Abstract
2-Phenethyl isothiocyanate (PEITC) is a natural product found as a conjugate in cruciferous vegetables. It has been reported to have preventative properties against lung cancer and to inhibit metabolic activation of tobacco carcinogens. In the present study, we evaluated the ability of PEITC to influence the metabolism of the human carcinogen 1,3-butadiene in current smokers in a phase II clinical trial with a crossover design. Urinary mercapturic acids of 1,3-butadiene were quantified at baseline and during PEITC treatment. Seventy-nine smokers were randomly assigned to one of two arms: PEITC followed by placebo, or placebo followed by PEITC. During the 1-week treatment period, each subject took PEITC (10 mg in 1 mL of olive oil, 4 times per day). There was a 1-week washout period between the PEITC and placebo periods. Oral ingestion of PEITC increased urinary levels of BD-mercapturic acids (MHBMA and DHBMA) by 11.1% and 3.7%, respectively, but these increases were not statistically significant (p = 0.17 and 0.64, respectively). A much stronger effect was observed among subjects with the null genotype of both GSTM1 and GSTT1: in these individuals, PEITC increased urinary levels of MHBMA by 58.7% (p = 0.004) and 90.0% (p = 0.001), respectively, but did not have a significant effect on urinary DHBMA. These results reveal a potentially protective effect of PEITC treatment with respect to the detoxification of 1,3-butadiene in cigarette smokers, specifically in those null for GSTT1, and provide further evidence in support of stronger chemopreventive effects from consumption of dietary isothiocyanates in these individuals.
Keywords: 2-Phenethyl isothiocyanate; 1,3-butadiene; glutathione-S-transferase; lung cancer; chemoprevention; clinical trial
Introduction
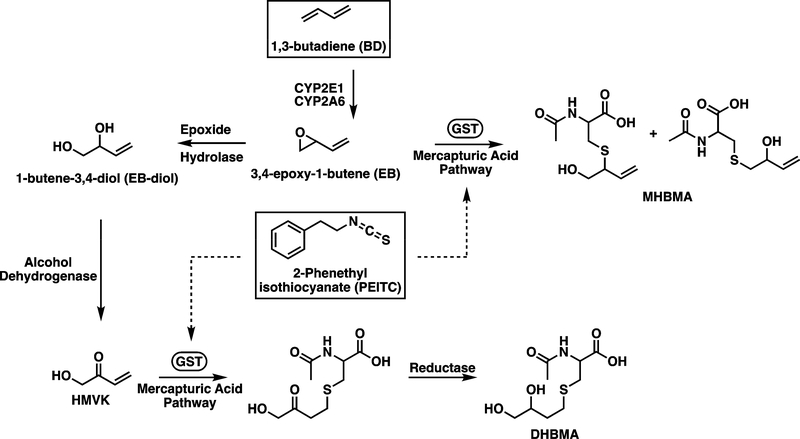
Isothiocyanates are a class of naturally occurring compounds found in fruits and vegetables that have been shown to have chemopreventive effects through multiple mechanisms of action (1,2). One such compound, 2-phenethyl isothiocyanate (PEITC, Figure 1), found in watercress, garden cress, radishes, and turnips (3), modulates multiple cancer-associated pathways, such as cell cycle arrest (4–8), NFκB (9,10), and apoptosis (8,11). PEITC can inhibit cytochrome P450 monoxygenases (CYP450s) involved in metabolic activation of carcinogens (12) and induce detoxifying enzymes such as glutathione-S-transferases (GSTs) (13). More specifically, treatment with PEITC inhibited lung carcinogenesis in laboratory mice and rats exposed to 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK), a potent tobacco-specific carcinogen (14–16). PEITC ingestion by current smokers has also been shown to decrease the metabolic activation of NNK to DNA-reactive species (17).
Figure 1.
Schematic showing the metabolism of BD; dotted lines represent induction of GSTs by PEITC.
Although the link between smoking and lung cancer is well established, smoking remains the most preventable cause of death in the world (18). Worldwide, there are approximately 1.1 billion smokers (19), with 80% and 50% of lung cancer deaths attributed to tobacco use in men and women, respectively (20). Among the 40 million smokers in the United States, cigarette smoking causes 84% of total lung cancer deaths in men and 79% of all lung cancer deaths in women (18,19). While smoking cessation is the best method of reducing smoking related lung cancer deaths, inhibition of the carcinogenic and genotoxic effects of cigarette smoke remains an important alternative route in the fight against smoking related deaths. The use of chemopreventive compounds naturally occurring in various food sources could provide a widely available and relatively inexpensive method of cancer prevention.
Recently, the effects of PEITC on the metabolism of cigarette smoke carcinogens and toxicants including benzene, acrolein, and crotonaldehyde were investigated by measuring their respective mercapturic acids, detoxification products formed through glutathione (GSH) conjugation via glutathione-S-transferases (GSTs) (21). A statistically significant increase in urinary concentrations of mercapturic acids formed from benzene and acrolein was observed when smokers were given PEITC, while no such increase was seen in urinary concentrations of the respective mercapturic acid formed from crotonaldehyde in all smokers (21). The effects of PEITC were more substantial in individuals null for GSTs mu 1 and theta 1 (GSTM1 and GSTT1); for these subjects, PEITC treatment significantly increased urinary concentrations of the mercapturic acids of benzene, acrolein, and crotonaldehyde, indicating that use of isothiocyanates could provide enhanced protection against lung carcinogenesis in individuals lacking these genes (21).
In this study, we investigated the effects of PEITC consumption on the metabolism of 1,3-butadiene (BD) in smokers. BD (Figure 1) is among one of the most abundant carcinogens present in cigarette smoke likely to contribute to the etiology of lung cancer,(22) with concentrations of 20–75 μg and 205–360 μg per cigarette in mainstream and sidestream smoke, respectively (23,24). Besides tobacco smoke, humans are exposed to BD in occupational settings in the production of synthetic rubber and polymers (25). BD has been classified as a known human carcinogen by the National Toxicology Program (NTP) and as a Group 1 agent by the International Agency for the Research on Cancer (IARC) (26,27). Occupational exposure to BD is associated with the development of leukemia and lymphoma, (25,28–34). The ability of BD to modify DNA bases depends on cytochrome P450-mediated formation of reactive metabolites such as 3,4-epoxy-1-butene (EB) and hydroxymethylvinyl ketone (HMVK). EB and HMVK undergo detoxification through conjugation with glutathione, a reaction catalyzed by GSTs, to ultimately form the mercapturic acids 1- and 2-(N-acetyl-L-cysteine-S-yl)-1-hydroxybut-3-ene (MHBMA) and N-acetyl-S-(3,4-dihydroxybutyl)-L-cysteine (DHBMA), respectively (Figure 1) (35–38). GSTs play an important role in the detoxification of BD epoxides (39–42). Oral supplementation with PEITC has been shown to facilitate glutathione conjugation of benzene and acrolein in individuals with GSTT1 and GSTM1 gene deletion (17). However, the effects of PEITC on the metabolic detoxification of BD have not been previously investigated.
Here, we employed quantitative HPLC-ESI−-MS/MS methods previously developed in our laboratory (41,43) to measure urinary concentrations of BD-mercapturic acids MHBMA and DHBMA (Figure 1) (35), in smokers supplemented with PEITC. Oral ingestion of PEITC induced statistically significant increases in urinary MHBMA concentrations in smokers lacking the GSTT1 gene or both the GSTT1 and GSTM1 genes. These results are consistent with epidemiological studies revealing a stronger protection by dietary PEITC in individuals lacking both GSTT1 and GSTM1 (44).
Experimental
Materials
LC-MS grade H2O, methanol, and acetonitrile were acquired from Fisher Scientific (Pittsburgh, PA). All other chemicals and solvents were obtained from Sigma-Aldrich (St. Louis, MO). MHBMA, DHBMA, 2H6-MHBMA, and 2H7-DHBMA were purchased from Toronto Research Chemicals (Toronto, Canada). Oasis HLB 96 well plates were procured from Waters Corp. (Milford, MA).
Study Design
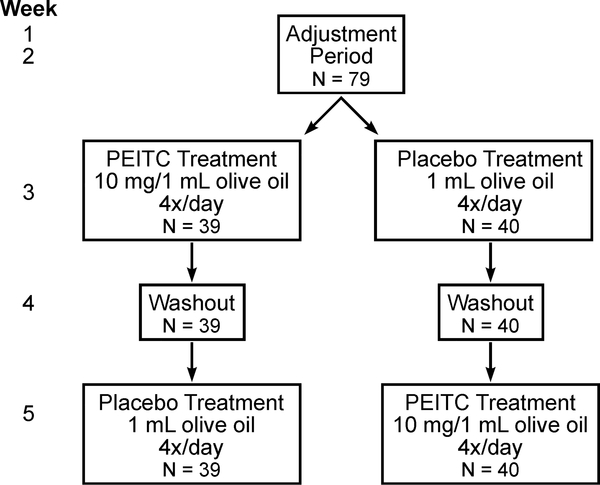
The study was a randomized, placebo-controlled, double-blind, phase II clinical trial with a crossover study design (Figure 2). Details of the study design can be found in prior publications (17,21). Over a duration of five weeks, qualified participants were asked to smoke cigarettes containing [pyridine-D4]NNK to allow for measurement of NNK metabolism. After an adjustment period of two weeks, individuals were placed into one of two treatment groups: those receiving PEITC before receiving a placebo or those receiving a placebo before receiving PEITC. PEITC (10 mg in 1 mL olive oil) was administered 4 times per day every 4 hours for 5 days during weeks three or five; the placebo (1 mL olive oil) was administered on the same schedule, either during weeks three or five. Week four consisted of a washout period, where participants did not receive PEITC or the placebo. This study was approved by the Institutional Review Boards of the University of Minnesota (0712M22651) and the University of Pittsburgh (PRO11110669). Patient studies were conducted in accordance with U.S. Common Rule ethical guidelines, and informed written consent was obtained from all subjects.
Figure 2.
Flow Diagram Outlining Study Design
Twenty-four hour urine samples were collected at the end of weeks two and four and on days three, four, and five of weeks three and five. For the purposes of this investigation, urine samples used for analysis were from weeks two and four and day five of weeks three and five. Total nicotine equivalents and total NNAL were quantified using high-throughput liquid chromatography-MS/MS assays described previously (37). Blood and buccal cell samples were collected at the end of each week. DNA from blood lymphocytes collected during week one was used to genotype GSTM1 and GSTT1. Details regarding genotyping methods can be found in prior publications (17,21,45).
HPLC-ESI−-MS/MS Analysis of Urinary MHBMA and DHBMA
Urinary concentrations of BD-mercapturic acids (MHBMA and DHBMA) were determined by isotope dilution HPLC-ESI−-MS/MS as described in our earlier publications (41–43). The method’s limits of detection (LOD) were 0.2 ng/mL urine and 5 ng/mL urine for MHBMA and DHBMA, respectively. One sample was discarded for having DHBMA values below the LOD of the method. Quality control (QC) samples were included three times per batch, fifteen times total, for the purposes of quality control and to account for any inter-batch variation. The mean coefficient of variation for these replicates was 11.0% and 11.8% for MHBMA and DHBMA, respectively.
Statistical Analyses
Urinary MHBMA and DHBMA concentrations were adjusted to creatinine by dividing each value by the appropriate creatinine value and by batch. The average value of the creatinine-adjusted outcomes of each batch were taken to get a1, …, a5 and calculated . To adjust for those in batch one, each MHBMA and DHBMA value was multiplied by and divided by a1. Similar processes were done for subjects in batches two through five. Therefore, final outcomes were defined as below:
These outcomes were log-transformed and back-transformed and presented as geometric means. In addition to adjusting for creatinine and batch, the models were adjusted by log-transformed creatinine-adjusted TNE (total nicotine equivalents). Eight subjects were removed from all analyses due to missing outcomes at baseline. One subject was removed from all analyses because the urinary DHBMA concentration was below the LOD of the method.
Baseline demographics and urinary biomarkers were summarized using means and standard deviations for continuous variables, frequencies and percentages for categorical variables, and geometric means and 95% confidence intervals for urinary biomarkers (Table 1). To determine if there were associations between variables at baseline and treatment sequence, Chi-square or Fisher’s exact tests were used for categorical variables, when appropriate, and Student’s t-tests were used for continuous variables.
Table 1.
Summary of study demographics and urinary biomarkers at baseline.
| Treatment Sequence Assignment | |||
|---|---|---|---|
| Characteristics or Biomarkers | PEITC-Placebo | Placebo-PEITC | P-valuea |
| Number of subjectsb | 39 | 40 | |
| Age (years), mean (SD) | 41.6 (10.5) | 40.8 (9.6) | 0.74 |
| Body mass index (kg/m2), mean (SD)d | 28.2 (4.8) | 28.2 (6.3) | 0.97 |
| Gender, n (%) | 0.31 | ||
| Male | 23 (59.0) | 19 (47.5) | |
| Female | 16 (41.0) | 21 (52.5) | |
| Race, n (%) | >0.99 | ||
| Black | 8 (20.5) | 9 (22.5) | |
| White | 26 (66.7) | 27 (67.5) | |
| Other | 5 (12.8) | 4 (10.0) | |
| Level of education, n (%) | 0.20 | ||
| High school or lower | 13 (33.3) | 19 (47.5) | |
| College or higher | 26 (66.7) | 21 (52.5) | |
| Cigarettes per day, mean (SD)e | 22.2 (9.4) | 21.1 (7.1) | 0.57 |
| Alcohol drinking, n (%)d | 0.69 | ||
| Never | 13 (34.2) | 17 (43.6) | |
| Monthly or less | 13 (34.2) | 12 (30.8) | |
| Weekly | 12 (31.6) | 10 (25.6) | |
| GSTM1 and GSTT1 genotypes, n (%) | 0.67 | ||
| Present and present | 19 (48.7) | 18 (45.0) | |
| Present and null | 3 (7.7) | 3 (7.5) | |
| Null and present | 13 (33.3) | 11 (27.5) | |
| Null and null | 4 (10.3) | 8 (20.0) | |
| Urinary biomarkersc, geometric mean (95% CI) | |||
| Total nicotine equivalents (TNE, nmol/mg Cr) | 45.7 (38.1, 54.8) | 59.3 (48.9, 71.8) | 0.05 |
| MHBMA (ng/mg Cr) | 15.2 (10.5, 22.1) | 13.4 (9.9, 18.3) | 0.60 |
| DHBMA (ng/mg Cr) | 753.3 (601.2, 943.9) | 642.7 (442.2, 934.1) | 0.46 |
P-value is for Student’s t-test for continuous variables and Chi-Square test or Fisher’s exact test for categorical variables.
There were 88 subjects, but nine were removed from all analyses: eight due to missing outcomes at baseline, and one due to values of DHBMA and LOD.
Urinary biomarkers are adjusted for creatinine and log-transformed. MHBMA and DHBMA were also adjusted for batch.
Two subjects were excluded from this analysis due to missing data.
Five subjects were excluded from this analysis due to missing data.
To determine whether there was an effect of PEITC on the urinary MHBMA or DHBMA concentrations, linear mixed models with random effects that also take into account period and sequence effects were used. Similar models were used to determine if there was an effect of PEITC treatment when stratified by GST genotype. An interaction term between treatment and GST genotype was also included to investigate if GST genotype modifies the effect of PEITC on the urinary MHBMA or DHBMA concentrations. Due to log-transformation of the outcomes, geometric means and percentage change are presented.
To investigate the relationship between GST genotype and the outcomes at baseline, a linear regression model was used with a covariate for GST genotype, and adjustment for creatinine-adjusted TNE. All reported p-values are two-sided and a significance level of 0.05 was used. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Characteristics of the study participants are detailed in Table 1. Between the two randomly assigned treatment sequences, e.g. PEITC-Placebo and Placebo-PEITC, there was no significant difference in age, body mass index (BMI), gender, race, level of education, amount of cigarettes smoked per day (CPD), or alcohol consumption. Of the 79 total participants, 53% were men, 67% were white, 22% were black, and the overall mean age was 41.2 years, with an average CPD smoked of 21.6. There were 7.6% of study participants with the GSTT1 null genotype, 30.4% with the GSTM1 null genotype, and 15.2% with the double null genotype. Overall, baseline levels of urinary MHBMA and DHBMA were not statistically significantly different between the two treatment groups (Table 1); there was a nearly significant difference between the two sequences for TNE (p = 0.05), which was still adjusted for in the later analyses.
We next examined the effects of PEITC treatment on urinary MHBMA and DHBMA in smokers. Comparison of PEITC treatment effect versus that of the placebo on urinary concentrations of MHBMA and DHBMA is summarized in Table 2. When compared to the placebo, urinary concentrations of both MHBMA and DHBMA increased with PEITC treatment (12.27 vs. 11.04 ng MHBMA/mg Cr and 593.81 vs. 572.91 ng DHBMA/mg Cr), but this increase was not statistically significant (p = 0.17 and 0.64, respectively).
Table 2.
Urinary MHBMA and DHBMA concentrations in smokers treated with PEITC and placebo.
| Geometric means | ||||
|---|---|---|---|---|
| Urinary biomarkersa | Placebo | PEITC | % Difference (95% CI) | P-valueb |
| MHBMA (ng/mg Cr) | 11.0 | 12.3 | 11.13 (−4.2, 28.9) | 0.17 |
| DHBMA (ng/mg Cr) | 572.9 | 593.8 | 3.7 (−10.9, 20.6) | 0.64 |
Urinary biomarkers were adjusted for creatinine and batch, and log-transformed.
Two-sided p-values were from mixed models that test the PEITC treatment effect, after adjusting for creatinine-adjusted TNE
A much stronger effect was observed when data was stratified by GSTM1 and GSTT1 genotype (Table 3). In participants null for GSTT1, PEITC increased urinary MHBMA levels by 58.7% as compared to the placebo (6.93 vs. 4.37 ng/mg Cr; p = 0.004). In participants null for both GSTT1 and GSTM1, PEITC treatment increased urinary MHBMA levels by 90.0% as compared to the placebo (5.0 vs. 2.6 ng/mg Cr; p = 0.001). PEITC treatment also resulted in a 19.5% increase of urinary MHBMA in individuals lacking GSTM1, although these differences were not statistically significant (p = 0.12). GSTT1 and GSTM1 genotype did not significantly influence PEITC treatment effect on urinary DHBMA, with the interaction terms for GSTT1, GSTM1, and both GSTT1/GSTM1 with treatment group being p = 0.67, 0.82, and 0.33, respectively (Table 3). Overall, these results indicate that oral ingestion of PEITC by smokers shifts BD metabolism towards detoxification via conjugation with glutathione, with more pronounced relative effects observed in individuals with null genotypes of GSTT1 alone or both GSTT1 and GSTTM1.
Table 3.
Effect of PEITC compared to placebo on urinary MHBMA and DHBMA, stratified by GST genotype.
| GST genotype | Na | Geometric mean | % Difference (95% CI) | P-valueb | P-valuec interaction | |
|---|---|---|---|---|---|---|
| Placebo | PEITC | |||||
| MHBMA (ng/mg Cr)d | ||||||
| GSTT1 | ||||||
| Null | 18 | 4.4 | 6.9 | 58.7 (17.1, 115.0) | 0.004 | 0.01 |
| Present | 61 | 14.5 | 14.4 | −0.4 (−15.24, 17.1) | 0.96 | |
| GSTM1 | ||||||
| Null | 36 | 11.4 | 13.6 | 19.5 (−4.4, 49.4) | 0.12 | 0.40 |
| Present | 43 | 10.7 | 11.3 | 5.2 (−13.7, 28.2) | 0.62 | |
| GSTT1 & GSTM1 | ||||||
| Both null | 12 | 2.6 | 5.0 | 90.0 (30.3, 176.9) | 0.001 | 0.01 |
| One present | 30 | 19.3 | 19.1 | −1.3 (−21.6, 24.2) | 0.91 | |
| Both present | 37 | 11.0 | 11.3 | 2.7 (−16.4, 26.0) | 0.80 | |
| DHBMA (ng/mg Cr)d | ||||||
| GSTT1 | ||||||
| Null | 18 | 713.1 | 696.2 | −2.4 (−29.3, 34.8) | 0.88 | 0.67 |
| Present | 61 | 536.6 | 567.9 | 5.8 (−10.9, 25.7) | 0.52 | |
| GSTM1 | ||||||
| Null | 36 | 596.6 | 631.4 | 5.8 (−15.8, 32.9) | 0.63 | 0.82 |
| Present | 43 | 553.1 | 565.1 | 2.2 (−16.7, 25.3) | 0.84 | |
| GSTT1 & GSTM1 | ||||||
| Both null | 12 | 716.7 | 613.7 | −14.4 (−42.5, 27.5) | 0.45 | 0.33 |
| One present | 30 | 580.2 | 688.9 | 18.7 (−6.9, 51.5) | 0.17 | |
| Both present | 37 | 528.0 | 523.1 | −0.9 (−20.3, 23.2) | 0.93 | |
Value of N varies based on missing outcomes or adjusting variables.
Two-sided p-values were from the mixed models that test PEITC effect on the change of MHBMA and DHBMA within each specific GST genotypes before and after PEITC intake, after adjusting for creatinine-adjusted TNE.
Two sided p-values were from the mixed models that test the interaction term between PEITC intake and GST genotype on the levels of MHBMA and DHBMA, after adjusting for creatinine-adjusted TNE.
Urinary biomarkers were adjusted for creatinine and batch, and log-transformed.
A weaker effect was observed for DHBMA, with limited influence of PEITC treatment on urinary metabolite concentrations (Table 3). Previous studies conducted in several laboratories indicate that DHBMA is only weakly associated with smoking and may have an endogenous source (37,43). Furthermore, urinary DHBMA levels were not affected by genetic polymorphisms in GSTT1 or other xenobiotic metabolism genes (41,42). Based on these observations, MHBMA appears to be a better biomarker of inter-individual differences in butadiene metabolism, despite its lower concentrations in human urine (37).
In untreated individuals at week two baseline, smokers with at least one copy of GSTT1 excreted 219.5% more MHBMA than in those with null GSTT1 genotype (p = 0.01, Table 4). In contrast, GSTM1 genotype did not have a significant effect on urinary MHBMA concentrations in smokers. These data support results from our previous publications showing the influence of GSTT1 genotype on BD metabolism to MHBMA (41–43). Furthermore, neither GSTT1 nor GSTM1 genotype appeared to have a significant effect on baseline levels of urinary DHBMA (p = 0.22 and 0.24, respectively, Table 4), though concentrations were higher in individuals null for either gene as compared to individuals with at least one copy. Participants null for GSTT1 excreted 891.3 ng DHBMA/mg Cr versus 645.9 ng/mg Cr in individuals with the gene present (Table 4); participants null for GSTM1 excreted 803.4 ng DHBMA/mg Cr versus 615.8 ng/mg Cr in individuals with the gene present (Table 4).
Table 4.
Effects of GST genotype on urinary MHBMA and DHBMA concentrations at week two baseline.
| GST genotype | N | Geometric mean (95% CI) | |
|---|---|---|---|
| MHBMA (ng/mg Cr)a | DHBMA (ng/mg Cr)a | ||
| GSTT1 | |||
| Null | 18 | 7.8 (4.9, 12.5) | 891.3 (568.8, 1396.8) |
| Present | 61 | 17.1 (13.3, 22.0) | 645.9 (507.5, 822.1) |
| % Difference | 219.5 (28.3, 375.6) | 72.5 (−56.6, 21.1) | |
| P-valueb | 0.01 | 0.22 | |
| GSTM1 | |||
| Null | 36 | 15.1 (10.7, 21.5) | 803.4 (584.3, 1104.5) |
| Present | 43 | 13.6 (9.9, 18.7) | 615.8 (460.6, 823.3) |
| % Difference | 90.0 (−44.4, 45.5) | 76.7 (−50.5, 18.8) | |
| P-valueb | 0.67 | 0.24 | |
Urinary biomarkers were adjusted for creatinine and batch, and log-transformed.
Two-sided p-values were from linear regression models that test the GST genotype effect on week 2 baseline values, after adjusting for creatinine-adjusted TNE.
Discussion
The chemopreventive properties of isothiocyanates are well documented, showing inhibition of carcinogenesis at multiple sites in rodents including mammary gland (46,47), lung (16,48–53), pancreatic (54), colon (55,56), skin (57), and liver tissues (58). In particular, PEITC has been found to attenuate carcinogenesis caused by a variety of chemical carcinogens including 7,12-dimethylbenz[a]anthracene (DBMA) (46), N-nitrosobenzylmethylamine (NBMA) (59,60), azoxymethane (AOM) (55,56), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (16,48,49).
Our results presented herein reveal that treatment with PEITC increases urinary concentrations of both MHBMA and DHBMA in smokers by 11.1% and 3.7% respectively; however, these increases were not statistically significant (Table 2). Further differentiation of the data by genotype, however, revealed that PEITC treatment significantly increased urinary MHBMA levels by 56.7% in individuals null for GSTT1 (Table 4). Given that individuals null for GSTT1 had the lowest baseline levels of urinary MHBMA (7.8 ng/mg Cr, Table 4), the results indicate that treatment with PEITC could provide a protective effect in those null for this genotype, possibly inducing other GSTs to catalyze detoxification of BD derived epoxides. Our previous work has shown that GSTT2 is able to catalyze the formation of an EB-GSH conjugate, the precursor to MHBMA, but further studies are warranted to determine the importance of this particular protein in metabolic detoxification of BD-derived reactive species (41). These findings provide additional support to prior work that shows the marked effect of GSTT1-null status on urinary MHBMA levels (41–43), and are similar to a recent study examining the effects of GSTT1-null status on mercapturic acids formed from metabolites of other chemical carcinogens, such as benzene, acrolein, and crotonaldehyde (21).
The effect of GSTM1 genotype on urinary MHBMA however, was less distinct. PEITC treatment increased MHBMA levels independent of GSTM1 genotype, and although individuals null for the gene saw a greater increase (19.5%) than those containing the gene (5.2%), the difference between treatment with PEITC and the placebo was not statistically significant (Table 4). Furthermore, there was no significant difference in baseline levels of MHBMA between individuals null for GSTM1 versus those where the gene was present (p = 0.67, Table 4); however, this could be due to a smaller sample size, as our previous study involving a much larger number of subjects (n = 1, 068 versus 79) did show significant differences among smokers when stratified by GSTM1 genotype (41). In that work, individuals with two, one, or no copies of the gene excreted urinary MHBMA concentrations of 5.5, 5.3, and 4.4 ng/mL urine, respectively (p < 0.0001) (41). Interestingly, the largest increase in urinary MHBMA by PEITC treatment was seen in individuals null for both GSTT1 and GSTM1 (89.97%, Table 4), still potentially indicating a protective effect, albeit small, in GSTM1-null individuals.
Unlike MHBMA, PEITC treatment did not have a significant effect on urinary DHBMA concentrations, even when stratified by GST genotype. However, prior work also indicates that GST genotype does not necessarily seem to influence excretion of this particular mercapturic acid. In the same aforementioned study containing 1,068 subjects, statistical analysis showed no significant effect on urinary DHBMA in smokers when data was stratified by GSTT1 or GSTM1 copy number (p = 0.226 and 0.94, respectively) (41); in an additional study containing 584 subjects, analyses also showed no significant effect on urinary DHBMA in smokers (p = 0.181) (42). These results could likely be due to the fact that DHBMA is typically a less sensitive biomarker of BD exposure from smoking as compared to MHBMA. In a smoking cessation study, urinary levels of MHBMA decreased 92% three days post cessation, whereas DHBMA levels only decreased 16% in the same amount of time; this disparity remained throughout the study (37). Additionally, urinary DHBMA in smokers is only about 35% higher than in nonsmokers, suggesting potential DHBMA formation from sources other than BD (37,61).
Because treatment with PEITC lasted only one week, there are potential limitations that accompany the interpretation of this data. Effects of long term treatment with PEITC on the urinary concentrations of mercapturic acids formed from carcinogen metabolites remainsto be evaluated; further investigation is warranted as PEITC itself can form conjugates with GSH (62), potentially acting as a scavenger of free GSH. Still, data from animal studies in which the effects of long term PEITC treatment in laboratory rodents were examined do support its use as chemopreventive agent. In multiple published studies (15,63), PEITC exhibited the ability to inhibit lung tumorogenesis: rats given PEITC in drinking water were completely protected from lung carcinogenesis induced by the tobacco specific carcinogen NNK in an 111 week-long study (15). NCr nude mice injected with colon cancer (SW260) cells previously treated with PEITC (2.5 μM) for six weeks showed delayed tumor growth and significantly decreased tumor weight compared to controls (64). In vitro work attributed these results to the ability of long term PEITC treatment to decrease DNA methylation at known anti-cancer gene loci, suggesting that this type of treatment could induce stable epigenetic modifications in tumor cells (64). Additional in vivo work in F344 rats compared the effect of PEITC treatment (as 0.1% of the animals’ diet) during initiation of multi-organ carcinogenesis over a period of four weeks to PEITC treatment post-initiation over a period of 22 weeks, and found that treatment during the initiation phase showed inhibitory effects of carcinogenesis in esophageal, kidney and liver tissues, whereas treatment during the post-initiation phase showed inhibitory effects in lung tissue (65). However, to our knowledge, there are no post-exposure studies demonstrating the long-term effects of PEITC after treatment is terminated.
Overall, this study is the first of its kind to examine the effect of PEITC treatment on BD metabolism in smokers. Our results suggest that ingestion of PEITC could provide a strongly protective effect against BD-mediated carcinogenesis in smokers null for GSTT1 or both GSTT1 and GSTM1. More broadly, these results support other work investigating the anticancer properties of dietary isothiocyanates and provide additional evidence that consumption of these compounds could provide a wide-reaching and cost effective method of cancer prevention.
Acknowledgments
This study was supported by the U.S. National Cancer Institute (P01 CA138338 to N. Tretyakova). Research reported in this publication was supported in part by National Institutes of Health grant P30 CA77598 utilizing the Biostatistics and Bioinformatics shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
References
- 1.Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug News Perspect 2005;18:445–51. [DOI] [PubMed] [Google Scholar]
- 2.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 2008;47 Suppl 2:73–88. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for the Research on Cancer. Cruciferous Vegetables, Isothiocyanates, and Indoles. Lyon, France: IARC; 2004. [Google Scholar]
- 4.Hasegawa T, Nishino H, Iwashima A. Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anticancer Drugs 1993;4:273–9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther 2003;2:1045–52. [PubMed] [Google Scholar]
- 6.Visanji JM, Duthie SJ, Pirie L, Thompson DG, Padfield PJ. Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J Nutr 2004;134:3121–6. [DOI] [PubMed] [Google Scholar]
- 7.Cheung KL, Khor TO, Yu S, Kong AN. PEITC induces G1 cell cycle arrest on HT-29 cells through the activation of p38 MAPK signaling pathway. AAPS J 2008;10:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao D, Johnson CS, Trump DL, Singh SV. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Cancer Ther 2004;3:567–75. [PubMed] [Google Scholar]
- 9.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 2004;21:661–70. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene 2005;24:4486–95. [DOI] [PubMed] [Google Scholar]
- 11.Hu R, Kim BR, Chen C, Hebbar V, Kong AN. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003;24:1361–7. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos 2001;29:1110–3. [PubMed] [Google Scholar]
- 13.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci 2006;79:1944–55. [DOI] [PubMed] [Google Scholar]
- 14.Jiao D, Smith TJ, Yang CS, Pittman B, Desai D, Amin S, et al. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis 1997;18:2143–7. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS, Trushin N, Rigotty J, Carmella SG, Borukhova A, Akerkar S, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev 1996;5:645–52. [PubMed] [Google Scholar]
- 16.Morse MA, Wang CX, Stoner GD, Mandal S, Conran PB, Amin SG, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res 1989;49:549–53. [PubMed] [Google Scholar]
- 17.Yuan JM, Stepanov I, Murphy SE, Wang R, Allen S, Jensen J, et al. Clinical Trial of 2-Phenethyl Isothiocyanate as an Inhibitor of Metabolic Activation of a Tobacco-Specific Lung Carcinogen in Cigarette Smokers. Cancer Prev Res 2016;9:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 19.Drope J, Schluger N, Cahn Z, Drope J, Hamill S, Islami F, et al. The Tobacco Atlas. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 20.American Cancer Society. Global Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 21.Yuan JM, Murphy SE, Stepanov I, Wang R, Carmella SG, Nelson HH, et al. 2-Phenethyl Isothiocyanate, Glutathione S-transferase M1 and T1 Polymorphisms, and Detoxification of Volatile Organic Carcinogens and Toxicants in Tobacco Smoke. Cancer Prev Res 2016;9:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control 2003;12:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis 1990;11:1863–8. [DOI] [PubMed] [Google Scholar]
- 24.International Agency for the Research on Cancer. Tobacco Smoke and Involuntary Smoking. Lyon, France: IARC; 2004. [Google Scholar]
- 25.Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, et al. Leukemia and cumulative exposure to butadiene, styrene and benzene among workers in the synthetic rubber industry. Toxicology 1996;113:190–202. [DOI] [PubMed] [Google Scholar]
- 26.International Agency for the Research on Cancer. 1,3-butadiene, ethylene oxide, and vinyl halides (vinyl flouride, vinyl chloride and vinyl bromide). Lyon, France: 2008. [Google Scholar]
- 27.National Toxicology Program. Report on Carcinogens. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Toxicology Progam; 2014. [Google Scholar]
- 28.Delzell E, Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R, et al. An updated study of mortality among North American synthetic rubber industry workers. Res Rep Health Eff Inst 2006;Research Report 132::1–63. [PubMed] [Google Scholar]
- 29.Matanoski G, Francis M, Correa-Villasenor A, Elliott E, Santos-Burgoa C, Schwartz L. Cancer epidemiology among styrene-butadiene rubber workers. IARC Sci Publ; 1993:363–74. [PubMed] [Google Scholar]
- 30.Matanoski G, Elliott E, Tao X, Francis M, Correa-Villasenor A, Santos-Burgoa C. Lymphohematopoietic cancers and butadiene and styrene exposure in synthetic rubber manufacture. Ann N Y Acad Sci 1997;837:157–69. [DOI] [PubMed] [Google Scholar]
- 31.Meinhardt TJ, Lemen RA, Crandall MS, Young RJ. Environmental epidemiologic investigation of the styrene-butadiene rubber industry. Mortality patterns with discussion of the hematopoietic and lymphatic malignancies. Scand J Work Environ Health 1982;8:250–9. [DOI] [PubMed] [Google Scholar]
- 32.Sathiakumar N, Delzell E, Hovinga M, Macaluso M, Julian JA, Larson R, et al. Mortality from cancer and other causes of death among synthetic rubber workers. Occup Environ Med 1998;55:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sathiakumar N, Delzell E. A follow-up study of mortality among women in the North American synthetic rubber industry. J Occup Environ Med 2009;51:1314–25. [DOI] [PubMed] [Google Scholar]
- 34.Sathiakumar N, Brill I, Delzell E. 1,3-butadiene, styrene and lung cancer among synthetic rubber industry workers. J Occup Environ Med 2009;51:1326–32. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin PJ, Burka LT, Bechtold WE, Dahl AR, Hoover MD, Chang IY, et al. Species differences in urinary butadiene metabolites; identification of 1,2-dihydroxy-4-(N-acetylcysteinyl)butane, a novel metabolite of butadiene. Carcinogenesis 1992;13:1633–8. [DOI] [PubMed] [Google Scholar]
- 36.Bechtold WE, Strunk MR, Chang IY, Ward JB Jr., Henderson RF Species differences in urinary butadiene metabolites: comparisons of metabolite ratios between mice, rats, and humans. Toxicol Appl Pharmacol 1994;127:44–9. [DOI] [PubMed] [Google Scholar]
- 37.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol 2009;22:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol Sci 2000;56:189–202. [DOI] [PubMed] [Google Scholar]
- 39.Fustinoni S, Soleo L, Warholm M, Begemann P, Rannug A, Neumann HG, et al. Influence of metabolic genotypes on biomarkers of exposure to 1,3-butadiene in humans. Cancer Epidemiol Biomarkers Prev 2002;11:1082–90. [PubMed] [Google Scholar]
- 40.Wiencke JK, Pemble S, Ketterer B, Kelsey KT. Gene deletion of glutathione S-transferase theta: correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol Biomarkers Prev 1995;4:253–9. [PubMed] [Google Scholar]
- 41.Boldry EJ, Patel YM, Kotapati S, Esades A, Park SL, Tiirikainen M, et al. Genetic determinants of 1,3-butadiene metabolism and detoxification in three populations of smokers with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev 2017;26:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SL, Kotapati S, Wilkens LR, Tiirikainen M, Murphy SE, Tretyakova N, et al. 1,3-Butadiene exposure and metabolism among Japanese American, Native Hawaiian, and White smokers. Cancer Epidemiol Biomarkers Prev 2014;23:2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotapati S, Esades A, Matter B, Le C, Tretyakova N. High throughput HPLC-ESI(−)-MS/MS methodology for mercapturic acid metabolites of 1,3-butadiene: Biomarkers of exposure and bioactivation. Chem Biol Interact 2015;241:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet 2000;356:724–9. [DOI] [PubMed] [Google Scholar]
- 45.Kelsey KT, Nelson HH, Wiencke JK, Smith CM, Levin S. The glutathione S-transferase theta and mu deletion polymorphisms in asbestosis. Am J Ind Med 1997;31:274–9. [DOI] [PubMed] [Google Scholar]
- 46.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst 1977;58:395–8. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A 1994;91:3147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung FL, Kelloff G, Steele V, Pittman B, Zang E, Jiao D, et al. Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res 1996;56:772–8. [PubMed] [Google Scholar]
- 49.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett 2000;150:49–56. [DOI] [PubMed] [Google Scholar]
- 50.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res 2002;62:2–7. [PubMed] [Google Scholar]
- 51.Lin JM, Amin S, Trushin N, Hecht SS. Effects of isothiocyanates on tumorigenesis by benzo[a]pyrene in murine tumor models. Cancer Lett 1993;74:151–9. [DOI] [PubMed] [Google Scholar]
- 52.Hecht SS, Kenney PM, Wang M, Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett 2002;187:87–94. [DOI] [PubMed] [Google Scholar]
- 53.Wattenberg LW. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis 1987;8:1971–3. [DOI] [PubMed] [Google Scholar]
- 54.Kuroiwa Y, Nishikawa A, Kitamura Y, Kanki K, Ishii Y, Umemura T, et al. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett 2006;241:275–80. [DOI] [PubMed] [Google Scholar]
- 55.Cheung KL, Khor TO, Huang MT, Kong AN. Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis 2010;31:880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis 2000;21:2287–91. [DOI] [PubMed] [Google Scholar]
- 57.Gills JJ, Jeffery EH, Matusheski NV, Moon RC, Lantvit DD, Pezzuto JM. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett 2006;236:72–9. [DOI] [PubMed] [Google Scholar]
- 58.Sugie S, Okumura A, Tanaka T, Mori H. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn J Cancer Res 1993;84:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, Wagner SA. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res 1991;51:2063–8. [PubMed] [Google Scholar]
- 60.Solt DB, Chang K, Helenowski I, Rademaker AW. Phenethyl isothiocyanate inhibits nitrosamine carcinogenesis in a model for study of oral cancer chemoprevention. Cancer Lett 2003;202:147–52. [DOI] [PubMed] [Google Scholar]
- 61.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res 2009;11:1216–25. [DOI] [PubMed] [Google Scholar]
- 62.Morris ME, Dave RA. Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: implications in breast cancer prevention. AAPS J 2014;16:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dayalan Naidu S, Suzuki T, Yamamoto M, Fahey JW, Dinkova-Kostova AT. Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1. Mol Nutr Food Res 2018;62:e1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park JE, Sun Y, Lim SK, Tam JP, Dekker M, Chen H, et al. Dietary phytochemical PEITC restricts tumor development via modulation of epigenetic writers and erasers. Sci Rep 2017;7:40569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa K, Futakuchi M, Hirose M, Boonyaphiphat P, Mizoguchi Y, Miki T, et al. Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, n-heptadecane-8–10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. Int J Cancer 1998;76:851–6. [DOI] [PubMed] [Google Scholar]