Significance
Calmodulin (CaM) regulation of cardiac NaV channels is vital for cardiac physiology and pathophysiology. Channelopathic mutations in NaV1.5 that disrupt CaM binding trigger two mechanistically divergent arrhythmia syndromes. Specifically, long QT syndrome 3 results from a gain-of-channel function, while Brugada syndrome stems from a loss-of-channel function. Yet, mechanisms that elicit seemingly paradoxical changes in channel function are unknown. Using single-channel analysis, we demonstrate that the disruption of CaM binding to NaV1.5 diminishes channel activity and enhances the propensity for persistent Na+ current, all resulting from a switch in the NaV inactivation mechanism. These findings reveal insights into the mechanism of CaM regulation of NaV channels as well as inform upon alterations in channel function that trigger life-threatening arrhythmias.
Keywords: Nav1.5, calmodulin, Brugada syndrome, long QT syndrome, ion channels
Abstract
In cardiomyocytes, NaV1.5 channels mediate initiation and fast propagation of action potentials. The Ca2+-binding protein calmodulin (CaM) serves as a de facto subunit of NaV1.5. Genetic studies and atomic structures suggest that this interaction is pathophysiologically critical, as human mutations within the NaV1.5 carboxy-terminus that disrupt CaM binding are linked to distinct forms of life-threatening arrhythmias, including long QT syndrome 3, a “gain-of-function” defect, and Brugada syndrome, a “loss-of-function” phenotype. Yet, how a common disruption in CaM binding engenders divergent effects on NaV1.5 gating is not fully understood, though vital for elucidating arrhythmogenic mechanisms and for developing new therapies. Here, using extensive single-channel analysis, we find that the disruption of Ca2+-free CaM preassociation with NaV1.5 exerts two disparate effects: 1) a decrease in the peak open probability and 2) an increase in persistent NaV openings. Mechanistically, these effects arise from a CaM-dependent switch in the NaV inactivation mechanism. Specifically, CaM-bound channels preferentially inactivate from the open state, while those devoid of CaM exhibit enhanced closed-state inactivation. Further enriching this scheme, for certain mutant NaV1.5, local Ca2+ fluctuations elicit a rapid recruitment of CaM that reverses the increase in persistent Na current, a factor that may promote beat-to-beat variability in late Na current. In all, these findings identify the elementary mechanism of CaM regulation of NaV1.5 and, in so doing, unravel a noncanonical role for CaM in tuning ion channel gating. Furthermore, our results furnish an in-depth molecular framework for understanding complex arrhythmogenic phenotypes of NaV1.5 channelopathies.
Voltage-gated sodium channels (NaV) are responsible for the initiation and spatial propagation of action potentials (AP) in excitable cells (1, 2). NaV channels undergo rapid activation that underlie the AP upstroke while ensuing inactivation permits AP repolarization. The NaV1.5 channel constitutes the predominant isoform in cardiomyocytes, whose pore-forming α-subunit is encoded by the SCN5A gene. NaV1.5 dysfunction underlies diverse forms of cardiac disease including cardiomyopathies, arrhythmias, and sudden death (3–6). Human mutations in NaV1.5 are associated with two forms of inherited arrhythmias–congenital long QT syndrome 3 (LQTS3) and Brugada syndrome (BrS) (7). LQTS3 stems from delayed or incomplete inactivation of NaV1.5 that causes persistent Na influx that prolongs AP repolarization—a “gain-of-function” phenotype (7–9). BrS predisposes patients to sudden death and is associated with a reduction in the peak Na current that may slow cardiac conduction or cause region-specific repolarization differences—a “loss-of-function” phenotype (10, 11). Genetic studies have identified an expanding array of mutations in multiple NaV1.5 domains, including the channel carboxy-terminus (CT) that is a hotspot for mutations linked to both LQTS3 and BrS (12, 13). This domain interacts with the Ca2+-binding protein calmodulin (CaM), suggesting that altered CaM regulation of NaV1.5 may be a common pathophysiological mechanism (12, 14–16). More broadly, human mutations in the homologous regions of neuronal NaV1.1 (17, 18), NaV1.2 (19, 20), and NaV1.6 (21) as well as skeletal muscle NaV1.4 (22) are linked to varied clinical phenotypes including epilepsy, autism spectrum disorder, neurodevelopmental delay, and myotonia (23). Taken together, a common NaV mechanistic deficit—defective CaM regulation—may underlie these diverse diseases.
CaM regulation of NaV channels is complex, isoform specific, and mediated by multiple interfaces within the channel (14–16). The NaV CT consists of a dual vestigial EF hand segment and a canonical CaM-binding “IQ” (isoleucine–glutamine) domain (24, 25) (Fig. 1A). The IQ domain of nearly all NaV channels binds to both Ca2+-free CaM (apoCaM) and Ca2+/CaM, similar to CaV channels (26–31). As CaM is typically a Ca2+-dependent regulator, much attention has been focused on elucidating Ca2+-dependent changes in NaV gating. For skeletal muscle NaV1.4, transient elevation in cytosolic Ca2+ causes a dynamic reduction in the peak current, a process reminiscent of Ca2+/CaM-dependent inactivation of CaV channels (32). Cardiac NaV1.5 by comparison exhibits no dynamic effect of Ca2+ on the peak current (32–34). Instead, sustained Ca2+ elevation has been shown to elicit a depolarizing shift in NaV1.5 steady-state inactivation (SSI or h∞), although the magnitude and the presence of a shift have been debated (32, 35). Additional CaM-binding sites have been identified in the channel amino terminus domain (36) and the III-IV linker near the isoleucine, phenylalanine, and methionine (IFM) motif that is well recognized for its role in fast inactivation (35, 37). However, recent cryogenic electron microscopy structures, biochemical, and functional analyses suggest that both the III-IV linker and the Domain IV voltage-sensing domain might instead interact with the channel CT in a state-dependent manner (38–43).
Fig. 1.
Absence of dynamic Ca2+/CaM effects on WT NaV1.5 SSI. (A, Left) Structure of NaV1.5 transmembrane domain (6UZ3) (70) juxtaposed with that of NaV1.5 CT–apoCaM complex (4OVN) (28). (Right) Arrhythmia-linked CT mutations highlighted in NaV1.5 CT–apoCaM structure (LQTS3, blue; BrS, magenta; mixed syndrome, purple). (B) Dynamic Ca2+-dependent changes in NaV1.5 SSI probed using Ca2+ photouncaging. Na currents specifying h∞ at ∼100 nM (Left) and ∼4 μM Ca2+ step (Right). (C) Population data for NaV1.5 SSI under low (black, Left) versus high (red, Right) intracellular Ca2+ reveal no differences (P = 0.55, paired t test). Dots and bars are mean ± SEM (n = 8 cells). (D) FRET two-hybrid analysis of Cerulean-tagged apoCaM interaction with various Venus-tagged NaV1.5 CT (WT, black; IQ/AA, red; S[1904]L, blue). Each dot is FRET efficiency measured from a single cell. Solid line fits show 1:1 binding isotherm.
Beyond Ca2+-dependent effects, the loss of apoCaM binding to the NaV1.5 IQ domain increases persistent current (34, 44), suggesting that apoCaM itself may be pathophysiologically relevant. Indeed, NaV1.5 mutations in the apoCaM-binding interface are associated with LQTS3 and atrial fibrillation (7), as well as a loss-of-function BrS phenotype and a mixed-syndrome phenotype whereby some patients present with BrS while others with LQTS3 (Fig. 1A) (13, 45). How alterations in CaM binding paradoxically elicits both gain-of-function and loss-of-function effects is not fully understood, though important to delineate pathophysiological mechanisms and for personalized therapies.
Here, using single- and multichannel recordings, we show that apoCaM binding elicits two distinct effects on NaV1.5 gating: 1) an increase in the peak channel open probability (PO/peak) and 2) a reduction in the normalized persistent channel open probability (Rpersist), consistent with previous studies (34, 44). The two effects may explain how mixed-syndrome mutations in the NaV1.5 CT produce either BrS or LQTS3 phenotypes. On one hand, the loss of apoCaM association may diminish PO/peak and induce BrS by shunting cardiac AP. On the other hand, increased Rpersist may prevent normal AP repolarization, resulting in LQTS3. Analysis of elementary mechanisms suggests that these changes relate to a switch in the state dependence of channel inactivation. Furthermore, dynamic changes in Ca2+ can inhibit persistent current for certain mutant NaV1.5 owing to enhanced Ca2+/CaM binding that occurs over the timescale of a cardiac AP. This effect may result in beat-to-beat variability in persistent Na current for some mutations. In all, these findings explain how a common deficit in CaM binding can contribute to distinct arrhythmogenic mechanisms.
Results
NaV1.5 SSI Is Unperturbed by Acute Cytosolic Ca2+ Elevation.
A prolonged increase in cytosolic Ca2+ has been suggested to shift the voltage dependence of NaV1.5 SSI or h∞ curve; however, it is unknown whether dynamic Ca2+ fluctuations as typical for cardiomyocytes might evoke similar effects. As such, we undertook Ca2+ photo-uncaging experiments with a modified voltage pulse protocol that measures h∞ curves within the same cell just before and after Ca2+ uncaging. Before Ca2+ uncaging, increasing the holding potentials (−120 to +30 mV) evoked peak currents of decreasing amplitudes because of SSI (Fig. 1 B, Left). Normalizing peak amplitudes of each pulse by the peak amplitude of the first pulse yielded a baseline h∞ curve at cytosolic [Ca2+]free of ∼100 nM (corresponding to diastolic Ca2+ in cardiomyocytes) as shown by the average h∞ curve obtained from multiple cells (Fig. 1 C, Left). A brief ultraviolet (UV) pulse elicited a step-like increase in intracellular [Ca2+]free up to ∼4 µM, beyond the peak systolic cytosolic Ca2+ levels observed in cardiomyocytes (Fig. 1 B, Right). The h∞ curve obtained following Ca2+ photo-uncaging, however, overlayed with that at low Ca2+ levels (Fig. 1 C, Right). Thus, NaV1.5 SSI appears to be insensitive to transient changes in cytosolic Ca2+ as would be observed during each cardiac beat.
Absent dynamic Ca2+ effects, we reasoned that the apoCaM interaction may be more consequential for NaV1.5 function. Building upon in vitro studies that show ∼50 nM affinity for apoCaM interaction with channel CT, we utilized a medium-throughput flow cytometry–based fluorescence resonance energy transfer (FRET) assay (46, 47) to demonstrate interaction between CaM and the wild-type (WT) NaV1.5 CT in live cells. We coexpressed Cerulean-tagged CaM as the FRET donor with Venus-tagged NaV1.5 CT as the FRET acceptor in HEK293 cells (Fig. 1D). Stochastic expression of the two interacting proteins yielded variable FRET efficiencies (ED) that were quantified using a flow cytometer from 5,000 to 20,000 individual cells. A saturating binding curve was constructed by correlating ED with the free acceptor concentration (Fig. 1D, black dots and fit). This maneuver yielded a relative binding affinity (Kd,EFF) indicative of strong baseline binding between CaM and the WT NaV1.5 CT in live cells (SI Appendix, Table S1). To dissect the functional consequences of apoCaM binding to NaV1.5, we utilized structure-guided and disease-linked mutations that diminish apoCaM interaction. We mutated the central IQ residues (I1908/Q1909) within the NaV1.5 IQ domain to double alanine (IQ/AA), which was shown to abolish apoCaM binding in in vitro studies (44, 48). Flow cytometry–based FRET analysis of the NaV1.5 IQ/AA mutant revealed diminished apoCaM binding (Fig. 1D, red dots and fit), with an ∼4,000-fold increase in Kd,EFF (SI Appendix, Table S1). We subsequently tested the channelopathic mutant S[1904]L that is linked to both BrS (13) and LQTS3 (9) based on its location within the IQ–CaM C-lobe interface. Flow cytometric FRET analysis between the S[1904]L CT and apoCaM demonstrated an ∼44-fold weaker affinity compared to WT (Fig. 1D, blue dots and fit; SI Appendix, Table S1).
Disruption of apoCaM Binding Diminishes NaV1.5 Peak Open Probability.
Having confirmed disruption of CaM binding by IQ/AA and S[1904]L mutations in live cells, we tested whether these mutations alter NaV1.5 gating. We first probed SSI (h∞) properties with whole-cell recordings. To discern apoCaM-dependent effects, we utilized high intracellular Ca2+ buffering in the pipette dialysate. A comparison of h∞ curves for WT channels versus IQ/AA and S[1904]L mutants revealed no appreciable shift in SSI (Fig. 2 A–C and SI Appendix, Fig. S1J). Diminishing cytosolic CaM levels by overexpressing a CaM chelator or enhancing cytosolic CaM by exogenous expression of recombinant CaM also did not alter the h∞ curves for WT, IQ/AA, and S[1904]L (Fig. 2 A–C and SI Appendix, Fig. S1J). By contrast, analysis of peak-current densities suggests CaM-dependent changes (SI Appendix, Fig. S1). Upon depletion of free CaM levels by overexpression of a CaM chelator, the WT channels showed reduced peak-current density (SI Appendix, Fig. S1 A and B). Similarly, both IQ/AA and S[1904]L mutants with defective CaM binding also showed an approximately threefold reduction in peak-current density compared to WT channels (SI Appendix, Fig. S1 A, D, and G). Exogeneous CaM overexpression with IQ/AA and S[1904]L mutants increased the peak-current density (SI Appendix, Fig. S1 F and I). Of note, we observed minimal differences in voltage dependence of activation between the three channel constructs at different CaM levels (SI Appendix, Fig. S1K). These findings suggest that apoCaM binding may be critical for baseline channel function. Alterations in peak current could result from changes in surface membrane trafficking, unitary conductance, or peak open probability (PO/peak), an ambiguity that may be readily resolved at the single-channel level.
Fig. 2.
Disruption of apoCaM preassociation diminishes peak PO of NaV1.5. (A) SSI (h∞) curve for WT NaV1.5 measured under endogenous (black), low (red), and high (blue) CaM levels. Low CaM levels were attained by overexpressing a CaM chelator. High CaM levels were attained by overexpressing recombinant CaM. (Inset) Voltage protocol. (B and C) Format as in A but for NaV1.5 IQ/AA (B) and S[1904]L (C) mutants. (D) Exemplar traces showing stochastic records of channel openings from a one-channel patch of NaV1.5 WT evoked in response to a depolarizing pulse to −10 mV. Solid line denotes zero-current baseline, and channel openings are downward deflections to the unitary current level (dashed line). (E) Ensemble average PO waveform from a single patch (147 sweeps) reveals a PO/peak ∼0.5. (F) Population PO/peak values are plotted as block dots and bars (mean ± SEM) as a function of the activating test pulse potential (n = 7 patches, 1,768 sweeps/voltage). The black line is the single Boltzmann fit for PO/peak–V relation with parameters: PO,max = 0.48; V1/2 = −36 mV, and slope factor (SF) = 9.5. (G–I) Format as in D–F but for NaV1.5 IQ/AA. (H) Ensemble PO average shown from a single patch with 120 sweeps. (I) Population data of NaV1.5 IQ/AA PO/peak–V relationship is shown by pale red dots (mean ± SEM, n = 5, 1,088 sweeps/voltage) and red line fit. Boltzmann fit parameters: PO,Max = 0.21; V1/2 = −45 mV; SF = 9.5. Gray line is the WT fit reproduced from F. (J–L) Format as in D–F but for NaV1.5 S[1904]L. (K) Ensemble PO average obtained from 254 sweeps in a single patch. (L) Population data for NaV1.5 S[1904]L PO/peak–V relationship is shown by pale red dots (mean ± SEM, n = 6, 1,365 sweeps/voltage) and red line fit. Boltzmann fit parameters for NaV1.5 S[1904]L: PO,Max = 0.26; V1/2 = −45 mV; SF = 9.5. Gray line is the WT fit reproduced from F. Statistical analysis: two-way ANOVA followed by post hoc Tukey’s multiple comparisons test, **P < 0.01 with WT channels as reference.
We undertook low-noise single-channel cell-attached recordings from HEK293 cells transfected with either NaV1.5 WT, IQ/AA, or S[1904]L mutant channels. Channel openings were elicited using step depolarizations from a holding potential of −120 mV, ensuring maximal channel availability (Fig. 1 A–C). Thus, measured alterations in peak PO reflect changes in channel openings from the resting state and not a shift in SSI. Exemplar traces in Fig. 2D show WT single-channel openings in response to a step depolarization to −10 mV. The unitary current levels at −10, −30, and −50 mV test voltages were estimated using amplitude histograms yielding a single-channel conductance of ∼18 pS (SI Appendix, Fig. S2A), consistent with previous single Na channel recordings (49, 50). The ensemble average current from ∼100 to 200 stochastic sweeps at each voltage was divided by the unitary current and the number of channels in the patch to obtain a time-resolved open probability (PO) waveform (Fig. 2E), reflecting rapid channel activation and inactivation. Population analysis of WT PO/peak obtained at three different voltages and across multiple cells and transfections revealed a maximal PO/peak of ∼0.5 at −10 mV test pulse (Fig. 2F). By comparison, IQ/AA and S[1904]L mutant channels showed a reduction in channel openings, although rare sojourns to the open state appear qualitatively prolonged (Fig. 2 G and J, respectively, quantified later; see Fig. 4). Single-channel conductance for both mutant channels were unperturbed compared to WT (SI Appendix, Fig. S2 C and E). However, the PO/peak for both IQ/AA and S[1904]L mutants were reduced by 2.5-fold as shown by ensemble average PO waveforms (Fig. 2 H and K) and the population data (Fig. 2 I and L). These results demonstrate that CT mutations disrupting apoCaM binding diminish PO/peak, an effect consistent with the loss-of-function BrS phenotype.
ApoCaM Binding Tunes Peak Open Probability of Mutant NaV1.5.
The reduction in NaV1.5 PO/peak by CT mutations hints that apoCaM preassociation may be a critical determinant for basal activity. However, two ambiguities obfuscate the effect of CaM on NaV1.5 activity. First, the reduced PO/peak may not directly relate to weakened apoCaM preassociation; instead, it may result from idiosyncratic structural alterations. Second, if weakened apoCaM binding is responsible for reduced PO/peak, then it is unknown whether CaM binding is obligatory for channel openings. The low residual activity of mutant channels may correspond to partial CaM occupancy of mutant channels.
We reasoned that if the reduction in PO/peak for CT mutants reflect a genuine loss of apoCaM binding, then overexpression of CaM with CT mutant channels would by mass action repopulate CT mutant channels and thereby reverse functional defects in PO/peak. Indeed, previous studies have shown that CaM overexpression can restore CaM preassociation to mutant CaV1.3 with diminished CaM-binding affinity (51). Single-channel recordings of both the IQ/AA and S[1904]L mutant in the presence of high CaM revealed gating behavior qualitatively similar to WT channels (IQ/AA, Fig. 3A; S[1904]L, Fig. 3E), with most sweeps featuring a single brief opening with fewer blank sweeps (quantified later, Fig. 4). Ensemble average PO waveforms showed increased PO/peak for both IQ/AA (Fig. 3C, blue) and S[1904]L (Fig. 3G, blue) mutant channels, reversing the effects seen at endogenous CaM levels. Population PO/peak–V relationship in the presence of CaM demonstrated statistically significant PO/peak increase compared to that measured under endogenous CaM conditions (Fig. 3 D and H, blue). By contrast, the single-channel conductance was unperturbed (SI Appendix, Fig. S2 D and F). The ability of heterologously expressed CaM to reverse deficits in PO/peak for CT mutants suggest that apoCaM interaction suffices for high basal channel activity.
Fig. 3.
ApoCaM binding tunes maximal PO of mutant NaV1.5 with reduced CaM binding. (A and B) Exemplar stochastic single-channel recordings of NaV1.5 IQ/AA under CaM-overexpressed (A) and CaM-depleted (B) conditions. (C) Ensemble PO average of NaV1.5 IQ/AA from a single patch under CaM-overexpressed (blue, 130 sweeps) and CaM-depleted (red, 276 sweeps) conditions. (D) Population NaV1.5 IQ/AA PO/peak–V relationship under CaM-overexpressed (blue dots and fit, n = 5, 539 sweeps/voltage) and CaM-depleted (red dots and fit, n = 7, 1,249 sweeps/voltage) conditions. Gray is the NaV1.5 IQ/AA fit under endogenous CaM levels reproduced from Fig. 2I. All dots and bars are mean ± SEM. Boltzmann fit parameters for CaM-overexpressed condition (blue): PO,max = 0.45; V1/2 = −38 mV; SF = 9.5. Boltzmann fit parameters for CaM-depleted condition (red): PO,max = 0.23; V1/2 = −40 mV; SF = 9.5. Statistical analysis: two-way ANOVA followed by post hoc Tukey’s multiple comparisons test, ***P < 0.001 comparing the CaM overexpression with the CaM-depleted condition. (E and F) Format as in A–D but for NaV1.5 S[1904]L mutant. (G) Ensemble PO average of NaV1.5 S[1904]L from a single patch under CaM-overexpressed (blue, 120 sweeps) and CaM-depleted (red, 218 sweeps) conditions. (H) Population NaV1.5 S[1904]L PO/peak–V relationship under CaM-overexpressed (blue dots and fit, n = 11, 2,496 sweeps/voltage) and CaM-depleted (red dots and fit, n = 8, 1,568 sweeps/voltage) conditions. Gray is the NaV1.5 S[1904]L fit under endogenous CaM levels reproduced from Fig. 2L. Boltzmann fit parameters for CaM-overexpressed condition (blue): PO,Max = 0.5; V1/2 = −43 mV; SF = 9.5. Boltzmann fit parameters for CaM-depleted condition (red): PO,Max = 0.18; V1/2 = −45 mV; SF = 9.5. Statistical analysis: two-way ANOVA followed by post hoc Tukey’s multiple comparisons test, **P < 0.01 and ***P < 0.001 comparing the CaM overexpression with the CaM-depleted condition.
Fig. 4.
Elementary mechanisms underlying CaM regulation of NaV1.5. (A and B) FL distributions for NaV1.5 WT (gray shaded area) and NaV1.5 IQ/AA mutant under CaM-overexpressed (pale blue shaded area) and CaM-depleted (rose shaded area) conditions. FL denotes the probability that the first opening occurred at time < t. Solid lines are fits of FL distributions generated by the model shown in Fig. 4I. The difference in FL distributions for NaV1.5 IQ/AA at low versus high CaM levels are statistically significant (P < 0.001, Kolmogorov–Smirnov [KS] test). (C and D) Histograms of OD distribution which correspond to the time spent in the open state for WT (gray shaded area, C) and IQ/AA under low (rose shaded area, D) and high (pale blue shaded area, D) CaM conditions. Solid lines are fits of OD distributions generated by the model shown in Fig. 4I. The OD distribution of IQ/AA is significantly prolonged under low CaM levels compared to CaM overexpression (D, P < 0.001, KS test). (E and F) Distribution of the number of openings per sweep for WT (black) and IQ/AA mutant channels at low (red) and high (blue) levels of ambient CaM. ***P < 0.001, test of proportion for comparing fraction of blank sweeps. (G and H) Conditional open probability (POO(t)) for WT (gray shaded area) and IQ/AA at low (rose shaded area) or high (pale blue shaded area) CaM levels. Solid lines are reproduction of the fits of OD distributions in C and D. For NaV1.5 IQ/AA, POO(t), distributions at low versus high CaM are statistically different (P < 0.001, KS test). (I) Summary of the effect of CaM on NaV1.5 gating. Absent CaM, channels preferentially inactivate from the closed state. Upon binding CaM, inactivation proceeds preferentially from the open state (α: rate of transition from the closed to open state, β: rate of transition from the open to closed state, kOI: rate of inactivation from the open state, kCI: rate of inactivation from the closed state, kOC: rate of channel closure, and kCO: rate of channel opening).
We next sought to determine whether residual channel activity observed for IQ/AA and S[1904]L mutations correspond to partial CaM binding as a result of high endogenous CaM levels in HEK293 cells. If so, the depletion of ambient CaM concentration by overexpression of a CaM chelator would result in further reduction in PO/peak (52). Single-channel records of IQ/AA following CaM depletion showed sparse but prolonged channel openings with many blank sweeps (Fig. 3B; quantified later, Fig. 4). Analysis of the sweeps revealed a unitary conductance of 17 pS and a PO/peak – V relationship ∼2.5-fold lower than that of WT (Fig. 3 C and D, red and SI Appendix, Fig. S2D). This relationship is nearly identical to the IQ/AA results obtained under endogenous levels of CaM, suggesting that IQ/AA mutant channels lack appreciable CaM binding at baseline. We observed a similar trend with the S[1904]L mutation, whereby channels exhibit a reduced PO/peak upon CaM chelation much as with endogenous CaM concentration (Fig. 3 F–H, red). Consistent with these findings, reducing ambient free CaM concentration also resulted in a modest (21.8%) reduction in PO/peak for WT channels (SI Appendix, Fig. S3). These results suggest that apoCaM preassociation is not obligatory for NaV openings; rather, it tunes baseline level of channel activity.
ApoCaM Interaction Switches State Dependence of NaV1.5 Inactivation.
We sought to leverage information from single-channel recordings to infer apoCaM-dependent elementary changes in NaV1.5 gating following the Aldrich–Corey–Stevens formalism. We analyzed one-channel patches to quantify the following: 1) FL(t), the first latency distribution describing the probability that the first opening will occur by time t [i.e., FL(t) = P(first opening ≤ t)]; 2) OD, the open-duration distribution that describes the total duration of each opening [i.e., OD(t) = P(duration of opening ≥ t)]; 3) NO, the number of openings in each sweep; and 4) POO (t + tj, tj), the conditional probability of observing a channel opening at time t+tj, if the channel were open at time tj. To dissect CaM-dependent effects, we compared properties of IQ/AA mutant channels at either low or high levels of CaM. We focused on three aspects of NaV1.5 gating in our analyses as conceptualized by the gating model drawn in Fig. 4I: 1): closed-state inactivation (kCI), 2) open-state inactivation (kOI), and 3) burst-like behavior.
We first examined close-state inactivation by analyzing FL distribution for NaV1.5 WT channels at −10 mV, which revealed a saturating exponential curve with a plateau value of FLmax ∼0.6. This indicates a ∼40% likelihood for the channel to inactivate without opening, that is, undergo closed-state inactivation (Fig. 4A). By comparison, the IQ/AA mutant channels at low CaM levels showed a decrease in the plateau value (FLmax ∼0.25) without a change in the time constant (Fig. 4B, red). This suggests that the rate constant for closed to open transitions are unaltered; rather, the rate of transition from the closed to inactivated state is enhanced. CaM overexpression reversed this change in FL for the IQ/AA mutant (Fig. 4B, blue), indicating that the change in FL distribution results from diminished CaM preassociation. A similar trend was observed with test pulse potentials of −30 and −50 mV (SI Appendix, Fig. S4). The S[1904]L mutant also exhibited similar changes in FL distribution at high versus low levels of CaM (SI Appendix, Fig. S5). In all, CaM-dependent alterations in FLmax are consistent with apoCaM binding reducing the propensity for closed-state inactivation by decreasing kCI (Fig. 4I).
We next scrutinized open-state inactivation with the OD distribution, which describes the time spent in the open state during each sojourn. For WT, the OD distribution was approximated by a single-exponential decay (Fig. 4C) with a time constant inversely related to the sum of rate constants for the channel leaving the open state to the closed state (kOC; Fig. 4I) and to the inactivated state (kOI; Fig. 4I). The OD distribution of IQ/AA under low CaM levels (Fig. 4D, red) was prolonged with a slower exponential decay, reflecting long openings in exemplar records (Fig. 3B). CaM overexpression with the IQ/AA mutant reduced the time constant for OD distribution, indicating briefer openings (Fig. 4D, blue) compared to low CaM levels. A similar trend was observed with −30 and −50 mV test pulse potentials (SI Appendix, Fig. S4) as well as in the S[1904]L mutant when the test pulse potential was −10 mV (SI Appendix, Fig. S5). Taken together, these findings suggest that apoCaM binding increases the rate of egress from the open state, which may result from either an increase in the rate of channel closure (kOC) or enhanced inactivation from the open state (kOI). To discern between these possibilities, we considered the distribution of the number of openings observed per each sweep (NO) for WT (Fig. 4E) and IQ/AA mutant channels (Fig. 4F). We reasoned that if apoCaM binding only increased kOC, then channels would flicker between open and closed states multiple times before inactivating. That is, an increase in kOC rate alone would cause a decrease in the likelihood of observing exactly one channel opening provided that the channels opened at least once. However, this was not the case. The proportion of sweeps with exactly one opening when blank sweeps are excluded [i.e., P(NO = 1 | NO ≥ 1)] was similar between the IQ/AA mutant under low (65%) and high CaM levels (68%) as well as WT channels (67%). Taken together, these results suggest that the prolonged OD seen in IQ/AA mutant channels at low CaM levels reflects decreased kOI. At the macroscopic level, this change would result in a slowed current decay kinetics upon CaM depletion. Indeed, a single-exponential fit of whole-cell current decay kinetics revealed prolonged decay kinetics at low ambient CaM levels as compared with CaM overexpression for WT NaV1.5 and both IQ/AA and S[1904]L mutant channels (SI Appendix, Fig. S6). In total, apoCaM binding likely enhances the rate of NaV1.5 open-state inactivation.
Lastly, we examined burst-like behavior by computing the conditional open probability, POO(t), that depends on the following: 1) how long the channel stays open from the initial opening and 2) the tendency for the channel to reopen following closure. The WT NaV1.5 POO(t) relation followed an exponential decay that was well approximated by the OD distribution (Fig. 4G), indicating that these channels favor complete inactivation after the first opening. By comparison, the POO(t) curve for the IQ/AA mutant at low CaM levels deviated from the OD distribution to a small pedestal value (Fig. 4H, red). With CaM overexpressed, this change was reversed for the IQ/AA mutant with the POO(t) curve being well approximated by the OD distribution (Fig. 4H, blue). The small but nonzero pedestal value for the POO(t) curve for the IQ/AA mutant at low CaM levels reflects a small fraction of sweeps exhibiting frequent channel reopenings or bursts (1.25% of sweeps from IQ/AA at low ambient CaM versus 0.3% at high CaM levels). These findings suggest that the disruption of CaM binding may lead to a small but pathophysiologically relevant persistent Na current, a possibility that will be subsequently evaluated.
In-depth analysis of the single-channel recordings suggests three distinct functional effects related to apoCaM preassociation on NaV1.5: 1) decreased rate of inactivation from the closed state (kCI), 2) increased rate of inactivation from the open state (kOI), and 3) decreased tendency for channels to exhibit burst-like behavior. To further corroborate these findings, we imposed a Markov model depicted in Fig. 4I. We fit FL, OD, and PO curves for WT and IQ/AA mutants holding α, β, kCO, and kOC values constant across all conditions while allowing kCI to increase and kOI to decrease as a consequence of loss of apoCaM binding (Fig. 4I, bar plot). This minimalistic model reasonably approximated the PO, FL, and OD curves across all constructs at three test voltages (solid line fits, Fig. 4 A–D, G, and H and SI Appendix, Figs. S4 and S5). Taken together, apoCaM-dependent changes in PO/peak reflect a switch in the state dependence of channel inactivation, with the loss of apoCaM binding biasing preferential closed-state inactivation.
ApoCaM Interaction with NaV1.5 Tunes Persistent Current.
Analysis of NaV1.5 singe-channel recordings show that the loss of apoCaM preassociation results in a pedestal level of the POO distribution, hinting at increased channel reopenings. Indeed, previous studies suggest that the loss of apoCaM preassociation increases a persistent Na current, an important arrhythmogenic substrate (44). To elucidate CaM-dependent changes in a persistent Na current, we undertook cell-attached multichannel recordings from either WT or mutant NaV1.5. Unlike whole-cell approaches that may be susceptible to a nonspecific membrane leak, late Na channel openings can be reliably detected with the multichannel approach, as the channel unitary conductance is sufficiently large to discern single openings from instrument noise (53).
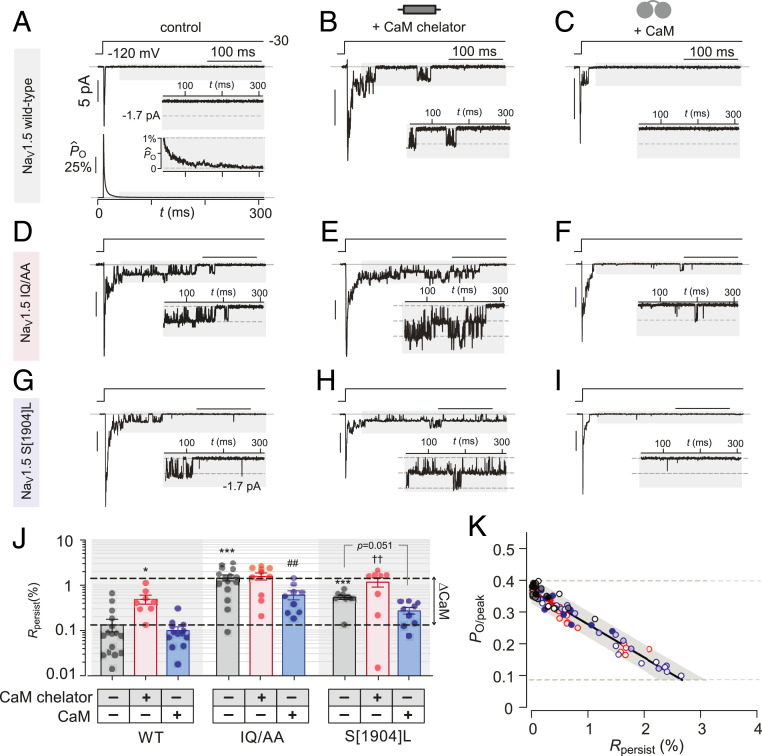
An exemplar stochastic record of WT NaV1.5 evoked in response to a 300-ms step depolarization to −30 mV shows rapid channel activation as indicted by stacked openings followed by rapid inactivation with no openings in the late phase, defined as following 50 ms of depolarization (Fig. 5 A, Top, gray shaded area and inset). For each patch, we averaged 50 to 100 stochastic traces to obtain an ensemble average current, which is subsequently normalized by the peak value to obtain the normalized waveform (Fig. 5 A, Bottom and SI Appendix, Fig. S7). As a metric for the persistent current, we quantified the average value of the normalized curve in the late phase for each patch (Rpersist = < PO > 50 ≤ t ≤ 300/PO/peak). This measurement is analogous to whole-cell measurement of late currents quantified as a ratio of the steady-state Na current normalized by the peak. WT channels exhibited a minimal persistent current as evident from population Rpersist values (Fig. 5J), indicating near complete channel inactivation. We subsequently probed whether the depletion of CaM binding to WT channels tune the persistent current by overexpressing a CaM chelator (Fig. 5B). This maneuver yielded channel openings in the late phase (Fig. 5B) and a statistically significant increase in Rpersist values (Fig. 5J). By contrast, CaM overexpression resulted in no change in Rpersist compared to control conditions (Fig. 5 C and J). These findings suggest that a loss of apoCaM interaction with NaV1.5 WT increases the persistent Na current.
Fig. 5.
ApoCaM interaction with NaV1.5 tunes likelihood for persistent channel openings. (A, Top) Representative multichannel record from NaV1.5 WT shows rapid activation and inactivation followed by rare openings in the late phase following 50 ms of depolarization (gray shaded region). (Inset) Enlarged late phase to better visualize NaV1.5 openings. (Bottom) Normalized ensemble average open probability (n = 16, 875 sweeps). (Inset) Enlarged normalized open probability in the late phase. (B and C) Format as in A Top but for NaV1.5 WT channels recorded under CaM-depleted (B) and CaM-overexpressed (C) conditions. (D–I) Format as in A–C but for NaV1.5 IQ/AA (D–F) and S[1904]L (G–I) mutants. (J) Quantification and population data for persistent channel openings for NaV1.5 WT, IQ/AA, and S[1904]L under different CaM concentrations (A–I). For each condition, a normalized ensemble open probability is calculated by averaging many sweeps (SI Appendix, Fig. S7). Rpersist is the average open probability in the late phase (gray shaded regions in A–I) normalized by the peak . Each bar and error, mean ± SEM. Statistical analysis: one-way ANOVA followed by Tukey multiple comparisons test. ***P < 0.001, **P < 0.01, *P < 0.05 compared to WT at endogenous CaM levels; ##P < 0.01 versus IQ/AA at endogenous CaM level; and ††P < 0.01 when compared to S1904L at endogenous CaM levels. (K) Correlation of Rpersist versus PO/peak under different CaM concentrations shows a linear relationship.
Thus informed, we measured the persistent current for the IQ/AA mutant NaV1.5 whose CaM binding ability is strongly diminished. Even at endogenous levels of CaM, the IQ/AA mutant exhibited a 10-fold increase in Rpersist (Fig. 5 D and J). Coexpression of the CaM chelator did not further increase the late current (Fig. 5 E and J), consistent with the absence of baseline CaM binding to these channels. Overexpression of CaM with the IQ/AA mutant, however, resulted in a partial inhibition of persistent channel activity (Fig. 5 F and J), presumably reflecting a partial restoration of CaM preassociation. To determine whether the loss of CaM preassociation due to channelopathic mutations can cause increased persistent activity, we probed the S[1904]L mutant. With endogenous levels of CaM, we observed an increase in persistent channel openings compared to WT (Fig. 5 G and J), reflecting weakened CaM binding. Decreasing ambient CaM levels further increased Rpersist values to NaV1.5 IQ/AA levels (Fig. 5 H and J), while CaM overexpression decreased the persistent current to near WT levels (Fig. 5 I and J). In-depth analysis suggests that changes in Rpersist pertain to altered CaM occupancy of channels (SI Appendix, Text). Taken together, these findings demonstrate that CaM binding to NaV1.5 tunes the likelihood of persistent channel openings.
If both PO/peak and Rpersist modulation relies on the preassociation of a single apoCaM to the channel CT, then we would expect the two entities to follow a linear relation, implicitly representing varied apoCaM binding. As Rpersist values were obtained from multichannel patches, we computed a corresponding PO/peak for all patches by estimating the number of channels in each patch using mean-variance analysis. Plotting Rpersist versus PO/peak demonstrated a linear relationship confirming that the preassociation of the very same CaM elicits both effects (Fig. 5K). Importantly, multiple CT channelopathic mutations variably weaken CaM binding. We expect that these mutations will result in a variable increase in the level of persistent Na current with up to an order of magnitude change.
Ca2+/CaM Interaction with NaV1.5 Tunes Persistent Current.
NaV CT channelopathies have been shown to differentially impact apoCaM versus Ca2+/CaM interaction. One possibility is that changes in intracellular Ca2+, as expected during cardiac systole, could impact the dynamic equilibrium of CaM association with NaV1.5. This change in the CaM-binding status of NaV1.5 could in turn dynamically tune persistent channel activity and thereby affect AP repolarization. To dissect this possibility, we employed flow cytometric FRET two-hybrid assays to determine Ca2+-dependent changes in CaM binding to the NaV1.5 WT, IQ/AA, and S[1904]L CT (Fig. 6A and SI Appendix, Table S1). Compared to its binding affinity in the apo-configuration, Ca2+-bound CaM features only a modest change in affinity for the WT NaV1.5 CT domain (Fig. 6B and SI Appendix, Table S1). By contrast, Ca2+/CaM exhibits an ∼35-fold increase in binding affinity over that of apoCaM for the IQ/AA mutant (Fig. 6B and SI Appendix, Table S1). The CT domain of the S[1904]L mutant demonstrated similar binding affinities to both apo- and Ca2+/CaM, with both being reduced compared to WT (Fig. 6B and SI Appendix, Table S1). These distinct patterns of apo- versus Ca2+/CaM binding to the mutant IQ domains are consistent with prior studies (24, 27, 38, 44).
Fig. 6.
Dynamic Ca2+ fluctuations tune persistent NaV channel openings. (A) FRET two-hybrid analysis probes the interaction of Venus-tagged NaV1.5 CT with Cerulean-tagged CaM at high Ca2+ levels. Black dots and fit, WT; red dots and fit, IQ/AA mutant; blue dots and fit, S[1904]L mutant. (B) Bar graph summary compares Ka,EFF = 1/Kd,EFF for Ca2+ versus apoCaM binding to WT NaV1.5 as well as IQ/AA and S[1904]L mutant. (C) Exemplar multichannel stochastic record from cell-attached recordings of NaV1.5 WT coexpressed with CaV2.1. NaV1.5 openings are evoked using the −35 mV voltage pulse. Ensuing +15 mV voltage pulse elicits Ca2+ channel openings (rose shaded area). A subsequent pulse to −35 mV is used to identify Ca2+-dependent changes in persistent NaV channel openings. (D) Normalized ensemble average waveform is computed from 293 sweeps (six patches). A comparison of the waveform before (black) and after (red) Ca2+ pulse reveals minimal differences in the late Na current. (E–H) Exemplar multichannel recordings and ensemble average waveforms of NaV1.5 IQ/AA (E and F) or S[1904]L (G and H) coexpressed with CaV2.1. Format same as C and D. Data obtained from 342 sweeps (seven patches) for NaV1.5 IQ/AA and 264 sweeps (seven patches). (I) Paired dot plot summarizes changes in Rpersist before (black dot, gray bar) and after (red dot, rose bar) Ca2+ influx. Only the IQ/AA mutant showed a consistent reduction in the late current following Ca2+ entry (**P < 0.01 by paired t test).
To probe dynamic Ca2+-dependent changes in the late Na+ current, we coexpressed NaV1.5 with CaV2.1 channels and undertook cell-attached multichannel recordings to determine whether Ca2+ spillover from neighboring Ca2+ channels perturb late NaV1.5 openings (32). We employed the voltage protocol shown in Fig. 6C, which enables persistent Na current quantification and a clear delineation between NaV1.5 versus CaV2.1 activity (SI Appendix, Fig. S8). As CaV2.1 requires strong depolarization to activate, NaV openings alone could be measured by a modest depolarizing “prepulse” to −35 mV (Fig. 6C, pulse i). The same voltage protocol applied to cells transfected with CaV2.1 alone exhibited no channel activity at −35 mV (SI Appendix, Fig. S8 A and B). We then applied an “intervening pulse” to +15 mV to activate CaV2.1, permitting local Ca2+ influx and spillover (Fig. 6C, pulse ii). Lastly, a “postpulse” to −35 mV enabled measurement of persistent NaV1.5 openings following local Ca2+ elevation (Fig. 6C, pulse iii). We expressed exogenous CaM to ensure that sufficient CaM is available to act on NaV1.5 as CaV2.1 also binds CaM with a high affinity.
Fig. 6 C and D displays an exemplar trace of the Na persistent current measured from WT channels with this protocol along with the normalized waveform from the ensemble average. The WT NaV1.5 persistent current following Ca2+ entry exhibited minimal change compared to that during the prepulse (Fig. 6 C, D, and I). By contrast, the IQ/AA mutant revealed an ∼10-fold reduction in persistent channel openings following Ca2+ influx via CaV2.1 (Fig. 6 E, F, and I). We further probed whether the persistent current reduction in IQ/AA was Ca2+ dependent by repeating the experiment in cells transfected with IQ/AA alone. Absent Ca2+ entry through CaV2.1, we observed no difference in persistent NaV openings of the IQ/AA mutant following the intervening pulse (SI Appendix, Fig. S8 C–E). This suggests that the reduction in the persistent current of IQ/AA reflects a genuine Ca2+-dependent effect. The S[1904]L mutant exhibited minimal change in the persistent current following Ca2+ spillover (Fig. 6 G–I). Mechanistically, Ca2+-dependent changes in late NaV openings correlate with Ca2+-dependent changes in CaM binding to NaV1.5 CT. Specifically, both WT and S[1904]L mutants exhibited a minimal change in CaM binding, whether in apo- or Ca2+-bound configuration. Functionally, late Na channel openings of both channels were insensitive to dynamic changes in Ca2+. By comparison, the IQ/AA mutant exhibits a 35-fold increase in CaM affinity in the presence of Ca2+. Functionally, this change results in a reduction in late channel openings following local Ca2+ spillover, presumably reflecting CaM recruitment to the channel. These findings suggest that dynamic Ca2+ fluctuations may tune late NaV openings; however, manifestation of this effect and its magnitude may be idiosyncratic depending on the precise mutation and the overall changes in apo- versus Ca2+/CaM binding.
Late NaV1.5 Openings Are Prolonged Similar to Inactivation-Deficient Channels.
Given the increase in the persistent Na current resulting from the loss of CaM binding and its potential importance to cardiac arrhythmogenesis, we further scrutinized biophysical mechanisms underlying the CaM-dependent late Na current. We calculated the conditional OD distributions for channel openings that occur within the late phase (greater than 50 ms after depolarization) for WT, IQ/AA, and S[1904]L channels (Fig. 7 A and C–F, pale blue) and compared to those in the early phase (20 ms of depolarization) (SI Appendix, Fig. S4 E and F). As late channel properties were determined from multichannel patches, we limited our analysis to openings that occur to the one-channel level. The conditional OD distributions obtained for WT, IQ/AA, and S[1904]L all appeared biexponential and prolonged compared to early openings (Fig. 7 A and C–F; blue shaded region and fit for late openings, versus gray solid line for early openings reproduced from SI Appendix, Fig. S4 E and F). The shared biexponential OD decay suggests that late openings may arise from two distinct open states: one open state from which inactivation stems normally and another from which inactivation is diminished corresponding to the persistent mode. To examine whether the slow component of the conditional OD distributions may reflect an “inactivation-deficient” open state, we undertook single-channel recordings of the mutant NaV1.5 with the phenylalanine residue of the IFM motif substituted with a glutamine residue (F1486Q; IFM/IQM). Consistent with previous studies showing that the IQM mutant is inactivation deficient (54, 55), this maneuver resulted in “burst-like” openings (SI Appendix, Fig. S9). The OD distribution of the IFM/IQM mutant exhibited prolonged decay that matched the time constant of the slow component of the conditional late-phase OD distribution in WT channels (Fig. 7 A and B, red fit). Thus, the slow component of the biexponential conditional OD distribution for late channel openings may correspond to an equivalent of an inactivation-deficient open state. The same pattern of fast and slow components time constant matching was also observed in the conditional OD distributions for the IQ/AA and S[1904]L channels under high and low CaM levels (Fig. 7 C–F). In all cases, the late-phase conditional OD distributions were well approximated as the sum of two exponentials (dark blue fit; Fig. 7 A and C–F): the first component with a time constant matching the OD distribution determined for each channel variant in the early phase (SI Appendix, Figs. S4F and S5E) and the second component having a time constant that matched the OD distribution for IFM/IQM mutant (Fig. 7B). Importantly, the IQ/AA and S[1904]L mutants at low CaM levels featured a higher fraction of the slow component (∼0.5) to WT (∼0.25) (Fig. 7 A, C, and E intercept of red fits), while CaM overexpression reduced the magnitude of the slow component in the mutant channels (intercept of red fits in Fig. 7C versus Fig. 7D and Fig. 7E versus Fig. 7F). These findings are consistent with the loss of CaM preassociation decreasing the channel propensity to enter an “inactivation-deficient” open state. Taken together, these results suggest that CaM tunes NaV1.5 occupancy of an alternate open state akin to inactivation-deficit channels, thereby modulating the propensity for the persistent openings.
Fig. 7.
In-depth analysis suggests a distinct open state associated with late Na current. (A) Pale blue shaded area shows the conditional OD histogram for NaV1.5 WT channel opening in the late phase (after 50 ms depolarization), which follows a multiexponential decay. The gray solid line is the OD fit for the WT channel opening in the early phase (reproduced from SI Appendix, Fig. S4E). The red solid line is a scaled OD fit for the IFM/IQM inactivation-deficient mutant opening (B). The blue solid line is a fit for the late-phase conditional OD by a weighted sum of the early phase and IFM/IQM OD distributions. (B) OD histogram (pale red shaded area) and fit (solid red line) of the IFM/IQM mutant opening. (C–F) Format as in A but for NaV1.5 IQ/AA or S[1904]L mutants at both low and high ambient CaM levels. Gray solid lines are OD fits for the respective mutant channel openings in the early phase (reproduced from SI Appendix, Figs. S4F and S5E). In all cases, the conditional OD distributions for late channel openings are multiexponential, with the slow component matching the OD distribution of inactivation-deficient (IFM/IQM) channels. (G) Summary of multifaceted modulation of NaV1.5 by CaM and potential molecular conformational changes. With CaM bound, the carboxy-terminus (CT) interacts with the III-IV linker and prevents premature translocation for the “IFM” inactivation particle to its receptor site near the S6 domain. Following voltage depolarization and channel opening, the III-IV linker may release from the CT and thereby trigger channel inactivation. Devoid of apoCaM, allosteric changes may cause the III-IV linker to be released from the CT. This change could result in premature translocation of the “IFM” motif to the S6 receptor site, manifesting as enhanced closed-state inactivation. Alternatively, with a low likelihood, the “IFM” motif may altogether fail to reach its receptor site, resulting in persistent channel openings.
In Silico Modeling of CaM Regulation of NaV1.5 on Cardiac AP.
Mechanistic analysis suggests that the disruption of apoCaM binding to NaV1.5 reduces the peak current and increases the persistent current. To discern the potential consequences of these changes, we modified the previously established ToR-ORd human ventricular action potential model to simulate the effect of reduced NaV1.5 apoCaM binding on cardiac AP morphology (56). We parameterized both the peak Na current amplitude and the persistent current amplitude as functions of the fraction of NaV1.5 bound to CaM (fb,CaM), while maintaining the empirical relationship between Rpersist and PO/peak as in Fig. 5K. Simulations performed at a low heart rate revealed that a reduction in CaM binding resulted in a nonlinear prolongation of both the epicardial and the endocardial AP duration (APD). A maximal 1.8-fold increase in epicardial APD90 was observed with fb,CaM ∼30% (SI Appendix, Fig. S10 A–C). This nonlinearly occurs as a reduction in fb,CaM increases Rpersist but also decreases PO/peak, causing nonlinear changes in the net late Na+ conductance. The increase in APD is consistent with the LQTS3 phenotype observed with CT channelopathies.
By comparison, the mechanism of BrS is thought to be a premature all-or-none repolarization of the epicardial but not endocardial AP, leading to dispersion of repolarization across the ventricular wall and an increased susceptibility for reentry (10, 11). Although diminished apoCaM binding reduced the peak Na current in our simulations, the concomitant enhancement in the persistent Na current provided a sufficient depolarizing influx to prevent premature AP repolarization. Thus, additional factors that tune the persistent Na current may be relevant for BrS pathogenesis of NaV CT channelopathies. One possibility is fibroblast growth factor (FGF) homologous factors (FGF12B/13), which interact with NaV1.5 in cardiomyocytes (57) and inhibit the persistent current for CT mutations (39, 53). FGF association with NaV1.5 CT mutants lacking apoCaM may lead to a reduction in peak Na current without a concurrent increase in persistent Na current (39). Simulations that account for the FGF effect demonstrate a loss of the AP dome in the epicardial but not endocardial AP in a heart rate–dependent manner (SI Appendix, Fig. S10 D–F). This behavior is consistent with a BrS phenotype. Altogether, these simulations indicate that the disruption of apoCaM binding can lead to both LQTS3 or BrS phenotypes, with the latter potentially requiring additional factors such as FGF12/13.
Discussion
CaM regulation of NaV channels is multifaceted and isoform specific with complex underlying molecular mechanisms (14, 15, 28, 58). This study provides an in-depth single-channel analysis of CaM regulation of cardiac NaV1.5. Having confirmed the absence of dynamic Ca2+-dependent regulation on SSI, we focused on elucidating effects of apoCaM on NaV1.5. Disruption of CaM preassociation via IQ/AA and mixed-syndrome S[1904]L mutations evoked two disparate functional effects: 1) an up to threefold reduction in peak open probability (PO/peak) and 2) an up to 10-fold increase in the likelihood for late or persistent Na channel openings (Rpersist). For both mutants, CaM overexpression reverses changes in both PO/peak and Rpersist by repopulating channels with CaM by mass action. The correlation of PO/peak versus Rpersist revealed a linear relationship suggesting that the binding of a single apoCaM to the channel CT mediates both functional effects. Furthermore, computer simulations suggest that these biophysical changes alter AP morphology consistent with either LQT3 or BrS phenotypes. These findings lend long-sought mechanistic insights into CaM regulation and bear important implications for arrhythmogenic mechanisms underlying NaV1.5 channelopathies.
Over the past two decades, CaM regulation of NaV channels has been subject to intense scrutiny with considerable effort devoted to identifying Ca2+-dependent effects of NaV1.5 gating (14, 15, 28, 58). Both functional manifestation and underlying mechanisms of Ca2+ regulation has remained controversial. Initial studies suggested that an EF hand in the NaV1.5 CT directly coordinates Ca2+ to alter SSI (59–61); however, subsequent structures excluded direct Ca2+ binding (30). Instead, CaM was purported to mediate Ca2+ effects (35). Multiple CaM-binding sites have been identified within the channel cytosolic domains including 1) an IQ domain in the channel CT that binds both apoCaM and Ca2+/CaM (24–26, 28, 29, 32), 2) a segment downstream of the IQ motif that interacts with Ca2+/CaM (26, 29), 3) two Ca2+/CaM binding regions within the III-IV linker (35, 37), and 4) the channel N terminus (36). The functional importance of CaM binding to distinct sites has been debated and typically involves variable Ca2+-induced shifts in SSI. Our results here demonstrate the absence of acute Ca2+ regulation of NaV1.5 SSI. One possibility is that the kinetics of Ca2+-induced shifts in SSI are slow (>1 s) and only present during pathophysiological conditions. It is possible that posttranslational changes could also accelerate Ca2+/CaM effects (62).
Beyond dynamic Ca2+ effects, apoCaM fulfills a structural role as a de facto subunit responsible for conformational changes (44). ApoCaM binding has been shown to tune baseline function of multiple ion channel families including CaV (58, 63), KV7 (64, 65), and ryanodine receptors (66, 67). Previous studies have shown that the disruption of CaM binding to NaV1.5 increases the persistent current (44). Our work extends this finding to the single-channel level and demonstrates that apoCaM binding also increases channel peak open probability. Interestingly, our results further show that either Ca2+/CaM or apoCaM binding to NaV1.5 CT suppresses persistent current. This phenomenon may have complex and mutation-specific pathophysiological consequences, as disease mutations with distinct apoCaM- versus Ca2+/CaM-binding affinities may yield a variable late current (27, 68). Ca2+ regulation of the late Na current may also be relevant for mutations outside of the CaM-binding segment (68, 69). One limitation of the present study is that we focused on mutations in the channel CT to identify the putative effects of CaM. It is possible that CaM-dependent changes in PO/peak and Rpersist may partially reflect CaM association with additional CaM-interacting domains as noted above. In particular, when considered in isolation, the IQ/AA mutation strongly disrupts apoCaM binding, yet CaM overexpression sufficed to reverse these changes to a large degree. It is possible that in the context of the holo channel, additional CaM-binding sites in the N terminus (36) and in the III-IV loop (35, 37) may synergistically augment both apo- and Ca2+/CaM interaction.
Mechanistically, CaM binding triggers a switch in state dependence of channel inactivation. With apoCaM bound, channels preferentially inactivate from the open state (OSi). Channels devoid of CaM exhibit enhanced closed-state inactivation (CSi) that results in an increased failure of channel openings and an increased propensity for late channel openings. Interestingly, the kinetics of late NaV1.5 openings match channels with a defective inactivation gate. Recent cryogenic electron microscopy (cryo-EM) and crystallographic structures hint at potential changes underlying the CaM-dependent switch in channel gating (41, 42, 70). A comparison of the NaVPaS structure with that of mammalian NaV1.5 shows a translocation of the III-IV linker from the proximal CT to a cleft adjoining the Domain IV (DIV) S6 gate (41, 42, 70). In vitro analysis also confirms a weak interaction between the proximal CT and the III-IV linker (38, 43, 71), with alterations at this interface modifying the late Na current (39, 40). Further cryo-EM studies of a hybrid NaV1.7-NaVPaS channel proposed a two-switch mechanism for NaV fast inactivation (42). At the resting state, the DIV gating charges interact with the CT (switch 1) to promote III-IV linker/CT interactions (switch 2) (Fig. 7I). Upon membrane depolarization, DIV gating charges move away from the CT and release switch 1. This destabilizes switch 2, weakening III-IV linker association with the CT, and frees the IFM motif to inactivate the channel. One possibility is that CaM regulation allosterically modifies the two-switch inactivation mechanism (Fig. 7I) by stabilizing switch 1 (DIV/CT) and destabilizing switch 2 (III-IV linker/CT). CaM stabilization of switch 1 may prevent CSi by slowing DIV activation, while the destabilization of switch 2 may enable OSi by promoting III-IV linker dissociation from the CT following the release of switch 1.
This study provides insights into potential pathogenic mechanisms underlying arrhythmogenic NaV1.5 CT channelopathies. Clinically, NaV1.5 channelopathies within the CaM-binding interface of the channel CT exhibits variable penetrance with some mutations linked to either BrS, LQTS3, or both (6, 7, 9–11, 13). Our findings highlight the multifaceted and seemingly paradoxical modulation of cardiac NaV channels by CaM. Altogether, our results provide an in-depth molecular framework for divergent arrhythmogenic mechanisms and furnish possible explanations for how certain channel CT mutations may yield mixed-syndrome phenotypes.
Materials and Methods
Molecular Biology.
The human NaV1.5 channel corresponds to clone M77235.1 (GenBank). The CT domain of NaV1.5 was PCR amplified and subcloned into a Zero Blunt TOPO II (Invitrogen) vector. To construct IQ/AA and S[1904]L mutations, we performed overlap extension PCR and ligated the mutated PCR product into the NaV1.5/pGW1 vector using KpnI and XbaI restriction sites. For experiments involving the CaM chelator, we utilized the Myosin Va IQ domains 3 to 6 attached to a Venus fluorophore (Ven-MyosinVa 4×IQ) in previous studies (52). Venus-tagged NaV1.5 CT and Cerulean-tagged CaM were constructed using the following strategy: We PCR amplified the CT of NaV1.5 with the initial residue I[1771] and ligated to the 3′ end of a Venus pcDNA3 plasmid following restriction digest using NotI and XbaI. Similarly, we PCR amplified CaM and ligated into the 3′ end of Cerulean pcDNA3 after restriction digest using NotI and XbaI.
Cell Culture and Transfection.
HEK293 cells were cultured on glass coverslips in 60 mm dishes and transfected using the Ca2+ phosphate method as previously described. For whole-cell patch clamp and cell-attached multichannel experiments, we cotransfected 4 to 8 μg complementary DNA (cDNA) encoding the desired channel variant with 4 µg Yellow Fluorescent Protein (YFP) and 1 μg simian virus 40 T antigen. Electrophysiology recordings were performed at room temperature 1 to 2 d following transfection.
For single-channel recordings, 1 μg NaV1.5 channel variant was transfected in 100 mm dishes using the Ca2+ phosphate method, and recordings were performed at room temperature ∼12 h after transfection. For experiments manipulating ambient CaM levels, either 10 to 20 μg Ven-MyosinVa 4×IQ DNA or 20 μg CaM DNA were used in CaM-depleted and CaM-overexpressed conditions, respectively.
Ca2+ Uncaging and Fluorescence Measurements.
Ca2+ uncaging experiments (32) were performed using a Nikon TE2000 inverted microscope with a Plan Fluor Apo 403 oil objective. Ca2+ was uncaged by ∼1.5 ms duration UV flashes (Cairn UV photolysis system). Flashes were driven by discharge of a 4,000 mF capacitor bank charged to 200 to 300 V. Photomultiplier tubes were shuttered during the UV pulse to prevent photo damage. For Ca2+ imaging, Fluo-4FF and Alexa 568 dyes (in fixed ratios) were dialyzed into cells and imaged with Argon laser excitation (514 nm). The autofluorescence of each cell was obtained before pipet dialysis. Single-cell fluorescence emission was isolated by a field-stop aperture. Dual-color fluorescence emission was obtained with a 545DCLP dichroic mirror paired with a 545/40 bandpass filter for Fluo-4FF and a 580 longpass (LP) filter for Alexa 568. Uncaging was conducted after ∼2 min dialysis. Steady-state [Ca2+] is measured 150 ms following UV pulse.
Whole-Cell Recordings.
Whole-cell recordings were obtained at room temperature with an Axopatch 200B Amplifier (Axon Instruments). Electrodes were made of borosilicate glass (World Precision Instruments, MTW 150-F4), yielding pipets of 1 to 2 MΩ resistance, which was compensated by >70%. Pipets were fabricated with a horizontal micropipette puller (model P-97, Sutter Instruments) and fire polished with a microforge (Narishige). Data acquisition utilized an ITC-18 (InstruTech) data acquisition unit controlled by custom MATLAB software (MathWorks). Currents were low-pass filtered at 2 kHz before digitization at several times that frequency. P/8 leak subtraction was used. Cells were maintained at a holding potential of −120 mV. The pipet solution contained (in millimolars): CsMeSO3, 114; CsCl, 5; MgCl2, 1; MgATP, 4; HEPES (pH 7.4), 10; and BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), 10; at 290 mOsm adjusted with glucose. The bath solution contained (in millimolars): TEA-MeSO3, 20; HEPES (pH 7.4), 10; NaCl, 140; CaCl2, 1.0; at 300 mOsm adjusted with TEA-MeSO3.
Single- and Multichannel Recordings.
Cell-attached patch clamp experiments were performed on HEK293 cells. The pipet solution contained the following (in millimolars): TeA-MeSO3, 30; NaCl, 140; CaCl2, 0.5; and Hepes, 10 (pH 7.4). The bath solution used for on-cell recordings of Na channels contained the following (in millimolars): K-glutamate, 132; KCl, 5; NaCl, 5; MgCl2, 3; EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), 2; glucose, 6; and Hepes, 10 (pH adjusted to 7.4). For experiments involving Ca2+ and Na channels, the pipet solution contained the following (in millimolars): TeA-MeSO3, 30; NaCl, 110; CaCl2, 10; and Hepes, 10 (pH 7.4). Data were acquired at room temperature using the integrating mode of an Axopatch 200A Amplifier (Axon Instruments). Patch pipettes (5–15 MΩ for single and 3–8 MΩ for multichannel) were pulled from ultra-thick walled borosilicate glass (BF200-116–10; Sutter Instruments) using a horizontal puller (P-97, Sutter Instruments), fire polished using a microforge (Narishige), and coated with Sylgard (Dow Corning). Data acquisition used an ITC-18 16-bit low-noise data acquisition interface (InstruTech) driven by custom MATLAB software (MathWorks). Currents were filtered at 5 kHz for single-channel or 2 kHz for multichannel with a four-pole Bessel filter and sampled at 200 kHz. The subtraction of capacitive transients were performed using an automated algorithm which fit the capacitive transient using convex optimization with L1 regularization. Discrete channel openings were considered as sparse outliers from the overall multiexponential capacitive transient. We applied the following constrained minimization:
Here, Imeasured is the raw input that includes the capacitive transient (Icap) and channel openings, D is a library of exponential functions with amplitude 1 and varying time constants, e is the amplitude coefficients associated with each exponential component, s is a vector representing an estimate of negative outliers, that is, channel openings that “corrupt” the desired leak current, and λ is a penalty term that ranged between 0.15 and 0.25. The actual current trace was obtained by subtracting the multiexponential fit from the raw data. For a majority of stochastic records, this leak subtraction algorithm sufficed. In rare cases, the capacitive transients were manually fit. Unitary current values were estimated using amplitude histograms. The number of channels were estimated by stacking, binomial analysis, and mean-variance analysis. For binomial analysis, we followed an optimization procedure, whereby we assumed the number of channels (, 1 ≤ n ≤ 15) and obtained an estimated PO waveform for each N value. With this estimate, the likelihood of observing N openings at each time point is specified by the binomial distribution. This theoretical value is compared with an empirical distribution computed from idealized current traces. The number of channels was estimated as the N value that yielded a minimum least squares error between the empirical distribution and binomial estimate and confirmed using mean-variance analysis.
For first latency and open-duration calculations, stochastic records were idealized, and sweeps recorded from identical constructs with number of channels n = 1 were pooled. It was assumed that data obtained from the identical construct followed the same statistical parameters, and therefore combining the data increased the sample size of single-channel analysis. Ensemble average PO waveforms were computed as the average of 80 to 100 stochastic records for each voltage. For all experiments, population data were estimated from at least three independent transfections. For conditional openduration measurements from multichannel records, we considered only openings to the one-channel level and also excluded channel openings whose initial time of opening was within 50 ms of voltage depolarization.
For multichannel recordings, the unitary current for each patch was estimated using an amplitude histogram following leak subtraction. Each stochastic trace was subsequently idealized. The ensemble average from 50 to 100 stochastic traces was computed for each patch and normalized to the peak value. The average late current for each patch (Rpersist) was computed as the average normalized PO following 50 ms of depolarization. For PO estimation from multichannel recordings, we used mean-variance analysis. The unitary current amplitude was specified using an amplitude histogram, leaving one free parameter, N, the number of channels in the patch. For statistical analysis of peak PO and Rpersist, we used one-way ANOVA followed by Tukey’s multiple comparisons test. Given that Rpersist values followed a log-normal distribution, statistical comparisons were performed with the logarithm of Rpersist.
Flow Cytometric FRET Two-Hybrid Assay.
Flow cytometric FRET assays were performed as described (47). Briefly, HEK293 cells (American Type Culture Collection CRL1573) were cultured in 12-well plates and transfected with polyethylenimine (PEI) 25 kDa linear polymer (Polysciences #2396602). For each experiment, we cotransfected the following: 1 μg Cerulean-tagged CaM, 2 μg Venus-tagged NaV1.5 CT construct, and 0.5 μg T antigen. The cDNA pairs were mixed together in 100 μl of serum-free Dulbecco’s Modified Eagle Medium media, and 5 μL PEI was added to each sterile tube. Following 15 min of incubation, PEI/cDNA mixtures were added to the 12-well plates, and cells were cultured for 2 d prior to experimentation. Protein synthesis inhibitor cycloheximide (100 μM) was added to cells 2 h prior to experimentation to halt synthesis of new fluorophores and allow existing fluorophores to fully mature. For the determination of Kd,EFF at low Ca2+ levels, cells were resuspended in Tyrode’s solution containing 0 mM Ca2+ (138 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM Hepes [pH 7.4 using NaOH], 0.2 mM NaHPO4, 1 mM EGTA, and 5 mM D-glucose). For the determination of Kd,EFF at high Ca2+ levels, cells were resuspended in Tyrode’s solution containing 10 mM Ca2+ (138 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM Hepes [pH 7.4 using NaOH], 0.2 mM NaHPO4 5 mM D-glucose, and 10 mM CaCl2) and 4 μM ionomycin (Sigma-Aldrich) was added 10 min prior to experimentation.
For FRET measurements, we utilized an LSR II (BD Biosciences) flow cytometer equipped with 405, 488, and 633 nm lasers for excitation and 18 different emission channels as previously described (47). Forward and side scatter signals were detected and used to gate for single and healthy cells. For determining FRET efficiency, we measured three distinct fluorescence signals: 1) Cerulean emission through direct excitation measured through the BV421 channel (excitation, 405 nm; emission, 450/50), 2) Venus emission from direct excitation measured via the fluorescein isothiocyanate channel (excitation, 405 nm; dichroic, 505 LP; emission, 525/50), and 3) Venus emission because of FRET via the BV510 channel (excitation, 405 nm; dichroic, 505 LP; emission, 525/50). These raw fluorescence measurements are used to obtain Vendirect (Venus emission because of direct excitation), Cerdirect (Cerulean emission because of direct excitation), and FRET efficiency measurements. Flow cytometric signals were collected at a medium flow rate (2,000 to 8,000 events/sec). Fluorescence data were exported as Flow Cytometry Standard 3.0 files for further processing and analysis using custom MATLAB functions (MathWorks). Analysis was performed as described (47). Briefly, we calculated the FRET efficiency ED = VenFRET/(VenFRET + fA/fD ∙ Cerdirect). Subsequently, the total concentration of donors and acceptors were calculated as ND = Cerdirect/(1 − ED) and NA = Vendirect/(gA/gD ∙ fA/fD). The instrument-specific parameters fA/fD and gA/gD are determined on the day of the experiments using a series of Cerulean–Venus dimers with known FRET efficiency. To construct FRET two-hybrid binding curves, we imposed a 1:1 binding isotherm as in previous studies (46). Specifically, we iteratively fit ED = Emax × [A]free/([A]free + Kd,eff) using the least squares method in which Afree is computed as NA – (ND – Dfree). The free concentration of the donor is computed as the following:
For each FRET pairs, Kd,EFF and ED,max and 95% CIs were obtained by an iterative constrained least squares fit. For all experiments, we show all individual cells obtained from at least two independent transfections.
Computational Ventricular Action Potential Modeling.
Computational modeling was performed with the ToR-ORd model simulated using MATLAB (56). We adjusted the model parameters to reflect the kinetic profile of the NaV1.5 PO waveform from our experiments both in the presence and absence of CaM, and the amplitude of the persistent current and PO/peak values were constrained to match the empirical relationship obtained in Fig. 5K. Specifically, we adjusted as follows: . Furthermore, the peak Na current conductance was adjusted as follow: , while the late Na current conductance was modified as . For simulations accounting for the effect of FGF12/13, the peak Na current conductance was adjusted as above. However, the late Na current conductance was modified as to maintain the overall ratio of peak to late Na conductance. Action potentials were simulated for 100 beats at various heart rates, and only the final two beats were considered for the measurement of APD.
Supplementary Material
Acknowledgments
We thank Dr. Richard Aldrich, Dr. L. Mario Amzel, Dr. Sandra Gabelli, Dr. Henry Colecraft, Dr. Steven O. Marx, Dr. Filip van Petegem, and Dr. Ivy Dick for valuable insight and feedback on this work. This study is supported by funding from National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke. Research supported in this publication was performed in the Columbia Center for Translational Immunology flow cytometry core, supported in part by the Office of the Director, NIH, under award S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest .
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025085118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Hille B., Ionic Channels of Excitable Membranes (Sinauer Associates, Sunderland, MA, 1984), pp. 226–248. [Google Scholar]
- 2.Catterall W. A., Lenaeus M. J., Gamal El-Din T. M., Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 60, 133–154 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Zimmer T., Surber R., SCN5A channelopathies–An update on mutations and mechanisms. Prog. Biophys. Mol. Biol. 98, 120–136 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Abriel H., Rougier J. S., Jalife J., Ion channel macromolecular complexes in cardiomyocytes: Roles in sudden cardiac death. Circ. Res. 116, 1971–1988 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan E., et al., Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J. Clin. Invest. 126, 112–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remme C. A., Cardiac sodium channelopathy associated with SCN5A mutations: Electrophysiological, molecular and genetic aspects. J. Physiol. 591, 4099–4116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilde A. A. M., Amin A. S., Clinical spectrum of SCN5A mutations: Long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin. Electrophysiol. 4, 569–579 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Bennett P. B., Yazawa K., Makita N., George A. L. Jr, Molecular mechanism for an inherited cardiac arrhythmia. Nature 376, 683–685 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Bankston J. R., et al., A novel LQT-3 mutation disrupts an inactivation gate complex with distinct rate-dependent phenotypic consequences. Channels (Austin) 1, 273–280 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Antzelevitch C., et al., Brugada syndrome: A decade of progress. Circ. Res. 91, 1114–1118 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C., The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J. Cardiovasc. Electrophysiol. 12, 268–272 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Kass R. S., Sodium channel inactivation in heart: A novel role of the carboxy-terminal domain. J. Cardiovasc. Electrophysiol. 17, S21–S25 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Kapplinger J. D., et al., An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 7, 33–46 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Petegem F., Lobo P. A., Ahern C. A., Seeing the forest through the trees: Towards a unified view on physiological calcium regulation of voltage-gated sodium channels. Biophys. J. 103, 2243–2251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitt G. S., Lee S. Y., Current view on regulation of voltage-gated sodium channels by calcium and auxiliary proteins. Protein Sci. 25, 1573–1584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabelli S. B., Yoder J. B., Tomaselli G. F., Amzel L. M., Calmodulin and Ca(2+) control of voltage gated Na(+) channels. Channels (Austin) 10, 45–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulley J. C., et al., SCN1A mutations and epilepsy. Hum. Mutat. 25, 535–542 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Satterstrom F. K.et al.; Autism Sequencing Consortium; iPSYCH-Broad Consortium , Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders S. J., et al., Progress in understanding and treating SCN2A-mediated disorders. Trends Neurosci. 41, 442–456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedrich U. B. S., Lauxmann S., Lerche H., SCN2A channelopathies: Mechanisms and models. Epilepsia 60, S68–S76 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Meisler M. H., et al., SCN8A encephalopathy: Research progress and prospects. Epilepsia 57, 1027–1035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horie R., et al., EF hand-like motif mutations of Nav1.4 C-terminus cause myotonic syndrome by impairing fast inactivation. Muscle Nerve 61, 808–814 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Cannon S. C., Sodium channelopathies of skeletal muscle. Handb. Exp. Pharmacol. 246, 309–330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldkamp M. D., Yu L., Shea M. A., Structural and energetic determinants of apo calmodulin binding to the IQ motif of the Na(V)1.2 voltage-dependent sodium channel. Structure 19, 733–747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chagot B., Chazin W. J., Solution NMR structure of Apo-calmodulin in complex with the IQ motif of human cardiac sodium channel NaV1.5. J. Mol. Biol. 406, 106–119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori M., Konno T., Morii T., Nagayama K., Imoto K., Regulatory interaction of sodium channel IQ-motif with calmodulin C-terminal lobe. Biochem. Biophys. Res. Commun. 307, 290–296 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Gardill B. R., Rivera-Acevedo R. E., Tung C. C., Van Petegem F., Crystal structures of Ca2+-calmodulin bound to NaV C-terminal regions suggest role for EF-hand domain in binding and inactivation. Proc. Natl. Acad. Sci. U.S.A. 116, 10763–10772 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabelli S. B., et al., Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nat. Commun. 5, 5126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoder J. B., et al., Ca2+-dependent regulation of sodium channels NaV1.4 and NaV1.5 is controlled by the post-IQ motif. Nat. Commun. 10, 1514 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C., Chung B. C., Yan H., Lee S. Y., Pitt G. S., Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure 20, 1167–1176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., et al., Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat. Commun. 5, 4896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Johny M., et al., Conservation of Ca2+/calmodulin regulation across Na and Ca2+ channels. Cell 157, 1657–1670 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deschênes I., et al., Isoform-specific modulation of voltage-gated Na(+) channels by calmodulin. Circ. Res. 90, E49–E57 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Kim J., et al., Calmodulin mediates Ca2+ sensitivity of sodium channels. J. Biol. Chem. 279, 45004–45012 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Sarhan M. F., Tung C. C., Van Petegem F., Ahern C. A., Crystallographic basis for calcium regulation of sodium channels. Proc. Natl. Acad. Sci. U.S.A. 109, 3558–3563 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z., et al., Calmodulin binds to the N-terminal domain of the cardiac sodium channel Nav1.5. Channels (Austin) 14, 268–286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson C. N., et al., A mechanism of calmodulin modulation of the human cardiac sodium channel. Structure 26, 683–694.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardill B. R., et al., The voltage-gated sodium channel EF-hands form an interaction with the III-IV linker that is disturbed by disease-causing mutations. Sci. Rep. 8, 4483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gade A. R., Marx S. O., Pitt G. S., An interaction between the III-IV linker and CTD in NaV1.5 confers regulation of inactivation by CaM and FHF. J. Gen. Physiol. 152, e201912434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters C. H., Watkins A. R., Poirier O. L., Ruben P. C., E1784K, the most common Brugada syndrome and long-QT syndrome type 3 mutant, disrupts sodium channel inactivation through two separate mechanisms. J. Gen. Physiol. 152, e202012595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H., et al., Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355, eaal4326 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Clairfeuille T., et al., Structural basis of α-scorpion toxin action on Nav channels. Science 363, eaav8573 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Glaaser I. W., et al., Perturbation of sodium channel structure by an inherited long QT syndrome mutation. Nat. Commun. 3, 706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan H., Wang C., Marx S. O., Pitt G. S., Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 149, 277–293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verkerk A. O., Amin A. S., Remme C. A., Disease modifiers of inherited SCN5A channelopathy. Front. Cardiovasc. Med. 5, 137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S. R., Sang L., Yue D. T., Uncovering aberrant mutant PKA function with flow cytometric FRET. Cell Rep. 14, 3019–3029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivas S., Hanif K., Chakouri N., Ben-Johny M., “Probing ion channel macromolecular interactions using fluorescence resonance energy transfer” in Methods in Enzymology, Minor D. L. Jr., Colecraft H. M., Eds. (Academic Press, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biswas S., DiSilvestre D., Tian Y., Halperin V. L., Tomaselli G. F., Calcium-mediated dual-mode regulation of cardiac sodium channel gating. Circ. Res. 104, 870–878 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanoye C. G., Lossin C., Rhodes T. H., George A. L. Jr, Single-channel properties of human NaV1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J. Gen. Physiol. 127, 1–14 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albitz R., Droogmans G., Nilius B., The conductance of single cardiac sodium channels from guinea pig depends on the intracellular sodium concentration. Biochim. Biophys. Acta 1068, 254–256 (1991). [DOI] [PubMed] [Google Scholar]
- 51.Ben Johny M., Yang P. S., Bazzazi H., Yue D. T., Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat. Commun. 4, 1717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee R., et al., Bilobal architecture is a requirement for calmodulin signaling to CaV1.3 channels. Proc. Natl. Acad. Sci. U.S.A. 115, E3026–E3035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrams J., et al., Fibroblast growth factor homologous factors tune arrhythmogenic late NaV1.5 current in calmodulin binding-deficient channels. JCI Insight 5, 141736 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kellenberger S., West J. W., Scheuer T., Catterall W. A., Molecular analysis of the putative inactivation particle in the inactivation gate of brain type IIA Na+ channels. J. Gen. Physiol. 109, 589–605 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartmann H. A., Tiedeman A. A., Chen S. F., Brown A. M., Kirsch G. E., Effects of III-IV linker mutations on human heart Na+ channel inactivation gating. Circ. Res. 75, 114–122 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Tomek J., et al., Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. eLife 8, e48890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hennessey J. A., et al., FGF12 is a candidate Brugada syndrome locus. Heart Rhythm 10, 1886–1894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Johny M., et al., Towards a unified theory of calmodulin regulation (calmodulation) of voltage-gated calcium and sodium channels. Curr. Mol. Pharmacol. 8, 188–205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wingo T. L., et al., An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat. Struct. Mol. Biol. 11, 219–225 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Chagot B., Potet F., Balser J. R., Chazin W. J., Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel NaV1.5. J. Biol. Chem. 284, 6436–6445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah V. N., et al., Calcium-dependent regulation of the voltage-gated sodium channel hH1: Intrinsic and extrinsic sensors use a common molecular switch. Proc. Natl. Acad. Sci. U.S.A. 103, 3592–3597 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashpole N. M., et al., Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J. Biol. Chem. 287, 19856–19869 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minor D. L. Jr, Findeisen F., Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation. Channels (Austin) 4, 459–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun J., MacKinnon R., Cryo-EM structure of a KCNQ1/CaM complex reveals insights into congenital long QT syndrome. Cell 169, 1042–1050.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang P. W., et al., Calmodulin acts as a state-dependent switch to control a cardiac potassium channel opening. Sci. Adv. 6, eabd6798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong D., et al., Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 572, 347–351 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Bers D. M., Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 37, 417–429 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Abdelsayed M., et al., Differential calcium sensitivity in NaV 1.5 mixed syndrome mutants. J. Physiol. 595, 6165–6186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potet F., Beckermann T. M., Kunic J. D., George A. L. Jr, Intracellular calcium attenuates late current conducted by mutant human cardiac sodium channels. Circ. Arrhythm. Electrophysiol. 8, 933–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang D., et al., Structure of the cardiac sodium channel. Cell 180, 122–134.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potet F., et al., Functional interactions between distinct sodium channel cytoplasmic domains through the action of calmodulin. J. Biol. Chem. 284, 8846–8854 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.