Abstract
Purpose
To evaluate the effect of dezocine on the postoperative ratio of Th1/Th2 cytokines in patients undergoing laparoscopic radical gastrectomy.
Patients and Methods
Sixty patients undergoing laparoscopic radical gastrectomy were randomly divided into two groups (n=30): dezocine group (Group D) and sufentanil group (Group S). They received patient-controlled intravenous analgesia (PCIA) after the operation with either dezocine 0.8 mg/kg (Group D) or sufentanil 2 µg/kg (Group S). Both groups also received ondansetron 8 mg diluted to 100 mL with saline. The primary outcome was the Th1/Th2 cytokines ratio at predetermined intervals, 30 min before the induction of general anaesthesia and 0, 12, 24 and 48 h after surgery. The secondary endpoints were patients’ pain scores, measured on a visual analogue scale (VAS) at predetermined intervals (0, 12, 24 and 48 h after surgery), and side effects at follow-up 48 h after surgery.
Results
The Th1/Th2 cytokines ratio in Group D was significantly higher than Group S (P<0.05) 12, 24 and 48 h after the operation. There were no significant differences in VAS pain scores between groups at 0, 12, 24 and 48 h after surgery (P>0.05). Compared to Group S, the incidence of postoperative nausea, vomiting and lethargy was significantly lower in Group D (P<0.05).
Conclusion
Dezocine increases the ratio of Th1/Th2 cytokines, relieves postoperative pain and causes fewer side effects in patients undergoing laparoscopic radical gastrectomy.
Keywords: dezocine, gastric cancer, laparoscopic radical operation, postoperative analgesia, Th1/Th2 cytokines
Introduction
Laparoscopic radical gastrectomy has become the preferred treatment for gastric cancer due to its advantages over traditional open procedures, including reduced trauma, less bleeding and faster recovery.1 However, moderate to severe postoperative pain can cause a strong stress response in patients, leading to decreased immune function and a greater risk of postoperative tumour recurrence and metastasis.2–4
Postoperative analgesia management is closely related to postoperative recovery, and some studies suggest that it is associated with the long-term prognosis of tumors.5 T helper (Th) lymphocytes play an important role in antitumour immunity. An imbalance of Th cells is related to metastasis and recurrence of malignant gastrointestinal tumours, especially adecreasedTh1/Th2 ratio.6 Multiple studies have shown that scientific and rational selection of postoperative analgesics can effectively relieve pain and reduce stress, perioperative complications and interference with the patient’s immune system and tumour cells, which directly and indirectly affect the prognosis.7,8 A good postoperative analgesic effect can improve the immunosuppressive state of the patient, promoting the differentiation and balance of Th cells and helping to maintain the balance of cellular immunity and humoral immunity.7–11
The most commonly used opioid analgesic in patient-controlled intravenous analgesia (PCIA) is sufentanil;11 however, it’s the adverse reactions associated with this drug are serious, and studies have shown that it inhibits immune function.10,12,13 As a new potent synthetic opioid analgesic, dezocine is mostly used for moderate to severe analgesia. It is characterised by complete activation of the κ receptor, spinal cord analgesia, mild sedation and respiratory depression. It is a μ receptor antagonist, so it presents a smaller risk of addiction.14,15 In addition, the latest research shows that dezocine can regulate immune function by affecting the activity of NK cells, CD4+ cells, CD8+ cells and dendritic cells.13,16,17 However, the effect of dezocine on Th cell differentiation remains unclear. Therefore, this study aimed to investigate the effect of dezocine on the ratio of Th1/Th2 cytokines in patients receiving postoperative analgesia following laparoscopic radical gastrectomy and provide a more reliable basis for perioperative analgesia of tumour patients.
Materials and Methods
Patients
The institutional Research Ethics Board approved the study protocol (KYLW-2019LW28) for this prospective, randomised, double-blind clinical trial. The study was registered at the Chinese Clinical Trial Registry (http://www.chictr.org.cn; ID: ChiCTR2000033754) on June 11, 2020. The authors adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting randomised controlled trials.
Study Protocol
After providing written informed consent, 60 patients classified as American Society of Anesthesiologists (ASA) physical status class I or II, aged 30–60 years and scheduled for laparoscopic radical gastrectomy between July and December 2020 in the Second Hospital of Shandong University were considered eligible to participate in the trial. The major exclusion criteria were as follows: preoperative cognitive dysfunction; history of severe hypertension or heart disease; severe respiratory disease; immune system disease; endocrine system disease; blood system disease; acute or chronic infectious disease; hepatic or renal dysfunction; recent antidepressant, sedative, analgesic or glucocorticoid use; allergy to the drugs used in the study; recent history of radiotherapy, chemotherapy or immunosuppressant application; perioperative blood transfusion; past history of abdominal surgery; transferred to open surgery; required reoperation or infection within 48 h after operation; failure to complete data collection; and refusal to participate in the study. Patients were randomly assigned in a blinded fashion (using a sealed opaque envelope system) to the dezocine group (Group D) or the sufentanil group (Group S). Before the study procedure, all patients received training on how to use the PCIA pump over the 48-h postoperative follow-up period and how to use the 10-cm visual analogue scale (VAS) to record pain (from 0, no pain, to 10, the worst imaginable pain). They were all encouraged to press the analgesia pump during the preoperative education session and postoperative follow-up periods. All patients fasted for at least 12 h before the procedure, and no premedication was administered before anaesthesia. Following their arrival in the operation room, patients received standard ASA and bispectral index (BIS) monitoring throughout the procedure. All patients received completely intravenous anaesthesia and mechanical ventilation. Anaesthesia was induced with 1.5–3.0 mg/kg of propofol, 0.3 µg/kg of sufentanil and 0.6 mg/kg of rocuronium. Anaesthesia was maintenance was performed with a continuous intravenous infusion of 3–10 mg/kg/h of propofol and 0.05–0.2 µg/kg/min of remifentanil. During surgery, heart rate (HR) was maintained at 60–100 beats/min, mean arterial pressure (MAP) was kept within ±20% of the baseline value, saturation of peripheral oxygen (SPO2) was 98–100%, end-tidal carbon dioxide (ETCO2) was 35–45 mmHg, and BIS was 40–60. Patients were given ondansetron 4 mg intravenously 10 min before the skin incision, supplemented with sufentanil 0.3 µg/kg. Rocuronium or vasoactive agents were administered as appropriate during the procedure. Propofol and remifentanil were stopped 15 min before the end of the operation, and no antagonist was used. After the patient’s steward score (0–6 points, ranging from an unresponsive, immobile patient whose airway requires maintenance to a fully recovered patient) was greater than 6 points, the tracheal tube was removed, and they were transferred to the postanaesthesia care unit for observation for 30 min before being sent back to the general ward. All operations were performed by the same surgical team. PCIA was initiated at the end of the surgery. The study medication and PCIA solutions were prepared by a nurse who did not participate in the study. The patients and all research staff who enrolled patients and collected study data were blinded to group assignment. The PCIA protocol was dezocine 0.8 mg/kg (Group D) or sufentanil 2 µg/kg (Group S) and ondansetron 8 mg diluted to 100 mL (background infusion volume was 2 mL/h, load volume was 5 mL, locking time was 15 min, and additional volume was 0.5 mL).
The primary outcome was the ratio of Th1/Th2 cytokines at predetermined intervals, 30 minutes before the induction of general anaesthesia and 0, 12, 24 and 48 h after surgery. Venous blood samples (3mL) were collected for the determination of serum concentrations of Th1 cytokines (IFN-γ and IL-2) and Th2 cytokines (IL-6 and IL-4), assessed by enzyme-linked immune sorbent assay. The Th1/Th2 cytokines equilibrium was evaluated based on the level of IFN-γ/IL-4, from which the Th1/Th2 cytokines ratio was calculated.18,19 The secondary endpoint was the VAS pain scores, which were measured at predetermined intervals (0, 12, 24 and 48 h) after surgery. If the patient-reported VAS score was ≥4, the analgesic pump was first pressed to add drugs, then the patient was re-evaluated after 15 min. If necessary, 3 μg sufentanil was intravenously injected as an emergency analgesic measure until the VAS score was<4. Emergency analgesia use of sufentanil and the dosage of intraoperative sufentanil and remifentanil were respectively recorded. Besides, the dosage of post-operative dezocine and sufentanil. Postoperative follow-up records were completed by the acute pain group, who were blinded to the group allocation. Furthermore, side effects such as lethargy, nausea and vomiting (PONV), pruritus, urinary retention, bradycardia (HR <50 beats/min) and respiratory depression (RR<10 beats/min lasting for more than 10 min) were recorded, along with other severe adverse reactions for the first 48 h postoperative. PONV was treated with ondansetron 4 mg, bradycardia was treated with atropine, and respiratory depression was treated with oxygen or naloxone until the patient’s RR was ≥15 beats/min. Moreover, patients with pruritus needed a lower dose of opioids, and patients with urinary retention required catheterisation. All patients were followed up for 48 h by the same resident anaesthesiologist who was blinded to the group allocation.
Statistical Analyses
The sample size was calculated using PASS 11.0 (NCSS-PASS 11, USA). According to the results of a preliminary experiment, the Th1/Th2 cytokines ratio, representing the major endpoint at 48 h after surgery, was 3.85±0.45 and 3.52±0.36 in Group D and Group S, respectively. The power for the endpoint was calculated based on a two-sample t-test with a significance level of 5% and β power of 0.20. The sample size of each group was calculated to be 24 cases; therefore, considering a 20% dropout rate, 60 patients (30 patients per group) would be sufficient in the present trial. SPSS 22.0 (SPSS Inc. Armonk, NY, USA) was used for all statistical analyses. Patient characteristics were compared by independent samples t-test or Mann–Whitney U-test for continuous variables and chi-squared test for categorical variables. The VAS scores and Th1/Th2 ratios were analysed by independent t-test or Mann–Whitney U-test, as appropriate. Analysis of variance was performed within groups for normally distributed variables. The Friedman test was used for repeated measures analysis of nonnormally distributed variables. The incidence of postoperative adverse events was compared by the chi-squared test or Fisher’s exact test. P-values less than 0.05 were considered statistically significant. Data are expressed as mean ± standard deviation (SD) or the number (proportion) as appropriate.
Results
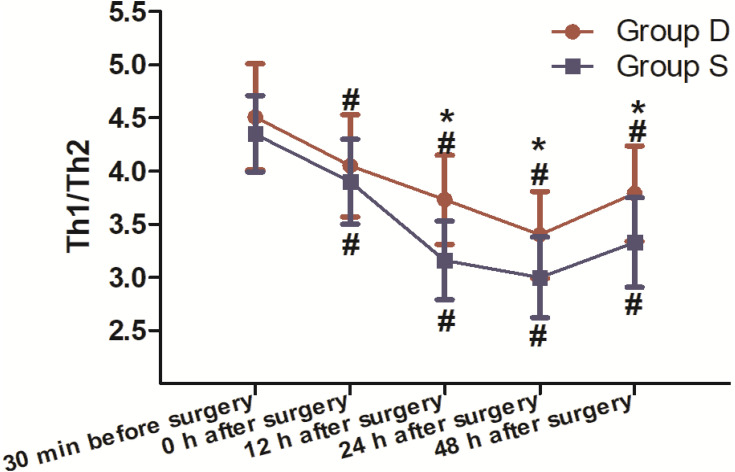
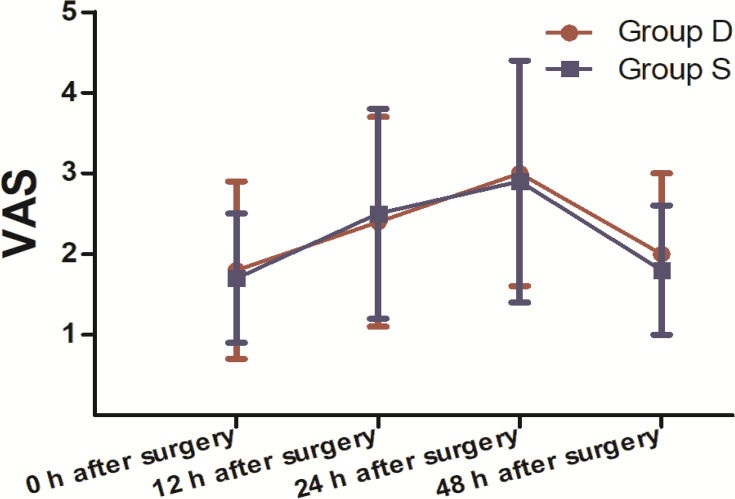
A total of 71 patients were recruited for this study. Of these patients, 60 completed the study and were included in the final statistical analysis. Eleven patients were ineligible as they did not meet the inclusion criteria or refused to participate (Figure 1). There were no clinically important differences between groups in terms of sex, age, ASA, body mass index (BMI), anaesthesia time, operative time, intraoperative sufentanil dosage, postoperative emergency sufentanil dosage or remifentanil dosage (Table 1). The dosage of post-operative dezocine (mg) and sufentanil (μg): 51.2± 12.8 vs 129.4±12.4. The ratio of Th1/Th2 cytokines in Group D was significantly higher than Group S (P<0.05) 12, 24 and 48 h after the operation (Figure 2). Compared to 30 min before surgery, the Th1/Th2 cytokines ratio in both groups was significantly lower (P<0.05) 0, 12, 24 and 48 h after the operation (Figure 2). There was no significant difference in the ratio of Th1/Th2 cytokines between the groups (P>0.05) 30 min before surgery and 0 h after surgery (Figure 2). There was no significant difference between the groups in VAS pain scores at 0, 12, 24 and 48 h postoperative (P>0.05; Figure 3). Moreover, compared to Group S, the incidence of lethargy and PONV was lower in Group D (P<0.05). Furthermore, there were no significant differences in the incidence of pruritus, urinary retention or respiratory depression between the groups (P>0.05), and no patients in the trial experienced bradycardia (Table 2).
Figure 1.
Flow diagram of the study.
Table 1.
Patient and Procedure Characteristics According to Study Group
| Characteristic | Group D (n = 30) | Group S (n = 30) | P value |
|---|---|---|---|
| Sex (n, male/female) | 16/14 | 13/17 | 0.273* |
| Age (years) | 54.4±4.8 | 56.0±3.9 | 0.175# |
| BMI (kg/m2) | 21.5±1.2 | 20.9±1.4 | 0.102# |
| ASA classification (n, I/II) | 17/13 | 12/18 | 0.801* |
| Anaesthesia time (min) | 193.0±20.2 | 203.4±22.1 | 0.064# |
| Operative time (min) | 171.2±14.2 | 177.7±26.2 | 0.232# |
| Intraoperative sufentanil dosage (μg) | 55.7±23.0 | 48.6±17.4 | 0.178# |
| Postoperative emergency sufentanil dosage (μg) | 5.2±0.5 | 5.4±0.9 | 0.195# |
| Remifentanil dosage (μg) | 1143.3±229.5 | 1007.3±198.8 | 0.519# |
Notes: Values are presented as mean ± SD or numbers of patients. *Fisher’s exact test for statistical analysis. #Independent samples t-test for statistical analysis.
Figure 2.
Evaluation of the ratio of Th1/Th2 cytokines between the two groups 30 min before the induction of general anaesthesia and 0, 12, 24 and 48 h after the operation (30 patients in each group, data are expressed as mean ± SD). *According to the Mann–Whitney U-test, the Th1/Th2 cytokines ratio in Group D was significantly higher than in Group S (P<0.05) 12, 24 and 48 h after the operation. #According to the Mann–Whitney U-test, compared to 30 min before the surgery, the Th1/Th2 cytokines ratio in the two groups was significantly lower (P<0.05) 0, 12, 24 and 48 h after the operation. According to the Mann–Whitney U-test, there was no significant difference in the Th1/Th2 cytokines ratio between groups (P>0.05) 30 min before surgery and 0 h after surgery.
Figure 3.
Evaluation of the patient-reported pain score (VAS) between the two groups during the postoperative period. Comparison of pain scores of both groups at 0, 12, 24 and 48 h after surgery (30 patients in each group, data are expressed as mean ± SD). There were no significant differences in pain scores between the two groups (according to the Mann–Whitney U-test) during the postoperative period (P>0.05).
Table 2.
Incidence of Adverse Reactions 48 h After Surgery
| Characteristic | Group D (n = 30) | Group S (n = 30) | P value |
|---|---|---|---|
| Lethargy, n (%) | 1 (3.3)* | 8 (12.5) | 0.031 |
| PONV, n (%) | 3 (10.0)* | 10 (33.3) | 0.033 |
| Pruritus, n (%) | 0 | 1 (3.3) | 0.310 |
| Urinary retention, n (%) | 0 | 1 (3.3) | 0.310 |
| Respiratory depression, n (%) | 0 | 2 (6.7) | 0.152 |
| Bradycardia, n (%) | 0 | 0 | – |
Notes: Data are expressed as the number and percentage.“-”No data. *P<0.05 compared to Group S.
Discussion
We conducted this study to investigate the effects of dezocine on postoperative analgesia and the Th1/Th2 cytokines ratio in patients undergoing laparoscopic radical gastrectomy. The present trial demonstrated that there were no significant differences in VAS pain scores between the two groups 0, 12, 24 and 48 h after surgery, indicating that dezocine can effectively reduce postoperative pain. Therefore, we can exclude a potential effect of differences in postoperative pain on the Th cell differentiation results. In previous studies of the analgesic properties of dezocine, the potency of 10 mg of dezocine was reported to be equal to 50 mg of meperidine and 10 mg of morphine, indicating that it might be an effective analgesic drug for the management of perioperative pain.11,20 Additionally, the spinal effect of dezocine, which occurs through interactions with κ receptors, produces a unique action in the treatment of visceral pain.21 This characteristic probably makes it more effective for relieving visceral pain after gastric cancer surgery. However, one meta-analysis reported that there is no significant difference between dezocine and morphine injections on the persistence of pain in patients with cancer among Chinese patients.22 A pilot study by Zhu et al13 showed that the analgesic efficacy of dezocine was similar to that of sufentanil. Dezocine combined with sufentanil can provide effective postoperative analgesia for PCIA in burn patients after escharectomy or tangential excision followed by autologous skin grafting.23 Th cells play a major role in antitumor immune processes, and each Th subtype is critical to maintain the immune balance of the body. Th1 and Th2 cells are differentiated from precursor Th0 cells, and the cross-regulation between Th1 and Th2 cell is important.24,25 Th1 cells can secrete IFN-γ and IL-2, mediate the cellular immune response, activate T lymphocytes and macrophages and reduce postoperative infection, which are the main mechanisms of antitumour immunity. Th2 cells can secrete IL-4 and IL-6, mediate humoral immunity, stimulate B lymphocyte proliferation, produce immunoglobulin and participate in humoral immunity.26,27 Under normal conditions, Th1 and Th2 cells are in a relatively balanced state; however, the bodies of patients with malignant tumours are in a state of immune escape, with a significant imbalance in cellular immunity and humoral immunity. In such situations, the Th1/Th2 balance is disturbed, which manifests as a drift of Th1 to Th2. This leads to the suppression of Th1-mediated cellular immunity, which mainly manifests in a predominance of Th2 cells, leading to a decline in the ability of the immune surveillance system to recognise and regulate tumour cells.28–30 Our findings show a significant decrease (P<0.05) in the ratio of Th1/Th2 cytokines postoperatively compared to before the operation. Additionally, those suggests a preference ofTh1 to Th2 drift, which indicates a disruption in cellular immune function and a decline in antitumour immunity after a series of stimulations during the perioperative period. A study by Gong et al31 revealed that Treg frequency and Foxp3 mRNA expression were decreased postoperatively compared to before the surgery. The most likely explanation is a reduction in cellular immune function due to various trauma stress reactions, such as trauma stress caused by surgery, anaesthetic factors, postoperative wound pain, visceral pain and the use of opioids, among others. Our study showed that there were no significant differences in Th1/Th2 cytokines values between the two groups 30 min before the surgery and immediately after surgery (0 hours, P>0.05). The results suggest that the immune function of the two groups was similar preoperatively and intraoperatively; therefore, an influence on postoperative immune function could be excluded. Compared to Group S, the ratio of Th1/Th2 cytokines in Group D was increased 12, 24 and 48 h after the operation (P<0.05). The outcomes indicate that the cellular immune status of Group D is reversed to Th1. We speculate that dezocine may be associated with lighter immune suppression and faster recovery after surgery. One study showed that dezocine was more beneficial to the recovery of the early postoperative immune function of patients with breast cancer undergoing postoperative analgesia after radical mastectomy.10 A study by Song et al17 revealed that dezocine can inhibit tumour metastasis by elevating CD8+ T cell proliferation and cytotoxicity. Zhu et al32 revealed that dezocine and ropivacaine infiltration anaesthesia can significantly inhibit the secretion of pain factors in patients undergoing open hepatectomy, as well as reduce the stress response after surgery, reduce immune function fluctuations and help promote postoperative recovery. A study by Jia et al33 demonstrated that the use of dezocine and remifentanil has a better analgesic effect and can effectively regulate the expression of inflammatory cytokines TNF-α and IL-6, Compared with midazolam-remifentanil intravenous anaesthesia. However, some studies have shown that the κ receptor does not affect the differentiation and function of immune cells.34 The first possible explanation for this is the close relationship of μ opioid receptors to immune regulation, as studies have shown that μ opioid receptors are expressed on the membranes of various immune cells and participate in the release of cytokines and cell mediators in the immune system, which plays a major role in immune regulation.35,36 Dezocine is a μ receptor antagonist, so it has little activity on the μ receptor. Therefore, it has been proposed that dezocine has a lower inhibitory effect on immune cells, which may be related to its antagonistic effect on the μ receptor.37 The second explanation is that dezocine may make Th0 cells preferentially differentiate into Th1 cells overTh2 cells; however, the mechanism of dezocine-mediated Th cell differentiation needs to be further studied. The perioperative use of dezocine, sufentanil, antibiotics and other drugs may cause adverse reactions such as lethargy, vomiting and respiratory depression, among others.35 Our findings demonstrate that the incidence of lethargy and PONV in Group D was significantly lower than the incidence in Group S. This difference was statistically significant (P<0.05), suggesting that dezocine might be an antagonist of the μ receptor, without μ receptor agonist side effect, capable of relaxing the gastrointestinal smooth muscle and reducing the incidence of PONV.36 Wang et al22 revealed that the rate of adverse drug reactions caused by dezocine injection was 56% lower than that caused by morphine injection. A study by Bian et al38 revealed that morphine and sufentanil may dose-dependently increase the contractile tension and contraction ability of isolated rat small intestine smooth muscle, but dezocine had no significant effect on intestinal smooth muscle contraction.
Limitations: The present study was conducted at a single centre, potentially confounding external validity. Although the desired statistical power was achieved, the follow-up time was limited to 48 h. Therefore, studies with a longer follow-up period are needed to confirm our results. In addition, we only measured IFN-γ and IL-4; therefore, more cytokines need to be measured to confirm our findings. Another possible limitation is that the specific mechanism of the effect of dezocine on the Th1/Th2 ratio is currently unclear, and this requires further study.
Conclusion
The present study suggests that dezocine may enhance the Th1 immune response so as to increase the ratio of Th1/Th2 cytokines; however, the mechanism underlying the effect of dezocine on the differentiation of Th1 remains unclear. In addition, dezocine has a definite analgesic effect in patients following laparoscopic radical gastric cancer surgery, with few adverse reactions. Furthermore, this study analysed the advantages and disadvantages of the two postoperative analgesic drugs, and our findings suggest that dezocine is more appropriate for the recovery of patients’ physical function and other long-term considerations.
Acknowledgments
This study was supported by clinical medicine science and technology innovation plan of Jinan Science and Technology Bureau, Shandong Province, China (No. 202019127).
Abbreviations
Th, T helper; Th1, T helper type 1; Th2, T helper type 2; IFN-γ, interferon-γ; IL-2, interleukin-2; IL-6, interleukin-6; IL-4, interleukin-4; VAS, visual analogue scale; PCIA, patient-controlled intravenous analgesia; ECG, Electrocardiogram; MAP, mean arterial pressure; SPO2, saturation of peripheral oxygen; RR, Respiration rate; ETCO2, end-tidal carbon dioxide; BIS, bispectral index; ASA, American Society of Anesthesiologists; BMI, body mass index; PONV, postoperative nausea and vomiting; SD, standard deviation.
Data Sharing Statement
The individual participant’s data that underlying the results reported in this article would be accessed with approval from the corresponding author after 3 months of publication. The study protocol and statistical analysis plan will also be available.
Ethical Approval
All procedures in this study were performed in accordance with the ethical standards, and the study received approval from the Ethics Committee (code of ethics KYLW-2019LW28 and ChiCTR code 2000033754). Information about the research project and possible complications were explained to all patients, and they were only included in the study after written informed consent was provided, in accordance with the Helsinki Declaration.
Author Contributions
Chang Feng and Man Feng conceived and designed the study. Qinli Feng and Zhuanglei Gao conducted the clinical trial. Yujie Chen, Ge Liu, and Juan Xiao performed the analysis and prepared the manuscript. Chang Feng and Man Feng critically revised the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Wang N, Zhou H, Song X, Wang J. Comparison of oxycodone and sufentanil for patient-controlled intravenous analgesia after laparoscopic radical gastrectomy: a randomized double-blind clinical trial. Anesth Essays Res. 2016;10(3):557–560. doi: 10.4103/0259-1162.186603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz S, Barlag H, Gerken M, et al. Laparoscopic surgery in patients with colon cancer: a population-based analysis. SurgEndosc. 2017;31(6):2586–2595. [DOI] [PubMed] [Google Scholar]
- 3.Hong S, Kim H, Park J. Analgesic effectiveness of rectus sheath block during open gastrectomy: a prospective double-blinded randomized controlled clinical trial. Medicine (Baltimore). 2019;98(15):e15159. doi: 10.1097/MD.0000000000015159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le-wendling L, Nin O, Capdevila X. Cancer recurrence and regional anesthesia: the theories, the data, and the future in outcomes. Pain Med. 2016;17(4):756–775. doi: 10.1111/pme.12893 [DOI] [PubMed] [Google Scholar]
- 5.Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124(5):1697–1708. doi: 10.1213/ANE.0000000000001652 [DOI] [PubMed] [Google Scholar]
- 6.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102(7):742–747. doi: 10.1002/jso.21725 [DOI] [PubMed] [Google Scholar]
- 7.Ma W, Wang K, Du J, et al. Multi-dose parecoxib provides an immune protective effect by balancing T helper 1(Th1), Th2, Th17 and regulatory T cytokines following laparoscopy in patients with cervical cancer. Mol Med Rep. 2015;11(4):2999–3008. doi: 10.3892/mmr.2014.3003 [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Kim NK, Baik SH, et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: arandomized study. Medicine (Baltimore). 2016;95(19):e3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren C, Zhang X, Liu Z, Li C, Zhang Z, Qi F. Effect of intraoperative and postoperative infusion of dexmedetomidine on the quality of postoperative analgesia in highly nicotine-dependent patients after thoracic surgery: a CONSORT-Prospective, Randomized, Controlled Trial. Medicine (Baltimore). 2015;94(32):e1329. doi: 10.1097/MD.0000000000001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Zhang X, Wang H, et al. Effects of dezocine and sufentanil for postoperative analgesia on activity of NK, CD4(+) and CD8(+) cells in patients with breast cancer. Oncol Lett. 2019;17(3):3392–3398. doi: 10.3892/ol.2019.9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Li L, Shen B, et al. A multicenter randomized double-blind prospectivestudy of the postoperative patient controlledintravenous analgesia effects of dezocinein elderly patients. Int J Clin Exp Med. 2014;7(3):530–539. [PMC free article] [PubMed] [Google Scholar]
- 12.Lindley P, Ding L, Danesi H, et al. Meta-analysis of the ease of care from a patients’perspective comparing fentanyl iontophoretic transdermal system versus morphine intravenous patient-controlled analgesia in postoperative pain management. J Perianesth Nurs. 2017;32(4):320–328. doi: 10.1016/j.jopan.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Chen Y, Huang S, Sun X. Interaction of analgesic effects of dezocine and sufentanil for relief of postoperative pain: apilot study. Drug Des Devel Ther. 2020;14:4717–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Huang XP, Yeliseev A, et al. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120(3):714–723. doi: 10.1097/ALN.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao XF, Ahsan MZ, Apryani E, et al. Dual μ-opioid receptor and norepinephrine reuptake mechanisms contribute to dezocine and tapentadol-induced mechanical antiallodynia in cancer pain. Eur J Pharmacol. 2020;876:173062. doi: 10.1016/j.ejphar.2020.173062 [DOI] [PubMed] [Google Scholar]
- 16.Feng C, Feng M, Jiao R, et al. Effect of dezocine on IL-12 and IL-10 secretion and lymphocyte activation by culturing dendritic cells from human umbilical cord blood. Eur J Pharmacol. 2017;l(796):110–114. doi: 10.1016/j.ejphar.2016.12.035 [DOI] [PubMed] [Google Scholar]
- 17.Song Q, Liu G, Liu D, Feng C. Dezocine promotes T lymphocyte activation and inhibits tumor metastasis after surgery in a mouse model. Invest New Drugs. 2020;38(5):1342–1349. doi: 10.1007/s10637-020-00921-6 [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Du L, Feng J, et al. Clinicopathological and prognostic significance of serum cytokine levels in breast cancer. Clin Lab. 2014;60:1145–1151. doi: 10.7754/Clin.Lab.2013.130738 [DOI] [PubMed] [Google Scholar]
- 19.Ghazy AA, Abu El-Nazar SY, Ghoneim HE, et al. Effect of murine exposure to gamma rays on the interplay between Th1 and Th2 lymphocytes. FrontPharmacol. 2015;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strain EC, Preston KL, Liebson IA, Bigelow GE. Opioid antagonist effects of dezocine in opioid-dependent humans. Clin Pharmacol Ther. 1996;60:206–217. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GF, Guo J, Qiu LL, et al. Effects of dezocine for the prevention of postoperative catheter-related bladderdiscomfort: a prospective randomized trial. Drug Des Devel Ther. 2019;13:1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Liu X, Wang J, et al. Comparison of the efficacy and safety between dezocine injection and morphine injection for persistence of pain in Chinese cancer patients: a meta-analysis. Biosci Rep. 2017;37(3). doi: 10.1042/BSR20170243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Min S, Wu B, Tang W. Application of patient-controlled intravenous analgesia of dezocine combined with sufentanil in burn patients after surgery. Zhonghua Shao Shang Za Zhi. 2015;31(1):48–51. [PubMed] [Google Scholar]
- 24.Haghshenas MR, Khademi B, Ashraf MJ, Ghaderi A, Erfani N. Helper and cytotoxic T-cell subsets (Th1, Th2, Tc1, and Tc2) in benign and malignant salivary gland tumors. Oral Dis. 2016;22(6):566–572. doi: 10.1111/odi.12496 [DOI] [PubMed] [Google Scholar]
- 25.Lin W, Zhang HL, Niu ZY, et al. The disease stage-associated imbalance of Th1/Th2 and Th17/Treg in uterine cervical cancer patients and their recovery with the reduction of tumor burden. BMC Womens Health. 2020;20(1):126. doi: 10.1186/s12905-020-00972-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheshtchin N, Gharagozloo M, Andalib A, Ghahiri A, Maracy MR, Rezaei A. The expression of Th1- and Th2-related chemokine receptors in women with recurrent miscarriage: the impact of lymphocyte immunotherapy. Am J Reprod Immunol. 2010;64(2):104–112. doi: 10.1111/j.1600-0897.2010.00829.x [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S, Yamada Y, Murakami H. Expression of Th1/Th2 cell-related chemokine receptors on CD4+ lymphocytes under physiological conditions. Int J Lab Hematol. 2020;42(1):68–76. doi: 10.1111/ijlh.13141 [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Liu H, Wang X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J Tradit Chin Med. 2014;34(6):641–645. doi: 10.1016/S0254-6272(15)30076-5 [DOI] [PubMed] [Google Scholar]
- 29.Bu X, Li M, Zhao Y, et al. Genetically engineered Newcastle disease virus expressing human interferon-λ1 induces apoptosis in gastric adenocarcinoma cells and modulates the Th1/Th2 immune response. Oncol Rep. 2016;36(3):1393–1402. doi: 10.3892/or.2016.4925 [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Liu J, Ge S. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol. 2019;10:624. doi: 10.3389/fphar.2019.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong L, Qin Q, Zhou L, et al. Effects of fentanyl anesthesia and sufentanilanesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. 2014;7(11):7708–7716. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu R, Du T, Gao H. Effects of dezocine and ropivacaine infiltration anesthesia on cellular immune function indicators, anesthesia recovery time and pain factors in patients with open liver resection. Cell Mol Biol (Noisy-Le-Grand). 2020;66(3):149–154. doi: 10.14715/cmb/2020.66.3.23 [DOI] [PubMed] [Google Scholar]
- 33.Jia Q, Tian F, Duan WN, et al. Effects of dezocine-remifentanil intravenous anaesthesia on perioperative signs, serum TNF-&aipha; and IL-6 in liver cancer patients undergoing radiofrequency ablation. J Coll Physicians Surg Pak. 2019;29(1):4–7. doi: 10.29271/jcpsp.2019.01.4 [DOI] [PubMed] [Google Scholar]
- 34.Du C, Duan Y, Wei W, et al. Kappa opioid receptor activation alleviates experimental autoimmune encephalomyelitis and promotes oligodendrocyte-mediated remyelination. Nat Commun. 2016;7(1):11120. doi: 10.1038/ncomms11120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Xu C, Wang X, Shi W. Comparison of the analgesic effects of dezocine, tramadol and butorphanol after cesarean section. Pak J Pharm Sci. 2018;31(5(Special)):2191–2195. [PubMed] [Google Scholar]
- 36.Beck TC, Hapstack MA, Beck KR, et al. Therapeutic potential of kappa opioid agonists. Pharmaceuticals(Basel). 2019;12(2):95. doi: 10.3390/ph12020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep. 2016;6(1):21514. doi: 10.1038/srep21514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bian X, Zhou R, Yang Y, et al. Divergent effect of dezocine, morphine and sufentanil on intestinal motor function in rats. Int J Med Sci. 2015;12(11):848–852. doi: 10.7150/ijms.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]