Abstract
Objective
This study examined the effect of the NF-κB pathway on tobacco smoke-elicited bladder epithelial–mesenchymal transition (EMT) and cancer stem cell (CSC) marker expression in vivo. The effect of diallyl trisulfide (DATS) treatment was also examined.
Methods
BALB/c mice were exposed to tobacco smoke and treated with an NF-κB inhibitor and DATS. Western blotting, quantitative real-time PCR, and immunohistochemical staining were used to detect the changes of relevant indices.
Results
Phosphorylated inhibitor of kappa-B kinase alpha/beta expression and p65 and p50 nuclear transcription were increased by tobacco smoke exposure, whereas inhibitor of kappa-B expression was decreased. In addition, tobacco smoke reduced the expression of epithelial markers but increased that of mesenchymal and CSC markers. Our study further demonstrated that tobacco smoke-mediated EMT and CSC marker expression were attenuated by inhibition of the NF-κB pathway. Moreover, DATS reversed tobacco smoke-induced NF-κB pathway activation, EMT, and the acquisition of CSC properties in bladder tissues.
Conclusions
These data suggested that the NF-κB pathway regulated tobacco smoke-induced bladder EMT, CSC marker expression, and the protective effects of DATS.
Keywords: Tobacco smoke, NF-κB, bladder cancer, IKKα/β, IκB, cancer stem cells, epithelial–mesenchymal transition, diallyl trisulfide
Introduction
Bladder cancer is a typical malignant cancer of the urinary system that is associated with approximately 429,793 new cases and 165,084 deaths each year.1,2 Smokers are reported to have a 4-fold higher risk of bladder cancer than non-smokers.3 Tobacco smoke is a major risk factor for bladder cancer, and great progress has been made in understanding the molecular mechanisms by which it promotes bladder cancer.4 However, the exact process of tobacco-smoke induced bladder cancer development remains largely unknown.
Epithelial–mesenchymal transition (EMT) contributes to cancer progression. In this process, cells lose epithelial characteristics and acquire mesenchymal features.5 EMT plays a crucial role in the occurrence and metastasis of cancer, and it can be activated by carcinogens.6 It has been documented that tobacco smoke exposure promotes EMT in cells and mice.7,8 Nonetheless, the potential molecular mechanisms by which tobacco smoke induces urocystic EMT are unclear. Tobacco smoke-induced EMT has been reported to play an important role in the early stages of carcinogenesis.4,9 Therefore, investigating the mechanisms by which tobacco smoke mediates bladder EMT may provide new insights into bladder cancer treatment and prevention.
Cancer stem cells (CSCs) comprise a subset of cells with unique characteristics, including the abilities to self-renew; generate tumor heterogeneity; and drive tumor growth, metastasis, and drug resistance.10 Increasing evidence indicates that many cancers, including bladder cancer, originate from CSCs.10,11 CSCs are closely related to the formation, metastasis, and recurrence of various tumors, including bladder tumors. CSCs are also resistant to chemotherapy and radiation therapy. Fortunately, the characteristics of CSCs can be regulated by multiple signaling pathways, including the NF-κB pathway.12,13
The NF-κB pathway plays important roles in several physiological and pathological processes, including inflammation, cell proliferation, apoptosis, EMT, and carcinogenesis. The NF-κB pathway is activated in a variety of cancers.14,15 Increasing studies have revealed the positive associations among the NF-κB pathway, EMT, and the acquisition of CSC-like properties.16,17 Studies illustrated that tobacco smoke activates the NF-κB pathway.18,19 However, no studies have investigated the role of the NF-κB pathway in tobacco smoke-induced bladder EMT and CSC property acquisition in vivo.
Diallyl trisulfide (DATS), a primary organ sulfur compound in garlic, is responsible for the therapeutic effects of garlic.20 Studies described several beneficial effects of DATS on human health, such as anti-inflammatory, anti-oxidant, and anti-cancer properties.21,22 Studies reported that DATS can regulate the activity of the NF-κB pathway.23,24 EMT and the acquisition of CSC properties are critical pathophysiological processes in cancer initiation that regulate early events in carcinogenesis. DATS may represent an effective chemotherapeutic agent that regulates EMT and the acquisition of CSC properties. However, its effects on tobacco smoke-associated urocystic EMT and CSC property acquisition have not been defined.
Thus, our study examined the regulation of the NF-κB pathway during tobacco smoke-triggered bladder EMT and CSC property acquisition and the preventive effects of DATS on bladder carcinogenesis in mice. Our findings may provide new insights into the pathogenesis of tobacco smoke-triggered bladder tumorigenesis.
Materials and methods
Chemicals and reagents
DATS was purchased from Sigma-Aldrich (St. Louis, MO, USA). Inhibitor of kappa-B kinase alpha/beta (p-IKKα/β), inhibitor of kappa B (IκB), p65, vimentin, E-cadherin, histone H3, and N-cadherin antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). CD44, Nanog, ALDH1A1, and Oct4 antibodies were obtained from Proteintech (Rosemont, IL, USA). ZO-1 and p50 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All primers used in the experiments were synthesized by Invitrogen (Carlsbad, CA, USA). The NF-κB pathway inhibitor pyrrolidinedithiocarbamate ammonium (PDTC) was acquired from Tocris Bioscience (Bristol, UK). Nucleoprotein and cytoplasmic protein extraction kits were procured from KeyGen (Nanjing, China).
Experimental animals and treatment
The Animal Research Center of Anhui Medical University (Hefei, China) provided 60 male BALB/c mice (4–6 weeks old) for this study. The animal studies were conducted in strict accordance with the recommendations of the Laboratory Animal Management Committee of Anhui Medical University, which also approved the study protocol (2018.03.01, No. LLSC 20180093). We followed the guide for the care and use of laboratory animals and the Equator network guidelines (animal pre-clinical studies) in caring for experimental animals, and we strived to reduce the number of animals used and minimize their suffering.25
Tobacco smoke exposure
Mice were randomly assigned into groups of six animals each. The control group was exposed to filtered air. The tobacco smoke group was exposed to tobacco smoke using a smoking device for 6 hours daily for 12 weeks. Smoke was drawn from a commonly consumed cigarette brand in China (containing 12 mg of tar and 1.1 mg of nicotine per cigarette) using a vacuum, and each cigarette took 5 minutes to be consumed. Smoke was delivered to whole-body exposure chambers with a target concentration of total particulate matter of 80 mg/m3. At the end of the experiment, the mice were sacrificed via suffocation.
Delivery of a specific NF-κB inhibitor in vivo
Mice were randomly assigned to groups of six animals each. Mice in the control group were exposed to filtered air. Mice in the tobacco smoke group were exposed to tobacco smoke. Mice in the DMSO group were injected with sterile DMSO and exposed to tobacco smoke, and mice in the PDTC group were injected with PDTC (10 mg/kg body weight) and exposed to tobacco smoke. PDTC was dissolved in sterile DMSO and injected intraperitoneally every other day.
DATS treatment
Mice were randomly divided into four groups of six animals each. Mice in the control group were exposed to air and intragastrically administered corn oil. Mice in the tobacco smoke group were exposed to tobacco smoke and treated intragastrically with corn oil. Mice in the tobacco smoke+DATS 5 mg/kg group were exposed to tobacco smoke and intragastrically administered 5 mg/kg DATS dissolved in corn oil. Mice in the tobacco smoke+DATS 10 mg/kg group were exposed to tobacco smoke and intragastrically administered 10 mg/kg DATS dissolved in corn oil.
Western blot analysis
Mouse bladder tissue proteins were lysed using nuclear and cytoplasmic extraction reagent kits. Equal amounts of proteins were subjected to 7.5% or 10% SDS-PAGE and immunoblotting as previous described.4
Quantitative real-time PCR
RNA extraction was conducted using TRIzol reagent (Invitrogen). Real-time PCR was performed as previously reported5 using an ABI 7300 real-time PCR detection system (Applied Biosystems, Foster City, CA, USA) and Power SYBR Green Master Mix (TaKaRa, Kusatsu, Shiga, Japan). Reverse transcription of RNA into cDNA was performed using reverse transcriptase (Promega, Madison, WI, USA). GAPDH expression served as the loading control in real-time PCR. The primers used for PCR are presented in Table 1.
Table 1.
Primer sequences.
| Gene name | Primer sequences (5′–3′) |
|---|---|
| E-cadherin | Forward 5′-TCGACACCCGATTCAAAGTGG-3′ |
| Reverse 5′-TTCCAGAAACGGAGGCCTGAT-3′ | |
| ZO-1 | Forward 5′-GCAGCCACAACCAATTCATAG-3′ |
| Reverse 5′-GCAGACGATGTTCATAGTTTC-3′ | |
| CK5 | Forward 5′-CTGGAGAGTAGTCTAGACCAAGCC-3′ Reverse 5′-GTTAGAACCAAAACAAAATTTGGG-3′ |
| Snail-1 | Forward 5′-TAC AGC GAG CTG CAG GAC TCT AAT-3′ Reverse 5′-AGG ACA GAG TCC CAG ATG AGC ATT-3′ |
| Vimentin | Forward 5′-CCTTGACATTGAGATTGCCA-3′ |
| Reverse 5′-GTATCAACCAGAGGGAGTGA-3′ | |
| N-cadherin | Forward 5′-ATCAAGTGCCATTAGCCAAG-3′ |
| Reverse 5′-CTGAGCAGTGAATGTTGTCA-3′ | |
| GAPDH | Forward 5′-GCTGCCCAACGCACCGAATA-3′ |
| Reverse 5′-GAGTCAACGGATTTGGTCGT-3′ |
Immunohistochemistry
After 12 weeks of tobacco smoke exposure, mouse bladder tissues were isolated and fixed in 4% formaldehyde for immunohistochemistry. Immunohistochemistry was performed to detect the expression of E-cadherin and vimentin as previous described.26
Statistical analysis
All data in this study were confirmed in at least three independent experiments, and these results were presented as the mean ± standard deviation. One-way analysis of variance and Fisher’s least significant difference were used to compare statistical differences between multiple groups. The unpaired Student’s t-test was also used for comparisons between two groups. Statistical analyses were performed using SPSS version 16.0 software (SPSS, Chicago, IL, USA). P < 0.05 denoted statistical significance.
Results
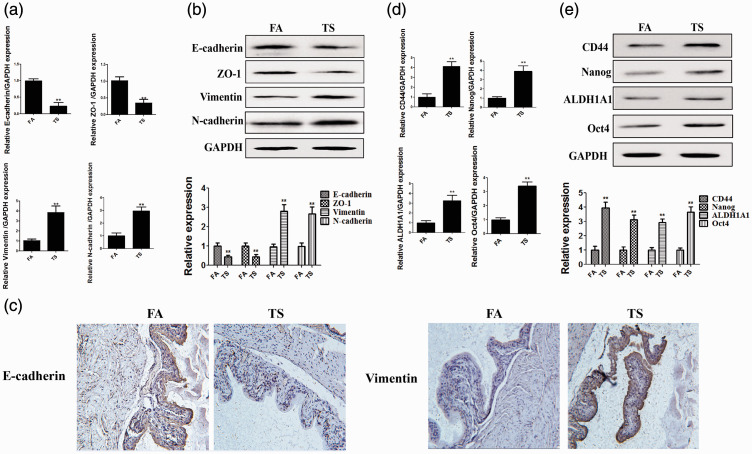
Tobacco smoke induced the abnormal expression of EMT and CSC markers in the mouse bladder
In the current study, we first examined whether tobacco smoke exposure induced bladder EMT in mice. After 12 weeks of tobacco smoke exposure, the results of real-time PCR illustrated that tobacco smoke exposure reduced E-cadherin, ZO-1, and CK5 mRNA levels, whereas Snail-1, vimentin, and N-cadherin expression was elevated (all P < 0.01, Figure 1a). Western blot analyses revealed that tobacco smoke reduced epithelial marker protein expression and increased the levels of mesenchymal markers (all P < 0.01, Figure 1b). Immunohistochemical staining illustrated that tobacco smoke increased vimentin expression and decreased E-cadherin expression (both P < 0.01, Figure 1c). Our data also revealed that tobacco smoke promoted the expression of CD44, Nanog, ALDH1A1, and Oct4 (all P < 0.01, Figure 1d).
Figure 1.
Tobacco smoke alters the expression of EMT and CSC markers. (a) Tobacco smoke exposure reduced the mRNA levels of epithelial markers and increased the levels of mesenchymal markers. (b) Tobacco smoke exposure induced alterations in the protein expression of EMT markers. (c) Immunohistochemistry illustrated that tobacco smoke decreased E-cadherin expression and increased vimentin expression. (d) Alterations in the mRNA expression of CSC markers induced by tobacco smoke. (e) Alterations in the protein expression of CSC markers induced by tobacco smoke. **P < 0.01, compared with FA.
EMT, epithelial–mesenchymal transition; CSC, cancer stem cell; FA, filtered air; TS, tobacco smoke.
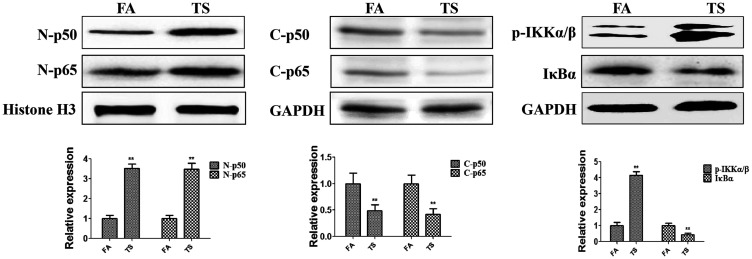
Tobacco smoke exposure increased NF-κB activation in the mouse bladder
To determine whether urocystic EMT induced by tobacco smoke is related to the NF-κB pathway, the levels of p65 and p50 in the mouse bladder were investigated. Western blotting indicated that tobacco smoke exposure increased the nuclear levels of p65 and p50 (both P < 0.01, Figure 2). Meanwhile, we found that p-IKKα/β expression was elevated in the cytoplasm following tobacco smoke exposure, and whereas IκBα expression was decreased (both P < 0.01, Figure 2). These results suggested that tobacco smoke-triggered bladder EMT was associated with the NF-κB pathway.
Figure 2.
Tobacco smoke exposure increased NF-κB pathway activity. Tobacco smoke effectively elevated the expression of p65, p50, and p-IKKα/β and decreased the expression of IκBα. **P < 0.01, compared with FA.
p-IKKα/β, phosphorylated inhibitor of kappa-B kinase alpha/beta; IκBα, inhibitor of kappa-B alpha; FA, filtered air; TS, tobacco smoke N-p50, nuclear p50; N-p65, nuclear p65; C-p50, cytoplasmic p50; C-p65, cytoplasmic p65.
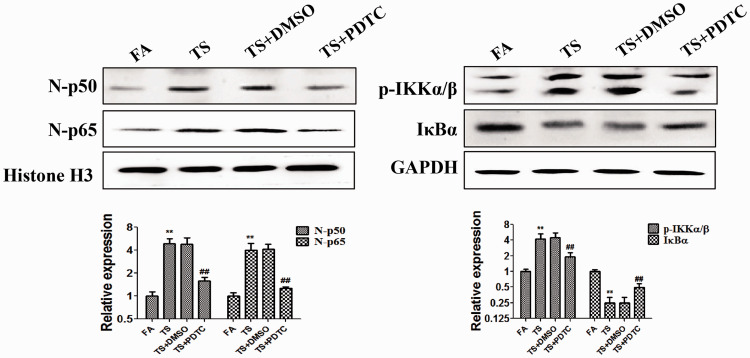
PDTC reversed tobacco smoke-mediated urocystic NF-κB activation
To verify the precise effect of the NF-κB pathway on tobacco smoke-elicited bladder EMT, BALB/c mice were treated with PDTC, a membrane-permeant inhibitor of NF-κB activation. After 12 weeks of treatment, western blotting illustrated that 10 mg/kg PDTC reversed tobacco smoke-induced expression changes of p65, p50, p-IKKα/β, and IκBα (all P < 0.01, Figure 3).
Figure 3.
PDTC inhibited tobacco smoke-induced NF-κB pathway activation. Western blotting revealed that PDTC reversed tobacco smoke-induced changes of p65, p50, p-IKKα/β, and IκB expression. **P < 0.01, compared with FA; ##P < 0.01, compared with TS+DMSO.
PDTC, pyrrolidinedithiocarbamate ammonium; p-IKKα/β, phosphorylated inhibitor of kappa-B kinase alpha/beta; IκBα, inhibitor of kappa-B alpha; FA, filtered air; TS, tobacco smoke; N-p50, nuclear p50; N-p65, nuclear p65.
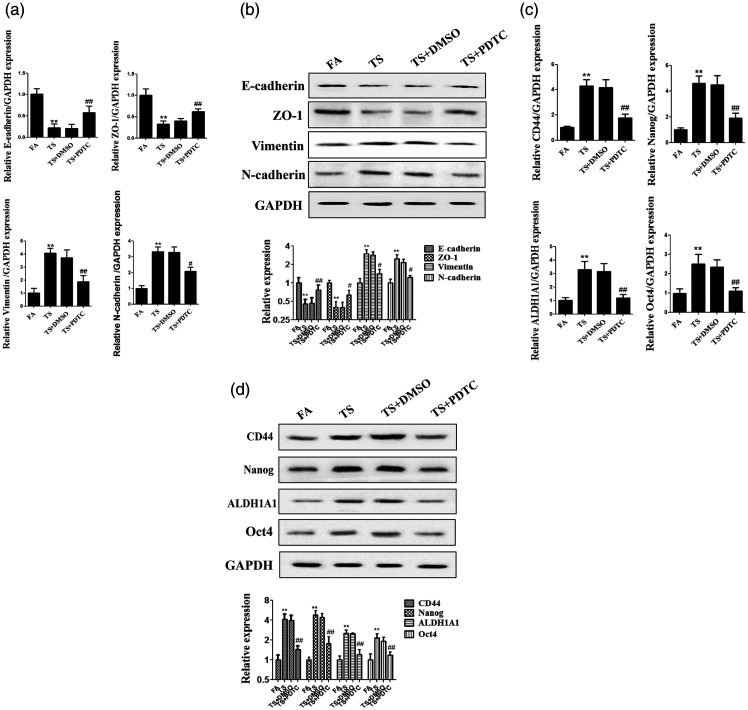
Tobacco smoke-triggered EMT and CSC property acquisition were reversed by PDTC
To determine the role of the NF-κB pathway in urocystic EMT caused by tobacco smoke, the expression of EMT markers was detected after treatment with PDTC and tobacco smoke. The results indicated that alterations in the levels of EMT markers induced by tobacco smoke, including the downregulation of E-cadherin, ZO-1, and CK5 and upregulation of Snail-1, vimentin, and N-cadherin, were significantly suppressed by PDTC (all P < 0.05, Figure 4a, b). We also found that PDTC reversed the tobacco smoke-induced abnormal expression of CSC markers (all P < 0.01, Figure 4c). These data indicated that inhibition of the NF-κB pathway reversed tobacco smoke-triggered EMT and CSC marker expression in vivo.
Figure 4.
NF-κB pathway suppression reversed tobacco smoke-induced changes of EMT and CSC marker expression. (a) Real-time PCR of EMT marker expression. (b) Western blotting of EMT marker expression. (c) Real-time PCR of CSC marker expression. (d) Western blotting of CSC marker expression. **P < 0.01, compared with FA; #P < 0.05, ##P < 0.01, compared with TS+DMSO.
EMT, epithelial–mesenchymal transition; CSC, cancer stem cell; FA, filtered air; TS, tobacco smoke; PDTC, pyrrolidinedithiocarbamate ammonium.
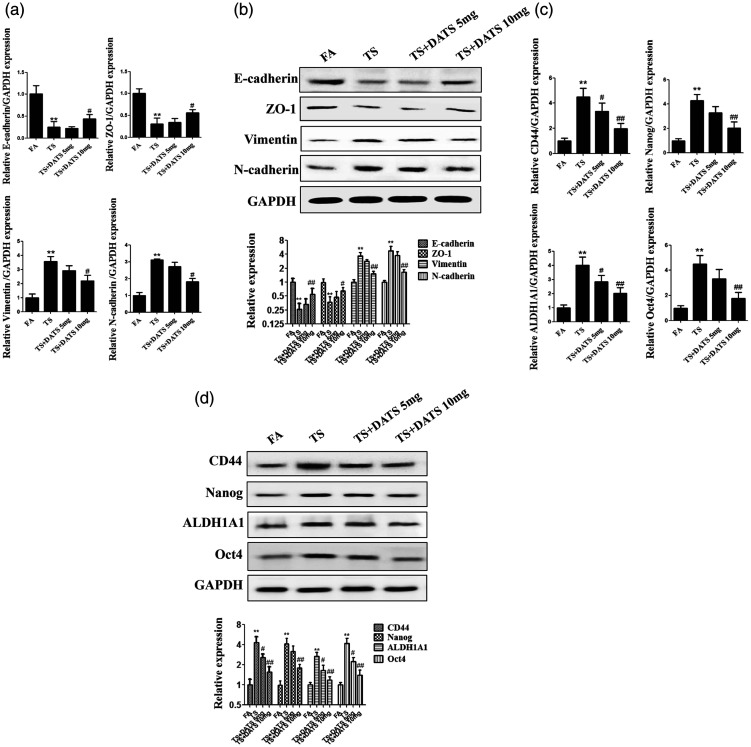
DATS attenuated tobacco smoke-mediated changes in EMT and CSC marker expression in the mouse bladder
To explore the effects of DATS on tobacco smoke-elicited EMT and CSC marker expression, mice were treated with DATS and then exposed to tobacco smoke. The results in Figure 5 indicated that 10 mg/kg DATS treatment attenuated the downregulation of E-cadherin, CK5, and ZO-1 and upregulation of vimentin, Snail-1, N-cadherin, CD44, Nanog, ALDH1A1, and Oct4 induced by tobacco smoke exposure (all P < 0.05). These results supported the preventive effects of DATS on tobacco smoke-induced changes in EMT and CSC marker expression in the mouse bladder.
Figure 5.
DATS attenuated tobacco smoke-mediated changes of urocystic EMT and CSC marker expression. (a) Real-time PCR of the mRNA levels of EMT markers. (b) Western blotting of EMT marker expression. (c) Real-time PCR of the mRNA levels of CSC markers. (d) Western blotting of CSC marker expression. **P < 0.01, compared with FA; #P < 0.05, ##P < 0.01, compared with TS+DMSO.
DATS, diallyl trisulfide; EMT, epithelial–mesenchymal transition; CSC, cancer stem cell; FA, filtered air; TS, tobacco smoke.
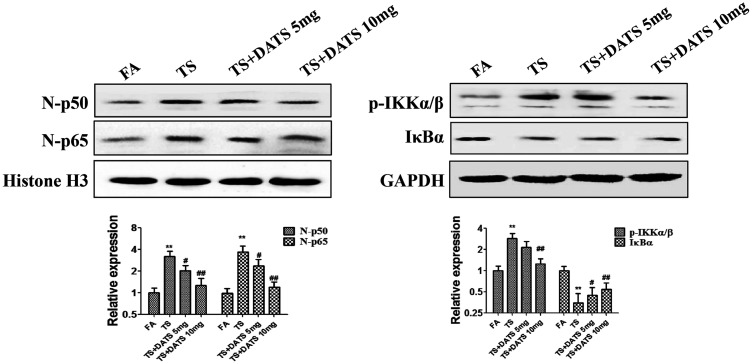
DATS reversed tobacco smoke-stimulated NF-κB pathway activation
To investigate the effects of DATS on tobacco smoke-induced NF-κB pathway activation, we further studied the alterations of p65, p50, p-IKKα/β, and IκB expression in the mouse bladder after DATS treatment. We found that daily treatment with 10 mg/kg DATS obviously reversed the protein expression changes induced by tobacco smoke exposure (all P < 0.05, Figure 6).
Figure 6.
DATS reversed tobacco smoke-induced activation of the NF-κB pathway. DATS attenuated tobacco smoke-stimulated NF-κB pathway activation in the mouse bladder. Western blotting was performed to analyze the expression changes of p65, p50, p-IKKα/β, and IκB.
DATS, diallyl trisulfide; EMT, epithelial–mesenchymal transition; CSC, cancer stem cell; FA, filtered air; TS, tobacco smoke; p-IKKα/β, phosphorylated inhibitor of kappa-B kinase alpha/beta; IκBα, inhibitor of kappa-B alpha.
Discussion
In recent years, many studies including our previous studies27–29 indicated that tobacco smoke is a leading cause of bladder cancer, and great progress has been made in understanding the mechanisms by which tobacco smoke exposure causes bladder cancer. However, little is known regarding the exact mechanism by which tobacco smoke promotes the initiation of bladder cancer. In this study, we found that tobacco smoke triggered EMT and the acquisition of CSC features in the BALB/c mouse bladder. Furthermore, our data indicated that DATS suppressed the NF-κB pathway, thereby preventing the changes in urocystic EMT and CSC marker expression induced by tobacco smoke in vivo.
EMT is a process in which epithelial cells undergo a phenotype change to mesenchymal cells, and it is crucially involved in the initiation and development of cancer. Previous studies reported that tobacco smoke could induce EMT, which regulates early events in many cancers.4,30 To determine whether the alterations of EMT occurred in the bladders of mice exposed to tobacco smoke, the expression of EMT markers was examined in our study. In accordance with previous reports, we found that tobacco smoke reduced the expression of E-cadherin, ZO-1, and CK5 and increased the expression of vimentin, Snail-1, and N-cadherin. These results suggested that tobacco smoke induced EMT in the mouse bladder. CSCs play plays a key role in the initiation and development of cancer. In the current study, we found that the expression of CD44, Nanog, ALDH1A1, and Oct4 was upregulated by tobacco smoke exposure. Our results suggested that tobacco smoke exposure promoted the acquisition of CSC features in the mouse bladder. In addition, we found that mice lost weight after exposure to tobacco smoke. However, the mechanisms by which tobacco smoke induce EMT and the acquisition of CSC characteristics are not well articulated.
Several signaling pathways are involved in EMT and the acquisition of CSC characteristics, including Wnt/β-catenin, HIF-2a, Notch, and some epigenetic pathways. The NF-κB pathway plays a critical role in multiple physiological processes and pathologies including cell proliferation, apoptosis, inflammation, and cancer initiation.31 Several studies suggested that the NF-κB pathway promotes EMT and the acquisition of CSC characteristics.32,33 However, few studies systematically analyzed tobacco smoke-triggered EMT and CSC property acquisition in the bladder. Our study results indicated that tobacco smoke-induced urocystic EMT and CSC phenotype acquisition were related to the upregulation of p65 and p50 in vivo. Meanwhile, tobacco smoke exposure increased the levels of p-IKKα/β and decreased IκB expression. These data implicated the NF-κB pathway in the induction of EMT and acquisition of CSC characteristics following tobacco smoke exposure in vivo.
As mentioned previously, tobacco smoke-induced urocystic EMT is associated with NF-κB pathway activation in vivo. To identify the role of the NF-κB pathway in EMT and the acquisition of CSC characteristics, mice were treated with PDTC, which has been reported to inhibit NF-κB pathway activation in a variety of cells.9,34 We found that PDTC suppressed tobacco smoke-induced p65, p50, and p-IKKα/β upregulation and IκB downregulation. Furthermore, suppression of the NF-κB pathway reversed tobacco smoke-mediated alterations in EMT and CSC marker levels, including the elevated expression of E-cadherin, ZO-1, and CK5 and decreased expression of vimentin, Snail-1, N-cadherin, CD44, Nanog, ALDH1A1, and Oct4. These results certified that the NF-κB pathway regulates bladder EMT and CSC feature changes induced by tobacco smoke.
It has been reported that diet modification and regular physical activity can prevent approximately one-third of cancers. Phytochemicals, especially food-derived phytochemicals, have demonstrated promise for preventing the initiation and development of cancer. The safety and anti-cancer activity of DATS have been confirmed in many types of cancer.10,35,36 The doses of DATS used in our study were 5 and 10 mg/kg body weight per day. The effects of DATS on tobacco smoke-induced bladder EMT and CSC marker expression were examined in mice. As presented in Figure 5, DATS (10 mg/kg) inhibited tobacco smoke-induced changes in EMT and CSC marker expression. We further explored whether DATS exerts its effects through the NF-κB pathway. The data revealed that tobacco smoke-triggered changes of p65, p50, p-IKKα/β, and IκB expression were significantly suppressed by 12 weeks of DATS treatment.
Our study illustrated that the NF-κB pathway positively regulated tobacco smoke-triggered bladder EMT/CSC marker changes and the in vivo effects of DATS on these changes. Our findings may provide new insights into the mechanisms and chemoprevention of bladder cancer induced by tobacco smoke exposure. However, the current study was an initial analysis, and we must conduct additional research before the findings can be applied to clinical practice.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 8157100782), the Key Project of Natural Science Research in Universities of Anhui Province (KJ2018A0209), the Foundation for Excellent Young Teachers of Jiangsu University, and the Research and Practice innovation program for graduate students of Jiangsu Province (No. KYCX20_3089).
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: Hao Geng and Zhaofeng Liang designed the research and wrote the paper. Lei Feng and Wenhao Guo performed research. Dongdong Xie and Liangkuan Bi analyzed data. Tao Zhang assisted with animal rearing and experimental operation. Professor Yu contributed to manuscript writing and revision.
ORCID iD: Dexin Yu https://orcid.org/0000-0001-9799-0837
References
- 1.Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017; 71: 96–108. [DOI] [PubMed] [Google Scholar]
- 2.Alifrangis C, McGovern U, Freeman A, et al. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol 2019; 16: 465–483. [DOI] [PubMed] [Google Scholar]
- 3.Chen LM, Nergard JC, Ni L, et al. Long-term exposure to cigarette smoke extract induces hypomethylation at the RUNX3 and IGF2-H19 loci in immortalized human urothelial cells. PLoS One 2013; 8: e65513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Z, Xie W, Wu R, et al . Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin. Int J Clin Exp Pathol 2015; 8: 4503–4513. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Lee S, Shin E, et al. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells 2020; 9: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindner P, Paul S, Eckstein M, et al. EMT transcription factor ZEB1 alters the epigenetic landscape of colorectal cancer cells. Cell Death Dis 2020; 11: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia H, Xue J, Xu H, et al. Andrographolide antagonizes the cigarette smoke-induced epithelial-mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR. Toxicology 2019; 422: 84–94. [DOI] [PubMed] [Google Scholar]
- 8.Xie C, Zhu J, Wang X, et al. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J Exp Clin Cancer Res 2019; 38: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P, Gao YJ, Liang HL. Effect of NF- kappa B inhibitor PDTC on VEGF and endostatin expression of mice with Lewis lung cancer. Asian Pac J Trop Med 2015; 8: 220–224. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Meng Y, Xie C, et al. Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/beta-catenin pathway. J Cell Biochem 2018; 119: 4134–4141. [DOI] [PubMed] [Google Scholar]
- 11.Liang Z, Lu L, Mao J, et al. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/beta-catenin. Cell Death Dis 2017; 8: e3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaltschmidt C, Banz-Jansen C, Benhidjeb T, et al. A Role for NF-kappaB in Organ Specific Cancer and Cancer Stem Cells. Cancers (Basel) 2019; 11: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong W, Chen A, Chao X, et al. Chrysin Inhibits Proinflammatory Factor-Induced EMT Phenotype and Cancer Stem Cell-Like Features in HeLa Cells by Blocking the NF-kappaB/Twist Axis. Cell Physiol Biochem 2019; 52: 1236–1250. [DOI] [PubMed] [Google Scholar]
- 14.Kaltschmidt B, Greiner JFW, Kadhim HM, et al. Subunit-Specific Role of NF-kappaB in Cancer. Biomedicines 2018; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedlinger T, Haas J, Busch J, et al. The Direct and Indirect Roles of NF-kappaB in Cancer: Lessons from Oncogenic Fusion Proteins and Knock-in Mice. Biomedicines 2018; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pires BR, Mencalha AL, Ferreira GM, et al. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS One 2017; 12: e0169622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiraiwa K, Matsuse M, Nakazawa Y, et al. JAK/STAT3 and NF-kappaB Signaling Pathways Regulate Cancer Stem-Cell Properties in Anaplastic Thyroid Cancer Cells. Thyroid 2019; 29: 674–682. [DOI] [PubMed] [Google Scholar]
- 18.Ma N, Deng TT, Wang Q, et al. Erythromycin Regulates Cigarette Smoke-Induced Proinflammatory Mediator Release Through Sirtuin 1-Nuclear Factor kappaB Axis in Macrophages and Mice Lungs. Pathobiology 2019; 86: 237–247. [DOI] [PubMed] [Google Scholar]
- 19.Wu YP, Cao C, Wu YF, et al. Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-kappaB activation. Toxicol Lett 2017; 270: 17–24. [DOI] [PubMed] [Google Scholar]
- 20.Uno S, Sakai M, Fujinari Y, et al. Diallyl Trisulfide Enhances Benzo[a]pyrene-induced CYP1A1 Expression and Metabolic Activation in Hepatic HepG2 Cells. Anticancer Res 2019; 39: 2369–2375. [DOI] [PubMed] [Google Scholar]
- 21.Puccinelli MT, Stan SD. Dietary Bioactive Diallyl Trisulfide in Cancer Prevention and Treatment. Int J Mol Sci 2017; 18: 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen SY, Tsai CY, Pai PY, et al. Diallyl trisulfide suppresses doxorubicin-induced cardiomyocyte apoptosis by inhibiting MAPK/NF-kappaB signaling through attenuation of ROS generation. Environ Toxicol 2018; 33: 93–103. [DOI] [PubMed] [Google Scholar]
- 23.Liang JJ, Li HR, Chen Y, et al. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-kappaB and Wnt pathways. Int Immunopharmacol 2019; 71: 132–138. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y, Lin S, Xing R, et al. Epigenetic Upregulation of Metallothionein 2A by Diallyl Trisulfide Enhances Chemosensitivity of Human Gastric Cancer Cells to Docetaxel Through Attenuating NF-kappaB Activation. Antioxid Redox Signal 2016; 24: 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US), 2011. PMID: 21595115. [PubMed]
- 26.Lu L, Chen J, Li M, et al. Betacarotene reverses tobacco smoke-induced gastric EMT via Notch pathway in vivo. Oncol Rep 2018; 39: 1867–1873. [DOI] [PubMed] [Google Scholar]
- 27.Min J, Geng H, Liu Z, et al. ERK5 regulates tobacco smoke-induced urocystic epithelialmesenchymal transition in BALB/c mice. Mol Med Rep 2017; 15: 3893–3897. [DOI] [PubMed] [Google Scholar]
- 28.Geng H, Zhao L, Liang Z, et al. Cigarette smoke extract-induced proliferation of normal human urothelial cells via the MAPK/AP-1 pathway. Oncol Lett 2017; 13: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng H, Zhao L, Liang Z, et al. ERK5 positively regulates cigarette smoke-induced urocystic epithelial-mesenchymal transition in SV40 immortalized human urothelial cells. Oncol Rep 2015; 34: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 30.Yu D, Geng H, Liu Z, et al. Cigarette smoke induced urocystic epithelial mesenchymal transition via MAPK pathways. Oncotarget 2017; 8: 8791–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soleimani A, Rahmani F, Ferns GA, et al. Role of the NF-kappaB signaling pathway in the pathogenesis of colorectal cancer. Gene 2020; 726: 144132. [DOI] [PubMed] [Google Scholar]
- 32.Shen T, Yang Z, Cheng X, et al. CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-kappaB signaling pathway. Oncol Rep 2017; 37: 2095–2100. [DOI] [PubMed] [Google Scholar]
- 33.Feng H, Lu JJ, Wang Y, et al. Osthole inhibited TGF beta-induced epithelial-mesenchymal transition (EMT) by suppressing NF-kappaB mediated Snail activation in lung cancer A549 cells. Cell Adh Migr 2017; 11: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat OM, Uday Kumar P, Harishankar N, et al. Interleukin-18-induced cell adhesion molecule expression is associated with feedback regulation by PPAR-gamma and NF-kappaB in Apo E-/- mice. Mol Cell Biochem 2017; 428: 119–128. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhao Y, Wei Z, et al. Targeting Thioredoxin System with an Organosulfur Compound, Diallyl Trisulfide (DATS), Attenuates Progression and Metastasis of Triple-Negative Breast Cancer (TNBC). Cell Physiol Biochem 2018; 50: 1945–1963. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Jiang L, Wang S, et al. Diallyl trisulfide protects the liver against hepatotoxicity induced by isoniazid and rifampin in mice by reducing oxidative stress and activating Kupffer cells. Toxicol Res (Camb) 2016; 5: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]