfunction. As a potential clinical issue, how does exogenously supplied PIP2 enter into cells? Flippases on the plasma membrane can import extracellular PIP2, but the process requires ATP, which is compromised in the capillaries of CADASIL SVD mice. Ca2+-dependent scramblase activity, independent of ATP, could also transport PIP2 to the inner leaflet of the plasma membrane. Understanding this transport process may be the key to unlocking the therapeutic potential of PIP2 supplementation.
The K+/Kir2.1-based neurovascular coupling mechanism appears to drive the initial response, on the 1-s timescale, for increased nutriments to a region in the brain (Fig. 1). Other aspects of neurovascular coupling work on longer timescales (19, 20). A recently discovered complementary mechanism is capillary-to-arteriole signaling triggered by Ca2+ influx through the transient receptor potential ankyrin 1 (TRPA1) channels in capillary endothelial cells (20). The vasodilatory signal initiated by TRPA1, which is essential for maintaining functional hyperemia during long-duration somatosensory stimuli, propagates through a biphasic mechanism that involves slow intercellular Ca2+ waves as well as the Kir2.1-dependent electrical conduction mechanism identified by Longden et al. (14, 20). Thus, PIP2 depletion likely impairs functional hyperemia in the brain that is orchestrated by different molecular sensors over multiple timescales. PIP2 depletion may prove to be a universal feature of cerebral SVDs.
Acknowledgments
We thank Beth Friedman and Xiang Ji for comments on a draft of the Commentary. Our neurovascular research programs are supported by grants from the National Heart, Lung, and Blood Institute (R35HL155008, R01HL122770, and R01HL146054 to S.E.), the National Institute of General Medical Sciences (P20GM130459 to S.E.), the National Institute of Mental Health (R01MH111438 to D.K.), and the National Institute of Neurological Disorders and Stroke (RF1NS110044 and R61NS115132 to S.E.; R35NS097265 to D.K.).
Footnotes
The authors declare no competing interest.
See companion article, “PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity,” 10.1073/pnas.2025998118.
References
- 1.Pantoni L., Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Bosetti F.et al.; “Small Blood Vessels: Big Health Problems” Workshop Participants , “Small blood vessels: Big health problems?”: Scientific recommendations of the National Institutes of Health Workshop. J. Am. Heart Assoc. 5, e004389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolsey T. A., et al., Neuronal units linked to microvascular modules in cerebral cortex: Response elements for imaging the brain. Cereb. Cortex 6, 647–660 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Attwell D., Laughlin S. B., An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Girouard H., Iadecola C., Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100, 328–335 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Attwell D., et al., Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinfeld D., et al., A guide to delineate the logic of neurovascular signaling in the brain. Front. Neuroenergetics 3, 1–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabertrand F., et al., PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity. Proc. Natl. Acad. Sci. U.S.A. 118, e2025998118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joutel A., et al., Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 120, 433–445 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih A. Y., et al., Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22, 204–218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devor A., et al., Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J. Neurosci. 28, 14347–14357 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould I. G., Tsai P., Kleinfeld D., Linninger A., The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J. Cereb. Blood Flow Metab. 37, 52–68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X., et al., Brain microvasculature has a common topology with local differences in geometry that match metabolic load. Neuron 109, 1168–1187.e13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longden T. A., et al., Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen R., et al., Cortex-wide changes in extracellular potassium ions parallel brain state transitions in awake behaving mice. Cell Rep. 28, 1182–1194.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harraz O. F., Longden T. A., Dabertrand F., Hill-Eubanks D., Nelson M. T., Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc. Natl. Acad. Sci. U.S.A. 115, E3569–E3577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harraz O. F., Hill-Eubanks D., Nelson M. T., PIP2: A critical regulator of vascular ion channels hiding in plain sight. Proc. Natl. Acad. Sci. U.S.A. 117, 20378–20389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mughal A., Harraz O. F., Gonzales A. L., Hill-Eubanks D., Nelson M. T., PIP2 improves cerebral blood flow in a mouse model of Alzheimer’s disease. Function (Oxf.) 2, b010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cauli B., et al., Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakore P., et al., Brain endothelial cell TRPA1 channels initiate neurovascular coupling. eLife 10, e63040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]