Abstract
The antimicrobial susceptibility of Helicobacter pylori strains isolated from HIV‐positive individuals is not well characterized. This study aimed to measure the prevalence and long‐term trends associated with primary H. pylori antibiotic resistance, evaluate correlations with antibiotic consumption, and compare predictors for H. pylori antibiotic resistance between HIV‐positive and HIV‐negative individuals. In this longitudinal registry study, we evaluated consecutive adults with and without HIV infection, naïve to H. pylori treatment, who underwent upper gastrointestinal endoscopy and had a positive H. pylori culture, with susceptibility testing available, between 2004 and 2015. Outpatient antibiotic consumption data were based on nationwide aggregated numbers. H. pylori was isolated from gastric biopsies of 3008/8321 patients, 181/477 (37.9%) were HIV‐positive and 2827/7844 (36.0%) HIV‐negative. Overall cohort mean prevalence of H. pylori primary antibiotic resistance was 11.1% for clarithromycin, 17.8% levofloxacin, and 39.4% metronidazole. The prevalence of H. pylori primary resistance was significantly higher for these three drugs in HIV‐positive individuals across the study period. Linear regression showed that the prevalence of clarithromycin and levofloxacin resistance correlated with the country aggregate daily dose consumption of macrolides and quinolones, respectively. Multivariable regression analysis showed that HIV infection is a strong independent risk factor for multiple H. pylori antibiotic resistance. In summary, HIV infection is a risk factor for carrying multi‐resistant H. pylori strains and this is correlated with antibiotic consumption. Empirical therapies should be avoided in HIV‐positive individuals. These data highlight the need to implement ongoing monitoring of H. pylori antimicrobial susceptibility among HIV‐positive individuals. The study is registered at ISRCTN registry, number 13466428: https://www.isrctn.com/ISRCTN13466428.
Keywords: anti‐bacterial agents, cohort studies, drug resistance, Helicobacter pylori, HIV, prevalence, risk factors
Primary antibiotic resistance was almost two‐fold more common among Helicobacter pylori strains isolated from HIV‐positive individuals over 12 years of observation: AIDS > AIDS‐free > HIV‐negative. This may be due to increased antibiotic consumptions and other factors. *p‐value after Bonferroni correction.

1. INTRODUCTION
Helicobacter pylori (H. pylori) and human immunodeficiency virus (HIV) infections are common worldwide, but the frequency of H. pylori infection among HIV‐positive individuals varies according to the region and population studied (Nevin et al., 2014). As an example, reported H. pylori infection prevalence rates are 23% and 51% among HIV‐positive individuals in Germany and Ghana, respectively (Eidt et al., 1995; Sarfo et al., 2015). Complications related to H. pylori infection, notably gastro‐duodenal ulcer, gastric adenocarcinoma, and mucosa‐associated lymphoid tissue (MALT) lymphoma, found in both HIV‐positive and HIV‐negative patients, could be avoided by treating H. pylori infection (Crowe, 2019). For decades, the standard of care for treating H. pylori infection has been triple therapy (TT) which combines a proton pump inhibitor (PPI) with two antibiotics (any two of clarithromycin [CLR], metronidazole [MET], amoxicillin [AMX], levofloxacin [LEV], or tetracycline [TET]), twice daily for at least 7–14 days (Malfertheiner et al., 2017; Thung et al., 2016). However, the failure rate for TT is rising in many parts of the world (Gisbert & Pajares, 2002; Glupczynski et al., 2001; Graham & Shiotani, 2008; Malfertheiner et al., 2017). The main reasons for this appear to be non‐compliance and antimicrobial resistance of H. pylori (Malfertheiner et al., 2002, 2017; Vakil & Megraud, 2007). Resistance to CLR has led to a 70% decline in TT eradication rates, while resistance to LEV and MET are responsible for 50% and 33% declines, respectively (Wong et al., 2003). The Maastricht V/Florence consensus conference has recommended that empirical TT containing CLR should be avoided when the prevalence threshold for CLR resistance is greater than or equal to 15% (Malfertheiner et al., 2017). Primary CLR resistance among adult patients is variable across countries. For example, CLR resistance rates were 5.6% in The Netherlands, 22% in Belgium, and 31.5% in Portugal in 2008–2009 (Thung et al., 2016). Helicobacter pylori antibiotic resistance rates are increasing globally and, at the same time, global antibiotic consumption increased by 36% between 2000 and 2010, mainly in developing countries (Thung et al., 2016).
In this regard, HIV‐positive individuals are a model of interest. Indeed, HIV, by chronically destroying CD4 T lymphocytes (CD4+ T), leads to a decline and impairment in immune function (Pantaleo, & Fauci, 1996). For this reason, HIV‐positive individuals are more susceptible to infections and, consequently, are exposed to antibiotic therapy for the treatment of acute infections as well as for chemoprevention against opportunistic infections (Panel on Opportunistic Infections in HIV‐Infected Adults & Adolescents, 2020). The extent to which this antibiotic exposure affects microbial susceptibility in HIV‐positive individuals is understudied. In a previous short‐term study from our center, we observed that H. pylori primary antibiotic‐resistant strains were more common among HIV‐positive compared to HIV‐negative individuals. Currently, there are few data available concerning recent levels and trends in H. pylori antibiotic resistance among HIV‐positive individuals (Gill et al., 2008). The goals of our study were to compare prevalence rates and long‐term trends in primary H. pylori antibiotic resistance among HIV‐positive and HIV‐negative individuals who underwent an upper gastrointestinal (GI) endoscopy for any medical reason in our hospital, to evaluate correlations with aggregate antibiotic consumption in the country, and to identify predictors for primary H. pylori antibiotic resistance among HIV‐positive individuals. This information may provide valuable data for decision‐making concerning the prescription of appropriate anti‐H. pylori drugs for H. pylori‐HIV‐co‐infected patients. The interim results of this work were published already in 2015 (Nkuize et al., 2015).
2. MATERIALS AND METHODS
This descriptive observational study was carried out at CHU Saint‐Pierre in Brussels, a general hospital that currently monitors more than 3000 HIV‐positive patients regularly and performs more than 3500 UGI endoscopies per annum.
We prospectively collected patient data in a registry from 1 January 2004 through 31 December 2015. Inclusion criteria were as follows: all consecutive HIV‐positive and HIV‐negative individuals, naïve to H. pylori treatment, aged ≥18 years of age that underwent upper GI endoscopy for any reason and for which H. pylori antimicrobial susceptibility test results were available. Exclusion criteria were as follows: denial or withdrawal of consent, coagulation disorders, and gastrectomy. Data collected on the day of inclusion were demographics (age, gender, and ethnicity); HIV status and HIV‐related parameters (duration of HIV infection, Center for Disease Control (CDC) stage, viral load, antiretroviral treatment, CD4 T lymphocyte cell count); and Toxoplasma gondii or Pneumocystis jiroveci chemoprevention or antimalarial drug use (including mefloquine, atovaquone, chloroquine, and primaquine) within 12 months before endoscopy because cross‐resistance has been described between trimethoprim‐sulfamethoxazole and sulfamides, and chloroquine and quinolones (Davidson et al., 2008; Gill et al., 2008). Bismuth compounds are rarely used in Belgium and were not recorded. The history of H. pylori treatment was obtained at the time of upper GI endoscopy, and at inclusion by anamnesis and, if not, from the patient's medical chart and the family medical doctor for all HIV‐positive and HIV‐negative individuals with antibiotic‐resistant strains of H. pylori. To take into account differences in the prevalence of antibiotic resistance between ethnicities and regions, region of origin was recorded, based on the patient's self‐identified ethnicity and geographic area of origin. The regions of origin were Eastern Europe, Western Europe, North Africa, Sub‐Saharan Africa, and Other, which included Latin and North America, Asia, and the Middle East. Similarly, a region of birth was classified following the same definition as a region of origin. Details are presented in Appendix 1.
Patients underwent upper GI endoscopy after fasting for 12 h or overnight. At least four gastric biopsy samples were taken (one from the angulus, one from the antrum, and two from the body). Samples were immediately transferred to Amies Agar Gel Collection and Transport Swabs (Copan Diagnostic Inc.) and placed in a refrigerator at −5 to +5°C before being sent within 24 h to the Department of Microbiology (Nkuize et al., 2015).
Susceptibility to CLR, MET, LEV, AMX, and TET was assessed under routine conditions using disk diffusion methods (Neo‐Sensitabs; Rosco, Taastrup, Denmark), and the minimum inhibitory concentration (MIC) determined by an agar dilution method. Isolates were classified as resistant with cut‐off values of ≥1 mg/L for CLR, >8 mg/L for MTZ, and >1 mg/L for LEV (Clinical & Laboratory Standards Institute, 2009; Miendje Deyi et al., 2011).
Data on outpatient antibiotic use were extracted from the database of the Agence Intermutualiste, which has compiled the health data of insured individuals for the full population in Belgium since 2002. Drug use data are registered following the Anatomical Therapeutic Chemical (ATC) classification. For this study, we first used ATC classification to select individuals with ambulatory consumption of antiretroviral (ARV) drugs (See details in Appendix 2). Patients were categorized according to their use of ARV (HIV‐positive subgroup) or no‐use of ARV (HIV‐negative subgroup); these subgroups were compared for macrolide (J01FA) and quinolone (J0MA) aggregated consumption expressed as defined daily dose per 1000 inhabitants per day/year for the whole insured Belgian population (Coenen et al., 2014) Metronidazole consumption and its correlation with MET resistance have not been studied given (i) there is currently some controversy regarding the reproducibility and benefit of the H. pylori metronidazole susceptibility test (Filipec Kanizaj et al., 2009; Malfertheiner et al., 2017), (b) it is taken by oral, rectal, or vaginal administration and, while oral dosing is consistent, rectal, or vaginal dose may vary.
2.1. Statistical analysis
Descriptive statistics are expressed as a median and interquartile range for continuous variables and as frequencies or proportions with 95% confidence intervals for categorical variables. Wilcoxon rank‐sum test was used for median comparisons. Fisher's exact test (2 groups) and Pearson's chi‐square test (>2 groups) were used for statistical analysis of frequency and proportion distributions. In univariable analysis, the strength of association was measured as odds ratio (OR) with a 95% confidence interval.
Risk factors for primary H. pylori resistance to one antibiotic and ≥two antibiotics in HIV‐positive patients and the whole cohort, respectively, were included in multivariable analysis (logistic regression) for any parameter with a p‐value < 0.1.
The following variables were included in the HIV‐positive subgroup model: age, sex, region of origin, region of birth, HIV duration, and treatment with antiretroviral or treatment with trimethoprim‐sulfamethoxazole, CDC stage, and CD4 nadir.
The following parameters were included in the whole cohort model: age, gender, ethnicity, country of birth, and HIV status.
To measure the period effect on H. pylori antibiotic resistance, the OR of frequency of H. pylori antibiotic resistance according to HIV status was determined within each period. The global effect of OR adjusted for the period was determined with the Mantel‐Hanszel method.
To study correlations between macrolide and quinolone consumption and primary H. pylori CLR and LEV resistance in the cohort, simple linear regression was used.
For the parameters studied, missing data were specified accordingly.
Analyses were performed using IBM SPSS Statistical software (v25 ‐ 08/2018 ‐ IBM Corporation). A bilateral p‐value of <0.05 was considered statistically significant.
3. RESULTS
The study population (Table 1) included 8321 patients (477 HIV‐positive and 7844 HIV‐negative), naïve for H. pylori treatment, and for whom an H. pylori culture was available between 2004 and 2015. Helicobacter pylori culture was positive in 3008 patients (36.1%): 181 (37.9%) HIV‐positive and 2827 (36.0%) HIV‐negative. See details in Appendix 4. The proportion of resistance to at least one antibiotic (CLR, LEV, or MET) was markedly greater in the HIV‐positive group (76.8%) than in the HIV‐negative group (52.4%) (OR = 3.01). A proportionally similar difference was observed for these three antibiotics analyzed separately (see Appendix 4). Data were incomplete in 127 individuals: 13/181(7.1%) and 114/2827 (4.0%) among HIV‐positive and HIV‐negative, respectively. When the analysis was restricted to individuals in the H. pylori‐positive subcohort for whom all three antibiotic tests were jointly available, proportionally similar differences and odds ratios were obtained.
TABLE 1.
Study population stratified according to HIV infection, Helicobacter pylori infection, and H. pylori susceptibility testing
| Parameters | HIV‐negative | HIV‐positive | Odds Ratio (95%CI) | Probability |
|---|---|---|---|---|
| Initial cohort: patients with H. pylori culture, total no. | 7844 | 477 | — | |
| H. pylori cohort: positive H. pylori culture and at least one antibiotic tested, no. (% of initial cohort) | 2827 (36.0%) | 181 (37.9%) | 1.09 (0.90–1.31) | 0.7 |
| Resistance to ≥1 antibiotic, no. (% of H. pylori + cohort) | 1481 (52.4%) | 139 (76.8%) | 3.01 (2.1–4.3) | <0.0001 |
| Clarithromycin, no./no. tests (% resistant) | 294/2720 (10.8%) | 29/176 (16.4%) | 1.62 (1.0–2.4) | 0.02 |
| Levofloxacin, no./no. tests (% resistant) | 480/2825 (16.9%) | 57/180 (31.6%) | 2.26 (1.6–3.1) | <0.0001 |
| Metronidazole, no./no. tests (% resistant) | 1083/2815 (38.4%) | 93/173 (54.9%) | 1.94 (1.4–2.6) | <0.0001 |
| H. pylori subcohort: subgroup of H. pylori + cohort with joint testing of clarithromycin, levofloxacin, and metronidazole, no. (% of initial cohort) | 2713 (34.6%) | 168 (35.2%) | 1.03 (0.8–1.2) | 0.7 |
| Resistance to ≥1 antibiotic, no. (% of H. pylori + subcohort) | 1367 (50.4%) | 126 (75.0%) | 2.95 (2.0–4.2) | <0.0001 |
| Clarithromycin, no./no. tests (% resistant) | 290/2713 (10.7%) | 26/168 (15.5%) | 1.52 (0.9–2.3) | 0.07 |
| Levofloxacin, no./no. tests (% resistant) | 438/2713 (16.1%) | 49/168 (29.2%) | 2.14 (1.5–3.0) | <0.0001 |
| Metronidazole, no./no. tests (% resistant) | 1025/2713 (37.8%) | 92/168 (54.8%) | 1.99 (1.4–2.7) | <0.0001 |
| >2 antibiotics, no. (% of HP + subcohort) | 345/2713 (12.7%) | 37 /168 (22.0) | 1.93 (1.3–2.8) | <0.0001 |
Figure 1 shows that the prevalence of H. pylori resistance to at least one of the three antibiotics (CLA, LEV, or MET) in HIV‐negative subjects was 50.4%. This proportion increased significantly to 71.3% in HIV‐positive AIDS‐free individuals and increased further to 92.6% in HIV‐positive AIDS individuals.
FIGURE 1.
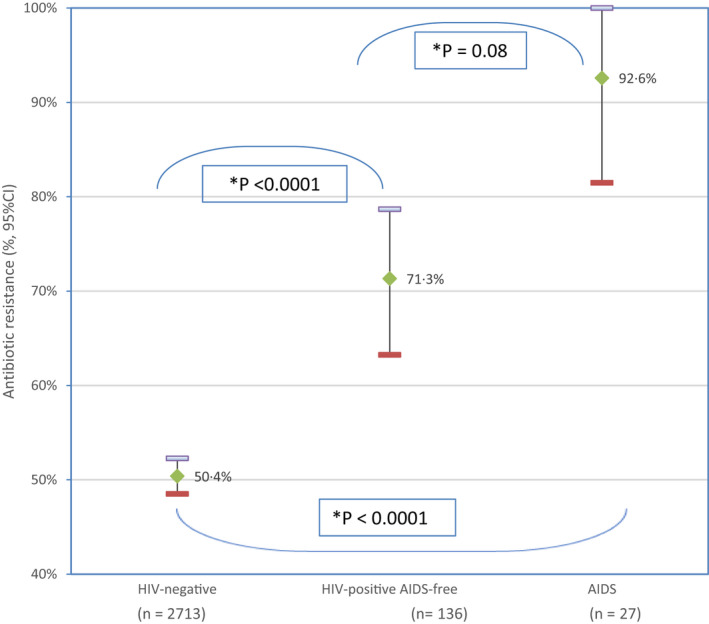
Prevalence and 95% confidence interval of primary Helicobacter pylori resistance to at least one antibiotic among HIV‐positive individuals with and without AIDS, and HIV‐uninfected patients. AIDS‐f, AIDS‐free. *p‐value after Bonferroni correction: p 1 = 0.08, p 2 < 0.0001, p 3 < 0.0001. The figure represents comparison according to AIDS status of the prevalence of H. pylori primary antibiotic resistance to at least one antibiotic among HIV‐negative and HIV‐positive individuals. The bars represent the statistic for each subgroup studied; the center representing the mean percentage, the top and bottom are the upper and lower boundary of the 95% confident interval. The frequency of H. pylori primary antibiotic resistance is higher in HIV‐positive individuals having AIDS than those who are AIDS‐free (P1), significantly higher than HIV‐negative individuals (P2). HIV‐positive AIDS‐free individuals carry significantly more H. pylori strains with primary antibiotic‐resistant than HIV‐negative individuals (P3)
Appendix 5 compares demographic and endoscopic data in both the HIV‐positive and HIV‐negative groups of the H. pylori‐positive cohort. HIV‐positive patients were significantly older and originated more frequently from sub‐Saharan Africa. The gender ratio was similar in both groups. Oesophageal candidiasis was more frequent (OR = 22.3) while gastric and duodenal ulcers were less frequent in the HIV‐positive group (OR = 0.52 and 0.50). Appendix 6 shows the demographics of HIV‐positive patients.
Table 2 compares the proportion of combined resistance to two or three antibiotics (CLR ± LEV ± MET) in the HIV‐positive and HIV‐negative groups. The proportion of resistance to any combination of two antibiotics was significantly greater in HIV‐positive versus HIV‐negative individuals (CLR‐MET: 8.2% vs. 4.7%, LEV‐MET: 15.1% vs. 8.5%, CLR‐LEV: 5.7% vs. 2.9%) with similar odds ratios of 1.82, 1.90, and 1.97, respectively. Triple antibiotic resistance was observed in 2.3% versus 1.5% (not significant). Finally, quadruple resistance was observed in 0.5% versus 0.0%.
TABLE 2.
Helicobacter pylori primary resistance to two or more antibiotics in HIV‐positive and ‐negative individuals
| Antibiotic resistance |
HIV‐negative n (%) |
HIV‐positive OR 95% CI n (%) |
|---|---|---|
| CLR‐MET | 128/2714 (4.7) | 14/169 (8.2) 1.82 (1.0–3.2)* |
| LEV‐MET | 241/2813 (8.5) | 26/172 (15.1) 1.90 (1.2–2.9)** |
| CLR‐LEV | 81/2719 (2.9) | 10/175 (5.7) 1.97 (1.0–3.8)*** |
| CLR‐LEV‐MET | 41/2713 (1.5) | 4/168 (2.3) 1.58 (0.5–4.4)**** |
| Two or more | 345/2713 (12.7) | 37/168 (22.0) 1.93 (1.3–2.8)***** |
p‐value 0.04.
p‐value 0.005.
p‐value 0.06.
p‐value 0.3.
p‐value 0.001.
Univariable analysis of the characteristics of the HIV‐positive group in relation to H. pylori resistance to antibiotics (Table 3) showed that CDC stage C had an important independent effect (OR = 5.02), that persisted after adjustment for all other potential confounding variables in logistic regression (OR = 4.34). The effect of HIV duration in univariable analysis (OR = 0.53) (p = 0.09) remained at the same level of significance in multivariable analysis (adjusted OR = 0.56) (p = 0.10). The other characteristics had no significant effect in univariable analysis (p > 0.10 to be included in the logistic analysis).
TABLE 3.
Risk factors for primary H. pylori antibiotic resistance in 168 HIV‐positive individuals
| Parameters | Category (number) | Number (%) | Unadj. OR, 95% CI | p‐value | Adj OR | 95% CI | p‐value |
|---|---|---|---|---|---|---|---|
|
Age (years) (n = 168) |
<50 versus >50 (n = 135) (n = 33) |
100 (74.0) versus 26 (78.7) | 0.6 | ||||
|
Gender (n = 168) |
F versus M (n = 83) (n = 85) |
66 (79.5) versus 60 (70.5) | 0.2 | ||||
|
Region of origin (n = 163) |
not‐SS versus SS (n = 66) (n = 97) |
50 (75.7) versus 72 (74.2) | 0.8 | ||||
|
Region of birth (n = 168) |
not‐SS versus SS (n = 71) (n = 97) |
53 (74.6) versus 73 (75.2) | 1.0 | ||||
|
HIV duration (years) (n = 153) |
<10 versus >10 (n = 102) (n = 51) |
79 (77.4) versus 33 (64.7) | 0.53 (0.2–1.1) | 0.09 | 1.79 | 0.8–3.7 | 0.1 |
|
ARV treat. duration (years) (n = 130) |
<10 versus >10 (n = 102) (n = 42) |
78 (76.4) versus 20 (71.4) | 0.6 | ||||
|
On ARV (n = 163) |
yes versus no (n = 121) (n = 42) |
91 (75.2) versus 31 (73.8) | 0.8 | ||||
|
On TMT‐SFX (n = 168) |
No versus yes (n = 123) (n = 45) |
90 (71.3) versus 36 (80.0) | 0.4 | ||||
|
CDC stage C (n = 163) |
No versus Yes (n = 136) (n = 27) |
97 (71.3) versus 25 (92.5) | 5.02 (1.1–22.2) | 0.02 | 0.23 | 0.06–0.9 | 0.04 |
|
CD4 nadir at HIV diagnosis (n = 146) |
>200 versus <200 (n = 91) (n = 55) |
67 (73.6) versus 42 (76.3) | 0.8 |
Abbreviations: 95% CI, 95% confidence interval; Adj OR, adjusted odds ratio; ARV antiretroviral; CDC, centers for disease control; F, female; M, male; not‐SS, not‐sub‐saharan; SS, sub‐Saharan; TMT‐SFX trimethoprim‐sulfamethoxazole; Unadj. OD, Unadjusted odds ratio.
Appendix 7 shows that, in univariable analysis, the OR of H. pylori resistance to at least one antibiotic in HIV‐positive versus HIV‐negative individuals was significantly associated with age, gender, ethnicity, country of birth, and HIV and these associations all remained significant after adjustment for confounding variables when logistic regression was performed. See Appendix 8 (‐A, ‐B, and ‐C) for data variations according to ethnicity, gender, and age.
Figure 2 shows the percentage of individuals carrying H. pylori strains that have primary resistance to at least one antibiotic in HIV‐negative and HIV‐positive individuals across four consecutive 3‐year periods from 2004 until 2015. Across each of the four periods, the percentages of H. pylori resistance to at least one antibiotic were statistically significantly greater in HIV‐positive individuals than in HIV‐negative patients; Fisher's exact test: p < 0.05. When all four periods were analyzed, the statistical significance by Pearson's chi‐square test was p < 0.0001. In HIV‐negative individuals, the percentages of variation of H. pylori primary resistance to at least one antibiotic during the four periods (21% and 24%) were not statistically significant (Pearson's chi‐square, p > 0.20). By contrast, in HIV‐positive individuals, these percentage variation differences were statistically significant across the four periods (Pearson's chi‐square, p = 0.019).
FIGURE 2.
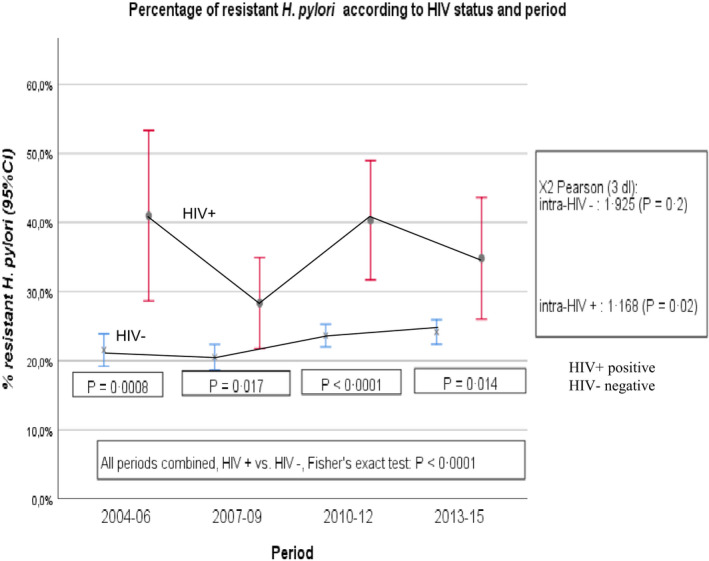
Evolution over time of the cohort prevalence and 95% confidence interval of primary Helicobacter pylori antibiotic resistance according to HIV status from 2004 to 2015
In a complementary analysis by linear regression for HIV‐positive individuals to define the variance due to the period, linear regression in HIV‐positive individuals was based on a percentage of H. pylori resistance to antibiotics (Y‐axis), and the year of observation (X‐axis). If we considered 1/1/2004 as the intercept (zero time), the linear regression equation was as follows: Y = −0.0022X + 0.3741.
The R 2 coefficient of Pearson's determinations (0.0204) and the slope coefficient (−0.0022±…) were not significant (p > 0.10). The slight decrease in the percentage of H. pylori resistance (−2.2% over 10 years) was not significant. The variation of the percentage of H. pylori resistance to an antibiotic in HIV‐positive patients is due to the absence of homogeneity of percentages (residual random variation), and not to a period effect. Supplemental data are presented in Appendix 9.
Figure 3 shows the relationship between H. pylori resistance in our cohort of HIV‐negative and HIV‐positive individuals as a dependent variable and defined daily dose (DDD) per 1000 inhabitants of antibiotics in the Belgian population and the Belgian HIV population as independent variables for both fluoroquinolone and macrolide antibiotic classes. For fluoroquinolones (Figure 3‐a), mean comparison by Student's t‐test was 9.122 (p < 0.0001) for DDD and 3.974 (p = 0.0003) for percent H. pylori resistance. For macrolides (Figure 3‐b), t‐test was 9.694 (p < 0.0001) for DDD and 3.401 (p = 0.001) for percent H. pylori resistance. No overlap of values was observed for DDD between the Belgian population and the Belgian HIV population. Slope coefficients of the linear regression between percent H. pylori resistance and DDD (Figure 3‐a,b) were significant for both antibiotics: fluoroquinolones: p = 0.002; macrolides: p < 0.0001. Details are presented in Appendix 10 (10‐A and 10‐B).
FIGURE 3.
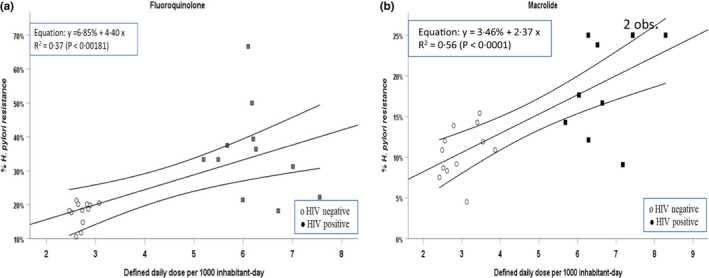
Yearly aggregated data for percent Helicobacter pylori resistance to antibiotics in local hospital HIV‐negative (white circles) and HIV‐positive individuals (gray and black squares) in relation to yearly aggregated data for defined daily dose (DDD) in the Belgian HIV‐negative and HIV‐positive population for fluoroquinolones (a) and macrolides (b). Each point represents yearly aggregated DDD per 1000 inhabitants from 2004 to 2015. Missing data for percent H. pylori resistance: fluoroquinolones [local hospital HIV‐positive individuals: 2012]; macrolides [local hospital HIV‐positive individuals: 2005 and 2007]. The linear regression model with 95% confidence intervals is illustrated
Appendix 3 shows the ecologic association of aggregate variables of national antibiotic consumption from 2004 to 2015 in HIV‐positive patients and HIV‐negative individuals. The evolution of yearly antibiotic consumption (fluoroquinolone, macrolide) for the Belgian HIV‐negative and ‐positive population is illustrated. For both classes of antibiotics, the DDD in the Belgian population was much higher in the HIV‐positive population than in the HIV‐negative Belgian population. Details are presented in Appendix 10 (10‐A and 10‐B).
4. DISCUSSION
This study provides insight into the prevalence of, and risk factors for, primary antibiotic resistance among H. pylori strains infecting HIV patients as well as correlations with aggregate antibiotic consumption, and the evolution of these over time. Primary antibiotic resistance was almost twofold more common among H. pylori strains isolated from HIV‐positive individuals over 12 years of observation, indicating that H. pylori strains isolated from HIV‐positive individuals can be classified as “usually antimicrobial‐resistant,” according to European Medicines Agency (EMA) recommendations and in agreement with our preliminary data (Malfertheiner et al., 2017; Nkuize et al., 2015). They were inconsistently susceptible to LEV, “usually resistant” to MET, and inconsistently susceptible to CLA. One major reason for this increased prevalence in primary H. pylori antibiotic resistance in HIV‐positive individuals is probably increased antibiotic exposure during antibiotherapy (Bell et al., 2014; Krucke et al., 2009; Megraud et al., 2013; Ngalani et al., 2019; Sarfo et al., 2015). Hence, we demonstrate (Appendix 10 and Appendix 3) that aggregate macrolide and quinolone consumption was two times more common among HIV‐positive individuals than their HIV‐negative counterparts from the national database of outpatient antibiotic use in Belgium during the period from 2004 to 2015. Furthermore, we found, for the whole cohort (Figure 3, ‐a and ‐b), a strong correlation between the prevalence of H. pylori primary resistance to clarithromycin and levofloxacin and the consumption of macrolides and quinolones, respectively, within the country. These data extend the known association between H. pylori primary resistance and antibiotic consumption in European countries to HIV‐positive individuals (Malfertheiner et al., 2017; Megraud et al., 2013). In previous studies, as well as in the Maastricht V/Florence consensus conference, there was no analysis of HIV‐positive individuals (Malfertheiner et al., 2017; Megraud et al., 2013).
In line with the above findings, we suggest that, in HIV‐positive individuals, CLR‐, LEV‐, and MET‐containing empirical first‐line and rescue treatments should be avoided because their resistance rate is over the threshold proposed by the Maastricht V/Florence Consensus Report. It has also been shown that primary LEV, CLA, and MET resistance undermines the efficacy of LEV‐, CLA‐, and MET‐containing triple therapy against H. pylori by 52%, 30%, and 70%, respectively. In this situation, with H. pylori primary resistance to multiple antibiotics, bismuth‐containing therapy has been proposed as the best empiric alternative treatment for the general population, although it is not widely available. Each of the above suggestions on LEV, MET, and CLR is well supported by studies in HIV‐negative patients. The magnitude of the impact of this resistance among HIV‐positive individuals is expected to be similar to that found among HIV‐negative patients but this hypothesis still needs to be confirmed (Bogaerts et al., 2006; Chey et al., 2017; Malfertheiner et al., 2017; Filipec Kanizaj et al., 2009; Glupczynski et al., 2001; Kouitcheu Mabeku et al., 2019; Malfertheiner et al., 2017; Raymond et al., 2010; Sugano et al., 2015; Thung et al., 2016; Wong et al., 2003). Our team is evaluating bismuth‐containing therapy in a subgroup of H. pylori ‐HIV co‐infected patients (In press).
Primary AMX and TET resistance were uncommon, <1% for each, in HIV‐positive as well as in HIV‐negative individuals, as shown in other European studies (Thung et al., 2016). In Africa and Latin America, both AMX and TET resistance seem to be more frequent in the general population. Data for H. pylori antimicrobial resistance among HIV‐positive individuals from other regions to compare with our findings are lacking (Kouitcheu Mabeku et al., 2019; Thung et al., 2016). Even so, HIV status can be added to the region or country of birth, ethnicity, age, and gender, which are known independent risk factors of H. pylori antibiotic resistance in the general population (Miendje Deyi et al., 2011; Thung et al., 2016).
Over the entire study period, there was a stable and persistent higher overall prevalence of primary H. pylori resistance among HIV‐positive individuals compared to HIV‐negative individuals (Figure 2; Appendix 9). The reason for this could be, in part, as outlined above, the persistent need for, or consumption of, antibiotics by HIV‐positive individuals. For instance, we demonstrate that aggregate antibiotic consumption remained high throughout the study periods among HIV‐positive individuals, but as a consequence of enormous therapeutic progress in HIV‐infection management (Saag et al., 2018), it started to decrease (Appendix 3) in the more recent period compared with the earlier period.
The reason why HIV‐positive AIDS‐free patients are also at risk of primary resistance is unclear (Figure 1). One hypothesis is that HIV and H. pylori generate oxidative stress within gastric mucosa (Butcher et al., 2017; Conway et al., 2014; Mouery et al., 2006; O'Rourke et al., 2003; Wells & Gaynor, 2006) and that this oxidative stress might be involved in the emergence of H. pylori antibiotic resistance (e.g., via DNA mutations or DNA transformation) (Lin et al., 2009). This might also explain why H. pylori infection is less frequently found in AIDS patients (e.g., by inducing a lower bacterial load or by triggering more coccoid forms) (Connolly et al., 2007; Lin et al., 2009; Roe et al., 1999). There are limitations to our study. First, the size of the ethnic subgroups was not balanced. Second, molecularly based methods rather than subculture could have provided additional information, such as that for bacterial load and strain diversity (Malfertheiner et al., 2017; Thung et al., 2016). The emerging test for H. pylori is costly, often limited to CLR and LEV resistance, and is not currently used in daily practice in many countries. Finally, around twenty percent of people living with HIV did not receive ARV in the early HAART era, and, therefore, had not been selected for nationwide antibiotic consumption analysis. Nevertheless, this weakness is overcome by the very large number of patients analyzed.
The strengths of the study include the long period of observation of an adult population from diverse ethnic origins, residing in the same geographic region, and the fact that our findings may have a direct clinical impact. Current recommendations state that AST should be done in case of second‐line treatment failure (Malfertheiner et al., 2017; Sugano et al., 2015). By contrast, given the finding of a high prevalence of multiple primary resistances to antibiotics among H. pylori strains isolated in HIV‐positive individuals in our setting, we would recommend performing an H. pylori diagnosis with an antimicrobial susceptibility test by culture or polymerase chain reaction from gastric biopsy, or, if available, by a fecal bacterial DNA investigation (THD fecal test) to guide first‐line H. pylori treatment in HIV‐positive individuals (Malfertheiner et al., 2017; Pero et al., 2019; Sugano et al., 2015). This may help to avoid the use of several H. pylori treatment courses and subsequent increases in resistance and dysbiosis of gut microbiota (Malfertheiner et al., 2017). Both HIV and H. pylori infections already impair the gut microbiota and unnecessary treatment increases life‐threatening primary antibiotic (AMX, CLR, and LEV) resistance for bacteria other than H. pylori (Iannone et al., 2018; Malfertheiner et al., 2017; Pero et al., 2019; Saxena et al., 2012). Furthermore, dysbiosis is associated with the development of numerous diseases, including neoplasia (Ferreira et al., 2018). Taken together, enhancing awareness of the medical community to the issue of H. pylori antibiotic resistance among HIV‐positive individuals is crucial and a subject of public health interest. This study also highlights that there is a need for guidelines and consensus focused on H. pylori care in HIV‐positive individuals.
In conclusion, our study demonstrates that HIV infection is a risk factor for carrying H. pylori strains with multiple antimicrobial resistances that persist over time, and correlate, in part, with antibiotic consumption in HIV‐positive individuals while other mechanisms might also be involved. Helicobacter pylori diagnostics using tools that also provide AST from gastric samples should be implemented to guide first‐line H. pylori infection treatment by avoiding empirical therapies in HIV‐positive individuals. Locally establishing the prevalence of antimicrobial resistance of H. pylori strains in HIV‐positive individuals may provide useful information. The distinction between individuals with and without HIV infection is mandatory in future epidemiological studies and consensus on H. pylori antibiotic resistance in a given population.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Marcel Nkuize: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Funding acquisition (equal); Investigation (lead); Methodology (lead); Project administration (lead); Resources (lead); Software (equal); Supervision (lead); Validation (lead); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Jean Vanderpas: Data curation (equal); Formal analysis (lead); Methodology (supporting); Software (equal); Supervision (supporting); Validation (equal); Visualization (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). Michel Buset: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Methodology (supporting); Project administration (supporting); Supervision (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Maria Gomez‐Galdon: Data curation (equal); Investigation (supporting); Project administration (supporting); Software (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (equal). Marc Delforge: Data curation (equal); Formal analysis (equal); Methodology (supporting); Resources (equal); Software (equal); Validation (equal); Visualization (equal); Writing‐review & editing (supporting). Véronique Yvette Miendje‐Deyi: Data curation (supporting); Investigation (equal); Project administration (supporting); Resources (equal); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Vinciane Muls: Data curation (supporting); Formal analysis (supporting); Resources (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐review & editing (supporting). Stephane De Wit: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (supporting); Methodology (equal); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (equal); Validation (supporting); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
ETHICS STATEMENT
The study was conducted following the Declaration of Helsinki and was approved by our local hospital ethics committee. All procedures described in the study were performed for routine medical purposes. Oral or written consent was obtained, following the evolution of the law throughout the study period.
ACKNOWLEDGMENTS
We acknowledge our nurses of the endoscopy unit and gastroenterologist colleagues who helped us throughout the study. The authors acknowledge the contribution of a medical writer, Sandy Field, Ph.D., for language editing and formatting of this document. This study was partly supported by a grant from Fondation de Recherche en Pathologie Digestive ASBL (non‐profit organization) for data collection.
APPENDIX 1. Ethnicity
Eastern European countries included Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and Northern Ireland.
Western European countries included Albania, Armenia, Belarus, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Estonia, Georgia, Hungary, Kosovo, Latvia, Lithuania, Macedonia, Moldova, Montenegro, Poland, Romania, Russia, Serbia, Slovakia, Slovenia, and Ukraine.
Sub‐Saharan African countries included Angola, Benin, Burkina Faso, Burundi, Cameroon, The Central African Republic, Chad, Republic of the Congo, The Democratic Republic of the Congo, Cote d'Ivoire, Djibouti, Equatorial Guinea, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea‐Bissau, Kenya, Liberia, Madagascar, Mali, Mauritania, Mauritius, Mozambique, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, South Africa, Sudan, Tanzania, Togo, Uganda, and Zimbabwe.
North African countries included Algeria, Libya, Morocco, Tunisia, Egypt, Mauritania, and Somalia.
Other countries included Asian countries (Bangladesh, Cambodia, China, India, Indonesia, Malaysia, Mongolia, Nepal, Philippines, Sri Lanka, Thailand, and Vietnam), Middle Eastern countries included (Afghanistan, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Pakistan, Qatar, Saudi Arabia, Syria, and Turkey), and Latin and North American countries (Argentina, Brazil, Chile, Colombia, Cuba, Dominican Republic, Ecuador, Mexico, Paraguay, Peru, Uruguay, and Venezuela, and Canada, the United States of America, and Haïti).
APPENDIX 2. ATC codes
The ATC classifications used to select individuals with ambulatory prescriptions of antiretrovirals ["J05AE," "J05AF," "J05AG," "J05AR," and "J05AX," and excluding CNK: 3054137, 1411354, 1411362, 767657, 776070, 3054137, and "J05AE11," "J05AE12," "J05AE14," "J05AF10," "J05AF08," "J05AX02," "J05AX05," "J05AX15," "J05AX30," as well as 5AF11 and 5AF12, which are not commercialized in Belgium].
The ATC classifications were used to select individuals with ambulatory prescriptions of antibiotics [including J01FA (macrolides), J01FA09 (CLA), and J01 M (quinolones), and J01MA02 (ciprofloxacin)].
APPENDIX 3.
FIGURE A1.
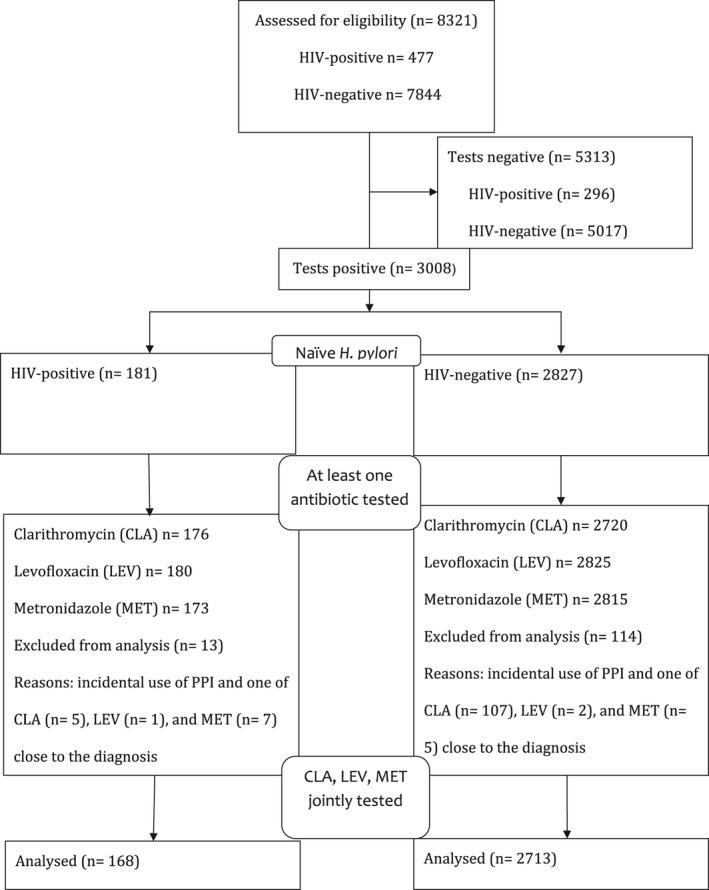
Yearly aggregated data for antibiotics consumption in HIV‐negative and HIV‐positive individuals. Antibiotic consumption as defined daily dose (DDD) in the Belgian HIV‐negative and HIV‐positive population for fluoroquinolones and macrolides. Each point represents yearly aggregated antibiotic consumption per 1000 inhabitants from 2004 to 2016
APPENDIX 4.
FIGURE A2.
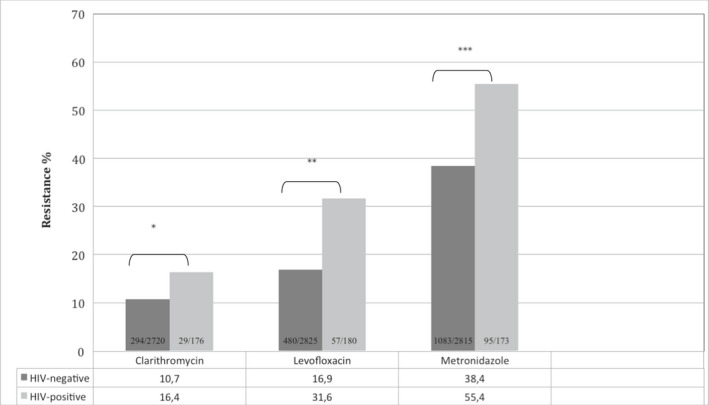
Cohort prevalence of H. pylori primary resistance to clarithromycin, levofloxacin, and metronidazole according to HIV status from 2004 to 2015. *p = 0.02, odds ratio and 95% confidence interval 1.62 (1.0–2.4). **p < 0.0001, odds ratio and 95% confidence interval 2.26 (1.6–3.1). ***p < 0.0001 odds ratio and 95% confidence interval 1.94 (1.4–2.6).
APPENDIX 5.
TABLE A1.
Cohort demographics according to HIV status
| Parameters | Overall | HIV‐negative | HIV‐positive | 95% CI | p‐value |
|---|---|---|---|---|---|
| (n = 3008) | (n = 2827) | (n = 181) | |||
| Age median | 38 | 38 | 41.3 | — | 0.002 |
| (Q1–Q3) | 28.0–48.0 | 28.0–48.0 | 33.0–49.0 | ||
| Gender | |||||
| Female | 1672 (55.5) | 1581 (55.9) | 91 (50.2) | 0.1 | |
| Male | 1336 (44.4) | 1246 (44.0) | 90 (49.7) | ||
| Region of origin | |||||
| Western Europe | 800 (26.6) | 757 (26.8) | 43 (23.7) | <0.0001 | |
| Eastern Europe | 244 (8.1) | 241 (8.5) | 3 (1.6) | ||
| North Africa | 929 (30.9) | 914 (32.4) | 15 (8.2) | ||
| Sub‐Saharan Africa | 665 (22.1) | 559 (19.8) | 106 (58.5) | ||
| Other | 363 (12.0) | 349 (12.3) | 14 (7.7) | ||
| Unknown | 7 (0.2) | 7 (0.2) | 0 (0.0) | ||
| Region of birth | |||||
| Not‐SS | 2343 (77.9) | 2268 (80.2) | 75 (41.4) | 5.73 (4.2–7.8) | <0.0001 |
| SS | 665 (22.1) | 559 (19.7) | 106 (58.5) | ||
| Endoscopic findings | n = 795 | n = 627 | n = 168 | ||
| Oesophageal candidiasis | 25 | 4 (0.6) | 21 (12.5) | 22.3 (7.5–66) | <0.0001 |
| Gastric ulcer | 128 | 111 (17.7) | 17 (10.1) | 0.52 (0.3–0.9) | 0.01 |
| Duodenal ulcer | 139 | 121 (19.3) | 18 (10.7) | 0.50 (0.2–0.8) | 0.008 |
APPENDIX 6.
TABLE A2.
Characteristics of HIV‐positive individuals
|
Parameters Age at HIV diagnosis (years) 32.0 (27.2–38.5) (median) (Q1–Q3) HIV duration (years) 6.5 (2.0–11.8) (median) (Q1–Q3) Ethnicity Not Sub‐Saharan 66 (40.4) Sub‐Saharan 97 (59.6) Risk factor n (%) Men sex men 41 (24.4) Heterosexual 103 (61.3) PWID 7 (4.1) Other 17 (10.2) CD4+ T count (cells/µl) Median (Q1–Q3) 482.0 (322.0–648.0) >350 110 (65.4) <350 42 (25.0) Unknown 16 (9.5) Nadir (Q1–Q3) 229.0 (134.0–357.0) Viral load (Q1–Q3) (copies/ml) 50 (20.−1110.0) CDC stage n (%) Stage C 27 (16.5) Not stage C 136 (83.4) Unknown 5 |
Abbreviations: PWID, people who inject drugs; Q1–Q3, first–third interquartile.
APPENDIX 7.
TABLE A3.
Cohort (n = 2881) baseline risk factors for carrying primary Helicobacter pylori strains resistant to two or more antibiotics
| Parameters | Category, number | Number (%) | Unadj. OR, 95% CI | p‐value | Adj. OR | 95% CI | p‐value |
|---|---|---|---|---|---|---|---|
| Age |
<50 versus >50 n = 2267 n = 614 |
262 (11.5) versus 120 (19.5) |
1.85 (1.4–2.3) |
<0.0001 | 2.06 | 1.6–2.6 | <0.0001 |
| Gender |
Female versus Male n = 1595 n = 1286 |
251 (15.7) versus 131 (10.1) |
0.60 (0.4–0.7) |
<0.0001 | 0.6 | 0.4–0.7 | <0.0001 |
| Region of origin | |||||||
| Western Europe | — | <0.0001 | 0.52 | 0.4–0.6 | <0.0001 | ||
| (n = 764) | 85 (11.1) | ||||||
| Eastern Europe (n = 238) | 35 (14.7) | ||||||
| North Africa (n = 889) | 77 (8.6) | ||||||
| Sub‐Saharan Africa (n = 637) | 130 (20.4) | ||||||
| Other | |||||||
| (n = 346) | 54 (15.6) | ||||||
| Region of birth | not‐SS versus SS | 252 (11.2) versus 130 (20.4) | 2.20 | <0.0001 | 1.97 | 1.0–2.3 | <0.0001 |
| n = 2244 n = 637 | (1.6–2.5) | ||||||
| HIV status | Neg versus Pos | 345 (12.7) versus 37 (22.0) | 1.93 | 0.001 | 1.57 | 1.0–2.3 | 0.02 |
| n = 2713 n = 168 | (1.3–2.8) | ||||||
Abbreviations: 95% CI, 95% confidence interval; Adj OR, adjusted odds ratio; Adj, adjusted; CI, confidence interval; Neg negative; not‐SS, not‐sub‐Saharan; OR, odds ratio; Pos positive; SS, sub‐Saharan; Unadj. OD, Unadjusted odd ratio.
APPENDIX 8.
TABLE A4.
Prevalence of Helicobacter pylori primary clarithromycin (8A), levofloxacin (8B), or metronidazole (8C) resistance according to HIV status, age, gender, and ethnicity
| Parameters | HIV‐negative | HIV‐positive | OR, 95% CI | p‐value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| 8A. Clarithromycin resistance a | ||||
| Age | 0.04 | |||
| <50 years | 274/2170 (9.8) | 22/140 (15.7) | 1.70 (1.0–2.7) | 0.4 |
| >50 years | 79/549 (14.3) | 7/36 (19.4) | — | |
| Gender | ||||
| Female | 191/1517 (12.5) | 12/87 (13.7) | — | 0.7 |
| Male | 102/1202 (8.4) | 17/89 (19.1) | 2.54 (1.4–4.4) | 0.003 |
| Ethnicity | ||||
| East European | 22/236 (9.3) | 1/3 (33.3) | — | 0.2 |
| West European | 97/725 (13.3) | 12/42 (28.5) | 2.59 (1.2–5.2) | 0.01 |
| Maghrebian | 92/876 (10.5) | 1/15 (6.6) | — | 1 |
| Sub‐Saharan | 49/543 (9.4) | 11/102 (10.7) | — | 0.5 |
| Other | 32/332 (9.6) | 4/14 (28.5) | 3.75 (1.1–12.6) | 0.04 |
| 8B. Levofloxacin resistance a | ||||
| Age | ||||
| <50 years | 320/2242 (14.2) | 41/142 (28.8) | 2.43 (1.6–3.5) | <0.0001 |
| >50 years | 160/583 (27.4) | 16/38 (42.1) | 1.92 (0.9–3.7) | 0.06 |
| Gender | ||||
| Female | 305/1580 (19.3) | 25/91 (27.4) | 1.58 (0.9–2.5) | 0.07 |
| Male | 175/1245 (14.0) | 32/89 (35.9) | 3.43 (2.1–5.4) | <0.0001 |
| Ethnicity | ||||
| East European | 34/241 (14.1) | 1/3 (33.3) | — | 0.3 |
| West European | 132/757 (17.4) | 13/43 (30.2) | 2.05 (1.0–4.0) | 0.04 |
| Maghrebian | 124/913 (13.5) | 4/14 (28.5) | — | 0.1 |
| Sub‐Saharan | 125/559 (22.3) | 36/106 (33.9) | 1.70 (1.0–2.6) | 0.01 |
| Other | 65/348 (18.6) | 3/14 (21.4) | — | 0.7 |
| 8C. Metronidazole resistance a | ||||
| Age | ||||
| <50 years | 855/2233 (38.2) | 78/136 (57.6) | 2.16 (1.5–3.0) | <0.0001 |
| >50 years | 226/582 (38.8) | 18/37 (48.6) | — | 0.2 |
| Gender | ||||
| Female | 635/1575 (40.3) | 56/86 (65.1) | 2.76 (1.7–4.3) | <0.0001 |
| Male | 446/1240 (35.9) | 40/87 (45.9) | 1.51 (0.9–2.3) | 0.06 |
| Ethnicity | ||||
| East European | 108/241 (44.8) | 1/2 (50.0) | — | 1 |
| West European | 227/754 (30.1) | 17/42 (40.4) | — | 0.1 |
| Maghrebian | 232/911 (25.4) | 8/15 (53.3) | 3.3 (1.1–9.3) | 0.01 |
| Sub‐Saharan | 318/555 (57.3) | 62/100 (62.0) | — | 0.4 |
| Other | 194/347 (55.9) | 8/14 (57.1) | — | 1 |
Abbreviations: 95% CI, 95% confidence interval; N, number; OR, odds ratio.
For the parameters age, gender, and ethnicity, given that there is no homogeneity of odds ratios between these parameters, the adjusted Mantel‐Haenszel odds ratio cannot be used.
APPENDIX 9.
TABLE A5.
Data for the study of the evolution over time of the Helicobacter pylori primary antibiotic resistance according to HIV status
| Parameter | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | p‐value |
|---|---|---|---|---|---|
| HIV‐negative | |||||
| (n) | 253/1175 | 378/1845 | 623/2637 | 528/2187 | 0.1 |
| Mean, 95% CI | 21.5 (19.2–23.9) | 20.5 (18.6–22.3) | 23.6 (22.0–25.3) | 24.1 (22.3–25.9) | |
| HIV‐positive | |||||
| (n) | 25/61 | 51/180 | 50/124 | 39/112 | 0.01 |
| Mean, 95% CI | 41.0 (28.6–53.3) | 28.3 (21.7–34.9) | 40.3 (31.7–49.0) | 34.8 (26.0–43.6) | |
| p‐value | 0.0008 | 0.01 | 0.00003 | 0.01 | |
APPENDIX 10.
TABLE A6.
Data for fluoroquinolone and macrolide consumption in the Belgian population from 2004 to 2016
| Year | DDD 1000 inhabitants‐year in the Belgian general population without HIV infection | DDD 1000 inhabitants‐year in the Belgian population living with HIV infection |
|---|---|---|
| 10‐A Fluoroquinolones | ||
| 2004 | 2.70 | 7.55 |
| 2005 | 2.61 | 6.10 |
| 2006 | 2.60 | 5.49 |
| 2007 | 2.50 | 6.71 |
| 2008 | 2.46 | 7.00 |
| 2009 | 2.73 | 6.20 |
| 2010 | 2.85 | 5.99 |
| 2011 | 2.89 | 6.17 |
| 2012 | 3.07 | 6.25 |
| 2013 | 2.72 | 5.67 |
| 2014 | 2.64 | 5.19 |
| 2015 | 2.82 | 5.70 |
| 2016 | 2.67 | 5.33 |
| 10‐B Macrolides | ||
| 2004 | 2.41 | 7.43 |
| 2005 | 2.51 | 7.76 |
| 2006 | 2.48 | 8.28 |
| 2007 | 2.61 | 7.31 |
| 2008 | 2.56 | 7.43 |
| 2009 | 2.86 | 6.28 |
| 2010 | 2.78 | 5.68 |
| 2011 | 3.12 | 6.03 |
| 2012 | 3.54 | 7.17 |
| 2013 | 3.39 | 6.27 |
| 2014 | 3.46 | 6.51 |
| 2015 | 3.86 | 6.64 |
| 2016 | 3.88 | 6.94 |
Abbreviation: DDD, defined daily dose per.
APPENDIX 11.
FIGURE A11.
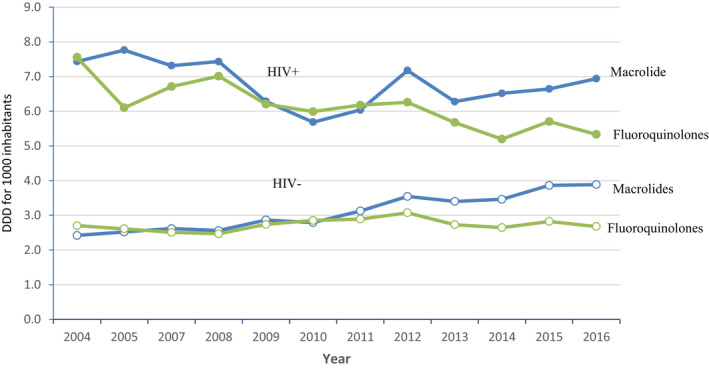
Fluoroquinolones and macrolides consumption, according to HIV status, in Belgium from 2004 until 2016
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article. Antibiotic consumption data analyzed during this study are the property of the Agence InterMutualiste (www.aim‐ima.be). The study is registered at ISRCTN registry, number 13466428: https://www.isrctn.com/ISRCTN13466428
REFERENCES
- Bell, B. G. , Schellevis, F. , Stobbering, E. , Goossens, H. , & Pringle, M. (2014). A systematic review and meta‐analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infectious Diseases, 14, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaerts, P. , Berhin, C. , Nizet, H. , & Glupczynski, Y. (2006). Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter, 11(5), 441–445. [DOI] [PubMed] [Google Scholar]
- Butcher, L. D. , Den Hartog, G. , Ernest, P. B. , & Crowe, S. E. (2017). Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cellular and Molecular Gastroenterology and Hepatology, 3(3), 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey, W. D. , Leontiadis, G. I. , Howden, C. W. , & Moss, S. F. (2017). ACG clinical guideline: Treatment of Helicobacter pylori infection. American Journal of Gastroenterology, 112(2), 212–239. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2009). Performance standards for antimicrobial susceptibility testing: 19th informational supplement (Vol. 29, no. 3, M100‐S19). Wayne, PA. Clinical and Laboratory Standards Institute. [Google Scholar]
- Coenen, S. , Gielen, B. , Blommaert, A. , Beutels, P. , Hens, N. , & Goossens, H. (2014). Appropriate international measures for outpatient antibiotic prescribing and consumption: Recommendations from a national data comparison of different measures. Journal of Antimicrobial Chemotherapy, 69(2), 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly, L. E. , Edelstein, P. H. , & Ramakrishnan, L. (2007). Why is long‐term required to treat tuberculosis? PLoS Med, 4(3), e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway, B. , & Tossonian, H. (2014). HIV/AIDS‐A model of chronic oxidative stress and immune activation. In Laher I. (Ed.), Systems biology of free radical and antioxidants (pp. 3217–3238). Springer. 10.1007/978-3-642-30018-9_149 [DOI] [Google Scholar]
- Crowe, S. E. (2019). Helicobacter pylori infection. New England Journal of Medicine, 380(12), 1158–1165. [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. , Davis, I. , Willey, B. M. , Rizg, K. , Bolotin, S. , Porter, V. , Polsky, J. , Daneman, N. , McGeer, A. , Yang, P. , Scolnik, D. , Rowsell, R. , Imas, O. , & Silverman, M. S. (2008). Antimalarial therapy selection for quinolone resistance among Escherichia coli in the absence of quinolone exposure, in Tropical South America. PLoS One, 3(7), e2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidt, S. , Schrappe, M. , & Fischer, R. (1995). Analysis of antral biopsy specimens for evidence of acquired mucosa‐associated lymphoid tissue in HIV1‐seropositive and HIV1‐negative patients . Scandinavian Journal of Gastroenterology, 30(7), 635–639. [DOI] [PubMed] [Google Scholar]
- Ferreira, R. M. , Pereira‐Marques, J. , Pinto‐Ribeiro, I. , Costa, J. L. , Carneiro, F. , Machado, J. C. , & Figueiredo, C. (2018). Gastric microbial community profiling reveals a dysbiotic cancer‐associated microbiota. Gut, 67(2), 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipec Kanizaj, T. , Katicic, M. , Skurla, B. , Ticak, M. , Plecko, V. , & Kalenic, S. (2009). Helicobacter pylori eradication therapy success regarding different treatment period based on clarithromycin or metronidazole triple‐therapy regimens. Helicobacter., 14(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Gill, C. J. , Mwanakasale, V. , Fox, M. P. , Chilengi, R. , Tembo, M. , Nsofwa, M. , Chalwe, V. , Mwananyanda, L. , Mukwamataba, D. , Malilwe, B. , Champo, D. , Macleod, W. B. , Thea, D. M. , & Hamer, D. H. (2008). Effect of presumptive cotrimoxazole prophylaxis on pneumococcal colonization rates, seroepidemiology and antibiotic resistance in Zambian infants: A longitudinal cohort study. Bulletin of the World Health Organization, 86(12), 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert, J. P. , & Pajares, J. M. (2002). Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor‐based triple therapies fail. Alimentary Pharmacology & Therapeutics, 16(6), 1047–1057. [DOI] [PubMed] [Google Scholar]
- Glupczynski, Y. , Mégraud, F. , Lopez‐Brea, M. , & Andersen, L. P. (2001). European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori . European Journal of Clinical Microbiology and Infectious Diseases, 20(11), 820–823. [DOI] [PubMed] [Google Scholar]
- Graham, D. Y. , & Shiotani, A. (2008). New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clinical Practice. Gastroenterology & Hepatology, 5(6), 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone, A. , Giorgio, F. , Russo, F. , Riezzo, G. , Girardi, B. , Pricci, M. , Palmer, S. C. , Barone, M. , Principi, M. , Strippoli, G. F. , Di Leo, A. , & Ierardi, E. (2018). New fecal test for non‐invasive Helicobacter pylori detection. World Journal of Gastroenterology, 24(27), 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouitcheu Mabeku, L. B. , Eyoum Bille, B. , Tepap Zemnou, C. , Tali Nguefack, L. D. , & Leundji, H. (2019). Broad spectrum resistance in Helicobacter pylori isolated from gastric biopsies of patients with dyspepsia in Cameroon and efflux‐mediated multiresistance detection in MDR isolates. BMC Infectious Diseases, 19(1), 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucke, G. W. , Grimes, D. E. , Grimes, R. M. , & Dang, T. D. (2009). Antibiotic resistance in the Staphylococcus aureus containing cutaneous abscesses of HIV patients. American Journal of Emergency Medicine, 27(3), 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, E. A. , Zhang, X. S. , Levine, S. M. , Gill, S. R. , Falush, D. , & Blaser, M. J. (2009). Natural transformation of Helicobacter pylori involves the integration of short fragments DNA interrupted by Gaps of variable size. PLoS Path, 5(3), e1000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfertheiner, P. , Megraud, F. , O'Morain, C. A. , Atherton, J. , Axon, A. T. , Bazzoli, F. , Gensini, G. F. , Gisbert, J. P. , Graham, D. Y. , Rokkas, T. , & El‐Omar, E. M. (2012). Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut, 61(5), 646–664. [DOI] [PubMed] [Google Scholar]
- Malfertheiner, P. , Megraud, F. , O'Morain, C. A. , Gisbert, J. P. , Kuipers, E. J. , Axon, A. T. , Bazzoli, F. , Gasbarrini, A. , Atherton, J. , Graham, D. Y. , Hunt, R. , Moayyedi, P. , Rokkas, T. , Rugge, M. , Selgrad, M. , Suerbaum, S. , Sugano, K. , El‐Omar, E. M. ; European Helicobacter and Microbiota Study Group and Consensus Panel (2017). Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut, 66(1), 6–30. [DOI] [PubMed] [Google Scholar]
- Malfertheiner, P. , Mégraud, F. , O'Morain, C. , Hungin, A. P. S. , Jones, R. , Axon, A. , Graham, D. Y. , Tytgat, G. , European Helicobacter Pylori Study Group (EHPSG) (2002). Current concepts in the management of Helicobacter pylori infection–the Maastricht 2–2000 Consensus Report. Alimentary Pharmacology & Therapeutics, 16(2), 167–180. [DOI] [PubMed] [Google Scholar]
- Megraud, F. , Coenen, S. , Versporten, A. , Kist, M. , Lopez‐Brea, M. , Hirschl, A. M. , Andersen, L. P. , Goossens, H. , & Glupczynski, Y. ; Study Group Participants (2013). Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut, 62(1), 34–42. [DOI] [PubMed] [Google Scholar]
- Miendje Deyi, V. Y. , Bontems, P. , Vanderpas, J. , De Koster, E. , Ntounda, R. , Van den Borre, C. , Cadranel, S. , & Burette, A. (2011). Routine survey determinations of resistance of Helicobacter pylori to antimicrobials over the last 20 years (1999 to 2009) in Belgium. Journal of Clinical Microbiology, 49(6), 2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouery, K. , Rader, B. A. , Gaynor, E. C. , & Guillemin, K. (2006). The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. Journal of Bacteriology, 188(15), 5494–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin, T. D. , Morgan, C. J. , Graham, D. Y. , & Genta, R. M. (2014). Helicobacter pylori gastritis in HIV‐infected patients: A review. Helicobacter, 19(5), 323–329. [DOI] [PubMed] [Google Scholar]
- Ngalani, O. J. T. , Mbaveng, A. T. , Marbou, W. J. T. , Ngai, R. Y. , & Kuete, V. (2019). Antibiotic resistance of enteric bacteria in HIV‐Infected patients at the Banka Ad‐Lucem Hospital, West region of Cameroon. Canadian Journal of Infectious Diseases and Medical Microbiology, 2019, 9381836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkuize, M. , De Wit, S. , Muls, V. , Delforge, M. , Miendje Deyi, V. Y. , Cadière, G. B. , & Buset, M. (2015). HIV‐Helicobacter pylori co‐infection: Antibiotic resistance, prevalence, and risk factors. PLoS One, 10(12), e0145119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkuize, M. , Vanderpas, J. , Buset, M. , Delforge, M. , Cadière, G.‐B. , De Wit, S. Failure to eradicate Helicobacter pylori infection is more frequent among HIV‐positive patients. HIV Medicine. 10.1111/hiv.13083. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- O'Rourke, E. J. , Chevalier, C. , Pinto, A. V. , Thiberge, J. M. , Ielpi, L. , Labigne, A. , & Radicella, J. P. (2003). Pathogen DNA as target for host‐generated oxidative stress: Role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proceedings of the National Academy of Sciences of the United States of America, 100(5), 2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Opportunistic Infections in HIV‐Infected Adults and Adolescents (2020). Guidelines for the prevention and treatment of opportunistic infections in HIV‐infected adults and adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. (Accessed February 11, 2020) [Google Scholar]
- Pantaleo, G. , Fauci, A. S. (1996). Immunopathogenesis of HIV infection. Annu Rev Microbiol, 50, 825‐854. [DOI] [PubMed] [Google Scholar]
- Pero, R. , Brancaccio, M. , Laneri, S. , Biasi, M. G. , Lombardo, B. , & Scudiero, O. (2019). A novel view of human Helicobacter pylori Infections: Interplay between microbiota and beta‐defensins. Biomolecules, 9(6), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, J. , Lamarque, D. , Kalach, N. , Chaussade, S. , & Burucoa, C. (2010). High level of antimicrobial resistance of French Helicobacter pylori isolates. Helicobacter, 15(1), 21–27. [DOI] [PubMed] [Google Scholar]
- Roe, I. H. , Son, S. H. , Oh, H. T. , Choi, J. , Shin, J. H. , Lee, J. H. , & Hah, Y. C. (1999). Changes in the evolution of the antigenic profiles and morphology during coccoid conversion of Helicobacter pylori . Korean Journal of Internal Medicine, 14(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag, M. S. , Benson, C. A. , Gandhi, R. T. , Hoy, J. F. , Landovitz, R. J. , Mugavero, M. J. , Sax, P. E. , Smith, D. M. , Thompson, M. A. , Buchbinder, S. P. , del Rio, C. , Eron, J. J. , Fätkenheuer, G. , Günthard, H. F. , Molina, J.‐M. , Jacobsen, D. M. , & Volberding, P. A. (2018). Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society‐USA Panel. JAMA, 320(4), 379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfo, F. S. , Eberhardt, K. A. , Dompreh, A. , Kuffour, E. O. , Soltau, M. , Schachscheider, M. , Drexler, J. F. , Eis‐Hübinger, A. M. , Häussinger, D. , Oteng‐Seifah, E. E. , Bedu‐Addo, G. , Phillips, R. O. , Norman, B. , Burchard, G. , & Feldt, T. (2015). Helicobacter pylori infection is associated with higher CD4 T cell counts and lower HIV‐1 viral loads in ART‐Naïve HIV‐positive patients in Ghana. PLoS One, 10(11), e0143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, D. , Li, Y. , Yang, L. , Pei, Z. , Poles, M. , Abrams, W. R. , & Malamud, D. (2012). Human microbiome and HIV/AIDS. Current HIV/AIDS Reports, 9(1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano, K. , Tack, J. , Kuipers, E. J. , Graham, D. Y. , El‐Omar, E. M. , Miura, S. , Haruma, K. , Asaka, M. , Uemura, N. , Malfertheiner, P. ; faculty members of Kyoto Global Consensus Conference (2015). Kyoto global consensus report on Helicobacter pylori gastritis. Gut, 64(9), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thung, I. , Aramin, H. , Vavinskaya, V. , Gupta, S. , Park, J. Y. , Crowe, S. E. , & Valasek, M. A. (2016). Review article: The global emergence of Helicobacter pylori antibiotic resistance. Alimentary Pharmacology & Therapeutics, 43(4), 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil, N. , & Megraud, F. (2007). Eradication therapy for Helicobacter pylori . Gastroenterology, 133(3), 985–1001. [DOI] [PubMed] [Google Scholar]
- Wells, D. H. , & Gaynor, E. (2006). Helicobacter pylori initiates the stringent response upon nutriment and pH downshift. Journal of Bacteriology, 188(10), 3726–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, W. M. , Gu, Q. , Wang, W. H. , Fung, F.‐ M.‐Y. , Berg, D. E. , Lai, K. C. , Xia, H.‐ H.‐X. , Hu, W. H. C. , Chan, C. K. , Chan, A.‐ O.‐O. , Yuen, M.‐F. , Hui, C.‐K. , Lam, S. K. , & Wong, B.‐ C.‐Y. (2003). Effects of primary metronidazole and clarithromycin resistance to Helicobacter pylori on omeprazole, metronidazole, and clarithromycin triple‐therapy regimen in a region with high rates of metronidazole resistance. Clinical Infectious Diseases, 37(7), 882–889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Antibiotic consumption data analyzed during this study are the property of the Agence InterMutualiste (www.aim‐ima.be). The study is registered at ISRCTN registry, number 13466428: https://www.isrctn.com/ISRCTN13466428


