Abstract
Objective
To investigate if co-transfection of human bone morphogenetic protein 2 (BMP-2, BMP2) and human fibroblast growth factor 2 (FGF2, FGF2) via chitosan nanoparticles promotes osteogenesis in human adipose tissue-derived stem cells (ADSCs) in vitro.
Materials and Methods
Recombinant BMP2 and/or FGF2 expression vectors were constructed and packaged into chitosan nanoparticles. The chitosan nanoparticles were characterized by atomic force microscopy. Gene and protein expression levels of BMP-2 and FGF2 in ADSCs in vitro were evaluated by real-time polymerase chain reaction (PCR), western blot, and enzyme-linked immunosorbent assay. Osteocalcin (OCN) and bone sialoprotein (BSP) gene expression were also evaluated by real-time PCR to assess osteogenesis.
Results
The prepared chitosan nanoparticles were spherical with a relatively homogenous size distribution. The BMP2 and FGF2 vectors were successfully transfected into ADSCs. BMP-2 and FGF2 mRNA and protein levels were significantly up-regulated in the co-transfection group compared with the control group. OCN and BSP mRNA levels were also significantly increased in the co-transfection group compared with cells transfected with BMP2 or FGF2 alone, suggesting that co-transfection significantly enhanced osteogenesis.
Conclusions
Co-transfection of human ADSCs with BMP2/FGF2 via chitosan nanoparticles efficiently promotes the osteogenic properties of ADSCs in vitro.
Keywords: Chitosan nanoparticle, bone morphogenetic protein-2, basic fibroblast growth factor, adipose tissue-derived stem cell, osteogenesis, osteocalcin, bone sialoprotein
Introduction
Trauma, infection, and cancer can cause bony defects, which can in turn lead to dysfunction and deformity, with an immense impact on patient quality of life.1,2 Traditional therapy for bone defects is generally ineffective; however, gene therapy, which aims to maintain local bone growth factors at a therapeutic concentration, is a promising method for boosting the healing of bone defects.3 Other advantages of gene therapy include the controlled release of target gene products, thereby maximizing local therapeutic effects and reducing systemic side effects.4 However, various techniques are available to deliver gene therapy, and there is still a lack of consensus regarding the optimal delivery system in the field of bone repair.
Re-vascularization is a crucial part of bone healing.5 Fibroblast growth factor 2 (FGF2, also known as basic FGF) is one of the most effective factors stimulating the migration and hyperplasia of capillary endothelial cells, the formation of capillary buds, and the secretion of plasminogen activators and collagenase.6 It also affects the degradation of the extracellular matrix in the wound,7 allowing capillaries to extend into the wound. Bone morphogenetic proteins (BMPs) are cytokines that have been proven to induce bone differentiation and are involved in osteogenesis. BMP-2 was the first BMP shown to induce differentiation towards cartilage and bone tissue in vivo, and the local application of exogenous BMP-2 protein or bone marrow-derived mesenchymal stem cells (BMSCs) transfected with the BMP-2 gene (BMP2) was shown to aid the repair of bone defects.8
Franceschi et al.9 found that the osteogenic potential could be significantly improved by increasing expression levels of BMP-2, -4, and -7 via adenovirus-mediated transfection. Similarly, Gromolak et al.10 found that in vitro treatment of sheep BMSCs with FGF2 and BMP-2 led to increased expression of osteocalcin (OCN) and collagen type I and a series of osteogenic-related gene markers. We hypothesized that co-expression of FGF2 and BMP-2 via an effective gene delivery system would enhance osteogenesis. Chitosan nanoparticles are a non-viral gene-transfer technology that can transduce multiple genes.11,12 We therefore evaluated the in vitro osteogenic properties of human adipose tissue-derived stem cells (ADSCs) co-transfected with a BMP2/FGF2 dual-gene system via chitosan nanoparticles.
Materials and Methods
Primary culture and identification of human ADSCs
Fat tissue was extracted from the omental fat of a healthy woman undergoing abdominal surgery. ADSCs were isolated by type I collagenase digestion, as described by Peng et al.13 Briefly, fatty tissues were separated and washed in phosphate-buffered saline (PBS), and cells were dissociated for 30 minutes with 0.75% type I collagenase (1710001; Gibco, MA, USA) in Dulbecco’s modified Eagle medium (DMEM; Gibco). After digestion, the digested cells were collected by centrifugation (4°C, 1000 × g for 10 minutes) and suspended in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 U/mL streptomycin (Sigma, MO, USA) in a humidified atmosphere containing 5% CO2 at 37°C. The cells were grown to approximately 80% confluence, digested with 0.25% pancreatic enzyme/0.02% ethylenediaminetetraacetic acid (EDTA) solution, and the reaction was stopped with DMEM containing FBS before passage. The study protocol was approved by the Institutional Ethics Committee of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine. The patient provided written consent for donating her fat tissues for research purposes.
To identify ADSCs, adherent P3 cells were harvested, washed, and suspended in fluorescence-activated cell sorting (FACS) buffer (Lonza, Basel, Switzerland). The cells were then incubated with human Fc block (564220, 1:10; BD Pharmingen, San Jose, CA, USA) for 15 minutes at 4°C followed by washing in FACS buffer twice, and incubated with anti-CD45-phycoerythrin, anti-CD31-allophycocyanin (APC), anti-CD34-fluorescein isothiocyanate (FITC), anti-CD44-APC, anti-CD29-FITC, and anti-CD105-APC for 45 minutes at 4°C, respectively. All the antibodies were purchased from BioLegend (San Diego, CA, USA). The cells were finally washed twice with FACS buffer and analyzed using a DxFLEX Flow Cytometer (Beckman Coulter Inc., Brea, CA, USA).
Construction of pEGFP-N2-hBMP-2/pTagRFP-C-hFGF2 recombinant expression vectors
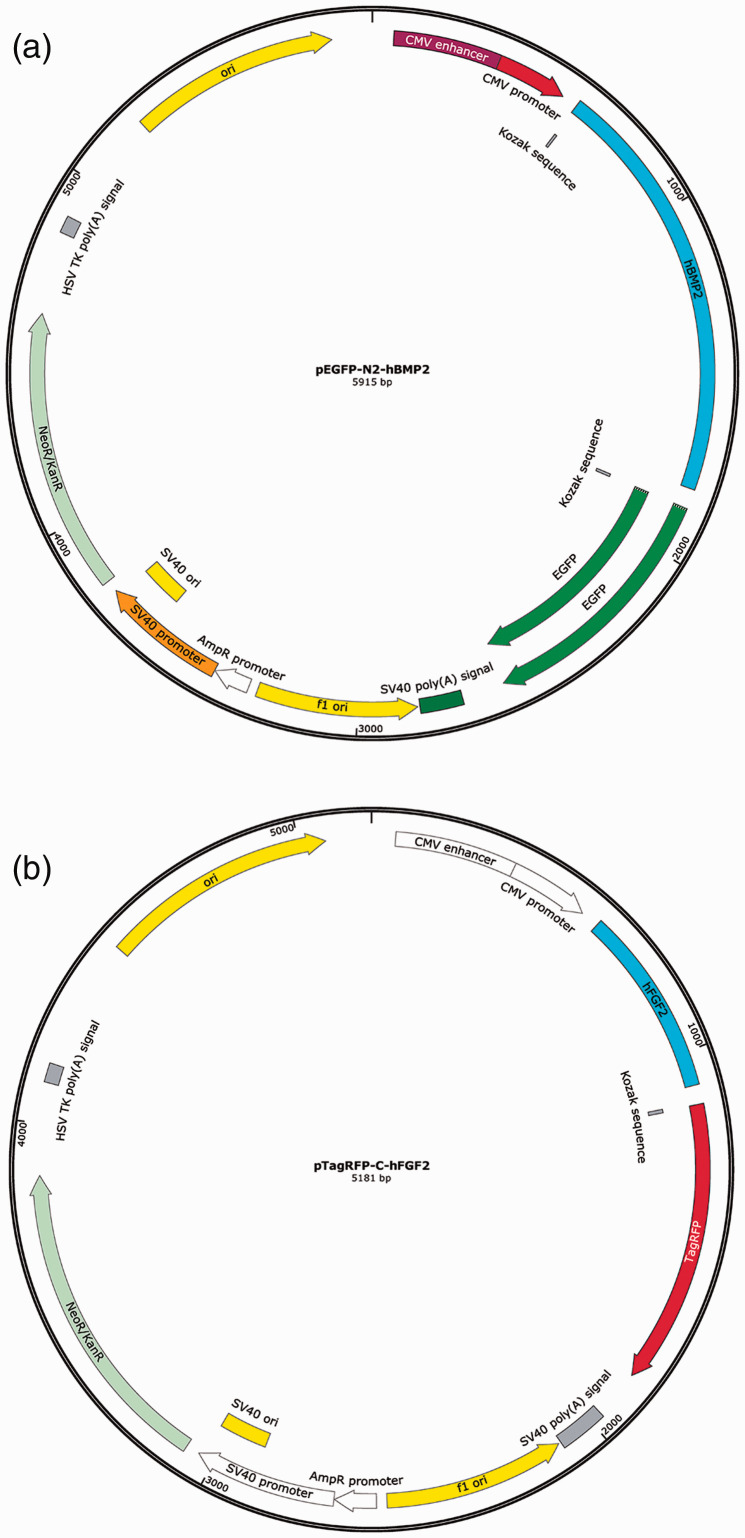
Full-length human BMP2/FGF2 were cloned by Shanghai Biological Engineering Ltd. (Shanghai, China). The recovered gene fragments were inserted into pEGFP-N2/pTagRFP-C expression vectors (Genscript, Hangzhou, China) (Figure 1) at a concentration ratio of 9:1 at 16°C for 8 hours using a Rapid DNA Ligation Kit (Thermo Fisher Scientific, MA, USA), according to the manufacturer’s instructions) and then transformed into competent Escherichia coli JM 109 for amplification. pEGFP-N2-hBMP-2/pTagRFP-C-hFGF2 recombinant plasmids were extracted from E. coli JM109 using a Mini Plasmid Purification Kit (Macherey-Nagel GmbH & Co., Germany) according to the manufacturer’s instructions. Recombinant plasmid vectors were verified by double restriction enzyme digestion: 1 μg of recombinant plasmid was digested by Bgl II and EcoR I at 37°C for 8 hours, and 5 μL of the digestion product was analyzed by agarose gel electrophoresis. Positive plasmids were also sequenced by TaKaRa Biotechnology Co., Ltd. (Dalian, China).
Figure 1.
Diagram showing structures of recombinant expression vectors for over-expression of BMP2 and FGF2 in vitro. Schematic structures of (a) pEGFP-N2-hBMP-2 and (b) pTagRFP-C-hFGF2.
Construction of plasmid packaging chitosan nanoparticles and transfection
The chitosan nanoparticles were prepared largely according to the double emulsification solvent evaporation method.14 Briefly, two kinds of chitosan (448869; Sigma, C8320; Solarbio, China) were dissolved in 1% acetic acid (0.3 mg/mL, final concentration) and 0.01 M NaOH solution was added to adjust the pH to 5 to 6. The chitosan solution was sterilized using a vacuum filter (0.22 μm) (A solution). The constructed plasmids (containing FGF2 and BMP2) were diluted in 100 μg/mL (B solution), and a 1:1 mixture of A and B solutions (containing either single or dual genes) was then used to obtain chitosan nanoparticles containing either the individual genes or both genes.
ADSCs were disseminated in 6-well plates at a concentration of 106 cells per well. After 12 to 20 hours, 50 µL chitosan nanoparticles were added to the medium for 6 hours. The ADSCs were examined by flow cytometry 48 hours after transfection to evaluate the transfection efficiency.
Physicochemical characterization of chitosan nanoparticles
Chitosan–plasmid nanoparticles were subjected to 0.8% agarose gel electrophoresis followed by ethidium bromide staining and ultraviolet imaging. For morphological observation, the chitosan-plasmid nanoparticles were diluted 1:15 with 30 mmol/L Na2SO4, fixed on clean mica, and dried with N2. The nanoparticles were then examined using an atomic force microscope (SPI3800N, DI instrument Nanoscope 3D Multimode SPM; Veeco, CA, USA) and dynamic light scattering (Malvern Instruments, Malvern, UK).
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from ADSCs and reverse transcribed to cDNA using cDNA Synthesis Kits (Takara, Tokyo, Japan) according to the manufacturer’s instructions. PCR primers were designed using Primer 5 software. The PCR primers (Nanjing Sirui Technology Co. Ltd.) used are listed in Table 1. Real-time PCR reactions were carried out using the MultiGene Gradient System (Labnet, NJ, USA) in a 96-well clear optical reaction plate with optical adhesive covers. Reactions were run in duplicate in 5 μL, including 2 μL of cDNA solution and 3 μL of a homemade target-specific mix composed of 5/6 2×Power SYBR Green Master Mix (Toyobo, Japan) and 1/6 100 mM primer solution. The PCR program was: 95°C for 10 minutes, followed by 40 cycles of 15 s at 95°C, and 1 minute at 60°C. PCR was carried out using a 7500 Fast Real-Time PCR system, and the data were analyzed with 7500 software v2.0.1 (both Applied Biosystems, Foster City, CA, USA).
Table 1.
Primer design for real-time polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| BMP-2 | CCAAGATGAACACAGCTGGTCACAGA | CCCACGTCACTGAAGTCCACG |
| FGF2 | GGAGAAGAGCGACCCTCACATCA | GCCAGGTAACGGTTAGCACACACT |
| 18S rRNA | GACTCAACACGGGAAACCTCAC | CCAGACAAATCGCTCCACCAAC |
| BSP | CGAACAAGGCATAAACGGCACCA | CTCCATTGTCTTCTCCGCTGCT |
| OCN | CAGTTCTGCTCCTCTCCAGGCA | CATCCATAGGGCTGGGAGGTCA |
BMP-2, bone morphogenetic protein-2; FGF2, fibroblast growth factor 2; BSP, bone sialoprotein; OCN, osteocalcin.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of human BMP-2 and FGF2 proteins in the supernatants of the cultured ADSCs were detected by ELISA (Invitrogen, MA, USA) according to the manufacturer’s instructions.
Western blot analysis
ADSCs were collected in RIPA buffer (containing 0.2% Triton X-100, 5 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 100 mmol/L NaF and 2 mmol/L Na3VO4) and lysed for 30 minutes on ice. Protein concentrations were assayed using a Bio-Rad protein kit (Hercules, CA, USA). Equal amounts of sample per well were loaded in a sodium dodecyl sulfate-polyacrylamide gel. The proteins were then transferred onto 0.45-μm pore-size positively-charged polyvinylidene difluoride membranes (Millipore, Germany) and blocked with 5% dry milk in PBS with 0.1% Tween-20 at room temperature. The blots were challenged with primary antibodies in blocking solution overnight at 4°C, washed three times with PBST (0.1% Tween-20), and challenged with horseradish peroxidase-conjugated goat anti-rabbit, rabbit anti-goat, or mouse anti-mouse antibodies, respectively, followed by detection with an enhanced chemiluminescent substrate (Pierce, Rockford, IL, USA). The primary antibodies were mouse monoclonal anti-BMP-2 (Abcam, Cambridge, MA, USA), rabbit polyclonal anti-FGF2 (Abcam), or mouse monoclonal anti-β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cell Counting Kit-8 (CCK-8) and lactate dehydrogenase (LDH) release assays
ADSCs were disseminated in 96-well plates at a concentration of 2000 cells per well and then transfected with BMP2 and/or FGF2. After 24 hours, the viability of the ADSCs and cytotoxic effects were determined using a CCK-8 kit (Dojindo, Japan) and a LDH release kit (Beyotime Institute of Biotechnology, Jiangsu, China), respectively, according to the manufacturer’s instructions.15,16
Statistical analysis
Data were analyzed using SPSS Statistics for Windows, Version 19.0 (SPSS Inc., Armonk, NY: IBM Corp, USA). Data were expressed as mean ± standard error of the mean. One-way ANOVA or two-way repeated measures ANOVA was used for multiple group comparisons. A probability of P < 0.05 was considered statistically significant.
Results
Physicochemical characterization of chitosan nanoparticles
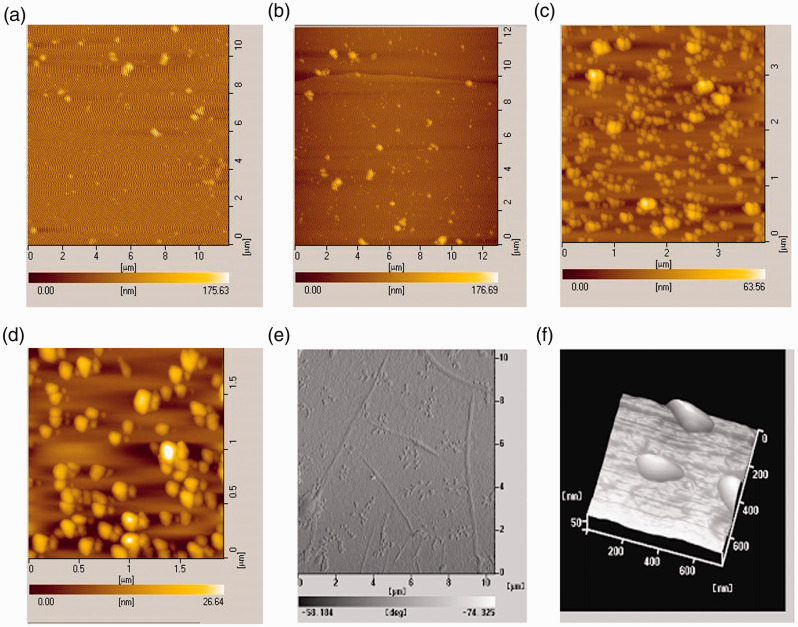
We characterized the physicochemical properties of the chitosan nanoparticles using nano-atomic force microscopy. The Solarbio chitosan nanoparticles were larger and showed more precipitation than the Sigma nanoparticles (Figure 2a-d). We therefore used the Sigma chitosan nanoparticles for all subsequent experiments. The chitosan–plasmid nanoparticles were irregularly spherical with a compact structure. The particle size was uniformly about 200 nm and the zeta potential was 30 mV, suggesting that the chitosan nanoparticles had been prepared successfully (Figure 2e and 2f).
Figure 2.
Physicochemical characterization of chitosan nanoparticles by nano-atomic force microscopy. (a, b) The Solarbio chitosan nanoparticles were larger and showed more precipitation; (c, d) the Sigma chitosan nanoparticles were smaller, with no precipitation. At higher definition, the chitosan–plasmid nanoparticles were generally spherical, but could also be irregular, with compact structure. The particle size was about 200 nm (e, f).
Human ADSC culture, identification, and transfection
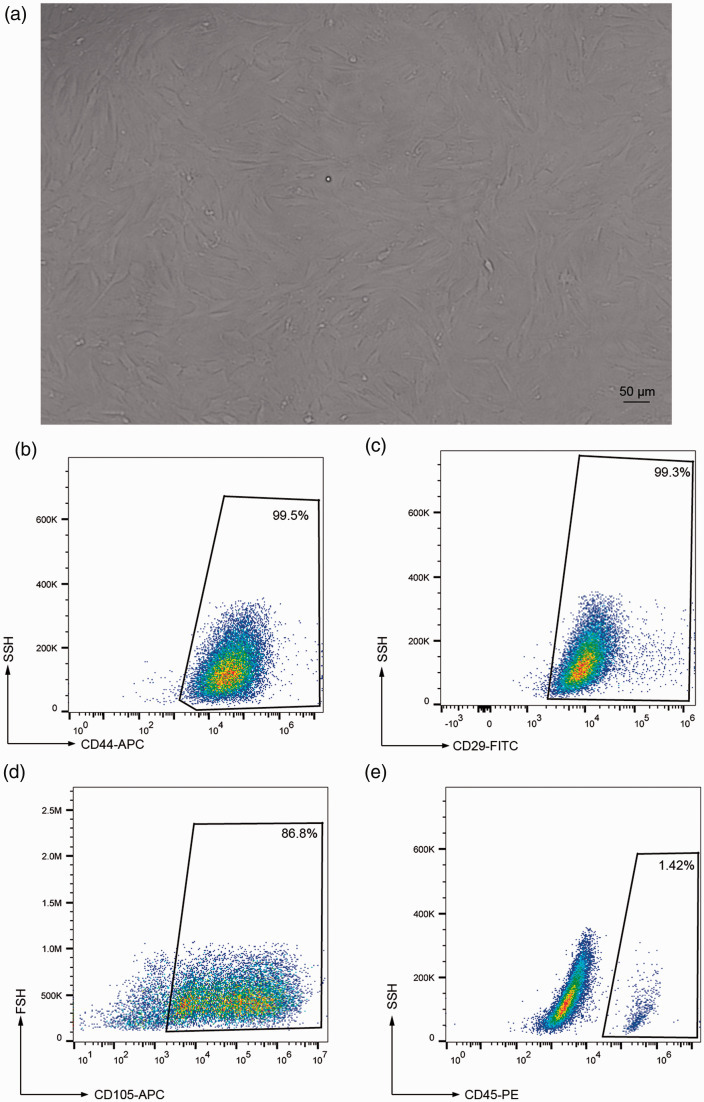
ADSCs were examined morphologically under an inverted microscope. After a further 7 days of culture, the ADSCs became spindle shaped and merged to form adherent cell clusters (Figure 3a). FACS analysis showed expression of the ADSC markers CD44, CD29, and CD105 in the cultured ADSCs (99.5%, 99.3%, and 86.8%, respectively) (Figure 3b-d), but only a few cells (1.42%) expressed the immune cell marker CD45 (Figure 3e). These results suggested successful culture of human ADSCs in vitro.
Figure 3.
Characterization of human adipose-derived stromal cells (ADSCs) in vitro. (a) ADSCs became spindle shaped and formed adherent clusters after 7 days in culture. (b-e) Fluorescence-activated cell sorting showed expression of CD44, CD29, CD105, and CD45 in cultured ADSCs, confirming their stem cell nature. Values expressed as mean ± standard error (n=4).
APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
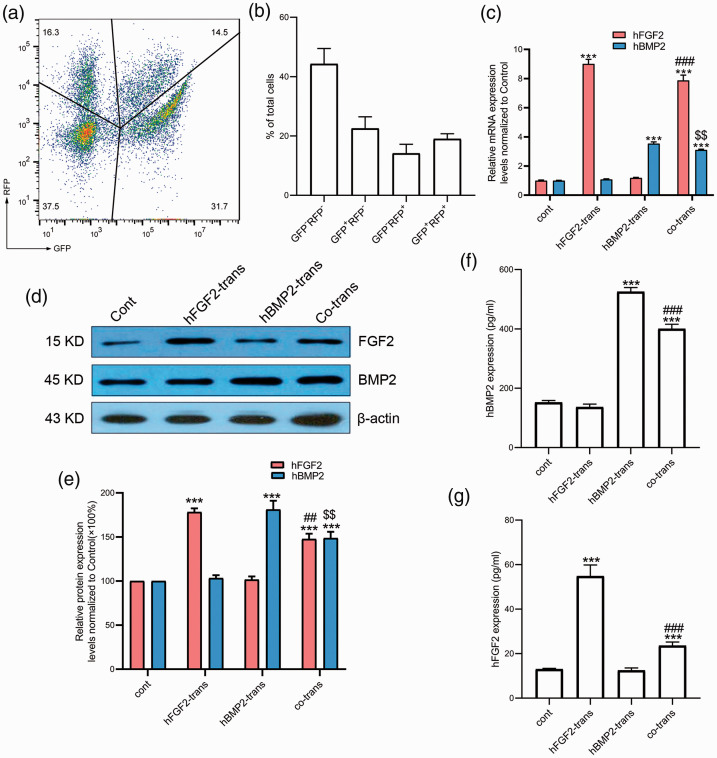
We also assessed the transfection efficiency by FACS analysis. After 48 hours of co-transfection, 19.03% ± 1.74% of cells expressed BMP2-EGFP and FGF2-RFP, 14.13% ± 3.06% only expressed FGF2-RFP, 22.53 ± 3.94% only expressed BMP-EGFP, and 44.30% ± 5.21% of cells expressed neither. These results indicated that both pEGFP-N2-hBMP-2 and pTagRFP-C-hFGF2 were successfully transfected (Figure 4a and 4b).
Figure 4.
Successful transfection of human adipose-derived stromal cells (ADSCs) with BMP2 and FGF2. (a, b) At 48 hours after transfection, pTagRFP-C-hFGF2 plasmid caused expression of red fluorescent protein (RFP) and pEGFP-N2-Hu-BMP2 plasmid caused expression of green fluorescent protein (GFP) in ADSCs. (c) Relative mRNA expression levels of FGF2 and BMP2 (n=6 from 3 independent experiments). ***P<0.001 vs control group, ###P<0.001 vs FGF2-transfected group, $$P<0.01 vs BMP2-transfected group. (d, e) Western blotting showed the protein expression levels of FGF2 and BMP-2 (n=4 from 2 independent experiments). ***P<0.001 vs control group, ##P<0.01 vs FGF2-transfected group, $$P<0.01 vs BMP2-transfected group. (f) Protein expression pattern of BMP-2 levels in cell supernatants (n=3 from 3 independent experiments) determined by enzyme-linked immunosorbent assay (ELISA). ***P<0.001 vs control group, ###P<0.001 vs BMP2-transfected group. (g) Protein expression pattern of the FGF2 in cell supernatants (n=3 from 3 independent experiments) determined by ELISA. ***P<0.001 vs control group, ###P<0.001 vs FGF2-transfected group. Values given as mean ± standard error. trans, transfected; cont, control; h, human.
FGF2 and BMP2 mRNA levels after transfection
To explore if the transfected ADSCs expressed BMP2 and/or FGF2, we detected the respective mRNA levels of these genes by real-time PCR. FGF2 and BMP2 mRNA levels were up-regulated in the co-transfection group compared with the control group (7.87-fold change, P < 0.001; 3.1-fold change, P < 0.001, respectively). However, the FGF2 and BMP2 mRNA levels were slightly lower than in the corresponding single-gene transfection groups (7.87 ± 0.15 vs 9.00 ± 0.13, P < 0.001; 3.11 ± 0.02 vs 3.54 ± 0.02, P < 0.01, respectively) (Figure 4c). These results indicated that transfection with the vectors containing FGF2 and BMP2 led to transcription of the respective genes in the ADSCs.
FGF2 and BMP-2 protein levels after transfection
FGF2 and BMP-2 protein expression levels were increased 1.47-fold and 1.49-fold in the co-transfection group compared with the control group (Figure 4d and 4e). However, the protein expression levels in the co-transfection group were significantly lower than in the corresponding single-gene transfection groups (147.67% ± 6% vs 178.3% ± 4.1%, P < 0.01; 148.7% ± 7.2% vs 181.22% ± 9.9%, P < 0.01, respectively). These results suggest that chitosan-mediated co-transfection of BMP2/FGF2 into human ADSCs resulted in expression of the respective proteins.
Secretion of FGF2 and BMP-2 in cell supernatant
We evaluated the release of FGF2 and BMP-2 into the cell supernatant by ELISA. Both FGF2 and BMP-2 protein expression levels were up-regulated in the co-transfection group compared with the control group, consistent with the results of western blotting. There was no significant difference in FGF2 levels between the BMP2-transfected and control groups, and no significant difference in BMP-2 levels between the FGF2-transfected and control groups (Figure 4f and 4g). This indicated that vector-induced expression of one factor did not influence the expression of the other factor in terms of local release, suggesting the importance of co-expression of BMP2 and FGF2.
Viability and osteogenic ability of ADSCs and assessment of cytotoxic effects after transfection with BMP2 and/or FGF2
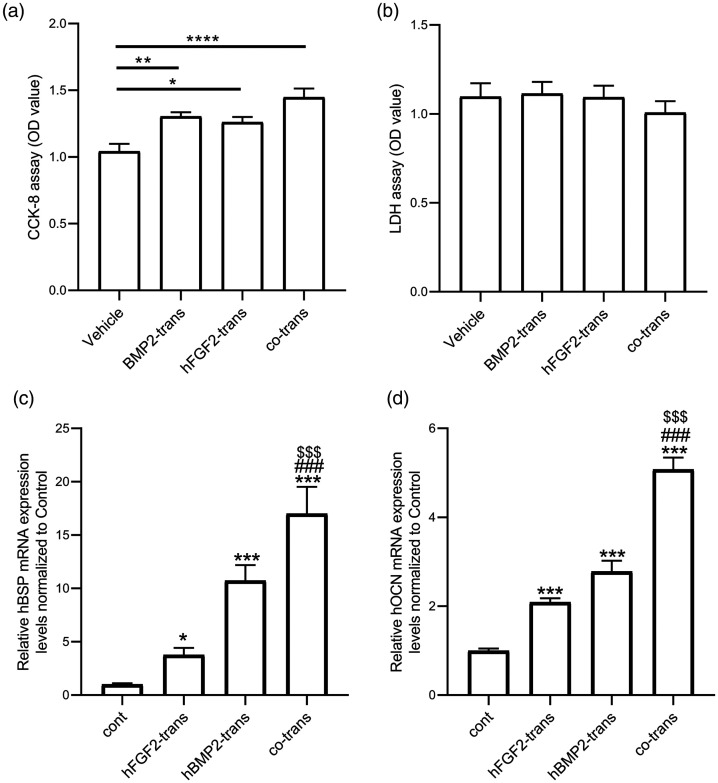
We explored the cell viability and cytotoxic effects of transfection on ADSCs by CCK-8 and LDH release assays, respectively. The viability of ADSCs increased significantly after transfection with BMP2 or FGF2, with the greatest effect following co-transfection with both genes (Figure 5a). In addition, there were no obvious toxic effects in ADSCs transfected with BMP2 and/or FGF2 (Figure 5b).
Figure 5.
Co-transfection of BMP2 and FGF2 via chitosan nanoparticles increased cell viability and osteogenic ability of human adipose-derived stromal cells (ADSCs) in vitro. The cell viability and cytotoxic effects on ADSCs were measured by (a) Cell Counting Kit-8 (CCK-8) and (b) lactate dehydrogenase (LDH) assays, respectively (n = 6 from 3 independent experiments). *P < 0.05, **P < 0.01, ****P < 0.0001. Relative mRNA expression levels of (c) osteocalcin (OCN) and (d) bone sialoprotein (BSP) (d) showed that co-transfection had a synergistic effect on osteogenesis, evidenced by higher expression of the osteogenesis markers BSP and OCN compared with single-gene transfection. Values given as mean ± standard error (n = 6 from 3 independent experiments). ***P < 0.001 vs control group, ###P < 0.001 vs FGF2-transfected group, $$$P < 0.001 vs BMP2-transfected group.
OD, optical density; trans, transfected; cont, control.
We evaluated the mRNA expression levels of OCN and BSP to explore the effects of gene co-transfection via chitosan nanoparticles on osteogenesis in ADSCs. OCN and BSP mRNA levels were significantly higher in the co-transfection group compared with the single-gene transfection groups and the control group, suggesting significant enhancement of osteogenesis (Figure 5c and 5d).
Discussion
Gene therapy for bone defects involves the local transfection of target cells with osteogenic genes that can be transcribed and translated into proteins. Expression of the proteins stimulates the target cells, thereby promoting bone formation via autocrine or paracrine mechanisms. Gene therapy ensures the targeted release of the gene product, maximizes the local therapeutic effect, and reduces systemic side effects. Moreover, several genes can be transduced together, leading to synergistic regulation of endogenous protein synthesis, and enhanced biological activity compared with the administration of exogenous recombinant proteins. Gene therapy is thus considered as the most promising therapy for maintaining an effective local therapeutic concentration of growth factors to aid the repair of partial bone defects.17–19
ADSCs isolated from the blood vessels of adipose tissue exhibit similar morphology, immunophenotype, and differentiation properties to mesenchymal stem cells from bone marrow (BMSCs) and umbilical cord blood. Numerous studies have shown that ADSCs have the potential to differentiate into fat, bone, cartilage, and muscle derived from mesoderm.20–22 Single-cell cloning experiments have confirmed the pluripotent differentiation of ADSCs.23,24 Although BMSCs were the first to be found to have osteogenic potential and were thus considered as seed cells for bone tissue engineering,25,26 the number of BMSCs in the bone marrow is limited, and although in vitro expansion can produce large numbers of cells, it also leads to the loss of stem cell characteristics and potential changes in the differentiation potential.27,28 Compared with BMSCs, ADSCs have several advantages, such as a wide variety of sources, rich content, little trauma to the donor during harvesting, and in vitro amplification capability.24,29 ADSCs have thus become an important source of seed cells for bone tissue engineering.
BMP-2 plays an important role in bone formation and healing.30–33 Moreover, Bouyer et al.34 and Tarek et al.35 developed effective delivery systems using film-coated poly(lactic-co-glycolic acid) (PLGA) and PLGA/magnesium hydroxide scaffolds to improve the in vivo bone regeneration potential of BMP-2. Recombinant human BMP-2 is the only drug approved by the U.S. Food and Drug Administration as an osteogenesis-promoting factor.32 Dragoo et al.36 first reported the deposition of large amounts of calcium in the extracellular matrix in vitro after virally mediated BMP2 gene transfection in human ADSCs. In vivo studies showed the formation of bone marrow cavity cells after transfection with BMP2, compared with fat-like tissue in the control group.37 OCT4 has been suggested to act as a downstream mediator for BMP-induced bone regeneration.38 Panetta et al.39 demonstrated that exogenous human ADSC recombinant human BMP-2 could significantly enhance osteoblast activity in vitro in a concentration-dependent manner. Down-regulation of the nuclear factor-κB signaling pathway was suggested to be involved in BMP-2-mediated repair of articular cartilage via upregulation of angiogenesis factors.40 Other BMP family proteins, including BMP-9, can also promote osteoblastic differentiation of MSCs both in vitro and in vivo, but probably via different mechanisms.41 FGF2 is a polypeptide that promotes cell growth and bone tissue repair, and exogenous FGF2 was shown to promote bone formation;42 however, exogenous FGF2 is easily degraded within the body, thus reducing its efficacy.43 The FGF2 gene can now be transfected into osteoblasts, leading to over-expression of FGF2 protein and the promotion of osteogenesis in vivo. FGF-2 and BMP-2 have demonstrated synergistic effects on bone induction in vivo via the extracellular signal-regulated kinase signaling pathway.44 Peroxisome proliferator-activated receptor-γ and Runt-related transcription factor 2 have been suggested to be the two major transcription factors involved at various intersecting signaling pathways regulating adipogenesis and osteogenesis.45 Interestingly, in addition to commonly recognized cell components such as osteoblasts and osteoclasts, emerging evidence also supports the role of macrophages as immunomodulators for the various cytokines involved during the bone regeneration process.46
Biomaterial scaffolds have been used extensively to deliver growth factors to induce new bone formation.47,48 Chitosan, as a non-viral gene vector, is both biocompatible and biodegradable, and is also a safe and easy-to-construct polymer molecule that can protect DNA from degradation.49 Most previous studies have been based on the expression of individual genes via viral vectors, while the main innovation of the current study involved the use of chitosan nanoparticles as a non-viral gene transfer vector for multiple genes, to produce a synergistic effect on osteoblasts. We successfully constructed high-performance chitosan nanoparticles carrying BMP2 and FGF2 genes, which could co-transfect both these genes into human ADSCs, resulting in BMP-2/FGF2 protein expression. Importantly, our results showed good synergy between the two plasmids, resulting in a significantly higher osteogenic index (i.e. BSP and OCN) compared with transfection with either gene alone.
This study had several limitations. First, we were not able to analyze the direct uptake of nanoparticles into the cells owing to our laboratory capacity. However, co-transfection of BMP2 and FGF2 via chitosan nanoparticles showed good efficiency, indirectly indicating the successful entry of the nanoparticles into the cells. Second, we could not assess the in vivo osteogenic efficacy of the chitosan nanoparticles co-transfected with BMP2 and FGF2 owing to the lack of a suitable animal model. However, further experiments are planned to address this in the future.
Conclusion
The results of this preliminary study suggest that co-transfection of BMP2 and FGF2 by chitosan nanoparticles into human ADSCs has a good synergistic osteogenic effect.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Zhejiang Provincial Medical and Healthy Science Foundation of China [grant number 2019ZD028], the National Key Research Program of China [grant number 2016YFC1101004], and the National Natural Science Foundation of China [grant number 81671918].
ORCID iDs: Ying Hu https://orcid.org/0000-0003-4558-9878
Wei-Qiang Tan https://orcid.org/0000-0003-4951-0960
References
- 1.Obremskey WT, Molina CS, Collinge C, et al. Current practice in the management of segmental bone defects among orthopaedic trauma surgeons. J Orthop Trauma 2013. [DOI] [PubMed]
- 2.Nair MB, Kretlow JD, Mikos AG, et al. Infection and tissue engineering in segmental bone defects–a mini review. Curr Opin Biotechnol 2011; 22: 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delhove J, Osenk I, Prichard I, et al. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther 2020; 31: 20–46. [DOI] [PubMed] [Google Scholar]
- 4.Hitti FL, Gonzalez-Alegre P, Lucas TH. Gene therapy for neurologic disease: a neurosurgical review. World Neurosurg 2019; 121: 261–273. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Yang S, Cao L, et al. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Mater Sci Eng C Mater Biol Appl 2020, 110: 110622. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Matinlinna JP, Tsoi JKH, et al. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J Orthop Translat 2020; 22: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchtova M, Oralova V, Aklian A, et al. Fibroblast growth factor and canonical WNT/beta-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage. Biochim Biophys Acta 2015; 1852: 839–850. [DOI] [PubMed] [Google Scholar]
- 8.Jiang N, He J, Zhang W, et al. Directed differentiation of BMSCs on structural/compositional gradient nanofibrous scaffolds for ligament-bone osteointegration. Mater Sci Eng C Mater Biol Appl 2020; 110: 110711. [DOI] [PubMed] [Google Scholar]
- 9.Franceschi RT, Yang S, Rutherford RB, et al. Gene therapy approaches for bone regeneration. Cells Tissues Organs 2004; 176: 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gromolak S, Krawczenko A, Antończyk A, et al. Biological characteristics and osteogenic differentiation of ovine bone marrow derived mesenchymal stem cells stimulated with FGF-2 and BMP-2. Int J Mol Sci 2020; 21: 9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T, Song X, Jing J, et al. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J Nanobiotechnology 2018; 16: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baghdan E, Pinnapireddy SR, Strehlow B, et al. Lipid coated chitosan-DNA nanoparticles for enhanced gene delivery. Int J Pharm 2018; 535: 473–479. [DOI] [PubMed] [Google Scholar]
- 13.Peng W, Gao T, Yang ZL, et al. Adipose-derived stem cells induced dendritic cells undergo tolerance and inhibit Th1 polarization. Cell Immunol 2012; 278: 152–157. [DOI] [PubMed] [Google Scholar]
- 14.Omwoyo WN, Ogutu B, Oloo F, et al. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int J Nanomedicine 2014; 9: 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Li H, Ren Y, et al. Local delivery of β-elemene improves locomotor functional recovery by alleviating endoplasmic reticulum stress and reducing neuronal apoptosis in rats with spinal cord injury. Cell Physiol Biochem 2018; 49: 595–609. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Li H, Yao Y, et al. β-Elemene enhances GAP-43 expression and neurite outgrowth by inhibiting RhoA kinase activation in rats with spinal cord injury. Neuroscience 2018; 383: 12–21. [DOI] [PubMed] [Google Scholar]
- 17.Bez M, Pelled G, Gazit D. BMP gene delivery for skeletal tissue regeneration. Bone 2020; 137: 115449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohrn MF, Auer-Grumbach M, Baron R, et al. Chance or challenge, spoilt for choice? New recommendations on diagnostic and therapeutic considerations in hereditary transthyretin amyloidosis with polyneuropathy: the German/Austrian position and review of the literature. J Neurol 2020. doi: 10.1007/s00415-020-09962-6. [DOI] [PMC free article] [PubMed]
- 19.Ilaltdinov AW, Gong Y, Leong DJ, et al. Advances in the development of gene therapy, noncoding RNA, and exosome-based treatments for tendinopathy. Ann N Y Acad Sci 2020. doi: 10.1111/nyas.14382. [DOI] [PubMed] [Google Scholar]
- 20.Kunze KN, Burnett RA, Wright-Chisem J, et al. Adipose-derived mesenchymal stem cell treatments and available formulations. Curr Rev Musculoskelet Med 2020; 13: 264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazini L, Rochette L, Admou B, et al. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci 2020; 21: 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla L, Yuan Y, Shayan R, et al. Fat therapeutics: the clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front Pharmacol 2020; 11: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazini L, Rochette L, Amine M, et al. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019; 20: 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W, Lin J, Zhao K, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med 2019; 47: 1722–1733. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Wei G, Gu Q, et al. Donor age and cell passage affect osteogenic ability of rat bone marrow mesenchymal stem cells. Cell Biochem Biophys 2015; 72: 543–549. [DOI] [PubMed] [Google Scholar]
- 26.El Refaey M, Watkins CP, Kennedy E, et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol 2015; 410: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palombella S, Lopa S, Gianola S, et al. Bone marrow-derived cell therapies to heal long-bone nonunions: a systematic review and meta-analysis-which is the best available treatment? Stem Cells Int 2019; 2019: 3715964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Ma J, Han J, et al. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res 2019; 11: 6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 29.Delanois RE, Etcheson JI, Sodhi N, et al. Biologic therapies for the treatment of knee osteoarthritis. J Arthroplasty 2019; 34: 801–813. [DOI] [PubMed] [Google Scholar]
- 30.Shakir S, MacIsaac ZM, Naran S, et al. Transforming growth factor beta 1 augments calvarial defect healing and promotes suture regeneration. Tissue Eng Part A 2015; 21: 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter RS, Goodrich LR, Frisbie DD, et al. Osteoblastic differentiation of human and equine adult bone marrow-derived mesenchymal stem cells when BMP-2 or BMP-7 homodimer genetic modification is compared to BMP-2/7 heterodimer genetic modification in the presence and absence of dexamethasone. J Orthop Res 2010; 28: 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsa A, Vahedi H, Goswami K, et al. Available findings fail to provide strong evidence of the role of bone morphogenic protein-2 in femoral head osteonecrosis. Arch Bone Jt Surg 2020; 8: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariscal G, Nunez JH, Barrios C, et al. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J Bone Miner Metab 2020; 38: 54–62. [DOI] [PubMed] [Google Scholar]
- 34.Bouyer M, Guillot R, Lavaud J, et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016; 104: 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedair TM, Lee CK, Kim DS, et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J Tissue Eng 2020; 11: 2041731420967591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragoo JL, Lieberman JR, Lee RS, et al. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg 2005; 115: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, Yamamoto N, Nojima T, et al. The process of bone regeneration from devitalization to revitalization after pedicle freezing with immunohistochemical and histological examination in rabbits. Cryobiology 2020; 92: 130–137. [DOI] [PubMed] [Google Scholar]
- 38.Kim SHL, Lee SS, Kim I, et al. Ectopic transient overexpression of OCT-4 facilitates BMP4-induced osteogenic transdifferentiation of human umbilical vein endothelial cells. J Tissue Eng 2020; 11: 2041731420909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panetta NJ, Gupta DM, Lee JK, et al. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg 2010; 125: 483–493. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Zang H, Zhou D. Bone morphogenetic protein-2 exhibits therapeutic benefits for osteonecrosis of the femoral head through induction of cartilage and bone cells. Exp Ther Med 2018; 15: 4298–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beederman M, Lamplot JD, Nan G, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng 2013; 6: 32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh MJ, Huang C, Lin CC, et al. Basic fibroblast growth factor promotes doxorubicin resistance in chondrosarcoma cells by affecting XRCC5 expression. Mol Carcinog 2020; 59: 293–303. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, Xu B, Wang Y, et al. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J Biomater Sci Polym Ed 2019; 30: 1591–1603. [DOI] [PubMed] [Google Scholar]
- 44.Song R, Wang D, Zeng R, et al. Synergistic effects of fibroblast growth factor-2 and bone morphogenetic protein-2 on bone induction. Mol Med Rep 2017; 16: 4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013; 2013: 684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu Y, Wang Z, Shi Y, et al. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Bioact Mater 2021; 6: 244–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King WJ, Krebsbach PH. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv Drug Deliv Rev 2012; 64: 1239–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Witte TM, Fratila-Apachitei LE, Zadpoor AA, et al. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 2018; 5: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veilleux D, Nelea M, Biniecki K, et al. Preparation of concentrated chitosan/DNA nanoparticle formulations by lyophilization for gene delivery at clinically relevant dosages. J Pharm Sci 2016; 105: 88–96. [DOI] [PubMed] [Google Scholar]