Abstract
Background
Intimate partner violence (IPV) includes any violence (physical, sexual or psychological/emotional) by a current or former partner. This review reflects the current understanding of IPV as a profoundly gendered issue, perpetrated most often by men against women. IPV may result in substantial physical and mental health impacts for survivors. Women affected by IPV are more likely to have contact with healthcare providers (HCPs) (e.g. nurses, doctors, midwives), even though women often do not disclose the violence. Training HCPs on IPV, including how to respond to survivors of IPV, is an important intervention to improve HCPs' knowledge, attitudes and practice, and subsequently the care and health outcomes for IPV survivors.
Objectives
To assess the effectiveness of training programmes that seek to improve HCPs' identification of and response to IPV against women, compared to no intervention, wait‐list, placebo or training as usual.
Search methods
We searched CENTRAL, MEDLINE, Embase and seven other databases up to June 2020. We also searched two clinical trials registries and relevant websites. In addition, we contacted primary authors of included studies to ask if they knew of any relevant studies not identified in the search. We evaluated the reference lists of all included studies and systematic reviews for inclusion. We applied no restrictions by search dates or language.
Selection criteria
All randomised and quasi‐randomised controlled trials comparing IPV training or educational programmes for HCPs compared with no training, wait‐list, training as usual, placebo, or a sub‐component of the intervention.
Data collection and analysis
We used standard methodological procedures outlined by Cochrane. Two review authors independently assessed studies for eligibility, undertook data extraction and assessed risks of bias. Where possible, we synthesised the effects of IPV training in a meta‐analysis. Other analyses were synthesised in a narrative manner. We assessed evidence certainty using the GRADE approach.
Main results
We included 19 trials involving 1662 participants. Three‐quarters of all studies were conducted in the USA, with single studies from Australia, Iran, Mexico, Turkey and the Netherlands. Twelve trials compared IPV training versus no training, and seven trials compared the effects of IPV training to training as usual or a sub‐component of the intervention in the comparison group, or both.
Study participants included 618 medical staff/students, 460 nurses/students, 348 dentists/students, 161 counsellors or psychologists/students, 70 midwives and 5 social workers. Studies were heterogeneous and varied across training content delivered, pedagogy and time to follow‐up (immediately post training to 24 months). The risk of bias assessment highlighted unclear reporting across many areas of bias. The GRADE assessment of the studies found that the certainty of the evidence for the primary outcomes was low to very low, with studies often reporting on perceived or self‐reported outcomes rather than actual HCPs' practices or outcomes for women. Eleven of the 19 included studies received some form of research grant funding to complete the research.
Within 12 months post‐intervention, the evidence suggests that compared to no intervention, wait‐list or placebo, IPV training:
· may improve HCPs' attitudes towards IPV survivors (standardised mean difference (SMD) 0.71, 95% CI 0.39 to 1.03; 8 studies, 641 participants; low‐certainty evidence);
· may have a large effect on HCPs' self‐perceived readiness to respond to IPV survivors, although the evidence was uncertain (SMD 2.44, 95% CI 1.51 to 3.37; 6 studies, 487 participants; very low‐certainty evidence);
· may have a large effect on HCPs' knowledge of IPV, although the evidence was uncertain (SMD 6.56, 95% CI 2.49 to 10.63; 3 studies, 239 participants; very low‐certainty evidence);
· may make little to no difference to HCPs' referral practices of women to support agencies, although this is based on only one study (with 49 clinics) assessed to be very low certainty;
· has an uncertain effect on HCPs' response behaviours (based on two studies of very low certainty), with one trial (with 27 participants) reporting that trained HCPs were more likely to successfully provide advice on safety planning during their interactions with standardised patients, and the other study (with 49 clinics) reporting no clear impact on safety planning practices;
· may improve identification of IPV at six months post‐training (RR 4.54, 95% CI 2.5 to 8.09) as in one study (with 54 participants), although three studies (with 48 participants) reported little to no effects of training on identification or documentation of IPV, or both.
No studies assessed the impact of training HCPs on the mental health of women survivors of IPV compared to no intervention, wait‐list or placebo.
When IPV training was compared to training as usual or a sub‐component of the intervention, or both, no clear effects were seen on HCPs' attitudes/beliefs, safety planning, and referral to services or mental health outcomes for women. Inconsistent results were seen for HCPs' readiness to respond (improvements in two out of three studies) and HCPs' IPV knowledge (improved in two out of four studies). One study found that IPV training improved HCPs' validation responses.
No adverse IPV‐related events were reported in any of the studies identified in this review.
Authors' conclusions
Overall, IPV training for HCPs may be effective for outcomes that are precursors to behaviour change. There is some, albeit weak evidence that IPV training may improve HCPs' attitudes towards IPV. Training may also improve IPV knowledge and HCPs' self‐perceived readiness to respond to those affected by IPV, although we are not certain about this evidence. Although supportive evidence is weak and inconsistent, training may improve HCPs' actual responses, including the use of safety planning, identification and documentation of IPV in women's case histories. The sustained effect of training on these outcomes beyond 12 months is undetermined. Our confidence in these findings is reduced by the substantial level of heterogeneity across studies and the unclear risk of bias around randomisation and blinding of participants, as well as high risk of bias from attrition in many studies. Further research is needed that overcomes these limitations, as well as assesses the impacts of IPV training on HCPs' behavioral outcomes and the well‐being of women survivors of IPV.
Keywords: Adult; Female; Humans; Bias; Dentists; Dentists/education; Health Personnel; Health Personnel/education; Intimate Partner Violence; Medical Staff; Medical Staff/education; Midwifery; Midwifery/education; Nursing Staff; Nursing Staff/education; Psychology; Psychology/education; Randomized Controlled Trials as Topic; Social Workers; Social Workers/education; Students, Health Occupations
Plain language summary
Training healthcare providers to respond to intimate partner violence against women
Review question
Does intimate partner violence (IPV) training for healthcare providers (HCPs) improve their:
· attitudes or beliefs, or both, towards IPV, · readiness to respond to those affected by IPV, · knowledge of IPV, · referral of women being subjected to IPV to specialist services, · actual response to women subjected to IPV (such as validation or safety planning), · identification and documentation of IPV, and · the mental health of survivors of IPV?
Background
Intimate partner violence is associated with a wide range of short‐ and long‐term physical and mental health problems. These include injuries and death, depression, anxiety, post‐traumatic stress disorder, unplanned/unwanted pregnancies and gynaecological problems, to name a few. Health problems can last beyond the duration of the violence and women who have experienced violence are more likely to seek health care compared to women who have never experienced violence.
Women are more likely to trust HCPs with a disclosure of violence. For some women, a healthcare setting may be one of the few places women can attend on their own. HCPs (such as nurses, doctors, midwives, etc.) are therefore ideally situated to identify and provide support for women affected by IPV. Many healthcare settings provide clinical guidelines or training or both on how to identify and respond to IPV. We wanted to find out what difference training makes to IPV‐related HCP attitudes, knowledge and response, including the care provided to women affected by IPV and whether it improved their health outcomes, including their mental health, or made a difference to their exposure to IPV.
Study characteristics
We found 19 trials comparing IPV training to no training, training as usual, or other trainings that were included in this review, with 1662 participants who were practising or student/trainee doctors, nurses, midwives, dentists, social workers and psychologists/counsellors. Three‐quarters of all studies were conducted in the USA, with single studies from Australia, Iran, Mexico, Turkey and the Netherlands. Most studies received some university or government financial support to complete the research.
Studies varied greatly in the kind of IPV training provided, in both content and delivery method. Studies differed in how they measured training outcomes and follow‐up time points. Most IPV training included types and definitions of IPV, prevalence and risk factors, and sought to challenge common myths and misinformation. Clinical scenarios were frequently used as learning tools, outlining typical patient presentations, and skills training involved learning how to ask women about IPV, how to respond by validating their experiences, document accurately, discuss safety planning and refer women to support services.
Key results with an assessment of the certainty of the evidence
Compared to no training, placebo or wait‐list, IPV training may have positive effects on HCPs' attitudes towards survivors of IPV. Training may improve their knowledge around, and readiness to respond to survivors of IPV, but the evidence is very uncertain. There is limited evidence that some types of IPV training can lead to improvements in identification, safety planning and documentation of IPV, but the findings are inconsistent, and most studies report little to no impact of training on these outcomes. Training may make little to no difference to referral practices. No studies with no training, placebo or wait‐list in the comparison group, assessed IPV survivors' mental health outcomes. No adverse effects of IPV training were reported in any of these studies.
The studies that compared training of HCPs to training as usual or a sub‐component of the training typically found no difference in HCPs' attitudes, safety planning, and referral to services or mental health outcomes for women. The evidence was inconsistent about provider readiness to respond, their actual response and changes in IPV knowledge.
Overall, the certainty of the evidence for the effectiveness of training HCPs in how to respond to IPV is low to very low. Future research should include higher‐quality trials, with greater clarity of methods that objectively measure outcomes (actual rather than perceived), with an emphasis on behaviour change in HCPs, and the well‐being of women survivors of IPV.
Up‐to‐dateness of the review
The evidence is current to June 2020.
Summary of findings
Summary of findings 1. Training to respond to intimate partner violence compared to no intervention, wait‐list or placebo on healthcare providers' attitudes towards, knowledge of and readiness to manage IPV, referrals for and response to IPV.
| Training to respond to intimate partner violence compared to no intervention, wait‐list, placebo in healthcare providers at less than 12 months after intervention | ||||||
|
Patient or population: physicians/doctors, medical staff, medical students, residents, nurses and nursing students, dentists and dental students, counsellors and psychology students
Setting: teaching and clinical practice settings such as universities, primary care clinics, clinical teaching hospitals/schools and online platforms Intervention: training to respond to intimate partner violence Comparison: no intervention, wait list, or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention, wait list, or /placebo | Risk with training to respond to intimate partner violence | |||||
| Healthcare providers’ attitudes/beliefs towards IPV Assessed with: Attitude Toward Battered Women Questionnaire; PREMIS‐ victim blaming or opinion subscale; Jefferson Scale of Physician Empathy, etc. | ‐ | The mean score in the intervention groups was 0.71 standard deviations higher (0.39 higher to 1.03 higher) | ‐ | 641 (8 RCTs) | ⊕⊕⊝⊝ Lowa,b | Higher scores indicate improved attitudes towards addressing IPV and helping IPV survivors. Training probably improves healthcare providers' (HCPs') attitudes towards survivors of intimate partner violence (IPV). The effect of training appears to be moderate. |
| Healthcare providers’ readiness to respond to/manage survivors of IPV Assessed with: self‐efficacy or perceived preparation subscale of the PREMIS; intended AVDR subscale of the subscale of the Domestic Violence Assessment Instrument | ‐ | The mean score in the intervention groups was 2.44standard deviations higher (1.51 higher to 3.37 higher) | ‐ | 487 (6 RCTs) | ⊕⊝⊝⊝ Verylowb,c,d | Higher scores indicate improved readiness to respond to IPV and helping IPV survivors. Training probably improves healthcare providers' readiness to respond to survivors of IPV. The effect appears to be large. A quasi‐RCT with 136 medical residents (and high risk of bias) did not provide data in a way that could be combined in the meta‐analysis. The authors report that compared to the control group, there was a statistically significant improvement from baseline to post‐training (P < 0.001) in HCPs' ability to explain correct interventions for survivors of IPV. |
| Healthcare providers knowledge or awareness about IPV Assessed with: Knowledge Test About Violence Against Women and the actual knowledge sub scale of the PREMIS | ‐ | The mean score in the intervention groups was 6.56standard deviations higher (2.49 higher to 10.63 higher) | ‐ | 239 (3 RCTs) | ⊕⊝⊝⊝ Verylowe,f,g | Higher scores indicate improved knowledge of IPV. Training probably improves healthcare providers' knowledge of IPV. The effect of the intervention is large. 2 other studies provided further support that training may improve HCPs' knowledge of IPV. 1 study with 23 medical residents compared change in percent of correct answers on 5 knowledge questions and report that the experimental group scored 17% more on average than the control group (P < 0.002). The risk of bias in this study was unclear. Another with 30 graduate students pursuing a counselling degree reported a Cohen's d of 0.42, indicating a medium effect of training on IPV knowledge. The risk of bias in this study was low. |
| Referrals made to support agencies, social workers or other specialised services Assessed with: a standardised researcher‐created checklist on office practices | 1 study reported on the referral practices of offices where intervention‐arm HCPs were based compared to referral practices of offices where the control‐arm HCPs were based. The study authors report that no difference in referral practices were seen between the 2 clusters. Physician referral rates were not measured. The study had a high risk of bias due to high and unequal attrition. | ‐ | 49 (1 RCT) | ‐ | ‐ | |
| Provider response to IPV: safety planning, counselling and validation of survivors’ feelings Assessed with: standardised patient reports of HCPs practicing at least 6 out of 8 on common safety planning items | Study population | RR 3.07 (0.96 to 9.77) | 29 (1 RCT) | ⊕⊝⊝⊝ Verylowg,h | Based on the 1 study reported here, we cannot conclude if training could improve healthcare providers' response to survivors of IPV. 1 other study, a cluster‐RCT, compared differences in self‐reported safety planning at the practice level. The authors report little to no difference in self‐reported safety planning between practices. The study had a high risk of bias due to high and unequal attrition. |
|
| 231 per 1000 | 571 per 1000 (238 to 851) | |||||
| Adverse outcomes | Not reported | ‐ | ‐ | ‐ | No data on harms are available | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
AVDR: Ask, Validate, Document and Refer; CI: confidence interval; HCP: Healthcare Provider; IPV: intimate partner violence; PREMIS: Physician Readiness to Manage Intimate Partner Violence; RCT: randomised controlled trial; RR: risk ratio. We interpreted the SMD using Cohen’s D, where we treated an SMD of about 0.2 as a small effect, an SMD of about 0.5 as a moderate effect, and a SMD of more than 0.8 as a large effect (Cohen 1977) | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for study limitations owing to unclear risk of bias from lack of blinding in all studies, high risk of bias from high and unequal attrition and lack of standardised tools in some studies, and high risk of bias due to lack of random sequence generation in one study. bDowngraded one level owing to inconsistency (heterogeneity in intervention and statistical heterogeneity (I2 > 75% and significant Chi2 test of heterogeneity has a low value and wide variance in point estimates across studies). cDowngraded one level for study limitations owing to unclear or high risk of bias from lack of blinding in all studies, high risk of bias from high and unequal attrition and lack of standardised tools in some studies. dDowngraded one level owing to indirectness (with respect to population and setting as only dentists, mental health professionals and graduate students and physicians were represented, and all the training took place online). eDowngraded one level owing to high risk of bias from high and unequal attrition in two out of three studies in the meta‐analysis, and high risk of bias due to lack of random sequence generation in one study. fDowngraded one level owing to indirectness (with respect to population and setting as only general practitioners/community physicians and nursing and counselling students were represented, and the training took place in university settings or online). gDowngraded two levels owing to imprecision. CIs are wide and clustering of events within individuals does not seem to be accounted for. Small sample sizes may be further contributing to this imprecision. hDowngraded one level owing to indirectness (with respect to population and setting, as GRADE criteria was only applied to one study).
Background
Description of the condition
Intimate partner violence (IPV) against women includes any violence (physical, sexual or psychological/emotional) or threats of such violence by a partner or a former partner, and is hereafter referred to as IPV or partner violence. In this Cochrane Review, we use the overarching definition of Heise 2002 (p 89), that refers to IPV as "any behaviour within an intimate relationship that causes physical, psychological or sexual harm to those in the relationship". Prevalence estimates based on data from 2000 to 2018 recently published by the World Health Organization (WHO) show that globally 27% of women and girls aged 15 to 49 years have been subjected to physical or sexual IPV or both in their lifetime (WHO 2021).
In addition to fatal (Stöckl 2013) and non‐fatal physical injuries that are a direct result of the inflicted violence, IPV that may involve psychological and sexual violence has been linked to a wide range of negative health outcomes, disorders or conditions such as sexually‐transmitted infections, HIV, unwanted and unintended pregnancies and unsafe abortions, gastrointestinal and gynaecological disorders, chronic diseases, harmful substance use, depression, post‐traumatic stress and other anxiety disorders, and other somatoform conditions (Campbell 2002; Coker 2002; WHO 2013a). Partner violence survivors are more likely to seek health care and to interact with healthcare providers (HCPs) than those who have not been exposed to IPV (WHO 2013b).
Healthcare providers are often a first point of call for women and are among those professionals they are most likely to trust with a disclosure (Feder 2006; Tarzia 2020). An empathetic well‐trained provider can validate women's experiences and help women access the support they need, often by connecting them with specialised services. Healthcare providers are in an ideal position to identify and provide care and support for women who have been victims of IPV, by linking them to other services, and potentially contributing to a reduction in violence and improved outcomes for women and their children (Aksan 2007; Bullock 1997; Garcia‐Moreno 2002; Garcia‐Moreno 2014; Kim 2002; Short 1998; WHO 2014). Healthcare providers can also play a central role in collecting and documenting evidence necessary for identification and legal action against the perpetrator of the violence (WHO 2013b). Furthermore, the healthcare sector presents a potential pathway to other services that survivors may need, including but not limited to, legal aid, social welfare or psychosocial support, and community resources targeted at addressing the needs of IPV survivors (WHO 2013b; WHO 2014). Healthcare providers may also be in a position to provide support to children exposed to violence within their families (Garcia‐Moreno 2014).
Despite widespread agreement on the role that HCPs can play in addressing IPV, many barriers can inhibit HCPs from adequately identifying and responding to women experiencing violence. Intimate partner violence training for HCPs could help overcome HCP‐related barriers to caring for women experiencing IPV. Tolerance for violence against women can result in low reporting rates of IPV. In some cultures, HCPs' attitudes towards violence against women and beliefs that place the responsibility for violence on the victim, act as a barrier to understanding that IPV is an issue that they need to address and provide appropriate care (Aksan 2007; Wood 1998; Zakar 2011). In addition, a HCP's own experience of violence can affect their ability to respond supportively to women experiencing IPV (Aksan 2007). A primary barrier to asking about IPV includes the belief (of HCPs) that by asking about IPV, HCPs will enter a personal and complex situation that they are unprepared to handle, due to inadequate training (Beynon 2012; Davidson 2001; Djikanovic 2010). Asking all women about IPV may increase identification by HCPs, although it has not been demonstrated to increase referrals by HCPs or uptake by women of support services, something that may be explained by an absence of adequate training in responding to IPV (O'Doherty 2015). Furthermore, it has been suggested that HCPs who lack adequate training in enquiring about and responding to IPV can cause harm; perhaps by advocating women leave an abusive relationship while failing to provide survivors with a safety plan or to take into account the survivor's perspective (Morse 2012). Responses like this may leave IPV survivors feeling helpless, guilty, isolated and at risk of further violence (Djikanovic 2010).
Training and education of HCPs in IPV is an important means of addressing several of these barriers and may lead to enhanced care and better health outcomes for survivors of IPV. In addition to these aspects and given the significant cost of IPV to a woman's family, community and society more broadly, HCP training has been proposed to be a cost‐effective and cost‐saving intervention from a societal perspective (Devine 2012). In this context, it is essential to evaluate the impact of training HCPs in how to respond to IPV against women, and to identify the characteristics of successful training interventions.
Description of the intervention
Training programmes should aim to increase HCPs' understanding and skill set related to providing care for women experiencing IPV. Training should provide HCPs with the knowledge and skills they need to investigate and respond appropriately to women experiencing IPV, including ensuring the safety and confidentiality of survivors (Garcia‐Moreno 2002; Garcia‐Moreno 2014). Training programmes examined in this review involve structured training that aimed to increase HCPs' knowledge about IPV (while targeting their existing beliefs and attitudes towards IPV) and aimed to improve the ability of HCPs to respond appropriately to survivors of IPV. Effective responses include knowledge of when and how to ask about violence, empathetic listening, validation of survivors' feelings, discussions around the violence and survivors’ readiness for change, first‐line psychological support, encouragement of safety‐promoting behaviours for IPV survivors, and identification and reporting of the violence, with improved documentation as well as referral of survivors of IPV to specialist agencies where they exist (Bair‐Merritt 2014; WHO 2014).
Training of HCPs in how they should respond to survivors of IPV was a central component of the structured interventions in this review. We considered any training intervention method and pedagogy. Some interventions explicitly identify themselves as based on AVDR (Asking, Validating, Documenting and Referral; Gerbert 2000). These typically listed the following four aspects of training:
Asking: routinely asking patients about partner violence, which should be done in a private setting, while ensuring confidentiality, and using a non‐judgemental and empathetic tone;
Validating: providing validating messages and compassionate statements that acknowledge that IPV is wrong, while confirming the worth of the woman;
Documenting: accurately documenting signs, symptoms and exact words of disclosures in writing or with photographs, or both; and
Making referrals: referring victims to social workers on‐site or to IPV advocates, or other relevant resources within the community.
Other programmes may identify this process as RADAR, which refers to Routine screening, Ask direct questions, Document your findings, Assess patient safety and Review patient options and referrals (Harwell 1998). This intervention typically involves three to six hours of trauma theory‐based training in IPV; it includes sessions with representatives of domestic violence (DV) agencies in the community, and has a similar aim of improving HCPs' ability to document IPV and carry out safety assessments and referrals for IPV survivors. Other studies have provided training on use of resource books and initiation of referrals in response to reporting of IPV on a screening form (Garg 2007). Still others have used computer‐assisted training, electronic reminders or practice "domestic violence advocates" (Feder 2011, p 1). Overall, a central component of the interventions involved training of HCPs in how to identify and respond to survivors of IPV.
Despite the focus on training HCPs on how to respond to survivors of IPV, there is noticeable variation in content, structure and duration of programmes. Training interventions use a wide variety of pedagogical techniques, including role‐plays, group discussions, lectures, experiential training and simulations, among others. They are delivered through a variety of methods, such as workshops, classroom‐based face‐to‐face teaching, online learning and seminars.
Training is sometimes tailored to accommodate the scenario in which HCPs encounter patients, for example, providers working in emergency services may be approached when survivors are seeking orders of protection (Morse 2012), and dentists are in a position to come across facial injuries that can be markers of IPV in women (Ochs 1996; Perciaccante 1999). Where available, the review extracts data on the method of delivery, pedagogical technique, content, frequency, duration, and intensity of the training intervention.
How the intervention might work
Increasing HCPs' awareness of the links between IPV exposure and presenting health issues (physical injury, medically unexplained symptoms, chronic health problems, ongoing mental health problems, etc.) may enhance provider self‐efficacy and understanding of the need to support patients with presenting complaints. Physicians who are trained in IPV during their residency, or who have received continuing education on IPV after licensing, are more likely to ask questions routinely and to identify victims of IPV (Sitterding 2003). Training interventions address HCPs' concerns about the lack of information on how to ask, and how to respond after identification, which is often a barrier to asking about and responding to IPV (Beynon 2012; Davidson 2001; Djikanovic 2010). Training interventions should go beyond addressing these barriers and should attempt to improve HCPs' knowledge, skills, attitudes, and behaviours related to caring for survivors of IPV. The theory of planned behaviour change (Azjen 1991) posits that behaviours are influenced mainly by an individual’s attitudes, subjective norms and perceived behavioural control, and that changes in these can lead to a successful change in the intended behaviour. Training interventions may influence beliefs around IPV that can lead to a change in attitudes towards IPV; may influence subjective norms around responding to IPV; and may increase knowledge of, and provide skills on, how to respond to IPV, thereby changing perceived behavioural control. Thus, changes in HCPs' knowledge and attitudes towards IPV may impact their behaviours/responses to women's disclosure, which in turn may affect the well‐being of IPV survivors. Being supported by a health system that provides ongoing HCP education and clinical support may enhance provider readiness to address IPV (Hegarty 2020; WHO 2017).
Why it is important to do this review
A recent systematic review (O'Doherty 2015) found that even though screening for IPV by healthcare providers can lead to increased identification of victims, overwhelming evidence shows that IPV screening does not increase the number of IPV survivors referred to specialist agencies. Encouraging disclosure of IPV and failing to respond adequately can threaten women's safety and weaken their confidence (Heron 2002). Many HCPs acknowledge that addressing IPV falls within the purview of their professional responsibility (Richardson 2001), although a lack of training on what to do following identification or disclosure remains a barrier to asking about IPV in the first place (Beynon 2012; Djikanovic 2010). Adequate training in both identification of and response to IPV disclosure may address this risk. However, even providers who have received training in IPV continue to feel underprepared to respond to survivors of IPV (Cohen 2002). It is therefore important to identify how to ensure that training in IPV improves identification/disclosure and HCPs' response, and then in turn women's health and well‐being outcomes. In this review we aimed to identify the characteristics of an effective training intervention that can successfully address the needs of HCPs and the women they care for.
No previous Cochrane Review has examined training interventions for HCPs on how to respond to IPV. O'Doherty 2015 assessed the impact of screening, not the impact of training providers in screening and responding. Note that studies in which the intervention group receives a screening tool, and the control group does not, fall outside the purview of this review. Previous systematic reviews on the topic of training are more than a decade old (Davidson 2001; Ramsay 2005). They included observational studies, as well as randomised controlled trials (RCTs) and quasi‐RCTs. The authors of these reviews concluded that overall no conclusive evidence showed the impact of IPV training or education on HCPs or on women survivors of IPV (Davidson 2001; Garcia‐Moreno 2002). Since that time, many studies have evaluated the impact of training HCPs in IPV. A systematic review conducted in 2011 as part of the background research for WHO guidelines on the health sectors' response towards interpersonal violence towards women (WHO 2013b), identified several new studies. This review did not include studies published before the year 2000, did not have a stringent inclusion criterion that was limited to only IPV training programmes, and did not adequately identify and include studies that were written in languages other than English.
Objectives
To assess the effectiveness of training programmes that seek to improve HCPs' identification of and response to IPV against women, compared to no intervention, wait‐list, placebo or usual care.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐RCTs (in which allocation is not truly random, for example, by date of birth, day of the week, alternate person).
Types of participants
All healthcare providers (HCPs) and HCP students (i.e. doctors, nurses, midwives, dentists, community health workers, medical social workers, dieticians, nutritionists, medical students or residents/fellows, healthcare assistants, paramedics, etc.) in any kind of healthcare setting (primary, secondary, tertiary or community setting), who directly provide health services. This review excluded studies of health professionals who are not direct providers of health care, such as hospital administrative staff and medical and health service managers.
Types of interventions
Any structured programme of (in‐service) training, including experiential training, workshops and educational programmes and sessions, delivered in‐person or virtually, in which the central component is aimed at improving HCPs' ability to identify and respond to IPV against women aged 16 years and older.
We excluded studies:
that addressed only screening for or identification of IPV, as these have been covered elsewhere (O'Doherty 2015);
that addressed training where the focus was on multiple types of violence, including rape and child abuse, and researchers did not specify that they included IPV; and
of training and educational interventions dealing with domestic violence (DV) that was not perpetrated by a partner or directed towards women in a current or past intimate relationship.
Comparisons
No training, wait‐list or placebo.
Training as usual (also referred to as 'treatment and usual' or 'usual care').
A sub‐component of the multi‐component intervention in the intervention arm. For example, the intervention arm includes component A + component B, compared to component B alone in the comparator arm, so as to allow the effect of component A to be assessed.
Types of outcome measures
Primary outcomes
Healthcare providers’ attitudes/beliefs towards IPV, measured on a scale such as the Domestic Violence Assessment Instrument (Danley 2004) or similar tools used by study authors.
Healthcare providers’ readiness to manage/respond to or support women survivors of IPV, measured on a scale such as the Physician Readiness to Manage Intimate partner violence Survey (PREMIS; Short 2006a) or similar tools.
Healthcare providers’ knowledge or awareness of IPV, measured on a scale such as the PREMIS (Short 2006a) or similar tools.
Referrals made to support agencies, social workers or other specialised services. These could have been self‐reported or documented by HCPs or by women survivors (or both) from medical records, or complete referrals measured by the use of referred services from the records of social workers or family/DV services.
Provider response to IPV: safety planning, counselling or validation of survivors' feelings, or both. This could have been assessed by means of self‐report or documented by HCPs or by women survivors (or both) from medical records.
Adverse outcomes for providers that may have included worsened attitudes, beliefs towards IPV or reduced readiness to manage IPV.
Secondary outcomes
Documentation or identification of IPV (or both) as part of routine data. This could have been self‐reported or documented by HCPs or by women survivors (or both) from medical records.
Mental health outcomes for women survivors of IPV. Depression measured by a standardised instrument such as the General Health Questionnaire (GHQ; Goldberg 1979), the Edinburgh Postnatal Depression Scale (EPDS; Murray 1990) or the Beck Depression Inventory (BDI; Beck 1974). Anxiety measured by a standardised instrument such as Spielberger’s State‐Trait Anxiety Inventory (STAI; Spielberger 1994), or similar tools used by study authors.
Adverse outcomes such as IPV‐related death, measured by medical records or vital data records (such as death certificate), or recurrence of IPV or injury after disclosure to a HCP, measured by standardised instruments such as the Composite Abuse Scale (CAS; Hegarty 1999; Hegarty 2005), the Revised Conflict Tactics Scales (CST2; Straus 1996) or the Women's Experience of Battering (WEB) scale (Smith 1999) or similar tools used by authors.
Search methods for identification of studies
We ran the first searches in April 2017 and ran top‐up searches in April 2019 and June 2020. We did not limit our searches by date or language but used a study methods filter, when appropriate, to identify RCTs and quasi‐RCTs (Lefebvre 2021).
Electronic searches
We searched the electronic databases and trials registers listed below from inception onwards:
Cochrane Register of Studies Online (CENTRAL) (crso.cochrane.org/, searched 2 June 2020)
MEDLINE Ovid (1946 to May Week 4 2020)
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 2 June 2020)
MEDLINE Epub Ahead of Print Ovid (searched 2 June 2020)
Embase Ovid (1974 to 1 June 2020)
ERIC EBSCOhost (1966 to 2 June 2020)
Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus EBSCO); 1937 to 2 June 2020)
PsycINFO Ovid (1806 to May Week 4 2020)
Cochrane Database of Systematic Reviews (Issue 6 2020; searched 2 June 2020)
Popline (Population Information Online; www.popline.org; searched 3 May 2019. This database service retired on 1 September 2019)
Latin American and Caribbean Health Sciences Literature (LILACS); lilacs.bvsalud.org/en; searched 2 June 2020)
African Index Medicus (AIM; indexmedicus.afro.who.int; searched 3 May 2019, service unavailable in June 2020)
World Health Organization Library and Information Networks for Knowledge (WHOLIS; www.who.int/en; searched 3 May 2019; service unavailable in June 2020)
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 3 May 2019, service unavailable in June 2020. Message on website 'Due to heavy traffic generated by the COVID‐19 outbreak, the ICTRP Search Portal is not responding from outside WHO temporarily')
ClinicalTrials.gov (clinicaltrials.gov; searched 2 June 2020)
The search strategies used for each database are reported in Appendix 1.
Searching other resources
We searched the resources listed below, to identify any additional studies.
Websites
We searched the following websites.
World Bank (www.worldbank.org; searched on 14 August 2018);
Violence Prevention (Centre for Public Health, Liverpool John Moores University; www.preventviolence.info; searched on 15 June 2019);
International Council of Nurses (ICN; www.icn.ch; searched on 15 June 2019);
Centers for Disease Control and Prevention (www.cdc.gov/injury; searched on 15 June 2019);
Centre for Public Health (cph.org.uk/expertise/violence) redirected to (www.ljmu.ac.uk/research/centres-and-institutes/public-health-institute; searched on 15 June 2019).
Reference lists
We searched the reference lists of relevant systematic reviews and all included studies.
Personal communication
We contacted the authors of included studies and other experts in the field to ask for details of any published or unpublished studies not identified by our searches or when we needed additional information to determine whether to include/exclude a study.
Data collection and analysis
In the following sections, we report only the methods that are used in the review. For methods that we had planned to use (Kalra 2017), readers are directed to Appendix 2 and the section on Differences between protocol and review.
Selection of studies
In line with the Cochrane Handbook (Higgiins 2021), two review authors (NK and LH or SR) independently applied the inclusion and exclusion criteria to titles and abstracts and full reports and resolved conflicts with the help of the third review author (LH or SR). For studies that were not excluded or when we had insufficient information to decide whether they met the inclusion criteria, we obtained full reports based on their abstract (Criteria for considering studies for this review). Two review authors (NK and LH or SR) reapplied the exclusion criteria to full reports and excluded from the review those that did not meet these criteria. The review team translated non‐English abstracts and full texts of studies where necessary, using Google Translate.
We describe the flow of studies by using a PRISMA flow chart (Moher 2009) (see Figure 1: PRISMA: Flow of studies diagram).
Data extraction and management
One member of the review team (NK and LH or SR) independently extracted descriptive details from full reports, and a second review author (LH, SR or NK) confirmed them. Review authors used a specially‐designed data collection form that was initially piloted and revised to ensure that relevant details were consistently collected from the full reports. We resolved conflicts in data extraction with the help of the third review author (LH or SR). We extracted data related to study population, design, intervention, randomisation methods, blinding, sample size, attrition and handling of missing data, other potential risks of bias, outcome measures, follow‐up duration and methods of analysis. We also extracted data specifying characteristics of the intervention and controls, such as content of the curriculum (e.g. Did it include addressing attitudes? Did it focus on providing counselling and psychological response training? Was it focused on skills like AVDR or identification only, duration and frequency, setting, training method and delivery mode?). We documented contextual factors and equity considerations when the information was available. For outcomes that were ambiguous or that were reported only in graphical form, we contacted the study authors for additional details.
We entered and managed extracted data on electronic data collection forms created in EPPI Reviewer software, version 4.0 (EPPI‐Reviewer 2010).
Assessment of risk of bias in included studies
Using the Cochrane risk of bias tool (Higgins 2011), two review authors (NK and LH or SR) independently assessed the risks of bias as low risk, high risk, or unclear risk for each included study across the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcomes, selective reporting, and other. More details of how risk of bias was assessed are in Appendix 3. We resolved disagreements through discussion and consultation with the third review author (SR or LH) as necessary.
We summarised the risk of bias for each study across domains. We present the results for each included study in a risk of bias table under the Characteristics of included studies and in a summary table and graph (Figure 2).
Measures of treatment effect
Binary data
For one study, which we report in Table 1, an odds ratio (OR) was provided. We used the data that were reported in that study to calculate the risk ratio (RR), which we report in the table.
Continuous outcome data
For continuous outcomes, we extract and pool differences of endpoint minus baseline values (meta‐analysis of difference‐in‐differences, which removes the component of between‐person variability from the analysis). When one study failed to report this information, we used and pooled the change score provided by the study. We entered outcome data on means, standard deviations and number of participants in each arm into an Excel sheet and into EPPI‐Reviewer 2010. As different scales were used to measure the same outcome, we synthesised results using the standardised mean difference (SMD) and 95% confidence intervals in random‐effects (DerSimonian 1986) models using Stata 16 software (Stata 2019) and Review Manager 2020, where the data permitted such a synthesis. In line with the specification in our protocol, we also report a fixed‐effect (Inverse variance) model for each meta‐analysis. However, due to the heterogeneity across trials and large Tau2 values, we consider the random‐effects model results to be our primary meta‐analysis results. We interpreted the SMD using Cohen’s D, whereby we interpreted about 0.2 as a small effect, an SMD of about 0.5 as a moderate effect, and a SMD of more than 0.8 as a large effect (Cohen 1977; Cohen 1988).
Multiple outcomes
If a study used more than one measure of the same outcome, we did not double‐count the data and gave preference to outcomes from a standardised scale.
Endpoint versus change scores
For continuous outcomes, we extracted and pooled differences of endpoint minus baseline values, where the latter were reported. If only change scores were available from the primary studies, we used these and relied on the assumption of good balance across the two arms at baseline.
Unit of analysis issues
Cluster‐RCTs
In the case of cluster‐RCTs, we assessed if the study authors had taken clustering into account in the data analyses. When an individual study had failed to conduct and report the proper analysis, we looked for the intra‐cluster correlation coefficients (ICCs) and found that these were not available in the studies where clustering had not been taken into account. In the absence of an ICC to borrow from other studies, we used the approach suggested by McKenzie 2016, that involves inflating the standard error of the estimated intervention effect (rather than reducing the sample size). This approach requires the calculation of a design effect, and therefore an estimate of the ICC. The adjustment is computed by multiplying the standard error by the square root of the design effect. The design effect can be calculated as 1+(M‐1)*ICC (where M is the mean cluster size). We explored some simulated examples using various combinations of M and the ICC. The 10% inflation is based on an M value of around 10 and ICC of 0.025 whilst the 30% inflation uses a very conservative value of ICC of 0.1. In the absence of a reliable estimate of the design effect, we conducted sensitivity analyses with and without inflating the standard errors (SEs) by 10% and 30% (Sensitivity analysis). In the meta‐analyses, we used the SE inflated by 10% for studies that did not correct for the ICC.
Multi‐arm trials
When a study involved more than one treatment group, including different individuals relevant to the review, we reported the multiple interventions in a narrative manner and used only the treatment group that was most compatible with the other interventions in the meta‐analysis. We retained the treatment arm with intervention components that were most similar to the treatment in other studies.
Dealing with missing data
We report on the extent and nature of missing data in the Risk of bias in included studies tables.
For each outcome, we extracted and reported potential reasons for missing data, where reported, how the missing data were handled (ignored, last observation carried forward (LOCF), statistical modelling, etc.), if specified, and the impact of missingness on review results. We also assessed and reported the risk of bias due to selective reporting of outcomes and attrition for each study. We contacted study authors for any unreported data. We ran a complete‐case analysis (by pooling only available data) on the assumption that the missing data are the same as the observed data.
Assessment of heterogeneity
Clinical heterogeneity
Although we had hoped to assess all studies together, we expected variation in studies due to type of provider, type of intervention (content of training, training technique, intensity and duration of intervention), and outcome measurement. Clinical diversity introduced by variation in the type of provider may be negligible to answer our overall question about all HCPs; however, clinical diversity in the type of training may need further exploration through subgroup analysis. Given this, we critically assessed the extent of this heterogeneity by performing subgroup analyses (see section on Subgroup analysis and investigation of heterogeneity).
Statistical heterogeneity
We assessed heterogeneity in statistical effects in each meta‐analysis by using the I2 statistic and evaluating the Chi2 test of homogeneity (Deeks 2021).
The Chi2 test has low power with few studies and small sample sizes, so although a statistically significant result may indicate some level of heterogeneity across studies, a non‐significant result does not indicate homogeneity. For this reason, we considered probability values less than 0.10 as statistically significant. Along with the Chi2 test, we quantified inconsistency of results through the I2 statistic, which is the proportion of variability in the effect of estimates due to heterogeneity, rather than to chance alone.
We performed both fixed‐effect and random‐effects models; with the latter, we provided an estimate of between‐study variance (Tau2).
Assessment of reporting biases
We graphically displayed funnel plots to assess asymmetry and investigate small‐study effects and other possible reasons (e.g. publication bias) for asymmetry.
Data synthesis
We present a narrative overview of intervention characteristics and findings.
We pooled results only when we expected minimal clinical heterogeneity (i.e. in the intervention, population and outcomes) between studies. We conducted a separate analysis of each outcome assessed at less than one year, at one to two years, and beyond two years. We conducted the meta‐analysis using the statistical software Stata 16 (Stata 2019). The main commands used were metan (for the meta analysis) and metafunnel (for the funnel plots).
When we detected substantial statistical heterogeneity (i.e. if the I2 value was greater than 50%), we used a random‐effects model to account for the heterogeneity. We reported and commented on the results of the model that was more relevant (random‐effects model where heterogeneity is formally incorporated into the pooled estimates) in our main results. However, in line with our protocol, we used both random‐effects (DerSimonian 1986) and fixed‐effect models (inverse variance method) to calculate the pooled intervention effect for each outcome, and we present the results in a sensitivity analysis. As we found substantial statistical heterogeneity in the main pooled results and since the true intervention effect size varies across studies, as well as keeping in mind our aim to generalise the results to similar populations, we use the results from the random‐effects model.
When we found large variation among types of interventions, comparisons and outcomes evaluated in the reports included in this review and it was not appropriate to conduct a statistical meta‐analysis, we described and synthesised study findings in a narrative manner. For example, we considered that the studies comparing intervention to no intervention, wait‐list or placebo were different to those that compared intervention to treatment as usual. We therefore did not combine these studies with different control arms in the same meta‐analysis. Similarly, the studies that compared intervention to ‘treatment as usual’ were clinically heterogeneous due to the wide variation in what was considered usual treatment and therefore were not combined in a meta‐analysis. We structured the narrative synthesis around outcomes. We were unable to synthesise data to comment on clinical significance based on the outcomes provided in the studies.
Subgroup analysis and investigation of heterogeneity
We explored clinical heterogeneity by conducting the subgroup analyses listed below.
Intervention type: we pooled together two or more studies that provided AVDR (including interventions that did not explicitly identify themselves as AVDR, such as RADAR or several others), and separately pooled those that addressed only a response to violence and did not address attitudes, beliefs or knowledge change, in order to identify the effective components of an intervention.
Duration of the intervention: We pooled together interventions that took less than one day, that required two to seven days of training, and that lasted longer than one week. If booster sessions were provided, we included their duration when calculating the duration of the intervention.
Mode of delivery: We pooled together two or more studies that reported on delivery modes, including computer‐based training or in‐person lectures.
Teaching technique: We pooled together two or more studies that used specific teaching techniques including role‐plays, group discussions, lectures, experiential training or simulations
Sensitivity analysis
We conducted sensitivity analyses to assess the robustness of the overall meta‐analysis to the following.
Model of meta‐analysis: we conducted both fixed‐effect and random‐effects meta‐analyses and presented both results when they differed.
ICC: for studies that did not account for clustering and where we could not borrow an ICC from other studies, we explored some simulated examples according to various combinations of mean and the ICC to account for clustering. Whilst the base case analysis is based on the 10% inflation of the SE of the study which did not consider the cluster effect, we have also explored two sensitivity analyses: one based on a scenario which did not inflate the SE of Short 2006b and another including a 30% inflation. See section on Differences between protocol and review for our justification for using this approach.
Outliers: where a study appears to be an obvious outlier, in line with the suggestion by Ryan 2016 we carried out sensitivity analyses with and without the study.
Summary of findings and assessment of the certainty of the evidence
We used the five GRADE criteria: study limitations, imprecision, inconsistency of results, indirectness of evidence and likelihood of publication bias (Guyatt 2011), to assess the overall quality of the body of evidence for each of the Primary outcomes within 12 months of the intervention (HCPs attitudes towards, knowledge of and readiness to manage IPV, referrals for and response to IPV). On the basis of design, we viewed the RCTs included in the review as providing high‐certainty evidence, but downgraded to moderate, low or very low certainty, depending on the presence of the aforementioned GRADE criteria.
One review author (NK) applied the GRADE criteria independently, and another (LH) reviewed them. No disagreements occurred. We used this information to populate the overall certainty of evidence section of the Table 1, that also included information on the number of participants, RR for dichotomous outcomes, SMD for continuous outcomes, number of studies and their design. We created this table by using the software developed by the GRADE working group: GRADEprofiler (GRADEpro GDT 2014), for the comparison: training to respond to IPV versus no intervention, wait‐list, or placebo.
Results
Description of studies
Results of the search
We identified a total of 11,413 records from searches of bibliographic databases, and 56 additional records from websites and reference checking of relevant studies and systematic reviews (Figure 1). We removed 3123 duplicates and screened 8346 titles and abstracts. Abstract screening revealed 8103 irrelevant records, leaving 243 for full‐text review. The review authors excluded 213 records that did not meet the review criteria (see Figure 1). We identified 19 studies (from 21 reports) for synthesis and possible meta‐analysis. We contacted 31 study authors for additional information, 10 of whom responded and provided further details to aid us in our evaluation of potential study eligibility for inclusion (Ayaba‐Apawu 2016; Abraham 2011; Dubowitz 2011; Feder 2011; Feigelman 2011; Harris 2002. Hegarty 2013; Jack 2019; McFarlane 2006; Short 2006b). From the 9 reports which remained, we identified 3 which are awaiting classification, and 6 ongoing studies.
1.
Study flow diagram.
Included studies
We include 19 studies in this review (from 21 reports). Fifteen of these are peer‐reviewed journal articles (Brienza 2005; Coonrod 2000; Danley 2004; Edwardsen 2006; Gupta 2017; Gürkan 2017; Haist 2007; Harris 2002; Hegarty 2013; Hsieh 2006; Lo Fo Wong 2006; Moskovic 2008; Sharps 2016; Short 2006b; Vakily 2017), and four are PhD theses (Ayaba‐Apawu 2016; Cutshall 2019; Mauch 1982; Ragland 1989).
The earliest trials were two unpublished PhD dissertations in the 1980s (Mauch 1982; Ragland 1989). Most of the studies (n = 12) were published in the 2000s. In the past decade, seven trials have been published, three of which were published in 2017 (Gupta 2017; Gürkan 2017; Vakily 2017).
Additional reports on studies by Gupta 2017 and Hegarty 2013 were found in their respective published protocols. We also received results for Hegarty 2013 through personal communication. Information for Short 2006b and Harris 2002 was obtained through correspondence with Harris 2002.
Location and healthcare settings
Most studies were conducted in the USA (14 studies), with the remaining single studies from Australia (Hegarty 2013), and Iran (Vakily 2017), Mexico (Gupta 2017), The Netherlands (Lo Fo Wong 2006), and Turkey (Gürkan 2017).
Healthcare provider training interventions occurred in various teaching and clinical settings. Universities were the most frequently‐reported location for training (seven studies: Ayaba‐Apawu 2016; Brienza 2005; Danley 2004; Edwardsen 2006; Hsieh 2006; Mauch 1982; Ragland 1989), followed by primary care clinics (five studies: Gupta 2017; Haist 2007; Hegarty 2013; Lo Fo Wong 2006; Sharps 2016), and clinical teaching hospitals/schools (four studies: Coonrod 2000; Gürkan 2017; Moskovic 2008; Vakily 2017). Three studies used online platforms to deliver and evaluate provider IPV training (Cutshall 2019; Harris 2002; Short 2006b).
Study designs
Study designs varied. Twelve studies were RCTs conducted at the individual participant level (Ayaba‐Apawu 2016; Brienza 2005; Coonrod 2000; Cutshall 2019; Danley 2004; Haist 2007; Harris 2002; Hsieh 2006; Mauch 1982; Moskovic 2008; Ragland 1989; Vakily 2017). Of the remaining seven studies, four were cluster‐RCTs (Gupta 2017; Hegarty 2013; Lo Fo Wong 2006; Short 2006b), and one apiece were a quasi‐RCT (Gürkan 2017), a quasi‐cluster‐RCT (Edwardsen 2006), and a combination of both individual randomisation in blocks along with randomisation of clusters (Sharps 2016).
Participants and sample size
The 19 included studies covered of 1662 HCPs, with the numbers of participants in each study ranging from 27 (Haist 2007) to 197 (Gupta 2017).
While a range of HCPs were included, medical staff were the most frequently studied (nine studies). This included medical residents/students (five studies: Brienza 2005; Coonrod 2000; Edwardsen 2006; Haist 2007; Moskovic 2008) and qualified physicians/doctors (four studies: Harris 2002; Hegarty 2013; Lo Fo Wong 2006; Short 2006b). The remaining providers included nurses, home visitors and nursing students (three studies: Gupta 2017; Gürkan 2017; Sharps 2016), dentists and dental students (two studies: Danley 2004; Hsieh 2006), counsellors (including social workers) or psychology students/graduates (four studies: Ayaba‐Apawu 2016; Cutshall 2019; Mauch 1982; Ragland 1989), and one study of midwives (Vakily 2017).
Healthcare provider participant socio‐demographic characteristics were poorly described in seven studies (Coonrod 2000; Edwardsen 2006; Gupta 2017; Hegarty 2013; Moskovic 2008; Sharps 2016; Vakily 2017). Only 12 of the 19 included studies clearly defined the sex of participants (Ayaba‐Apawu 2016; Brienza 2005; Cutshall 2019; Danley 2004; Gürkan 2017; Haist 2007; Harris 2002; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Ragland 1989; Short 2006b). Eleven of the 19 studies included healthcare students still in training (Ayaba‐Apawu 2016; Brienza 2005; Coonrod 2000; Danley 2004; Edwardsen 2006; Gürkan 2017; Haist 2007; Hsieh 2006; Mauch 1982; Moskovic 2008; Ragland 1989), who had limited or no previous IPV training prior to the intervention.
IPV training interventions
Training content
Few studies provided comprehensive detail on IPV training content, with significant variation noted across trials. Studies commonly included some form of general information about IPV (types and definitions, prevalence and risk factors) to challenge myths and provider misinformation (Ayaba‐Apawu 2016; Brienza 2005; Cutshall 2019; Edwardsen 2006; Gupta 2017; Haist 2007; Hegarty 2013; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Ragland 1989; Sharps 2016; Short 2006b; Vakily 2017). Some also covered historical and cultural aspects of IPV (Cutshall 2019; Mauch 1982; Ragland 1989; Vakily 2017), including sex role socialisation (Mauch 1982; Ragland 1989). Ragland 1989 showed media clips (news, movies/television) to emphasise community attitudes towards abused women.
Expanded IPV training content included exploration of common clinical presentations/impact on women’s health (Gupta 2017; Gürkan 2017; Haist 2007; Hegarty 2013; Lo Fo Wong 2006; Short 2006b), including perinatal health (Sharps 2016), reproductive coercion (Gupta 2017), and profiles on perpetrators and effects of partner violence on children (Lo Fo Wong 2006).
Some studies then followed up with clinical practice requirements, most frequently providing training in AVDR: Asking about IPV in order to identify IPV survivors, providing Validating responses, ensuring accurate Documentation, and Referralto specialist services. In particular, Danley 2004 and Hsieh 2006 used this AVDR framework explicitly in the development of their tutorial to train dentists on IPV. Danley 2004 reported delivering content on AVDR without any mention of foundational theory on IPV, such as common clinical presentations and impacts of IPV. Hsieh 2006 included how to identify signs of IPV in dental patients and then used an interactive, multimedia AVDR tutorial.
Other authors based their provider training around some or all of these core (AVDR) elements. The most common element of training (based on what was mentioned by the study authors) was on IPV identification through asking or routine screening (Ayaba‐Apawu 2016; Brienza 2005; Coonrod 2000; Danley 2004; Edwardsen 2006; Gupta 2017; Gürkan 2017; Haist 2007; Harris 2002; Hsieh 2006; Lo Fo Wong 2006; Sharps 2016; Short 2006b; Vakily 2017). Only a third of studies reported training providers on accurate documentation of IPV (Ayaba‐Apawu 2016; Danley 2004; Edwardsen 2006; Gürkan 2017; Harris 2002; Hsieh 2006; Sharps 2016). More than half of the included studies mentioned that they provided training on how to validate survivor experiences (Brienza 2005; Danley 2004; Edwardsen 2006; Gupta 2017; Harris 2002; Hegarty 2013; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Ragland 1989; Short 2006b). A small sub‐set of these explicitly mentioned that they provided some kind of training in counselling (Gupta 2017; Hegarty 2013; Lo Fo Wong 2006; Mauch 1982). Over half of all included studies explicitly mentioned training on referral options, including information about local women’s shelters (Ayaba‐Apawu 2016; Brienza 2005; Gupta 2017; Gürkan 2017; Harris 2002; Lo Fo Wong 2006; Sharps 2016; Danley 2004; Edwardsen 2006; Hsieh 2006; Short 2006b).
Moskovic 2008 provided little detail on the didactic content of IPV training but included an experiential component (termed "outreach" (p 1043) by the authors) in the intervention, where medical students delivered the curriculum to adolescents on dating violence. A women’s DV safe shelter experience was offered to medical residents in Brienza 2005, to augment their IPV education by attending group evening sessions, where women survivors discussed their experiences and the impact of IPV on their work, health and children.
Edwardsen 2006 described the testing of a mnemonic to aid medical student IPV identification and management. All students received one hour of training on IPV, which included exposure to the mnemonic SCRAPED (Identification of IPV:Suspicion/screen, Central injuries, Repetitive, Abuse stated, Possessive partner, Explanation inconsistent, Direct questions; and Management of IPV: Safety, Crime reported, Referral, Acknowledgement, Protocols, Evidence collection, Documentation) and a model interview with an IPV survivor. The intervention arm then completed a smaller one‐hour workshop, which included detailed instruction and practice asking simulated patients about IPV, while using a laminated copy of the SCRAPED mnemonic. Controls received ‘standard teaching methods’ and obtained the mnemonic after the workshop. In a family home‐visiting IPV intervention that focused on prevention and early intervention, Sharps 2016 tested the Domestic Violence Enhanced Home Visitation Program (DOVE), which included training nurses to empower women by providing information, risk assessment, safety planning, emphasising her options and supporting her decision‐making and autonomy.
Other common content included explicitly addressing HCPs' beliefs or attitudes towards IPV (Ayaba‐Apawu 2016; Lo Fo Wong 2006), understanding the barriers to identifying IPV (Brienza 2005; Lo Fo Wong 2006), and IPV survivor barriers to presenting and disclosing violence (Brienza 2005; Mauch 1982). Also included were safety planning and risk assessment (Ayaba‐Apawu 2016; Gupta 2017; Gürkan 2017; Haist 2007; Sharps 2016; Short 2006b), use of clinical guidelines and screening tools/methods (Gürkan 2017; Haist 2007; Harris 2002; Lo Fo Wong 2006; Sharps 2016), and training on mandatory reporting/legislative requirements (Cutshall 2019; Gupta 2017; Gürkan 2017; Haist 2007; Harris 2002; Lo Fo Wong 2006; Mauch 1982; Short 2006b).
Women’s ‘readiness to change’ their IPV relationship was included by Hegarty 2013 and Short 2006b in their online medical training programmes. Hegarty 2013 used motivational interviewing, tailoring a women‐centred approach to care with an emphasis on assessing women’s readiness rather than using a structured approach like AVDR alone, while Short 2006b developed an interactive case study to emphasise the concept of behaviour change and women’s readiness.
IPV training duration and methods
IPV training time frames ranged from 15‐minute, brief education sessions (Danley 2004; Hsieh 2006) to three days of intensive IPV training with clinic follow‐up visits by educators, to support HCPs' practice skills (Gupta 2017). More than half of the included studies (n = 9) offered two hours or less of IPV training. The remaining studies delivered approximately three (Ayaba‐Apawu 2016), four (Vakily 2017), five (Mauch 1982), six (Moskovic 2008), eight (Gürkan 2017; Hegarty 2013; Sharps 2016) and 15 (Cutshall 2019) hours of training, respectively. Hegarty 2013 and Sharps 2016 implemented provider training prior to and in addition to broader supportive systems‐level interventions. Cutshall 2019 offered 15 hours of online, interactive problem‐based learning to licensed professional counsellors, social workers and psychologists, delivered over three five‐hour modules. Short 2006b provided online continuous medical education (CME) to practising primary care physicians in the USA that included four to 16 hours of IPV learning. Most physicians (65%) completed the minimum time frame to obtain four CME points (Short 2006b), with only two physicians completing all online modules to obtain the full 16 CME points that count towards the physicians' continued licensing requirements. Two studies included booster training sessions, one at three months after training (Gupta 2017) and the other annually (Sharps 2016).
IPV training methods, as part of the interventions, were very heterogeneous. The varied level of detail provided in papers on IPV training made synthesis challenging. Most applied a didactic portion of the training and combined it with other varied pedagogical methods. Delivery of the intervention through group work was more common (12 studies: Brienza 2005; Coonrod 2000; Cutshall 2019; Edwardsen 2006; Gupta 2017; Gürkan 2017; Haist 2007; Lo Fo Wong 2006; Mauch 1982; Moskovic 2008; Ragland 1989; Sharps 2016) than individually‐delivered content, which was usually accessed online (six studies: Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hegarty 2013; Hsieh 2006; Short 2006b). One study (Vakily 2017) offered both methods, where team learning was followed up by viewing online content in individual sessions.
A variety of teaching methods were used to educate HCPs on IPV, and the skills required to respond to survivors of IPV effectively. Lecture or didactic information sessions, often combined with role‐play, were the most common (Ayaba‐Apawu 2016; Brienza 2005; Gupta 2017; Gürkan 2017; Hegarty 2013; Lo Fo Wong 2006; Mauch 1982; Sharps 2016), where participants worked in small groups and undertook the role of the HCP or patient (or used simulated patients) and practised asking about violence and responding in line with best‐practice methods. Nine studies used video footage in educational sessions to reinforce didactic content, depict survivor voices and model sound counselling skills (Brienza 2005; Coonrod 2000; Gupta 2017; Gürkan 2017; Hsieh 2006; Mauch 1982; Ragland 1989; Short 2006b; Vakily 2017). Case studies/scenarios were also used and reflected common clinical presentations (Harris 2002; Lo Fo Wong 2006; Short 2006b; Vakily 2017).
Clinical case studies or vignettes, familiar to trainees, were frequently used as learning tools. These were included as part of group work or online sessions (Gürkan 2017; Harris 2002; Lo Fo Wong 2006; Ragland 1989; Short 2006b; Vakily 2017). Seven studies used interactive online multi‐media methods (Cutshall 2019; Danley 2004; Harris 2002; Hegarty 2013; Hsieh 2006; Short 2006b; Vakily 2017).
Vakily 2017 offered a compact disc (CD) of IPV content (general information, case reports and videos) to the intervention‐group midwives, who also received the same content in didactic form. Harris 2002 and Short 2006b used online case studies of common clinical situations to reinforce best practice for existing medical practitioners. After watching the scenarios, users answered questions on how to respond and were provided with correct answers. Information also included online resources and referral options. Short 2006b used the online platform to deliver 17 typical, interactive, clinical IPV cases. These included simulated cases that often present in specialty areas: family medicine, mental health services, paediatrics and obstetrics and gynaecology.
Danley 2004 and Hsieh 2006 used a (brief) online AVDR teaching method for dentists. The tailored interactive resource depicts a clinical interaction between practising dentist and a patient (actors) who presents with facial trauma. Online users ask the virtual patient questions and they respond in various ways. The dentist then guides the user on their interaction and practice. Training methods evolve as authors engage with technology and advanced pedagogy. Hegarty 2013 used online distance education training combined with four teleconference sessions for doctors that were followed up with clinic visits for role‐play skills with simulated patients.
Other aspects of IPV training included the provision of readings (Ayaba‐Apawu 2016; Coonrod 2000; Edwardsen 2006; Hegarty 2013), and modelling best‐practice interviewing/counselling of patients, either by the educator (Mauch 1982) or using simulated patients (Edwardsen 2006; Haist 2007; Hegarty 2013). In a novel approach, Moskovic 2008 provided didactic IPV training to all medical students included in the trial. In addition, intervention‐group students delivered community‐based education to high school students on dating violence and relationship conflict, which reinforced their IPV learning.
Comparisons
Twelve studies compared IPV training with no training (Ayaba‐Apawu 2016; Cutshall 2019; Gürkan 2017; Lo Fo Wong 2006; Mauch 1982), placebo (Coonrod 2000; Haist 2007; Ragland 1989), or a wait‐list control group (Danley 2004; Harris 2002; Hsieh 2006; Short 2006b).
In three studies the control arm received some form of intervention that was described as usual care: Gupta 2017 (one day training); Sharps 2016 (usual care); and Vakily 2017 (traditional training).
For another four RCTs (Brienza 2005; Edwardsen 2006; Hegarty 2013; Moskovic 2008), an intervention with multiple components was provided in the intervention arm and one of the sub‐components of that intervention was provided alone in the comparator arm. This type of study tests the impact of component A of an intervention by implementing component A + component B in the intervention arm, versus only component B in the control arm. Brienza 2005 tested experiential learning in a women’s safety shelter + workshop seminar versus workshop seminar alone. Edwardsen 2006 tested the effect of a mnemonic technique + lecture/simulated patient versus lecture/simulated patient alone. Hegarty 2013 tested the impact of the Healthy Relationship Training program focused on responding to IPV survivors + a basic IPV education versus basic IPV education alone. Moskovic 2008 tested the impact of outreach education to adolescents + didactic training on IPV versus didactic training on IPV alone.
Measurement of outcomes
Primary outcomes
'Attitudes or Beliefs about IPV' were assessed in 10 studies using scales or subscales. The most commonly‐reported scales to measure attitudes or beliefs towards IPV were the Attitude Toward Battered Women Questionnaire (Mauch 1982; Ragland 1989) and the victim‐understanding subscale of the Physician Readiness to Manage Intimate Partner Violence Survey (PREMIS) (Ayaba‐Apawu 2016; Harris 2002; Short 2006b). Other measures used to assess HCPs' attitudes and beliefs towards IPV were: the Attitudes Towards Domestic Violence Scale (ATDVS) (Gürkan 2017); the attitude subscale of the Domestic Violence Assessment Instrument (Danley 2004); and the attitude subscale of the Jefferson Scale of Physician Empathy (Hsieh 2006). Brienza 2005 used an attitude subscale of an instrument adapted from the Health Care Provider Survey for Domestic Violence, while Vakily 2017 developed a standardised 15‐item attitude measure for their study.
Ten studies assessed HCPs' 'readiness to manage/respond to survivors of IPV'. One study assessed this using a nine‐item (self‐developed) scale, which they called HCP confidence and attitude (Moskovic 2008); however, the items appear to assess medical students' confidence in their ability to address IPV, and discuss, recognise and respond to IPV. The most common scales to assess HCPs' readiness to respond to IPV were the self‐efficacy (Harris 2002; Short 2006b) or perceived preparation (Ayaba‐Apawu 2016; Cutshall 2019; Hegarty 2013) subscales of the PREMIS, and the intended asking, validating, documenting and referring subscale of the Domestic Violence Assessment Instrument (Danley 2004; Hsieh 2006). Gürkan 2017 used HCP responses to open‐ended questions following a 'Written case study of violence against women' (WCSVAW) to determine percentage change in correct responses between intervention and control. Brienza 2005 assessed self‐perceived skills and resource awareness using an adaptation of the Health Care Provider Survey for Domestic Violence instrument.
HCPs' 'knowledge of IPV' was mostly assessed using questions that appear to have been developed by the authors (Coonrod 2000; Gürkan 2017; Moskovic 2008; Vakily 2017). A few studies used subscales, such as Brienza 2005 who assessed knowledge of IPV using a seven‐item subscale of the Health Care Provider Survey for Domestic Violence instrument, and Ayaba‐Apawu 2016; Cutshall 2019; Hegarty 2013 and Short 2006b, who reported on actual and/or perceived knowledge using the PREMIS (we only used results from the actual knowledge subscale and not perceived knowledge for this outcome).
'Referrals' provided by HCPs were assessed in three of the 19 included studies by measuring women’s use of community resources (as a result of HCP referrals) (Gupta 2017), the instances of offering referrals to simulated patients (Edwardsen 2006), or by asking an office manager about practice‐level referral relationships and if referrals of women to IPV services was routine or if there was evidence of contact with IPV service providers (even though only some HCPs from the practices were allocated to the control or intervention arm) (Short 2006b).
Four of the 19 studies in this review evaluated HCPs' 'response to IPV' through the actual provision of safety planning or counselling and/or validation of survivors’ feelings using standardised patient reports or reports of assessors or women survivors of IPV (Edwardsen 2006; Gupta 2017; Haist 2007; Short 2006b). Edwardsen 2006 used a standardised checklist completed by simulated patients to evaluate HCP students’ provision of validation and counselling using the question “Acknowledg[ing] the violence and provid[ing] empathy” (Edwardsen 2006, p 63), yes or no, and the question “Was safety of the patient addressed” (Edwardsen 2006, p 65) to assess safety planning. Haist 2007 also used standardised patients to examine the effects of training. Immediately after their appointment with the medical resident, the simulation patient completed a checklist that included an eight‐item measurement of the safety plan counselling provided. Short 2006b also evaluated the provision of safety planning using trained assessors and a standardised checklist; however, this was done at the level of the full practice. The checklist evaluated office safety planning using the question “Safety planning for IPV victims done” (Short 2006b, p 32) but it is unclear how exactly this was assessed. Gupta 2017 examined women’s use of safety planning behaviours three months and 15 months after providers attended a training intervention.
Secondary outcomes
Six of the 19 included studies reported on 'documentation or identification of partner violence' as an outcome of IPV training (Brienza 2005; Coonrod 2000; Edwardsen 2006; Gürkan 2017; Haist 2007; Lo Fo Wong 2006). Two studies asked HCPs to self‐report on the frequency of their documentation or identification of IPV (Brienza 2005; Coonrod 2000). Two studies used a standardised insinuated patient approach where standardised patients reported or encounters were video‐recorded and coded to assess IPV identification (Edwardsen 2006; Haist 2007). Lo Fo Wong 2006 looked at doctors' IPV incident‐reporting forms and (confirmed/not confirmed) cases. Gürkan 2017 used the WCSVAW to assess correct student nurse diagnoses of physical, psychological and verbal violence at two months post‐training. The tool "prepared by specialists in the project" was a survivor story describing various types of violence with subsequent questions asking students the correct diagnosis and clinical response. Correct answers were coded as higher scores.
Two studies looked at the impact of interventions on "mental health outcomes of women survivors of IPV" as well as on adverse outcomes such as "rates of IPV" (Gupta 2017; Sharps 2016). Gupta 2017 reported using the SF‐12 (mental) quality‐of‐life measure to assess mental health and the physical and sexual IPV instrument from the WHO Multi‐Country Study on Domestic Violence and Women’s Health to assess IPV. Sharps 2016 reported that they used the Edinburgh Postnatal Depression Scale (EPDS) to assess the mental health of survivors of IPV and the Conflict Tactics Scale 2 to assess IPV.
Funding
Of the 19 included studies, 11 received some form of formal funding from internal sources such as university research funding (Edwardsen 2006; Gürkan 2017; Haist 2007; Vakily 2017) or external Government grant funding bodies (Danley 2004; Hegarty 2013; Hsieh 2006; Sharps 2016), including those focused on women’s health (Moskovic 2008) or mental health (Harris 2002; Short 2006b). One study reported receiving some funding from an anonymous donor (Gupta 2017), and one from a health insurance company research grant (Lo Fo Wong 2006). Four studies were PhD dissertations with no funding source identified (Ayaba‐Apawu 2016; Cutshall 2019; Mauch 1982; Ragland 1989), and two studies did not include information about funding sources (although both declared no conflict of interest) (Brienza 2005; Coonrod 2000).
Excluded studies
We excluded 213 ineligible studies after reviewing their full texts, for the reasons given in Figure 1. From these, we selected 18 RCTs and cluster‐RCTs to report in the Characteristics of excluded studies tables. These studies appeared to meet the eligibility criteria, but on closer inspection did not, mostly on the basis of the intervention. They contained a training component but, in addition to training HCPs, a system change or an additional component was also implemented in the intervention arm; the impact of training alone therefore could not be assessed. Among these, we also list one quasi‐experimental study for similar reasons. We only included HCP training interventions that did not involve more complex systems interventions, as inclusion of confounding elements would have prevented us from clearly determining training benefits. Some studies were excluded because they did not assess the impact of training intervention, while others did not assess the impact of training on IPV specifically.
Studies awaiting classification
For another three studies (Abraham 2001; Abraham 2011; Hill 2016), we were unable to make a decision about eligibility due to a lack of data in the study reports, and a lack of response from the study authors to our request for data. For Abraham 2001; Abraham 2011 we were unable to determine what type of violence the HCPs were trained in. Details of these studies can be found in the Characteristics of studies awaiting classification tables.
Ongoing studies
We identified six protocols for RCTs (NCT00257296; NCT01028118; Pallitto 2016) or cluster‐RCTs (Fernández 2006; NCT03259646; Ruijne 2017) for which we were unable to find the full‐text reports. Most involved delivering mental health and empowerment‐based interventions to patients who were survivors of IPV, while others involved training on referral pathways and increasing knowledge of providers. Control groups appeared to receive standard care, except in one study where it was not specified, and in another where it was explicitly stated that they received no IPV training. We contacted the study authors for further information where the end date was not provided or had already passed. For one of these studies (Fernández 2006) the abstract reported that the protocol had been suspended. Details of these studies can be found in the Characteristics of ongoing studies tables.
Risk of bias in included studies
We provide a graphical summary of the risk of bias assessment in Figure 2. More details can be found in the risk of bias tables (beneath the Characteristics of included studies tables).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated seven studies at low risk of bias due to sequence generation as they used computer‐generated randomisation or randomisation using random number tables (Brienza 2005Coonrod 2000; Danley 2004; Gupta 2017; Hegarty 2013; Hsieh 2006; Moskovic 2008). We considered another study, Ayaba‐Apawu 2016, to be at low risk of bias also, as it randomised participants using a lottery/picking from a bowl method. For 10 studies, the risk of bias was unclear, as the method for generating the random sequence was not specified (Cutshall 2019; Edwardsen 2006; Haist 2007; Harris 2002; Lo Fo Wong 2006; Mauch 1982; Ragland 1989; Sharps 2016; Short 2006b; Vakily 2017). One study was at high risk of bias due to their sequence generation as they used a quasi‐random method of allocation (Gürkan 2017).
Randomisation was carried out after recruitment, at one point in time using computer‐generated random numbers or random‐number tables in five trials and therefore unclear allocation concealment was not a concern in these studies (Ayaba‐Apawu 2016; Danley 2004; Hsieh 2006; Lo Fo Wong 2006; Moskovic 2008). The allocation concealment process was not adequately described in thirteen trials (Brienza 2005; Cutshall 2019; Edwardsen 2006; Gürkan 2017; Haist 2007; Harris 2002; Hegarty 2013; Mauch 1982; Ragland 1989; Sharps 2016; Short 2006b; Vakily 2017). One study indicated that those who recruited and screened participants into the trial were not blind to allocation. Hence the risk of allocation bias for this study (Gupta 2017) was considered high.
Blinding
Blinding of participants was potentially difficult in these trials and 13 studies did not specify whether participants were blind to allocation (Brienza 2005; Danley 2004; Gürkan 2017; Haist 2007; Harris 2002; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Moskovic 2008; Ragland 1989; Sharps 2016; Short 2006b; Vakily 2017) so we rated the risk of bias for these studies as unclear. One study compared the intervention to treatment as usual and stated that the women were blind to allocation of the clinic they presented at. However, HCPs trained at the clinic were not blind to allocation (Gupta 2017). We therefore judged the risk of bias for this study as unclear. Two studies had a low risk of bias from blinding of participants. They stated that they had compared the intervention to another treatment/placebo while attempting to maintain masking of participants (Coonrod 2000; Edwardsen 2006). Participants seemed to be aware of allocation and study process in two studies (Ayaba‐Apawu 2016; Cutshall 2019) and were rated as high risk of performance bias. Only one study explicitly stated that blinding was not carried out (Hegarty 2013) and was also rated as high risk of bias.
For 15 studies, we judged the risk of detection bias as being low, due to the outcomes being self‐reported by participants (Ayaba‐Apawu 2016; Brienza 2005; Coonrod 2000; Cutshall 2019; Danley 2004; Gürkan 2017; Harris 2002; Hegarty 2013; Hsieh 2006; Mauch 1982; Moskovic 2008; Ragland 1989; Sharps 2016; Short 2006b; Vakily 2017). In two studies assessors were blind to allocation and so we judged the risk of bias to be low (Edwardsen 2006; Haist 2007). In one study it was unclear if assessors were blind to allocation (Lo Fo Wong 2006). In another study, the risk of detection bias was low for women's self‐reported outcomes as they were blind to allocation, but overall the risk of detection bias was unclear as HCPs were not blind to allocation (Gupta 2017).
Incomplete outcome data
Six studies had a high risk of bias from high attrition of more than 20% (Brienza 2005; Gupta 2017; Gürkan 2017; Harris 2002; Sharps 2016; Short 2006b). Loss to follow‐up was low in 12 studies, with rates ranging from 0 to 20% (Ayaba‐Apawu 2016; Coonrod 2000; Cutshall 2019; Danley 2004; Edwardsen 2006; Haist 2007; Hegarty 2013; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Moskovic 2008; Ragland 1989). Loss to follow‐up was unclear in one study (Vakily 2017).
Selective reporting
Sixteen studies were rated as having a low risk of bias for selective reporting. Fifteen studies did not register or refer to a protocol, but they reported the results for all outcomes mentioned in the Methods sections (Ayaba‐Apawu 2016; Brienza 2005; Coonrod 2000; Cutshall 2019; Danley 2004; Gürkan 2017; Haist 2007; Harris 2002; Hsieh 2006; Lo Fo Wong 2006; Mauch 1982; Moskovic 2008; Ragland 1989; Sharps 2016; Vakily 2017); one study published a protocol and discussed all results listed in the protocol (Gupta 2017). We rated two studies as having unclear risk of bias for selective reporting, as one of them did not mention any outcomes in the Methods section and hence we were unable to assess if outcomes were reported selectively (Edwardsen 2006), the other reported results briefly and the authors had to be contacted to obtain adequate information (Short 2006b). We rated one study as being at high risk of bias from selective reporting as we found evidence of selective reporting compared to the protocol; we contacted the authors for these results which were provided and included in the review (Hegarty 2013).
Other potential sources of bias
For 12 studies other potential sources of bias were unclear, as they either did not adequately report their power calculation, and/or whether their measurement tool was standardised and/or whether they were able to contain contamination of the control group. Small sample sizes that could impact the studies’ ability to detect an effect was a potential source of concern in six studies (Brienza 2005; Coonrod 2000; Edwardsen 2006; Haist 2007; Lo Fo Wong 2006; Ragland 1989). Unclear reporting on whether the measures used were standardised or not was another source of potential bias in five studies (Coonrod 2000; Danley 2004; Gürkan 2017; Hsieh 2006; Mauch 1982). Four studies did not provide any intra‐cluster correlations and hence it was not clear whether they accounted for clustering in their analysis or power calculation (Edwardsen 2006; Lo Fo Wong 2006; Short 2006b; Vakily 2017). Potential contamination and spillover was another potential risk in one study (Gürkan 2017).
Effects of interventions
See: Table 1
Comparison 1. Training to respond to intimate partner violence compared to no intervention, wait‐list or placebo
Twelve studies compared IPV training with no training (Ayaba‐Apawu 2016; Gürkan 2017; Lo Fo Wong 2006; Mauch 1982), placebo (Coonrod 2000; Haist 2007; Ragland 1989), or a wait‐list control group (Cutshall 2019; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b).
The Table 1 presents an overview of the studies that report on the primary outcomes and compare IPV training to a comparison group with no intervention, wait‐list or placebo. Most studies have outcomes that relate to building knowledge, confidence or skills in participants that act as potential precursors to the main objectives of this review – namely identification and response.
Primary outcomes
Healthcare providers' attitudes or beliefs towards IPV
Six individually‐randomised trials, one quasi‐randomised trial and one cluster‐randomised trial reported on the effects of training HCPs to respond to IPV compared to no training or wait‐list or placebo on attitude of HCPs towards IPV (Ayaba‐Apawu 2016; Danley 2004; Gürkan 2017; Harris 2002; Hsieh 2006; Mauch 1982; Ragland 1989; Short 2006b).
The pooled effect estimates of these eight studies suggests that compared to no training in responding to IPV, training HCPs to respond to IPV improved their attitudes towards IPV at zero to 12 months after training (standardised mean difference (SMD) 0.71, 95% confidence interval (CI) 0.39 to 1.03; P < 0.001, Tau2 = 0.14, I2 = 78%; 8 studies, 641 participants; low‐certainty evidence; Figure 3; Table 2). The SMD effect size indicates that training interventions appear to have a moderate‐to‐large effect on improving HCPs' attitudes/beliefs around IPV (Cohen 1977). In line with the analysis plan outlined in the Unit of analysis issues, this meta‐analysis (and all others) involved inflating the standard error for Short 2006b by 10%, as the study did not account for ICC.
3.
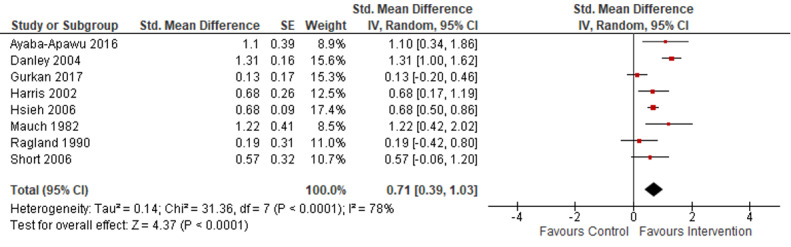
Analysis 1.1: impact of no training in responding to IPV vs training HCPs to respond to IPV on their attitudes towards IPV at zero to 12 months after training
Footnotes
HCP: healthcare providers; IPV: intimate partner violence; IV: inverse variance; SE: standard error; Std: standardised.
1. Comparison 1. Training versus no training, wait‐list, or placebo: attitudes.
| Outcome or subgroup title | No of studies | No of participants | Statistical method | Effect size (95% CI) |
| HCP attitudes (all interventions) | 8 | 641 | Std mean difference (IV, random, 95% CI) | 0.71 (0.39 to 1.03) |
| HCP attitudes (all interventions) | 8 | 641 | Std mean difference (IV, fixed, 95% CI) | 0.70 (0.58 to 0.83) |
| HCP attitudes (no inflation of standard error for Short 2006b) | 8 | 641 | Std mean difference (IV, random, 95% CI) | 0.71 (0.39 to 1.03) |
| HCP attitudes (no inflation of standard error for Short 2006b) | 8 | 641 | Std mean difference (IV, fixed, 95% CI) | 0.70 (0.58 to 0.83) |
| HCP attitudes (30% inflation of standard error for Short 2006b) | 8 | 641 | Std mean difference (IV, random, 95% CI) | 0.71 (0.39 to 1.04) |
| HCP attitudes (30% inflation of standard error for Short 2006b) | 8 | 641 | Std mean difference (IV, fixed, 95% CI) | 0.70 (0.58 to 0.83) |
| ADVR or online ‐ individual training | 5 | 434 | Std mean difference (IV, random, 95% CI) | 0.87 (0.54 to 1.20) |
| Information and validation training | 2 | 71 | Std mean difference (IV, random, 95% CI) | 0.67 (−0.34 to 1.68) |
| < 1 day of training | 7 | 505 | Std mean difference (IV, random, 95% CI) | 0.82 (0.52 to 1.12) |
| Didactic technique: online computer‐based individual training | 6 | 476 | Std mean difference (IV, random, 95% CI) | 0.78 (0.46 to 1.10) |
| Didactic technique: in‐person group discussion/group‐based training | 2 | 165 | Std mean difference (IV, random, 95% CI) | 0.61 (−0.44 to 1.67) |
| Didactic technique: role play | 4 | 454 | Std mean difference (IV, random, 95% CI) | 0.80 (0.30 to 1.29) |
| Didactic technique: case studies | 3 | 251 | Std mean difference (IV, random, 95% CI) | 0.41 (0.03 to 0.78) |
| ADVR: Asking, validating, documenting and referral; CI: confidence interval; HCP: healthcare professional; No: number; Std: standardised; IV: inverse variance | ||||
We determined the overall certainty of the evidence for this outcome to be low, due to unclear risk of bias from lack of blinding in all studies, high risk of bias from high and unequal attrition, lack of standardised tools in some studies, and high risk of bias due to lack of random sequence generation in one study.
Short 2006b reported follow‐up results at 12 months after training and that the improvement in physician attitude towards survivors of IPV was sustained at this time point.
Healthcare providers' readiness to manage
Of the 12 studies that compared training to no intervention, wait‐list or placebo, seven studies reported on HCPs' readiness to respond (Ayaba‐Apawu 2016; Cutshall 2019; Danley 2004; Gürkan 2017; Harris 2002; Hsieh 2006; Short 2006b). Six studies used scales such as PREMIS (Ayaba‐Apawu 2016; Cutshall 2019; Harris 2002; Short 2006b) and the intended asking, validating, documenting and referring subscale of the Domestic Violence Assessment Instrument (Danley 2004; Hsieh 2006) to assess HCPs' readiness to respond to IPV. These six studies reported data that could be pooled in a meta‐analysis (Ayaba‐Apawu 2016; Cutshall 2019; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b). The pooled effect estimate of these six studies suggests that compared to no training in responding to IPV, training HCPs to respond to IPV improved their self‐perceived readiness to respond to survivors of IPV at zero to 12 months after training (SMD 2.44, 95% CI 1.51 to 3.37; P < 0.001, Tau2 = 1.17, I2 = 94%; 6 RCTs, 487 participants; very low‐certainty evidence; Figure 4; Table 3). The size of the effect of training interventions on improving HCP self‐perceived readiness to manage/respond to survivors of IPV appears to be large (Cohen 1977). In line with the analysis plan outlined in the Unit of analysis issues, this meta‐analysis involved inflating the standard error for Short 2006b by 10%, as the study did not account for ICC.
4.
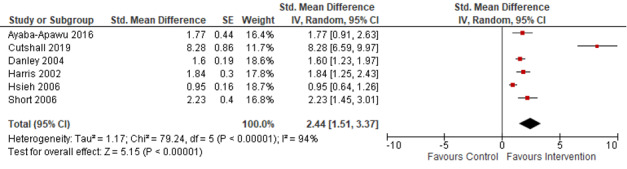
Analysis 1.2: impact of no training in responding to IPV vs training HCPs to respond to IPV on their readiness to respond to IPV at zero to 12 months after training
Footnotes
HCP: healthcare providers; IPV: intimate partner violence; IV: inverse variance; SE: standard error; Std: standardised.
2. Comparison 1. Training versus no training, wait‐list, or placebo: readiness to respond.
| Outcome or subgroup title | No of studies | No of participants | Statistical method | Effect size (95% CI) |
| HCP readiness to respond | 6 | 487 | Std mean difference (IV, random, 95% CI) | 2.44 (1.51 to 3.37) |
| HCP readiness to respond | 6 | 487 | Std mean difference (IV, fixed, 95% CI) | 1.50 (1.30 to 1.71) |
| HCP readiness to respond (no inflation of standard error for Short 2006b) | 6 | 487 | Std mean difference (IV, random, 95% CI) | 2.43 (1.51 to 3.35) |
| HCP readiness to respond (no inflation of standard error for Short 2006b) | 6 | 487 | Std mean difference (IV, fixed, 95% CI) | 1.51 (1.31 to 1.72) |
| HCP readiness to respond (30% inflation of standard error for Short 2006b) | 6 | 487 | Std mean difference (IV, random, 95% CI) | 2.44 (1.50 to 3.39) |
| HCP readiness to respond (30% inflation of standard error for Short 2006b) | 6 | 487 | Std mean difference (IV, fixed, 95% CI) | 1.49 (1.28 to 1.70) |
| HCP readiness to respond (without Cutshall 2019) | 5 | 434 | Std mean difference (IV, random, 95% CI) | 1.61 (1.14 to 2.07) |
| ADVR training | 5 | 434 | Std mean difference (IV, random, 95% CI) | 1.61 (1.14 to 2.07) |
| < 1 day of training | 5 | 434 | Std mean difference (IV, random, 95% CI) | 1.61 (1.14 to 2.07) |
| Didactic technique: role play | 2 | 289 | Std mean difference (IV, random, 95% CI) | 1.27 (0.63 to 1.90) |
| Didactic technique: case studies | 2 | 115 | Std mean difference (IV, random, 95% CI) | 1.98 (1.51 to 2.45) |
| Didactic technique: case studies | 2 | 115 | Std mean difference (IV, fixed, 95% CI) | 1.98 (1.51 to 2.45) |
| CI: confidence interval; HCP: healthcare professional; No: number; Std: standardised; IV: inverse variance | ||||
Gürkan 2017 reported results that could not be combined in the meta‐analysis. The study was a quasi‐randomised trial and used HCP responses to open‐ended questions following a Written Case Study of Violence Against Women to determine percentage change in correct responses between intervention and control. They report that there was a statistically significant improvement from baseline to post‐training (P < 0.001) in the intervention‐group members' ability to explain some of the correct interventions compared to no significant change from baseline to post‐training in the control group. However, the study does not compare intervention group to control group directly at post‐test.
Short 2006b also reported follow‐up results at 12 months after training and reported that there continued to be a significant difference (P = 0.013) between the intervention and control groups in physician readiness to respond to survivors of IPV as measured by the self‐efficacy subscale of the PREMIS.
The overall certainty of evidence for the studies reporting on this outcome was determined to be very low. This was due to high heterogeneity in the types of interventions pooled and high statistical heterogeneity (I2 > 75% and significant Chi2 test of heterogeneity has a low value and wide variance in point estimates across studies) in the meta‐analysis. There was also unclear risk of bias from lack of blinding of participants in all studies, high risk of bias from high and unequal attrition, and lack of standardised tools in some studies. Furthermore, the results may not be generalisable to all HCPs, as only counselling students, mental health professionals, dentists and physicians were represented in the studies reporting on this outcome, and all the trainings took place online.
Healthcare providers' knowledge or awareness of IPV
Five studies compared IPV training of HCPs to a no‐training, wait‐list or placebo group and reported on HCPs' knowledge (Ayaba‐Apawu 2016; Coonrod 2000; Cutshall 2019; Gürkan 2017; Short 2006b). Gürkan 2017 used what appears to be a non‐validated researcher‐developed instrument ‐ Knowledge Test About Violence Against Women (KTVAW), to measure HCP post‐training knowledge. Ayaba‐Apawu 2016; Cutshall 2019 and Short 2006b reported on actual or perceived knowledge, or both, using the PREMIS tool.
Only three studies provided suitable data for meta‐analysis (Cutshall 2019; Gürkan 2017; Short 2006b). Despite inflating the standard error for Short 2006b, the pooled effects for the three studies indicate that IPV training compared to no training improves provider IPV knowledge, up to six months post‐training (SMD 6.56, 95% CI 2.49 to 10.63; P = 0.002, Tau2 = 9.17, I2 = 98%; 3 RCTs, 239 participants; very low‐certainty evidence; Figure 5; Table 4). The effect of training interventions on improving HCPs' knowledge/awareness of IPV appears to be large (Cohen 1977). However, the certainty of the evidence is very low. This is mostly due to the imprecision of the results, high risk of bias from high and unequal attrition in two out of three studies in the meta‐analysis, and high risk of bias due to lack of random sequence generation in one study. It is also difficult to generalise these findings, as only general practitioners/community physicians, mental health professionals and nursing students were represented in the studies.
5.
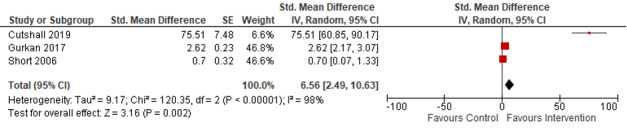
Analysis 1.3: impact of no training in responding to IPV vs training HCPs to respond to IPV on their knowledge of IPV at zero to 12 months after training
Footnotes
HCP: healthcare providers; IPV: intimate partner violence; IV: inverse variance; SE: standard error; Std: standardised.
3. Comparison 1. Training versus no training, wait‐list, or placebo: knowledge.
| Outcome or subgroup title | No of studies | No. of participants | Statistical method | Effect size (95% CI) |
| HCP knowledge | 3 | 239 | Std mean difference (IV, random, 95% CI) | 6.56 (2.49 to 10.63) |
| HCP knowledge | 3 | 239 | Std mean difference (IV, fixed, 95% CI) | 2.01 (1.65 to 2.38) |
| HCP knowledge (no inflation of standard error for Short 2006b) | 3 | 239 | Std mean difference (IV, random, 95% CI) | 6.19 (2.30 to 10.08) |
| HCP knowledge (no inflation of standard error for Short 2006b) | 3 | 239 | Std mean difference (IV, fixed, 95% CI) | 1.92 (1.57 to 2.27) |
| HCP knowledge (30% inflation of standard error for Short 2006b) | 3 | 239 | Std mean difference (IV, random, 95% CI) | 7.36 (2.90 to 11.82) |
| HCP knowledge (30% inflation of standard error for Short 2006b) | 3 | 239 | Std mean difference (IV, fixed, 95% CI) | 2.16 (1.77 to 2.54) |
| HCP knowledge (without Cutshall 2019) | 2 | 186 | Std mean difference (IV, random, 95% CI) | 1.67 (−0.21 to 3.55) |
| HCP knowledge (without Cutshall 2019) | 2 | 186 | Std mean difference (IV, fixed, 95% CI) | 1.210 (0.887 to 1.533) |
| Training in information and response | 2 | 189 | Std mean difference (IV, random, 95% CI) | 38.68 (−32.75 to 110.11) |
| > 1 day of training | 2 | 189 | Std mean difference (IV, random, 95% CI) | 38.68 (−32.75 to 110.11) |
| Online training | 2 | 103 | Std mean difference (IV, random, 95% CI) | 37.73 (−35.58 to 111.04) |
| Didactic technique: group discussion | 2 | 189 | Std mean difference (IV, random, 95% CI) | 38.68 (−32.75 to 110.11) |
| CI: confidence interval; No: number; Std: standardised; IV: inverse variance | ||||
Two studies provided results that could not be combined in the meta‐analysis. Change in knowledge scores were calculated by Coonrod 2000 using five researcher‐developed true/false questions. Compared with the placebo group, IPV knowledge improved significantly in the intervention group after controlling for baseline knowledge scores (P = 0.002). Ayaba‐Apawu 2016 also reports a post‐training improvement in the intervention group's knowledge scores on the PREMIS scale compared to the control group (Cohen‘s d is 0.42, P = 0.001).
Sustained improvement in HCPs' knowledge (at 12 months post‐training) were reported by Short 2006b, but this was not statistically significant at the P < 0.05 level (P = 0.06).
Referrals made to support agencies, social workers or specialised services
One study with no intervention, wait‐list or placebo in the comparison group reported on referring women to IPV services (Short 2006b). This cluster‐RCT evaluated an online minimum four‐hour IPV training programme compared with no training. The study evaluated the provision of referrals of the whole practice, where trained HCPs were based during a practice site visit at baseline, six and 12 months post‐intervention using trained assessors and a standardised researcher‐created checklist. The overall office practice activities rather than individual provider behaviour were assessed with “52 participating physicians (29 control and 23 study) and 49 offices (30 control and 19 study) as study participants” (Short 2006b, p 12). Results were not statistically significant at any time point and the study authors attributed this in part to the fact that there was usually an average of four practitioners in the clinic and usually only one had participated in the training.
Provider response to IPV: safety planning, counselling and/or validation of survivors' feelings
Two of the 12 studies with no intervention in the comparison group evaluated the provider’s response to IPV through the actual provision of safety planning or counselling or validation of survivors’ feelings, or both (Haist 2007; Short 2006b). The findings have been separated into the sub‐themes of a) counselling or validation of survivors’ feelings, or both; and b) safety planning.
Counselling or validation of IPV survivors’ feelings, or both
No study provided data on this outcome.
Safety planning
Of the 12 studies that compared training to no intervention, wait‐list or placebo, only two studies investigated safety planning in the outcome of provider response to IPV (Haist 2007; Short 2006b). The data from these studies could not be combined for meta‐analysis as they measured different outcomes.
Short 2006b measured provider safety planning with women by office clusters, and Haist 2007 used provider provision of safety planning with simulated patients. Haist 2007 used standardised patients to examine effects of training on internal medical residents (n = 27) working in continuity clinics at approximately one month and six months post‐training. Training in a two‐hour DV workshop (intervention) or a chronic pain workshop (control) was provided using interactive learning and simulated patients (IPV for intervention and chronic pain for control) followed by group discussion with a faculty expert (Haist 2007).
Up to four standardised patients were randomly inserted into the residents’ consultation lists, approximately one and six months after the training (Haist 2007). Standardised patients presented with either depressive or injury symptoms related to IPV. Immediately after their appointment with the medical resident, the simulation patient completed a checklist that included an eight‐item measurement of the safety‐plan counselling provided. The checklist was developed by faculty based on relevant literature. Residents who correctly discussed at least six of the eight safety‐plan items were deemed successful in safety planning (Haist 2007). Intervention‐group participants were more likely to successfully provide safety planning during their interactions with standardised patients (P = 0.04) (odds ratio (OR) 4.44, 95% CI 1.04 to 19.0) (Haist 2007).
Short 2006b evaluated the provision of safety planning versus no intervention using a cluster‐RCT where training was provided on average to one HCP in a community‐based practice but a full practice’s behaviour was evaluated during a practice site visit at baseline, six and 12 months after training, using trained assessors and a standardised checklist. The checklist evaluation of office safety planning used the question “Safety planning for IPV victims done” (p 32) but it is unclear how this was assessed. Results reported little or no impact at any time point.
The certainty of the evidence is very low, owing to imprecision, as CIs are wide and overlap no effect when the risk ratio is calculated (RR 3.07, 95% CI 0.96 to 9.77) for Haist 2007. Clustering of events within individuals does not seem to be accounted for, and the small sample size may further contribute to this imprecision. Furthermore, the findings have limited generalisability by population and setting.
Adverse outcomes for providers
None reported.
Secondary outcomes
Documentation or identification of IPV (or both)
Four studies reported having no IPV training or a wait‐list/placebo group in the comparison arm of the study (Coonrod 2000; Gürkan 2017; Haist 2007; Lo Fo Wong 2006). Coonrod 2000 had residents self‐report diagnoses of IPV at nine to 12 months after the intervention, but the measurement tool they used to diagnose was not described. Proportional group differences between arms did not reach statistical significance (P = 0.07).
A survivor story‐based tool describing various types of violence was used by Gürkan 2017 to assess student nurse diagnoses of physical, psychological and verbal violence at two months post‐training. Correct answers to questions asking students about diagnosis and clinical response to IPV were coded as higher scores. Improved scores were reported in both groups post‐training, with the authors not finding differences between groups (Gürkan 2017).
A more objective measure was used by Haist 2007, who compared a DV workshop for residents with a control group that received a chronic‐pain workshop using insinuated standardised patients to report back to researchers on residents' identification of IPV. At one to seven months post‐training, the study did not find a statistically significant difference (P = 0.86) between groups in identification of insinuated standardised patients presenting with case scenarios indicative of having experienced IPV.
Only Lo Fo Wong 2006 found significant improvements in identification rates post‐training in doctors' IPV incident‐reporting forms and (confirmed/not confirmed) cases analysed six months after training. The study reports on two intervention groups (focus group with full training or focus group only) and one control (no intervention). We considered the focus group with full training as our primary intervention and only report on this group compared to the no‐intervention group. The study reported that intervention‐group HCPs identified patients/survivors of IPV at more than four times the rate of controls (RR 4.54, 95% CI 2.5 to 8.09; P < 0.001; 54 participants) (Lo Fo Wong 2006).
Mental health outcomes of women survivors of IPV
No study provided data on this outcome. While Ragland 1989 reported on HCPs' perception of the IPV survivor’s mental health, they were testing whether training impacted the perception and therefore the results cannot be extrapolated to actual impacts on survivors' mental health.
Adverse outcomes for women such as IPV‐related death, or recurrence of IPV or injury
No study provided data on this outcome.
Subgroup analyses
We explored clinical heterogeneity by conducting the subgroup analyses listed below.
Primary outcomes
HCP attitude/belief towards IPV
Intervention type
Of the eight studies that looked at the effectiveness of training HCPs to respond to IPV compared to no intervention, five studies described the components of their training programmes as providing training on AVDR (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b). A meta‐analysis of these studies found a significantly positive impact of AVDR training on HCPs' attitudes towards IPV (SMD 0.87, 95% CI 0.54 to 1.20; P < 0.001, Tau2 = 0.09, I2 = 70%; 5 RCTs, 434 participants; Table 2). Two studies specified that their training focused on providing information about IPV and training HCPs on how to provide validation to survivors of IPV (Mauch 1982; Ragland 1989). The pooled results from these two studies found little to no impact of training on HCP attitude towards IPV (SMD 0.67, 95% CI −0.34 to 1.68; P = 0.19, Tau2 = 0.39, I2 = 75%, 2 RCTS, 71 participants; Table 2).
Duration of the intervention
Seven studies had interventions that were delivered in sessions that lasted less than 24 hours (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006; Mauch 1982; Ragland 1989; Short 2006b). Gürkan 2017 had an intervention that was a total of eight hours but was delivered across eight sessions of one hour each. Compared to the control arm where no training was received, the meta‐analysis of the seven studies where the training interventions were delivered in one go found significant improvements in HCP attitudes towards IPV in the intervention arm (SMD 0.82, 95% CI 0.52 to 1.12; P < 0.001, Tau2 = 0.10, I2 = 67%; 7 studies, 505 participants; Table 2).
Booster sessions
No study reported on this.
Mode of delivery
Six studies (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b;Ragland 1989) reported their training as being provided online and individually. A meta‐analysis of these studies found a significantly positive impact of online training on HCPs’ attitudes towards IPV (SMD 0.78, 95% CI 0.46 to 1.10; P < 0.001, Tau2 = 0.10, I2 = 76.5%, 6 RCTS, 476 participants; Table 2). Two studies described their training as group‐based and provided in‐person (Gürkan 2017; Mauch 1982). A meta‐analysis of these studies found little to no impact of in‐person group training on HCPs’ attitudes towards IPV (SMD 0.61, 95% CI −0.44 to 1.67; Tau2 = 0.50, I2 = 83.3%; 1 RCT and 1 quasi‐RCT, 165 participants; Table 2).
Didactic technique
Four studies (Danley 2004; Gürkan 2017; Hsieh 2006; Mauch 1982) used role play as a technique in their training. They found a positive impact of this training on HCPs' attitudes towards IPV compared to no intervention (SMD 0.80, 95% CI 0.30 to 1.29; P = 0.002, Tau2 = 0.22, I2 = 88.9%; 3 RCTs and 1 quasi‐RCT, 454 participants; Table 2). As mentioned in the mode‐of‐delivery section, two studies described their training as group‐based, and involved group discussions (Gürkan 2017; Mauch 1982). A meta‐analysis of these studies found little to no impact of in‐person group training on HCPs' attitudes towards IPV (SMD 0.61, 95% CI −0.44 to 1.67; Tau2 = 0.50, I2 = 83.3%; 1 RCT and 1 quasi‐RCT, 165 participants; Table 2). Three studies (Gürkan 2017; Harris 2002; Short 2006b) used case studies in their training to teach HCPs about IPV. Compared to no training, they found some improvements in HCPs' attitudes towards IPV as a result of training (SMD 0.41, 95% CI 0.03 to 0.78; P = 0.04, Tau2 = 0.05, I2 = 46.6%; 2 RCTS and 1 quasi‐RCT, 251 participants; Table 2).
HCP readiness to manage/respond to survivors of IPV
Intervention type
Of the six studies that looked at the effectiveness of training HCPs to respond to IPV compared to no intervention, five studies described the components of their training programmes as providing training on AVDR (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b). A meta‐analysis of these studies found a that AVDR training improved HCPs' readiness to respond to IPV (SMD 1.61, 95% CI 1.14 to 2.07; P < 0.001, Tau2 = 20, I2 = 75%; 5 RCTS, 434 participants, Table 3).
Duration of the intervention
The same five studies (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006; Short 2006b) also provide training in a session that lasted less than a day, and found that even with this short duration there was an improvement in HCPs' readiness to respond to IPV (SMD 1.61, 95% CI 1.14 to 2.07; P < 0.001, Tau2 = 20, I2 = 75%; 5 RCTS, 434 participants; Table 3).
Booster sessions
No study reported on this.
Didactic technique
Three studies that reported on readiness to manage also reported using role‐play techniques in their training (Danley 2004; Gürkan 2017; Hsieh 2006). Of these, two reported outcomes in a way that could be statistically combined (Danley 2004; Hsieh 2006). The meta‐analysis of these two studies found a statistically significant improvement in HCPs’ self‐reported readiness to respond compared to no training (SMD 1.27, 95% CI 0.63 to 1.90; P = 0.00, Tau2 = 0.18, I2 = 85%; 2 RCTs, 289 participants; Table 3). Gürkan 2017 reports that there was a statistically significant improvement from baseline to post‐training (P < 0.001) in the intervention‐group members' ability to explain some of the correct interventions compared to no significant change from baseline to post‐training in the control group. Two studies reported using a case‐study approach in their training (Harris 2002; Short 2006b). The meta‐analysis of these two studies also found a statistically significant improvement in HCPs’ self‐reported readiness to respond compared to no training (SMD 1.98, 95% CI 1.51 to 2.45; P < 0.001, Tau2 = 0.00, I2 = 0.0%; 2 RCTs, 115 participants; Table 3).
HCP knowledge of IPV
Intervention type
Two of the three studies (Cutshall 2019; Gürkan 2017) in the meta‐analysis reported that their training focused on providing information and response. The subgroup analysis finds little to no impact of this type of training on HCP knowledge of IPV (SMD 38.68, 95% CI −32.75 to 110.11; P = 0.29, Tau2 = 2628.47, I2 = 99%; 1 RCT and 1 quasi‐RCT, 189 participants; Table 4).
Duration of the intervention
The same studies (Cutshall 2019; Gürkan 2017) also delivered the intervention in more than one session/day. Little to no impacts of the intervention were seen.
Booster sessions
No study reported on this.
Mode of delivery
Two of the three studies (Cutshall 2019;Short 2006b) reported providing their training online. A subgroup analysis found little to no impact of this training (SMD 37.73, 95% CI −35.58 to 111.04; P = 0.31, Tau2 = 2770.24, I2 = 99%; 2 RCTs, 103 participants; Table 4).
Didactic technique
Cutshall 2019 and Gürkan 2017 used interactive group‐based training approaches. As mentioned before, these had little to no impact on HCPs' IPV knowledge (SMD 38.68, 95% CI −32.75 to 110.11; P = 0.29, Tau2 = 2628.47, I2 = 99%; 1 RCT and 1 quasi‐RCT, 189 participants; Table 4).
Other outcomes:
Subgroup analyses were not possible for the primary outcomes of referrals (to support agencies, social workers or specialised services); safety planning, counselling or validation; adverse outcomes for providers; and the secondary outcomes of documentation or identification (or both); mental health of women survivors; adverse outcomes for women (IPV‐related death or recurrence of IPV or injury).
Sensitivity analyses
We re‐ran the three meta‐analyses (main results and the subgroup analyses) assuming a fixed‐effect model and the results remained similar to the random‐effects model's results (see both results reported in Additional tables).
We also explored the impact of not adjusting for ICC by not inflating and by inflating the standard error for studies that do not report ICC by 10% and 30% for each outcome (Table 2; Table 3; Table 4). This did not impact the results of the meta‐analyses in any substantive way. However, to be conservative we have used the SE inflated by 10% in all analyses.
We further explored the impact of removing Cutshall 2019 on the results for HCP knowledge, as it appeared to be an outlier. After removing this study, there was little or no impact of training on HCP knowledge of IPV (SMD 1.67, 95% CI −0.21 to 3.55; P = 0.08, Tau2 = 1.77, I2 = 96%; 1 cluster‐RCT and 1 quasi‐RCT, 186 participants; Table 4).
Comparison 2. Training to respond to intimate‐partner violence compared to standard care or a sub‐component of the intervention
A total of seven studies tested an intervention compared to an active intervention in the control arm. In three of these seven studies the control arm received some form of intervention that was described as standard or usual care: Gupta 2017 (one day training); Sharps 2016 (usual care); and Vakily 2017 (traditional full training but not using a CD).
For another four RCTs (Brienza 2005; Edwardsen 2006; Hegarty 2013; Moskovic 2008), the intervention arm consisted of more than one component and in the comparator arm a sub‐component of the intervention was provided. This type of study tests the impact of component A of an intervention by implementing component A + sub‐component B in the intervention arm, versus just sub‐component B in the control arm. Brienza 2005 tested experiential learning in a women’s safety shelter + workshop seminar versus workshop seminar alone. Edwardsen 2006 tested the effect of a mnemonic technique + lecture/simulated patient versus lecture/simulated patient alone. Hegarty 2013 tested the impact of the Healthy Relationship Training programme focused on responding to IPV survivors + a basic IPV education versus basic IPV education alone. Moskovic 2008 tested the impact of outreach education to adolescents + didactic training on IPV versus didactic training on IPV alone.
Primary outcomes
Healthcare providers' attitudes or beliefs towards IPV
Out of the seven studies in this review where the control arm received some form of intervention, two studies reported on whether training had an impact on HCP attitudes (Brienza 2005; Vakily 2017).
Both the studies that compared training HCPs to respond to IPV to a sub‐part of the intervention/standard care in the control group found no significant differences between intervention and control groups. Brienza 2005 used an attitudes subscale of an instrument adapted from the Health Care Provider Survey for Domestic Violence. At six to 12 months after intervention they found no statistically significant differences in HCPs' attitudes between the intervention and control arms (P < 0.9). Vakily 2017 developed a standardised 15‐item attitude measure for this study. They did not find any significant effect of delivering the intervention through a CD compared to traditional in‐person training on attitudes towards IPV at two months after intervention (P = 0.3).
Moskovic 2008 reported on readiness to respond, but termed it 'HCP confidence and attitude' in their paper. The results from their study are therefore discussed under the next outcome, 'Readiness to respond'.
Healthcare providers' readiness to manage
Out of the seven studies where the control arm received some form of intervention, three studies reported on HCPs' readiness to respond to IPV (Brienza 2005; Hegarty 2013; Moskovic 2008).
Brienza 2005 tested the impact of a visit to a local women’s safety shelter (in addition to group training which was provided in both intervention and control arms) at six to 12 months post‐training. They found no impact of the visit on providers' self‐perceived skills (P < 0.3) or resource awareness (P < 0.8) assessed by an adaptation of the Health Care Provider Survey for Domestic Violence instrument.
Two studies assessed impacts immediately after training. Hegarty 2013 assessed the impact of the Healthy Relationship Training programme (in addition to a basic IPV education pack provided in both intervention and control arms) immediately post‐training using the Perceived Preparation subscale of the PREMIS. They found that intervention‐arm doctors were more likely to report greater self‐perceived preparedness to respond to and manage IPV (P < 0.001). Moskovic 2008 assessed the impact of providing outreach training to high school students (didactic training in IPV was provided in both intervention and control arms) on medical students' confidence in their ability to address IPV, discuss, recognise and respond to IPV, measured by a nine‐item (self‐developed) scale. They found that outreach improved HCPs' confidence immediately after training (P ≤ 0.002).
Healthcare providers' knowledge or awareness of IPV
Four studies (Brienza 2005; Hegarty 2013; Moskovic 2008; Vakily 2017) included various forms of IPV training in the comparison arms of their studies and were excluded from the meta‐analysis. Brienza 2005 and Moskovic 2008 provided IPV training to both arms, with the intervention group also having an experiential/outreach component as part of their training. All medical residents in Brienza 2005 completed IPV training, with intervention‐group residents also having a clinical placement at a women’s DV shelter. Residents self‐reported their knowledge of IPV on an adapted Health Care Provider Survey for Domestic Violence instrument (seven‐question knowledge subscale). Compared with controls, the intervention group showed significant improvement in pre‐post IPV knowledge scores (P = 0.04) (Brienza 2005). Moskovic 2008 included didactic IPV training in both arms of the RCT and a dating‐violence teaching opportunity for the intervention group alone, where medical students delivered a community outreach programme to adolescents, two to three weeks after training. A researcher‐generated 34‐item measure assessed IPV knowledge, pre‐ and two to three weeks post‐training (26 true/false and eight multiple‐choice questions where correct answers received a score of one). Mean knowledge scores in the ‘didactic plus outreach’ group did not differ significantly from the ‘didactic only’ students (P = 0.277) (Moskovic 2008).
In addition to comprehensive IPV training provided to general practitioners (GPs) in the intervention group, Hegarty 2013 sent a basic IPV education pack (in the mail) to control GPs who also received continuous professional development points for completing training. In unpublished data provided by the authors, GP knowledge differences across arms failed to reach significance (P = 0.328) (Hegarty 2013). Vakily 2017 provided didactic DV training to midwives in both study groups, but the intervention group also received the same DV training content via a CD for later revision. Vakily 2017 assessed knowledge using an awareness measurement tool consisting of 24 multiple‐choice questions. At two months post‐training, midwives mean awareness scores of DV in the CD group were higher (indicating greater awareness) than those in the training‐only arm (P < 0.001).
Referrals made to support agencies, social workers or specialised services
Two studies evaluated the impact of training where additional training was compared with training as usual (Gupta 2017), or a sub‐component of the IPV training in order to isolate the effects of training using a mnemonic to guide patient care (Edwardsen 2006).
Edwardsen 2006 evaluated participants' provision of referral to the simulated patient with two trained assessors individually reviewing the simulated patient interview and answering the question “Was a referral to the community women’s advocacy agency provided?” (p 65). Upon immediate follow‐up after training, they found no significant difference between the two groups (P = 0.10) (Edwardsen 2006).
Gupta 2017 presented outcomes for women that were associated with care provided by intervention‐group nurses who had extensive “training on screening for IPV, providing supportive referrals, and assessing for health and safety risks” compared with the control‐group nurses who received “one‐day training focused on sensitizing staff to IPV as a health issue and referral cards to give to women”. The study evaluated women’s use of community resources across three time points (baseline, three months and 15 months) with use of resources potentially associated with provision of referrals. Baseline and three‐month comparison (services ever used at baseline compared with services used in the past three months, at three months post‐intervention) demonstrated statistically significantly increased use of community services in both arms, but no significant difference between arms at three months after intervention (OR 0.13, 95% CI −0.05 to 0.32; P = 0.17). However, women in the control arm reported significantly increased use of community resources (β 0.20; 95% CI 0.08 to 0.31; P < 0.01) at 15 months post‐intervention.
Provider response to IPV: safety planning, counselling or validation of survivors' feelings, or both
Two of the seven studies in this comparison evaluated the provider’s response to IPV through the actual provision of safety planning or counselling or validation of survivors’ feelings, or both (Edwardsen 2006; Gupta 2017). We have separated the findings into the sub‐themes of a) counselling or validation of survivors’ feelings, or both; and b) safety planning.
Counselling or validation of IPV survivors’ feelings, or both
Only one study (Edwardsen 2006) reported outcomes related to actual counselling or validation of survivors’ feelings (or both) after disclosure of IPV. This study reported significant positive outcomes associated with training of first‐year medical students (with only 25 students in each arm randomly evaluated) on validation and counselling using a mnemonic technique with women survivors of IPV. The control group received training based on usual practice, and the mnemonic was only provided at the end of the session without any specific training on its use (Edwardsen 2006, p 62). Both groups were able to practise with simulated patients prior to the evaluation interview. The effects of the intervention were evaluated only once, immediately after the education session. On the same day, after training, students participated in an audio‐visually recorded evaluation interview with a simulated patient. The research team used a standardised checklist to evaluate the students’ performance. Participants’ provision of validation and counselling was evaluated through “acknowledg[ing] the violence and provid[ing] empathy” (p 63): yes or no. There was a significant improvement in the intervention group (P = 0.027) in provision of an empathetic statement, 82% versus 45% (OR 4.95, 95% CI 1.06 to 26.21). The study authors suggest that providing instruction on using the mnemonic improves outcomes compared with only having access to the mnemonic without training on its use (Edwardsen 2006, p 66).
Safety planning
Two studies evaluated the impact of training interventions where additional training was compared with a) training as usual (Gupta 2017), or b) IPV training versus a sub‐component of training to test the use of a mnemonic techniques to guide patient care (Edwardsen 2006).
Edwardsen 2006 investigated the impact of mnemonic‐based training on medical students’ performance with simulated patients using the standardised checklist question: “Was safety of the patient addressed” (p 65) during a performance evaluation interview immediately after training. Edwardsen 2006 did not find a significant impact of mnemonic‐based training compared to training without a mnemonic on implementation of safety planning immediately after training (P = 0.54).
The second study, comparing an intervention to usual care (Gupta 2017), examined women’s use of safety planning behaviours three months and 15 months after providers attended a training intervention. The intervention included IPV screening, “supportive care and safety planning and harm reduction counselling” (p 3, Gupta 2017) from nurses who had received a three‐day training session that included “topics related to IPV, safety planning, reproductive coercion, and community resource referrals”. Improvements in safety planning behaviours (women reported ever having undertaken safety planning behaviours at baseline compared with implementation of safety planning behaviours within the last three months at three months post‐intervention) were seen only in the control arm, with a significant improvement for control participants in the treatment and time interaction (β 0.41; 95% CI 0.02 to 0.79; P = 0.04). Gupta 2017 also reported a statistically significant increase in implementation of safety behaviours for women in both intervention and control arms of the trial at 15 months and found no significant difference in intention‐to‐treat and time interaction analysis for the use of safety behaviours over the previous 12 months (P = 0.10).
Adverse outcomes for providers
None reported.
Secondary outcomes
Documentation or identification of IPV (or both)
Two studies with a sub‐component of the intervention in the comparison group reported on the impacts of training on documentation or identification of IPV. One study reported on both IPV identification and documentation as stand‐alone outcomes (Brienza 2005). Medical residents responded to a five‐point Likert scale (anchors ‐ never to always) survey question on medical documentation of partner violence. While documentation and identification/screening rates increased across both case and control groups at six months follow‐up (always or sometimes documented), there was no statistically significant difference in either outcome across groups (data not provided) (Brienza 2005).
Edwardsen 2006 video‐recorded medical student clinical encounters with simulated patients and coded content to assess IPV identification. Proportions identified in the intervention group were higher, compared to controls, but not significantly different between the two arms (P = 0.22).
Mental health outcomes of women survivors of IPV
Two studies (Gupta 2017; Sharps 2016) looked at the impact of nurse‐delivered programmes on mental health outcomes of women who reported experiencing IPV in the past year. Gupta 2017 compared the effectiveness of nurse‐delivered sessions that included IPV screening, supportive referrals, health/safety risk assessments plus standard care versus standard/usual care including integrated IPV screening in the control arm on women’s mental quality of life. To do this they used the SF‐12 mental quality‐of‐life measure (physical quality of life was not assessed) at three months and 15 months post‐intervention. They found that women in the intervention arm reported improvements in mental quality of life at three months post‐intervention (P = 0.03), although no statistically significant differences were seen between intervention and control arms at 15 months (P = 0.19) in an intention‐to‐treat analysis.
Sharps 2016 compared the brochure‐based DOVE programme, which focused on training nurse practitioners to inform and empower women about IPV during home visits versus home visits alone. They assessed differences in depression symptomology, measured by the Edinburgh Postnatal Depression Scale (EPDS) at three, six, 12 and 24 months, and found no significant difference between groups at any time point (P > 0.05 at all time points).
Adverse outcomes for women such as IPV‐related death, or recurrence of IPV or injury
Gupta 2017 reported on IPV using the WHO Multi‐Country Study on Domestic Violence and Women’s Health physical and sexual IPV instrument. While they found no significant difference between groups on rates of past year IPV (P = 0.10), no adverse events were found in either arm. Sharps 2016 assessed IPV using the Conflict Tactics Scale 2 (CTS2) at one, three, six, 12, 18, and 24 month and found a greater difference in reduction in IPV in the intervention arm compared to the control arm (P < 0.01). No study reported an increase in IPV recurrence. No studies reported on IPV‐related deaths or injuries.
Discussion
Summary of main results
Nineteen studies with 1662 healthcare providers (HCPs) satisfied our inclusion criteria (Criteria for considering studies for this review). Of these 19 studies, 12 compared HCP training to a group receiving no intervention or a placebo (for example, training in a different topic), and seven compared training to training as usual (for example, the training of HCPs that would normally occur within the intervention where the trial is occurring) or some sub‐component of the intervention was administered in the comparison group, or both.
When comparing HCP training to no intervention or placebo, we found a positive effect of training on attitudes at less than one year after training. We also found that HCP training is likely to improve self‐reported readiness to respond and HCPs' knowledge of IPV up until 12 months post‐intervention. However, the certainty of this finding is very low (See GRADE in Table 1) as it is based on one study that followed up these outcomes at 12 months. In this study, the effects of training on attitudes and readiness to respond were sustained at one year, while outcomes for knowledge were not sustained.
Only one study looked at the impact of training versus wait‐list or no training on referrals provided to survivors of IPV. However, while the intervention was not provided to all HCPs in a practice, the assessment was carried out at the practice level, and no impacts were seen. Based on the poor quality of these data, we are uncertain about the impact of training on referral provided to survivors of IPV. Two studies looked at the impact of training versus wait‐list or no training on HCPs' response to survivors of IPV, but were too diverse to combine in a meta‐analysis. One study reported that HCPs in the intervention arm were more likely to provide safety planning, while the second study that looked at outcomes at the practice level (while training only a few providers in the practice), saw little to no impact of the training on this outcome. Overall, the evidence is very uncertain about the effect of training on HCP response to survivors of IPV.
Out of the 12 studies with no intervention or wait‐list in the control group, only four studies looked at the impact of training on HCPs' documentation or identification of IPV. Only one of these four studies reported positive impacts of training on identification or documentation of IPV at six months post‐intervention.
Subgroup analysis comparing brief IPV training (less than one day) on asking, documenting, validating and responding versus wait‐list or no training across studies, all reported similar improvements in pooled analysis for the outcomes of attitudes and self‐reported readiness to respond. The evidence so far does not appear to support group in‐person training for HCP attitude improvements, but there was evidence in favour of online programmes that provide training on all AVDR components. Too few studies in each subgroup prevented further subgroup analysis.
When comparing HCP training to standard care or a sub‐component of the intervention across all outcomes, we mostly found no evidence of additional effectiveness of the trainings that were being tested. Seven out of the 19 studies compared the training of HCPs to ‘usual care’ or tested a specific component of an intervention by comparing it to the remainder of the intervention. Four out of the 19 included studies assessed the effectiveness of varying specific components of training programmes compared to implementing the intervention programme without those specific components that were being tested. Three compared a training intervention to ‘usual care’. These studies involved a variety of interventions in the comparison group and could not be combined in any statistical analysis. Two studies that had an active comparison group found no additional/relative impact of training interventions or of the specific component of intervention that they were testing on HCPs' attitudes. Of the three studies with active comparison groups that looked at HCPs' readiness to respond to survivors of IPV, two assessed readiness to respond immediately after training and found impacts on self‐reported readiness to respond; but the one study that assessed effects after six to 12 months did not find any impacts.
Amongst the studies with active comparison groups, two looked at the impact of outreach training on knowledge; while one found relative impacts of this type of training, the other did not. One study found an impact of training using a group training plus CD‐based method versus using group‐based in‐person training alone on IPV knowledge of HCPs. Another compared a more comprehensive training to a basic package on IPV and found no difference in post‐test IPV knowledge.
Two studies that assessed impacts of the sub‐component of training or advanced training versus usual care (i.e. an active comparison group) found no impact of these on referral or on provider response towards survivors of IPV. One study found an increase in referral use that was sustained in the ‘usual care’ (control) arm at 15 months post‐intervention, while an improvement in safety behaviours was seen in the ‘usual care’ (control) arm at three months post‐intervention but was not sustained at 15 months. However, in one of these two studies, the intervention arm saw a significant increase in validation measured by empathetic statements provided by HCPs. Two studies looked at impacts of training on identification compared to a sub‐component of the intervention and found no evidence of impact on identification rates. The two studies that looked at impacts on the mental‐health outcomes of IPV survivors found no evidence for improvement in mental health outcomes in the intervention arm at more than one year after intervention.
No study reported any adverse effects of the interventions.
Overall completeness and applicability of evidence
Sixteen of the 19 studies were carried out in high‐income countries. with the remaining three conducted in Iran (Vakily 2017), Turkey (Gürkan 2017) and Mexico (Gupta 2017), all of which are categorised as upper‐middle‐income countries. This means we cannot generalise our findings to low‐income country settings, as other factors may impact on the effects of training HCPs around IPV. In addition to the limited research from low‐ and middle‐income countries, the context within the high‐income countries was not adequately described, making it difficult to assess if the training programmes would be culturally appropriate in settings outside a high‐income context. Many different interventions were explored and many used internet‐based online training that may be more difficult to implement in some low‐resource settings where internet access is challenging.
A variety of HCPs were represented across the studies. These included medical students and residents, physicians/doctors, nurses, midwives, and dentists. Paraprofessionals and paramedics were not represented. For some outcomes only a few types of provider were represented, and this impacted the GRADE rating for the outcome. We also did not have adequate description of the characteristics of those who were trained. The interventions were mostly implemented in teaching hospitals or university settings. Five studies were carried out in primary‐care clinics and three were delivered online. Limited types of settings were explored and very few looked at practice‐based settings.
A limited number of studies carried out follow‐up assessments that were longer than one year (Gupta 2017; Sharps 2016) and four studies reported on outcomes almost immediately after the intervention was delivered (Ayaba‐Apawu 2016; Danley 2004; Harris 2002; Hsieh 2006). We could not carry out a large number of our planned sensitivity analyses and subgroup analyses due to the small number of studies reporting on each outcome. Overall, more research is needed of a higher quality, across high‐, middle‐ and lower‐income countries, with longer follow‐up periods conducted in a variety of healthcare settings.
Quality of the evidence
For each primary outcome for the main comparison of no intervention/placebo/wait‐list, we also assessed the certainty of the evidence using the GRADE approach (Guyatt 2011). We judged the certainty of the evidence as varying between very low and low, based on five criteria: study limitations, imprecision, inconsistency of results, indirectness of evidence and likelihood of publication bias. This means that we are not fully confident that these effect estimates are consistent with the true effect, and further high‐quality research is likely to have an impact on our estimates of effects across all outcomes.
We assessed the risks of bias from sequence generation, allocation, blinding, incomplete outcome data and selective reporting. Most studies did not adequately describe the randomisation process or the allocation concealment process. Masking of participants is difficult with training interventions, due to the behavioural nature of the interventions. This issue was especially prevalent across almost all studies included in the review. Lack of masking of participants may have a substantial impact on results, particularly as most outcomes, such as attitudes and readiness to manage, were self‐reported and subjective. In addition, many studies did not report whether they were using standardised, validated instruments to measure these self‐reported outcomes. Low power to detect an effect due to small sample sizes was another concern in a few studies in this review.
There was great variation in the type of training programmes delivered, which can contribute to the variation in effect estimates. The heterogeneity in training programmes and populations potentially contributed to the high I2 value, which also impacted the GRADE assessments. We had planned to conduct subgroup analyses to explore some known sources of heterogeneity, but there were too few studies per outcome to allow for meaningful subgroup analyses for all outcomes, and the heterogeneity in the subgroup analyses remained high for most of the analyses that we did carry out. In addition to heterogeneity in treatment, there was also large variation in what is meant by ‘usual care’, which also impacts the relative effects of an intervention when comparing it to a ‘usual care’ comparison group.
We visually assessed asymmetry and publication bias with a graphical assessment of the funnel plots (analyses not shown, funnel plots available upon request). For HCPs' readiness to respond, a data point was outside the funnel. It is also possible that this publication bias is driven by one study which was an unpublished dissertation that was only completed in 2019, and hence it is reasonable that it had not already been published at the time of this review. We planned to formally investigate funnel plot asymmetry and reporting bias however we did not have enough studies in any of our meta‐analyses to do this, please see Appendix 2 for unused methods.
Potential biases in the review process
While we carried out comprehensive searches, we were unable to get further details on a number of studies (Characteristics of studies awaiting classification), and hence were unable to determine whether they were eligible to be included in the review. We included one study (Hegarty 2013) that provided a systems intervention, after our personal correspondence with the author, who informed us that one follow‐up period was carried out prior to the systems‐change component being implemented. The results of only this follow‐up period are included in our review as we do not include interventions with systems‐change components, and hence other outcomes that were assessed after the systems‐level intervention was implemented were not included. Despite our attempts, we were unable to contact and follow up with the authors of other systems‐change interventions, to check if they had done anything similar. As our focus was on training of HCPs, systems‐change studies have been excluded from our review, as the effects of the intervention may relate to the systems change rather than training of HCPs to respond to women affected by IPV. A comprehensive review of research associated with systems‐changes would be helpful in the future, since training embedded in a broader systems approach is known to be most effective.
We pooled a variety of training interventions together when comparing to no intervention/placebo/wait‐list and the heterogeneity in treatments makes us less likely to develop a nuanced understanding of which aspects of the training work, in which setting and for whom. Reporting on the intervention characteristics was inadequate to allow us to understand or even narratively report what aspects/types of the intervention are effective. In a second comparison we narratively combined studies that compared training to other active interventions in the control arm, and here it is possible that the heterogeneity in comparison is responsible for the presence or absence of effects. For example, when an active intervention component is tested against ‘treatment as usually occurs within the trial setting’, the features and components of the treatment as usual are potentially responsible for the presence or absence of effect of the intervention being tested.
We presumed that if a component of the intervention was also provided to the control group, we would be seeing the impact of the additional part of the intervention that was provided to the intervention group only. However, this may not have been the case, as there could have been an interaction effect between the additional intervention provided to the intervention group only and the sub‐component of the intervention that was provided to both arms; that effect may not hold if only the additional component is provided.
Some studies could not be pooled as they did not provide data that could be combined with others. This could potentially bias the meta‐analysis and hence the review.
Agreements and disagreements with other studies or reviews
Four reviews (two systematic, one scoping and one integrative) have investigated the impact of training on different populations of healthcare professionals or students, or both. Their inclusion criteria differ from ours and hence they are not directly comparable. However, in line with the findings of our Cochrane Review, these four reviews reported that training generally improved HCP knowledge (Crombie 2017; Sammut 2021; Sawyer 2016; Zaher 2014), and some improvements were seen also in provider attitudes and to a lesser extent, behaviours or women’s outcomes. Similar to this Cochrane Review, all four previous reviews reported variable types of training, duration and content, and relatively low‐certainty research, with short follow‐up time frames. None of these reviews reported adverse outcomes from training.
Unlike our review, which examined the impacts of training across a broad range of HCPs and students, the four previous reviews focused on specific HCP or student groups and had a variety of inclusion criteria for studies. Zaher 2014 examined nine RCTs in a systematic review that evaluated "educational interventions among physicians and provided data on the effects of the interventions" (p 618). Sawyer 2016 also undertook a systematic review exploring the effects of educational interventions related to responding to women experiencing IPV for allied healthcare professionals and students, including nurses and midwives but excluding physicians. They included 18 studies that used RCTs, pre/post‐test or two‐group non‐randomised designs. Crombie 2017 undertook a scoping review examining the impact of training on nurses and midwives (practising and students). Twenty qualitative, quantitative and mixed‐methods studies were included. Sammut 2021 carried out a systematic review that identified 17 qualitative, quantitative or mixed‐method studies that explored educational strategies for teaching IPV care to prequalified HCPs.
Like this Cochrane Review, these four reviews all identified significant diversity in the type of training and outcomes being measured. The studies in Sawyer 2016's review used a variety of training methods (online, face‐to‐face, didactic and simulation), varied in durations (15 minutes to 10 weeks), and content (signs and symptoms, causes, routine screening or case finding, and response to disclosure), and had different outcomes (knowledge, attitudes, skills, and behaviours) being assessed. Crombie 2017 reported that training sessions for nurses and midwives mostly occurred post‐registration and were provided as part of continuing professional development in a variety of methods, and with varying content.
The type and duration of training had variable effects across studies within the reviews, although the general consensus, similar to our findings, was that training was associated with an improvement in attitudes, and often knowledge, as well as a smaller, positive effect on the HCPs' (self‐perceived) readiness to respond and actual behaviours, with few studies of women’s outcomes after healthcare professional training. Crombie 2017 reported positive or no changes in nurse/midwife attitudes associated with training. Zaher 2014 found some variation in outcomes associated with the type of training, noting that workshops and brief education sessions or online training with problem‐based learning seemed to improve knowledge, while online training or the use of simulated patients also improved skills in responding to women experiencing IPV, particularly in terms of identifying these women.
Similarly, Sammut 2021 found that interactive educational strategies and those which included practical application of learning were more effective than didactic teaching approaches alone, for increasing knowledge and improving attitudes. As with our review, Sawyer 2016 reported that providers’ attitudes were demonstrated to improve with training and the intention to improve practice (a potential measure of readiness to respond) also improved. Actual practice was only evaluated in one study within the Sawyer 2016 review, while our review only assessed outcomes that indicated actual changes in HCP response behaviours, such as actual referrals or safety planning by HCPs.
Across the previous reviews, training was usually associated with increased knowledge (self‐reported or actual) and awareness of resources or professional responsibility. This is echoed in our findings where training enhanced HCP outcomes such as knowledge and provider readiness to respond.
Zaher 2014 and Crombie 2017 found that incorporating training with systems support had more positive outcomes than training alone, but systems training interventions were outside the scope of our review.
As with our review, almost all studies in previous reviews only included short‐term outcomes (less than one year), with a focus on HCP outcomes rather than directly on women’s outcomes. Studies across these reviews were usually of low certainty and short duration. For example, Crombie 2017 noted that most studies in their review only measured short‐term outcomes, with a focus on nurse/midwife changes rather than outcomes affecting women, and Sawyer 2016, in line with our findings, also explicitly stated that studies were generally of low quality with methodological issues that limit confidence in the findings.
Authors' conclusions
Implications for practice.
The evidence presented here broadly supports IPV training for HCPs. There is some evidence that suggests training HCPs in IPV may contribute to improved attitudes (low‐certainty evidence), and training may improve HCPs' knowledge and self‐reported readiness to respond to survivors of IPV, but the evidence is very uncertain. Given that HCPs are often a point of possible support for women affected by violence, HCP training that addresses negative attitudes, increases knowledge of IPV and improves their perceived readiness to respond to survivors is likely to do more good than harm across multiple contexts. This is in line with the World Health Organisation's current guidelines for the health sectors' response to violence against women, which identifies training of HCPs as necessary if not sufficient to improving women’s access to supportive response. The guidelines suggest that training of individual providers needs to be supported by wider systemic changes and should be ongoing rather than a one‐off training. See for example WHO curriculum (WHO 2019).
The findings of studies that assess the impact of training on improving HCPs' actual response to survivors of IPV, as well as impacts on documentation and identification of IPV, are inconsistent. A poorly‐designed training programme can lead to increased awareness about, for example, asking women about IPV without sufficient awareness about the importance of ensuring confidentiality and safety, which could potentially be harmful. Asking should only be done when certain requirements are met: people have been trained on how to provide as a minimum a first‐line response, confidentiality can be maintained and a protocol/standard operating procedure and a referral pathway are in place. Training is therefore necessary but not sufficient, and needs to be supported by an institution‐wide readiness to support the provider to address IPV. It is therefore important to assess the content and setting of the one intervention that is impactful and ensure that implemented programmes are in line with the ones that were tested and found to be successful. Based on only one study, we find that there is little to no evidence that training can increase referrals to services for survivors of IPV. However, absence of evidence should not deter the practice of training HCPs on IPV, as no harms or negative impacts were reported.
It is unclear if these effects of training are sustained in the long term. Evidence suggests that more experiential training approaches (e.g. use of role plays or case studies) that focus on all aspects of provider response (Asking, Validating, Documenting and Referral) may be an effective way to train HCPs. However, while training may be helpful, particularly for provider‐level outcomes, more information is needed on what is the most effective type of training approach, content, duration and intensity. System‐wide changes are needed to support individual providers, and training needs to go beyond one‐off interventions (as is often the case in research), to be continuous, with regular referrals as well as mentoring and support.
Implications for research.
Further research is needed to:
address gaps in the existing literature, particularly for training HCPs in low‐ and middle‐income countries and training HCPs to respond to those experiencing IPV, taking into account intersectionality, gender diversity and cultural diversity;
provide better‐certainty evidence through high‐quality trials; and
throw light on the nature of the interventions that are most likely to be effective (for example, through training, screening, systems change processes).
Evidence from low‐income countries is particularly lacking. A greater diversity in HCP type and setting is needed and future studies should be carried out in practice settings and with a range of providers. While this review has focused on IPV against women, reflecting the current research and practice, future reviews could also consider IPV training across a broader population of men, women and gender‐diverse victims and perpetrators. Replication studies are needed of successful interventions identified in this review, as well as studies that assess similar types of interventions. In addition, it is important that future studies clearly describe the intervention and its characteristics (including training content) and active components.
Future research can address gaps in the literature by looking at outcomes that are not systematically addressed by the current literature, such as the impact of training HCPs compared to no training, wait‐list, placebo on: safety planning, referrals made to support agencies/services, identification and documentation of IPV, women’s mental health, as well as adverse outcomes such as rates of IPV or other harms to women. Future research should add to evidence on the sustainability of the impacts of training. For example, no individual papers reported knowledge outcomes past one year, and few studies included training booster/refresher sessions, limiting our understanding of the ingredients necessary to sustain the effects of IPV training on providers' or women's outcomes.
Future research should also provide better‐certainty evidence that addresses the lack of provider masking by offering a placebo training in the comparison group. Studies should identify and prespecify their outcomes in a protocol, and use standardised and validated instruments to measure attitudes and readiness to respond. For more HCP practice‐related changes, objective measures, such as actual documentation and identification of IPV in case files, should be assessed, instead of provider self‐reports. A standardised patient approach may also be sufficient. Future studies should be mindful of the sample size needed to estimate an effect.
A review of the qualitative evidence would be useful, to provide insight into content, duration and method of delivery of the training, and how these impact learning and uptake of such training. Future studies should also consider the availability of support and supervision, and test the impacts of refresher/booster sessions, rather than seeing training as a one‐off intervention.
History
Protocol first published: Issue 2, 2017
Acknowledgements
We are grateful to the Cochrane Developmental, Psychosocial and Learning Problems Group and to its Managing Editor, Co‐ordinating Editor and Deputy Managing Editor for insightful comments, guidance and support provided. We would like to thank Margaret Anderson, the Review Group's Information Specialist, for guidance in developing the search strategy and for carrying out an updated search for us. We would also like to acknowledge the importance of feedback provided by external reviewers of the protocol in shaping this review.
The CRG Editorial Team are grateful to the following peer reviewers for their time and comments: Laura S Sadowski, MD, MPH, Cook County Health, Chicago (IL); Dr Virginia Minogue, Ireland; and also to the one peer reviewer who chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials(CENTRAL); Cochrane Register of Studies Online (http://crso.cochrane.org/)
#1(Domestic Violence):MH #2(Battered Women):MH #3(Intimate Partner Violence ):MH #4(Spouse Abuse):MH #5((abus* adj3 wom*n) OR (wom*n adj3 abus*)):TI,AB #6((abus* adj3 spous*) or (spous* adj3 abus*)):TI,AB #7((abus* adj3 partner*) OR (partner* adj3 abus*)):TI,AB #8(((abus* adj3 (wife or wives)) OR ((wife or wives) adj3 abus*))):TI,AB #9((abused adj3 mother*) OR (mother* adj3 abused)):TI,AB #10((batter* adj3 wom*n) OR (wom*n adj3 batter*)):TI,AB #11(((batter* adj3 (wife or wives)) OR ((wife or wives) adj3 batter*))):TI,AB #12((batter** adj3 spous*) or (spous* adj3 batter*)):TI,AB #13((batter* adj3 partner*) OR (partner* adj3 partner*)):TI,AB #14((wife or wives) adj3 violen*) OR (violen* adj3 (wife or wives)):TI,AB #15((partner* adj3 violen*) or (violen* adj3 partner*)):TI,AB #16((spous* adj3 violen*) or (violen* adj3 spous*)):TI,AB #17(IPV NOT polio*):TI,AB #18((domestic adj3 (abus* or violen*)) OR ((abus* or violen*) adj3 domestic)):TI,AB #19((relationship* adj3 violen*) OR (violen* adj3 relationship*)):TI,AB #20((famil* adj3 violen*) OR (violen* adj3 famil*)):TI,AB #21((intimat* adj3 violen*) OR (violen* adj3 intimat*)):TI,AB #22(dating violence or date rape):TI,AB #23((gender adj3 (abuse* or violen*)) OR ((abuse* or violen*) adj3 gender*)):TI,AB #24MESH DESCRIPTOR education EXPLODE ALL TREES #25MESH DESCRIPTOR teaching EXPLODE ALL TREES #26MESH DESCRIPTOR Inservice Training EXPLODE ALL TREES #27MESH DESCRIPTOR Health Personnel EXPLODE ALL TREES #28MESH DESCRIPTOR health Knowledge, Attitudes, Practice #29(Clinical Competence):MH #30(((staff or professional) adj3 development) OR (development adj3 (staff or professional))):TI,AB #31instruct*:TI,AB #32(train or trained or training):TI,AB #33untrained:TI,AB #34curricul*:TI,AB #35(educate* or education*):TI,AB #36teach*:TI,AB #37seminar*:TI,AB #38tutorial*:TI,AB #39lecture*:TI,AB #40program*:TI,AB #41(workshop* or work shop*):TI,AB #42(web* OR internet* OR online or on‐line or computer based or computer assist*):TI,AB #43lesson*:TI,AB #44#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 #45#24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 #46#44 AND #45
MEDLINE Ovid
Combined Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) strategy used August 2017
1. Battered Women/ 2. Domestic Violence/ 3. Spouse Abuse/ 4. Intimate Partner Violence/ 5. (abus$ adj3 wom#n).tw,kf. 6. (abus$ adj3 spous$).tw,kf. 7. (abus$ adj3 partner$).tw,kf. 8. (abus$ adj3 (wife or wives)).tw,kf. 9. (abused adj3 mother$).tw,kf. 10. (batter$ adj3 wom#n).tw,kf. 11. (batter$ adj3 (wife or wives)).tw,kf. 12. (batter$ adj3 spous$).tw,kf. 13. (batter$ adj3 partner$).tw,kf. 14. ((wife or wives) adj3 violen$).tw,kf. 15. (partner$ adj3 violen$).tw,kf. 16. (spous$ adj3 violen$).tw,kf. 17. (IPV not polio$).tw,kf. 18. (domestic adj3 (abus$ or violen$)).tw,kf. 19. (relationship$ adj3 violen$).tw,kf. 20. (famil$ adj3 violen$).tw,kf. 21. (intimate adj3 violen$).tw,kf. 22. ((dat$ adj3 violence) or date rape).tw,kf. 23. (gender adj3 violence).tw,kf. 24. or/1‐23 25. exp education/ 26. exp Teaching/ 27. exp Inservice Training/ 28. exp Health Personnel/ed [Education] 29. Health Knowledge, Attitudes, Practice/ 30. Clinical Competence/ 31. ((staff or professional) adj3 development).tw,kf. 32. exp video‐audio media/ 33. lesson$.tw,kf. 34. instruct$.tw,kf. 35. (train or trained or training).tw,kf. 36. untrained.tw,kf. 37. curricul$.tw,kf. 38. (educate$ or education$).tw,kf. 39. teach$.tw,kf. 40. seminar$.tw,kf. 41. tutorial$.tw,kf. 42. lecture$.tw,kf. 43. program$.tw,kf. 44. (workshop$ or work‐shop$).tw,kf. 45. (class or classes).tw,kf. 46. (online or on‐line or computer based or computer assist$).tw,kf. 47. or/25‐46 48. randomized controlled trial.pt. 49. controlled clinical trial.pt. 50. randomi#ed.ab. 51. placebo$.ab. 52. drug therapy.fs. 53. randomly.ab. 54. trial.ab. 55. groups.ab. 56. or/48‐55 57. exp animals/ not humans.sh. 58. 56 not 57 59. 24 and 47 and 58
MEDLINE strategy used May 2019 and June 2020
1 Battered Women/ 2 Domestic Violence/ 3 exp Intimate Partner Violence/ 4 (abus$ adj3 wom#n).tw,kf. 5 (abus$ adj3 spous$).tw,kf. 6 (abus$ adj3 partner$).tw,kf. 7 (abus$ adj3 (wife or wives)).tw,kf. 8 (abused adj3 mother$).tw,kf. 9 (batter$ adj3 wom#n).tw,kf. 10 (batter$ adj3 (wife or wives)).tw,kf. 11 (batter$ adj3 spous$).tw,kf. 12 (batter$ adj3 partner$).tw,kf. 13 ((wife or wives) adj3 violen$).tw,kf. 14 (partner$ adj3 violen$).tw,kf. 15 (spous$ adj3 violen$).tw,kf. 16 (IPV not polio$).tw,kf. 17 (domestic adj3 (abus$ or violen$)).tw,kf. 18 (relationship$ adj3 violen$).tw,kf. 19 (famil$ adj3 violen$).tw,kf. 20 (intimate adj3 violen$).tw,kf. 21 ((dat$ adj3 violence) or date rape).tw,kf. 22 (gender adj3 violence).tw,kf. 23 or/1‐22 24 exp education/ 25 exp Teaching/ 26 exp Inservice Training/ 27 exp Health Personnel/ed [Education] 28 Health Knowledge, Attitudes, Practice/ 29 Clinical Competence/ 30 ((staff or professional) adj3 development).tw,kf. 31 exp video‐audio media/ 32 lesson$.tw,kf. 33 instruct$.tw,kf. 34 (train or trained or training).tw,kf. 35 untrained.tw,kf. 36 curricul$.tw,kf. 37 (educate$ or education$).tw,kf. 38 teach$.tw,kf. 39 seminar$.tw,kf. 40 tutorial$.tw,kf. 41 lecture$.tw,kf. 42 program$.tw,kf. 43 (workshop$ or work‐shop$).tw,kf. 44 (class or classes).tw,kf. 45 (online or on‐line or computer based or computer assist$).tw,kf. 46 or/24‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomi#ed.ab. 50 placebo$.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab 55 or/47‐54 56 exp animals/ not humans.sh. 57 55 not 56 58 23 and 46 and 57
MEDLINE In‐Process & Other Non‐Indexed Citations (Ovid)
Strategy used August 2019 and June 2020
1 (abus$ adj3 wom#n).tw,kf. 2 (abus$ adj3 spous$).tw,kf. 3 (abus$ adj3 partner$).tw,kf. 4 (abus$ adj3 (wife or wives)).tw,kf. 5 (abused adj3 mother$).tw,kf. 6 (batter$ adj3 wom#n).tw,kf. 7 (batter$ adj3 (wife or wives)).tw,kf. 8 (batter$ adj3 spous$).tw,kf. 9 (batter$ adj3 partner$).tw,kf. 10 ((wife or wives) adj3 violen$).tw,kf. 11 (partner$ adj3 violen$).tw,kf. ) 12 (spous$ adj3 violen$).tw,kf. 13 (IPV not polio$).tw,kf. 14 (domestic adj1 (abus$ or violen$)).tw,kf. 15 (relationship$ adj3 violen$).tw,kf. 16 (famil$ adj3 violen$).tw,kf. 17 (intimate adj3 violen$).tw,kf. 18 ((dat$ adj3 violence) or date rape).tw,kf. 19 (gender adj3 violence).tw,kf. 20 or/1‐19 21 ((staff or professional) adj3 development).tw,kf. 22 lesson$.tw,kf. 23 instruct$.tw,kf. 24 (train or trained or training).tw,kf. 25 untrained.tw,kf. ( 26 curricul$.tw,kf. 27 (educate$ or education$).tw,kf. 28 teach$.tw,kf. 29 seminar$.tw,kf. 30 tutorial$.tw,kf. 31 lecture$.tw,kf. 32 program$.tw,kf. 33 (workshop$ or work shop$).tw,kf. 34 (class or classes).tw,kf. 35 (online or on‐line or computer based or computer assist$).tw,kf. 36 or/22‐35 37 20 and 36 38 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 39 37 and 38
MEDLINE(R) Epub Ahead of Print
Strategy used August 2019 and June 2020
1 (abus$ adj3 wom#n).tw,kf. 2 (abus$ adj3 spous$).tw,kf. 3 (abus$ adj3 partner$).tw,kf. 4 (abus$ adj3 (wife or wives)).tw,kf. 5 (abused adj3 mother$).tw,kf. 6 (batter$ adj3 wom#n).tw,kf. 7 (batter$ adj3 (wife or wives)).tw,kf. 8 (batter$ adj3 spous$).tw,kf. 9 (batter$ adj3 partner$).tw,kf. 10 ((wife or wives) adj3 violen$).tw,kf. 11 (partner$ adj3 violen$).tw,kf. ) 12 (spous$ adj3 violen$).tw,kf. 13 (IPV not polio$).tw,kf. 14 (domestic adj1 (abus$ or violen$)).tw,kf. 15 (relationship$ adj3 violen$).tw,kf. 16 (famil$ adj3 violen$).tw,kf. 17 (intimate adj3 violen$).tw,kf. 18 ((dat$ adj3 violence) or date rape).tw,kf. 19 (gender adj3 violence).tw,kf. 20 or/1‐19 21 ((staff or professional) adj3 development).tw,kf. 22 lesson$.tw,kf. 23 instruct$.tw,kf. 24 (train or trained or training).tw,kf. 25 untrained.tw,kf. ( 26 curricul$.tw,kf. 27 (educate$ or education$).tw,kf. 28 teach$.tw,kf. 29 seminar$.tw,kf. 30 tutorial$.tw,kf. 31 lecture$.tw,kf. 32 program$.tw,kf. 33 (workshop$ or work shop$).tw,kf. 34 (class or classes).tw,kf. 35 (online or on‐line or computer based or computer assist$).tw,kf. 36 or/22‐35 37 20 and 36 38 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 39 37 and 38
Embase
Embase (Embase.com ) strategy used August 2017
(('partner violence'/exp OR 'partner violence') OR 'battered woman'/exp OR 'gender based violence'/exp OR 'family violence'/exp OR 'domestic violence'/de OR (abus* NEAR/3 wom?n):ab,ti OR (abus* NEAR/3 spous*):ab,ti OR (abus* NEAR/3 partner*):ab,ti OR (abus* NEAR/3 (wife OR wives)):ab,ti OR (abused NEAR/3 mother):ab,ti OR (batter* NEAR/3 wom?n):ab,ti OR (batter* NEAR/3 (wife OR wives)):ab,ti OR (batter* NEAR/3 spous*):ab,ti OR (batter* NEAR/3 partner*):ab,ti OR ((wife OR wives) NEAR/3 violen*):ab,ti OR (partner* NEAR/3 violen*):ab,ti OR (spous* NEAR/3 violen*):ab,ti OR (ipv:ab,ti NOT polio*:ab,ti) OR (relationship* NEAR/3 violen*):ab,ti OR (domestic NEAR/3 violen*):ab,ti OR (domestic NEAR/3 abus*):ab,ti OR (famil* NEAR/3 violen*):ab,ti OR (intimate NEAR/3 violen*):ab,ti OR (dat* NEAR/3 violence):ab,ti OR 'date rape':ab,ti OR (gender NEAR/3 violence):ab,ti) AND (('education'/exp OR 'education') OR ('teaching'/exp OR 'teaching') OR 'inservice training'/exp OR 'training'/de OR 'clinical competence'/de OR (professional NEAR/3 development):ab,ti OR (staff NEAR/3 development):ab,ti OR lesson*:ab,ti OR instruct*:ab,ti OR (train:ab,ti OR trained:ab,ti) OR curricul*:ab,ti OR educate*:ab,ti OR teach*:ab,ti OR seminar*:ab,ti OR tutorial*:ab,ti OR lecture*:ab,ti OR program*:ab,ti OR (workshop*:ab,ti OR (work AND shop*:ab,ti)) OR (class:ab,ti OR classes:ab,ti) OR (online:ab,ti OR 'on line':ab,ti OR 'computer based':ab,ti OR 'computer assist*':ab,ti) OR untrained:ab,ti) AND ('crossover procedure'/de OR 'double blind procedure'/de OR 'randomized controlled trial'/de OR 'single‐blind procedure'/de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR (cross NEXT/1 over*):de,ab,ti OR placebo*:de,ab,ti OR (doubl* NEAR/1 blind*):de,ab,ti OR (singl* NEAR/1 blind*):de,ab,ti OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti
Embase Ovid strategy used August 2019 and June 2020
1 domestic violence/ 2 battered woman/ 3 partner violence/ 4 gender based violence/ 5 family violence/ 6 (abus$ adj3 wom#n).tw,kw. 7 (abus$ adj3 spous$).tw,kw. 8 (abus$ adj3 partner$).tw,kw. 9 (abus$ adj3 (wife or wives)).tw,kw. 10 (abused adj3 mother$).tw,kw. 11 (batter$ adj3 wom#n).tw,kw. 12 (batter$ adj3 (wife or wives)).tw,kw. 13 (batter$ adj3 spous$).tw,kw. 14 (batter$ adj3 partner$).tw,kw. 15 ((wife or wives) adj3 violen$).tw,kw. 16 (partner$ adj3 violen$).tw,kw. 17 (spous$ adj3 violen$).tw,kw. 18 (IPV not polio$).tw,kw. 19 (domestic adj1 (abus$ or violen$)).tw,kw. 20 (relationship$ adj3 violen$).tw,kw. 21 (famil$ adj3 violen$).tw,kw. 22 (intimate adj3 violen$).tw,kw. 23 ((dat$ adj3 violence) or date rape).tw,kw. 24 (gender adj3 violence).tw,kw. 25 or/1‐24 26 exp education/ 27 exp teaching/ 28 exp in service training/ 29 clinical competence/ 30 ((staff or professional) adj3 development).tw,kw. 31 lesson$.tw,kw. 32 instruct$.tw,kw. 33 (train or trained or training).tw,kw. 34 untrained.tw,kw. 35 curricul$.tw,kw. 36 (educate$ or education$).tw,kw. 37 teach$.tw,kw. 38 seminar$.tw,kw. 39 tutorial$.tw,kw. 40 lecture$.tw,kw. 41 program$.tw,kw. 42 (workshop$ or work shop$).tw,kw. 43 (class or classes).tw,kw. 44 (online or on‐line or computer based or computer assist$).tw,kw. 45 or/26‐44 46 Randomized controlled trial/ 47 controlled clinical trial/ 48 Single blind procedure/ 49 Double blind procedure/ 50 triple blind procedure/ 51 Crossover procedure/ 52 (crossover or cross‐over).tw. 53 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 54 Placebo/ 55 placebo.tw. 56 prospective.tw. 57 factorial$.tw. 58 random$.tw. 59 assign$.ab. 60 allocat$.tw. 61 volunteer$.ab. 62 or/46‐61 63 25 and 45 and 62
ERIC EBSCOhost
S38 S15 AND S34 AND S37 S37 S35 OR S36 S36 TI (random* or trial* or experiment* or PROSPECTIVE* OR longitudinal or BLIND* or CONTROL*) OR AB (random* or trial* or experiment* or PROSPECTIVE* OR longitudinal or BLIND* or CONTROL*) S35 DE "Meta Analysis" OR DE "Evaluation Research" OR DE "Control Groups" OR DE "Experimental Groups" OR DE "Longitudinal Studies" OR DE "Followup Studies" OR DE "Program Effectiveness" OR DE "Program Evaluation" S34 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S33 TI(web* or internet* or online or on‐line or "computer based" or "computer assisted") OR AB(web* or internet* or online or on‐line or "computer based" or "computer assisted") S32 TI(class or classes) OR AB(class or classes) S31 TI(workshop* or work‐shop*) OR AB(workshop* or work‐shop*) S30 TI(program* or module*) OR AB(program* or module*) S29 TI(lecture*) OR AB(lecture*) S28 TI(tutorial*) OR AB(tutorial*) S27 TI(seminar*) OR AB(seminar*) S26 TI(teach*) OR AB(teach*) S25 TI(educate* or education*) OR AB(educate* or education*) S24 TI(curricul*) OR AB(curricul*) S23 TI(untrained) OR AB(untrained) S22 TI (train or trained or training) OR AB(train or trained or training) S21 TI(instruct*) OR AB(instruct*) S20 TI(lesson*) OR AB(lesson*) S19 TI((staff or professional) N1 development) OR AB((staff or professional) N1 development) S18 DE "Professional Development" OR DE "Professional Education" S17 DE "Curriculum" S16 DE "Medical Education" OR DE "Graduate Medical Education" OR DE "Nursing Education" OR DE "Pharmaceutical Education" OR DE "Professional Education" OR DE "Allied Health Occupations Education" S15 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 S14 TI (gender N3 (abuse* or violen*)) OR AB(gender N3 (abuse* or violen*)) S13 TI ((dat* N3 violen*) or date rape) OR AB((dat* N3 violen*) or date rape) S12 TI(intimate N3 violen*) OR AB(intimate N3 violen*) S11 TI(famil* N3 violen*)OR AB(famil* N3 violen*) S10 TI(relationship* N3 violen*) OR AB(relationship* N3 violen*) S9 TI(domestic N3 (abus* or violen*)) OR AB(domestic N3 (abus* or violen*)) S8 TI(IPV not polio*) OR AB(IPV not polio*) S7 TI(spous* N3 violen*) OR AB(spous* N3 violen*) S6 TI(partner* N3 violen*) OR AB(partner* N3 violen*) S5 TI((wife or wives) N3 violen*) OR AB((wife or wives) N3 violen*) S4 TI (battered N3 (wom*n or mother*or spous* or partner or wife or wives*)) OR AB(battered N3 (wom*n or mother*or spous* or partner or wife or wives*)) S3 TI (abus* N3 (wom*n or mother*or spous* or partner or wife or wives*)) OR AB (abus* N3 (wom*n or mother*or spous* or partner or wife or wives*)) S2 (DE "Family Violence") S1 (DE "Violence" AND DE "Females")
CINAHL Plus EBSCOhost
CINAHL strategy used August 2017
(MM "Intimate Partner Violence") OR (MM "Domestic Violence+") OR (MM "Dating Violence") OR (MM "Battered Women") OR (TX abus* N3 wom*n) OR (TX abus* N3 spous*) OR (TX abus* N3 partner*) OR (TX abus* N3 wife) OR (TX abus* N3 wives) OR (TX batter* N3 spous*) OR (TX batter* N3 partner*) OR (TX wife N3 violen*) OR (TX wives N3 violen*) OR (TX partner* N3 violen*) OR (TX spous* N3 violen*) OR (TX domestic N3 violen*) OR (TX domestic N3 abus*) OR (TX relationship* N3 violen*) OR (TX famil* N3 violen*) OR (TX intimate N3 violen*) OR (TX dat* N3 violence) OR (TX “date rape”) OR (TX gender N3 violence)
AND
(MM "Education+") OR (MM "Adult Education") OR (MM "Education, Clinical+") OR (MM "Education, Health Sciences+") OR (MM "Learning Methods+") OR (MM "Teaching+") OR (MM "Teaching Materials+") OR (MM "Teaching Methods+") OR (MM "Clinical Competence+") OR (TX professional N3 development) OR (TX staff N3 development) OR (TX lesson*) OR (TX instruct*) OR (TX train) OR (TX trained) OR (TX untrained) OR (TX curricul*) OR (TX educate*) OR (TX teach*) OR (TX seminar*) OR (TX tutorial*) OR (TX lecture*) OR (TX program*) OR (TX workshop*) OR (TX work‐shop*) OR (TX class) OR (TX classes) OR (TX online) OR (TX on‐line) OR (TX “computer based”) OR (TX computer assist*)
AND
(MM "Clinical Trials+") OR (MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (TX “randomi*ed controlled trial”) OR (TI experiment*) OR (TI “clinical trial*”) OR (TI control* N2 trial*) OR (AB experiment*) OR (AB trial) OR (AB single N2 mask*) OR (AB double N2 blind*) OR (AB double N2 mask*) OR (AB single N2 blind*) OR (AB random* N1 assign*) OR (AB random* N1 allocat*) OR (AB randomi*ed) OR (AB group N1 assign*) OR (AB group N1 allocat*)
CINAHL strategy used May 2019 and June 2020
Lines 34 to 56 form the Cochrane CINAHL Plus RCT filter (Glanville 2019)
S1(MH "Intimate Partner Violence") S2(MH "Domestic Violence") S3TI (abus* N3 (wom*n or mother*or spous* or partner or wife or wives*)) OR AB (abus* N3 (wom*n or mother*or spous* or partner or wife or wives*)) S4TI (battered N3 (wom*n or mother*or spous* or partner or wife or wives*)) OR AB(battered N3 (wom*n or mother*or spous* or partner or wife or wives*) S5TI((wife or wives) N3 violen*) OR AB((wife or wives) N3 violen*) S6TI(partner* N3 violen*) OR AB(partner* N3 violen*) S8TI(IPV not polio*) OR AB(IPV not polio*) S9TI(domestic N3 (abus* or violen*)) OR AB(domestic N3 (abus* or violen*)) S10TI(relationship* N3 violen*) OR AB(relationship* N3 violen*) S11TI(famil* N3 violen*)OR AB(famil* N3 violen*) S12TI(intimate N3 violen*) OR AB(intimate N3 violen*) S13TI ((dat* N3 violen*) or "date rape") OR AB((dat* N3 violen*) or "date rape") S14TI (gender N3 (abuse* or violen*)) OR AB(gender N3 (abuse* or violen*))h S15S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 Database ‐ CINAHL Plus22,141 S16(MH "Curriculum+") OR (MH "Education, Clinical") OR (MH "Education, Health Sciences+") OR (MH "Education, Non‐Traditional") OR (MH "Schools, Health Occupations+") OR (MH "Staff Development") S17TI((staff or professional) N1 development) OR AB((staff or professional) N1 development) S18TI(lesson*) OR AB(lesson*) S19TI(instruct*) OR AB(instruct*) S20TI (train or trained or training) OR AB(train or trained or training) S21TI(untrained) OR AB(untrained) S22TI(curricul*) OR AB(curricul*) S23TI(educate* or education*) OR AB(educate* or education*) S24TI(teach*) OR AB(teach*) S25TI(seminar*) OR AB(seminar*) S26TI(tutorial*) OR AB(tutorial*) S27TI(lecture*) OR AB(lecture*) S28TI(program* or module*) OR AB(program* or module*) S29TI(workshop* or work‐shop*) OR AB(workshop* or work‐shop*) S30TI(class or classes) OR AB(class or classes) S31TI(web* or internet* or online or on‐line or "computer based" or "computer assisted") OR AB(web* or internet* or online or on‐line or "computer based" or "computer assisted") S32S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33S15 AND S32 S34MH ("Randomized Controlled Trials") S35(MH "Double‐Blind Studies") S36(MH "Single‐Blind Studies") S37(MH "Random Assignment") S38(MH "Pretest‐Posttest Design") S39MH ("Cluster Sample") S40TI (randomised OR randomized) S41AB (random*) S42TI (trial) S43(MH "Sample Size") AND AB (assigned OR allocated OR control) S44MH (Placebos) S45PT (Randomized Controlled Trial) S46AB (control W5 group) S47MH ("Crossover Design") OR MH ("Comparative Studies") S48AB (cluster W3 RCT) S49(MH "Animals+") S50MH ("Animal Studies") S51TI (animal model*) S52S49 OR S50 OR S51 S53MH ("Human") S54S52 NOT S53 S55S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 S56S55 NOT S54 S57S33 AND S56
PsycINFO Ovid
1 domestic violence/ 2 battered females/ 3 intimate partner violence/ 4 (abus$ adj3 spous$).tw,id. 5 (abus$ adj3 partner$).tw,id. 6 (abus$ adj3 (wife or wives)).tw,id. 7 (abused adj3 mother$).tw,id. 8 (abus$ adj3 (female$ or wom#n)).tw,id. 9 (batter$ adj3 (female$ or wom#n)).tw,id. 10 (batter$ adj3 (wife or wives)).tw,id. 11 (batter$ adj3 spous$).tw,id. 12 (batter$ adj3 partner$).tw,id. 13 ((wife or wives) adj3 violen$).tw,id. 14 (partner$ adj3 violen$).tw,id. 15 (spous$ adj3 violen$).tw,id. 16 (IPV not polio$).tw,id. 17 (domestic adj3 (abus$ or violen$)).tw,id. 18 (relationship$ adj3 violen$).tw,id. 19 (famil$ adj3 violen$).tw,id. 20 (intimate adj3 violen$).tw,id. 21 ((dat$ adj3 violence) or date rape).tw,id. 22 (gender adj3 violence).tw,id. 23 or/1‐22 24 exp education/ 25 exp teaching/ 26 training/ 27 exp personnel training/ 28 professional development/ 29 professional competence/ 30 exp Knowledge Level/ 31 ((staff or professional) adj3 development).tw,id. 32 (web$ or internet or online or on‐line or computer based or computer assist$).tw,id. 33 (class or classes or curricul$ or educate$ or education$ or instruct$ or lecture$ or lesson$ or module$ or program$ or seminar$ or teach or train or trained or training or tutorial$ or untrained or workshop$ or work‐shop$).tw,id. 34 or/24‐33 35 clinical trials/ 36 experimental design/ 37 placebo/ 38 Experiment controls/ 39 ((clinic$ or control$) adj (study or trial$ or experiment$)).tw,id. 40 (randomiz$ or randomis$ or randomly).tw,id. 41 exp program evaluation/ 42 treatment effectiveness evaluation/ 43 ((compar$ or control$ or experiment$ or treat$ or TAU) adj3 (subjects or group$ or participants)).tw,id. 44 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw,id. 45 or/35‐44 46 23 and 34 and 45
Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library
#1(mh "Domestic Violence") #2(mh "Battered Women") #3(mh "Intimate Partner Violence") #4(mh "Spouse Abuse") #5(abus* Near/3 wom*n):ti,ab #6(abus* Near/3 spous*):ti,ab #7(abus* Near/3 partner*):ti,ab #8(abus* Near/3 (wife or wives)):ti,ab #9(abused Near/3 mother*):ti,ab #10(batter* Near/3 wom*n):ti,ab #11(batter* Near/3 (wife or wives)):ti,ab #12(batter* Near/3 spous*):ti,ab #13(batter* Near/3 partner*):ti,ab #14((wife or wives) Near/3 violen*):ti,ab #15(partner* Near/3 violen*):ti,ab #16(spous* Near/3 violen*):ti,ab #17(IPV NOT polio*):ti,ab #18(domestic Near/3 (abus* or violen*)):ti,ab #19(relationship* Near/3 violen*):ti,ab #20(famil* Near/3 violen*):ti,ab #21(intimat* Near/3 violen*):ti,ab #22("dating violence" or "date rape"):ti,ab #23(gender Near/3 (abuse* or violen*)):ti,ab #24#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 #25(mh education) #26(mh teaching) #27(mh "Inservice Training") #28(mh "Health Personnel") #29(mh "Health Knowledge, Attitudes, Practice") #30(mh "Clinical Competence") #31(((staff or professional) Near/3 development) #32instruct*:ti,ab #33(train or trained or training):ti,ab #34untrained:ti,ab #35curricul*:ti,ab #36(educate* or education*):ti,ab #37teach*:ti,ab #38seminar*:ti,ab #39tutorial*:ti,ab #40lecture*:ti,ab #41program*:ti,ab #42(workshop* or work NEXT shop*):ti,ab #43(web* OR internet* OR online or on NEXT line or computer NEXT based or computer NEXT assist*):ti,ab #44lesson*:ti,ab #45#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 #46#24 AND #45
Popline
POPLINE website was retired on September 1, 2019.
("Domestic Violence") OR ("Battered Woman" ~3) OR ("Battered Women" ~3) OR ("abused woman" ~3) OR ("abused spouse" ~3) OR ("abused partner" ~3) OR ("abused wife" ~3) OR ("abused wives" ~3) OR ("abuse woman" ~3) OR ("abuse spouse" ~3) OR ("abuse partner" ~3) OR ("abuse wife" ~3) OR ("abuse wives" ~3) OR ("battered spouse" ~3) OR ("battered partner"~3) OR ("batter spouse" ~3) OR ("batter partner"~3) OR ("wife violence" ~3) OR ("wives violence" ~3) OR ("partner violence" ~3) OR ("spouse violence" ~3) OR ("domestic violence" ~3) OR ("domestic abuse" ~3) OR ("relationship violence" ~3) OR ("family violence" ~3) OR ("intimate violence" ~3) OR ("intimate violent" ~3) OR ("date violence" ~3) OR ("dating violence" ~3) OR ("date rape" ~3) OR ("gender violence" ~3)
(Education) OR ("Adult Education" ~3) OR ("Education Clinical" ~3) OR ("Education Health" ~3) OR ("Learning Methods" ~3) OR ("Teaching Materials" ~3) OR ("Teaching Methods" ~3) OR ("Clinical Competence" ~3) OR ("professional development" ~3) OR ("staff development" ~3) OR (lesson*) OR (instruct*) OR (train) OR (training) OR (trained) OR (untrained) OR (curricul*) OR (educat*) OR (teach*) OR (seminar*) OR (tutorial*) OR (lecture*) OR (program*) OR (workshop*) OR (work‐shop*) OR (class) OR (classes) OR (online) OR (on‐line) OR ("computer based") OR ("computer‐based") OR (computer assist*)
("Clinical Trial") OR ("Random Assignment") OR (experiment*) OR (clinical trial*) OR (control* trial*) OR ("single mask" ~2) OR ("double blind" ~2) OR ("double mask" ~2) OR ("single blind" ~2) OR ("single masked" ~2) OR ("double blinded" ~2) OR ("double masked" ~2) OR ("single blinded" ~2) OR ("single masking" ~2) OR ("double blinding" ~2) OR ("double masking" ~2) OR ("single blind" ~2) OR (random* assign*) OR (random* allocat*) OR (randomi*ed) OR (group assign*) OR (group allocat*) OR (RCT) OR (placebo*) OR (randomi*ed) OR (randomly)
LILACS
(tw:(violen* OR abuse* OR batter* )) AND (tw:(partner* OR domestic OR gender* OR intimate OR woman OR women OR girl* OR wife* OR wives OR mother* OR female*)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials")) OR (mh:("Violence" OR "Spouse Abuse" OR "Battered Women" )) AND (mh:( "Health Knowledge, Attitudes, Practice" OR "Public Health" OR "Nursing")) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
WHOLIS database
‘violence’ ‘train’ ‘violence training’
WHO International Clinical Trials Registry Platform (ICTRP)
CONDITION violence OR battering OR abuse OR battered OR abused AND INTERVENTION| training OR train OR education OR educate OR teach OR teaching OR educating OR development OR lesson OR workshop OR lecture OR program or class
ClinicalTrials.gov
CONDITION violence OR battering OR abuse OR battered OR abused AND INTERVENTION training OR train OR education OR educate OR teach OR teaching OR educating OR development OR lesson OR workshop OR lecture OR program or class
AIM database
term used: ‘violence’
Website searches
World Bank (www.worldbank.org)
Key words used: Healthcare provider AND violence against women AND training
Violence Prevention (Centre for Public Health, Liverpool John Moores University; www.preventviolence.info).
Searched for Healthcare provider AND violence against women AND training
International Council of Nurses (ICN; www.icn.ch)
Search terms: Healthcare provider AND violence against women AND training
Centers for Disease Control and Prevention (www.cdc.gov/injury and stacks.cdc.gov)
Search terms: RCT AND violence AND training AND healthcare provider site:cdc.gov
Appendix 2. Unused methods
Measures of treatment effect
Binary outcome data
For binary outcomes, we had planned to use risk ratios (RRs) provided by study authors for the meta‐analysis and to present these with 95% confidence intervals (CIs). However, no binary data were synthesised in a meta‐analysis.
Continuous outcome data
For continuous outcomes, we had planned to use the mean difference (MD) if outcomes were measured across studies on the same/similar scales. Since this was not the case, we reported SMDs throughout.
Unit of analysis issues
Cluster RCTs
For studies that did not report the ICC, we had planned to borrow one(s) from similar studies or from external sources, but we did not find suitable estimates of ICC.
Cross‐over trials
Owing to potential carry‐over effects of training, cross‐over studies may be inappropriate for assessing the impact of training interventions. If a study had used a cross‐over design, and depending on how results were reported, we had planned to appropriately re‐analyse them (if necessary, we had planned to borrow correlation coefficients from other sources), and make all assumptions that we made explicit and subject to a sensitivity analysis.
Dealing with missing data
Where the standard deviation was not provided by a study and could not be obtained from its authors, we had planned to impute it by calculating the median of available standard deviations. However, this was not necessary.
Assessment of heterogeneity > Methodological heterogeneity
We had planned to explore variation due to the quality of studies (as measured by the ’Risk of bias’ tool) by conducting a sensitivity analysis (See sensitivity analysis below).
Assessment of reporting biases
We had planned to formally investigate funnel plot asymmetry and reporting bias using Beggs and regression‐based Egger's test (Egger 1997), where possible. However, we were not able to do this as fewer than 10 studies were included in all meta‐analyses. We had also planned to use a 'trim and fill' method to further investigate reasons for asymmetry. This would have involved removing smaller studies that led to the asymmetry of the funnel plot and performing the meta‐analysis with remaining studies. This was also not done due to the small number of studies.
Subgroup analysis and investigation of heterogeneity
We had planned to pool together studies with booster sessions if two or more studies had reported this. However, we did not find booster sessions in two or more studies in any of the meta‐analyses that we conducted.
Sensitivity analysis
Study quality and attrition: we had planned to pool only studies at low risk of bias to see if their results differ. We had planned to explore the variation due to the certainty of studies (as measured by the risk of bias tool) by conducting a sensitivity analysis. However, we did not implement this, as we did not identify subgroups where risk of bias was low across all criteria.
Intracluster correlation coefficient (ICC): we had planned to carry out a deterministic sensitivity analysis by varying borrowed values of the ICC for cluster‐RCTs that do not reporting the ICC value. However, we did not find suitable estimates of ICC and have not been able to identify similar studies to borrow it from. We therefore could only inflate standard errors where appropriate.
Correlation coefficients: we had planned to carry out a deterministic sensitivity analysis by varying borrowed correlation coefficients for cross‐over studies that not reporting correlation coefficients.
Imputed missing data: we had planned to remove studies that had imputed missing data and synthesise studies that provided complete information.
Imputed standard deviations: we had planned to remove studies with imputed standard deviations and compare them versus a meta‐analysis of studies that reported standard deviations.
Appendix 3. Criteria used to assess risk of bias
Sequence generation
We considered this as follows.
Low risk of bias: random number tables or computed‐generated random numbers were used.
High risk of bias: a non‐random component was used in the sequence generation process (e.g. sequence generated on the basis of hospital or clinic record numbers, day or time of visit, etc.).
Unclear risk of bias: the process was not adequately described to permit a judgement of high or low risk of bias.
Allocation concealment
We considered this as follows.
Low risk of bias: The unit of allocation and analysis was a cluster, allocation was done at one point in time and an adequate description of the allocation process (i.e. use of random number tables, flip a coin, etc.) was provided. If allocation was at the individual level, acceptable methods of allocation concealment include sequentially numbered, opaque, sealed envelopes (SNOSE); numbered or coded containers; central randomisation (e.g. by telephone to a trials office); or another method whose description contained elements convincing of concealment (e.g. a secure computer‐assisted method);
High risk of bias: allocation was carried out in a manner whereby those responsible for admitting participants into trial arms could detect the upcoming assignment.
Unclear risk of bias: the study did not provide an adequate description to permit a judgement of high or low risk of bias.
Blinding of participants and personnel
We considered whether participants and personnel were blinded to group allocation as follows.
Low risk of bias: participants were unaware of the assignment.
High risk of bias: HCPs or participants had knowledge of group assignment and this knowledge could have interfered with reporting of outcomes (i.e. behavioural outcomes or other self‐reported outcomes). If the outcome was objective, such as mortality, we considered the risk of bias from the lack of blinding to be low.
Unclear risk of bias: no adequate information was provided to permit a judgement of high or low risk of bias.
Blinding of outcome assessors
We considered whether outcome assessors were unaware of which participant was allocated to which group as follows.
Low risk of bias: assessors or investigators were unaware of the assignment.
High risk of bias: as knowledge of group assignment could interfere with assessment of outcomes (i.e. behavioural outcomes or other self‐reported outcomes) by an external assessor, we considered outcomes that were self‐reported by a participant or a HCP or that were objectively assessed, such as mortality, at low risk of bias.
Unclear risk of bias: no adequate information was provided to permit a judgement of high or low risk of bias.
Incomplete outcome (attrition bias)
When available, we collected and reported proportions of loss at follow‐up in each arm of included studies, with the aim of critically assessing imbalance in attrition between arms. We considered whether missing data were balanced across groups as follows.
Low risk of bias: dropout rate was less than 20% of participants in both arms.
High risk of bias: attrition was greater than 20% in either arm or was unequal in numbers across the arms of a trial, and no adequate explanation was provided. In the case of cluster randomisation, we considered dropping out of any cluster as presenting high risk of bias. However we also took into consideration the reasons for attrition and missing data.
Unclear risk of bias: the dropout rate was not mentioned in the study.
Selective reporting
We considered this as follows.
Low risk of bias: all relevant outcomes from the Methods section or protocol (when a trial protocol is registered) were reported in the Results section of the study.
High risk of bias: study authors reported only some outcomes. In particular, the outcome of interest was not reported.
Unclear risk of bias: no adequate information was provided to permit a judgement of high or low risk of bias.
Other
We considered other potential sources of bias as follows.
Low risk of bias: no other risk was identified.
High risk of bias: other potential sources of bias, such as lack of accounting for clustering in the case of cluster‐RCTs, were identified. We also considered the reliability and validity of outcome measures, and assessed factors such as directness of the research (i.e. how different the population, intervention and outcome are from the review question).
Unclear risk of bias: information regarding other potential sources of bias was insufficient to permit a judgement of high or low risk of bias.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ayaba‐Apawu 2016.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: graduate students |
|
| Participants |
Healthcare provider: graduate students pursuing a master's degree in counselling Other: N/A Location/Setting: mid‐west Urban University, USA Sample size: n = 30 (intervention group n = 15, control group n = 15) Number of withdrawals/dropouts: nil Sex: 2 men and 28 women Mean age: 33.32 years (range 24 ‐ 57 years) Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 15): IPV educational materials—focused on knowledge, asking, safety, documentation and referral—were emailed to participants; materials included 3 Powerpoint presentations with information on IPV, self‐reflective exercises and reflective questions Control (n = 15): no intervention |
|
| Outcomes |
Primary outcomes
Secondary outcomes: not reported Timing of outcome assessment: 2 weeks after baseline, immediately after intervention |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...were randomly assigned to the experimental group and the control group using ―pick from the bowl technique” (p 45) |
| Allocation concealment (selection bias) | Low risk | Comment: allocation at 1 point in time, using chits with numbers pulled from a bowl after recruitment into the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Comment: Participants seemed to be aware of allocation and study process Quotes: "All respondents attended a pre‐study information meeting where the study procedures and the duration of the study were discussed” (p 45)”; and “self‐reporting nature of the ―PREMIS, there is the possibility of a ―social desirability threat to external validity” (p 49) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes were self‐reported and therefore blinding of assessors is unlikely to impact detection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All PREMIS results that were measured were reported |
| Other bias | Low risk | Comment: The study appears to be free from other sources of bias |
Brienza 2005.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: individual participants were randomised using a random‐numbers table to either the workshop seminar alone (controls) or the workshop seminar plus shelter experience (cases) |
|
| Participants |
Healthcare provider: medical residents Other: N/A Location/Setting: university‐based, primary care internal medicine residents from the USA Sample size: n = 36 participants. Numbers in each arm not reported Number of withdrawals/dropouts: n = 14. 22 participants completed both pre‐ and post‐test and included in analysis. Dropout numbers in each arm not reported Sex: 16 men, 20 women Mean age: 31.1 (SD 2.8) years Inclusion criteria: residents were eligible for the study if assigned to the outpatient ambulatory block rotation during academic year 2001 – 2002 Exclusion criteria: not reported |
|
| Interventions | Number of participants within intervention and control not reported Interventions:
Control: IPV training only |
|
| Outcomes |
Primary outcomes: measured with 43‐item survey; majority of questions (n = 33) based on the Health Care Provider Survey for Domestic Violence instrument
Secondary outcomes: not reported Timing of outcome assessment: mean duration between administration of the pre‐ and post‐surveys was 7.5 months (range 6 – 12 months) |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: work completed while Dr Brienza and Ms Ladouceur were affiliated with the Department of Internal Medicine at Yale University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible residents were randomized using a random numbers table to either the workshop seminar alone (controls) or the workshop seminar plus shelter experience (cases)" (p 536) |
| Allocation concealment (selection bias) | Unclear risk |
Comment: the authors do not report sufficient information for an assessment to be made Quote: “Residents were eligible for the study if assigned to the outpatient ambulatory block rotation during academic year 2001–2002. Eligible residents were randomized using a random numbers table to either the workshop seminar alone (controls) or the workshop seminar plus shelter experience (cases)" (p 536) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes measured were self‐reported and not subjective to interpretation by the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 39% attrition. 14 out of 36 participants did not respond/complete post survey |
| Selective reporting (reporting bias) | Low risk | Comment: no mention of a protocol, but all outcomes in the Methods sections were reported on. Results for documentation and screening are not adequately presented and only statistical significance is reported |
| Other bias | Unclear risk |
Comment: extremely small sample size and no power calculations were provided Quote: “Our study is limited by the small sample size and concomitant limited power to detect differences.” (p 539) Comment: low risk of measurement related bias as they used a validated scale Quote: “The majority of survey items were adapted from the Health Care Provider Survey for Domestic Violence, a validated, Likert‐scale provider survey. All 3 of these surveys demonstrated content validity and responsiveness to IPV educational interventions; 2 showed high test‐retest reliability; and 1 showed adequate internal consistency. To confirm our survey’s content validity, we circulated it to experts in IPV, who agreed that it included the most important constructs of adult IPV.” (p 537) |
Coonrod 2000.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: individual residents beginning their training in 1995 or 1996 were randomly assigned to attend, at their hospital orientation; residents randomised (using a computer and stratifying by sex and specialty) before recruitment |
|
| Participants |
Healthcare provider: medical residents Other: N/A Location/Setting: hospital: medical Residents at Maricopa Medical Center (a 500‐bed county hospital in Phoenix), USA Sample size: n = 102 (intervention group n = 53, control group n = 49) Number of withdrawals/dropouts: 14 (intervention n = 9, control n = 5) Sex: not reported Mean age: not reported Inclusion criteria: medical residents attending hospital orientation Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 53): IPV training; 2 different group‐based medical resident training interventions over 2 years (1995 and 1996) developed by authors:
Control (n = 49): education on unrelated topic to DV |
|
| Outcomes |
Primary outcomes: identification or screening (actual), measured with self‐reported diagnosis of DV Secondary outcomes: change in knowledge; measured with 5 true or false questions Timing of outcome assessment: baseline and 9 ‐ 12 months postintervention |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: all authors are at the Maricopa Medical Center, Phoenix, Arizona |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For logistic reasons, we had randomized the residents (using a computer and stratifying by sex and specialty) before recruitment." (p 55) |
| Allocation concealment (selection bias) | Unclear risk | Comment: randomisation appears to have been carried out at 1 point in time and was done using a computer. However, researchers/investigators may be aware of the intended allocation before the participants were recruited. Allocation concealment was not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To blind the participants to the purpose of our study, we presented it as a test of different educational interventions; we did not reveal our specific interest in domestic violence education." (p 56) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: unclear blinding of assessors but outcomes measured were self‐reported and not subjective to interpretation by external assessors Quote: "The individuals who contacted the residents for follow‐up were blinded to both the residents’ group assignments and our study’s hypothesis." (p 56) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low risk as less than 20% dropped out of the study |
| Selective reporting (reporting bias) | Low risk | Comment: no mention of study protocol or trial registration, but both outcomes that are discussed in the Methods are reported upon in the Results |
| Other bias | Unclear risk |
Comment: potentially small sample size to detect difference Quote: "A final limitation concerns statistical significance, which limits the ability to see "statistical significance", given the absolute and relative rates of domestic violence diagnosis found" (p 57) Comment: no information on validation of measurement tools; Less than 20% dropouts but the numbers in Results do not match the table in the text |
Cutshall 2019.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: not reported |
|
| Participants |
Healthcare provider: newly‐licensed mental health professionals, such as licensed clinical social workers; licensed professional counsellors; licensed marriage and family therapists; licensed clinical psychologists Other: N/A Location/Setting: online delivery to healthcare providers at home or the office in the USA Sample size: n = 53 (intervention group n = 26, control group n = 27) Number of withdrawals/dropouts: nil Sex: 20 men, 31 women, 2 non‐binary Mean age: 7 = 20 ‐ 29 years; 29 = 30 ‐ 39 years; 11 = 40 ‐ 49 years; 5 = 50 ‐ 59 years; 1 = 60 ‐ 69 years Inclusion criteria: less than 5 years clinical experience, have treated at least 1 victim of IPV Exclusion criteria: if healthcare provider had any additional/specialised coursework in trauma practice of IPV or sexual violence outside of the graduate programme, licensed longer than 5 years |
|
| Interventions |
Intervention (n = 26): online only, interactive web‐based IPV and sexual violence training; total of 15 hours (3 x 5‐hour sessions offered over 3 consecutive weeks); 3 modules (module 1: introduction to IPV and rape culture ‐ definitions, prevalence, myths; module 2: marginalised populations (e.g. children, military); and module 3: knowledge of IPV and sexual violence reporting, rape culture, consent, advocacy skills, confidence levels in providing therapeutic intervention and follow‐up care) Control (n = 27): wait‐list |
|
| Outcomes |
Primary outcomes
Secondary outcomes: not reported Timing of outcome assessment: 1 follow‐up at 4 weeks after baseline |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A waitlist‐controlled design was used, and participants were randomized into either the educational intervention group (15‐hour training) or the waitlist control group”. (p 49) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided on allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Comment: High risk of performance bias as outcomes were self‐reported and participants were aware of allocation status Quote: "Participants who met inclusion criteria were informed that they would be randomly assigned to either one of two treatment groups”. (p 61) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: self‐reported outcomes used Quote: “I administered a pre‐test using the following outcome measures: the personal assessment for advocates working with victims of sexual violence and the PREMIS previously noted.” (p 62) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Adherence to the waitlist control, educational training intervention, post‐test, and questionnaires was at 100% adherence and retention in both the initial intervention group and the waitlist intervention group.” (p 68) |
| Selective reporting (reporting bias) | Low risk | Comment: all relevant outcomes from the Methods section were reported in the Results section of the study |
| Other bias | Low risk | Comment: no other risks identified |
Danley 2004.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: computer‐generated random lists at 1 point in time |
|
| Participants |
Healthcare provider: second to fourth year dental students and dental faculty Other: N/A Location/Setting: educational Institute, California, USA Sample size: n = 174 (2 x intervention groups: pre‐post only n = 56; post‐test only n = 59. Total intervention group n = 115, control group n = 59). (161 dental students; 13 dentists) Number of withdrawals/dropouts: not reported Sex: 92 men, 82 women Mean age: not reported Inclusion criteria: dental students with clinical experience and faculty members from the University of California San Francisco (UCSF), and the University of the Pacific (UOP), dental schools. At UCSF, fourth‐year dental school and third‐ and fourth‐year students were recruited, while at UOP, three‐year dental school, and second‐ and third‐year students were recruited Exclusion criteria: not reported |
|
| Interventions |
Interventions (n = 115)
Control (n = 59): wait list received AVDR online interactive tutorial at post‐test |
|
| Outcomes |
Primary outcomes: measured by 16 questions from 24‐item Domestic Violence Assessment Instrument delivered via the computer
Secondary outcomes: not reported Timing of outcome assessment: immediately after training |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: The development and testing of AVDR tutorial was funded by UCSF Comprehensive Oral Health Research Center of Discovery, which was funded by grant R01 DE13058 from the National Institute of Dental and Craniofacial Research Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized to one of the three study groups by the computer." (p 68 and 69) |
| Allocation concealment (selection bias) | Low risk | Comment: appears to be carried out at 1 point after recruitment using a computer‐generated method and therefore concealment of allocation sequence is not a concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the study does not explicitly address this criterion but outcomes were self‐reported and were not subject to interpretation by an external assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data and no reported attrition |
| Selective reporting (reporting bias) | Low risk | Comment: no mention of a protocol but results were presented for all outcomes mentioned in the Methods section |
| Other bias | Unclear risk | Comment: no information was provided on the validation of the measurement tools |
Edwardsen 2006.
| Study characteristics | ||
| Methods |
Design: quasi‐cluster RCT. Post‐test only Unit of randomisation: unclear; "We randomly assigned half of the small‐group facilitators (by room number) to an intervention group…” (quote, p 63) |
|
| Participants |
Healthcare provider: first‐year medical students Other: N/A Location/Setting: Rochester University, USA Sample size
Number of withdrawals/dropouts
Sex: described sample as gender‐balanced across groups but numbers not reported Mean age: not reported Inclusion criteria: first‐year medical student class at the University of Rochester, School of Medicine, in 1997 Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 25): 2‐hour faculty‐facilitated IPV training using standardised patients (SP) and mnemonic; small‐group sessions structured on how to interview SP using the SCRAPED mnemonic, which provides guidance on how to identify (Suspicion/screen, Central injuries, Repetitive, Abuse stated, Possessive partner, Explanation inconsistent, Direct questions) and manage (Safety, Crime reported, Referral, Acknowledgement, Protocols, Evidence collection, Documentation) IPV Control (n = 25): standard teaching methods; students provided with IPV training (including SP activity) and SCRAPED mnemonic, but facilitators given no instruction on how to run IPV training session in control group; students not provided with in‐depth tutorial discussion or hands‐on practice with mnemonic |
|
| Outcomes |
Primary outcomes: measured with post‐evaluation questionnaire and review of taped student interviews with SP
Secondary outcomes: not reported Timing of outcome assessment: immediately post‐training |
|
| Notes |
Study start date: 1997 Study end date: not reported Funding source: funded by a development grant from the Department of Emergency Medicine, University of Rochester School of Medicine Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: randomisation process was not explicitly specified in the report Quote: "We randomly assigned half of the small‐group facilitators (by room number) to an intervention group in which they attended an additional training session prior to their small‐group discussion meetings with the students" (p 63) |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: both arms received training. Students were asked not to discuss the intervention until after the evaluation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research team assessed the videotaped interviews blinded to the intervention status of the interviewees" (p 64) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 25 in each arm were assessed and 3 ‐ 4 dropped out. Attrition was less than 20% |
| Selective reporting (reporting bias) | Unclear risk | Comment: no mention of a protocol. The Methods section does not specify any outcomes. There is not enough information to make a clear judgement |
| Other bias | Unclear risk |
Comment: does not seem to account for clustering by standardised patients; potentially small sample size Quote: “We believe that the main reason for the failure to detect significant differences between the intervention and control groups is the size of the study, although it is certainly possible that systematic differences among small groups and/or SPs could have been contributing factors” (p 67); (validity of measurement is less of a concern as standardised patients were used). |
Gupta 2017.
| Study characteristics | ||
| Methods |
Design: cluster‐RCT. Clinic‐based intervention to address IPV. Women attending clinics were recruited to the study and nurses trained to deliver the intervention. Pre‐post and follow‐up survey of women in study Unit of randomisation: computer‐generated random lists at 1 point in time |
|
| Participants |
Healthcare provider: nurses Other: women attending primary care clinics Location/Setting: primary care clinics in Mexico City, Mexico Sample size
Number of withdrawals/dropouts
Sex: not reported Mean age: women = 30.12 (SD 7.28) years. Nurses' ages not reported Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 480): nurse‐delivered intervention to women, which involved: (1) integrated IPV and health‐screening assessment; (2) supportive care; (3) safety planning and harm reduction counselling, including reproductive health concerns; (4) assisted referrals; and (5) a booster counselling session at 3 months after screening and counselling session; intervention‐clinic nurses received 3 days of intensive training on screening for IPV and reproductive coercion (plus 3 follow‐up clinic visits for practice), providing supportive referrals, and assessing for health and safety risks Control (n = 470): women received standard care, referral card with information on IPV and available Ministry of Housing and community services; control‐clinic nurses received a 1‐day training that focused mainly on sensitising nurses and training them on using the abuse assessment and providing women with a referral card (standard training) |
|
| Outcomes |
Primary outcomes: women's physical and sexual IPV (time point 1: IPV experienced in past 12 months; time point 2: IPV in past 3 months; and time point 3: IPV in past 15 months), measured using affirmative response to binary measure from the WHO Multicounty Study on DV and Women’s Health Secondary outcomes
Timing of outcome assessment: baseline, 3 and 15 months |
|
| Notes |
Study start date: 2012 Study end date: 2015 Funding source: The study was funded by an anonymous donor administered by the Vanguard Charitable Endowment Program. Based on the stipulations set forth by the donor, the study authors were not permitted to disclose the funder (PI: JG). The work was supported, in part, by Yale University’s Center for Interdisciplinary Research on AIDS (CIRA), through grants from the National Institute of Mental Health, Paul Cleary, PhD, Principal Investigator (P30MH062294). The funders had no role in study design, data collection, analysis, interpretation, or writing of the report Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The 42 health centers were stratified by zone and district, and randomly assigned to treatment or control using STATA" (p 3) "... centers were assigned random numbers in Excel and sorted from smallest to largest; health centers were selected based on city zone and in order of their random number" (p 130). |
| Allocation concealment (selection bias) | High risk | Comment: although clusters were randomised, women were recruited from each cluster and it appears that research assistants recruiting women into the study were not blind to allocation; allocation may not have been done at 1 point in time |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participants were blinded to their study arm, while nurses, clinic staff, and the research team were not" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "However, data were collected through a computer‐assisted survey which has been shown to improve response rates to sensitive behaviours. Participants completed the survey in a private space in the clinic and were able to listen to the questions in Spanish through headphones and answer on the keyboard. Research assistants were available during this period in case the participant had any questions." (p 5 Falb et al., 2014 protocol) Comment: the risk is low for outcomes related to IPV survivors because the assessment is self‐report and participants were blinded. But the risk is unclear for HCPs as nurses, clinic staff, and the research team were not blind to their own allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: attrition rate was > 20%, so we considered there to be high risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes listed in the protocol were discussed in the published report |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias and accounted for clustering |
Gürkan 2017.
| Study characteristics | ||
| Methods |
Design: quasi‐RCT. Pre‐post test Unit of randomisation: individual students randomised with the systematic randomisation method |
|
| Participants |
Healthcare provider: first‐ and second‐year student nurses Other: N/A Location/Setting: nursing school in Turkey Sample size: n = 190 (intervention group n = 95, control group n = 95) Number of withdrawals/dropouts: n = 54 (intervention group n = 32, control group n = 22) Sex: 27 men, 109 women (based on final participant numbers in analysis) Mean age: intervention = 19 (SD 1.5) years; control = 19.5 (SD 2.0) years Inclusion criteria: willing to participate in the study and had not previously attended any education programme about combating violence against women Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 95): 'Peer education programme on combating violence against women (VAW)' delivered to 1st‐ and 2nd‐year student nurses who received 8 x 1‐hour sessions of peer IPV education provided by trained 3rd‐year nursing students; training involved identification and response to IPV using computer presentations, training videos, group activities (including games and role‐playing with fictional cases) and ability to call educators for follow‐up Control (n = 95): no training programme |
|
| Outcomes |
Primary outcomes: measures on pre‐post evaluation questionnaire
Secondary outcomes: not reported Timing of outcome assessment: 2 months post‐training |
|
| Notes |
Study start date: April 2012 Study end date: June 2013 Funding source: This work was supported by the Marmara University Scientific Research Projects Commission (grant numbers SAGA 200611) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Comment: the randomisation process used in this study was quasi‐random: the sequence was generated on the basis of odd and even names. Individual students randomised with the systematic randomisation method; names corresponding to uneven numbers recruited to intervention group and those corresponding to even numbers to control group Quote: "190 participants were randomised with the systematic randomisation method. The names corresponding to uneven numbers were recruited into the intervention group and those corresponding to even numbers into the control group (95 participants per group)" (p 48) |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no blinding of participants or researchers discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors were not blinded but the knowledge and attitude outcomes were self‐reported by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: more than 20% in both arms Quote: "190 participants were randomised...However, only 136 participants (intervention group: n = 63, control group: n = 73) were included in the study. A total of 54 participants withdrew from the study (Had not regularly attended a peer education program on combating VAW. Did not respond to post‐training questionnaires)." (p 48) Comment: missing data re: attrition on Consort diagram (Figure: research scheme. Supplementary 1) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but the published reports include all expected outcomes mentioned in the Methods section |
| Other bias | Unclear risk |
Comment: knowledge and skill outcome measure is potentially not validated Quote: “...a story of a woman who experienced different types of violence is described in her own words. There are two open‐ended questions following the case study in the Written Case Study of Violence Against Women (WCSVAW). The answers obtained from the participants were compared with the expected answers and assessed quantitatively” (p 49) Comment: potential contamination between groups discussed in limitations Quote: "No measures were taken to prevent interaction between the experimental and control groups" (p 52) |
Haist 2007.
| Study characteristics | ||
| Methods |
Design: RCT. Post‐test only Unit of randomisation: unclear |
|
| Participants |
Healthcare provider: internal medical residents Other: N/A Location/Setting: healthcare provider clinic or office in the USA Sample size: n = 27 (intervention group n = 14, control group n = 13) Number of withdrawals/dropouts: nil Sex: 15 men, 12 women Mean age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 14): 2‐hour IPV group‐based interactive workshop using standardised patient (SP) and group discussion delivered by faculty; 1 resident interviewed each SP in front of the group for 10 to 15 minutes; after the SP exercises, faculty reviewed cases and led discussions on the topic; students were informed that between 1 and 4 SPs representing DV or chronic pain (or both) would be insinuated into their medical clinic 1 to 6 months after the IPV workshop Control (n = 13): chronic pain workshop |
|
| Outcomes |
Primary outcomes
SP report to research co‐ordinator immediately post‐clinic visit Checklist and safety plan success presume SP identification of dependent outcomes: 1. DV identification: did the resident identify the SP DV problems?; 2. DV checklist success: did the resident score 75% or higher on all DV‐relevant items from the SP checklist (11 or more of 14 checklist items for the injured DV case and 9 or more of 12 for the depressed DV case)?; 3. DV safety plan counselling success: did the resident score 75% or higher on the 8‐item safety plan (common to both the depressed and injured DV case scenarios)? Secondary outcomes: prior resident DV training and knowledge collected at baseline but no results reported Timing of outcome assessment: 1 ‐ 7 months post‐DV training |
|
| Notes |
Study start date: 2003 Study end date: 2004 Funding source: This project is supported in part by a grant from the University of Kentucky Center for Research on Violence Against Women Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: the authors do not report sufficient information for an assessment to be made Quote: "Residents were randomly assigned to either the DV or control workshop" (p 337) |
| Allocation concealment (selection bias) | Unclear risk |
Comment: Investigators/researchers may not have been aware of the randomisation sequence before allocation as allocation appears to be after recruitment. But it is unclear how exactly the allocation was carried out Quote:"During the orientation, residents were told that between one and four SPs representing DV and/or chronic pain would be insinuated into their continuity clinic 1 to 6 months after their participation in the workshop" (p 337) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "There could have been dissemination of DV workshop information from DV workshop residents to chronic pain workshop residents" (p 341) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: assessors/simulated patients were blind to allocation Quote: "The Simulated Patients were unaware of resident workshop assignment" (p 338) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk |
Comment: the study protocol is not available, but published reports include all expected outcomes Quote: "Because checklist success and safety plan success presume DV identification, these variables represent an overall measure of clinical success combining identification and counseling" (p 338) |
| Other bias | Unclear risk |
Quote:"Finally, the sample size was relatively small; however, a small sample size would have likely resulted in a Type II error (i.e. falsely accepting the null hypothesis), yet we found differences between our intervention and control groups" (p 341) Comment: But for some outcomes an effect was not detected and there is a potential risk for this study to be underpowered to detect a difference due to low sample sizes |
Harris 2002.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: not reported |
|
| Participants |
Healthcare provider: physicians Other: N/A Location/Setting: online education for Kansas physicians from the USA Sample size: n = 99 (intervention group n = 50, control group n = 49) Number of withdrawals/dropouts: n = 34 (intervention n = 22, control n = 12) Sex: 45 men, 20 women (based on final participant numbers in analysis) Mean age: intervention = 43.2 (SD 9.2) years; control = 43.7 (SD 10.9) years Inclusion criteria: members of the Kansas Medical Association and practitioners of a primary care specialty or members of certain specialties likely to care for DV patients who had less than 1 hour of IPV training in the last year Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 50): password‐protected 2‐hour scenario‐based online DV educational programme; included a series of case studies in which different aspects of DV were gradually revealed as the user worked through the case; online resources and referral options provided Control (n = 49): no DV training |
|
| Outcomes |
Primary outcomes: measured on 56‐item pre‐post evaluation questionnaire with 5‐point Likert responses
Secondary outcomes: not reported Timing of outcome assessment: up to 6 weeks post training |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: supported by grant 1R43‐MH62233 from the US National Institute of Mental Health (NIMH); opinions and assertions contained therein represent those of the authors and not the NIMH Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: unclear about blinding of assessors but outcomes were self‐report and so not subjective to assessor interpretation |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: greater than 20% attrition Quote: "Sixty‐five (66%) of the 99 eligible physicians completed both the pretest and posttest survey. This group was the study population" (p 290) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but published reports include all expected outcomes. However, adequate details were not provided and we obtained outcome details from the authors |
| Other bias | Low risk | Comment: unclear about adequacy of sample size to detect meaningful difference in some outcomes. But in all outcomes of relevance to this review, the sample size seemed adequate |
Hegarty 2013.
| Study characteristics | ||
| Methods |
Design: Cluster‐RCT. Clinic‐based intervention to address IPV. Women attending clinics were recruited to the study and doctors trained to deliver the intervention. Pre‐post survey of doctors in the study Unit of randomisation: 1 family doctor per clinic; computer‐generated random lists at one point in time |
|
| Participants |
Healthcare provider: family doctors Other: women who have screened positive as fearful of partner Location/Setting: general practice primary care clinics in Melbourne, Australia Sample size
Number of withdrawals/dropouts
Sex: 32 female doctors (intervention n = 14 (56%); control n = 18 (67%)), 20 male doctors Mean age: doctors = 48.1 (SD 8.1) years; women = 38.5 (SD 8.1) years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention (n = 162)
Control (n = 162)
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Only doctor’s education outcomes were included in our review, as we do not include interventions with systems‐change components and hence the above‐mentioned primary and secondary outcomes that were assessed after the systems‐ level intervention was implemented were not included. Doctor’s education outcomes used: Author provided unpublished pre‐post Physician Readiness to Manage IPV Survey (PREMIS) scores, as not reported in WEAVE primary outcomes paper; knowledge (actual knowledge), measured with the PREMIS tool (8 knowledge questions, of which 7 were multiple choice, and 1 with sub‐questions and responses as true, false, or do not know); and perceived knowledge, assessed with PREMIS tool (14 perceived knowledge questions on a 7‐point Likert scale) Timing of outcome assessment: doctors educational outcomes assessed at 6 ‐ 8 weeks post‐training; women’s outcomes assessed at baseline and 6 and 12 months |
|
| Notes |
Study start date: 2008 Study end date: 2013 Funding source: Australian National Health and Medical Research Council Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"A statistician who was otherwise not involved in the study follow‐up, generated a random allocation sequence in Stata, stratified by location of each doctor’s practice (urban vs rural), with random permuted block sizes of two and four within each stratum" (p 252) |
| Allocation concealment (selection bias) | Unclear risk | Comment: Unclear if concealment of randomisation was done adequately after randomisation was carried out. (The unit of randomisation is the unit of analysis for this review and hence for the purpose of risk of bias, it is considered an individual RCT, not a cluster RCT) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Comment: High risk of performance bias as outcomes were self‐reported and participants were aware of which group they were allocated to Quote: "Because of the nature of the intervention, neither doctors nor patients could be masked to intervention." (p 252) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: unclear about blinding of assessors but outcomes were not subjective to assessor interpretation as the included outcomes from this study were self‐reported Quote:"...but study investigators and researchers following‐up patients and entering and analysing data were masked to allocation." (p 252) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: no missing primary outcome data. All secondary outcomes apart from Readiness to Change were reported Quote: " We have not yet analysed the open‐ended questions (at 6 months and 12 months) about readiness for change." (p 252) |
| Selective reporting (reporting bias) | High risk | Comment: while the trial was registered and the study protocol was published and available, not all outcomes in the protocol were reported and information on GP participant DV education‐knowledge and attitudes assessed through PREMIS were not reported. We had to contact the author for details ‐ results were provided |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Hsieh 2006.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: computer‐generated randomisation with laptops for completion of training as strata |
|
| Participants |
Healthcare provider: dentists and dental residents Other: N/A Location/Setting: San Francisco, USA Sample size: n = 174 (intervention group n = 86, control group n = 88) Number of withdrawals/dropouts: nil Sex: 104 men, 70 women Mean age: not reported Inclusion criteria: dentists attending professional development sessions; dental residents in University of California San Francisco (UCSF) dental clinics; those practising in the USA and engaged in at least 20 hours of outpatient care a week Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 86): dentists watched the 15‐minute online interactive tutorial video, Ask, Validate, Document, Refer (ADVR), on how to identify signs of abuse and how to respond using the AVDR approach Control (n = 88): wait‐list controls completed a pre‐ and post‐test; they only received the ADVR interactive tutorial post‐test |
|
| Outcomes |
Primary outcomes: measures collected via laptop computer
Of these, 2 domains were relevant: Beliefs about DV (e.g. "If a victim does not disclose the abuse, there is nothing I can do to help") and Attitudes about DV (e.g. "How much do you feel it is within dentists' role to ask pts about...")
Secondary outcomes: not reported Timing of outcome assessment: immediately after training |
|
| Notes |
Study start date: 2003 Study end date: not reported Funding source: The development and testing of ADVR tutorial was funded by UCSF Comprehensive Oral Health Research Center of Discovery, which was funded by grant R01 DE13058 from the National Institute of Dental and Craniofacial Research Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"These components of the trial were delivered on laptop computers, which randomly assigned the participants to control and experimental groups, resulting in randomization with laptops as strata." (p 598) |
| Allocation concealment (selection bias) | Low risk |
Comment: allocation concealment was not explicitly discussed, but randomisation was completed by the computer at 1 time after recruitment and enrolment into the study Quote:"These components of the trial were delivered on laptop computers, which randomly assigned the participants to control and experimental groups, resulting in randomization with laptops as strata." (p 598) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: unclear about blinding of assessors but outcomes were not subjective to assessor interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no consort diagram, but no attrition reported |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but there appears to be no missing prespecified outcomes |
| Other bias | Unclear risk | Comment: no power analysis mentioned but appears to be adequately powered to detect differences in key outcomes. The Asking, validating, documenting, referring: AVDR scale may not be validated |
Lo Fo Wong 2006.
| Study characteristics | ||
| Methods |
Design: cluster‐RCT. Post‐test only Unit of randomisation: family practice clinics |
|
| Participants |
Healthcare provider: family doctors Other: N/A Location/Setting: family practice clinics in Rotterdam and surrounding areas, Netherlands Sample size
Number of withdrawals/dropouts
Sex: 26 men, 28 women Mean age: 15 doctors < 40 years; 20 doctors 40 ‐ 50 years; 19 doctors > 50 years Inclusion criteria: registered doctors in Rotterdam and the surrounding areas Exclusion criteria: not reported |
|
| Interventions |
Interventions (n = 37)
Control (n = 17): no IPV training or focus group |
|
| Outcomes |
Primary outcomes
Secondary outcomes: number of patients with whom the doctor had non‐obvious reasons to suspect or discuss abuse Timing of outcome assessment: over 6 months post‐training |
|
| Notes |
Study start date: 2002 Study end date: 2003 Funding source: This project received a research grant from Theia Foundation, Zilveren Kruis Achmea Health Insurance (project number 200173) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The research assistant, blinded against the participants’ name and that of the group practice or health centre, executed the randomisation by sequential assignment of a number to a group." (pp 250‐1) Quote. "After one male participant in the full‐training group fell ill, he had to be moved to the control group." (p 251) |
| Allocation concealment (selection bias) | Low risk |
Comment: Randomisation was carried out at 1 time point after recruitment into the study Quote: "The research assistant, blinded against the participants’ name and that of the group practice or health centre, executed the randomisation by sequential assignment of a number to a group." (pp 250‐1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk |
Comment: the impact of clustering was not explored Quote:"...we did not take clustering into account in recruiting our final sample, mainly because of the cluster size, resulting in a somewhat underpowered study." (p 255) |
Mauch 1982.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: not reported |
|
| Participants |
Healthcare provider: trainee counselling and psychology graduates Other: N/A Location/Setting: University of Missouri‐Columbia, USA Sample size: n = 41 (2 x intervention groups: Counselling Battered Women Training Program (CBWTP) n = 13, reading only n = 12. Total intervention group n = 25, control group n = 16) Number of withdrawals/dropouts: not reported Sex: 11 men, 30 women Mean age: 26.7 years; range 22 ‐ 48 years Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Interventions (n = 25)
Control(n = 16): no readings or CBWTP |
|
| Outcomes |
Primary outcomes: attitudes (actual attitudes and beliefs), measured using the Attitude Toward Battered Women Questionnaire (57‐item survey assessed on 7‐point Likert scale) Secondary outcomes: not reported Timing of outcome assessment: 4 weeks after training |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: no funding identified; PhD dissertation Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: unclear about blinding of assessors but outcomes were not subjective to assessor interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: complete case analysis ‐ no attrition post‐training |
| Selective reporting (reporting bias) | Low risk | Comment: only 1 outcome measured and reported |
| Other bias | Unclear risk | Comment: significant amount of information missing to make call on risk of bias. The study does not address many criteria; validity and reliability of the self‐developed instrument to measure attitudes are not explicitly stated |
Moskovic 2008.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post study Unit of randomisation: computer‐generated random lists at 1 point in time |
|
| Participants |
Healthcare provider: medical students Other: N/A Location/Setting: educational institutions in the USA Sample size: n = 117 (intervention group n = 58, control group n = 59) Number of withdrawals/dropouts: nil Sex: not reported Mean age: not reported Inclusion criteria: first‐ to third‐year students attending 4 medical school sites in the USA Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 58): didactic plus outreach; medical students had a 3‐hour didactic interactive training session (delivered by an IPV advocate) on delivery of the 'In Touch with Teens' (dating violence) curriculum and delivered 3 x 1‐hour outreach education sessions to high school students over 2 ‐ 3 weeks Control (n = 59): 3‐hour didactic training only |
|
| Outcomes |
Primary outcomes: pre‐post survey completed by students
Secondary outcomes: not reported Timing of outcome assessment: 2 time points; time point 1 at immediately after didactic training (knowledge), and time point 2 at 3 weeks once didactic plus outreach students completed high school training (knowledge and attitudes) |
|
| Notes |
Study start date: 2005 Study end date: not reported Funding source: This study was funded by the US Department of Health and Human Services, Office on Women’s Health Conflicts of interest: The author Dr Bigby received honoraria from Time Inc, and has been a consultant with Pfizer Inc and Lily, neither of which provided funding for or were involved in this study. No other authors report any potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: computer‐generated random lists at 1 point in time Quote: "Students were stratified based on background experience working with teens and IPV prevention and assigned using computer‐generated random numbers to a “didactic only” (control group) or a “didactic plus outreach” high school training experience" (p 1044). |
| Allocation concealment (selection bias) | Low risk |
Comment: Allocation was done at 1 point in time after recruitment and hence investigators/researchers are not likely to know the allocation before assignment Quote: "Students were stratified based on background experience working with teens and IPV prevention and assigned using computer‐generated random numbers to a “didactic only” (control group) or a “didactic plus outreach” high school training experience" (p 1044) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Comment: No information provided regarding blinding for research personnel. For students it is possible that there is risk of bias from being aware of allocation into an intervention group. Efforts to blind participants are not adequately described. Quote: "Students were aware that they were participating in a study and that group assignment was random" (p 1044) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: self‐reported outcomes used and therefore low risk of bias from external assessment Quote: "Knowledge of IPV was assessed by 26 true–false and 8 multiple‐choice items that were scored dichotomously. Students’ attitudes about the general importance of addressing IPV and their confidence in addressing IPV and working with adolescents were assessed using 15 items that students rated on 6‐point scale from 1 = strongly disagree to 6 = strongly agree." (p 1045) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Less than 20% attrition Quote:"Of the 123 medical students who were initially enrolled, 117 completed the study" (p 1045) |
| Selective reporting (reporting bias) | Low risk |
Comment: the study protocol is not available, but it is likely that published reports include all expected outcomes Quote:"OBJECTIVE: To determine whether the experience of serving as educators in a community‐based adolescent IPV prevention program improves medical students’ knowledge, skills, and attitudes towards victims of IPV, beyond that of didactic training." (p 1043) Comment: When skill is measured as 'confidence' to treat then all primary outcomes are considered as reported |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Ragland 1989.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: not reported |
|
| Participants |
Healthcare provider: clinical psychology graduate students Other: N/A Location/Setting: Hofstra and Adelphi University, New York, USA Sample size: n = 42 (intervention group n = 21, control group n = 21) Number of withdrawals/dropouts: not reported Sex: 19 men, 23 women Mean age: 26.55 years; range 22 ‐ 42 years Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 21): videotape training group included a 30‐minute video with information on IPV, assessment and treatment; knowledge about battered women, attitudes about sex roles and battered women, and skills necessary for counselling women were included; used sections from media to show cultural norms and violence against women attitudes Control (n = 21): watched a 30‐minute video from '48 Hours' television programme about working women’s problems finding day care for children |
|
| Outcomes |
Primary outcomes
Secondary outcomes: not reported Timing of outcome assessment: 1 week after training |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: no funding identified; PhD dissertation Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: incomplete information provided about randomisation Quote: "After volunteering to participate in the study, subjects were randomly assigned to one of two groups: The Videotape Training Group and the No‐Training" (p 78) |
| Allocation concealment (selection bias) | Unclear risk | Comment: incomplete information provided about randomisation and whether allocation was done at 1 point in time for all those who volunteer and are recruited in the study or whether sequence was available immediately after participant volunteered but before recruitment into the study is not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: both control and intervention participants view a 30‐minute video, but there is no further information provided on how participants or researchers are blinded to the outcomes, although questionnaires were used to collect data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: self‐reported outcomes, so low risk of bias from assessment from external assessors Quote: "I understand that I will be asked to view a 30‐minute videotape and complete three questionnaires the first week, and I will be asked to complete two questionnaires one week later" (pp 83‐5) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition reported. |
| Selective reporting (reporting bias) | Low risk | Quote:"The present study attempted to assess the effects of a videotape training program on male and female graduate students' attitudes about battered women and their assessment of battered women's mental health. The two dependent measures used in this study were the Attitude Toward Battered Women Questionnaire and a mental health assessment questionnaire (adapted from Accomondo, 1979). Both of the dependent measures were given at pretest and posttest" (p 94) |
| Other bias | Unclear risk |
Comment: small sample size and no information provided on power Quote: “The videotape training program did not appear to affect the assessment of an abused woman. These results may have been due to small sample size, however, since only half of the subjects in each training condition (training, no‐training) received a vignette containing violence against a woman.” (p 146) |
Sharps 2016.
| Study characteristics | ||
| Methods |
Design: individual and cluster‐RCT. Mothers recruited to the study and nurse home visitors trained to deliver the intervention. Post‐test only survey of mothers in the study Unit of randomisation: urban participants randomised individually; rural participants randomised by cluster‐according to health care agency |
|
| Participants |
Healthcare provider: home visitors (HV) (community health nurses, community health workers supervised by nurses) Other: pregnant women Location/Setting: 13 rural, midwest and 1 urban east coast health departments in the USA. Training for HV was in the primary care organisation. Intervention for women was delivered in their homes Sample size
Number of withdrawals/dropouts
Sex: not reported Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 124 women)
Control (n = 115 women)
|
|
| Outcomes |
Primary outcomes: IPV, measured using the Conflict Tactics Scale 2; at baseline, women were asked about violence in the past year, and at subsequent data collection time points, asked if these acts had occurred since the previous data collection time point Secondary outcomes: women’s mental health outcomes (actual), measured with the Edinburgh Postnatal Depression Scale (10‐item scale used to measure depressive symptoms in the perinatal period) Timing of outcome assessment: women’s outcomes measured at baseline and 1, 3, 6, 12, 18 and 24 months after delivery |
|
| Notes |
Study start date: 2006 Study end date: 2014 Funding source: This study was supported by grant (number R01009093) from the National Institutes of Health/National Institute of Nursing Research (NIH/NINR). NIH/NINR had no role or made no contribution to the scientific design of the study, implementation, data collection, or analysis and made no contribution to this article Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomization procedures varied by site. At the urban health department site, participants were randomized using computer‐generated number assignments in blocks" (p 1131) Comment: Information about rural randomisation not clearly provided Quote: "In the rural sites, there were 13 rural health agencies that participated. Cluster randomization was used to assign seven health agencies to deliver the DOVE intervention and six health agencies were designated as UC. Cluster randomization was necessary in the rural sites because each health agency was small enough that intervention drift was a plausible threat if women were the unit of randomization" (p 1131) |
| Allocation concealment (selection bias) | Unclear risk |
Comment: No information on blinding of those carrying out the random allocation was provided Quote: "The data managers, database development team, and statistical analysis team members were blinded to group assignment" (p 1131) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Comment: incomplete information provided for urban participants Quote: rural participants ‐ "The data managers, database development team, and statistical analysis team members were blinded to group assignment" (p 1131) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: outcomes were self‐reported by women and hence the risk of bias from lack of masking of assessor was already determined to be low. However, researchers/assessors also appeared to be masked to allocation status Quote:"All data were collected by research nurses who were not associated with delivering the DOVE intervention. There were no changes to study outcome measures after the trial commenced" (p 1132) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: greater than 20% attrition rate Quote: "239 women were randomized to DOVE (n = 124) or UC (n = 115) and completed the baseline assessment. A proportion (22.6%) of women did not have a chance to complete their 18‐ or 24‐month assessments" (p 1132) |
| Selective reporting (reporting bias) | Low risk | Comment: results for the primary outcomes mentioned in the Methods section were reported in the paper |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Short 2006b.
| Study characteristics | ||
| Methods |
Design: cluster‐RCT Unit of randomisation: all physicians in an office assigned to the same study or control group |
|
| Participants |
Healthcare provider: physicians Other: N/A Location/Setting: community practice settings in Arizona and Missouri, USA Sample size
Number of withdrawals/dropouts
Sex: 52% ‐ 56% men Mean age: 47 years Inclusion criteria: community physician specialists in internal medicine, family medicine, paediatrics, obstetrics and gynaecology, and psychiatry in Kansas City and Phoenix, USA; physicians in private (non‐university, non government) practice in the medical specialty, in a group of 7 or fewer physicians, and who have internet access Exclusion criteria: not reported |
|
| Interventions |
Intervention (n = 44): 4 ‐ 16 hours of asynchronous interactive online IPV e‐teaching; content included 17 case studies that simulated typical presentations; to receive 4 hours of continuing medical education credit, physicians had to complete minimum cases for their specialty (3 ‐ 4) and the ‘readiness to change’ case Control (n = 37): no online IPV training |
|
| Outcomes |
Primary outcomes: measured on paper‐based PREMIS survey tool
Secondary outcomes: not reported Timing of outcome assessment: 6 and 12 months post‐training |
|
| Notes |
Study start date: 2003 Study end date: 2006 Funding source: The development of the online continuing medical education programme and the research study were supported by a small business innovation and research grant (R44‐MH62233) from the National Institute of Mental Health Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: incomplete information provided Quote: "Physicians were randomly assigned to the CME (study) or to the control group, stratified by city, after completing the initial KABB survey and site visit" (p 182) |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: self‐reported outcomes and therefore risk of bias from lack of blinding of assessors is low Quote: "Physician IPV KABB was measured via a self‐administered, paper‐based survey tool, physician readiness to manage intimate partner violence survey (PREMIS)" (p 183) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: > 20% attrition Quote:"85 physicians initially agreed to participate in the study; however, only 81 physicians completed the first PREMIS surveys. Forty‐four of these physicians were randomly assigned to take the online CME. When the study ended approximately 12 months later, 61% of the physicians had been retained through all three phases" (p 183) |
| Selective reporting (reporting bias) | Unclear risk | Comment: results for the primary outcomes mentioned in the Methods section were briefly reported in the paper. Adequate data were not provided and some extra results were obtained from the author |
| Other bias | Unclear risk |
Comment: power calculations and analyses do not appear to account for intra‐cluster correlation Quote:"Power calculations were conducted for a two‐group repeated measures design with three time points and an unbalanced design with 52 cases (29 control and 23 study cases). Considering means ranging from 3.5 to 4.5 in the intervention group, and remaining consistent at 3.5 in the comparison group, standard deviations of 1 in both groups, and correlations of 0.5 between levels of the repeated measures, the power to detect moderate effects of 0.20 was 0.88" (p 183) |
Vakily 2017.
| Study characteristics | ||
| Methods |
Design: RCT. Pre‐post survey Unit of randomisation: not reported |
|
| Participants |
Healthcare provider: midwives Other: N/A Location/Setting: health centres and hospitals in Isfahan, Iran Sample size: n = 70 (intervention group n = 35, control group n = 35) Number of withdrawals/dropouts: not reported Sex: not reported Mean age: 26.55 years; range 22 ‐ 42 years Inclusion criteria: bachelor's or master's degree in Midwifery, exclusive employment in health and treatment centres to provide services for women of fertility ages, willingness to participate in the study, and not having received any training courses regarding DV in the past Exclusion criteria: not participating in training sessions for the group‐based training method, not studying or insufficiently studying the training CD for the CD training method, unwillingness to co‐operate during the study, and receiving information about DV from other resources during the study period |
|
| Interventions |
Intervention (n = 35): CD training; midwives completed group training (training content: DV prevalence in Iran, cultural aspects, causes, presenting symptoms, DV screening, treatment and role of midwife) and then given a CD of DV content (CD contents included case reports, questions, images, and videos); group training was over 4 hours (2 x 2‐hour sessions in 1 day) Control (n = 35): group training conducted over 4 hours; verbally delivered by the researcher; included varied teaching methods and DV content similar to CD training group |
|
| Outcomes |
Primary outcomes
Secondary outcomes: not reported Timing of outcome assessment: baseline and 2 months after training |
|
| Notes |
Study start date: not reported Study end date: not reported Funding source: Isfahan University of Medical Sciences, research proposal number 394658 Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: incomplete information provided Quote: "After finalizing the selection of the centers, midwives were chosen through simple sampling based on the inclusion criteria. Afterward, samples were divided into two groups of group training and CD training using random allocation method; meaning that two separate lists were recorded for the health and treatment centers after assigning codes to the participants. Then, midwives were randomly allocated to the Group Training (with code “A”) and CD Training (with code “B”)" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: self‐reported by participants and therefore risk of bias from lack of blinding of assessors is low Quote: "Data collecting tool was a questionnaire that was completed by the studied groups at two stages (before the intervention and 2 months after the intervention)" (p 3) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the authors do not report sufficient information for an assessment to be made |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes were reported in the paper |
| Other bias | Unclear risk | Comment: did not report accounting for intracluster correlations in the analysis |
CD: compact disc; DV: domestic violence; DVA: domestic violence and abuse; IPV: intimate partner violence; N/A: not applicable; PI: Principal investigator; PREMIS: Physician Readiness to Manage Intimate Partner Violence Survey; PhD: Doctorate of Philosophy; RCT: randomised controlled trial; SD: standard deviation; SP: standardised patient; vs: versus; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arrab 2018 | Did not assess the impacts of training alone: assessed a systems intervention (also excluded on design as it is a quasi‐experimental study) |
| Campbell 2001 | Did not assess the impacts of training alone: assessed a systems intervention that did not assess the impacts of training alone (included training administrators) |
| Carroll 2005 | Did not assess the impacts of training: assessed the impact of a screening intervention (the Alpha form) |
| Cripe 2010 | Did not assess the impacts of training: evaluation of an empowerment intervention and not an evaluation of training healthcare providers |
| Dubowitz 2011 | Did not assess the impacts of training alone: social worker spent a half‐ or full day per week in the intervention group |
| Duggan 2007 | Did not assess the impacts of training HCPs to respond to IPV: training in child abuse |
| Feder 2011 | Did not assess the impacts of training alone: intervention group included administrative staff who received a 1‐hour training session on confidentiality and patient safety and IRIS information materials |
| Feigelman 2011 | Did not assess the impacts of training alone: social worker was available to the intervention group |
| Garg 2007 | Did not assess the impacts of training alone: study carried out screening for IPV by research assistants |
| Jack 2019 | Inadequate comparison group: correspondence with the author confirmed that the control group received universal screening for IPV |
| McFarlane 2006 | Inadequate comparison group: author correspondence confirmed that both intervention and control arms received training interventions. The study aimed to assess the impact of case management, not training |
| Miller 2011 | Did not assess the impacts of training alone: intervention included materials (card) aimed to serve as an intervention for patients |
| Miller 2016 | Did not assess the impacts of training alone: training is not the main component of the intervention, and the study focuses on student education and counselling |
| Miller 2017 | Did not assess the impacts of training alone: included a palm‐sized information brochure on health risks of IPV and safety planning in the intervention arm |
| Nagler 1993 | Did not assess the impacts of training HCPs to respond to survivors of IPV: primarily a bystander intervention carried out with medical students to prevent acquaintance rape |
| Thompson 2000 | Did not assess the impacts of training alone: intervention arm received DV brochures in restrooms, posters, cue cards, questionnaires, bi‐monthly newsletter, etc. |
| Vijayalakshmi 2021 | Intervention has a focus on family violence. Did not assess IPV: assessed family violence involving all family members, not just intimate partners |
| Zachor 2108 | Did not assess the impacts of training on HCPs alone: included administrative staff in clinic and did not separate results |
DV: domestic violence; HPCs: healthcare providers; IPV: intimate partner violence; IRIS: identification and referral to improve safety
Characteristics of studies awaiting classification [ordered by study ID]
Abraham 2001.
| Methods |
Design: unclear (potential quasi‐RCT). Pre‐post test study Unit of randomisation: not reported |
| Participants |
Healthcare provider: second‐year medical students and residents Other: N/A Location/setting: adolescent clinic of tertiary paediatric hospital Sample size: n = 56 Number of withdrawals/dropouts: not reported Sex: not reported Mean age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention (n = 26): 3‐hour workshop that included a didactic lecture, teen panel, role‐playing with teen, health educators, and a feedback session. The lecture included an overview of media, firearm, interpersonal, and sexual violence among teens. Risk factors for teen violence, such as drug use, media influences, peer group, and poor self‐esteem. At end of clinical rotation, all participants saw 1 ‐ 2 adolescent SPs who completed a 14‐item evaluation form that included items on patient perceptions, provider identification and management of violence‐related problems and use of Fights/Injuries/Sexual Violence/Threats/Self‐Defence strategies (FISTS) screening tool. SP used a Likert scale to rate student interpersonal skills Control (n = 30): standard ambulatory clinic manual with articles on violence prevention |
| Outcomes |
Primary outcomes: adapted from the AAP Ambulatory Care Quality Improvement Program survey (50‐item questionnaire asking about training status, history of previous violence prevention education, if they routinely inquire about violence with adolescent patients, how student values the issue, their self‐efficacy and outcome expectations)
Secondary outcomes: not reported Timing of outcome assessment: baseline (pre‐student medical rotation) and 4 weeks (post‐rotation) |
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: not reported |
Abraham 2011.
| Methods |
Design: unclear (potential quasi‐RCT). Publication is a research abstract poster Pre‐post survey Unit of randomisation: not reported |
| Participants |
Healthcare provider: third‐year medical students assigned to the paediatric clerkship Location/Setting: not reported Sample size: 158 Number of withdrawals/dropouts: not reported Sex: not reported Mean age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention (n = 73): 2‐hour communication workshop involving an abbreviated lecture and an educational encounter with trained teen actors, which included small‐group role‐play scenarios. Teens provided feedback on discussions of confidentiality, use of body language, screening, and ability to identify and manage teen complaint Control (n = 85): standard 1‐hour lecture on taking a confidential, adolescent psychosocial history based on the SSHADES (Strengths, School, Home, Activities, Diet, Drugs, Emotions, Sexuality, Spirituality, and Safety) screening technique |
| Outcomes |
Primary outcomes: adapted from the AAP Ambulatory Care Quality Improvement Program survey
Secondary outcomes: not reported Time of outcome assessment: pre‐ and post‐rotation (rotation time frame unclear) |
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: not reported |
Hill 2016.
| Methods |
Design: unclear (potential RCT). Publication is an abstract of a dissertation Unit of randomisation: not reported |
| Participants |
Healthcare provider: college‐based healthcare providers Location/Setting: not reported Sample size: 44 Number of withdrawals/dropouts: not reported Sex: not reported Mean age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention (n = not available): asynchronous educational intervention Control (n = not available): not reported |
| Outcomes |
Primary outcomes: assessed using PREMIS
Secondary outcomes: not reported Time of outcome assessment: not reported |
| Notes |
Study start date: not reported Study end date: not reported Funding source: not reported Conflicts of interest: not reported |
AAP: American Academy of Pediatrics; n: sample size; PREMIS: Physician Readiness to Manage Intimate Partner Violence Survey; RCT: randomised controlled trial; SP: standardised patient
Characteristics of ongoing studies [ordered by study ID]
Fernández 2006.
| Study name |
English title: Protocol to evaluate the effectiveness of a consciousness‐raising and training intervention for primary care professionals, in order to improve detection of DV Originaly title: Protocolo para la evaluación de la efectividad de una intervención sensibilizadora y formativa en profesionales de atención primaria para la mejora de la detección de la violencia doméstica |
| Methods |
Design: cluster‐RCT Unit of randomisation: basic care team |
| Participants |
Healthcare provider: basic care team ‐ primary care physician and nurse Other: N/A Location/Setting: primary care centres in Spain Sample size: n = 136 Nubmer of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention (n = 68): a short training programme with homogeneous training content, aimed at raising the awareness of health professionals and teaching them how to identify risk factors, situations of special vulnerability and alarm signals. The programme also aims to provide health professionals with tools to make the clinical interview easier, when they suspect mistreatment, and how to tackle a case once it is detected Control (n = 68): training as usual |
| Outcomes |
Primary outcomes: number of cases of DV detected during the study Secondary outcomes: not reported Timing of outcome assessment: baseline, 3, 6, 9 months postintervention |
| Starting date | 2006 |
| Contact information | Email:mfernandeza@meditex.es |
| Notes | Comment: protocol written in Spanish |
NCT00257296.
| Study name | Evaluation of an intimate partner violence screening‐intervention |
| Methods |
Design: RCT Unit of randomisation: not reported |
| Participants |
Healthcare provider: not reported Other: women aged 18 ‐ 45 years, seen at study sites for primary care Location/Setting: obstetrics‐gynaecology clinics or general medical care in internal medicine clinics in USA Sample size: n = 471 Number of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: women aged 18 ‐ 45 years attending primary care study sites during study periods, English‐speaking, able to separate from accompanying person(s) and willing to participate Exclusion criteria: too ill, unable to separate from accompanying person(s), unable to speak English, refuses to participate |
| Interventions |
Intervention (n = not available): a computer‐based screening tool for IPV and provision of a multi‐faceted intervention based on the needs of the woman and the services she would like to use Control (n = not available): service as usual, including screening for IPV and providing enhanced usual care |
| Outcomes |
Primary outcomes: not reported Secondary outcomes: not reported Timing of outcome assessment: baseline, 1 week, 1, 3, 6, 9 months postintervention |
| Starting date | 22 November 2005 |
| Contact information |
Principal investigator: Louise‐Anne McNutt Address: University at Albany |
| Notes |
Registered at:clinicaltrials.gov/ct2/show/NCT00257296 Comment: potentially suspended |
NCT01028118.
| Study name |
Public title: Violence against women and consequences during climacteric's phase (DV) Official title: Domestic and sexual violence against women: consequences for climacteric´s phase |
| Methods |
Design: RCT Unit of randomisation: unclear |
| Participants |
Healthcare provider: unclear; but some mention of trained professionals Other: women (40 ‐ 65 years old) who were or are victims of domestic or sexual violence, or both Location/Setting: outpatient clinic for Endocrines Gynecology and Climactery, University of Sao Paulo General Hospital, Brazil Sample size: 300 Number of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: women (aged 40 to 65 years) who were or are victims of domestic or sexual violence, or both Exclusion criteria: women who were not victims of domestic or sexual violence, or both; those not aged between 40 to 65 years |
| Interventions |
Intervention (n = not available): asking about life experience with violence and cognitive behaviour therapy Control (n = not available): unclear |
| Outcomes |
Primary outcomes: unclear Secondary outcomes: unclear but appears to be depression and fibromyalgia or sexual dysfunction in women Timing of outcome assessment: unclear |
| Starting date | 9 December 2009 |
| Contact information |
Principal investigator: Sandra Dircinha TA Moraes Address: university of Sao Paulo General Hospital |
| Notes | Registered at:clinicaltrials.gov/ct2/show/NCT01028118 |
NCT03259646.
| Study name | The IPV provider network: engaging the healthcare provider response to interpersonal violence against women |
| Methods |
Design: cluster‐RCT Unit of randomisation: unclear |
| Participants |
Healthcare provider: unclear Other: women aged 18 ‐ 59 years old seeking health care at a partner clinic Location/Setting: USA Sample size: 6272 Number of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: seeking healthcare at one of the partner clinics, able to complete an online survey on a safe device (e.g. tablet) in English or Spanish, has a safe email address or phone number and is not acutely ill Exclusion criteria: male, not seeking health care at partner clinics, < 18 years, > 59 years, cannot read or speak English or Spanish, no access to safe devices for completing online survey, no safe email or phone number, is acutely ill |
| Interventions |
Intervention (n = not available): integration into the clinic setting IPV/sexual assault screening, universal education, trauma informed counselling, warm referrals (e.g. provider/staff contact advocacy programme with survivor) to local IPV/Sexual assault advocacy agencies, and access to the evidence‐based myPlan safety decision aid application Control (n = not available): standard clinical practice |
| Outcomes |
Primary outcomes
Secondary outcomes
Timing of outcome assessment: unclear |
| Starting date | 26 May 2017 |
| Contact information | Email:nglass1@jhu.edu |
| Notes | Registered at:clinicaltrials.gov/ct2/show/NCT03259646 |
Pallitto 2016.
| Study name | Testing a counselling intervention in antenatal care for women experiencing partner violence: a study protocol for a randomised controlled trial in Johannesburg, South Africa |
| Methods |
Design: RCT Unit of randomisation: individuals using a block randomisation procedure |
| Participants |
Healthcare provider: nurses Other: pregnant women aged 18 years old and less than 33 weeks gestation Location/Setting: 3 antenatal clinics in Johannesburg, South Africa Sample size: 504 Number of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: at least 18 years old and less than 33 weeks gestation, able to communicate in one of the most common local languages (English, Sotho, or Zulu), have experienced physical or sexual violence by their current or most recent partner in the past 12 months, and no immediate safety risk on screening Exclusion criteria: not reported |
| Interventions |
Intervention (n = not available): nurse‐led, 2‐session empowerment counselling intervention for women; and, for nurses, 30‐hour technical training of nurse researchers aimed at improving IPV knowledge and awareness and how it is related to maternal and child health Control (n = not available): enhanced control condition (referral list to local resources) |
| Outcomes |
Primary outcomes: physical or sexual (or both) IPV Secondary outcomes
Timing of outcome assessment: unclear |
| Starting date | Unclear, but the protocol was published in 2016 |
| Contact information |
Email: mailto: pallittoc@who.int Address: Department of Reproductive Health and Research, WHO |
| Notes |
Ruijne 2017.
| Study name | Detection of domestic violence by community mental health teams: a multi‐centre, cluster‐randomised controlled trial |
| Methods |
Design: cluster‐RCT Unit of randomisation: community mental health teams |
| Participants |
Healthcare provider: community mental health teams Location/Setting: community mental health clinics in municipalities of Rotterdam and The Hague, The Netherlands Sample size: 24 total teams Number of withdrawals/dropouts: N/A Sex: N/A Mean age: N/A Inclusion criteria: not reported Exclusion criteria: teams providing care to patients < 18 years, with more than 20% of the employees working over different teams, specialised in one specific mental illness (e.g. autism), and without a functioning electronic patient file system or with < 12 months of historical data at the start of the intervention |
| Interventions |
Intervention (n = 12): the intervention consists of
Control (n = 12): no additional training in DVA |
| Outcomes |
Primary outcomes: rate of detected cases of recent or any history of DVA Secondary outcomes: assessed using PREMIS
Timing of outcome assessment: baseline, 6 and 12 months |
| Starting date | Unclear; but the protocol was published in 2017 |
| Contact information | Email:r.ruijne@erasmusmc.nl |
| Notes |
DV: domestic violence; DVA: domestic violence and abuse; IPV: intimate partner violence; N/A: not applicable; n: sample size; RCT: randomised controlled trial; PREMIS: Physician Readiness to Manage Intimate Partner Violence Survey; WHO: World Health Organization
Differences between protocol and review
Review author team
We added two new authors to the review since the protocol was published: Dr(s) Leesa Hooker and Sonia Reisenhofer.
Objectives
Our objective previously said:“To assess the effectiveness of training programmes that seek to improve healthcare providers' identification of, and response to, IPV against women.” To be more specific and provide greater clarity, it now says: “To assess the effectiveness of training programmes that seek to improve healthcare providers' identification of and response to IPV against women compared to no intervention, wait‐list, placebo or usual care.”
Types of outcome measures
We considered 'knowledge' and 'readiness to respond' as two separate outcomes in the review. At the outset we had anticipated they would be reported together in a combined scale, but all studies provided subscale scores.
We also treated validating/counselling and safety planning as separate sub‐outcomes of 'providers’ response to IPV'.
Search methods
-
We did not carry out our planned searches of:
IndMed—the Indexing of Indian Medical Journals—as we could not access the web site;
the Maternal and Child Health Library at Georgetown, as no relevant topic listing was identified; and
the International Confederation of Midwives, as no search feature was identified on the website.
We expanded our MEDLINE search strategy that was specified in the protocol to include the major heading 'intimate partner violence/'.
The following databases were unavailable when we searched in 2020 (Popline, ICTRP, African Index Medicus Database and WHOLIS) and so no top‐up search was carried out for these.
We did not include the websites listed in Searching other resources in the top‐up search because no relevant studies were identified by previous searches.
We had planned to request support from a colleague (Igor Toskin) to translate studies reported in languages outside the purview of the authors of this review but did not do so and used Google Translate instead.
Assessment of risk of bias in included studies
In the protocol (Kalra 2017), we said that we would consider a study at low risk of bias for allocation concealment if: “the unit of allocation is a team and an adequate description of the allocation process (i.e. use of random number tables, flip a coin, etc.) is provided”. However, in the case of Gupta 2017, we rated the study at unclear risk of selection bias despite it meeting this criterion, because although clusters were allocated, research assistants who were potentially not blinded to allocation were recruiting women from within these clusters. We therefore revised these criteria to specify low risk of bias as follows: “the unit of allocation and analysis was a cluster, allocation was done at one point in time and an adequate description of the allocation process (i.e. use of random number tables, flip a coin, etc.) was provided".
Measures of treatment effects
Continuous outcomes
To aid interpretability of the SMD, we used the rule of thumb provided by Cohen 1988, where we interpreted an SMD of about 0.2 as a small effect, an SMD of about 0.5 as a moderate effect, and a SMD of more than 0.8 as a large effect (Cohen 1977).
Endpoint versus change scores
For continuous outcomes, we had planned to extract and pool endpoint data and adjust for baseline values, when possible. Given the small number of studies included in the meta‐analysis of the various outcomes, we could not perform this type of meta‐regression. Instead, we used an approach based on the meta‐analysis of difference‐in‐differences. This has been possible as all the studies reported endpoint and baseline values (with standard deviations/errors), with the exception of one study, which directly reported change scores. Where only change scores were reported, we planned to attempt to calculate endpoint data using baseline data, if available, and report these. However, as only one study reported change scores, we used these directly and relied on the assumption of good balance across the two arms.
Unit of analysis issues > Cluster‐RCTs
We had planned to correct the analysis of any study that failed to carry out proper adjustment of clustering by using intra‐cluster correlation coefficients (ICCs). For studies that did not report the ICC, we planned to borrow one(s) from similar studies or from external sources. However, we did not find suitable estimates of ICCs and were not able to identify similar studies to borrow from. We therefore used the second approach as suggested by McKenzie 2016, inflating the standard error of the estimated intervention effect (rather than reducing the sample size). This approach requires a calculation of a design effect, and therefore an estimate of the ICC. The adjustment is computed by multiplying the standard error by the square root of the design effect. The design effect can be easily calculated as 1+(M‐1)*ICC [with M as the mean cluster size]. We explored some simulated examples according to various combinations of M and the ICC. The 10% inflation is based on a cluster size of around 10 and ICC of 0.025, whilst the 30% multiplier considers a very conservative value of ICC up to 0.1. We ran analyses according to no adjustment and according to the inflation factors as above, to assess the robustness of the pooled results. .
Sensitivity analyses
Outliers: where a study appeared to be an obvious outlier, in line with the suggestion by Ryan 2016, we carried out sensitivity analyses with and without the study.
ICC: we carried out a sensitivity analysis by inflating the SEs of cluster RCTs that did not account for clustering by 10% and 30%, and reported the results of the SE inflated by 10%, where appropriate.
Effects of interventions
We had planned to structure the narrative synthesis around study characteristics in an attempt to link specific intervention components to impacts. However, this was not possible and instead we structured it around outcomes, as there were not enough studies of similar enough interventions that reported on the same outcomes to structure the narrative synthesis by intervention characteristics. However, where more than one similar intervention reported on the same outcome and could be combined in a meta‐analysis, we did carry out subgroup meta‐analysis by intervention type and we discuss these under subgroup analyses. We also continued to analyse separately studies where an additional component of an intervention was being tested (e.g. delivering a mnemonic technique, or using compact disks) and where the comparison group received treatment as usual or all other aspects of the same intervention without the aspect that was being tested. In this sub‐set of studies that was testing specific components, it was easier to link the particular characteristic of the intervention to our outcomes of interest.
Contributions of authors
The idea for this review came from CGM. NK developed the protocol, and CGM and GLDT reviewed it. NK, LH and SR contributed to double‐screening of studies, extraction of data and appraising the risk of bias for this review. NK developed the data extraction strategy. NK and LH retrieved study reports and wrote to study authors for additional data. GLDT carried out the statistical analyses in this review with support from NK. LH and SR contributed to the resolution of disagreements in study selection, data extraction, and assessment of risks of bias. NK, LH, and SR drafted the review. NK completed the GRADE assessments, LH confirmed the GRADE assessments, and SR arbitrated any disagreements in the event they occurred. CGM reviewed, edited and provided comments on the review. All review authors read and approved the final manuscript. CGM is the guarantor of the review.
Sources of support
Internal sources
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme on Research and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO) , Switzerland
Financial support for NK
-
The World Bank Group’s Umbrella Facility for Gender Equality, USA
Financial support for NK
External sources
None, Other
Declarations of interest
Naira Kalra: was funded by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme on Research and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO) and by the World Bank Umbrella Facility for Gender Equality (UFGE). The findings, interpretations, and conclusions expressed in this paper are entirely those of the authors. They do not necessarily represent the views of the International Bank for Reconstruction and Development/World Bank and its affiliated organizations, or those of the Executive Directors of the World Bank or the governments they represent.
Leesa Hooker: none known. Sonia Reisenhofer: none known. Gian Luca Di Tanna: none known. Claudia Garcia‐Moreno: is a staff member of the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, a cosponsored programme implemented by the World Health Organization (WHO). This work received funding from the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO). HRP receives public funding from various governmental institutions and foundations, however no funder played any role in the development of this work. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) or the World Health Organization.
New
References
References to studies included in this review
Ayaba‐Apawu 2016 {published data only}
- Ayaba-Apawu AT. Intimate Partner Violence (ipv) Counselor Education: Exploring Opinions, Knowledge And PerceivedPreparedness To Counsel Ipv Clients [PhD Thesis]. Detroit (MI): Wayne State University, 2016. [Google Scholar]
- Hooker L. Cochrane review query-Alvis Talata Ayaba-Apawu [personal communication]. Email to: John Pietrofesa 9 November 2020.
Brienza 2005 {published data only}
- Brienza RS, Whitman L, Ladouceur L, Green ML. Evaluation of a women's safe shelter experience to teach internal medicine residents about intimate partner violence: a randomized controlled trial. Journal of General Internal Medicine 2005;20(6):536-40. [DOI: 10.1111/j.1525-1497.2005.0100.x] [PMC1490142] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coonrod 2000 {published data only}
- Coonrod DV, Bay CR, Rowley BD, Del Mar NB, Gabriele L, Tessman TD, et al. A randomized controlled study of brief interventions to teach residents about domestic violence. Academic Medicine 2000;75(1):55-7. [DOI: 10.1097/00001888-200001000-00014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cutshall 2019 {published data only}
- Cutshall SL. Rape Culture, Intimate Partner Violence and Sexual Violence: A Pilot Study of an Educational Intervention for Mental Health Professionals [PhD Thesis]. Santa Barbara (CA): Fielding Graduate University, 2019. [Google Scholar]
Danley 2004 {published data only}
- Danley D, Gansky SA, Chow D, Gerbert B. Preparing dental students to recognize and respond to domestic violence: the impact of a brief tutorial. Journal of the American Dental Association 2004;135(1):67-73. [DOI: 10.14219/jada.archive.2004.0022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwardsen 2006 {published data only}
- Edwardsen EA, Morse DS, Frankel RM. Structured practice opportunities with a mnemonic affect medical student interviewing skills for intimate partner violence. Teaching and Learning in Medicine 2006;18(1):62-8. [DOI: 10.1207/s15328015tlm1801_13] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Falb K, Diaz-Olavarrieta C, Campos PA, Valades J, Cardenas R, Carino G, et al. Evaluating a health care provider delivered intervention to reduce intimate partner violence and mitigate associated health risks: study protocol for a randomized controlled trial in Mexico City. BMC Public Health 2014;14:772. [DOI: 10.1186/1471-2458-14-772] [PMCID: PMC4127193] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Falb KL, Ponta O, Xuan Z, Campos PA, Gomez AA, et al. A nurse-delivered, clinic-based intervention to address intimate partner violence among low-income women in Mexico City: findings from a cluster randomized controlled trial. BMC Medicine 2017;15:128. [DOI: 10.1186/s12916-017-0880-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gürkan 2017 {published data only}
- Gürkan ÖC, Kömürcü N. The effect of a peer education program on combating violence against women: a randomized controlled study. Nurse Education Today 2017;57:47-53. [DOI: 10.1016/j.nedt.2017.07.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haist 2007 {published data only}
- Haist SA, Wilson JF, Lineberry MJ, Griffith CH. A randomized controlled trial using insinuated standardized patients to assess residents' domestic violence skills following a two-hour workshop. Teaching and Learning in Medicine 2007;19(4):336-42. [DOI: 10.1080/10401330701542495] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2002 {published data only}
- Harris JM Jr, Kutob RM, Surprenant ZJ, Maiuro RD, Delate TA. Can internet-based education improve physician confidence in dealing with domestic violence? Family Medicine 2002;34(4):287-92. [PMID: ] [PubMed] [Google Scholar]
- Hooker L. Request for raw data on attitude and knowledge outcomes [personal communication]. Email to: J Harris 05 November 2018.
Hegarty 2013 {published data only}
- Hegarty K, Gunn JM, O'Doherty LJ, Taft A, Chondros P, Feder G, et al. Women’s evaluation of abuse and violence care in general practice: a cluster randomised controlled trial (WEAVE). BMC Public Health 2010;10:2. [DOI: 10.1186/1471-2458-10-2] [PMCID: PMC2823699] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty K, O'Doherty L, Taft A, Chondros P, Brown S, Valpied J, et al. Screening and counselling in the primary care setting for women who have experienced intimate partner violence (WEAVE): a cluster randomised controlled trial. Lancet 2013;382(9888):249-58. [DOI: 10.1016/S0140-6736(13)60052-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hooker L. Request for PREMIS data not published in primary outcomes paper [personal communication]. Email to: K Hegarty 23 November 2018.
Hsieh 2006 {published data only}
- Hsieh NK, Herzig K, Gansky SA, Danley D, Gerbert B. Changing dentists' knowledge, attitudes and behavior regarding domestic violence through an interactive multimedia tutorial. Journal of the American Dental Association 2006;137(5):596-603. [DOI: 10.14219/jada.archive.2006.0254] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lo Fo Wong 2006 {published data only}
- Lo Fo Wong S, Wester F, Mol SS, Lagro-Janssen TL. Increased awareness of intimate partner abuse after training: a randomised controlled trial. British Journal of General Practice 2006;56(525):249-57. [PMC1832231] [bjgp.org/content/bjgp/56/525/249.full.pdf] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Mauch 1982 {published data only}
- Mauch PA. The Effects of Training on Attitudes of Counselors about Battered Women [PhD Thesis]. Columbia (MS): University of Missouri, 1982. [Google Scholar]
Moskovic 2008 {published data only}
- Moskovic CS, Guiton G, Chirra A, Núñez AE, Bigby JA, Stahl C, et al. Impact of participation in a community-based intimate partner violence prevention program on medical students: a multi-center study. Journal of General Internal Medicine 2008;23(7):1043-7. [DOI: 10.1007/s11606-008-0624-y] [PMC2517914] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenhofer S. Request for measures used on participant confidence [personal communication]. Email to: C Moskovic 23 May 2019.
Ragland 1989 {published data only}
- Ragland DJ. Marital Violence and Psychotherapeutic Issues: An Examination of the Effects of a Videotape Training Program on Therapist Attitudes About an Assessment of Battered Women [PhD thesis]. New York (NY): Hofstra University, 1989. [Google Scholar]
Sharps 2016 {published data only}
- Sharps PW, Bullock LF, Campbell JC, Alhusen JL, Ghazarian SR, Bhandari SS, et al. Domestic violence enhanced perinatal home visits: the DOVE randomized clinical trial. Journal of Women's Health 2016;25(11):1129-38. [DOI: 10.1089/jwh.2015.5547] [PMC5116686] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Short 2006b {published data only}
- Hooker L. Request for raw data on all outcome measures [personal communication]. Email to: J Harris 05 October 2018.
- Short LM, Surprenant ZJ, Harris JM Jr. A community-based trial of an online intimate partner violence CME program. American Journal of Preventive Medicine 2006;30(2):181-5. [DOI: 10.1016/j.amepre.2005.10.012] [PMC1413953] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakily 2017 {published data only}
- Vakily M, Noroozi M, Yamani N. Comparing the effect of group-based and compact disk-based training on midwives' knowledge and attitude toward domestic violence in women of reproductive age. Journal of Education and Health Promotion 2017;6:70. [DOI: 10.4103/jehp.jehp_44_16] [PMC5561673] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arrab 2018 {published data only}
- Arrab MM, Ibrahim HS. Effect of educational training intervention on overcoming nurses' barriers to screening intimate partner violence against women in outpatient clinics. American Journal of Nursing Research 2018;6(4):198-207. [DOI: 10.12691/ajnr-6-4-8] [DOI] [Google Scholar]
Campbell 2001 {published data only}
- Campbell JC, Coben JH, McLoughlin E, Dearwater S, Nah G, Glass N, et al. An evaluation of a system-change training model to improve emergency department response to battered women. Academic Emergency Medicine 2001;8(2):131-8. [DOI: 10.1111/j.1553-2712.2001.tb01277.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carroll 2005 {published data only}
- Carroll JC, Reid AJ, Biringer A, Midmer D, Glazier RH, Wilson L, et al. Effectiveness of the antenatal psychosocial health assessment (ALPHA) form in detecting psychosocial concerns: a randomized controlled trial. Canadian Medical Association Journal 2005;173(3):253-9. [DOI: 10.1503/cmaj.1040610] [PMC1180654 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cripe 2010 {published data only}
- Cripe SM, Sanchez SE, Sanchez E, Quintanilla BA, Alarcon CH, Gelaye B, et al. Intimate partner violence during pregnancy: a pilot intervention program in Lima, Peru. Journal of Interpersonal Violence 2010;25(11):2054-76. [DOI: 10.1177/0886260509354517] [PMC3741342 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubowitz 2011 {published data only}
- Dubowitz H, Lane WG, Semiatin JN, Magder LS, Venepally M, Jans M. The safe environment for every kid model: impact on pediatric primary care professionals. Pediatrics 2011;127(4):e962-70. [DOI: 10.1542/peds.2010-1845] [PMC3387892 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz H. Re: request for raw data for the IPV scale for each time point [personal communication]. Email to: N Kalra 06 November 2018.
- Kalra N. Request for raw data for the IPV scale for each time point [personal communication]. Email to: H Dubowitz 20 October 2018.
Duggan 2007 {published data only}
- Duggan A, Caldera D, Rodriguez K, Burrell L, Rohde C, Crowne SS. Impact of a statewide home visiting program to prevent child abuse. Child Abuse & Neglect 2007;31(8):801-27. [DOI: 10.1016/j.chiabu.2006.06.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feder 2011 {published data only}ISRCTN74012786
- Devine A, Spencer A, Eldridge S, Norman R, Feder G. Cost-effectiveness of Identification and Referral to Improve Safety (IRIS), a domestic violence training and support programme for primary care: a modelling study based on a randomised controlled trial. BMJ Open 2012;2(3):1-8. [doi:10.1136/bmjopen-2012-001008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder G, Agnew Davies R, Baird K, Dunne D, Eldridge S, Griffiths C, et al. Identification and referral to improve safety (IRIS) of women experiencing domestic violence with a primary care training and support programme: a cluster randomised controlled trial. Lancet 2011;378(9805):1788-95. [DOI: 10.1016/S01406736(11)61179-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Feder G. Re: request for raw IRIS data on GP knowledge [personal communication]. Email to: L Hooker 12 December 2018.
- Gregory A, Ramsay J, Agnew-Davies R, Baird K, Devine A, Dunne D, et al. Primary care Identification and Referral to Improve Safety of women experiencing domestic violence (IRIS): protocol for a pragmatic randomised controlled trial. BMC Public Health 2010;10:54. [DOI: 10.1186/1471-2458-10-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker L. Request for raw IRIS data on GP knowledge [personal communication]. Email to: G Feder 29 October 2018.
Feigelman 2011 {published data only}
- Feigelman S, Dubowitz H, Lane W, Grube L, Kim J. Training pediatric residents in a primary care clinic to help address psychosocial problems and prevent child maltreatment. Academic Pediatrics 2011;11(6):474-80. [DOI: 10.1016/j.acap.2011.07.005] [PMC5482713 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigelman S. Re: request for raw IPV data including mean scores across arm and three time points [personal communication]. Email to: N Kalra 29 November 2018.
- Kalra N. Request for raw IPV data including mean scores across arm and three time points [personal communication]. Email to: S Feigelman 11 October 2018.
Garg 2007 {published data only}
- Garg A, Butz AM, Dworkin PH, Lewis RA, Thompson RE, Serwint JR. Improving the management of family psychosocial problems at low-income children’s well-child care visits: the WE CARE project. Pediatrics 2007;120(3):547-58. [DOI: 10.1542/peds.2007-0398] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jack 2019 {published data only}
- Hooker L. Request for data on prespecified secondary outcomes [personal communication]. Email to: S Jack 25 June 2019.
- Jack S. Re: request for data on prespecified secondary outcomes [personal communication]. Email to: Hooker L 6 August 2019.
- Jack SM, Boyle M, McKee C, Ford-Gilboe M, Wathen CN, Scribano P, et al. Effect of addition of an intimate partner violence intervention to a nurse home visitation program on maternal quality of life: a randomized clinical trial. JAMA 2019;321(16):1576-85. [DOI: 10.1001/jama.2019.3211] [PMC6487547 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarlane 2006 {published data only}
- Hooker L. Request for information re: nurses who received IPV training [personal communication]. Email to: J McFarlane 23 May 2019.
- McFarlane J. Re: request for information re: nurses who received IPV training [personal communication]. Email to: L Hooker 18 July 2019.
- McFarlane JM, Groff JY, O'Brien JA, Watson K. Secondary prevention of intimate partner violence: a randomized controlled trial. Nursing Research 2006;55(1):52-61. [DOI: 10.1097/00006199-200601000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2011 {published data only}
- Miller E, Decker MR, McCauley HL, Tancredi DJ, Levenson RR, Waldman J, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011;83(3):274-80. [DOI: 10.1016/j.contraception.2010.07.013] [PMC3052939 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2016 {published data only}
- Miller E, Tancredi DJ, Decker MR, McCauley HL, Jones KA, Anderson H, et al. A family planning clinic-based intervention to address reproductive coercion: a cluster randomized controlled trial. Contraception 2016;94(1):58-67. [DOI: 10.1016/j.contraception.2016.02.009] [PMC4884549 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi DJ, Silverman JG, Decker MR, McCauley HL, Anderson HA, Jones KA, et al. Cluster ranomized controlled trial protocol: addressing reproductive coercion in health setting (ARCHES). BMC Women's Health 2015;15:57. [DOI: 10.1186/s12905-015-0216-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2017 {published data only}
- Miller E, McCauley H, Decker MR, Levenson R, Zelazny S, Jones KA, et al. Implementation of a family planning clinic–based partner violence and reproductive coercion intervention: provider and patient perspectives. Perspectives on Sexual and Reproductive Health 2017;49(2):85-93. [DOI: 10.1363/psrh.12021] [PMC5453817 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagler 1993 {published data only}
- Nagler MS. Effect of an Acquaintance Rape Prevention Program on Rape Attitudes, Knowledge, Intent and Sexual Assault Reporting [PhD Thesis]. Long Island (NY): Hofstra University, 1993. [Google Scholar]
Thompson 2000 {published data only}
- Thompson RS, Rivara FP, Thompson DC, Barlow WE, Sugg NK, Maiuro RD, et al. Identification and management of domestic violence a randomized trial. American Journal of Preventative Medicine 2000;19(4):253-63. [DOI: 10.1016/s0749-3797(00)00231-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vijayalakshmi 2021 {published data only}
- Vijayalakshmi P, Sailaxmi G, Nikhil Reddy SS, Marimuthu P, Suresh BM. Effectiveness of a module based training on nurses’ attitude towards social norms and beliefs that support abuse among women with mental illness. Community Mental Health Journal 2021;57(1):161-6. [DOI: 10.1007/s10597-020-00628-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zachor 2108 {published data only}
- Zachor H, Chang JC, Zelazny S, Jones KA, Miller E. Training reproductive health providers to talk about intimate partner violence and reproductive coercion: an exploratory study. Health Education Research 2018;33(2):175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abraham 2001 {published data only}
- Abraham A, Cheng TL, Wright JL, Addlestone I, Huang Z, Greenberg L. Assessing an educational intervention to improve physician violence screening skills. Pediatrics 2001;107(5):e68. [DOI: 10.1542/peds.107.5.e68] [PMID: ] [DOI] [PubMed] [Google Scholar]
Abraham 2011 {published data only}
- Abraham A, Hawkins K, Chen R. An educational intervention to improve physician interviewing skills of adolescent patients during the pediatric clerkship. Journal of Adolescent Health 2011;48(S2):234-5. [DOI: 10.1016/j.jadohealth.2010.11.079] [Abstract #33] [DOI] [Google Scholar]
- Kalra N. Query about your 2011 study AN EDUCATIONAL INTERVENTION TO IMPROVE PHYSICIAN INTERVIEWING SKILLS OF ADOLESCENT PATIENTS DURING THE PEDIATRIC CLERKSHIP [personal communication]. Email to: A Abraham 14 September 2018.
Hill 2016 {published data only}
- Hill SK. Effectiveness of an Educational Intervention on the Intimate Partner Violence Screening Behaviors of College-Based Health Care Providers. Electronic Theses and Dissertations 2016. [https://dc.etsu.edu/etd/3091]
References to ongoing studies
Fernández 2006 {published data only}
- Fernández Alonso MC, Herrero Velázquez S, Cordero Guevara JA, Madereuelo Fernández JÁ, González Castro ML, ISFVIDAP Group. Protocol to evaluate the effectiveness of a consciousness-raising and training intervention for primary care professionals, in order to improve detection of domestic violence [Protocolo para la evaluación de la efectividad de una intervención sensibilizadora y formativa en profesionales de atención primaria para la mejora de la detección de la violencia doméstica (ISFVIDAP)]. Atención Primaria 2006;38(3):168-73. [DOI: 10.1157/13090973] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00257296 {published data only}
- NCT00257296. Evaluation of an intimate partner violence screening-intervention. clinicaltrials.gov/ct2/show/NCT00257296 (first received 21 November 2005).
NCT01028118 {published data only}
- NCT01028118. Violence against women and consequences during climacteric´s phase (DV) [Domestic and sexual violence against women: consequences for climacteric´s phase]. clinicaltrials.gov/ct2/show/NCT01028118 (first received 7 December 2009).
NCT03259646 {published data only}
- NCT03259646. The IPV provider network: engaging the health care provider response to interpersonal violence against women. clinicaltrials.gov/ct2/show/NCT03259646 (first received 21 August 2017).
Pallitto 2016 {published data only}ISRCTN35969343
- Pallitto C, García-Moreno C, Stöeckl H, Hatcher A, MacPhail C, Mokoatle K, et al. Testing a counselling intervention in antenatal care for women experiencing partner violence: a study protocol for a randomized controlled trial in Johannesburg, South Africa. BMC Health Services Research 2016;16(1):630. [DOI: 10.1186/s12913-016-1872-x] [PMC5097399 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruijne 2017 {published data only}ISRCTN14115257
- Ruijne RE, Howard LM, Trevillion K, Jongejan FE, Garofalo C, Bogaerts S, et al. Detection of domestic violence by community mental health teams: a multi-center, cluster randomized controlled trial. BMC Psychiatry 2017;17(1):288. [DOI: 10.1186/s12888-017-1399-7] [28784096] [PMC5545838 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aksan 2007
- Aksan HA, Aksu F. The training needs of Turkish emergency department personnel regarding intimate partner violence. BMC Public Health 2007;7:350. [DOI: 10.1186/1471-2458-7-350] [PMC2241616] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azjen 1991
- Azjen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50(2):179-211. [DOI: 10.1016/0749-5978(91)90020-T] [DOI] [Google Scholar]
Bair‐Merritt 2014
- Bair-Merritt MH, Lewis-O’Connor A, Goel S, Amato P, Ismailji T, Jelley M, et al. Primary care–based interventions for intimate partner violence: a systematic review. American Journal of Preventive Medicine 2014;46(2):188-94. [DOI: 10.1016/j.amepre.2013.10.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Beamesderfer A. Assessment of depression: the Depression Inventory. Psychological Measurements in Psychopharmacology 1974;7:151-69. [DOI: 10.1159/000395074] [DOI] [PubMed] [Google Scholar]
Beynon 2012
- Beynon CE, Gutmanis AI, Tutty LM, Wathen CN, MacMillan HL. Why physicians and nurses ask (or don’t) about partner violence: a qualitative analysis. BMC Public Health 2012;12:473. [DOI: 10.1186/1471-2458-12-473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bullock 1997
- Bullock K. Domestic violence training at an inner-city hospital found helpful. Journal of Emergency Nursing 1997;23(4):299-300. [DOI: ] [DOI] [PubMed] [Google Scholar]
Campbell 2002
- Campbell JC. Health consequences of intimate partner violence. Lancet 2002;359(9314):1331-6. [10.1016/S0140-6736(02)08336-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1977
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York (NY): Academic Press Inc, 1977. [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Cohen 2002
- Cohen F, Salmon ME, Stobo JD (editors). Confronting Chronic Neglect: The Education and Training of Health Professionals on Family Violence. Washington (DC): The National Academies Press, 2002. [PubMed] [Google Scholar]
Coker 2002
- Coker AL, Davis KE, Arias I, Desai S, Sanderson M, Brandt HM, et al. Physical and mental health effects of intimate partner violence for men and women. American Journal of Preventive Medicine 2002;23(4):260-8. [DOI: 10.1016/S0749-3797(02)00514-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Crombie 2017
- Crombie N, Hooker L, Reisenhofer S. Nurse and midwifery education and intimate partner violence: a scoping review. Journal of Clinical Nursing 2017;26(15-6):2100-25. [DOI: 10.1111/jocn.13376] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davidson 2001
- Davidson LL, Grisso JA, Garcia-Moreno C, Garcia J, King VJ, Marchant S. Training programs for healthcare professionals in domestic violence. Journal of Women’s Health & Gender-based Medicine 2001;10(10):953-69. [DOI: 10.1089/152460901317193530] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Devine 2012
- Devine A, Spencer A, Eldridge S, Norman R, Feder G. Cost-effectiveness of Identification and Referral to Improve Safety (IRIS), a domestic violence training and support programme for primary care: a modelling study based on a randomised controlled trial. BMJ Open 2012;2(3):1-8. [doi:10.1136/bmjopen-2012-001008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Djikanovic 2010
- Djikanovic B, Celik H, Simic S, Matejic B, Cucic V. Health professionals’ perceptions of intimate partner violence against women in Serbia: opportunities and barriers for response improvement. Patient Education and Counseling 2010;80(1):88-93. [DOI: 10.1016/j.pec.2009.09.028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPPI‐Reviewer 2010 [Computer program]
- EPPI-Reviewer 4: software for research synthesis. EPPI-Centre Software. Thomas J, Brunton J, Graziosi S. London: Social Science Research Unit, University College London, Institute of Education, 2010.
Feder 2006
- Feder GS, Hutson M, Ramsay J, Taket AR. Women exposed to intimate partner violence: expectations and experiences when they encounter health care professionals: a meta-analysis of qualitative studies. Archives of Internal Medicine 2006;166(1):22-37. [DOI: 10.1001/archinte.166.1.22] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Moreno 2002
- García-Moreno C. Dilemmas and opportunities for an appropriate health-service response to violence against women. Lancet 2002;359(9316):1509-14. [DOI: 10.1016/S0140-6736(02)08417-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Moreno 2014
- García-Moreno C, Hegarty K, D'Oliveira AF, Koziol-McLain J, Colombini M, Feder G. The health-systems response to violence against women. Lancet 2015;385(9977):1567-79. [DOI: 10.1016/S0140-6736(14)61837-7] [DOI] [PubMed] [Google Scholar]
Gerbert 2000
- Gerbert B, Moe J, Caspers N, Salber P, Feldman M, Herzig K, et al. Simplifying physicians' response to domestic violence. Western Journal of Medicine 2000;172(5):329-31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI: 10.1111/hir.12251] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldberg 1979
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychological Medicine 1979;9(1):139-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADEpro GDT. Version September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harwell 1998
- Harwell TS, Casten RJ, Armstrong KA, Dempsey S, Coons HL, Davis M. Results of a domestic violence training program offered to the staff of urban community health centers. Evaluation Committee of the Philadelphia Family Violence Working Group. American Journal of Preventive Medicine 1998;15(3):235–42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hegarty 1999
- Hegarty K, Sheehan M, Schonfeld C. A multidimensional definition of partner abuse: development and preliminary validation of the Composite Abuse Scale. Journal of Family Violence 1999;14(4):399-415. [DOI: 10.1023/A:1022834215681] [DOI] [Google Scholar]
Hegarty 2005
- Hegarty K, Bush R, Sheehan M. The Composite Abuse Scale: further development and assessment of reliability and validity of a multidimensional partner abuse measure in clinical settings. Violence and Victims 2005;20(5):529-47. [PMID: ] [PubMed] [Google Scholar]
Hegarty 2020
- Hegarty K, McKibbin G, Hameed M, Koziol-McLain J, Feder G, Tarzia L, et al. Health practitioners' readiness to address domestic violence and abuse: a qualitative meta-synthesis. PLOS One 2020;15(6):e0234067. [DOI: 10.1371/journal.pone.0234067] [PMCID: PMC7297351] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heise 2002
- Heise L, Garcia Moreno C. Chapter 4: Violence by intimate partners. In: Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R, editors(s). World Report on Violence and Health. Geneva (CH): World Health Organization, 2002:87-122. [Google Scholar]
Heron 2002
- Heron SL, Kellermann AL. Screening for intimate partner violence in the emergency department: where do we go from here? Annals of Emergency Medicine 2002;40(5):493-5. [DOI: 10.1067/mem.2002.128828] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgiins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February, 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Kim 2002
- Kim J, Motsei M. ‘‘Women enjoy punishment’’: attitudes and experiences of gender-based violence among PHC nurses in rural South Africa. Social Science & Medicine 2002;54(8):1243-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated Fenruary 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
McKenzie 2016
- McKenzie J, Ryan R, Di Tann GL, Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Review Group: cluster randomised controlled trials; December 2016. cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/clusterrcts_revising_december_1st.pdf (accessed 12 August 2020). [DOI: 10.26181/5b57d08a7ad22] [DOI]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morse 2012
- Morse DS, Lafleur R, Fogarty CT, Mittal M, Cerulli C. "They told me to leave": how health care providers address intimate partner violence. Journal of the American Board of Family Medicine 2012;25(3):333-42. [DOI: 10.3122/jabfm.2012.03.110193] [ PMC3388120] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1990
- Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. British Journal of Psychiatry 1990;157:288-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Doherty 2015
- O'Doherty L, Hegarty K, Ramsay J, Davidson LL, Feder G, Taft A. Screening women for intimate partner violence in healthcare settings. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD007007. [DOI: 10.1002/14651858.CD007007.pub3] [PMC6599831] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochs 1996
- Ochs HA, Neuenschwander MC, Dodson TB. Are head, neck and facial injuries markers of domestic violence? Journal of the American Dental Association 1996;127(6):757-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perciaccante 1999
- Perciaccante VJ, Ochs HA, Dodson TB. Head, neck, and facial injuries as markers of domestic violence in women. Journal of Oral and Maxillofacial Surgery 1999;57(7):760-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramsay 2005
- Ramsay J, Rivas C, Feder G. Interventions to Reduce Violence and Promote the Physical and Psychosocial Well-being of Women Who Experience Partner Abuse: A Systematic Review. London (UK): Barts and The London Queen Mary's School of Medicine and Dentistry, 2005. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Nordic Cochrane Centre, Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Richardson 2001
- Richardson J, Feder G, Eldridge S, Chung WS, Coid J, Moorey S. Women who experience domestic violence and women survivors of childhood sexual abuse: a survey of health professionals’ attitudes and clinical practice. British Journal of General Practice 2001;51(467):468-70. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. Cochrane Consumers and Communication Group: meta-analysis; December 2016. ccrg.cochrane.org/author-resources (accessed 30 November 2020). [DOI: 10.26181/5b57d2b96b326] [DOI]
Sammut 2021
Sawyer 2016
- Sawyer S, Coles J, Williams A, Williams B. A systematic review of intimate partner violence educational interventions delivered to allied health care practitioners. Medical Education 2016;50(11):1107-21. [DOI: 10.1111/medu.13108] [PMID: ] [DOI] [PubMed] [Google Scholar]
Short 1998
- Short LM, Johnson D, Osattin A. Recommended components of health care provider training programs on intimate partner violence. American Journal of Preventive Medicine 1998;14(4):283-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Short 2006a
- Short LM, Alpert E, Harris JM, Surprenant ZJ. PREMIS: a comprehensive and reliable tool for measuring physician readiness to manage IPV. American Journal of Preventive Medicine 2006;30(2):173–80 2006;30(2):173-80. [DOI: 10.1016/j.amepre.2005.10.009] [NIHMS8976] [PMC1451776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sitterding 2003
- Sitterding HA, Adera T, Shields-Fobbs E. Spouse/partner violence education as a predictor of screening practices among physicians. Journal of Continuing Education in the Health Profession 2003;23(1):54-63. [DOI: 10.1002/chp.1340230109] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 1999
- Smith PH, Smith JB, Earp JA. Beyond the measurement trap. A reconstructed conceptualization and measurement of woman battering. Psychology of Women Quarterly 1999;23(1):177-93. [DOI: 10.1111/j.1471-6402.1999.tb00350.x] [DOI] [Google Scholar]
Spielberger 1994
- Spielberger CD, Sydeman SJ. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: Maruish ME, editors(s). The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1994:292-321. [Google Scholar]
Stata 2019 [Computer program]
- Stata. Stata, Version 16. College Station, TX, USA: StataCorp, 2019. Available at www.stata.com.
Stöckl 2013
- Stöckl H, Devries K, Rotstein A, Abraham N, Campbell J, Watts C, et al. The global prevalence of intimate partner homicide: a systematic review. Lancet 2013;382(9895):859-65. [DOI: 10.1016/S0140-6736(13)61030-2] [DOI] [PubMed] [Google Scholar]
Straus 1996
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scale (CTS2): development and preliminary psychometric data. Journal of Family Issues 1996;17(3):283-316. [DOI: 10.1177/019251396017003001] [DOI] [Google Scholar]
Tarzia 2020
- Tarzia L, Bohren M, Cameron J, Garcia-Moreno C, O'Doherty L, Fiolet R, et al. Women’s experiences and expectations after disclosure of intimate partner abuse to a healthcare provider: A qualitative meta-synthesis. BMJ Open 2020;10(11):e041339. [DOI: 10.1136/bmjopen-2020-041339] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013a
- World Health Organization. Global and Regional Estimates of Violence Against Women. Prevalence and Health Effects of Intimate Partner Violence and Non-Partner Sexual Violence. Geneva (CH): WHO, 2013. [Google Scholar]
WHO 2013b
- World Health Organization. Responding to intimate partner violence and sexual violence against women. WHO clinical and policy guidelines; 2013. apps.who.int/iris/bitstream/10665/85240/1/9789241548595_eng.pdf 2013 (accessed 19 February 2016). [PubMed]
WHO 2014
- World Health Organization. Health Care for Women Subjected to Intimate Partner Violence or Sexual Violence — A Clinical Handbook. Geneva (CH): World Health Organization, 2014. [Google Scholar]
WHO 2017
- World Health Organization. Strengthening health systems to respond to women subjected to intimate partner violence or sexual violence: a manual for health managers; 2017. Available at www.who.int/reproductivehealth/publications/violence/vaw-health-systems-manual/en/.
WHO 2019
- World Health Organization. Caring for women subjected to violence: a WHO curriculum for training health-care providers; 2019. Available at www.who.int/reproductivehealth/publications/caring-for-women-subject-to-violence/en/.
WHO 2021
- World Health Organization. Violence against women prevalence estimates, 2018. Global, regional and national prevalence estimates for intimate partner violence against women and global and regional prevalence estimates for non-partner sexual violence against women; 2021. Available at www.who.int/publications/i/item/violence-against-women-prevalence-estimates.
Wood 1998
- Wood K, Maforah F, Jewkes R. ‘‘He forced me to love him’’: putting violence on adolescent sexual health agendas. Social Science & Medicine 1998;47(2):233-42. [DOI: 10.1016/S0277-9536(98)00057-4] [PMID: 9720642] [DOI] [PubMed] [Google Scholar]
Zaher 2014
- Zaher E, Keogh K, Ratnapalan S. Effect of domestic violence training systematic review of randomized controlled trials. Canadian Family Physician [Le Médecin de Famille Canadien] 2014;60(7):618-24. [PMC4096259] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Zakar 2011
- Zakar R, Zakar MZ, Kraemer A. Primary health care physicians' response to the victims of spousal violence against women in Pakistan. Health Care for Women International 2011;32(9):811–32. [DOI: 10.1080/07399332.2011.569042] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kalra 2017
- Kalra N, Di Tanna GL, García-Moreno C. Training healthcare providers to respond to intimate partner violence against women. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012423. [DOI: 10.1002/14651858.CD012423] [PMC6464528] [DOI] [PMC free article] [PubMed] [Google Scholar]


