Abstract
Objective
Relying on tau-PET imaging, this cross-sectional study explored whether memory impairment is linked to the presence of concomitant tau pathology in individuals with cerebral amyloid angiopathy (CAA).
Methods
Forty-six patients with probable CAA underwent a neuropsychological examination and an MRI for quantification of structural markers of cerebral small vessel disease. A subset of these participants also completed a [11C]-Pittsburgh compound B (n = 39) and [18F]-flortaucipir (n = 40) PET for in vivo estimation of amyloid and tau burden, respectively. Participants were classified as amnestic or nonamnestic on the basis of neuropsychological performance. Statistical analyses were performed to examine differences in cognition, structural markers of cerebral small vessel disease, and amyloid- and tau-PET retention between participants with amnestic and those with nonamnestic CAA.
Results
Patients with probable CAA with an amnestic presentation displayed a globally more severe profile of cognitive impairment, smaller hippocampal volume (p < 0.001), and increased tau-PET binding in regions susceptible to Alzheimer disease neurodegeneration (p = 0.003) compared to their nonamnestic counterparts. Amnestic and nonamnestic patients with CAA did not differ on any other MRI markers or on amyloid-PET binding. In a generalized linear model including all evaluated neuroimaging markers, tau-PET retention (β = −0.85, p = 0.001) and hippocampal volume (β = 0.64 p = 0.01) were the only significant predictors of memory performance. The cognitive profile of patients with CAA with an elevated tau-PET retention was distinctly characterized by a significantly lower performance on the memory domain (p = 0.004).
Conclusions
These results suggest that the presence of objective memory impairment in patients with probable CAA could serve as a marker for underlying tau pathology.
Classification of Evidence
This study provides Class II evidence that tau-PET retention is related to the presence of objective memory impairment in patients with CAA.
Cerebral amyloid angiopathy (CAA), a condition characterized by the microvascular deposition of amyloid in leptomeningeal and cortical vessels, is a key contributor to vascular cognitive impairment and dementia.1 While CAA is a common accompanying feature of Alzheimer disease,2–4 it is an independent neuropathologic condition, with more than half of CAA cases presenting without concomitant Alzheimer disease–related tau pathology.5 Clinically, differentiating patients with CAA presenting with or without concomitant tau pathology could have important implications for therapeutic approaches and prognosis.
Previous studies in patients with Alzheimer disease have shown that memory performance is closely related to the severity of tau-mediated neuropathologic alterations in the medial temporal lobes and predicts impending cognitive decline.6–8 Given this evidence, the presence of objective memory deficits in patients with CAA could provide relevant information on underlying neuropathologic processes and hint to an increased severity of concomitant tau pathology.
In the present study, we compare neuroimaging features of patients with probable CAA presenting with or without objective memory impairment. Leveraging recent advances in PET, we further contrast levels of amyloid ([11C]-Pittsburgh compound-B amyloid-PET) and tau ([18F]-flortaucipir tau-PET) aggregation in patients with CAA with and without memory impairment to examine the presumed nature and severity of underlying neuropathologic processes in these 2 groups. We specifically hypothesized that the presence of memory impairment in individuals with CAA is associated with an increased likelihood of concomitant tau pathology and would hence be reflected by increased tau PET binding and medial temporal lobe neurodegeneration.
Methods
The primary research question of this study was to determine whether objective memory impairment was related to neuroimaging markers of tau pathology, including tau-PET retention and medial temporal lobe neurodegeneration, in asymptomatic individuals without dementia with CAA (Class II evidence).
Study Design
This is a cross-sectional study from a single-center prospective memory clinic research cohort.
Standard Protocol Approvals, Registrations, and Patient Consents
All research procedures were reviewed and approved by the Institutional Review Board of Massachusetts General Hospital. Written informed consent was obtained from all participants, and research procedures were conducted in accordance with the Declaration of Helsinki.
Participants
Participants were drawn from an ongoing research cohort of patients with CAA at Massachusetts General Hospital. Participants enrolled in this cohort were recruited from a memory clinic affiliated with the Massachusetts Alzheimer's Disease Research Center. Inclusion criteria for cohort enrollment were: suspected diagnosis of CAA based on clinical neuroimaging, age >55 years (no upper limit), ability and willingness to provide written informed consent, no history of symptomatic or asymptomatic intracerebral hemorrhage, no contraindication for MRI (e.g., cranial metallic implant, cardiac pacemaker, severe claustrophobia), and absence of unstable medical illness (e.g., unstable angina, advanced renal or liver failure).
For the present study, we selected participants who (1) were enrolled in the cohort between November 2016 and July 2019; (2) completed a research MRI; (3) completed a neuropsychological evaluation; (4) met criteria for mild cognitive impairment (MCI), as determined from neurologic and neuropsychological examinations confirming the presence of objective cognitive deficits and the absence of functional impairment in daily life activities9; and (5) fulfilled the modified Boston criteria for probable CAA,10,11 as determined from MRI findings confirming the presence of multiple cerebral microbleeds restricted to lobar, cortical, or corticosubcortical regions or a single lobar, cortical, or corticosubcortical microbleed accompanied by evidence of cortical superficial siderosis. A subgroup of included participants completed PET scans for in vivo estimation of amyloid ([11C]-Pittsburgh compound B amyloid-PET, n = 39) and tau ([18F]-flortaucipir tau-PET, n = 40) pathology. A flowchart summarizing the selection process of study participants is presented in figure 1.
Figure 1. Flowchart Illustrating the Selection of Study Participants.
Flowchart illustrating the selection of study participants from a larger study cohort (n = 78). Participants were classified as having probable cerebral amyloid angiopathy (CAA) or not on the basis of the modified Boston criteria.10,11 Three participants could not be classified due to an absence of research MRI and thus were excluded. MCI = mild cognitive impairment; PiB = Pittsburgh compound B.
Neuropsychological Evaluation and Characterization of Amnestic Status
All participants completed a detailed neuropsychological evaluation. Global cognitive status was assessed with the Mini-Mental State Examination (MMSE).12 Using standardized neuropsychological tests, we assessed the following cognitive domains: (1) memory (Hopkins Verbal Learning Test, total immediate recall and delayed recall; Free and Cued Selective Reminding Test, free recall and free and cued recall)13,14; (2) attention/processing speed (Wechsler Adult Intelligence Scale–III Digit Symbol-Coding; Trail Making Test A, Digit Span Forward)15,16; (3) language/semantics (Boston Naming Test, 15 items, Semantic Fluency, Animals)17,18; (4) executive function (Controlled Oral Word Association Test, Trail Making Test B, Digit Span Backward)15,16,18; and (5) visuospatial processing (Benton Facial Recognition Test, short form; Benton Judgment of Line Orientation, 30 items).19 With the use of published normative data, the raw performance on each test was converted to a z score adjusted for age, sex, and education. The z scores were averaged across tests included in a given cognitive domain to create domain-specific composite scores.
Participants with CAA were classified as amnestic or nonamnestic with the use of a previously recommended comprehensive criteria approach.20 Precisely, participants with at least 2 performances falling below a z score of −1.0 within the memory domain were categorized as amnestic (amnestic CAA), while other participants were classified as nonamnestic (nonamnestic CAA).
MRI Acquisition
All participants included in the study completed a 3T research MRI (Siemens Healthcare, Magnetom Prisma-Fit) at the Massachusetts General Hospital with a 32-channel head coil. The MRI procedure was performed within 3 months of the neuropsychological evaluation (median 0.0 days). The MRI protocol included a T1-weighted sagittal multiecho magnetization-prepared rapid acquisition with gradient echo (repetition time 2,510 milliseconds, echo time 1.69 milliseconds, voxel size 1 × 1 × 1 mm), fluid-attenuated inversion recovery (repetition time 5,000 milliseconds, echo time 356 milliseconds, voxel size 0.94 × 0.94 × 0.9 mm), and susceptibility-weighted imaging (repetition time 30 milliseconds, echo time 20 milliseconds, voxel size 0.86 × 0.86 × 1.4 mm).
Quantification of MRI Markers of Cerebral Small Vessel Disease and Neurodegeneration
MRI markers of cerebral small vessel disease were quantified in accordance with published expert guidelines (Standards for Reporting Vascular Changes on Neuroimaging recommendations).21 In brief, cerebral microbleeds were identified on susceptibility-weighted MRIs and counted across the brain to obtain a total cerebral microbleeds count. Lacunes of presumed vascular origin (i.e., lacunes) were identified on fluid-attenuated inversion recovery images as ovaloid areas of signal hypointensity and counted across the brain. The presence of cortical superficial siderosis was assessed on susceptibility-weighted MRIs according to newly proposed criteria22 and transformed into a dichotomous variable according to its presence or absence. The FreeSurfer volumetric pipeline (version 6.0)23 was used to automatically segment T1 anatomical MRIs (i.e., magnetization-prepared rapid acquisition with gradient echo) and to obtain the volume of white matter hyperintensity (i.e., white matter hypointensities), the total brain volume (i.e., brain segmentation volume), the left and right hippocampal volumes, and the estimated total intracranial volume. Automated segmentations were visually inspected to ensure validity. To account for variation in brain sizes, the volume of white matter hyperintensity was normalized to the total brain volume: (white matter hyperintensity volume/total brain volume) × 100. To obtain an estimation of global brain atrophy, the brain parenchymal fraction was estimated by computing the ratio of the total brain volume to the total intracranial volume: (total brain volume/estimated total intracranial volume) × 100. Finally, to obtain an estimation of medial temporal lobe atrophy, we computed the ratio of the bilateral hippocampal volume to the total intracranial volume: normalized total hippocampal volume = [(right + left hippocampal volumes)/estimated total intracranial volume] × 1,000.
PET Acquisition
PET scans were acquired on a Siemens/CTI (Knoxville, TN) ECAT HR+ (3-dimensional mode, 63 image planes, 15.2-cm axial field of view, 5.6-mm transaxial resolution, 2.4-mm slice interval) at the Massachusetts General Hospital. PET scans were performed within 6 months of the neuropsychological evaluation (median 69.0 days for amyloid-PET and 69.0 days for tau-PET) and MRI scan (median 66.0 days for amyloid-PET and 69.2 days for tau-PET). For amyloid-PET imaging, [11C]-Pittsburgh compound B was prepared at the Massachusetts General Hospital, and PET data were acquired according to previously published protocols.24 Briefly, 8.5 to 15 mCi of [11C]-Pittsburgh compound B was injected as a bolus followed immediately by a 60- to 70-minute dynamic acquisition in 69 frames. For tau-PET imaging, [18F]-flortaucipir was prepared at the Massachusetts General Hospital, and PET data were acquired from 80 to 100 minutes (4 × 5-minute frames) after a 9.0- to 11.0-mCi bolus injection.25
Analysis of PET Data
PET data were reconstructed, corrected for attenuation, and inspected to verify adequate count statistics and absence of significant head motion. Postacquisition processing was performed with a FreeSurfer-based pipeline (version 6.0). PET images were first aligned to FreeSurfer-processed T1 images, and measurements were made in regions of interest (ROIs) defined by the Desikan-Killiany atlas.26
For quantification of amyloid load, [11C]-Pittsburgh compound B amyloid-PET–specific binding was expressed as the distribution volume ratio (DVR) using the Logan graphical method applied to data collected 40 to 60 minutes after injection, with the cerebellar cortex as the reference region.27,28 DVRs were then extracted in an aggregate ROI consisting of the frontal, lateral temporal/parietal, and retrosplenial cortex (FLR). This ROI was selected on the basis of previous publications demonstrating an increased Pittsburgh compound B-PET retention in these regions in both sporadic Alzheimer disease and CAA.29,30 Finally, using a previously established cutoff score showing increased sensitivity to the early detection of abnormal β-amyloid levels (i.e., FLR DVR 1.08), participants were classified as either positive or negative amyloid-PET.31 In the analyses, we used amyloid-PET FLR DVRs not corrected for partial volume effects. However, to ensure that the lack of partial volume correction did not bias our results, we also examined DVRs corrected for partial volume effects using an established approach.32,33
For quantification of tau load, [18F]-flortaucipir tau-PET–specific binding was expressed as standardized uptake value ratios (SUVRs) based on data collected 80 to100 minutes after injection, with the cerebellar cortex used as the reference region. Following a previously described method, SUVR measurements were extracted from an aggregate ROI comprising Alzheimer disease cortical signature regions (ADCortSig SUVR), including the entorhinal cortex, fusiform gyrus, inferior, middle, and superior temporal gyri, superior and inferior parietal lobules, posterior cingulate, and precuneus.34,35 Prior work has demonstrated that these regions are affected at an early stage in Alzheimer disease and are associated with increased levels of tau pathology.34,35 As a supplementary analysis, tau-PET SUVRs were also extracted from other potential ROIs.36 Finally, using a previously established cutoff score (i.e., ADCortSig SUVR 1.19), we classified participants as either positive or negative tau-PET.35 Tau-PET SUVRs presented in the main text were not corrected for partial volume. However, to ensure that this did not bias our results, tau-PET SUVRs corrected for partial volume using an established approach were also examined.32,33
Statistical Analysis
Statistical analyses were performed with the SPSS version 24.0 (SPSS Inc, Chicago, IL) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
We compared demographic, clinical, and cognitive characteristics between individuals with amnestic and those with nonamnestic CAA using χ2 tests for dichotomous variables and 1-way analyses of variance for continuous variables. Because of their nonparametric distributions, group differences in MRI markers of cerebral small vessel disease were assessed with generalized linear models (GLMs), with age and sex as additional covariates. For count variables (cerebral microbleeds count, lacunes count), we fitted GLMs with a Poisson distribution. For nonparametric continuous variables (normalized white matter hyperintensity volume, brain parenchymal fraction, normalized total hippocampal volume), we fitted GLMs with a gamma distribution and a log link function. Finally, for dichotomous variables (presence of cortical superficial siderosis), we fitted the GLM with a binomial distribution.
In participants with available PET data, group differences in amyloid-PET (FLR DVR) and tau-PET (ADCortSig SUVR) retention were analyzed with GLMs fitted with a gamma distribution and a log link function. To ensure that group differences were not driven by confounds associated with the severity of cerebral small vessel disease or demographic features, we also compared amyloid- and tau-PET retention between amnestic and nonamnestic CAA in similar GLMs adjusting for all quantified MRI markers of cerebral small vessel disease, age, and sex. Finally, group differences in the frequency of participants with positive amyloid- or tau-PET were assessed in GLMs with a binomial distribution.
The association between memory performance and all neuroimaging markers was first assessed in separate univariate GLMs with a gaussian distribution. The statistical significance of each model was adjusted for the total numbers of univariate tests performed, according to the Bonferroni correction (corrected p value = p value × 8, for the 8 tests performed). The combined influence of PET (amyloid-PET FLR DVR, tau-PET ADCortSig SUVR) and MRI markers on memory performance was also assessed in a multivariate GLM fitted with a gaussian distribution. Because cognitive scores were already adjusted for, age, sex, and education level, these variables were not additionally included in the model. To obtain an estimation of the relative importance of each individual marker with regard to the explained variance in memory performance, we applied a model decomposition method using the relaimpo R package with the glm function (version 1.1-1).37
Finally, in separate GLMs, we contrasted scores across cognitive domains between participants with CAA with a positive and negative tau-PET. The statistical significance of each model was adjusted for the total numbers of tests performed according to the Bonferroni correction (corrected p value = p value × 6, for the 6 tests performed). Because cognitive scores were already corrected for age, sex, and education, these variables were not included in the models.
Classification of Evidence
This study provides Class II evidence that tau-PET retention is related to the presence of objective memory impairment in individuals with CAA.
Data Availability
Anonymized data can be made available to other researchers on request and with adequate justification.
Results
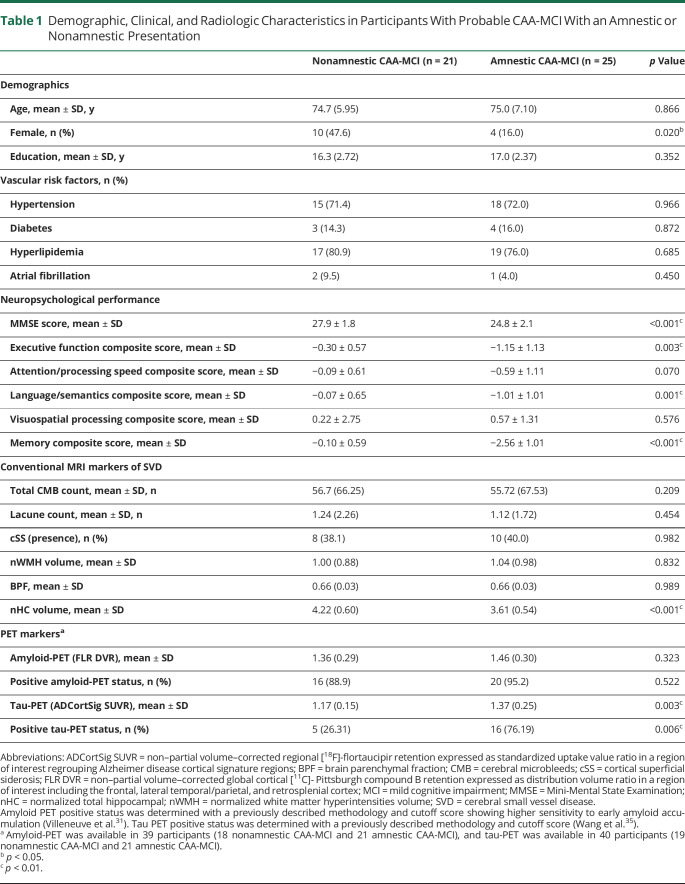
According to our predefined criteria, 25 participants with CAA had an amnestic presentation and 21 were nonamnestic. Between-group differences in demographic, clinical, and cognitive characteristics, as well as in all quantified MRI markers of cerebral small vessel disease, are presented in table 1. Overall, participants with amnestic and nonamnestic CAA did not significantly differ in age, level of education, and vascular risk profile. There was, however, an increased representation of females in the nonamnestic CAA group (χ2 = 5.39, p = 0.02). In addition to a reduced performance on the memory domain (F1,44 = 95.47, p < 0.001), amnestic CAA showed a reduced performance on the MMSE (F1,44 = 35.89, p < 0.001), as well as on the language/semantics domain (F1,44 = 13.23, p = 0.001) and executive function (F1,44 = 9.87, p = 0.003) domains. After adjustment for age and sex, participants with amnestic CAA had a lower normalized total hippocampal volume (t = 4.06, p < 0 .001) but did not significantly differ from participants with nonamnestic CAA on any other structural MRI markers (p > 0.05 for all).
Table 1.
Demographic, Clinical, and Radiologic Characteristics in Participants With Probable CAA-MCI With an Amnestic or Nonamnestic Presentation
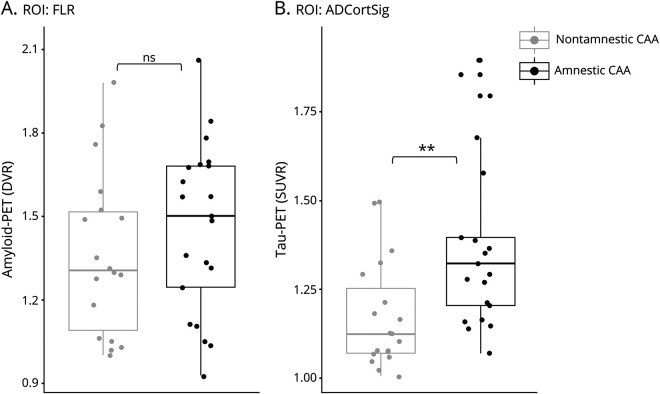
The results of analysis comparing participants with amnestic and nonamnestic CAA on amyloid- and tau-PET retention are summarized in figure 2. Amyloid-PET was available in 39 participants (18 with nonamnestic and 21 with amnestic CAA), and tau-PET was available in 40 (19 with nonamnestic and 21 with amnestic CAA). While amyloid-PET retention was globally elevated in participants with CAA, there was no difference in FLR DVR values between the amnestic and nonamnestic groups (t = −1.00, p = 0.323). Group differences in amyloid-PET FLR DVR remained nonsignificant after controlling for the presence of MRI markers of cerebral small vessel disease, age, and sex (t = −0.71, p = 0.485) or when using FLR DVR values corrected for partial volume effect (t = −1.14, p = 0.261). Tau-PET retention (ADCortSig SUVR) was increased in participants with amnestic compared to those with nonamnestic CAA (t = −3.13, p = 0.003). Group differences in tau-PET retention in the ADCortSig region remained significant after controlling for the presence of MRI markers of cerebral small vessel disease, age, and sex (t = −2.71, p = 0.01). In those with amnestic CAA, a significant increase in tau-PET retention was observed when ADCortSig SUVRs both corrected and not corrected for partial volume effect were used and across multiple other regions affected in Alzheimer disease36 (table 2).
Figure 2. Differences in Tau- and Amyloid-PET Retention Based on Amnestic Status in Individuals With CAA.
Boxplots representing the dispersion of (A) amyloid-PET retention expressed as distribution volume ratio (DVR) in a region of interest (ROI) including the frontal, lateral temporal/parietal, and retrosplenial cortex (FLR) and (B) tau-PET retention expressed as standardized uptake value ratio (SUVR) in an ROI regrouping Alzheimer disease cortical signature regions (ADCortSig). Participants with probable cerebral amyloid angiopathy (CAA) with a nonamnestic presentation are represented in gray; those with an amnestic presentation are represented in black. ns = not statistically significant, **p < 0.01.
Table 2.
Regional [18F]-Flortaucipir PET Retention in Individuals With CAA and Comparison of SUVRs Corrected or Not for Partial Volume Effect
In univariate models, lower memory performance in participants with CAA was associated with greater tau-PET ADCortSig SUVR (β = −0.77, corrected p = 0.006; figure 3A) and lower normalized hippocampal volume (β = 0.73, corrected p = 0.005; figure 3B) but not with any other quantified neuroimaging markers (table 3A). A GLM evaluating the combined influence of all quantified MRI and PET markers on memory performance was significant and explained an estimated 60% (p < 0.001) of the variance in scores on the memory composite (table 3B). Tau-PET ADCortSig SUVR (β = −0.85, p = 0.001) and normalized hippocampal volume (β = 0.64, p = 0.01) were the only variables significantly contributing to the model fit, while the influence of global cortical amyloid-PET retention and other quantified MRI markers was not significant. Evaluation of the relative explanatory importance of each regressor in the model (figure 3C) revealed that tau-PET retention accounted for nearly half of the variance in memory performance explained by the model (relative importance 47%), while hippocampal volume accounted for nearly a third of the variance (relative importance 32.7%). A separate multivariate model including only tau-PET retention and normalized hippocampal volume (table 3C) showed that these 2 markers accounted, by themselves, for an estimated 45% (p < 0.001) of the variance in memory performance.
Figure 3. Associations Between Imaging Markers and Memory in Individuals With Probable CAA.
Results of univariate generalized linear models between memory performance and (A) tau-PET retention expressed as standardized uptake value ratio (SUVR) in a region of interest regrouping Alzheimer disease cortical signature regions (ADCortSig) or (B) normalized total hippocampal volume (nHC). Dashed lines represent the 95% confidence intervals. (C) Bar graph representing the relative contribution of each evaluated regressor to the multivariate generalized linear model evaluating the contribution of neuroimaging markers on memory performance, using the LMG metric computed with the R package relaimpo (U. Grömping, 2006). Metrics are normalized to sum to 100%. Lines represent 95% confidence intervals after 1,000 bootstrapping replications. BPF = brain parenchymal fraction; CAA = cerebral amyloid angiopathy; CMB = cerebral microbleed; cSS = cortical superficial siderosis; DVR = distribution volume ratio; FLR = frontal, lateral temporal/parietal, and retrosplenial cortex; nWMH = normalized white matter hyperintensity volume. *p < 0.05; **p < 0.01.
Table 3.
Summary of Generalized Linear Models Predicting Memory Performance in Individuals With CAA
To complement our analyses, the effect of tau-PET status on the cognitive profile of individuals with CAA was evaluated (figure 4). Participants with CAA with a positive tau-PET (n = 21) had a lower performance on the memory domain (t = −3.72, corrected p = 0.004) compared to participants with a negative tau-PET (n = 19). Performance on other cognitive domains did not differ significantly between groups after correction for multiple comparisons.
Figure 4. Effect of Tau PET Status on the Cognitive Profile of Participants With Probable CAA.
Bar graph contrasting the cognitive profile of participants with cerebral amyloid angiopathy (CAA) presenting with a positive or negative tau-PET. Tau-PET status was determined from the [18F]-flortaucipir standardized uptake value ratio in a region of interest comprising previously described Alzheimer disease cortical signature regions and using a previously established cutoff score.35 The p values were adjusted for multiple comparisons as per the Bonferroni correction. *Corrected p < 0.05; **corrected p < 0.01.
Discussion
This comprehensive neuroimaging study explored the correlates of the amnestic presentation in participants fulfilling diagnostic criteria for MCI and probable CAA (CAA-MCI). In this cohort of patients with probable CAA, the amnestic status was associated with increased tau-PET retention, greater medial temporal neurodegeneration, and a globally more severe profile of cognitive impairment. Our results thus suggest that memory impairment in this population could serve as a marker for concomitant tau pathology and disease severity.
The central finding of this study is that patients with amnestic CAA show an increased tau-PET retention in regions susceptible to pathologic tau accumulation in Alzheimer disease (ADCortSig) and a reduced hippocampal volume compared to their nonamnestic counterparts. Patients with amnestic and nonamnestic CAA did not differ on any structural MRI markers of cerebral small vessel disease or on amyloid-PET retention. Tau accumulation and hippocampal atrophy are well-established markers of Alzheimer disease that have not been strongly associated with pure forms of CAA.38,39 Our findings thus suggest that amnestic patients with CAA present with a mixed neuropathologic profile, consistent with the concomitant presence of both CAA-mediated cerebrovascular alterations and tau pathology.
Participants with CAA in our sample presented elevated levels of amyloid binding on [11C]-Pittsburgh compound B amyloid-PET imaging, with a mean FLR DVR of 1.42 (SD 0.30).31 Relying on a previously established cutoff sensitive to early amyloid accumulation,31 we classified the vast majority of individuals with CAA in our sample as amyloid-PET positive. This is consistent with the pathologic nature of CAA, characterized by the cerebrovascular accumulation of amyloid. However, 3 individuals with CAA (2 nonamnestic and 1 amnestic) in our sample were classified as amyloid-PET negative on the basis of this cutoff. Cutoff scores to define amyloid-PET positivity are derived from varied clinical populations and methodologies, with significant discrepancy across laboratories.40 A previous meta-analysis examining the accuracy of amyloid-PET in the diagnosis of CAA suggests an overall pooled sensitivity of 79% and specificity of 78%.41 The significance of negative amyloid-PET in patients with CAA remains ambiguous and could reflect the limited sensitivity of these cutoff scores in defining pathologic levels of amyloid accumulation. To ensure that the presence of these amyloid-PET negative participants did not influence our results, we performed all analyses without these participants and found that our findings remained highly consistent (data not presented). In addition to elevated amyloid retention on PET imaging, nearly half of the studied individuals with CAA presented with elevated [18F]-flortaucipir tau-PET retention in regions susceptible to Alzheimer disease neurodegeneration (i.e., ADCortSig). This finding is in accord with the high comorbidity between CAA and Alzheimer pathology.2–4 Patients with amnestic CAA presented with higher tau-PET retention in ADCortSig than patients with nonamnestic CAA. The mean tau-PET retention in ADCortSig of patients with amnestic CAA (mean [SD] 1.37 [0.25]) was highly consistent with levels previously reported in amyloid-positive patients with Alzheimer disease (mean [SD] 1.3 [0.3]).35 In contrast, mean SUVR values in nonamnestic CAA (mean [SD] 1.17 [0.15]) were comparable to levels previously reported in amyloid-negative controls (mean [SD] 1.1 [0.1]).35 Our findings might thus indicate an increased prevalence of comorbid Alzheimer disease neuropathology in amnestic as opposed to nonamnestic patients with CAA.
Our results further demonstrate that greater tau-PET retention is significantly and independently related to lower memory performance in CAA, even after accounting for conventional MRI markers of cerebral small vessel disease and amyloid-PET retention. Tau-PET retention was the most important predictor contributing to the explained variance in memory performance in a GLM including all evaluated neuroimaging markers, with a relative contribution approaching 50%. Hippocampal volume was the only other quantified neuroimaging marker significantly contributing to the variance in memory performance. In contrast, amyloid-PET retention and structural MRI markers of cerebral small vessel disease did not significantly contribute to the variance in memory performance in these individuals with CAA. Our results align with those from a recent postmortem study showing significant associations between episodic memory and Braak stages of neurofibrillary pathology in the presence of CAA.42 Our findings also intersect with a recent study emphasizing the central role of tau-PET retention with regard to cognitive symptoms in patients with subcortical vascular cognitive impairment43 and indicate that memory impairment in CAA likely arises in parallel with tau accumulation and neurodegeneration.
In addition to memory impairment, individuals with amnestic CAA exhibited more severe global cognitive impairment (i.e., MMSE score) and more pronounced deficits across multiple cognitive domains, including language/semantics and executive function. The increased severity of cognitive impairments in those with amnestic CAA might be related to the underlying presence of mixed pathology (i.e., vascular and tau), as opposed to a predominantly vascular profile. This interpretation is consistent with findings from recent pathologic studies demonstrating greater cognitive impairment in aging individuals presenting both tau and vascular pathologies.42,44 Alternatively, the increased severity of cognitive deficits in persons with amnestic CAA could indicate that memory deficits arise at a more advanced stage of cognitive impairment in CAA.45 Longitudinal studies to track the progression of cognitive dysfunctions and neuropathologic processes in patients with CAA are needed to shed light on this question.
Finally, to further examine the relationship between memory and tau pathology in CAA, we contrasted the cognitive profile of participants with positive vs negative tau-PET, as determined with the use of a previously established cutoff value.35 The cognitive profile of patients with CAA with positive tau-PET was distinct from that of individuals with negative tau-PET and predominantly characterized by an increased severity of memory impairment.
While amyloid-PET has previously been used to characterize amyloid burden in patients with CAA,41 reports using tau-PET in this population are sparse. A recent study46 performed both amyloid and tau-PET in 3 individuals with probable CAA and found higher regional tau-PET retention in regions affected predominantly by cerebral microbleeds and cortical superficial siderosis. However, results presented in this study were limited by the small sample size and the lack of quantitative examination of associations between PET retention and clinical measures. The strength of the current study lies in the combined in vivo quantification of amyloid and tau pathology using PET in a larger sample of individuals with probable CAA, together with a characterization of cognitive correlates. Our initial results suggest that memory performance is related to tau pathology and medial temporal lobe neurodegeneration in patients with CAA. In the advent of tau therapeutic strategies, these findings could potentially inform clinical management and future clinical trials.
Nonetheless, our study has several limitations. First, the diagnosis of CAA in our sample, as well as the evaluation of amyloid and tau pathology, was obtained with neuroimaging and not confirmed via histopathology. This may lead to imprecision in the diagnosis of CAA. However, to favor diagnostic accuracy, we selected only individuals fulfilling the revised Boston Criteria for probable CAA, as opposed to individuals with possible CAA. Previous validation studies have demonstrated that this set of criteria show a high specificity for pathologic CAA.11,47 The absence of neuropathologic data also limits characterization of the exact nature of amyloid and tau pathology. Although PET scans are informative, they provide an indirect and imprecise measure of both forms of pathologies. For instance, the signal derived from [11C]-Pittsburgh compound B amyloid-PET does not allow parenchymal to be distinguished from vascular amyloid deposition.41 Regarding tau-PET, tracers for imaging tau pathology have been associated with off-target binding,48 leading to a lower specificity in the signal. Furthermore, while we classified participants into positive or negative tau- and amyloid-PET using previously published methods and cutoff values,31,35 there is currently little consensus in the field on the best approach to define abnormal levels of tau or amyloid pathology based on PET imaging.40,48 The selection of these cutoff scores remains arbitrary and potentially influenced our results. A second limitation associated with our study is the small sample size. This hindered the investigation of the full clinical spectrum of MCI, including the specific characterization of participants presenting single-domain amnestic or nonamnestic MCI. It also limited the evaluation of other factors potentially influencing the clinical and radiologic presentation of CAA such as the APOE genotype. Finally, the present study comprised patients with CAA from a memory clinic population with no history of intracerebral hemorrhage. It is possible that our results are influenced by our sample selection and would differ in patients with CAA with a history of intracerebral hemorrhage or other overt cerebrovascular diseases.
Our study provides initial and hypothesis-generating evidence for a link between memory impairment and tau pathology in CAA. Future research using different methodologies, including histopathologic data, is needed to replicate these initial results and to provide further evidence for the relevance of memory deficits as an indicator of concomitant tau pathology in patients with CAA. Studies using larger and more diverse samples would allow a deeper understanding of factors influencing the neuroimaging and cognitive presentation of CAA. Longitudinal studies are also necessary to shed light on whether memory impairment in CAA is linked to a distinct disease trajectory. Follow-up research building on the present findings is now warranted to further characterize the diagnostic value of the amnestic presentation, alone or in conjunction with other relevant biomarkers (e.g., CSF tau, plasma tau, APOE genotype), as a clinical marker of tau pathology in patients with CAA.
Our study demonstrates that the presence of memory impairment in patients with CAA is a promising marker of tau pathology and medial temporal lobe integrity. In the absence of advanced neuroimaging technologies, this accessible clinical marker could provide relevant information on the severity and nature of underlying pathologic mechanisms in this heterogeneous population.
Acknowledgment
The authors thank all the participants who contributed their time for participating to this research project. They also thank Vanessa A. Gonzalez for her efforts in coordinating the study and collecting the data. They thank the Biostatistical Consulting group of Harvard Catalyst for support with this work.
Glossary
- ADCortSig
Alzheimer disease cortical signature region
- CAA
cerebral amyloid angiopathy
- DVR
distribution volume ratio
- FLR
frontal, lateral temporal/parietal, and retrosplenial cortex
- GLM
generalized linear model
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- ROI
region of interest
- SUVR
standardized uptake value ratio
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This research was supported by the NIH (grants R01AG047975, R01NS104130, P50AG005134, and K23AG02872605). D.S. received postdoctoral fellowships from the Fonds de recherche du Québec–Santé (Canada, award 254389) and the American Heart Association (award 20POST35110047). This work was conducted with support from Harvard Catalyst|The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the NIH.
Disclosure
The author reports on disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011745 for full disclosures.
References
- 1.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Demen 2015;11:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Reuck J. The impact of cerebral amyloid angiopathy in various neurodegenerative dementia syndromes: a neuropathological study. Neurol Res Int 2019;2019:7247325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person‐specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018;83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thal DR, Griffin WST, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathologica 2008;115:599–609. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011;70:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003;60:729–736. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer J, Gosche K, Riley K, Markesbery W, Snowdon D. Delayed recall, hippocampal volume and Alzheimer neuropathology: findings from the Nun Study. Neurology 2004;62:428–432. [DOI] [PubMed] [Google Scholar]
- 8.Lekeu F, Magis D, Marique P, et al. The California Verbal Learning Test and other standard clinical neuropsychological tests to predict conversion from mild memory impairment to dementia. J Clin Exp Neuropsychol 2010;32:164–173. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 10.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke 2018;49:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychologist 1991;5:125–142. [Google Scholar]
- 14.Buschke H. Cued recall in amnesia. J Clin Exp Neuropsychol 1984;6:433–440. [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–214. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corp; 1997. [Google Scholar]
- 17.Fastenau PS, Denburg NL, Mauer BA. Parallel short forms for the Boston Naming Test: psychometric properties and norms for older adults. J Clin Exp Neuropsychol 1998;20:828–834. [DOI] [PubMed] [Google Scholar]
- 18.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177. [PubMed] [Google Scholar]
- 19.Benton AL, Abigail B, Sivan AB, Hamsher Kd, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford: Oxford University Press; 1994. [Google Scholar]
- 20.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138:2126–2139. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 24.Becker JA, Hedden T, Carmasin J, et al. Amyloid‐β associated cortical thinning in clinically normal elderly. Ann Neurol 2011;69:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 27.Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time: activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990;10:740–747. [DOI] [PubMed] [Google Scholar]
- 29.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol Official J Am Neurol Assoc Child Neurol Soc 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 30.Mintun M, Larossa G, Sheline Y, et al. [11C] PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 31.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain 2015;138:2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greve DN, Salat DH, Bowen SL, et al. Different partial volume correction methods lead to different conclusions: an 18F-FDG-PET study of aging. Neuroimage 2016;132:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39:904–911. [PubMed] [Google Scholar]
- 34.Wang L, Benzinger TL, Hassenstab J, et al. Spatially distinct atrophy is linked to β-amyloid and tau in preclinical Alzheimer disease. Neurology 2015;84:1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Benzinger TL, Su Y, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol 2016;73:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho H, Choi JY, Hwang MS, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 2016;87:375–383. [DOI] [PubMed] [Google Scholar]
- 37.Grömping U. Relative importance for linear regression in R: the package relaimpo. J Stat Softw 2006;17:1–27. [Google Scholar]
- 38.Fotiadis P, van Rooden S, van der Grond J, et al. Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol 2016;15:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natté R, Maat‐Schieman ML, Haan J, Bornebroek M, Roos RA, Van Duinen SG. Dementia in hereditary cerebral hemorrhage with amyloidosis‐Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol 2001;50:765–772. [DOI] [PubMed] [Google Scholar]
- 40.Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Demen 2015;11:1–15.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charidimou A, Farid K, Baron J-C. Amyloid-PET in sporadic cerebral amyloid angiopathy: a diagnostic accuracy meta-analysis. Neurology 2017;89:1490–1498. [DOI] [PubMed] [Google Scholar]
- 42.Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ. Braak stage, cerebral amyloid angiopathy, and cognitive decline in early Alzheimer's disease. J Alzheimer's Dis 2020;74:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Park S, Cho H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol 2018;75:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wennberg AM, Whitwell JL, Tosakulwong N, et al. The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging 2019;77:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You Y, Perkins A, Cisternas P, et al. Tau as a mediator of neurotoxicity associated to cerebral amyloid angiopathy. Acta Neuropathol Commun 2019;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HJ, Cho H, Werring DJ, et al. 18F-AV-1451 PET imaging in three patients with probable cerebral amyloid angiopathy. J Alzheimers Dis 2017;57:711–716. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Ramirez S, Romero J-R, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Demen 2015;11:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leuzy A, Chiotis K, Lemoine L, et al. Tau PET imaging in neurodegenerative tauopathies: still a challenge. Mol Psychiatry 2019;24:1112–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D. A coefficient of determination for generalized linear models. Am Statistician 2017;71:310–316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available to other researchers on request and with adequate justification.