Abstract
Objective:
Awareness with paralysis (AWP) is a devastating complication for mechanically ventilated patients and risks long-term psychological morbidity. Data from the emergency department (ED) demonstrate a high rate of longer-acting neuromuscular blocking agent (NMBA) use, delayed analgosedation, and a lack of sedation depth monitoring. These practices are discordant to recommendations for preventing AWP. Despite this, AWP has not been rigorously studied in the ED population. Our objective was to assess the prevalence of AWP in mechanically ventilated ED patients.
Methods:
This was a single-center, prospective, observational cohort study on 383 mechanically ventilated ED patients. After extubation, we assessed patients for AWP using the modified Brice questionnaire. Three expert reviewers independently adjudicated AWP. We report the prevalence of AWP (primary outcome); the secondary outcome was perceived threat, a mediator for development of post-traumatic stress disorder.
Results:
The prevalence of AWP was 2.6% (10/383). Exposure to rocuronium at any time point in the ED was significantly different between patients who experienced AWP (70%) versus the rest of the cohort (31.4%) (unadjusted odds ratio, 5.1; 95% confidence interval [CI], 1.30 to 20.1). Patients experiencing AWP had higher mean (standard deviation) values on the threat perception scale, denoting a higher degree of perceived threat, as compared to patients that did not experience AWP [13.4 (7.7) vs. 8.5 (6.2), mean difference 4.9; 95% CI 0.94 to 8.8.
Conclusions:
AWP occurs in a significant minority of mechanically ventilated ED patients. Potential associations of AWP with ED care and increased perceived threat warrant further evaluation.
INTRODUCTION
Background
Awareness with recall of paralysis (AWP) is the recollection of sensory perceptions while under the influence of a neuromuscular blocking agent (NMBA). Studies examining outcomes of patients who experience AWP in the operating room (OR) have documented disturbing longterm psychological sequelae occurring in up to 70% of cases, including post-traumatic stress disorder (PTSD), clinical depression, and complex phobias.1–4
Prospective studies have estimated prevalence of AWP during general anesthesia to be approximately 0.1-0.2%;5,6 this figure approaches 1.0% in high-risk patients given only intravenous anesthesia.7 Risk factors for higher prevalence and greater severity of AWP in the OR include: 1) intravenous anesthetic approach (versus use of inhaled anesthetics),8,9 2) underdosing of anesthesia,10 3) administration of longer-acting NMBAs,2,5,11 and 4) lack of protocolized sedation depth monitoring.3 While extensive research has been conducted on AWP in the OR, this has yet to extend to other areas, such as the emergency department (ED), potentially placing mechanically ventilated patients at higher risk for this complication.
In the United States, clinicians have historically managed mechanically ventilated ED patients in a way that could predispose them to AWP.12,13 These patients exclusively receive intravenous analgosedation and are frequently under-dosed.14 This includes induction agents during rapid sequence intubation (RSI), particularly in obese patients.15,16 Several studies have shown that 10-54% of ventilated patients receive no sedation after RSI,14,17–21 and there can be substantial delay (up to 50 minutes) in the provision of post-intubation sedation.18,22 Approximately 90% of patients receive NMBAs for intubation in the ED, with an increasing use of longer-acting agents (e.g. rocuronium) as opposed to succinylcholine.23 After intubation, approximately 10-25% of mechanically ventilated ED patients receive additional, longer-acting NMBAs without any increase in sedation.18,19 Literature has demonstrated that for ED patients receiving longer-acting NMBAs such as rocuronium, post-intubation sedation is initiated at lower doses and with greater delays compared to those who receive succinylcholine.21,22 Finally, a lack of protocol-driven management of sedation is common, and up to 33% of mechanically ventilated ED patients receive no sedation depth assessment.18,19
Importance
These data describe a historical precedent of management in the ED that is discordant to recommendations for prevention of AWP. However, only a few small studies have examined awareness in this vulnerable cohort. Four prospective cohort studies (combined n = 123) assessed for recall of intubation and demonstrated a prevalence ranging from 6-50%.24–27 This prior research on AWP in ED patients is limited secondary to small sample sizes, methodological limitations, and use of non-validated and never-before used questionnaires to assess for awareness. Despite a lack of studies examining AWP in ED patients, prior data regarding analgosedation practices suggest that these patients could be at a higher risk for AWP and justify the conduct of more rigorous studies.
Goals of This Investigation
To address this critical knowledge gap, we conducted the ED-AWARENESS Study to estimate the prevalence of AWP in mechanically ventilated ED patients.
METHODS
Study Design & Setting
We conducted a single-center, prospective cohort study from June 2019 to May 2020 at a large (annual ED volume ~ 90,000 patients visits) academic, residency-affiliated, tertiary care center in St. Louis, Missouri. These results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Appendix E1).28 Our institutional review board approved this study and waived requirement to obtain a signed informed consent form; the study team obtained verbal informed consent from each subject. A detailed description of the methods has been published.29
Selection of Participants
The study team prospectively identified mechanically ventilated patients via an automated screening alert and enrolled consecutively, 24 hours per day. Patients were eligible if they were aged 18 years or older and underwent mechanical ventilation via an endotracheal tube in the ED. Intubation could have occurred either in the ED or prior to arrival, such as prehospital or at a transferring facility. Exclusion criteria were: 1) death before discontinuation of mechanical ventilation, 2) presence of neurological injury with residual deficit that precluded assessment for AWP (e.g. cerebrovascular accident, traumatic brain injury, cardiac arrest with hypoxic brain injury), 3) transfer to another facility, and 4) attrition or refusal to answer the questionnaire.
Methods of Measurement
All measurements and clinical data were gathered from chart review and collated using REDCap electronic data capture tools.30,31 All variables were objective and easily abstracted from the electronic medical record. A trained team member entered data from the electronic medical record into REDCap. This team member was also experienced in the methodology, given prior experience extracting similar data.19 We performed data quality control using both automatic and manual methods, and controlled REDCap fields by enforcing reference ranges for all data entered (e.g. plausible ranges for all values). A second team member performed periodic monitoring throughout the study on 20% of REDCap patient records. Prior to statistical analysis, the complete database was electronically searched for out-of-range and implausible values, and all flagged data were rechecked in the electronic chart to ensure accuracy.
Baseline characteristics included: age, gender, race, weight, height, pre-existing comorbidities, initial ED vital signs, and laboratory values. Comorbid conditions included dementia, diabetes mellitus, cirrhosis, heart failure, end-stage renal disease, chronic obstructive pulmonary disease, immunosuppression, malignancy, alcohol abuse, and psychiatric illness (i.e. schizophrenia, bipolar disorder, major depression, or generalized anxiety disorder). Select laboratory values included lactate, creatinine, bilirubin, platelets, hemoglobin, and blood gases. ED length of stay and data related to mechanical ventilation were collected.
All sedation-related data in the ED were collected, including induction agents and NMBAs used to facilitate intubation. Post-intubation medications related to analgosedation included opiates, benzodiazepines, propofol, ketamine, etomidate, haloperidol, quetiapine, and all NMBAs. We recorded sedation depth using the Richmond Agitation-Sedation Scale (RASS) per routine care. When more than one sedation depth was recorded, the median value was used. In patients that did not have an ED RASS score recorded, the first RASS score from the intensive care unit (ICU) was used as a surrogate, consistent with prior approaches.18,19 Data were also collected from the first 48 hours of ICU stay, including all analgesics, sedatives, NMBAs, sedation depth, and delirium assessments using the Confusion Assessment Method for the ICU (CAM-ICU) per routine care. The incidence of acute brain dysfunction, ventilator-free days, ICU- and hospital-free days were also tracked.
Outcomes
The primary outcome was AWP. In the assessment of the primary outcome, an important distinction had to be recognized with respect to the management goals for anesthetized patients in the OR (the only clinical arena where AWP has been rigorously studied) compared to critically ill mechanically ventilated patients. In the OR, the goal is to typically achieve unconsciousness and a lack of movement during a course of periodic painful stimuli. In contrast, data from patients in the ED and ICU demonstrate that light levels of sedation are associated with improved outcome18,19,32–34 Therefore, memory and recall of events is not only expected in mechanically ventilated patients, but in general is considered beneficial. This is in stark contrast to memories of awareness of paralysis, which carries substantial, negative psychological sequelae.35–39 To aid in distinguishing AWP from the appropriate recall of memories while mechanically ventilated, a combination of questions from the Brice questionnaire and the ICU Memory Tool were used (Appendix E2). The Brice questionnaire is the preferred method of evaluating for AWP,3,40–42 and the ICU Memory Tool is a validated questionnaire to assess memory of events in critically ill patients.43–45
To be considered for a possible AWP event, patients had to report memories of the period between losing consciousness and waking up (Brice questionnaire item #3 answered as ‘yes’), report a sensation/feeling of wakeful paralysis, and have documented NMBA administration. If a patient did not report memories of the period between losing consciousness and waking up but did report memories of wakeful paralysis before losing consciousness (e.g. recall of intubation), and had documented NMBA administration, then they were also considered for a possible AWP event. Events related to waking up during neuromuscular blockade and experiencing AWP before unconsciousness were considered equivalent. The study team assessed for AWP after extubation and prior to hospital discharge. During the final two months of the study, due to university-mandated clinical research restrictions related to the COVID-19 pandemic, AWP was assessed via telephone follow up after hospital discharge. AWP was independently adjudicated by three expert reviewers who were provided patient responses to the questionnaire, qualitative reports of patient experiences, and pertinent clinical information, including data regarding analgesics, sedatives, and NMBA. In assessing whether AWP occurred, the reviewers were instructed to consider such things as details and consistency of the reported memories, along with pertinent clinical information, such as type or dose of NMBA (Appendix E3). Due to the somewhat subjective nature in assessing for AWP these instructions were used to provide some standardization for adjudicators regarding the background of the study, how awareness and memories were assessed for, and to make sure they were looking at the accounts through a similar lens. Each expert reviewer adjudicated events as either no AWP, possible AWP, or definite AWP. The primary outcome of AWP was determined when at least two experts were in agreement. If all experts had held opposing views, then it was planned for a fourth reviewer to assist in the adjudication process.40 The use of a fourth reviewer was not needed.
The secondary clinical outcome was perceived threat, which was assessed with a previously validated measurement tool (scale 0 to 21 with higher scores denoting a greater degree of perceived threat).46,47 A link between AWP and perceived threat exists because perceived threat (conceptualized as a self-measured sense of life endangerment and personal vulnerability) during a medical emergency has previously been identified as a mediator (i.e. on the causal pathway) for the development of PTSD symptoms.46–49
Analysis
Patient characteristics are reported using descriptive statistics and frequency distributions. Data normality was assessed by inspection of Q-Q plots and the Kolmogorov-Smirnov test.
AWP was calculated as the proportion of patients with either possible or definite awareness events. The agreement among adjudicators of AWP events was assessed with the use of a two-way, random effects, intraclass correlation coefficient for absolute agreement according to the following: 0= no AWP, 1= possible AWP, and 2= definite AWP.
We previously published a detailed rationale regarding our sample size.29 Given the observational design of the study, the primary outcome is more descriptive rather than a hypothesis test between two groups. Prior to conduct of the study, we noted a dearth of literature regarding AWP from the ED domain, which raised the potential that no events would be detected. However, we noted that patients receiving intravenous (not inhaled) anesthesia in the OR had a prevalence of AWP approaching 1% during routine care.9 Since data demonstrate that our population could be at even higher risk, we estimated a prevalence of 1-2%, recognizing that the sample size needed to be large enough to observe an event with a high degree of probability and with sufficient precision. We decided a priori to enroll patients for approximately 12 months in order to accrue an adequate sample size and reduce the chance that any seasonal trends would skew the data. Based on our prior work in mechanically ventilated ED patients, we expected 2.1 patients per day to satisfy inclusion criteria, and estimated approximately half would ultimately be excluded, leaving just over one patient per day enrolled (n= 383).18,19,50–52 With a sample size of 383, if only one AWP event were detected, the corresponding event rate of 0.26% is similar to that seen in the OR, where sedation depth is monitored more diligently.3 Based on known risk factors for AWP and prior literature regarding ED sedation practices, we were confident that the sample size would be large enough to observe at least one event with sufficient precision.
RESULTS
Characteristics of Study Subjects
Figure 1 shows the study flow and final study population. Baseline characteristics are reported in Table 1.
Figure 1.
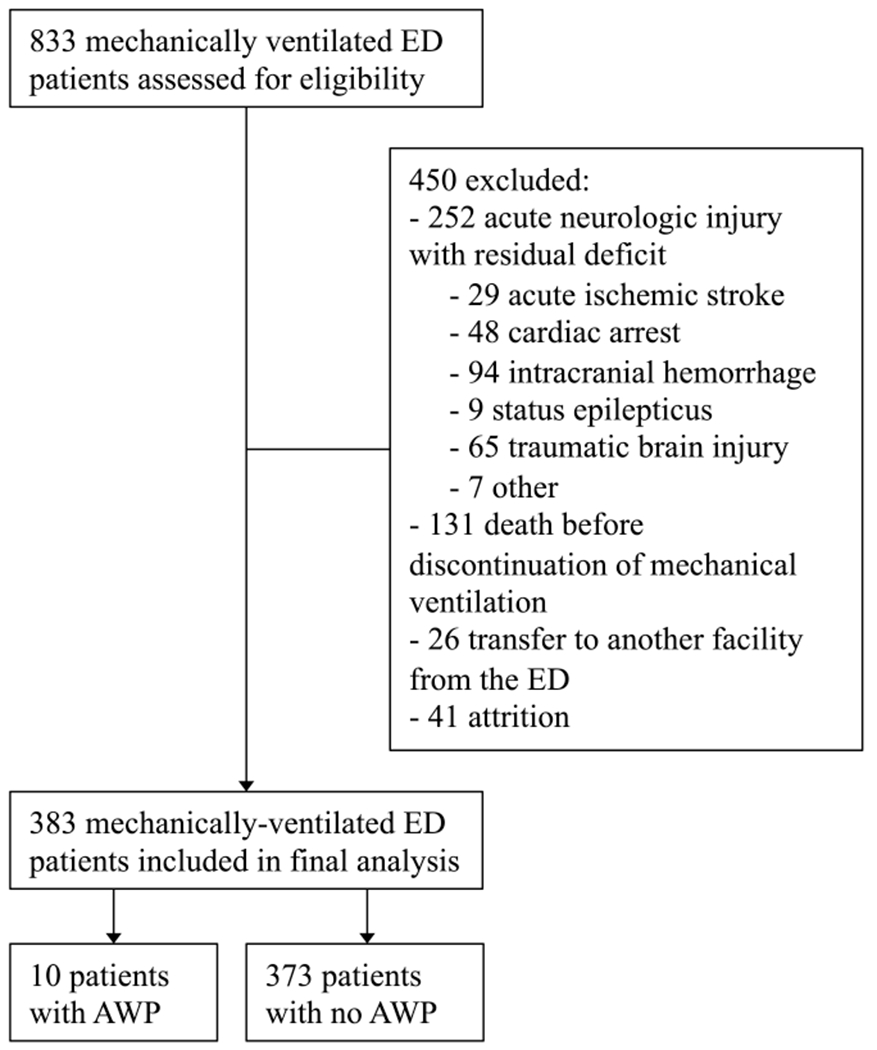
Study flow and final study population. AWP, Awareness with paralysis.
Table 1.
Characteristics of included study participants.
| Baseline Characteristics | All Subjects (n = 383) |
Patients with AWP (n = 10) |
Patients without AWP (n = 373) |
|---|---|---|---|
| Age (years)2 | 54 (37-63) | 65 (53-67) | 53 (37-63) |
| Female, n (%) | 132 (35) | 4 (40) | 128 (34) |
| BMI2 | 26.9 (22.3-31.7) | 30.4 (22.9-36.4) | 26.6 (22.3-31.6) |
| Race, n (%) | |||
| Black | 224 (59) | 3 (30) | 221 (59) |
| White | 151 (38) | 7 (70) | 144 (38) |
| Asian | 5 (2) | 0 | 5 (2) |
| Not Reported | 3 (1) | 0 | 3 (1) |
| Comorbidities, n (%) | |||
| Dementia | 9 (2) | 0 | 9 (2) |
| Diabetes Mellitus | 86 (23) | 3 (30) | 83 (22) |
| Cirrhosis | 8 (2) | 0 | 8 (2) |
| Heart Failure | 76 (20) | 2 (20) | 74 (20) |
| ESRD | 28 (7) | 1 (10) | 27 (7) |
| COPD | 71 (19) | 3 (30) | 68 (18) |
| Immunosuppression | 14 (4) | 2 (20) | 12 (3) |
| Malignancy | 41 (11) | 1 (10) | 40 (11) |
| Alcohol Abuse | 44 (12) | 0 | 44 (12) |
| Psychiatrica | 71 (19) | 2 (20) | 69 (19) |
| Intubation Data, n (%) | |||
| Location of Intubation | |||
| Emergency Department | 309 (81) | 9 (90) | 300 (80) |
| Transferring Facility | 44 (11) | 1 (10) | 43 (12) |
| Prehospital | 30 (8) | 0 | 30 (8) |
| Indication for Intubation | |||
| Trauma | 106 (28) | 4 (40) | 102 (27) |
| Medical | 277 (72) | 6 (60) | 271 (73) |
| Temperature (°C)2 | 36.5 (36.0-36.9) | 36.6 (36.0-37.1) | 36.5 (36.0-36.9) |
| Heart Rate (bpm)1 | 99 (25) | 92 (24) | 99 (25) |
| Mean Arterial Pressure (mmHg)1 | 98.8 (24.4) | 105.6 (28.2) | 98.7 (24.3) |
| Lactate (mmol/L)2 | 2.8 (1.6-5.1) | 2.4 (1.4-3.1) | 2.8 (1.6-5.2) |
| Creatinine (mg/dL)2 | 1.1 (0.9-1.5) | 1.5 (1.0-1.6) | 1.1 (0.9-1.5) |
| Bilirubin (mg/dL)2 | 0.4 (0.3-0.7) | 0.2 (0.2-0.5) | 0.4 (0.3-0.8) |
| SOFA2 | 2.0 (0-4.0) | 2.5 (1.8-4.2) | 2.0 (0-4.0) |
| ED Process of Care Variables | |||
| Length of Stay (hours)2 | 5.1 (3.3-7.0) | 4.1 (3.0-5.8) | 5.2 (3.3-7.0) |
| Vasopressor Infusion, n (%) | 86 (23) | 3 (30) | 83 (22) |
AWP = awareness with paralysis, BMI = body mass index, ESRD = end-stage renal disease, COPD = chronic obstructive pulmonary disease, SOFA = Sequential Organ Failure Assessment
Psychiatric if diagnosed with schizophrenia, bipolar, major depression, or generalized anxiety disorder
Continuous variables are reported as mean (standard deviation)
Continuous variables are reported as median (interquartile range)
Main Results
There were 383 patients included in the study. Seven percent (27/383) reported memories of wakeful paralysis and were assessed for AWP. Adjudicators of AWP events had high agreement (intraclass correlation coefficient, 0.72; 95% CI 0.55 to 0.85). After adjudication, the prevalence of possible or definite AWP was 2.6% (10/383; 95% CI, 1.3-4.7%). Clinical summaries, analgosedation data, and adjudication information for the 10 patients with possible or definite AWP are presented in Table 2. The summaries for all 27 patients reporting wakeful paralysis is available in Table E1. A description of analgosedation practices in the ED (RSI and post-intubation sedation) is presented in Table 3. There was no documented NMBA use for thirty-eight (9.9%) patients. The prevalence of possible or definite AWP among patients with documented NMBA exposure was 2.9% (10/345; 95% CI, 1.4-5.3%). Exposure to rocuronium at any time point in the ED (i.e. combining RSI and post-intubation) was significantly different between patients who experienced AWP (70%) versus the rest of the cohort (31.4%) (odds ratio, 5.1; 95% confidence interval [CI], 1.30 to 20.1).
Table 2.
Patients reporting wakeful paralysis and adjudicated to have possible or definite awareness with paralysis.
| ID | Gender, Age (yr) (Weight) | Drugs for Intubation | Post-Intubation Analgosedation | Clinical Scenario | Reported Memory / Awareness Experience | Determination of Awareness (definite, possible, no) | |
|---|---|---|---|---|---|---|---|
| 3 Expert Reviewers | Overall | ||||||
| 1 | M, 67 (62 kg) | Ketamine 100 mg Rocuronium 60 mg |
Fentanyl infusion | Brought to ED for dyspnea, failed NIPPV | - No memory of anything in between losing consciousness and waking up. - Answered ‘yes’ to question regarding a sensation of feeling paralyzed while mechanically ventilated. Remembered feeling scared, tried to open eyes and move, but couldn’t. Patient had no further details regarding this memory. |
Possible Possible Possible |
Possible |
| 3 | F, 65 (110 kg) | Etomidate 30 mg Rocuronium 100 mg |
Propofol infusion | Altered mental status, DKA, and possible seizure | - Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like she was paralyzed. - Stated she felt as though she woke up once in the ED but couldn’t move anything except for maybe her fingers. She felt she could open her eyes and saw lights but could not move the rest of her body. Reported that she remembered voices and specific conversations, and remembered her bed being pushed/transported. Believed her event took place in ED because she remembered a lot of commotion occurring when she had her moment of awareness, and that she had significant pain. Patient stated that then after a short time everything went dark again. |
Definite Definite Possible |
Definite |
| 4 | M, 72 (60 kg) | Ketamine 100 mg Succinylcholine 60 mg |
Fentanyl, propofol, and midazolam infusions One dose ketamine (80 mg) and etomidate (10 mg) after intubation |
Brought to ED for dyspnea, failed NIPPV | - No memory of anything in between losing consciousness and waking up. - Patient did not remember anything after losing consciousness but described procedural awareness of intubation (“I remember the breathing tube going in”). Stated “I had a mask on my face”, then he was given medications. Before he went to sleep, he remembered “something opening my mouth up and the tube going in my mouth and down my throat”. - Stated that this experience was his worst memory of his entire period of mechanical ventilation. |
Possible Possible Definite |
Possible |
| 5 | M, 64 (84 kg) | Ketamine 80 mg Rocuronium 70 mg |
Fentanyl, propofol infusions One dose ketamine (10 mg) after intubation |
Angioedema-fiberoptic nasotracheal intubation with multiple attempts | - No memory of anything in between losing consciousness and waking up. Reported last memory was of recall of the procedure of intubation. - When asked if he ever felt the sensation of feeling paralyzed, he answered “yes”. Stated “I came to the ER because my tongue was swollen. I remember them putting the breathing tube down, but I could not move. I remember the breathing tube actually going in and being panicked. It was terrible and traumatic. I was panicking inside. Then I went to sleep.” - Stated his worst memory was “being paralyzed and remembering it.” |
Definite Definite Definite |
Definite |
| 11 | M, 37 (68 kg) | Etomidate 20 mg Succinylcholine 200 mg |
Propofol boluses, then infusion. Given 20 mg vecuronium after intubation |
Fall from a roof and sustained bilateral lower extremity fractures, including an open distal tibia fracture/dislocation. Intubated in ED of transferring facility. |
- Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like he was paralyzed. - He remembers waking up with someone pulling very hard on his injured leg, which caused severe pain. He thinks he was in the emergency room. The patient said this was the worst pain he has ever had and was unbearable, and said he felt “scarred” by going through such intense pain. Reported that he tried to move but could not. He remembers hearing alarms, hearing and seeing 3-4 people standing around his bed and one person pulling hard on his injured leg. - Records note that patient’s open fracture/dislocation was reduced in the ED after intubation and prior to transfer; reported “spike in blood pressure” during this event. |
Possible Definite Definite |
Definite |
| 18 | F, 67 (114 kg) | Etomidate 20 mg Succinylcholine 100 mg |
Fentanyl and propofol infusions | Fell and had open tib/fib fracture with extensive blood loss | - No memory of anything in between losing consciousness and waking up. Reported last memory before unconsciousness was being in the ED, asking for pain medication. - Answered ‘yes’ to question regarding a sensation of feeling paralyzed while mechanically ventilated. - Stated that she remembered the physicians telling her they need to put in a breathing tube, and they began to give her medication through the IV. For “about a minute” she experienced paralysis where she “couldn’t move anything, not even my eyes.” She said that then she passed out. |
Possible No Possible |
Possible |
| 20 | F, 57 (80 kg) | Ketamine 100 mg Rocuronium 100 mg |
Propofol infusion | Inhalation injury after house fire. Intubated in ED, then bronchoscopy performed. |
- Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like she was paralyzed. - Said she remembered, “coming into the ER after the fire and my throat hurt. They said they were worried about my breathing, so they needed to put a breathing tube in.” Said that when she woke up, she couldn’t move but could hear people talking about “putting a camera down to look in my lungs”. She felt a lot of pain in the back of her throat and inside her chest from something going down the tube. “When I woke up, it felt like the same room and I heard the same voices. I felt that pain inside my chest before I went to sleep again.” - Said her worst memory was waking up and not being able to move and feeling the pain of endotracheal tube being suctioned. |
Definite Definite Possible |
Definite |
| 21 | M, 41 (92 kg) | Ketamine 100 mg Succinylcholine 100 mg |
Fentanyl and midazolam infusions; one bolus dose of rocuronium 100 mg (“patient biting tube”) | Pedestrian struck by car; open tibia fracture was reduced, splinted and placed in traction after intubation | - Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like he was paralyzed. - Patient states he was in the emergency department and heard a female nurse say, “we are trying to bring you back, just stay calm.” He remembers laying on the bed and feeling like he was in a dream, but he knew that this ‘dream’ was actually happening in real life. He stated he felt out of his own body and couldn’t move. He stated he couldn’t even open his eyes or breathe on his own. He states he saw himself on the bed almost like he was outside his own body. He said he had a breathing tube in his throat and was trying to move and talk but couldn’t. - He stated the worst part about having the breathing tube and being aware in the ED was: “I felt like if I stopped breathing, I would die right there on the bed, and it would be all over.” |
Definite Definite Possible |
Definite |
| 23 | M, 67 (96 kg) | Etomidate 20 mg Succinylcholine 100 mg |
Fentanyl and propofol infusions | Altered mental status and severe cervical stenosis | - Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like he was paralyzed. - Patient stated he remembers being in a hospital room, he thinks it was the emergency department, and the breathing tube was being inserted into his throat while he was awake. The patient remembers he didn’t think he could move. However, the patient stated that he was able to open his eyes and look around at people and felt like he was able to turn his head “a little bit.” |
Definite Definite Possible |
Definite |
| 27 | F, 65 (68 kg) | Etomidate 20 mg Rocuronium 90 mg |
Fentanyl and propofol infusions | Altered with COPD, hypercapnia and hypoxia, failed NIPPV | - Reported remembering things in between losing consciousness and waking up and reported a sensation of not being able to move, like she was paralyzed. - “I was in the ER and I had a mask blowing air into my mouth to help me breath. I remember the doctors telling me that I would need to be put on the breathing machine. When I woke up, I was lying flat and I could hear everybody’s voices around me. I tried to move and breath but could not and it was terrifying. I heard people in the room talking and I remember seeing the curtains and the lights in the room. I don’t know how long this lasted but it felt like forever. Then I went to sleep again and the next thing I remember was waking up in the room in the ICU.” - States her worst memory was waking up and not being able to move. |
Definite Definite Definite |
Definite |
M = male, F = female, ED = emergency department, NIPPV = non-invasive positive pressure ventilation, DKA = diabetic ketoacidosis, COPD = chronic obstructive pulmonary disease
Table 3.
Emergency department analgosedation variables.
| Variable | Patients with AWP (n = 10) |
Patients without AWP (n = 373) |
Between-Group Difference (95% CI) |
|---|---|---|---|
| RSI Variables | |||
| Induction Agent | |||
| Etomidate, n (%) | 5 (50) | 173 (46.4) | 3.62 (−27.8 to 35.0) |
| Dose (mg) | 20 (20-25) | 20 (20-20) | 0.60 (−4.63 to 5.82) |
| Weight-Based Dose (mg/kg) | 0.27 (0.19-0.29) | 0.28 (0.24-0.34) | 0.05 (−0.3 to 0.13) |
| Ketamine, n (%) | 5 (50) | 126 (33.8) | 16.2 (−15.1 to 47.6) |
| Dose (mg) | 100 (90-100) | 100 (100-150) | 38.1 (23.9 - 52.3) |
| Weight-Based Dose (mg/kg) | 1.25 (1.02-1.64) | 1.43 (1.10-1.97) | 0.36 (−0.37 to 1.08) |
| Midazolam, n (%) | 0 | 10 (2.7) | −2.68 (−4.32 to −1.04) |
| Dose (mg) | - | 8.9 (5.1) | - |
| Weight-Based Dose (mg/kg) | - | 0.11 (0.06) | - |
| Propofol, n (%) | 0 | 10 (2.7) | −2.68 (−4.32 to −1.04) |
| Dose (mg) | - | 129 (52) | - |
| Weight-Based Dose (mg/kg) | - | 1.47 (0.78) | - |
| None, n (%) | 0 | 21 (5.6) | −5.63 (−7.97 to −3.29) |
| Paralytic | |||
| Succinylcholine, n (%) | 5 (50) | 196 (52.5) | −2.55 (−34.0 to 28.9) |
| Dose (mg) | 100 (100-160) | 100 (100-100) | −21.9 (−75.7 to 31.8) |
| Weight-Based Dose (mg/kg) | 1.08 (0.96-2.47) | 1.23 (1.06-1.52) | −0.27 (−1.35 to 0.82) |
| Rocuronium, n (%) | 5 (50) | 102 (27.3) | 22.7 (−8.7 to 54.0) |
| Dose (mg) | 90 (65-100) | 100 (80-100) | 10.9 (−15.5 to 37.3) |
| Weight-Based Dose (mg/kg) | 1.05 (0.22) | 1.17 (0.33) | 0.12 (−0.18 to 0.42) |
| None, n (%) | 0 | 39 (10.5) | −10.5 (−13.6 to −7.35) |
| ED Post-Intubation Variables | |||
| Fentanyl, n (%) | 8 (80) | 294 (78.8) | 1.18 (−24.0 to 26.3) |
| Cumulative Dose (mcg) | 250 (138-300) | 288 (150-500) | 115 (−118 to 348) |
| Weight-Based Dose (mcg/kg) | 2.8 (1.8-4.4) | 3.4 (2.0-6.4) | 1.64 (−1.39 to 4.67) |
| Propofol, n (%) | 8 (80) | 267 (71.6) | 8.42 (−16.8 to 33.6) |
| Cumulative Dose (mg) | 660 (290-1340) | 510 (290-980) | −391 (−1382 to 600) |
| Weight-Based Dose (mg/kg) | 7.5 (3.8-11.9) | 6.6 (3.6-12.0) | −5.6 (−19.3 to 80.0) |
| Midazolam, n (%) | 2 (20) | 128 (34.3) | −14.3 (−39.6 to 10.9) |
| Cumulative Dose (mg) | 6 (6-6) | 5 (4-10) | 1.4 (−6.9 to 9.7) |
| Weight-Based Dose (mg/kg) | 0.08 | 0.07 (0.04-0.13) | 0.01 (−0.09 to 0.11) |
| Lorazepam, n (%) | 0 | 46 (12.3) | −12.3 (−15.7 to −9.0) |
| Cumulative Dose (mg) | - | 2 (2-4) | - |
| Weight-Based Dose (mg/kg) | - | 0.03 (0.02-0.06) | - |
| Ketamine, n (%) | 3 (30) | 57 (15.3) | 14.7 (−13.9 to 43.4) |
| Cumulative Dose (mg) | 70 | 100 (60-118) | 54 (−42 to 150) |
| Weight-Based Dose (mg/kg) | 0.83 (0.63) | 1.41 (0.88) | 0.58 (−0.45 to 1.61) |
| Rocuroniuma, n (%) | 2 (20) | 22 (5.9) | 14.1 (−10.8 to 39.0) |
| Cumulative Dose (mg) | 100 (100-100) | 100 (50-100) | −11 (−103 to 81) |
| Weight-Based Dose (mg/kg) | 1.09 | 1.11 (0.47) | - |
| Sedation Depth Variables | |||
| Median RASS in ED | −1.5 (−2.3 to 1.3) | −1.7 (−3 to 0) | −0.8 (−2.1 to 0.5) |
| Deep Sedation, n (%) | 2 (20) | 146 (39) | −19.1 (−44.4 to 6.14) |
AWP = awareness with paralysis, CI = confidence interval, RSI = rapid sequence intubation, ED = emergency department, RASS = Richmond Agitation-Sedation Scale
Refers to paralytic given as additional bolus after RSI
Continuous variables are reported as mean (standard deviation) and median (interquartile range).
Patients experiencing AWP had higher mean (standard deviation) values on the threat perception scale, denoting a higher degree of perceived threat, as compared to patients that did not experience AWP [13.4 (7.7) vs. 8.5 (6.2), mean difference 4.9; 95% CI 0.94 to 8.8].
LIMITATIONS
This study has several limitations. While it is the largest non-OR study to date focusing on AWP, the overall sample is small and derived from a single center. Therefore, all results from this observational single-center cohort study with ten events for the outcome of interest are exploratory and hypothesis-generating only. Our design also limits generalizability to other centers and could lead to an overestimation of the true event rate for AWP. While our rigorous methodology in adjudicating AWP and similar prevalence as a recent multi-center ICU-based trial enhance face validity of our results,53 larger, multicenter studies from the ED are needed. Large, prospective multicenter cohort studies would provide a higher number of AWP cases, which could provide more reliable estimates of ED-based factors associated with AWP, and allow for the conduct of interventional trials going forward. There is also some subjectivity in the assessment of AWP and interpretation of our results should take into account the fact that unmeasured variables (e.g. inducing false memories) could confound responses given by participants. However, we are encouraged by the fact that good agreement existed between the independent reviewers and a fourth reviewer was never needed during the adjudication process. Further, the objective demonstration of higher perceived threat suggests the patients’ experiences of AWP were indeed real. Patients with definite and possible AWP were combined in the assessment of the total event rate. This approach has been done in major trials from the OR which demonstrated similar reports of distress among patients with definite versus possible awareness.41 However, this raises the possibility that our reported event rate is inflated. However, seven cases of definite AWP (1.8%) remains worrisome and meaningful. The exclusion of a large number of neurologically injured patients could have also inflated the event rate. However, even if all eligible patients were included as the denominator, the resulting prevalence of AWP (1.2%) is still factors higher than that seen in other domains, placing thousands of patients at risk annually. With respect to excluded patients, 9.1% (n= 41) of exclusions were due to attrition. As these patients were not administered the questionnaire or included in the analysis, we cannot be sure that their characterisitics, treatment, or possible event rate for AWP is not systematically different from our study population. The receipt of a NMBA was a requirement for consideration of an AWP event. Thirty-eight patients never received a NMBA, and exclusion of these patients would increase the event rate to 2.9%. We elected to use 383 as the denominator to err on the side of conservative estimates, and because our over-arching goal was to inform practicing clinicians regarding AWP across a full spectrum of patients requiring mechanical ventilation in the ED. Because AWP in the ED has not been rigorously examined before, our research methods are largely extrapolated from similar studies in the OR, e.g. the utilization of the modified Brice questionnaire.42 While these methods are the current standard for assessing AWP, the modified Brice questionnaire may not perform in the same manner for our cohort as when applied to post-surgical patients. We therefore made extensive efforts to separate memories from wakeful paralysis. We also did not serially assess patients for AWP, as some OR-based studies have done. 40,41 In preparing for this study, we did not feel this necessary because all patients would be interviewed typically after multiple days on the mechanical ventilator and days after exposure to neuromuscular blockers, which would encompass multiple interview periods from OR-based studies. Based on prior literature from the OR, had we interviewed at day 30, there is a chance that we could have uncovered more cases of AWP, which is a consideration for future studies.
DISCUSSION
AWP is a potentially devastating but largely preventable complication of mechanical ventilation that has only been well studied in the OR.5,6 Rigorous studies examining this complication have yet to be performed in the ED. Research on analgosedation practices for mechanically ventilated ED patients demonstrate a pattern of delayed intravenous sedation,14–22 frequent administration of longer-acting NMBAs,18,19,23 and an overall lack of protocolized sedation monitoring,18,19 all of which are known risk factors for AWP.3 To address this gap in the literature, we conducted a single-center, prospective, cohort study on mechanically ventilated ED patients to determine the prevalence of AWP and explore risk factors and adverse psychological effects related to this complication. There are several important findings.
First, the prevalence of AWP in our cohort was 2.6%, a figure substantially higher than that reported from the OR, and comparable to the prevalence reported from a recent ICU-based study regarding neuromuscular blockers in acute respiratory distress syndrome (1.8%).53 Clinical summaries demonstrate AWP events related to both endotracheal intubation and the postintubation phase of care, including vivid memories of painful procedures performed in the ED. While this event rate may seem low, when considering the shear volume of patients intubated in the ED, this could translate into more than 6,000 annual cases of AWP related to the ED.12,13 The estimated prevalence of AWP in the ED from four prior studies was substantially higher than our estimate, ranging from 6-50%.24–27 We believe these estimates were likely inflated secondary to: 1) methodological limitations, including non-validated questionnaires to assess for AWP, 2) small sample sizes (combined n = 123), and 3) inconsistent and non-standard definitions of AWP. To try and avoid these limitations, we used the modified Brice questionnaire, the preferred method of assessing for AWP and powered our study to detect a prevalence of 1-2%. Finally, we defined AWP specifically as recall of wakeful paralysis with record of administration of an NMBA. All clinical data and questionnaire responses were adjudicated independently by three experts to make all AWP determinations rigorous.
Second, exposure to rocuronium in the ED was significantly different between patients who experienced AWP versus the rest of the cohort. These findings are biologically plausible and congruent with prior work, as studies from the OR demonstrate that longer-acting NMBAs are an important risk factor for AWP.2,5,11 In this study, all patients with AWP events which appear temporally associated to the post-intubation phase of care had a longer-acting NMBA administered. The use of rocuronium in the ED has increased substantially in recent years, and prior work demonstrates that these paralyzed patients typically receive less analgesia and sedation, lower doses, and in a delayed fashion when compared to patients receiving succinylcholine.21–23 As sedation depth cannot reliably be monitored clinically during periods of neuromuscular blockade, our results suggest that clinicians should be cognizant that rocuronium use could increase patient-centered complications related to a vulnerable time period of care. However, until larger studies are conducted, we urge caution in interpreting these results and they should be viewed as exploratory and hypothesis-generating.
Our last significant finding concerns the psychological sequelae attributed to experiencing AWP. Historically, patients reporting AWP from the OR have been at risk for a number of adverse psychological conditions, most notably PTSD but also major depression and complex phobias.1–4 We found that in our cohort, patients experiencing AWP had a higher degree of perceived threat, as compared to patients that did not experience AWP. Perceived threat is defined as a measure of the patient’s perceived vulnerability during the hospital stay and after discharge, and the literature shows that perceived threat is common in critically-ill patients and is predictive of developing PTSD.49,54–56 While the subjective accounts provided by the patients demonstrate the negative consequences of AWP, elevated perceived threat also shows objectively an increased risk of adverse psychological effects, including PTSD. This underscores the importance of further studying AWP in the ED and instituting interventions to protect patients from this complication and the commensurate psychological sequelae that can result.
In conclusion, AWP had a prevalence of 2.6% in this cohort of mechanically ventilated ED patients and was associated with rocuronium exposure in the ED. Given the known consequences attributed to AWP, future studies are warranted in order to further quantify this complication in the ED population and explore targeted interventions to reduce the risk of AWP in this vulnerable cohort.
Supplementary Material
Acknowledgments
Financial Support
RDP received funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344, PI: Jay F Piccirillo, MD, Project Title: Washington University Institute of Clinical and Translational Sciences. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. WW was supported by the NIH T35 NHLBI Training Grant, Grant Number 5T35HL007815, PI: Koong-Nah Chung, PhD. BMF was supported by a grant-in-aid from the Division of Clinical and Translational Research of the Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings
A partial interim analysis of this work was accepted for presentation at the Society for Academic Emergency Medicine annual meeting in Denver, Colorado in May of 2020.
Conflicts of Interest
None.
REFERENCES
- 1.Cook TM, Andrade J, Bogod DG, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: patient experiences, human factors, sedation, consent, and medicolegal issues. British Journal of Anaesthesia. 2014;113(4):560–574. [DOI] [PubMed] [Google Scholar]
- 2.Pandit JJ, Andrade J, Bogod DG, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. British Journal of Anaesthesia. 2014;113(4):549–559. [DOI] [PubMed] [Google Scholar]
- 3.Avidan MS, Mashour GA. Prevention of intraoperative awareness with explicit recall: making sense of the evidence. Anesthesiology. 2013;118(2):449–456. [DOI] [PubMed] [Google Scholar]
- 4.Leslie K, Chan MTV, Myles PS, Forbes A, McCulloch TJ. Posttraumatic stress disorder in aware patients from the B-aware trial. Anesthesia and Analgesia. 2010;110(3):823–828. [DOI] [PubMed] [Google Scholar]
- 5.Sandin RH, Enlund G, Samuelsson P, Lennmarken C. Awareness during anaesthesia: a prospective case study. Lancet. 2000;355(9205):707–711. [DOI] [PubMed] [Google Scholar]
- 6.Sebel PS, Bowdle TA, Ghoneim MM, et al. The incidence of awareness during anesthesia: a multicenter United States study. Anesthesia and Analgesia. 2004;99(3):833–839. [DOI] [PubMed] [Google Scholar]
- 7.Errando CL, Sigl JC, Robles M, et al. Awareness with recall during general anaesthesia: a prospective observational evaluation of 4001 patients. British Journal of Anaesthesia. 2008;101(2): 178–185. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto Y, Nogami Y, Harada K, Tsubokawa T, Masui K. Awareness during anesthesia: the results of a questionnaire survey in Japan. Journal of Anesthesia. 2011;25(1):72–77. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Xu L, Ma YQ, et al. Bispectral index monitoring prevent awareness during total intravenous anesthesia: a prospective, randomized, double-blinded, multi-center controlled trial. Chinese Medical Journal. 2011;124(22):3664–3669. [PubMed] [Google Scholar]
- 10.Tasbihgou SR, Vogels MF, Absalom AR. Accidental awareness during general anaesthesia - a narrative review. Anaesthesia. 2018;73(1):112–122. [DOI] [PubMed] [Google Scholar]
- 11.Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesthesia and Analgesia. 2009;108(2):527–535. [DOI] [PubMed] [Google Scholar]
- 12.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Critical Care Medicine. 2010;38(10): 1947–1953. [DOI] [PubMed] [Google Scholar]
- 13.Easter BD, Fischer C, Fisher J. The use of mechanical ventilation in the ED. American Journal of Emergency Medicine. 2012;30(7): 1183–1188. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo JB, Butler AS, Lindsell CJ, Venkat A. Inadequate provision of postintubation anxiolysis and analgesia in the ED. American Journal of Emergency Medicine. 2008;26(4):469–472. [DOI] [PubMed] [Google Scholar]
- 15.Bhat R, Mazer-Amirshahi M, Sun C, et al. Accuracy of rapid sequence intubation medication dosing in obese patients intubated in the ED. American Journal of Emergency Medicine. 2016;34(12):2423–2425. [DOI] [PubMed] [Google Scholar]
- 16.Traylor B, Patanwala A, Sakles J, Erstad B. Under-dosing of etomidate for rapid sequence intubation in the emergency department. Current Drug Safety. 2013;8(4):253–256. [DOI] [PubMed] [Google Scholar]
- 17.Weingart GS, Carlson JN, Callaway CW, Frank R, Wang HE. Estimates of sedation in patients undergoing endotracheal intubation in US EDs. American Journal of Emergency Medicine. 2013;31(1):222–226. [DOI] [PubMed] [Google Scholar]
- 18.Stephens RJ, Ablordeppey E, Drewry AM, et al. Analgosedation practices and the impact of sedation depth on clinical outcomes among patients requiring mechanical ventilation in the ED: a cohort study. Chest. 2017;152(5):963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller BM, Roberts BW, Mohr NM, et al. The ED-SED study: a multicenter, prospective cohort study of practice patterns and clinical outcomes associated with Emergency Department SEDation for mechanically ventilated patients. Critical Care Medicine. 2019;47(11):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nengchu P Reducing the risk of inadequate sedation during rapid sequence intubation in the emergency department setting. Journal of the American Pharmacists Association. 2014;54(2):e217–e217. [Google Scholar]
- 21.Korinek JD, Thomas RM, Goddard LA, St John AE, Sakles JC, Patanwala AE. Comparison of rocuronium and succinylcholine on postintubation sedative and analgesic dosing in the emergency department. European Journal of Emergency Medicine. 2014;21(3):206–211. [DOI] [PubMed] [Google Scholar]
- 22.Watt JM, Amini A, Traylor BR, Amini R, Sakles JC, Patanwala AE. Effect of paralytic type on time to post-intubation sedative use in the emergency department. Emergency Medicine Journal. 2013;30(11):893–895. [DOI] [PubMed] [Google Scholar]
- 23.Brown CA, Bair AE, Pallin DJ, Walls RM. Techniques, success, and adverse events of emergency department adult intubations. Annals of Emergency Medicine. 2015;65(4):363–370.e1. [DOI] [PubMed] [Google Scholar]
- 24.Puller J, Juhasz K, Zerkle S, Woodley M, Lewandowski T, Kaminsky J. PRIER: patient recall in emergency rapid sequence intubation. Annals of Emergency Medicine. 2017;70(4):S11. [Google Scholar]
- 25.Miner JR, Friewald S, Haug E, Biros M. Bispectral electroencephalogram analysis of pharmacologically paralyzed patients in the emergency department. Annals of Emergency Medicine. 2004;43(2):293–294. [DOI] [PubMed] [Google Scholar]
- 26.Smith B, Bishop R. Incidence of recall of emergency intubation: a preliminary report. Emergency Medicine. 1998;10(3):223–225. [Google Scholar]
- 27.Kimball D, Kincaide RC, Ives C, Henderson S. Rapid sequence intubation from the patient’s perspective. Western Journal of Emergency Medicine. 2011;12(4):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Annals of Internal Medicine. 2007;147(8):573. [DOI] [PubMed] [Google Scholar]
- 29.Pappal RD, Roberts BW, Mohr NM, et al. Protocol for a prospective, observational cohort study of awareness in mechanically ventilated patients admitted from the emergency department: the ED-AWARENESS study. BMJ Open. 2019;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American Journal of Respiratory and Critical Care Medicine. 2012;186(8):724–731. [DOI] [PubMed] [Google Scholar]
- 33.Shehabi Y, Bellomo R, Kadiman S, et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality. Critical Care Medicine. 2018;46(6):850–859. [DOI] [PubMed] [Google Scholar]
- 34.Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Medicine. 2013;39(5):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finlay T, Parke T. Awareness in the emergency department: a patient’s story. Journal of the Intensive Care Society. 2016;17(2):175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan LJ, Bailey H. Bispectral index (BIS) monitoring of ICU patients on continuous infusion of sedatives and paralytics reduces sedative drug utilization and cost. Critical Care. 2000;4:P190. [Google Scholar]
- 37.Wagner BKJ, Zavotsky KE, Sweeney JB, Palmeri BA, Hammond JS. Patient recall of therapeutic paralysis in a surgical critical care unit. Pharmacotherapy. 1998;18(2 I):358–363. [PubMed] [Google Scholar]
- 38.Ballard N, Robley L, Barrett D, Fraser D, Mendoza I. Patients’ recollections of therapeutic paralysis in the intensive care unit. American Journal of Critical Care. 2006;15(1):86–94. [PubMed] [Google Scholar]
- 39.Johnson K, Cheung R, Johnson S, Roberts M, Niblett J, Manson D. Therapeutic paralysis of critically ill trauma patients: perceptions of patients and their family members. American Journal of Critical Care. 1999;8(1):490–498. [PubMed] [Google Scholar]
- 40.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. New England Journal of Medicine. 2008;358(11):1097–1108. [DOI] [PubMed] [Google Scholar]
- 41.Avidan MS, Jacobsohn E, Glick D, et al. Prevention of intraoperative awareness in a high-risk surgical population. New England Journal of Medicine. 2011;365(7):591–600. [DOI] [PubMed] [Google Scholar]
- 42.Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. British Journal of Anaesthesia. 1970;42(6):535–542. [DOI] [PubMed] [Google Scholar]
- 43.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Critical Care Medicine. 2001;29(3):573–580. [DOI] [PubMed] [Google Scholar]
- 44.Samuelson KAM, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients? a 2-month follow-up study. Acta Anaesthesiologica Scandinavica. 2007;51(6):671–678. [DOI] [PubMed] [Google Scholar]
- 45.Svenningsen H Associations between sedation, delirium and post-traumatic stress disorder and their impact on quality of life and memories following discharge from an intensive care unit. Danish Medical Journal. 2013;60(4):B4630. [PubMed] [Google Scholar]
- 46.Cornelius T, Agarwal S, Garcia O, Chaplin W, Edmondson D, Chang BP. Development and validation of a measure to assess patients’ threat perceptions in the emergency department. Academic Emergency Medicine. 2018;25(10):1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moss J, Roberts MB, Shea L, et al. Association between perceived threat and the development of posttraumatic stress disorder symptoms in patients with life-threatening medical emergencies. Academic Emergency Medicine. 2020;27(2):109–116. [DOI] [PubMed] [Google Scholar]
- 48.Heir T, Blix I, Knatten CK. Thinking that one’s life was in danger: perceived life threat in individuals directly or indirectly exposed to terror. British Journal of Psychiatry. 2016;209(4):306–310. [DOI] [PubMed] [Google Scholar]
- 49.Meli L, Kautz M, Julian J, Edmondson D, Sumner JA. The role of perceived threat during emergency department cardiac evaluation and the age-posttraumatic stress disorder link. Journal of Behavioral Medicine. 2018;41(3):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuller BM, Ferguson IT, Mohr NM, et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Annals of Emergency Medicine. 2017;70(3):406–418.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuller BM, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: An observational study. Academic Emergency Medicine. 2013;20(7):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuller BM, Mohr NM, Miller CN, et al. Mechanical ventilation and ARDS in the ED: a multicenter, observational, prospective, cross-sectional study. Chest. 2015;148(2):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. New England Journal of Medicine. 2019;380(21):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiedemar L, Schmid J-P, Müller J, et al. Prevalence and predictors of posttraumatic stress disorder in patients with acute myocardial infarction. Heart & Lung. 2008;37(2):113–121. [DOI] [PubMed] [Google Scholar]
- 55.Davydow DS, Zatzick Douglas, Hough CL, Katon WJ. A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. General Hospital Psychiatry. 2013;35(3):226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moss J, Roberts MB, Shea L, et al. Healthcare provider compassion is associated with lower PTSD symptoms among patients with life-threatening medical emergencies: a prospective cohort study. Intensive Care Medicine. 2019;45(6):815–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


