Abstract
Objectives
Ferroptosis is caused by iron-dependent lipid peroxide accumulation, the sensitivity of which might be regulated by acyl-CoA synthetase long chain family member 4 (ACSL4). Non-small-cell lung cancer (NSCLC) can resist oxidative stress and reduce the sensitivity of tumor cells to ferroptosis by changing the expression of some proteins. Mechanisms involving ferroptosis sensitivity in NSCLC are not fully understood.
Methods
A dual-luciferase reporter assay was used to confirm a targeting relationship between long non-coding (lnc)RNA NEAT1 and ACSL4. Overexpression and silencing assays of NEAT1 function were used to determine its roles in cell death (by TUNEL staining) and lipid peroxidation (by malondialdehyde levels). Expression of ferroptosis-related proteins (SLCA11, GPX4, and TFR4) was evaluated by western blot in NSCLC cells treated or not with the ferroptosis inducer erastin.
Results
Erastin-induced cell death was positively correlated with ACSL4 level. NEAT1 regulated levels of ACSL4 and proteins related to the ferroptosis and classical apoptosis pathways. Levels of ACSL4, SLC7A11, and GPX4 were decreased more by NEAT1 silencing plus erastin than by erastin alone.
Conclusion
NEAT1 regulates ferroptosis and ferroptosis sensitivity, with the latter depending on ACSL4, suggesting that targeting NEAT1 or ACSL4 may be a viable therapeutic approach to the treatment of NSCLC.
Keywords: Non-small-cell lung cancer, ferroptosis, ferroptosis sensitivity, NEAT1, ACSL4, long non-coding RNA
Introduction
Lung cancer is one of the most common causes of cancer-related death in the world, and non-small-cell lung cancer (NSCLC), as the main subtype of lung cancer, accounts for 70% to 80% of all cases.1 Recent studies have found that long non-coding (lnc)RNAs play an important role in many biological processes, including cell proliferation, metastasis, and differentiation.2–5 Lipid peroxidation is thought to be one factor causing apoptosis and necrosis. Ferroptosis is a newly discovered type of iron-dependent programmed cell death, which is different from cell apoptosis, cell necrosis, and autophagy. With the development of research into the mechanism of ferroptosis, it has been shown that lipid peroxidation can induce ferroptosis (and iron overload). Studies have shown that NSCLC can resist oxidative stress and reduce the sensitivity of tumor cells to ferroptosis by affecting the expression of certain functional proteins.6
Acyl-CoA synthetase long chain family member 4 (ACSL4) is a fatty acid-activating enzyme that esterifies CoA into free fatty acids, depending on adenosine triphosphate to play its role.7 ACSL4 influences ferroptosis sensitivity by regulating cellular lipid composition.8 Erastin, a ferroptosis induction factor, inhibits uptake of cystine through the cystine/glutamate reverse transporter (system Xc−), resulting in glutathione peroxidase 4 (GPX4) deficiency, glutathione (GSH) depletion, endoplasmic reticulum stress, and ultimately, induced iron-dependent oxidative death.9,10
Nuclear enriched transcript 1 (NEAT1) is a lncRNA with a cancer-promoting effect and a perinuclear locus. It is closely associated with the biological behavior of tumor cells, including proliferation, cell cycle, and apoptosis.11 A number of studies have shown that lncRNA NEAT1 is highly expressed in NSCLC (although some have suggested that it is not), and that NEAT1 plays an overall role in promoting cancer.5,12 It has been found by database prediction (binding sites were predicted through Starbase database; http://bigd.big.ac.cn/databasecommons/database/id/169) that NEAT1 and ACSL4 have a direct binding target region, which suggests that targeted binding of NEAT1 to ACSL4 inhibits the normal expression of ACSL4, resulting in sensitivity to ferroptosis the above manifestations. This study was designed to evaluate whether NEAT1 regulates ferroptosis sensitivity via ACSL4.
Materials and methods
Ethics statement
This study did not involve human participants, so informed consent was not required. In addition, no approval was required from an institutional review board.
Cell lines
The normal bronchial cell line HBE and the NSCLC cell lines A549, NCI-H1975, SK-MES-1, and PLA-801D (Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI-1640 medium (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C with 5% CO2.
Real time-quantitative PCR
Total RNA was extracted by TRIzol method. After digestion of cells with trypsin, the supernatant was collected after centrifugation at 12,900 × g and 4°C for 15 minutes. Total RNA was extracted by adding 750 µL of TRIzol reagent (Invitrogen/Thermo Fisher Scientific). Reverse transcription was performed using the TaKaRa reverse transcription kit (TaKaRa, Dalian, China) to obtain cDNA of the cells, which was later used as a template for detecting gene expression. The primers for NEAT1 were as follows: forward: 5′-TTGGGACAGTFFACGTGTGG-3′; reverse: 5′-TCAAGTCCAGCAGAGCA-3′. The primers for GAPDH were as follows: forward: 5′-TGACGTGCCGCCTGGAGAAC-3′; reverse: 5′-CCGGCATCGAAGGTGGAAGAG-3′. The relative expression of NEAT1 was calculated using the 2−ΔΔCt method (ΔCt = Ct NEAT1 − Ct GADPH, ΔΔCt = ΔCt experimental group − ΔCt control), where Ct is the cycle threshold.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed according to the TUNEL staining instructions (Beyotime Biotechnology, Shanghai, China). Five non-overlapping visual fields were randomly selected from each section to count A549 cells under a 200-fold field of vision; brownish-yellow particles in the nucleus indicated apoptotic cells. TUNEL-positive apoptotic cells appear brown and nuclei stained with hematoxylin appear blue. TUNEL-positive cells usually cannot be stained with hematoxylin.
Western blot
Cultured or transfected A549 cells were lysed with radioimmunoprecipitation assay (RIPA) buffer solution and the protein was quantified by bicinchoninic acid (BCA) method. Then, the proteins were separated by 10% sodium dodecyl sulfate-PAGE electrophoresis. After transfer, the membrane was incubated in 5% skimmed milk for 1 hour. Anti-SLC7A11 (1:2000), anti-GPX4 (1:1000), anti-TFR1 (1:1000), anti-Bax (1:1000), anti-Bcl-2 (1:2000), anti-caspase3 (1:1000) and anti-GAPDH (1:5000) primary antibodies (Abcam, Cambridge, UK) were incubated with the membrane at 4°C overnight. Then, horseradish peroxidase-labeled secondary antibody (Abcam) was added and incubated at 37°C for 1 hour. Finally, ECL luminescent solution (ltra High Sensitivity ECL kit (Glpbio, Montclair, CA, USA) was used to develop the color. The gray levels of target protein were analyzed using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA), and the relative levels of target protein relative to GAPDH were calculated.
Plasmid transfection
Short hairpin (Sh)RNA-NEAT1 was constructed and cloned into U6/GFP/Neo plasmid (ShRNA-NEAT1-1, Oligo1, 5′-ACCTCGATGCCACAACGCAGATTGATTCAAGAGATCAATCTGCGTTGTGGCATCTT-3′, Oligo2, 5′-CAAAAAGATGCCACAACGCAGATTGATCTCTTGAATCAATCTGCGTTGTGGCATCG-3′, ShRNA- NEAT1-2, Oligo1, 5′-ACCTCGAAGATCCCTAAGCTGTAGAATCAAGAGTTCTACAGCTTAGGGATCTTCTT-3′, Oligo2, 5′-CAAAAAGAAGATCCCTAAGCTGTAGAACTCTTGATTCTACAGCTTAGGGATCTTCG-3′; Gemma, Shanghai, China), and OverExp-NEAT1 was constructed and cloned into pcDNA3.1 plasmids (Gemma). Then, A549 cells in logarithmic phase were seeded into a six-well plate. When the cells reached 70% to 80% confluency, they were transfected with NEAT1 knockdown (ShRNA-NEAT1) or overexpression (OverExp-NEAT1) plasmids (0.2 µg) using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The negative control used scrambled RNA or empty plasmids. After 24 hours, erastin (10 µM) was added to the cells and incubated for an additional 24 hours.
Dual-luciferase reporter gene assay
A549 cells at logarithmic phase were seeded in a 24-well plate. When the cells reached 80% confluency, 100 ng of ACSL4 wild type (WT) or ACSL4 mutant (MUT) luciferase vector and 50 nmol/L NEAT1 WT or empty plasmids were added and incubated for 48 hours after transfection. Luciferase activity was evaluated according to the dual-luciferase report analysis system (Luciferase Reporter Gene Assay Kit, Yuanpinghao Biotechnology Co. Ltd., Beijing, China). Each experiment was independently repeated at least three times.
CCK8 assay
A549 cells at logarithmic growth stage were digested with trypsin (Solebol, Beijing, China) and then seeded into a 96-well plate. Then, 10 µL of CCK-8 reaction solution (Glpbio) was added to each well 24 hours after transfection. After cells were cultured in an incubator for 3 hours, absorbance at 450 nm was detected using a microplate reader (Tecan, Männedorf, Switzerland).
EdU staining
A549 cells were seeded into a 96-well plate. After the cells underwent shRNA-NEAT1 or OverExp-NEAT1 transfection for 24 hours, EdU (5-ethynyl-2′-deoxyuridine) labeling, cell fixation, infiltration, detection, and DNA restaining were carried out according to the instructions of the YF 488 click-iT EdU Imaging kit (US Everbright, Xiangcheng, China). Finally, the culture plates were observed under an inverted fluorescence microscope, and 3 fields were randomly selected for photography.
Malondialdehyde levels
A549 cells were seeded into cell slides and allowed to adhere to the wall for 24 hours. After transfection, malondialdehyde (MDA) activity was detected using the thiobarbituric acid method and the Lipid Peroxidation MDA Assay Kit (Beyotime Biotechnology).
Determination of iron levels
After different group treatments (without erastin induction: control, vector, shRNA-NEAT1-1, OverExp-NEAT1-1; with erastin induction: control, vector, shRNA-NEAT1-1, OverExp-NEAT1-1), the iron content was detected in accordance with the manufacturer’s guidance (Abcam). A mixture of iron assay buffer and cells were homogenized and centrifuged at 16,000 × g and 4°C for 10 minutes. Iron levels were evaluated by detection of absorbance at 593 nm using a microplate reader (BioTek, Winooski, VT, USA).
Statistical analysis
Experimental results are shown as means ± standard deviations (SD). Student’s t-test was used to compare the differences between two groups, and p < 0.05 was considered significant. All experiments were independently conducted at least three times. All statistical analyses were conducted using GraphPad 7.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
ACSL4 was positively related to ferroptosis in NSCLC cells
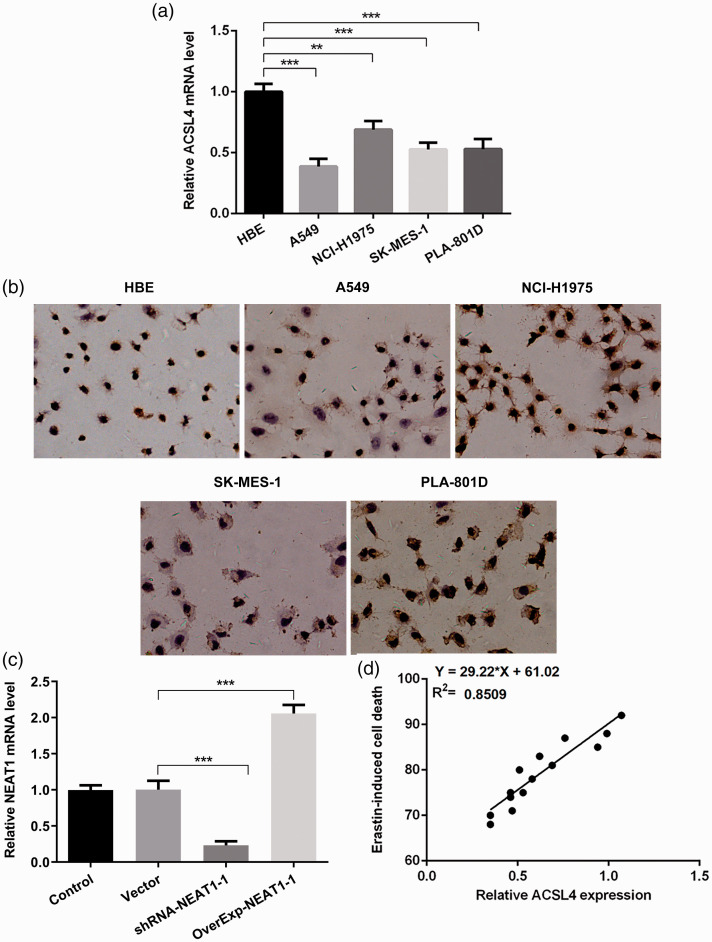
ACSL4 is considered an essential proferroptotic gene.8 We used different normal (HBE) and NSCLC cell lines to analyze ACSL4 expression. ACSL4 expression was significantly reduced (p < 0.001) in NSCLC cell lines compared with HBE cells (Figure 1a). Furthermore, the level of ACSL4 was lower in A549 cells than in other NSCLC cell lines. Subsequently, we used the ferroptosis inducer erastin (10 µM) to trigger ferroptosis of NSCLC cells. Specifically, NSCLC cells underwent different levels of death (Figure 1b), and we observed that ACSL4 was positively related to cell death induced by erastin in NSCLC cells. The lower the expression of ACSL4, the lower the extent of ferroptosis (p < 0.001; Figure 1c).
Figure 1.
(a) Reverse transcription quantitative (RT-q)PCR analysis of acyl-CoA synthetase long chain family member 4 (ACSL4) expression in normal bronchial epithelial cells (HBE) and non-small-cell lung cancer (NSCLC) cell lines (A549, NCI-H1975, SK-MES-1, and PLA-801D). (b) Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was used to assess cell death levels after erastin intervention in the cell lines. (c) Correlation analysis of both ACSL4 and ferroptosis induced by erastin. **p < 0.01, ***p < 0.001.
NEAT1 influenced the death and MDA levels of A549 cells induced by erastin
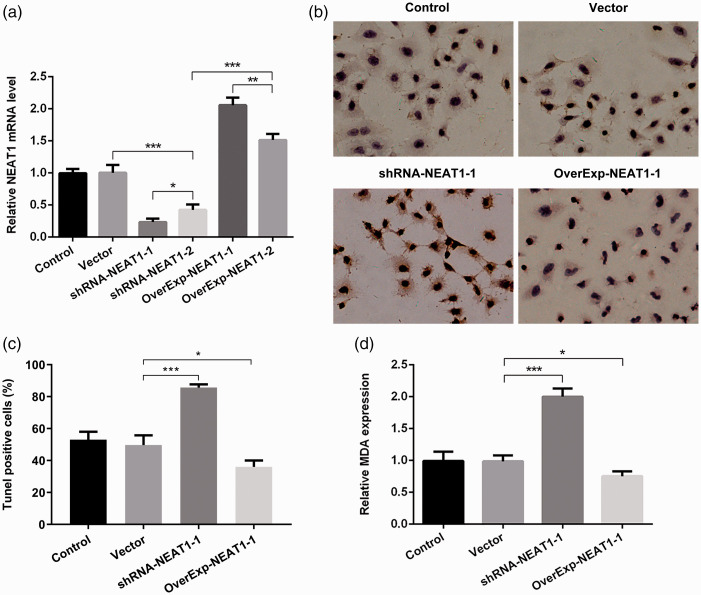
We next investigated whether NEAT1 affected erastin-mediated ferroptosis in A549 cells; we constructed NEAT1 knockdown and overexpression vectors to clarify the functions of NEAT1. As shown in Figure 2a, ShRNA-NEAT1-1 had a greater effect (p < 0.05) on silencing NEAT1 than ShRNA-NEAT1-2, whereas OverExp-NEAT1-1 had a greater effect on overexpressing NEAT1 than OverExp-NEAT1-2. Therefore, ShRNA-NEAT1-1 and OverExp-NEAT1-1 were used for subsequent experiments. Because lipid peroxidation is a hallmark of ferroptosis, we assessed levels of lipid peroxidation in A549 cells. NEAT1 knockdown and overexpression greatly increased and decreased cell death (TUNEL staining, p < 0.05; Figure 2b, c), respectively, and affected MDA levels (p < 0.05; Figure 2d) in A549 cells, further confirming the link between NEAT1 and ferroptosis induction.
Figure 2.
(a) Reverse transcription quantitative (RT-q)PCR analysis of NEAT1 levels after transfection of different plasmids. (b, c, d) NEAT1 knockdown (shRNA-NEAT1-1) or overexpression (OverExp-NEAT1-1) was shown to induce or decrease the sensitivity, respectively, of A549 cells to erastin induction through analysis of (C) cell apoptosis (TUNEL staining) and (d) malondialdehyde (MDA) levels. *p < 0.05, **p < 0.01, ***p < 0.001.
NEAT1 regulated expression levels of nodal proteins of ferroptosis and classical apoptotic pathways
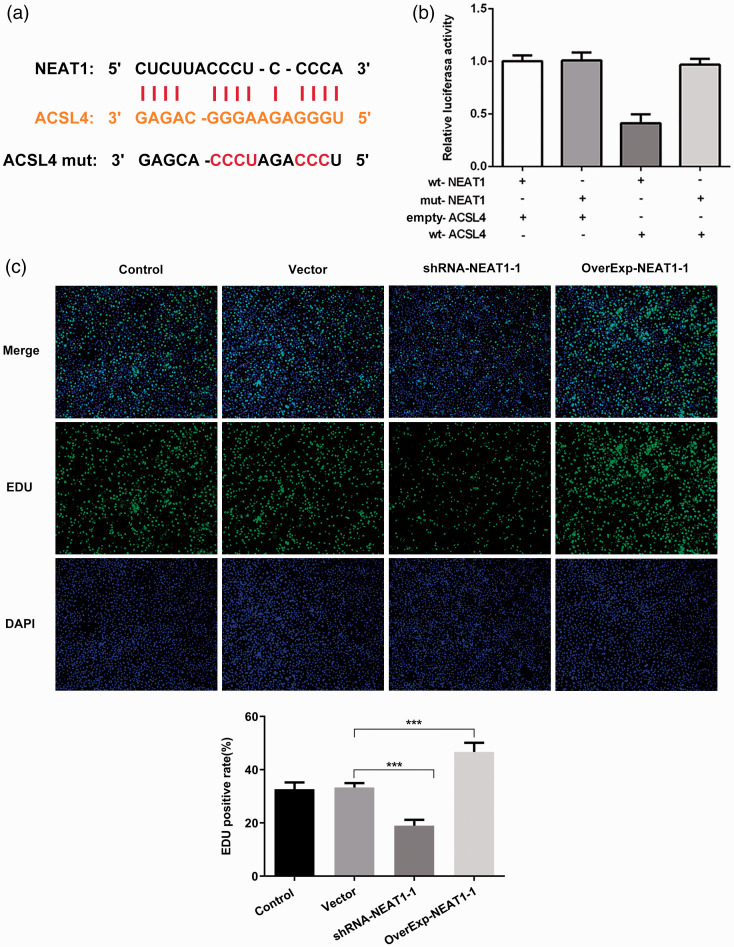
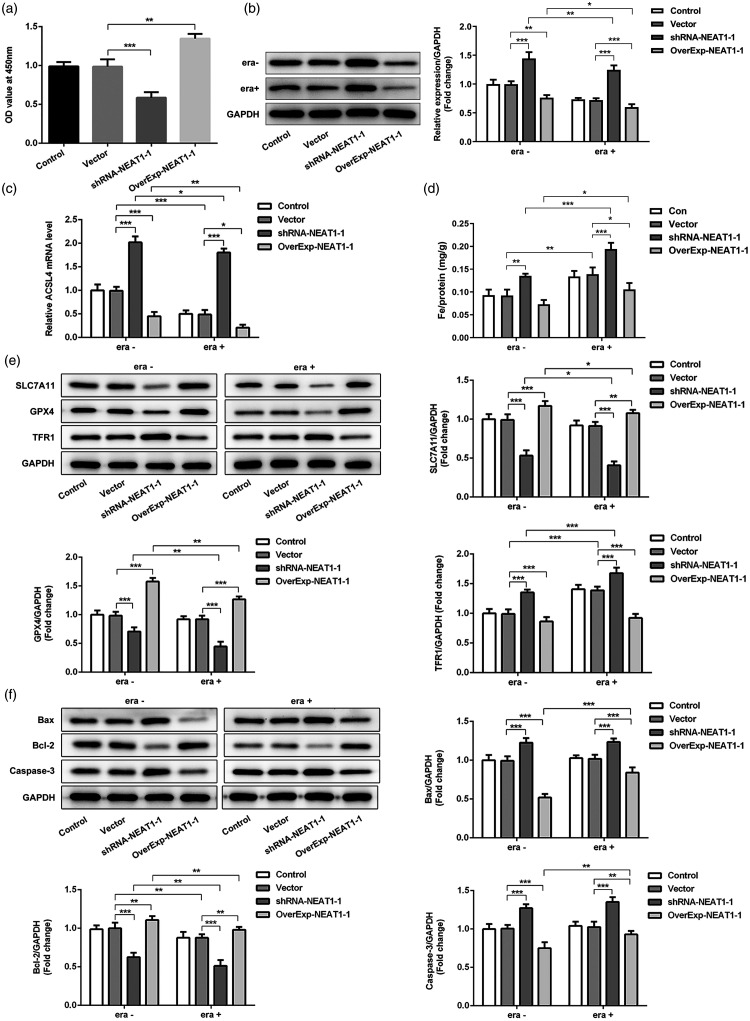
To further evaluate the role of NEAT1 in the ferroptosis pathway, we used bioinformatics analysis and found that NEAT1 and ACSL4 have targeted regions that directly bind (Figure 3a). We then designed a dual-luciferase reporter gene assay and confirmed that NEAT1 could bind to ACSL4 (p < 0.01; Figure 3b). We showed that NEAT1 overexpression or silencing increased or suppressed cell proliferation, respectively, as detected by EdU staining (p < 0.001; Figure 3c) and cell viability (CCK-8 assay; Figure 4a). Specifically, erastin treatment markedly reduced ACSL4 protein and mRNA levels in the control group, and NEAT1 regulated ACSL4 protein and mRNA levels (Figure 4b, c). Then, we detected expression of nodal proteins associated with the ferroptosis and classical apoptosis pathways. Notably, regulation of NEAT1 in the iron intake pathway (iron ion and TFR1, with high expression, mediating ferroptosis) and the glutathione peroxidase pathway (SLC7A11 and GPX4, with low expression, mediating ferroptosis) affected the tolerance of tumor cells to ferroptosis (Figure 4d, e). In addition, erastin had a minor effect on classical apoptosis pathway-related proteins (Bax, Bcl-2, and caspase-3). The expression of Bax and caspase-3 was increased whereas Bcl-2 levels were reduced when NEAT1 silencing was induced in A549 cells with or without erastin induction (p < 0.01). NEAT1 overexpression induced the opposite effects in A549 cells with or without erastin treatment (p < 0.01 (Figure 4f).
Figure 3.
(a, b) The targeting relationship of NEAT1 and ACSL4 was determined by dual-luciferase reporter gene assay. (c) Cell proliferation was detected by EdU staining. ***p < 0.001. Wt, wild type; mut, mutant; shRNA-NEAT1, A549 cells were transfected with NEAT1 silencing plasmid; OverExp-NEAT1-1, A549 cells were transfected with NEAT1 overexpressing plasmid.
Figure 4.
(a) Cell viability was analyzed by using the CCK-8 assay after NEAT1 silencing (short hairpin [sh]RNA-NEAT1-1) or overexpression (OverExp-NEAT1-1) intervention. (b, c) Detection of ASCL4 protein and mRNA levels through western blot and reverse transcription quantitative (RT-q)PCR. (d) Levels of iron (Fe) in A549 cells with (era+) or without (era−) erastin induction. (e) Immunoblot analysis of SLC7A11, GPX4, and TFR1 expression after NEAT1 silencing or overexpression. (f) Analysis of apoptosis-related proteins Bax, Bcl-2, and caspase-3 affected by ferroptosis induction by erastin after NEAT1 silencing or overexpression. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In this study, our aim was to identify an essential regulatory role of NEAT1 in ferroptotic cell death. The dual-luciferase reporter assay revealed that NEAT1 could bind to ACSL4 mRNA. We further found that NEAT1 regulated the protein expression of ACSL4. It was recently reported that ACSL4 levels reflect ferroptosis sensitivity and that ACSL4 reduces the sensitivity of cells to ferroptosis induction.8 Accumulating evidence indicates that the incidence and progression of lung cancer might be attributed to ferroptosis.13 Nevertheless, the ferroptosis sensitivity of some tumor cells has been shown to be weak.14
In the present study, 10 µM erastin was used to induce ferroptosis. We observed that the sensitivity of A549 cells to erastin-induced ferroptosis was lower than that of other NSCLC cell lines. In addition, erastin induction did not significantly affect SLC7A11 or GPX4 levels in the control group. GPX4 is a versatile, selenium-based glutathione peroxidase that can effectively repair cell damage caused by lipid oxidation; it is a major inhibitor of ferroptosis.15–17 It tightly controls ferroptosis, the inactivation of which could contribute to an increase in uncontrolled lipid peroxidation, inducing cell death.15 However, the levels of SLCA11 and GPX4 were decreased much more by NEAT1 silencing with erastin treatment than without erastin, indicating that NEAT1 silencing changed the sensitivity of A549 cells to erastin. A study found that NEAT1 regulated iron content in A549 cells with or without erastin induction, facilitating the generation of superoxide radicals through the Fenton reaction, which could cause lipid peroxidation.8 An increase in MDA levels (reflecting the degree of membrane lipid peroxidation18) after NEAT1 silencing implied an increase in intracellular lipid peroxidation induced by NEAT1 knockdown. The inhibition of GPX4 activity could trigger ferroptosis.19 Furthermore, GPX4 levels affect the sensitivity of cells to erastin.20 This result indicated that NEAT1 silencing could enhance the ferroptosis sensitivity of cells to erastin.
NEAT1 overexpression did not alter the sensitivity of A549 cells to erastin in the expression of TFR1. Protein levels of TFR1 were elevated after erastin administration in the control group. TFR1, often considered a specific marker of ferroptosis, is necessary for iron input into cells, and it is regulated by the intracellular iron concentration.21 Erastin has been reported to enhance TFR1 levels.22–24 However, NEAT1 silencing increased the response of A549 cells to erastin. We also found that NEAT1 affected the classical apoptosis pathway in A549 cells through analysis of Bax, Bcl-2, and caspase-3 expression levels. Erastin treatment did not affect intracellular Bcl2, Bax, and caspase-3 activities. As reported in other studies, ferroptosis is not dependent on the classical apoptotic pathway. Moreover, it does not require caspase or Bax/Bak, which regulate mitochondrial outer membrane permeability, and it is not affected by other cell death inhibitors.25–27 A regulatory effect of NEAT1 on cell apoptosis and proliferation has been found in some studies.28 However, this regulatory mechanism of NEAT1 in ferroptosis requires further confirmation in an in vivo study, which is a limitation of the present study.
In summary, we identified roles of NEAT1 in ferroptosis and demonstrated the sensitivity of ferroptosis to erastin induction. ACSL4 appears to mediate the function of NEAT1 in the ferroptosis process. Targeting NEAT1 or ACSL4 might represent a potential therapeutic intervention in NSCLC, a possibility that deserves further exploration.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Aiwen Liu https://orcid.org/0000-0001-5057-166X
References
- 1.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016; 5: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gernapudi R, Wolfson B, Zhang Y, et al. MicroRNA 140 promotes expression of long noncoding RNA NEAT1 in adipogenesis. Mol Cell Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Gu W, Zhu X. NEAT1 mediates paclitaxel-resistance of non-small cell of lung cancer through activation of Akt/mTOR signalling pathway. J Drug Target 2019; 27: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 4.Dong D, Mu Z, Wang W, et al. Prognostic role of long noncoding RNA NEAT1 in various carcinomas: a meta-analysis. Onco Targets Ther 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Mo Q, Wan X, et al. NEAT1/hsa‐mir‐98‐5p/MAPK6 axis is involved in non–small‐cell lung cancer development. J Cell Biochem 2019; 120: 2836–2846. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez SW, Sviderskiy VO, Terzi EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017; 551: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaco ME, Creighton CJ, Lee P, et al. Expression of long-chain fatty acyl-CoA synthetase 4 in breast and prostate cancers is associated with sex steroid hormone receptor negativity. Transl Oncol 2010; 3: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017; 13: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berghe TV, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15: 135–147. [DOI] [PubMed] [Google Scholar]
- 10.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 2018. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Zhang Q, Wang Q, et al. NEAT1 paraspeckle promotes human hepatocellular carcinoma progression by strengthening IL-6/STAT3 signaling. Oncoimmunology 2018; 7: e1503913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan LJ, Zhong TF, Tang RX, et al. Upregulation and clinicopathological significance of long non-coding NEAT1 RNA in NSCLC tissues. Asian Pac J Cancer Prev 2015; 16: 2851–2855. [DOI] [PubMed] [Google Scholar]
- 13.Gai C, Yu M, Li Z, et al. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J Cell Physiol 2020; 235: 3329–3339. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Xie Y, Cao L, et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2015; 2: e1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014; 16: 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 1999; 27: 951–965. [DOI] [PubMed] [Google Scholar]
- 17.Hambright WS, Fonseca RS, Chen L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 2017; 12: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 2017; 524: 13–30. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Sui S, Wang L, et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol 2020; 235: 3425–3437. [DOI] [PubMed] [Google Scholar]
- 20.Imai H, Matsuoka M, Kumagai T, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 2017; 403: 143–170. [DOI] [PubMed] [Google Scholar]
- 21.Qian Z, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 2002; 54: 561–587. [DOI] [PubMed] [Google Scholar]
- 22.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 2019; 10: 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai T, Lei P, Zhou H, Liang R, Sun Y. Sigmareceptor protects against ferroptosis in hepatocellular carcinoma cells. Journal of Cellular and Molecular Medicine 2019; 23: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng H, Schorpp K, Jin J, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep 2020; 30: 3411–3423.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ 2016; 23: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Guo P, Xie X, et al . Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med 2017; 21: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do Van B, Gouel F, Jonneaux A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis 2016; 94: 169–178. [DOI] [PubMed] [Google Scholar]
- 28.Zhou HY, Wei Q, Cao HY, et al. Long non-coding RNA NEAT1 promotes myocardiocyte apoptosis and suppresses proliferation via regulation of miR-129-5p. J Cardiovasc Pharmacol 2019; 74: 1. [DOI] [PubMed] [Google Scholar]