Abstract
This paper is to examine the relationship between anti-cyclic citrullinated peptide (anti-CCP) antibody titers and the development of interstitial lung disease (ILD) in patients with and without rheumatoid arthritis (RA). A retrospective investigation was conducted on all adult patients tested for anti-CCP between January 1, 2007, and December 31, 2012, in a university healthcare system. Patients with specified exposures or conditions known to cause ILD were excluded. The prevalence of ILD was compared between those with and without a positive CCP. The study population was then divided into four titer groups based on anti-CCP titers: negative, low titer, moderate titer, high titer. Fisher’s exact tests compared the prevalence of ILD among the anti-CCP titer groups. Multivariate logistic regression examined the association between anti-CCP and ILD while controlling for confounders. These analyses were repeated in two subgroups: a “confirmed RA” subgroup and an “unconfirmed RA” subgroup. Two thousand and thirty patients met inclusion criteria and 453 of those had confirmed RA. Progressively higher anti-CCP titer groups developed an increasingly higher prevalence of ILD (p < 0.01). When adjusting for age, tobacco, and a diagnosis of RA, higher anti-CCP titer groups continued to correlate with an increased prevalence of ILD (OR 1.47, 95% CI 1.10–1.96, p < 0.001). This study is the first to show that progressively higher anti-CCP titers correlate with increasing prevalence of ILD, even when adjusting for confounders.
Keywords: Interstitial lung disease (ILD), Rheumatoid arthritis (RA), Rheumatology
Introduction/objectives
Rheumatoid arthritis (RA) is an autoimmune arthritis characterized by symmetric, destructive polyarthritis and multiple extra-articular manifestations [1]. One of these extra-articular manifestations is interstitial lung disease (ILD) which is reported in 4.5–7.7% of patients with RA [2,3] and is characterized by dyspnea, dry cough, and characteristic findings on chest imaging or pulmonary function tests [4].
To our knowledge, five studies have evaluated the association between anti-cyclic citrullinated peptide (anti-CCP) antibodies and ILD with conflicting results. Inui et al. reported no difference in the anti-CCP antibody titer between a group of 18 RA patients with ILD (RA-ILD) and a group of 36 RA patients without ILD [5]. In contrast, Chen et al. described higher anti-CCP titers in RA patients with ILD compared to RA patients without ILD in two independent cohorts of 86 American patients and 133 Chinese patients, respectively [6]. Giles et al. also found higher anti-CCP titers in patients with ILD compared to those without ILD in their cohort of 177 RA patients [7]. A study of 230 patients with RA-ILD in the UK had a higher median CCP titer compared to matched RA subjects without ILD [2]. Similar findings were reported for a Greek study comparing 11 patients with RA and pulmonary fibrosis with 125 patients who had RA alone [8].
No investigation has examined whether the extent of the anti-CCP titer elevation modulates the prevalence of ILD in RA patients and no studies have examined this relationship in patients without RA. ILD that precedes articular manifestations in RA is recognized, but poorly studied, and previous investigations may have excluded these patients [4]. Researchers have also demonstrated an increased proportion of chronic obstructive pulmonary disease in RA [9]. Other lung conditions, such as lung cancer, were not found to be associated with RA after adjusting for confounders [10]. These studies, however, did not evaluate for an association of these conditions with anti-CCP antibodies.
In this investigation, we investigate whether the mean anti-CCP titer differs in those with and without ILD and whether progressively higher anti-CCP titers modulate the prevalence of ILD. We are the first to report these outcomes in patients with and without confirmed RA.
Materials and methods
This investigation was approved by the institutional review board (#205696). Records from all adult patients in a university healthcare system tested with the Euro-Diagnostica EDIA anti-cyclic citrullinated antibody ELISA assay™ between January 1, 2007, and December 31, 2012, were reviewed. This anti-CCP assay measures serum CCP between 0 and 300 U/mL. All values > 300 U/mL were recorded as “> 300.” Values between 0 and 5 U/mL are categorized as negative while values ≥ 6 are positive.
Subjects were included in the study if they were tested for anti-CCP antibodies during the study time period and did not meet the exclusion criteria. Subjects were categorized as having RA if they met the 2010 American College of Rheumatology criteria for RA [11] determined by chart review or if they were diagnosed by an expert rheumatologist, and they are collectively referred to as the “confirmed RA subgroup.” Subjects tested for anti-CCP who did not meet the criteria for diagnosis of RA at the time of our chart review were referred to as the “unconfirmed RA subgroup,” acknowledging the fact that some subset of these patients may go on to develop overt disease at a later time. The combination of these two groups for the purpose of analyses is referred to as the “total cohort.”
Subjects were categorized as having ILD if they met any of the following criteria: characteristic findings on computed tomography (CT) scan, the combination of bilateral interstitial infiltrates on chest radiograph and low DLCO with restrictive pattern on PFTs, or the diagnosis of ILD by an expert pulmonologist. To determine whether higher anti-CCP titers were associated with higher risk of ILD, anti-CCP results were stratified into four groups, which were defined prior to the collection of any data: 0–5 U/mL (negative titer), 6–150 U/mL (low titer), 151–299 U/mL (moderate titer), and 300 U/mL or greater (high titer). These groups were chosen because 5 U/mL is the cutoff for a negative test, 300 U/mL is the cutoff for largest titer recorded by the lab, and 150 U/mL is roughly halfway between.
Subjects were excluded if they were diagnosed with the following known causes of ILD: systemic lupus erythematosus, dermatomyositis, polymyositis, scleroderma, Sjogren syndrome, overlap syndrome, mixed connective tissue disease, sarcoidosis, exposure to silica, or therapeutic radiation to the chest as determined by ICD9 billing code or chart review.
As shown in Table 1, the total cohort tends to be female, elderly, Caucasian, and with frequency of rheumatoid factor positivity of 30.9%. The RA cohort tends to be female, elderly, and with an average RF of 228.9 IU/mL. The unconfirmed RA cohort tends to be female, elderly, with an average RF of 37.5 IU/mL.
Table 1.
Demographic data for all subjects included in this investigation
| Total cohort (n = 2030) |
Confirmed RA subgroup (n = 453) |
Unconfirmed RA subgroup (n = 1577) |
|
|---|---|---|---|
| Female, % Age, years Ethnicity, % | 79.2 (77.4–81.0) 56.6 (55.9–57.3) |
80.6 (76.9–84.2) 59.6 (58.2–61.1) |
78.7 (76.7–80.7) 55.7 (54.9–56.5) |
| Caucasian | 68.1 (66.1–70.2) | 62.5 (58.0–67.0) | 69.8 (67.5–72.0) |
| African American | 14.2 (12.7–15.7) | 19.2 (15.6–22.8) | 12.8 (11.1–14.4) |
| Hispanic | 1.8 (1.2–2.4) | 1.3 (0.27–2.4) | 2.0 (1.3–2.7) |
| Asian | 2.0 (1.4–2.6) | 1.8 (0.5–3.0) | 2.0 (1.3–2.7) |
| Native Hawaiian | 0.1 (0–0.2) | 0.2 (0–0.6) | 0.1 (0–0.2) |
| Other | 11.3 (9.9–12.7) | 11.7 (8.7–14.7) | 11.2 (9.6–12.7) |
| Not stated | 2.5 (1.8–3.1) | 3.3 (1.7–5.0) | 2.2 (1.5–3.0) |
| ILD, % | 2.8 (2.0–3.5) | 6.0 (3.8–8.1) | 1.8 (1.1–2.5) |
| Anti-CCP, U/mL | 29.5 (26.0–33.0) | 113 (101.4–124.7) | 5.5 (3.8–7.3) |
| Rheumatoid factor positive, % | 44.8 (39.3–50.3) | 69.7 (54.6–84.8) | 19.3 (16.6–22.0) |
| Rheumatoid factor, IU/mL** | 81.8 (67.6–96.0) | 228.9 (181.4–276.4) | 37.5 (26.8–48.2) |
| Erythrocytic sedimentation rate, mm | 25.1 (23.8–26.3) | 34.9 (32.1–37.6) | 22.0 (20.7–23.3) |
| C-reactive protein, mg/dL | 1.6 (1.4–1.8) | 2.0 (1.6–2.4) | 1.5 (1.2–1.7) |
| Hemoglobin, g/dL | 12.9 (12.8–13.0) | 12.7 (12.5–12.9) | 13.0 (12.9–13.0) |
| Serum albumin, g/dL | 3.7 (3.7–3.7) | 3.6 (3.6–3.7) | 3.8 (3.7–3.8) |
| Platelets, K/uL | 262.3 (258.2–266.4) | 276.6 (268.6–284.6) | 257.1 (252.3–261.9) |
Values are the percentage or mean value followed by 95% confidence interval
The lowest reported value is 20 IU/mL. All values reported as < 20 IU/mL were counted as 19 IU/mL
Two-sample T test or Fisher’s exact test was used for the comparison of patient characteristics. Simple logistic regression was performed to screen for potential confounders of ILD including age, race, gender, tobacco exposure, anti-CCP titer, rheumatoid factor titer, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), hemoglobin, mean corpuscular volume, platelet count, and serum albumin. Then multivariate logistic regression was fitted to examine the association between ILD and anti-CCP while controlling for confounders. All statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Two thousand and thirty patients met the inclusion criteria, composing the “total cohort” (Table 1). The mean anti-CCP was 29.7 U/mL and the prevalence of ILD was 2.8%. Four hundred and fifty-three subjects (22%) were confirmed to have a diagnosis of RA, comprising the “confirmed RA subgroup.” The confirmed RA subgroup had a higher mean anti-CCP (113 U/mL) and a higher prevalence of ILD (6.0%). One thousand and five hundred seventy-seven subjects did not meet the criteria for the diagnosis of RA and make up the “unconfirmed RA subgroup.” This subgroup had a lower mean anti-CCP titer (5.5 U/mL) and a lower prevalence of ILD (1.8%).
In the total cohort and confirmed RA subgroup, mean anti-CCP titers were higher in subjects with ILD compared to those without ILD (89.7 U/mL vs 28.0 U/mL in the total cohort, p < 0.001; 162.4 U/mL vs 109.9 U/mL in the confirmed RA subgroup, p = 0.035). When evaluated in the unconfirmed RA subgroup, the difference in CCP titers was not statistically significant (21.93 U/mL vs 5.52 U/mL, p = 0.26).
In the total cohort, a higher proportion of anti-CCP-positive patients (> 5 U/mL) developed ILD compared to anti-CCP-negative (0–5 U/mL) patients (6.79% vs 1.87%, p < 0.001). Similar findings were observed in the confirmed RA and unconfirmed RA subgroups, but did not achieve statistical significance (confirmed RA subgroup: 7.12% vs 3.47%, p = 0.14; unconfirmed RA subgroup: 2.08% vs 1.71%, p = 0.091). A higher proportion of patients with ILD had a positive rheumatoid factor (65.2% vs 29.9% in Chi-square analysis, p < 0.001).
Subjects were divided into four titer groups by their anti-CCP level. In the total cohort, when comparing the prevalence of ILD by titer group, the prevalence of ILD increased progressively with higher anti-CCP titers (1.90%, 4.92%, 8.82%, and 10.3% in the negative, low, moderate, and high titer groups, respectively, p < 0.001; Fig. 1).
Fig. 1.
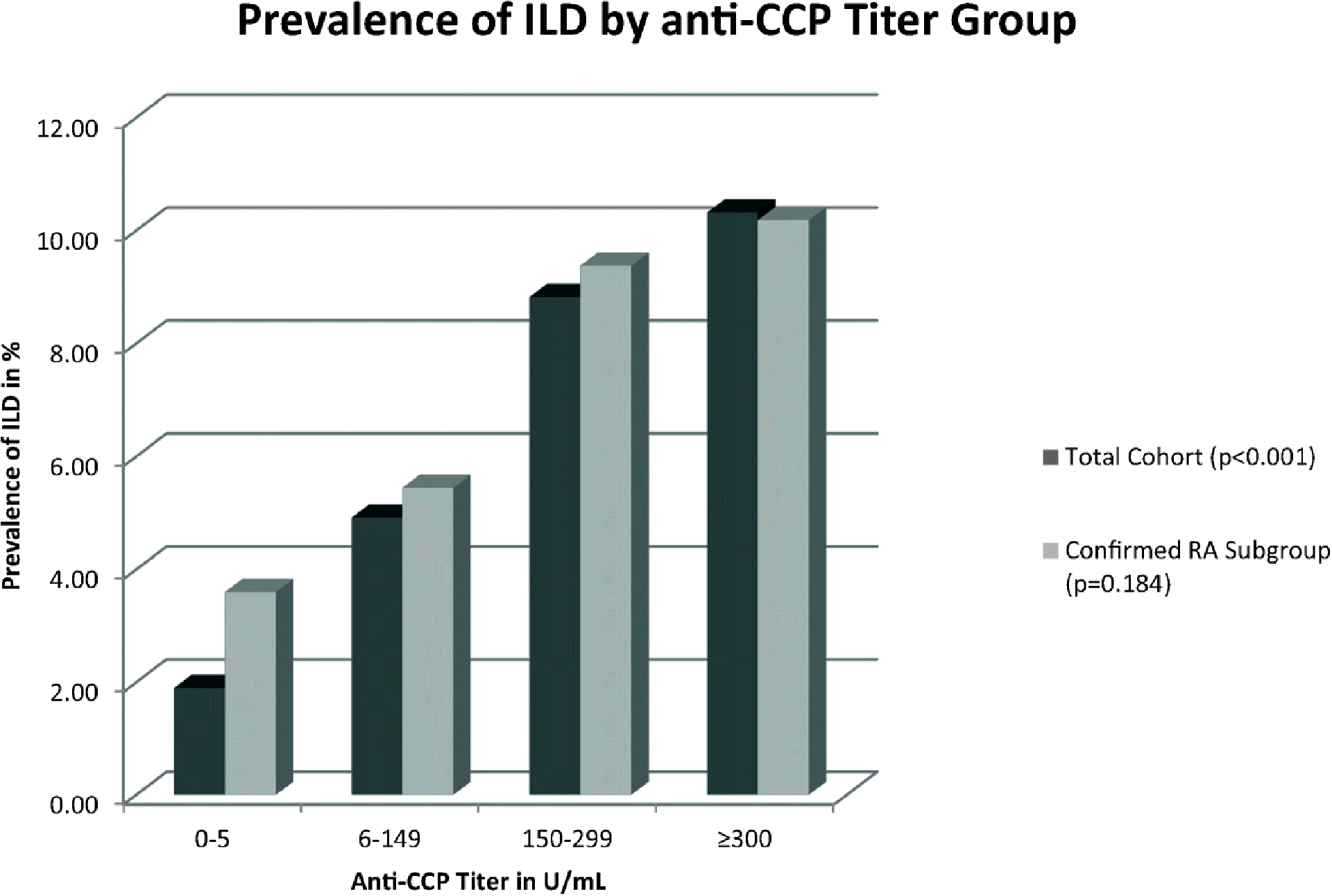
Prevalence of ILD by anti- CCP titer group.
Using Fisher’s exact test, only the total cohort achieved statistical significance (p < 0.001)
In the total cohort, increasing the risk for developing ILD was demonstrated in higher anti-CCP titer groups (low titer group vs negative anti-CCP: OR 2.59, CI 1.21–5.52, p = 0.014; moderate titer group vs negative anti-CCP: OR 4.64, CI 1.35–15.9, p = 0.015; high titer group vs negative anti-CCP: OR 5.42, CI 2.77–10.63, p < 0.001). Factors associated with the development of ILD in the total cohort included higher anti-CCP titer group, age, tobacco use, and a diagnosis of RA (Table 2). When adjusting for age, tobacco, and diagnosis of RA, a higher anti-CCP titer group continued to correlate with an increased risk for ILD (OR 1.47, 95% CI 1.10–1.96, p < 0.001; Table 3).
Table 2.
Odds ratio for the presence of interstitial lung disease, simple logistic regression
| Total cohort | Confirmed RA subgroup | Unconfirmed RA subgroup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | p value | 95% CI | Odds ratio | p value | 95% CI | Odds ratio | p value | 95% CI | |||
| Age | 1.06 | < 0.01 | 1.04–1.08 | 1.04 | < 0.01 | 1.01–1.07 | 1.06 | < 0.01 | 1.04–1.09 | ||
| Tobacco | 2.23 | < 0.01 | 1.35–3.68 | 3.7 | < 0.01 | 1.62–8.45 | 1.4 | 0.35 | 0.70–2.79 | ||
| CRP | 1.01 | 0.17 | 1.0–1.02 | 1 | 0.79 | 0.99–1.02 | 1.01 | 0.38 | 0.99–1.03 | ||
| RA | 3.38 | < 0.01 | 1.98–5.78 | Not applicable | Not applicable | ||||||
| Male gender* | 0.86 | 0.65 | 0.46–1.62 | 1.07 | 0.9 | 0.39–2.90 | 0.7 | 0.4 | 0.31–1.60 | ||
| Asian, Hispanic, and Native Hawaiian races** | 0.94 | 0.94 | 0.22–3.99 | 1.12 | 0.92 | 0.14–9.01 | 0.86 | 0.88 | 0.11–6.48 | ||
| African American race** | 1.04 | 0.92 | 0.48–2.25 | 0.95 | 0.93 | 0.34–2.67 | 0.82 | 0.75 | 0.24–2.78 | ||
| Other or not stated race** | 1.21 | 0.61 | 0.58–2.54 | 0.98 | 0.97 | 0.32–3.01 | 1.31 | 0.56 | 0.49–3.53 | ||
| Anti-CCP 6–150 U/mL compared to anti-CCP 0–5 U/mL | 2.59 | 0.01 | 1.21–5.52 | 1.51 | 0.48 | 0.48–4.74 | 1.59 | 0.65 | 0.21–12.07 | ||
| anti-CCP 150–300 U/mL compared to anti-CCP 0—5 U/mL | 4.64 | 0.02 | 1.35–15.93 | 2.61 | 0.21 | 0.59–11.47 | † | ||||
| anti-CCP >300 U/mL compared to anti-CCP 0–5 U/mL | 5.43 | < 0.01 | 2.77–10.64 | 2.83 | 0.06 | 0.96–8.39 | 6.38 | 0.02 | 1.41–28.90 | ||
Compared to female gender
Compared to Caucasian race
Of two patients in this category, neither developed ILD
Table 3.
Adjusted odds ratio for presence of interstitial lung disease
| Total cohort | Confirmed RA subgroup | Unconfirmed RA subgroup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | p value | 95% CI | OR | p value | 95% CI | OR | p value | 95% CI | |||
| Age | 1.052 | < 0.001 | 1.031–1.072 | 1.036 | 0.021 | 1.005–1.068 | 1.063 | < 0.001 | 1.035–1.091 | ||
| Tobacco | 1.896 | 0.019 | 1.113–3.229 | 3.468 | 0.005 | 1.456–8.259 | 1.221 | 0.582 | 0.599–2.487 | ||
| RA | 1.613 | 0.187 | 0.793–3.279 | Not applicable | Not applicable | ||||||
| Anti-CCP quartile* | 1.47 | 0.009 | 1.101–1.965 | 1.407 | 0.047 | 1.005–1.969 | 1.648 | 0.055 | 0.988–2.747 | ||
Odds ratio is reported for every increase in quartile group of anti-CCP U/mL defined as 0–5 U/mL (negative titer), 6–149 U/mL (low titer), 150–299 U/ mL (moderate titer), and 300 U/mL or greater (high titer)
In the confirmed RA subgroup, the prevalence of ILD also increased with higher anti-CCP titer groups, but did not achieve statistical significance (p = 0.184; Fig. 1). Additionally, a progressively higher odds ratio was observed with higher anti-CCP titer groups but did not achieve statistical significance (Table 2). Factors included in the model included higher anti-CCP titer group, age, and tobacco use, but only a higher anti-CCP titer group correlated with an increased risk for the development of ILD when adjusting for confounders (OR 1.41, 95% CI 1.005–1.969, p = 0.047; Table 3).
In the unconfirmed RA subgroup, the prevalence of ILD increased with higher anti-CCP titer groups but did not reach statistical significance (p = 0.072). A higher odds ratio for developing ILD was observed in the high titer anti-CCP group (Table 2). With simple logistic regression, both anti-CCP titer group and age were associated with the development of ILD (Table 2), but when adjusting for age and tobacco, the odds ratio did not achieve statistical significance for anti-CCP (p = 0.55; Table 3).
Although race did not emerge as a predictor of ILD in any cohorts, an additional analysis analyzed the relationship between race and anti-CCP positivity. Caucasian race demonstrated the lowest CCP positivity (15.1%) while Hispanic and African American races demonstrated higher rates (21.6% and 27.1%, respectively). A chi-squared analysis established a significant association between race and anti-CCP positivity (p < 0.001).
Conclusion
Previous investigations have reported a difference in mean anti-CCP titers between RA patients with and without ILD [2,5,6,7,8]. Uniquely, this study further explores this association in two ways: it divides subjects into anti-CCP titer groups to evaluate whether the prevalence of ILD increases as anti-CCP increases and it evaluates this question in three groups: all patients tested for anti-CCP, patients with RA, and patients without confirmed RA. This is important not only because ILD can precede articular symptoms in RA, but also because of the limitations of a retrospective cohort design.
This investigation demonstrates that patients with RA-ILD have higher mean anti-CCP titers compared to RA patients without ILD. To our knowledge, our confirmed RA subgroup of 453 patients is the largest sample of RA patients that has been studied to answer this question.
In our total cohort of 2030 patients, the data demonstrates a higher mean anti-CCP in those with ILD compared to those without ILD. This is a novel finding. Our data also demonstrates a higher prevalence of ILD in patients with positive anti-CCP titers compared to those with negative anti-CCP titers. To better characterize this relationship, we studied how the prevalence of ILD changes with anti-CCP titer.
This study demonstrates progressively higher ILD prevalence with higher anti-CCP titers in our total cohort. Furthermore, there is a progressively higher odds ratio of developing ILD as anti-CCP titer increases in the total cohort. These findings have not been previously reported. Although these trends remained apparent in the RA cohort, they were not statistically significant.
To reconcile these statistically disparate results, it is helpful to observe two trends that are apparent in Fig. 1. One reason for the lack of statistical significance in the confirmed RA subgroup is the higher prevalence of ILD in the negative titer group. This reflects the higher prevalence of ILD in all RA patients. Second, a relationship between anti-CCP titer and ILD prevalence is suggested, but may need to be demonstrated in an even larger sample size to achieve statistical significance.
Univariate analysis demonstrated that age, tobacco use, and the diagnosis of RA predicted the development of ILD. CRP, age, and diagnosis of RA have previously been observed to predict ILD and may reflect either disease activity or disease duration [3,12]. Studies have shown that tobacco directly increases anti-CCP titers, especially in those with the HLA-DRB1 shared epitope [13]. Smoking is also a known cause of ILD [14]. However, the multivariate analysis shows that even when adjusting for these factors, a higher anti-CCP titer group correlates with an increased prevalence of ILD. Interestingly, this was true in both the total cohort and the RA subgroup.
There are limitations to this study. First, there is a relatively short observation period of at most 7.5 years between CCP testing and the final chart review to determine if ILD was present, which may underestimate the final prevalence of ILD. Another limitation of this investigation is the problem of confirming whether or not subjects were diagnosed with RA by chart review. Specifically, in the unconfirmed RA subgroup, 9 of the 29 subjects diagnosed with ILD were never examined by a rheumatologist, so it is not possible to say whether some of these patients would have met the ACR criteria at the time of anti-CCP testing or at a time in the future.
A strength of our study is the large total cohort of 2030 patients. Other known causes of ILD such as other autoimmune diseases and exposures associated with the development of ILD were excluded, leaving a more pristine group of RA patients in the confirmed RA subgroup. Additionally, this investigation is the largest study to date that reports a fundamental relationship between anti-CCP and ILD prevalence.
Our observations of a progressively higher ILD prevalence in the setting of higher anti-CCP titers and progressively higher odds ratios for developing ILD as anti-CCP titer increases have not been previously reported. This association exists not only in patients with confirmed RA, but also in a combined cohort of patients with confirmed RA or possible RA. If these observations were confirmed in larger studies, the clinical value of the anti-CCP antibodies would be expanded from simply diagnosing RA to stratifying the risk of developing ILD.
Footnotes
Disclosures None
References
- 1.Shah AS (2012) Rheumatoid arthritis. In: Longo DE (ed) Harrison’s Principles of Internal Medicine 18th edition. McGraw-Hill, New York, p 2739 [Google Scholar]
- 2.Kelly C et al. (2014) Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics - a large multicentre UK study. Rheumatology 53:1676–1682 [DOI] [PubMed] [Google Scholar]
- 3.Bongartz T et al. (2010) Incidence and mortality of interstitial lung disease in rheumatoid arthritis. Arthritis Rheumatol 62(6):1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EJ HC (2009) Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 5(136):1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inui N et al. (2008) Anti-cyclic citrullinated peptide antibodies in diseases associated with rheumatoid arthritis. Clin Biochem 41: 1075–1077 [DOI] [PubMed] [Google Scholar]
- 6.Chen J et al. (2015) Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 67(1):28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giles JT et al. (2013) Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexious I et al. (2008) Anti-cyclic citrullinated peptide-2 (CCP2) autoantibodies and extra-articular manifestations in Greek patients with rheumatoid arthritis. Clin Rheumatol 27: 511–513 [DOI] [PubMed] [Google Scholar]
- 9.Bieber V et al. (2013) Autoimmune smoke and fire–coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross- sectional analysis. Immunol Res 56(2–3):261–266 [DOI] [PubMed] [Google Scholar]
- 10.Dagan A et al. (2017) Coexistent malignant conditions in rheumatoid arthritis - a population-based cross-sectional study. Int J Clin Pract 71:e12929. [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D et al. (2010) 2010 Rheumatoid arthritis classification criteria. Arthritis Rheumatol 62(9):2569–2581 [DOI] [PubMed] [Google Scholar]
- 12.Mori S et al. (2012) Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 106(11):1591–1599 [DOI] [PubMed] [Google Scholar]
- 13.Linn-Rasker SP et al. (2006) Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis 3(65):366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassallo RRJ (2012) Smoking-related interstitial lung diseases. Clin Chest Med 33:165–178 [DOI] [PubMed] [Google Scholar]


